SYNOPSIS
Very preterm infants are commonly exposed to a chronic, often asymptomatic chorioamnionitis that is diagnosed only after delivery by histologic evaluation of the placenta. The reported effects of these exposures on fetal lungs are inconsistent because exposure to different organisms, durations of exposure, and fetal/maternal responses impact outcomes. In experimental models, chorioamnionitis can both injure and mature the fetal lung and cause immune nodulation. Postnatal care strategies also change how chorioamnionitis relates to clinical outcomes such as BPD.
Keywords: Respiratory Distress Syndrome, Bronchopulmonary Dysplasia, Lung Development, Lung Maturation, Lung Injury
CHORIOAMNIONITIS – A MULTIFACETED FETAL EXPOSURE
Overview
There is no consensus about the relationships between chorioamnionitis and three pulmonary outcomes of concern for preterm infants – respiratory distress syndrome (RDS), pneumonia/sepsis, and bronchopulmonary dysplasia (BPD). The difficulty in defining clear relationships results from the multiple variables contributing to the antenatal exposures, the postnatal exposures and care strategies that contribute to the diagnoses of the short-term outcomes of RDS and pneumonia/sepsis, and the longer term outcome of BPD. Multivariant analyses of large data sets are imperfect tools to define relationships because of the inter-related nature of the variables, the poorly defined fetal exposures and the imprecision of diagnosis of diseases such as RDS and BPD. This review will attempt to capture a sense of the disarray in the field based on the clinical data. In contrast, research with animal models provides solid information about how experimental chorioamnionitis can impact the fetal lung. The combination of an appreciation of the clinical complexity and the experimental effects of chorioamnionitis provides insight into how individual preterm infants present and respond to therapy. The focus of this review will be the lungs of preterm infants born at less than 32 weeks gestation, during the period of saccular expansion and prior to alveolarization.
Diagnosis
Chorioamnionitis can be diagnosed before the infant is born by findings such as maternal fever, elevated white blood cell count, tender uterus, and by amniotic fluid analyses for bacteria, inflammatory mediators, or inflammatory cells. Histologic chorioamnionitis is a post-delivery diagnosis that is graded by the amount of inflammatory cells and the amount of necrosis in the chorioamnion (1). The diagnosis of clinical chorioamnionitis does not reliably predict the presence or severity of histologic chorioamnionitis, which is far more common following very preterm birth. Furthermore, the diagnosis of histologic chorioamnionitis is not reproducible between pathologists (1).
The diagnosis of chorioamnionitis correlates with the clinical presentation of a pregnancy at risk for very preterm delivery. Preterm premature rupture of membranes is a surrogate for chorioamnionitis with a high concordance. Preterm labor of unknown cause or with a short cervix is frequently associated with chorioamnionitis (2). In various populations, 50–70% of women delivering very preterm infants will have chorioamnionitis with the incidence increasing as gestation at delivery decreases (2, 3).
The Organisms
The organisms associated with early gestational delivery can be single species or polymicrobial aerobic and anabolic isolates that generally are vaginal flora (Table 1) (4). More than 50% of amniotic fluids collected by amniocentesis or at Cesarean delivery from women with preterm premature rupture of membranes were positive for Ureaplasma (5). These organisms are not usually considered to be pathogens. The generally accepted pathway to the subclinical histologic chorioamnionitis associated with very preterm birth is a diffuse ascending colonization of the endometrial-chorionic space with extension into the fetal membranes, the amniotic fluid, and ultimately the fetus (6). Recent pathological analyses suggest another route may be more common. A localized epithelial colonization of the endometrium may breach the chorioamnion locally, contaminating the amniotic fluid with subsequent extension to the fetal membranes and fetus (7). The identification of organisms associated with chorioamnionitis by culture does not capture the entire population of organisms as more non-culturable organisms can be identified by PCR (8).
Table 1.
Organisms Cultured from Chorion in Association with Preterm Deliveries*
| Organism Type | |||
|---|---|---|---|
| Ureaplasma/Mycoplasma | Aerobes | Anaerobes | % of Culture Positive Placentas* |
|
| |||
| + | − | − | 9 |
| − | + | − | 30 |
| − | − | + | 21 |
| + | + | − | 3 |
| + | − | + | 4 |
| − | + | + | 28 |
| + | + | + | 6 |
51% of 1365 placentas were culture positive
Data from Onderdonk, et al., (4)
Duration of Fetal Exposure
Ureaplasma parvum and Mycoplasma hominis are the organisms most frequently associated with very early gestation deliveries (4, 5) (Box 1). These organisms are normal vaginal flora in 67% of women of reproductive age (9). However, Ureaplasma also were identified in about 12% of 433 amniotic fluids collected for genetic analysis from normal pregnancies prior to 20 weeks gestation (10, 11). Only 7% of the Ureaplasma positive amniotic fluids were associated with preterm delivery. This behavior of Ureaplasma in human pregnancy is similar to experimental infection in fetal sheep. Fetal sheep exposed to intra-amniotic Ureaplasma at 50d gestation have high titers of organisms continuously for 100d to term with a 20% fetal loss (12). Some animals have chorioamnionitis and others do not despite persistence of the organism. There is no information about the potential for the multiple other organisms associated with prematurity to cause prolonged fetal exposures. Presumably, women with preterm labors that progress to ruptured membranes and then delayed delivery carry fetuses with bacterial exposures for days to months.
Box 1. Variables Contributing to Chorioamnionitis.
Organisms – single microbes and polymicrobial
Duration of exposure in utero
Intensity of maternal and fetal inflammatory responses
Rupture of membranes
Therapies that modulate infection – antibiotics and antenatal corticosteroids
The Fetal Exposure
It makes intuitive sense that the fetal response to chorioamnionitis will depend on the organisms and the duration of the exposure. However, for early gestation deliveries, there is almost no clinical information correlating organism or duration of exposure to fetal response. The exposure is complex as both the bacteria and products of the inflammation bathe the chorioamnion (fetal tissue) and the fetal skin. The fetal gut is exposed from swallowed amniotic fluid (6). Amniotic fluid also mixes with fetal lung fluid by fetal breathing causing a lung exposure (13). Thus, the fetal lung is just one organ that can respond to chorioamnionitis. Funisitis is an infiltration of fetal inflammatory cells around the vessels of the cord, indicating a systemic fetal response and perhaps a longer duration of fetal exposure or more pathogenic organisms (14).
RESPONSES OF THE FETAL LUNG TO CHORIOAMNIONITIS – CLINICAL
An Overview
The instinctive response of the clinician to a diffuse lung exposure to bacteria and inflammatory products is to assume the outcome will be an acute pneumonia. In the preterm, pneumonia caused by pathogens often is accompanied by sepsis because of immature innate immune defenses. However, for the 9595 infants with birth gestations of 22–28 weeks cared for in the NICHD Neonatal Research Network between 2003 and 2007, only 2% had early onset sepsis diagnosed by positive blood culture (15). The diagnosis of pneumonia was so infrequent that it was not reported. Nevertheless, 25% of the population had rupture of membranes >24 hr before delivery and 48% had a pathological diagnosis of chorioamnionitis. Although many of these infants must have had a fetal pulmonary exposure, no clinical syndrome of lung infection was recognized. However, tracheal aspirates from infants exposed to chorioamnionitis and collected at intubation shortly after birth contain elevated pro-inflammatory cells, cytokines, and prostaglandis as indicators of a fetal lung response (16, 17). Ureaplasma of antenatal origin can be cultured or identified by PCR from 20–45% of these preterm infants (18, 19). Thus, these very preterm infants have frequent but generally “silent” lung exposures to infection/inflammation.
Chorioamnionitis and RDS
A decreased incidence of RDS was associated in 1974 with preterm prolonged rupture of the membranes, a surrogate marker for chorioamnionitis (20). Watterberg and associates (16) then reported that ventilated preterm infants exposed to histologic chorioamnionitis had a lower incidence of RDS, but a higher incidence of BPD than infants not exposed to chorioamnionitis. The exposure to histologic chorioamnionitis decreased the incidence of RDS for a consecutive series of 446 preterm births of less than 32 weeks gestational age, but histologic chorioamnionitis with isolation of Ureaplasma or Mycoplasma from cord blood did not correlate with a decreased risk of RDS (14, 21). Lahra and coworkers (22) also noted in a population of 724 preterm infants that RDS was decreased for infants exposed to histologic chorioamnionitis (odds ratio [OR] 0.49, 95% confidence interval [95% CI] 0.31–0.78) or chorioamnionitis plus funisitis (OR 0.23, 95% CI 0.15–0.35) relative to no chorioamnionitis. This group also reported their 13-year experience that histologic chorioamnionitis (with or without funisitis) was associated with a decreased risk of BPD (OR 0.58, 95% CI 0.51–0.67) (3).
In contrast, there are other reports associating chorioamnionitis with poor pulmonary and other outcomes. Hitti and associates (23) reported that high levels of TNFα in amniotic fluid predicted prolonged postnatal ventilation, suggesting early and persistent lung injury from chorioamnionitis. Ramsey and colleagues (24) also demonstrated that chorioamnionitis increased neonatal morbidities. Laughon and coworkers (25) cultured 1340 placentas of infants born before 28 weeks of gestation and found no association between histologic chorioamnionitis, funisitis, or specific organisms and the initial oxygen requirements of the infants. They did not report the diagnosis of RDS specifically. The Canadian Neonatal Network also reported that clinical chorioamnionitis was not predictive of RDS (26).
These discrepant RDS outcomes for very preterm infants exposed to chorioamnionitis need to be understood within the context of the following complexities:
The diagnosis of chorioamnionitis was imprecise and did not include information about the duration, intensity, or organisms contributing to the exposure.
No attempt was made to make a specific diagnosis of surfactant deficiency as a cause of the respiratory distress.
The diagnosis of RDS was imprecise in very preterm infants as most of the infants will have some respiratory adaptation problems soon after birth.
The severity of the respiratory distress was not calibrated, and there are important differences between severe and mild RDS.
Surfactant treatment and mechanical ventilation at birth will interfere with assigning a diagnosis of RDS.
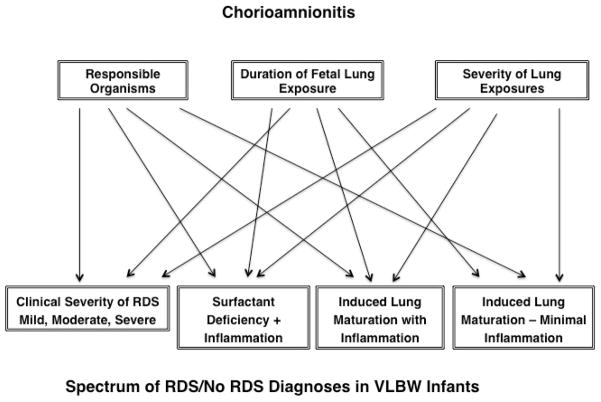
A clinical indicator for how chorioamnionitis may confound outcomes is found in the report by Been, et al. (27) in which infants with RDS and exposed to chorioamnionitis have good responses to surfactant treatment while infants exposed to chorioamnionitis and with funisitis have poor responses to surfactant. The major confounder for a comparison of the incidence of an outcome such as RDS between groups of VLBW infants is the comparison group. As all VLBW deliveries are associated with abnormalities, there is no “normal” comparison group. The sketch in Fig. 1 illustrates the problem of correlation of chorioamnionitis with an outcome such as RDS. Chorioamnionitis can be associated with very severe “RDS” – likely the combination of surfactant deficiency and a diffuse pneumonia/inflammation with a non-culturable organism. Chorioamnionitis also can result in an infant without RDS where the signature of the chorioamnionitis could be detected only by analysis of tracheal aspirate collected shortly after birth.
Figure 1.
Pathways from several of the variables contributing to chorioamnionitis and the clinical severity of RDS, lung maturation and inflammation.
Chorioamnionitis and BPD
The relationship between chorioamnionitis and BPD is as clouded as is the relationship of chorioamnionitis with RDS, and for similar reasons. Following the seminal paper by Watterberg, et al. (16) in 1996 that associated chorioamnionitis with a decrease in RDS and an increase in BPD in ventilated infants, multiple reports showed that chorioamnionitis increased the risk of BPD. Schelonka, et al. (19) reported a meta-analysis in 2005 that Ureaplasma in association with chorioamnionitis increased the risk of BPD in infants (OR 1.6, 95% CI 1.1–2.3), but the effect was greater in smaller than larger studies. A recent systemic review of 59 studies including over 15,000 infants concluded that BPD was increased by chorioamnionitis (OR 1.89, 95% CI 1.56–2.3) (28). However, this estimate decreased to an OR 1.58, 95% CI 1.11–2.24 with adjustments for birth weight differences. The relationship was significant only for histologic chorioamnionitis.
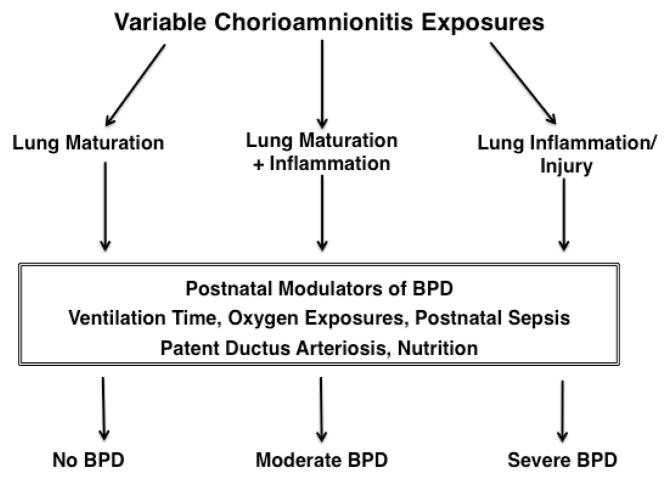
In contrast, Laughon et al. (25) found no relationship between BPD and histologic chorioamnionitis for 1340 infants born before 28 weeks gestational age. A report from the Canadian Neonatal Network also found no association between clinical chorioamnionitis and BPD (26). BPD is caused primarily by mechanical ventilation and oxygen exposure with potent modulators being other occurrences during postnatal care such as a patent ductus arteriosus and postnatal sepsis (29, 30). Three reports highlight the complexities of these associations. Van Marter, et al. (31) reported that chorioamnionitis decreased BPD (OR 0.2) in ventilated preterm infants unless they were ventilated for more than 7 days or had postnatal sepsis, which increased the risk of BPD (odds ratios of about 3.0). Lahra, et al. (3) also found that BPD was decreased in a population of 761 infants by histologic chorioamnionitis, but chorioamnionitis plus postnatal sepsis increased the risk. The characteristic of the chorioamnionitis also altered the association. For example, in a cohort of 446 singleton deliveries there was no increase in BPD with histologic chorioamnionitis, but a cord blood culture positive for Ureaplasma or Mycoplasma increased the BPD rate almost 3-fold in the same cohort (14, 21). BPD is a complicated lung development/injury/repair syndrome with multiple postnatal factors contributing to its occurrence and progression. Some chorioamnionitis exposures may protect the infant from BPD by decreasing the severity of RDS (lung maturation) while other types of exposures may promote BPD by initiating a progressive inflammatory response. Some of these possibilities are illustrated in Fig. 2.
Figure 2.
Flow diagram for the relationships between a chorioamnionitis exposure, postnatal variables that modulate the risk of BPD and the BPD outcome.
PROOF OF PRINCIPAL: LUNG EFFECTS OF CHORIOAMNIONITIS IN ANIMAL MODELS
Chorioamnionitis and Lung Inflammation
Intra-amniotic injections of pro-inflammatory mediators such as E. coli lipopolysaccharide (LPS/endotoxin), IL-1 or single live organisms such as Ureaplasma are used to create chorioamnionitis – generally defined as inflammation of the fetal membranes (12, 32). Intra-amniotic injections of LPS or pro-inflammatory mediators cause an acute lung inflammation in fetal sheep and mouse lungs (33, 34). This lung inflammation is characterized by:
A mediator dose-dependent recruitment of primarily neutrophils to the lung parenchyma that can be detected within hours of the intra-amniotic exposure (35).
A large increase in expression of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and MCP-1 (33, 34).
The mediator must have direct contact with the fetal lung – demonstrated by surgical isolation of the lung from the amniotic fluid (13).
A sequence of early injury that includes apoptosis, proliferation, and microvascular injury (36, 37).
The inflammatory response is signaled via NFkB activation in lung monocytes in fetal mouse lungs (38).
In the fetal sheep, the inflammation from intra-amniotic LPS does not cause a severe pneumonia or consolidation, even with repetitive or direct applications of LPS via the fetal trachea (13, 39). The initial inflammatory response to intra-amniotic injection of live Ureaplasma induces less neutrophil recruitment and cytokine expression than does LPS in the fetal sheep lung (40). Inflammation is substantial following Ureaplasma exposure of fetal mouse and monkey lungs based on the limited information available (41, 42). Ureaplasma are readily recoverable from the fetal lungs and fetal lung fluid (43). Although chorioamnionitis causes lung inflammation in experimental models, that inflammation does not progress to severe pneumonia, consistent with the infrequent diagnosis of pneumonia in VLBW infants shortly after birth (15). The fetus has a well-developed ability to suppress and modulate inflammation.
Consequences of Lung Inflammation
Induced Lung Maturation
Bry, et al. (44, 45) demonstrated that intra-amniotic injection of IL-1 or LPS induced early lung maturation in rabbits as indicated by increased mRNA for the surfactant proteins and increases in pressure-volume curves. Intra-amniotic LPS increases the numbers of type II cells and the expression of mRNA for surfactant proteins via NFkB dependent pathways in fetal mouse lungs (46). In fetal sheep, the lung maturation response is characterized by:
An increase in the mRNA for the surfactant proteins within 24 hours of the LPS or IL-1 exposure (47).
An increase in surfactant lipids in the airspaces and improved pressure-volume curves after 4–5 days, with maximal responses at 7–15 days (48).
A maturational response that requires inflammation and contact of the pro-inflammatory mediator with the fetal airways (13, 49).
Inflammation can initiate lung maturation as early as 60 days gestation (term is 150 days) (50).
Lung maturation without an increase in fetal plasma cortisol (48).
Maturation of immature monocytes to alveolar macrophages in the fetal lung via induction of granulocyte-macrophage colony stimulating factor and the transcription factor PU.1 (51).
These remarkable effects of exposure of the fetal lungs to chorioamnionitis and caused by mediators such as LPS or IL-1 can change postnatal lung function with resultant severe RDS to no RDS (48). Ureaplasma also induces surfactant protein mRNA and more modest and less consistent increases in pressure-volume curves in sheep without maturing monocytes to alveolar macrophages (40, 52). Thus, the lung exposures to clinical chorioamnionitis may result in variable effects on lung maturation, depending on the organism and the duration of the exposure.
Lung Injury from Chorioamnionitis
The flip side of the potential benefits of induced lung maturation is injury to the developing lung from inflammation. The diffuse, but relatively modest inflammation followed by the injury responses of apoptosis and proliferation indicates a prototypic injury response (36). Fetal sheep exposed to intra-amniotic LPS have persistently activated leucocytes in the airways, recruitment of CD3 positive lymphocytes to lung tissue, increased expression of toll-like receptors 2 and 4, decreased caveolin-1 expression, and changes in multiple other signaling pathways (53–55). The net effects in the intact fetal sheep are changes in lung collagen and elastin with a transient inhibition of alveolar septation and an increase in vascular wall thickness (56, 57). In mechanistic in vitro studies using explants from mouse lungs, LPS activates NFκB, which interferes with FGF-10 and integrin expression resulting in decreased airspace branching (58). Macrophages resident in the fetal lung tissue mediate the activation of NFκB signaling that inhibits multiple genes critical to lung development with thickening of the lung interstitium and decreased airway branching (38). Although LPS exposure disrupts alveolar septation and vascular development, the fetal sheep lung can continue to develop and “grow through” continued LPS exposure (39).
Short interval Ureaplasma exposures cause acute inflammation in Rhesus monkey lungs (42) and 2 to 3 day fetal exposures caused chorioamnionitis and lung colonization in preterm baboons (59). Following delivery and ventilation for 14 days, some animals cleared the Ureaplasma from the airways with improving lung function, while others had persistent colonization and worse lung function. At 14 days, the Ureaplasma infected animals had greater pro-fibrotic responses and fibrosis (60). In a mouse model, intra-amniotic Ureaplasma increased the postnatal oxygen induced lung injury (41). Prolonged exposure of fetal sheep to Ureaplasma had no demonstrable effect on a 3 hour ventilation mediated lung injury (61).
These experiences demonstrate inconsistent interactive effects between chorioamnionitis-induced lung injury and postnatal lung function. A reasonable interpretation is that fetal lung inflammation caused by chorioamnionitis can promote progression toward BPD by initiating alveolar simplification and by interfering with multiple signaling pathways involved in lung development. But, the effects will depend on the bacterial agent and the other characteristics of the exposure, which are not known in clinical practice.
CHORIOAMNIONITIS AND IMMUNE MODULATION IN THE FETAL LUNG
An Overview
Studies of immune modulation in the fetal lung have been limited to the fetal sheep to date. The advantages of this model is that the fetuses can be repetitively exposed over months and subsequently allowed to deliver for the evaluation of total effects later in life (62). The fetus is generally considered to have depressed immune/inflammatory responses because of incomplete development of immune defense systems and lack of immune challenges such that response systems are naïve. Within the context of the infectious causes of the chronic and indolent chorioamnionitis frequently associated with very preterm delivery, the fetus can respond to pro-inflammatory mediators such as LPS (a TLR4 agonist), IL-1, and live Ureaplasma (Box 2). The lung response is a low-grade inflammation (recruitment of granulocytes, cytokine expression) followed by a resolution of the acute inflammation with minimal effects on lung development other than induced lung maturation. However, this lung response cannot be understood in isolation from other fetal responses to chorioamnionitis that are being described in animal models. The fetal gut has injury, developmental, and immunomodulatory responses to intra-amniotic LPS or IL-1 (63). The fetal skin has a diffuse inflammatory response with increased cytokine expression on exposure to intra-amniotic LPS (64). Surprisingly, the systemic effects are modest – the acute phase reactant serum amyloid A increases in the liver and there are small changes in white blood cell counts and platelets, but no significant increase in plasma cortisol in fetal sheep (48, 65). Fetal humans exposed to chorioamnionitis do have increased cortisol levels in cord blood, perhaps because of stress related to preterm labor (66). Changes in immune status of the fetal lung probably reflect lung specific effects from direct contact with the organisms and inflammatory products in the amniotic fluid and systemic responses to skin and gut exposures.
Box 2. Immune Modulation by Chorioamnionitis in the Fetal Sheep Lung.
primary response to LPS that is low grade
Maturation of monocytes to alveolar macrophages
No persistent or secondary inflammatory responses to continuous or repeated LPS (endotoxin tolerance)
A “cross tolerance” for a mediator different from the initial mediator
Increases in innate host defense proteins – SP-A, SP-D
The fetal sheep lung contains very few monocytic linage cells or mature alveolar macrophages (67), which is in contrast to the fetal mouse lung that contains more mature monocytes (38). The human fetal lung is thought to be like the sheep lung with the recruitment and maturation of monocytes to alveolar macrophages after delivery. Intra-amniotic LPS induces GM-CSF mRNA in the fetal lung and increased GM-CSF protein, which is a known inducer of the transcription factor PU.1 and monocyte to macrophage maturation (51). Mature appearing alveolar macrophages are induced in the fetal lung at 80% gestation in the fetal sheep. Fetal exposure to live Ureaplasma does not mature monocytes to macrophages (40).
The monocytes/macrophages recovered from fetal lung tissue of control animals have minimal oxidant or IL-6 secretory responses to challenge with LPS in vitro (68). Seven days after intra-amniotic LPS, the cells produce oxidants and IL-6 comparably to alveolar macrophages from adult sheep. These cells that were minimally responsive to TLR4 and other TLR agonists become responsive to in vitro challenge to multiple TLR agonists after intra-amniotic LPS, a phenomenon referred to as cross tolerance (69). Fetal lung monocytes increased their potential to respond to infectious challenges following exposure to LPS/chorioamnionitis.
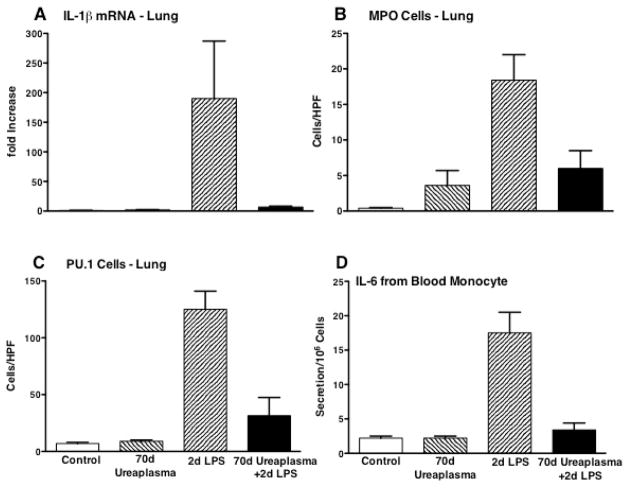
In vivo, a second fetal exposure to intra-amniotic LPS or IL-1 will not increase inflammatory cells or cytokine expression in the fetal lung – an endotoxin tolerance type response (70). Monocytes/macrophages recovered after a second intra-amniotic LPS exposure also have severely blunted responses to in vitro exposure to multiple TLR agonists. A striking example is the effect of chronic fetal Ureaplasma colonization on the lung responses to intra-amniotic LPS. Animals exposed to live Ureaplasma 70 days before preterm delivery have high titers of Ureaplasma in their amniotic fluid and lung, but with a minimal inflammatory response as indicated by cytokine expression or myeloperoxide positive cells relative to controls (71) (Fig. 3). Intra-amniotic LPS given 2 days before delivery induced a large inflammatory response in the lung. The maturational indicator for monocytes – PU.1 increased as did IL-6 secretion by blood monocytes in vitro. However, the intra-amniotic LPS had no effect on the lungs chronically colonized with Ureaplasma. Chorioamnionitis also increases other components of the very complex innate immune system. Two examples are the large increases in surfactant-A and surfactant-D proteins by intra-amniotic LPS (54).
Figure 3.
Intra-amniotic Ureaplasma given 70d prior to a 2nd intra-amniotic exposure to E. coli lipopolysaccharide (LPS) suppressed the LPS responses in lungs of preterm fetal sheep. The chronic fetal exposure to Ureaplasma (A) suppressed IL-1β mRNA in the fetal lung, (B) decreased recruitment of myeloperoxidase positive cells (MPO) to the lung, (C) prevented the expression of the macrophage maturation transcription factor PU.1 in lung, and (D) decreased the release of IL-6 from blood monocytes challenged in culture with LPS. Data from Kallapur, et al..(71)
A Perspective on Immune Modulation
The fetus is capable of complex immune modulation despite the immaturity of the immune system. The fetal lung is a primary organ receiving the signals that result in immune/inflammatory responses in that it is in direct contact with the chorioamnionitis via the amniotic fluid. The lung is also an “end organ” for both beneficial and adverse immune/inflammatory responses as it is the target of ventilation and oxygen mediated injuries following delivery. The importance of specific fetal immune modulations from chorioamnionitis on lung outcomes such as BPD remain speculative.
CHORIOAMNIONITIS AND ANTENATAL CORTICOSTEROIDS
Antenatal corticosteroids are given to more than 80% of the women at risk of preterm delivery before 30 weeks gestation and the majority of these women will have undiagnosed (histologic) chorioamnionitis (25, 72). The current recommendation is to give antenatal corticosteroids with preterm labor or preterm rupture of membranes because the treatment decreases the incidence of RDS, intraventricular hemorrhage, and death (73). In clinical series, antenatal corticosteroids are of benefit for preterm deliveries that in retrospect had associated histologic chorioamnionitis (74). A recent analysis of observational studies identified the benefit of corticosteroid treatment for women with chorioamnionitis (75). Antenatal corticosteroids also decrease the fetal inflammatory response syndrome in preterm infants exposed to histologic chorioamnionitis (74).
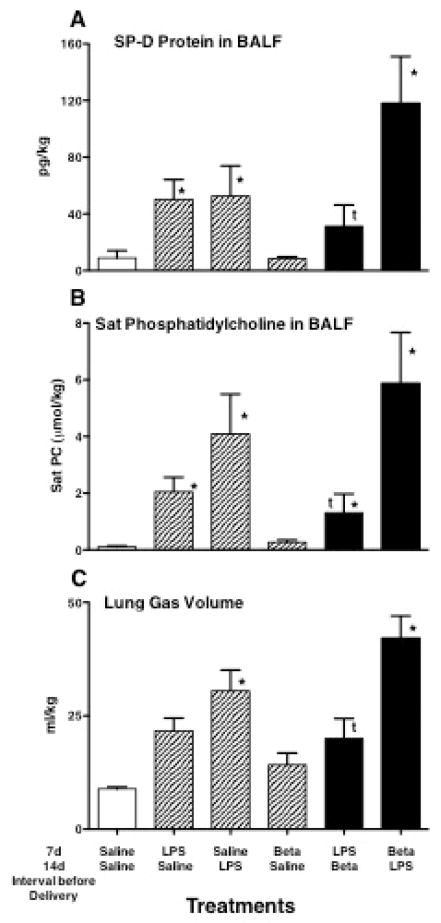
Although there is no clinical information about how corticosteroids might influence chorioamnionitis, the corticosteroids could suppress inflammation – a potential benefit, or increase the risk of progressive infection – a potential risk. Maternal treatment with betamethasone initially suppressed the inflammation caused by intra-amniotic endotoxin in the chorioamnion and lungs of fetal sheep (76, 77). Inflammatory cells and pro-inflammatory cytokine expression were suppressed for about 2 days after the betamethasone treatment, but subsequently inflammation was increased in the lungs of lambs exposed to both maternal betamethasone and intra-amniotic endotoxin relative to endotoxin alone 5 and 15 days after the exposures (77). Lung maturation was greater in lambs exposed to both betamethasone and endotoxin given together than to either treatment alone (78). The more likely clinical scenarios are corticosteroid treatments of women with chronic, indolent chorioamnionitis or women who develop chorioamnionitis after corticosteroid treatments. Fetal sheep exposed to maternal betamethasone and/or intra-amniotic LPS 7 and 14 days before preterm delivery have quite different indicators of lung maturation depending on the order of the exposures (Fig. 4) (54). The innate host defense protein SP-D was increased by the LPS, but not betamethasone. The combination LPS given 7 days before betamethasone caused the largest increase in SP-D. Similarly, the largest increases in saturated phosphatidylcholine, the major surfactant lipid, occurred if the LPS exposure preceded the maternal betamethasone exposure. This combination also had the greatest effect on the maximal lung gas volume. These results with fetal sheep support the clinical observations that betamethasone can further decrease RDS in the presence of histologic chorioamnionitis (74). In fetal sheep the lung maturational response to endotoxin was larger and more uniform than was the response to betamethasone. Betamethasone also augmented the lung maturation induced by chronic fetal Ureaplasma colonization (79).
Figure 4.
Indicators of lung maturation following intra-amniotic E. coli lipopolysaccharide (LPS) and/or maternal betamethasone (Beta) given 7 or 14d prior to preterm delivery at 120d gestational age. (A,B) The indicators surfactant protein-D (SP-D), and saturated (Sat) phosphatidylcholine in bronchoalveolar lavage fluid (BALF) increased with LPS exposure, but increased more when the order of exposure was LPS given 14d and Beta given 7d before delivery. (C) The lung gas volumes measured with air inflation to 40 cm H2O pressure reflected the amounts of saturated phosphatidylcholine. Data from Kuypers, et al. (54). *p<0.05 relative to control tP<0.05 LPS/Beta relative to Beta/LPS
The increased inflammation in the fetal sheep lungs that occurred 5 to 15 days after simultaneous betamethasone and endotoxin exposures is a potential concern. Such effects have not been apparent clinically, but they have not been carefully evaluated. A potential mechanism to explain the increased inflammation is that both betamethasone and the endotoxin “mature” an immature inflammatory system. Blood monocytes from fetal sheep have decreased responses in vitro to endotoxin stimulation relative to monocytes from adult sheep (80). However, 7 days after the fetal exposures, the monocytes respond to endotoxin in vitro similarly to monocytes from adult sheep. Maternal betamethasone initially suppresses the fetal monocyte function, but function is increased 7 days after the maternal treatment (81). These results illustrate just how complex interactions between exposures may be clinically. Repetitive courses of betamethasone treatments may be a concern particularly when chorioamnionitis is present. The clinical dilemma is that histologic chorioamnionitis is a retrospective diagnosis of a clinically silent process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123:1314–1319. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 4.Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198:110, e111–117. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 5.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, Yoon BH. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203:211, e211–218. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Laboratory investigation; a journal of technical methods and pathology. 2009;89:924–936. doi: 10.1038/labinvest.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS ONE. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viscardi RM. Ureaplasma species: role in diseases of prematurity. Clinics in perinatology. 2010;37:393–409. doi: 10.1016/j.clp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 11.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 12.Dando SJ, Nitsos I, Kallapur SG, Newnham JP, Polglase GR, Pillow JJ, Jobe AH, Timms P, Knox CL. The Role of the Multiple Banded Antigen of Ureaplasma parvum in Intra-Amniotic Infection: Major Virulence Factor or Decoy? PloS one. 2012;7:e29856. doi: 10.1371/journal.pone.0029856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss TJ, Nitsos I, Kramer BW, Ikegami M, Newnham J, Jobe A. Intra-amniotic endotoxin induces lung maturation by direct effects on the developing respiratory tract in preterm sheep. American Journal of Obstetrics and Gynecology. 2002;187:1059–1065. doi: 10.1067/mob.2002.126296. [DOI] [PubMed] [Google Scholar]
- 14.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195:803–808. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 15.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, 3rd, Watterberg KL, Saha S, Das A, Higgins RD. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 17.De Dooy J, Colpaert C, Schuerwegh A, Bridts C, Van Der Planken M, Ieven M, De Clerck L, Stevens W, Mahieu L. Relationship between histologic chorioamnionitis and early inflammatory variables in blood, tracheal aspirates, and endotracheal colonization in preterm infants. Pediatr Res. 2003;54:113–119. doi: 10.1203/01.PDR.0000069702.25801.D1. [DOI] [PubMed] [Google Scholar]
- 18.Kasper DC, Mechtler TP, Bohm J, Petricevic L, Gleiss A, Spergser J, Witt A, Herkner KR, Berger A. In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. Journal of perinatal medicine. 2011;39:331–336. doi: 10.1515/jpm.2011.022. [DOI] [PubMed] [Google Scholar]
- 19.Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24:1033–1039. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]
- 20.Richardson CJ, Pomerance JJ, Cunningham MD, Gluck L. Acceleration of fetal lung maturation following prolonged rupture of the membranes. Am J Obstet Gynecol. 1974;118:1115–1118. doi: 10.1016/0002-9378(74)90691-7. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, Hauth JC. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198:43, e41–45. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahra MM, Beeby PJ, Jeffery HE. Maternal versus fetal inflammation and respiratory distress syndrome: a 10-year hospital cohort study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F13–16. doi: 10.1136/adc.2007.135889. [DOI] [PubMed] [Google Scholar]
- 23.Hitti J, Krohn MA, Patton DL, Tarczy-Hornoch P, Hillier SL, Cassen EM, Eschenbach DA. Amniotic fluid tumor necrosis factor-alpha and the risk of respiratory distress syndrome among preterm infants. Am J Obstet Gynecol. 1997;177:50–56. doi: 10.1016/s0002-9378(97)70437-x. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey PS, Lieman JM, Brumfield CG, Carlo W. Chorioamnionitis increases neonatal morbidity in pregnancies complicated by preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:1162–1166. doi: 10.1016/j.ajog.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 25.Laughon M, Allred EN, Bose C, O’Shea TM, Van Marter LJ, Ehrenkranz RA, Leviton A. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics. 2009;123:1124–1131. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. American Journal of Obstetrics and Gynecology. 2009;200:372, e371–376. doi: 10.1016/j.ajog.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Been JV, Rours IG, Kornelisse RF, Jonkers F, Krieger RR, Zimmernan LJ. Chorioamnionitis alters the repsonse to surfactant in preterm infants. Journal of Pediatrics. 2010;156:10–15. e11. doi: 10.1016/j.jpeds.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 28.Hartling L, Liang Y, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Archives of disease in childhood Fetal and neonatal edition. 2012;97:F8–F17. doi: 10.1136/adc.2010.210187. [DOI] [PubMed] [Google Scholar]
- 29.Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003;8:63–71. doi: 10.1016/s1084-2756(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 30.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, Stoll BJ, Buchter S, Laptook AR, Ehrenkranz RA, Cotten CM, Wilson-Costello DE, Shankaran S, Van Meurs KP, Davis AS, Gantz MG, Finer NN, Yoder BA, Faix RG, Carlo WA, Schibler KR, Newman NS, Rich W, Das A, Higgins RD, Walsh MC. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. American journal of respiratory and critical care medicine. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, Martin C. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 32.Berry CA, Nitsos I, Hillman NH, Pillow JJ, Polglase GR, Kramer BW, Kemp MW, Newnham JP, Jobe AH, Kallapur SG. Interleukin-1 in lipopolysaccharide induced chorioamnionitis in the fetal sheep. Reproductive sciences. 2011;18:1092–1102. doi: 10.1177/1933719111404609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince LS, Dieperink HI, Okoh VO, Fierro-Perez GA, Lallone RL. Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev Dyn. 2005;233:553–561. doi: 10.1002/dvdy.20362. [DOI] [PubMed] [Google Scholar]
- 34.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski C. Intra-amniotic endotoxin: Chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol. 2001;280:L527–L536. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- 35.Kramer BW, Moss TJ, Willet K, Newnham J, Sly P, Kallapur SG, Ikegami M, Jobe A. Dose and time response after intra-amniotic endotoxin in preterm lambs. American Journal of Respiratory and Critical Care Medicine. 2001;164:982–988. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]
- 36.Kramer BW, Kramer S, Ikegami M, Jobe A. Injury, inflammation and remodeling in fetal sheep lung after intra-amniotic endotoxin. American Journal of Physiology Lung Cellular & Molecular Physiology. 2002;283:L452–L459. doi: 10.1152/ajplung.00407.2001. [DOI] [PubMed] [Google Scholar]
- 37.Kallapur SG, Bachurski C, Le Cras TD, Joshi S, Ikegami M, Jobe AH. Vascular injury and remodeling following intra-amniotic endotoxin in the preterm lamb lung. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2004;287:L1178–L1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 38.Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LS. NF-kappaB signaling in fetal lung macrophages disrupts airway morphogenesis. Journal of immunology. 2011;187:2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallapur SG, Nitsos I, Moss TJM, Kramer BW, Newnham J, Ikegami M, Jobe AH. Chronic endotoxin exposure does not cause sustained structural abnormalities in the fetal sheep lungs. American Journal of Physiology Lung Cellular & Molecular Physiology. 2005;288:L966–L974. doi: 10.1152/ajplung.00389.2004. [DOI] [PubMed] [Google Scholar]
- 40.Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, Kuypers E, Newnham JP, Jobe AH, Kramer BW. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol. 2010;299:L852–860. doi: 10.1152/ajplung.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thebaud B. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatric research. 2009;65:430–436. doi: 10.1203/PDR.0b013e31819984ce. [DOI] [PubMed] [Google Scholar]
- 42.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 43.Knox CL, Dando SJ, Nitsos I, Kallapur SG, Jobe AH, Payton D, Moss TJ, Newnham JP. The severity of chorioamnionitis in pregnant sheep is associated with in vivo variation of the surface-exposed multiple-banded antigen/gene of Ureaplasma parvum. Biol Reprod. 2010;83:415–426. doi: 10.1095/biolreprod.109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest. 1997;99:2992–2999. doi: 10.1172/JCI119494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bry K, Lappalainen U. Intra-amniotic endotoxin accelerates lung maturation in fetal rabbits. Acta Paediatr. 2001;90:74–80. doi: 10.1080/080352501750064914. [DOI] [PubMed] [Google Scholar]
- 46.Prince LS, Okoh VO, Moninger TO, Matalon S. Lipopolysaccharide increases alveolar type II cell number in fetal mouse lungs through Toll-like receptor 4 and NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2004;287:L999–1006. doi: 10.1152/ajplung.00111.2004. [DOI] [PubMed] [Google Scholar]
- 47.Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant components and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280:L279–L285. doi: 10.1152/ajplung.2001.280.2.L279. [DOI] [PubMed] [Google Scholar]
- 48.Jobe AH, Newnham JP, Willet KE, Moss TJ, Ervin MG, Padbury JF, Sly PD, Ikegami M. Endotoxin induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respirt Crit Care Med. 2000;162:1656–1661. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- 49.Kallapur SG, Moss JTM, Newnham JP, Ikegami M, Jobe AH. Recruited inflammatory cells mediate endotoxin-induced lung maturation in preterm fetal lambs. American Journal of Respiratory and Critical Care Medicine. 2005;172:1315–1321. doi: 10.1164/rccm.200506-1007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moss TM, Newnham J, Willet K, Kramer BW, Jobe A, Ikegami M. Early gestational Intra-amniotic endotoxin: Lung function, surfactant and morphometry. American Journal of Respiratory and Critical Care Medicine. 2002;165:805–811. doi: 10.1164/ajrccm.165.6.2108053. [DOI] [PubMed] [Google Scholar]
- 51.Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, Kallapur SG. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L345–353. doi: 10.1152/ajplung.00003.2007. [DOI] [PubMed] [Google Scholar]
- 52.Moss TJM, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, Ikegami M, Jobe AH. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. American Journal of Obstetrics and Gynecology. 2008;198:e1–e8. doi: 10.1016/j.ajog.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheah FC, Pillow JJ, Kramer BW, Polglase GR, Nitsos I, Newnham JP, Jobe AH, Kallapur SG. Airway inflammatory cell responses to intra-amniotic lipopolysaccharide in a sheep model of chorioamnionitis. Am J Physiol Lung Cell Mol Physiol. 2009;296:L384–393. doi: 10.1152/ajplung.90547.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuypers E, Collins JJ, Kramer BW, Ofman G, Nitsos I, Pillow JJ, Polglase GR, Kemp MW, Newnham JP, Gavilanes AW, Nowacki R, Ikegami M, Jobe AH, Kallapur SG. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. American journal of physiology Lung cellular and molecular physiology. 2012;302:L380–L389. doi: 10.1152/ajplung.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunzmann S, Collins JJ, Yang Y, Uhlig S, Kallapur SG, Speer CP, Jobe AH, Kramer BW. Antenatal inflammation reduces expression of caveolin-1 and influences multiple signaling pathways in preterm fetal lungs. American journal of respiratory cell and molecular biology. 2011;45:969–976. doi: 10.1165/rcmb.2010-0519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willet KE, Jobe AH, Ikegami M, Kovar J, Sly PD. Lung morphometry after repetitive antenatal glucocorticoid treatment in preterm sheep. American Journal Respiratory and Critical Care Medicine. 2001;163:1437–1443. doi: 10.1164/ajrccm.163.6.2003098. [DOI] [PubMed] [Google Scholar]
- 57.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes following intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1178–L1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 58.Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L550–558. doi: 10.1152/ajplung.00329.2006. [DOI] [PubMed] [Google Scholar]
- 59.Yoder BA, Coalson JJ, Winter VT, Siler-Khodr T, Duffy LB, Cassell GH. Effects of antenatal colonization with ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res. 2003;54:797–807. doi: 10.1203/01.PDR.0000091284.84322.16. [DOI] [PubMed] [Google Scholar]
- 60.Viscardi RM, Atamas SP, Luzina IG, Hasday JD, He JR, Sime PJ, Coalson JJ, Yoder BA. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr Res. 2006;60:141–146. doi: 10.1203/01.pdr.0000228322.73777.05. [DOI] [PubMed] [Google Scholar]
- 61.Polglase GR, Hillman NH, Pillow JJ, Nitsos I, Newnham JP, Knox CLJ, Kallapur SG, Jobe AH. Ventilation mediated injury following preterm delivery of Ureaplasma colonized fetal lambs: In revision with minor comments. 2010. [DOI] [PubMed] [Google Scholar]
- 62.Lee AJX, Lambertmont VAC, Pillow JJ, Polglase GR, Nitsos I, Newnham JP, Beilharz MW, Kallapur SG, Jobe AH, Kramer BW. Fetal responses to lipopolysaccharide-induced chorioamnionitis alter immune and airway responses in 7-week-old sheep. American Journal of Obstetrics and Gynecology. doi: 10.1016/j.ajog.2010.11.015. Epub: 2011/01/25. [DOI] [PubMed] [Google Scholar]
- 63.Wolfs TG, Kallapur SG, Polglase GR, Pillow JJ, Nitsos I, Newnham JP, Chougnet CA, Kroon E, Spierings J, Willems CH, Jobe AH, Kramer BW. IL-1alpha mediated chorioamnionitis induces depletion of FoxP3+ cells and ileal inflammation in the ovine fetal gut. PloS one. 2011;6:e18355. doi: 10.1371/journal.pone.0018355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kemp MW, Saito M, Nitsos I, Jobe AH, Kallapur S, Newnham JP. Exposure to In Utero Lipopolysaccharide Induces Inflammation in the Fetal Ovine Skin. Reprod Sci. 2010;18:88–98. doi: 10.1177/1933719110380470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson TC, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG. Pulmonary and systemic induction of SAA3 after ventilation and endotoxin in preterm lambs. Pediatr Res. 2005;58:1204–1209. doi: 10.1203/01.pdr.0000185269.93228.29. [DOI] [PubMed] [Google Scholar]
- 66.Watterberg KL. Adrenocortical function and dysfunction in the fetus and neonate. Seminars in Neonatology. 2004;9:13–21. doi: 10.1016/j.siny.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Kramer BW, Jobe AH, Ikegami M. Monocyte function in preterm, term, and adult sheep. Pediatr Res. 2003;54:52–57. doi: 10.1203/01.PDR.0000066621.11877.33. [DOI] [PubMed] [Google Scholar]
- 68.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced Chorioamnionitis Modulates Innate Immunity of Monocytes in Preterm Sheep. Am J Respir Crit Care Med. 2005;171:73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- 69.Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–107. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 70.Kallapur SG, Jobe AH, Ball MK, Nitsos I, Moss TJ, Hillman NH, Newnham JP, Kramer BW. Pulmonary and Systemic Endotoxin Tolerance in Preterm Fetal Sheep Exposed to Chorioamnionitis. J Immunol. 2007;179:8491–8499. doi: 10.4049/jimmunol.179.12.8491. [DOI] [PubMed] [Google Scholar]
- 71.Kallapur SG, Kramer BW, Knox CL, Berry CA, Collins JJ, Kemp MW, Nitsos I, Polglase GR, Robinson J, Hillman NH, Newnham JP, Chougnet C, Jobe AH. Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. Journal of immunology. 2011;187:2688–2695. doi: 10.4049/jimmunol.1100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 73.Harding JE, Pang J, Knight DB, Liggins GC. Do antenatal corticosteroids help in the setting of preterm rupture of membranes? Am J Obstet Gynecol. 2001;184:131–139. doi: 10.1067/mob.2001.108331. [DOI] [PubMed] [Google Scholar]
- 74.Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol. 2006;195:1020–1024. doi: 10.1016/j.ajog.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 75.Been JV, Degraeuwe PL, Kramer BW, Zimmermann LJ. Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. BJOG. 2011;118:113–122. doi: 10.1111/j.1471-0528.2010.02751.x. [DOI] [PubMed] [Google Scholar]
- 76.Newnham J, Kallapur SG, Kramer BW, Moss TJM, Nitsos I, Ikegami M, Jobe AH. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. American Journal of Obstetrics and Gynecology. 2003;189:1458–1466. doi: 10.1067/s0002-9378(03)00758-0. [DOI] [PubMed] [Google Scholar]
- 77.Kallapur SG, Kramer BW, Moss TJ, Newnham JP, Jobe AH, Ikegami M, Bachurski CJ. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L633–642. doi: 10.1152/ajplung.00344.2002. [DOI] [PubMed] [Google Scholar]
- 78.Newnham JP, Moss TJ, Padbury JF, Willet KE, Ikegami M, Ervin MG, Sly P, Jobe AH. The interactive effects of endotoxin with prenatal glucocorticoids on short-term lung function in sheep. Am J Ob Gyn. 2001;185:190–197. doi: 10.1067/mob.2001.114500. [DOI] [PubMed] [Google Scholar]
- 79.Moss TJM, Nitsos I, Knox CL, Polglase G, Kallapur SG, Ikegami M, Jobe AH, Newnham JP. Ureaplasma colonization of amniotic fluid and efficacy of antenatal corticosteroids for preterm lung maturation in sheep. American Journal of Obstetrics and Gynecology. 2008;200:96, e91–96. doi: 10.1016/j.ajog.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171:73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- 81.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res. 2004;55:764–768. doi: 10.1203/01.PDR.0000120678.72485.19. [DOI] [PubMed] [Google Scholar]