Abstract
Aim: In this study, we evaluated the effect of capsaicin on the interaction of redox-sensitive thioredoxin (Trx)/apoptosis signal-regulating kinase 1 (ASK1) in pancreatic cancer cells. Results: Capsaicin treatment downregulated Trx and increased the phosphorylation (activation) of ASK1 at Thr845 and kinase activity in AsPC-1 and BxPC-3 cells. Capsaicin treatment also activated downstream effector molecules MKK4/7, caspase-9, and caspase-3. Antioxidants tiron or PEG-catalase blocked the activation of ASK1 cascade by capsaicin and protected the cells from apoptosis, indicating the involvement of reactive oxygen species in the activation of ASK1. Our results further revealed that Trx overexpression suppressed the effects of capsaicin, whereas ASK1 overexpression enhanced the apoptosis-inducing effects of capsaicin. β-mercaptoethanol, a reducing agent, blocked capsaicin-mediated activation of ASK1, indicating that Trx-ASK1 complex exists and requires reducing conditions in the cell. On the other hand, the Trx inhibitor (1-chloro-2-4-dinitrobenzene) increased capsaicin-induced ASK1 kinase activity, suggesting that Trx inhibition by capsaicin is essential for ASK1 activation. Oral administration of 5 mg capsaicin/kg body weight substantially suppressed the growth of tumors in xenograft and orthotopic mouse model. Tumors from capsaicin-treated mice showed reduced levels of Trx, increased phosphorylation of ASK1 at Thr845, and cleavage of caspase-3 and poly (ADP-ribose) polymerase. Innovation: Our results for the first time demonstrated a new perspective that Trx-ASK1 complex can be targeted by capsaicin in pancreatic cancer. Conclusion: Capsaicin reduces Trx expression and dissociates Trx-ASK1 complex resulting in the activation of ASK1 and downstream effectors leading to apoptosis in pancreatic tumor cells in vitro and in vivo. Antioxid. Redox Signal. 17, 1417–1432.
Introduction
Reactive oxygen species (ROS) play an essential role in the regulation of normal physiology of a cell, including cell proliferation, cell survival, and apoptotic cell death. However, excessive ROS cause severe damage to cellular components, including DNA, protein, and lipid. Apoptosis signal-regulating kinase 1 (ASK1), a member of mitogen-activated protein kinase kinase kinase family, is activated mostly by ROS. Previous study showed that ASK1 is activated in the cells by various stimuli, including oxidative stress, tumor necrosis factor-α, endoplasmic reticulum stress, serum withdrawal, and chemotherapeutic agents (9). Activated ASK1 further activates both MKK4/MKK7-c-Jun NH2-terminal kinase (JNK)- and MKK3/MKK6-p-38 MAPK-signaling cascade (11). Dominant negative ASK1, which is a catalytically inactive mutant, inhibits apoptosis induced by stress signals such as tumor necrosis factor-α and H2O2 (5, 11, 33). Few studies have shown that ASK1 plays a critical role in oxidative stress-induced apoptosis (5, 33). The ASK1 activity is regulated by multiple mechanisms, such as phosphorylation, oligomerization, and protein–protein interaction. ASK1 shows its activity when it is phosphorylated at Thr845 (26). On the other hand, phosphorylation of ASK1 at Ser-83 by AKT/PKC attenuates its activity (15). This indicates that ASK1 is a key player in apoptosis signaling, particularly through oxidative stress, but the molecular mechanism by which ASK1 transmits the oxidative stress-induced signals in pancreatic cancer cells remains unclear.
Thioredoxin (Trx) is a cellular redox enzyme that plays an essential role in regulation of cell growth, apoptosis, and activation (29). Trx acts as a direct inhibitor of ASK1 by binding to the N-terminal noncatalytic region of ASK1 (amino acids 1 to 655) (18, 33). Oxidation of Trx is induced by ROS through a disulfide bridge between Cys32 and Cys35, and dissociates it from ASK1, resulting in the activation of ASK1 (33). Trx protects the cells against hydrogen peroxide (H2O2), tumor necrosis factor (TNF)-α, and cis-diamminedichloroplatinum (II)-induced cytotoxicity (21, 27, 36). Overexpression of Trx has been shown in several tumor types, including pancreatic cancer (28). It appears that apoptotic stimuli such as oxidative stress activate ASK1 in part by oxidizing Trx to release ASK1 from the Trx-ASK1 complex. Therefore, dissociation of the Trx-ASK1 complex leading to the activation of ASK1 would be a viable option for inducing apoptosis in cancer cells.
Innovation.
Intracellular antioxidant thioredoxin (Trx) negatively regulates apoptosis signal-regulating kinase 1 (ASK1), a MAPKKK, by keeping the reducing environment. Reactive oxygen species, mainly hydrogen peroxide, inactivates Trx, which in turn dissociates from ASK1, leading to apoptosis by an ASK1-dependent process. Hence, the Trx/ASK1 complex can be targeted to induce apoptosis and can be the focus of the study in pancreatic cancer cells. Trx was significantly reduced by capsaicin, resulting in the activation of ASK1 mainly by phosphorylation at Thr845. Increasing the reducing environment by β-mercaptoethanol blocked the activation of ASK1 and hence related apoptosis in pancreatic cancer cells. Ectopic expression of Trx decreased, whereas ASK1 increased the apoptosis-inducing effects of capsaicin in cancer cells. Oral administration of 5 mg capsaicin/kg body weight resulted in the regression of implanted pancreatic tumors in two different mouse models. The tumors of capsaicin-treated mice demonstrated reduced Trx, increased ASK1, and cleavage of caspase-3 and poly (ADP-ribose) polymerase, indicating apoptosis in agreement of in vitro observations. The Trx-ASK1 complex can be targeted for therapy in pancreatic cancer.
Capsaicin, a homovanillic acid derivative (N-vanillyl-8-methyl-nonenamide), has been used in South Asian and Latin-American countries (6, 20, 24, 46). Capsaicin is being used as a chemopreventive and therapeutic agent against certain mutagens and carcinogens (23, 34, 35, 40, 41, 45). Capsaicin has also been shown to prevent several types of cancer by different mechanisms (12, 13, 16, 19, 25). Moreover, capsaicin has been used to treat pain, inflammation, and a variety of diseases, including diabetic neuropathy, rheumatoid arthritis, postmastectomy pain syndrome, cluster headaches, and herpes zoster. (8, 22, 38, 44). In our previous study, we have shown that capsaicin-induced apoptosis in pancreatic cancer cells was associated with the generation of ROS and persistent disruption of mitochondrial membrane potential without affecting the normal cells (30, 48). In the present study, we examined the mechanism by which capsaicin-mediated oxidative stress inhibits Trx and activates ASK1, leading to apoptosis in pancreatic cancer cells in vitro and in vivo.
Results
Capsaicin triggers apoptosis by activating ASK1 cascade in pancreatic cancer cells
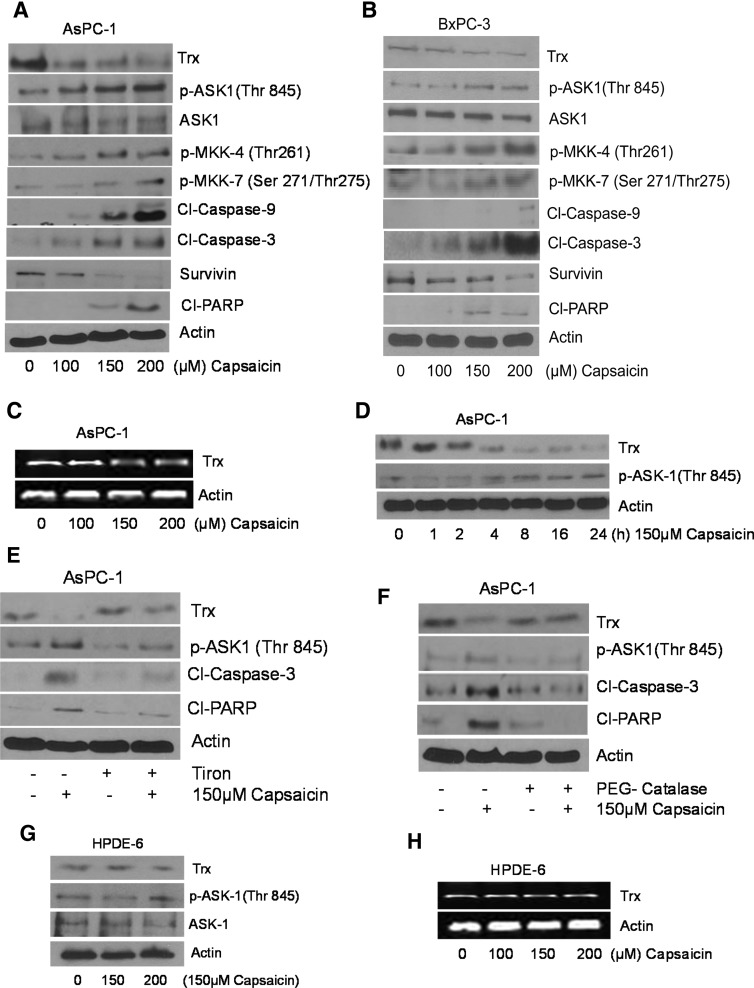
To determine the effect of capsaicin on ASK1 and Trx, we treated BxPC-3 and AsPC-1 cells with various concentrations of capsaicin for 24 h. Capsaicin treatment substantially reduced Trx expression in both the cell lines in a concentration-dependent manner (Fig. 1A, B). Furthermore, our results show that capsaicin treatment increased the phosphorylation of ASK1 at Thr845 and MKK-4(Thr261)/7(Ser271/Thr275) (Fig. 1A, B). The expression of survivin was also drastically decreased by capsaicin treatment (Fig. 1A, B). The cleavage of caspase-9, caspase-3, and poly (ADP-ribose) polymerase (PARP) was observed by capsaicin treatment in both the cell lines, indicating apoptosis (Fig. 1A, B). Capsaicin treatment also reduced the mRNA levels of Trx, suggesting that inhibition of Trx was at the transcriptional level (Fig. 1C). In a time-dependent study, downregulation of Trx started around 4 h of capsaicin treatment concomitant with the phosphorylation of ASK1 at the same time, indicating that inhibition of Trx correlates well with ASK1 activation (Fig. 1D). However, cleavage of caspase-3 and PARP was observed at 16 h as shown by us in our previous study (30). These results suggest that early changes in Trx and ASK1 preceded apoptosis in our model. In our previous study, we observed that capsaicin causes ROS generation in pancreatic cancer cells (30). In the present study, we wanted to know whether capsaicin-mediated ROS were involved in the activation of the ASK1 cascade. We therefore treated the cells with antioxidants tiron (10 mM) or PEG-catalase (500 U/ml) before capsaicin treatment. Capsaicin treatment failed to activate ASK1 or reduce the expression of Trx in the cells that were pretreated with tiron or PEG-catalase (Fig. 1E, F). The cleavage of caspase-3 and PARP by capsaicin was also blocked by antioxidants, indicating that capsaicin-mediated ROS generation was involved in the inhibition of Trx and activation of the ASK-1 cascade, leading to apoptosis. Capsaicin treatment failed to modulate the mRNA or protein expression of Trx and phosphorylation of ASK1 in normal HPDE-6 cells, indicating that the effects of capsaicin were specific to cancer cells (Fig. 1G, H).
FIG. 1.
Capsaicin triggers apoptosis by activating apoptosis signal-regulating kinase 1 (ASK1) cascade in pancreatic cancer cells. (A) AsPC-1 and (B) BxPC-3 cells were treated with various concentrations of capsaicin for 24 h. Cells were lyzed, and the total lysate was prepared as described in the Materials and Methods section. Representative immunnoblots showed the effect of capsaicin treatment on the phosphorylation of p-ASK1 (Thr 845), p-MKK-4 (Thr261), p-MKK-7 (Ser271/Thr275), as well as protein levels of thioredoxin (Trx), ASK-1, survivin, and cleavage of caspase-9/3 and PARP. The same blots were stripped and reprobed for actin to ensure equal protein loading. (C) AsPC-1 cells were treated with different concentrations of capsaicin, and total RNA was isolated with Trizol. Total RNA was analyzed for Trx. Actin was used as an internal control. (D) AsPC-1 cells were treated with 150 μM capsaicin at different time points and immunoblotted with Trx and p-ASK-1 (Thr845). The same blots were stripped and reprobed for actin. In another experiment, AsPC-1 cells were treated with antioxidants (E) 10 mM tiron and (F) 500 U/ml PEG-catalase for 1 h followed by 150 μM of capsaicin for 24 h and immunoblotted with Trx, p-ASK 1(Thr845), Cl-caspase-3, and cleaved poly (ADP-ribose) polymerase (Cl-PARP), respectively. The same blot was stripped and reprobed for actin to ensure equal protein loading. (G) Normal human pancreatic ductal epithelial (HPDE-6) cells were treated with various concentrations of capsaicin and immunoblotted with Trx, p-ASK-1 (Thr845), and ASK1. The same blot was stripped and reprobed for actin to ensure equal protein loading. (H) HPDE-6 cells were treated with various concentration of capsaicin, and total RNA was analyzed for Trx. These experiments were performed three times independently, and similar results were obtained.
Effect of capsaicin on ASK1 cascade in cells overexpressing ASK1 or Trx
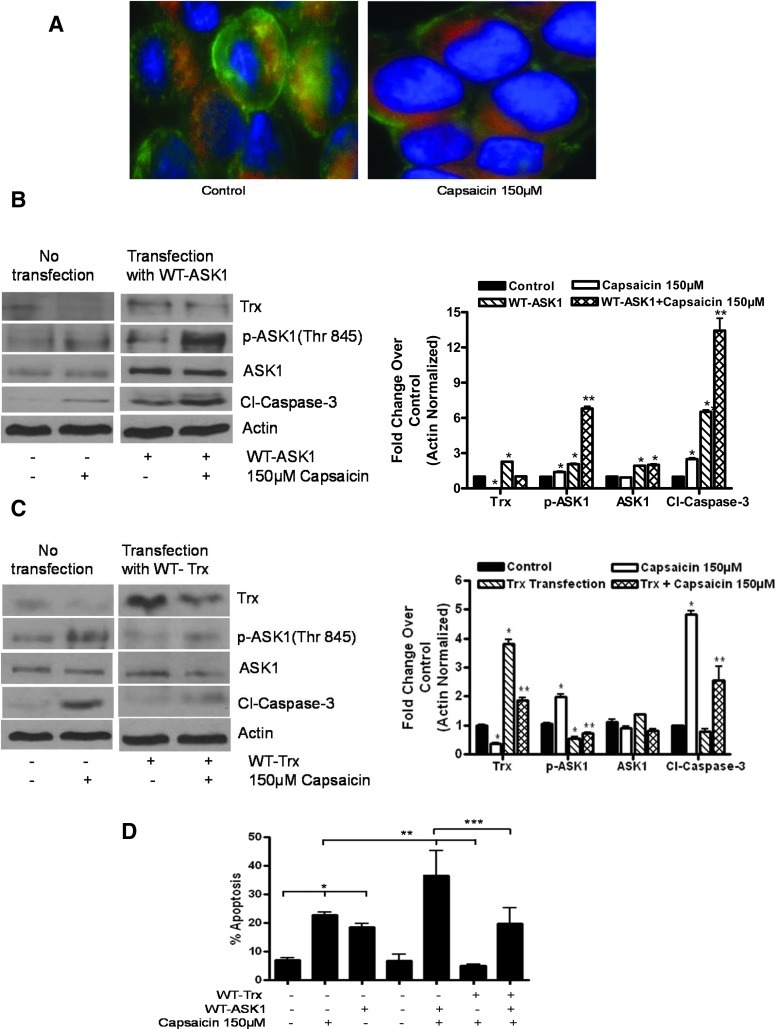
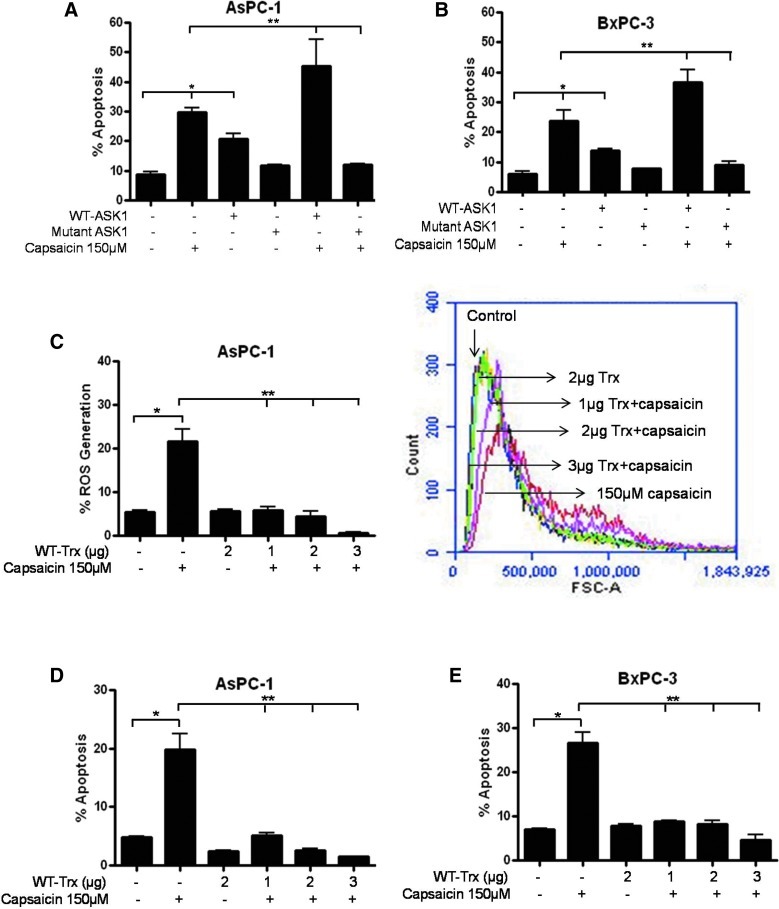
To confirm the phosphorylation of ASK1, control and capsaicin-treated cells were analyzed by fluorescence microscopy. As can be seen in Figure 2A, red fluorescence due to phosphorylated ASK1 was observed in capsaicin-treated cells as compared to control cells. To confirm whether capsaicin-induced apoptosis was caused by inhibition of Trx and activation of ASK1 by phosphorylation at Thr845, AsPC-1 cells were transiently transfected with hemagglutinin (HA)-tagged WT-ASK1 and Flag-tagged WT-Trx plasmid and treated with 150 μM capsaicin for 24 h (Fig. 2B, C). Our results demonstrate that overexpression of WT-ASK1 increased capsaicin-induced p-ASK1 (Thr845) and cleavage of caspase-3 (Fig. 2B), indicating that ASK1 overexpression increases capsaicin-induced apoptosis. On the other hand, Trx overexpression suppressed capsaicin-mediated p-ASK-1 (Thr845) and cleavage of caspase-3 (Fig. 2C), indicating that Trx overexpression blocks capsaicin-induced apoptosis. The role of activated ASK1 in inducing apoptosis was further confirmed by the Annexin-V assay. Cells transfected with WT-ASK1 and treated with capsaicin exhibited increased apoptosis as compared capsaicin treatment alone (Fig. 2D). On the other hand, Trx overexpression suppressed capsaicin-induced apoptosis (Fig. 2D).
FIG. 2.
Effect of capsaicin on ASK1 cascade in cells overexpressing ASK1 or Trx. (A) AsPC-1 cells were treated with dimethyl sulfoxide (DMSO) or 150 μM of capsaicin, immunostained with p-ASK1 Thr845 (red) and actin (green) antibodies, and visualized under a fluorescence microscope (Olympus Inc.). AsPC-1 cells were transiently transfected with 2 μg of (B) hemagglutinin (HA)-tagged WT-ASK1 or (C) Flag-tagged WT-Trx using the FuGENE transfection reagent for 24 h, and the transfected cells were treated with DMSO or 150 μM capsaicin for 24 h. The expressions of Trx, p-ASK1 (Thr845), and ASK1 and cleavage of caspase-3 were evaluated by immunoblotting. Each blot was stripped and reprobed with antiactin antibody to ensure equal protein loading. Bar diagram shows the quantitation of respective western blots normalized with actin. (D) Apoptosis was determined using annexin-V/fluorescein isothiocyanate (FITC) and propidium iodide in AsPC-1 cells transfected with HA-tagged WT-ASK1 or Flag-tagged Trx as described above and then treated with DMSO or 150 μM of capsaicin for 24 h. Western blot experiments were performed three times independently with similar observations made in each experiment. Values are means±SD of three independent experiments. *Statistically different compared with control (p<0.05), **statistically different when compared with capsaicin treatment (p<0.05), and ***statistically different when compared with capsaicin treatment and WT-ASK1 transfection (p<0.05), as analyzed by the one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars).
Capsaicin disrupts the interaction of Trx with ASK1 and activates ASK1
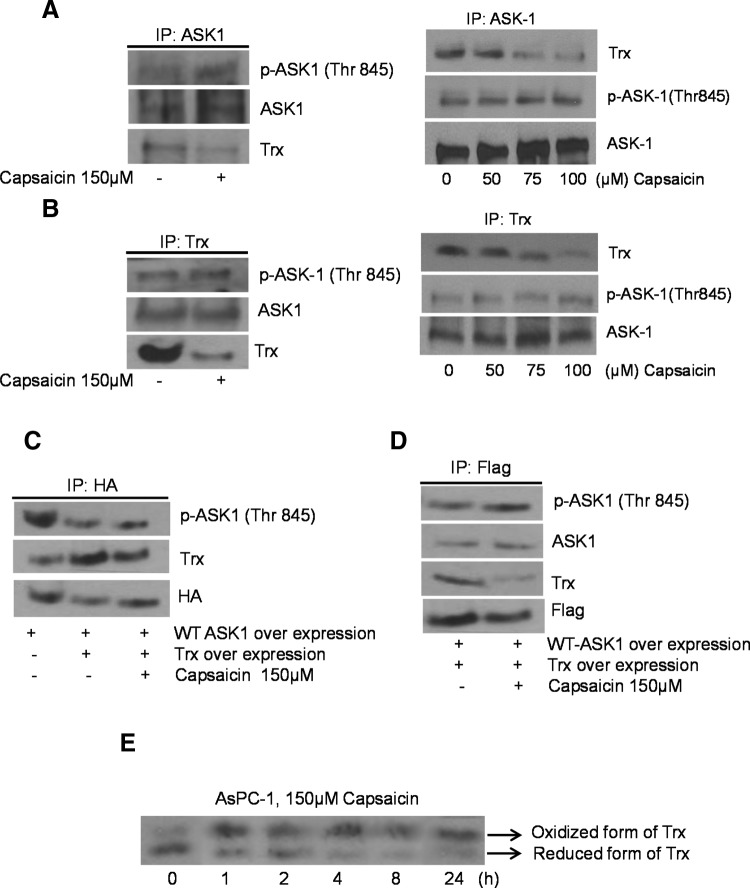
We next wanted to evaluate the effect of capsaicin on the interaction of Trx and ASK1. AsPC-1 cells were treated with dimethyl sulfoxide (DMSO) or various concentrations of capsaicin and immunoprecipitated with the anti-ASK1 or anti-Trx antibody. As shown in Figure 3A, when immunoprecipitated with anti-ASK1, capsaicin treatment decreased the expression of Trx and increased the phosphorylation of ASK1 at Thr845 as compared to control in a concentration-dependent manner. On the other hand, when immunoprecipitated with anti-Trx, capsaicin treatment decreased the expression level of Trx without significantly affecting the protein or phosphorylation level of ASK1 (Fig. 3B). To further confirm the effect of capsaicin on the dissociation of ASK1 from Trx, cells were cotransfected with HA-tagged WT-ASK1 and Flag-tagged WT-Trx and then treated with capsaicin. Treated and control cells were immunoprecipitated with anti-HA-tagged or anti-Flag-tagged antibodies. Our results show that Trx overexpression suppressed the activation of ASK1 in the cells transfected with WT-ASK1. On the other hand, capsaicin treatment enhanced the activation of ASK1, but decreased Trx in the cells transfected with WT-ASK1 and WT-Trx (Fig. 3C). Similar observations were made in Figure 3D, except that a modest increase in the phosphorylation of ASK1 was observed by capsaicin treatment in the cells immunoprecipitated with the anti-Flag antibody. The exact reason behind this discrepancy in our result is not clear at this point and warrants further investigation. These findings suggest that capsaicin dissociates the Trx-ASK1 complex and releases and activates ASK1 by phosphorylation at Thr845. Next we sought to determine whether capsaicin treatment oxidizes Trx. The redox western blotting was used to determine the relative amount of oxidized and reduced Trx by capsaicin treatment in pancreatic cancer cells. As shown in Figure 3E, capsaicin treatment decreased the reduced form and increased the oxidized form of Trx when compared to the control in a time-dependent manner. The cells treated with 150 μM capsaicin showed mainly two immunoreactive bands. The reduced form (carboxymethylated Trx) was depicted as the lower band, whereas partially carboxymethylated Trx, the upper band, was depicted as the oxidized form. Oxidation of Trx by capsaicin treatment was as early as 1 h, which sustained up to 24 h. The reduced form of Trx started decreasing after 4 h of capsaicin treatment (Fig. 3E). These results indicate that capsaicin treatment causes early and significant changes in the redox status of Trx in pancreatic cancer cells.
FIG. 3.
Capsaicin disrupts the interaction of Trx with ASK1 and activates ASK1. AsPC-1 cells were treated with DMSO or various concentrations of capsaicin for 24 h and immunoprecipitated with (A) anti-ASK1 or (B) anti-Trx antibodies overnight, resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotted with anti-p-ASK1 (Thr845) or ASK1 or anti-Trx antibody. To further confirm the interaction of Trx with ASK1, AsPC-1 cells were transiently transfected with constructs encoding (C) ASK1 fused to C-terminal HA (2 μg) or (D) Flag-tagged Trx plasmid (Flag) (2 μg), treated with DMSO or 150 μM capsaicin and immunoprecipitated with anti-HA or anti-Flag antibody. The blots were probed with anti-p-ASK1 (Thr845), or ASK1 or anti-Trx or HA or Flag antibody. (E) AsPC-1 cells were treated with 150 μM capsaicin at different time points and carboxymetylated in a buffer containing 50 mM Tris/HCl pH 8.3, 3 mM ethylenediaminetetraacetic acid, 5 mM iodoacetic acid, 6 M guanidine, HCl, and 0.5% Triton X-100 for 30 min at 37°C in the dark. Trx present in the cell lysates with different degrees of carboxymethylation was separated by native gel electrophoresis and analyzed by immunoblotting, representing oxidized and reduced form of Trx. The experiments were repeated three times with similar results obtained.
Effect of capsaicin on ASK1 kinase activity and apoptosis in AsPC-1 cells
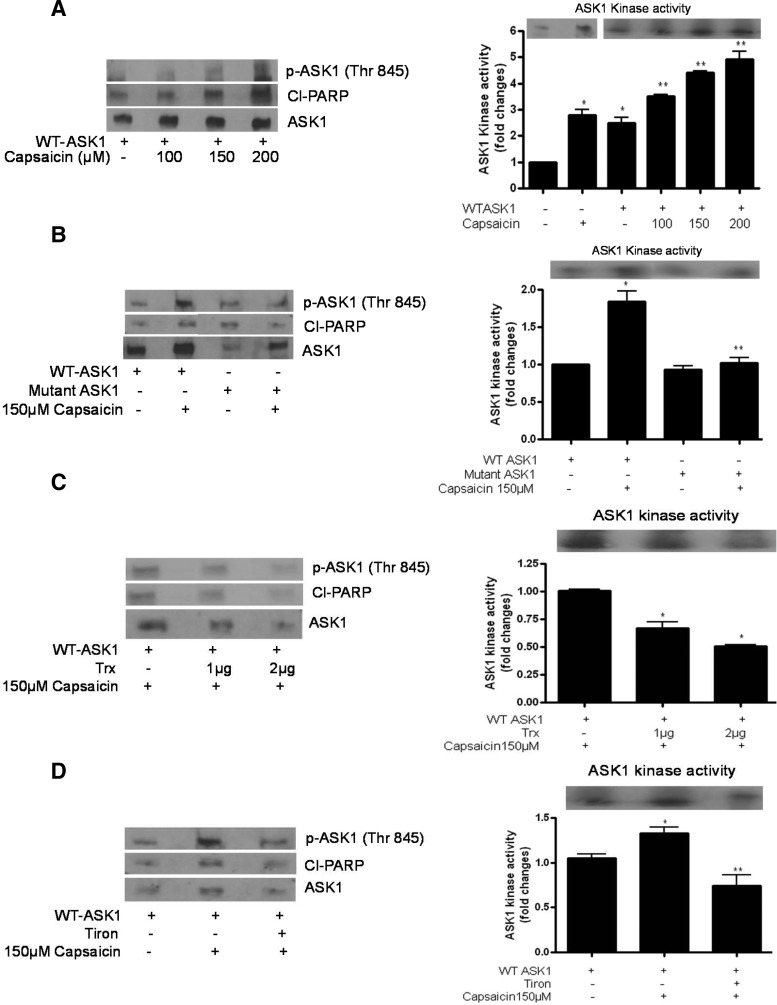
To determine the biochemical effects of Trx on the activation of ASK1 by capsaicin, AsPC-1 cells were transiently transfected with HA-tagged WT-ASK1 or HA-tagged mutant ASK1 or Flag-tagged Trx and treated with DMSO or 150 μM capsaicin for 24 h. Treated and control cells were immunoprecipitated with the ASK1 antibody, and the ASK-1 kinase assay was performed using myelin basic protein (MBP) as a direct substrate. To further correlate changes in ASK1 kinase activity with apoptosis, phosphorylation of ASK1 and cleavage of PARP were also evaluated in these experiments. As shown in Figure 4A, capsaicin treatment increased ASK1 kinase activity, phosphorylation of ASK1 at Thr845, and cleavage of PARP, in a concentration-dependent manner. ASK1 kinase activity was quantified and presented as a bar diagram in the right panel. To confirm these results, cells were transfected with a kinase dead mutant of ASK1. Capsaicin treatment failed to increase the kinase activity of mutant ASK1 or cleavage of PARP (Fig. 4B), indicating that functional and active ASK1 is required for ASK1-mediated apoptosis. Since Trx is known to block the activation of ASK1 as well as apoptosis, we next sought to determine if ectopic expression of Trx would block the increase in ASK1 kinase activity and apoptosis by capsaicin. As expected, Trx overexpression substantially blocked capsaicin-mediated increase in ASK1 kinase activity, phosphorylation of ASK1, and cleavage of PARP (Fig. 4C). In addition, antioxidant tiron also blocked capsaicin-mediated ASK1 kinase activity, phosphorylation of ASK1, and cleavage of PARP (Fig. 4D), indicating the involvement of ROS in the inhibition of Trx and activation of ASK1 resulting in apoptosis.
FIG. 4.
Effect of capsaicin on ASK1 kinase activity. AsPC-1 cells were transiently transfected with (A) HA-tagged WT-ASK1 (2 μg) or (B) HA-tagged WT-ASK1 and HA-tagged mutant ASK1 (2 μg) or (C) HA-tagged WT-ASK1 (2 μg) and Flag-tagged Trx (1 μg or 2 μg) or (D) HA-tagged WT-ASK1 expression plasmid followed by 1 h treatment with 10 mM tiron. Transfected cells were treated with DMSO or 150 μM of capsaicin for 24 h. Kinase activity of ASK1 was determined by the immune complex-coupled kinase assay using myelin basic protein (MBP) as a direct substrate and 0.5 μCi of [γ32P] ATP. MBP phosphorylation was detected using autoradiography. To determine phosphorylation of ASK1 and apoptosis in the same sample, the upper part of the membrane (>50 kDa) was cut out and immunoblotted with anti-p-ASK-1 (Thr 845) and Cl-PARP antibody. Bar diagram shows the quantitation of ASK1 kinase activity. These experiments were performed three times independently with similar observations made in each experiment. Values are means±SD of three individual experiments. *Statistically different when compared with control (p<0.05) and **statistically different when compared with capsaicin treatment between mutant ASK1 and WT-ASK1 treatment or WT-ASK1 with capsaicin treatment (p<0.05), as analyzed by one-way ANOVA followed by the Bonferroni post-hoc test.
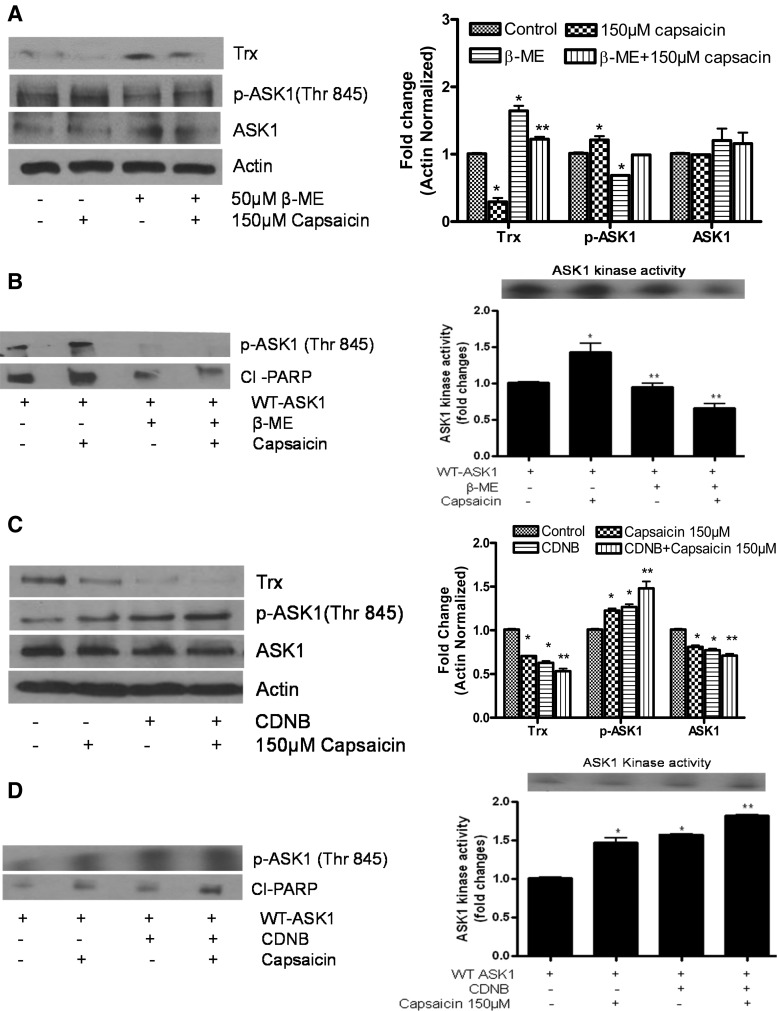
Activation of ASK1 by capsaicin is attenuable by a reducing agent and augmented by a Trx inhibitor
Trx-ASK1 association depends on the reducing condition in the cell. Since capsaicin causes ROS generation and disrupts redox homeostasis, cells were treated with the reducing agent β-mercaptoethanol (β-ME) to see if a reducing environment could block the deleterious effects of capsaicin. As shown in Figure 5A and B, β-ME treatment significantly blocked capsaicin-mediated increase in ASK1 kinase activity and phosphorylation of ASK1, indicating that the Trx-ASK1 complex depends on the reducing condition in the cell. These results were further confirmed using dithiothreitol (DTT), which is another reducing agent (Supplementary Fig. S1B; Supplementary Data are available online at www.liebertpub.com/ars). Since we observed that Trx overexpression suppressed ASK1 kinase activity, ASK1 phosphorylation, and apoptosis, we next wanted to see whether the Trx-specific inhibitor 1-chloro-2-4-dinitrobenzene (CDNB) would increase ASK1 activity and apoptosis. Cells were treated with CDNB before capsaicin treatment. As expected, CDNB treatment significantly depleted Trx and increased the phosphorylation and kinase activity of ASK1 and cleavage of PARP (Fig. 5C, D), suggesting that capsaicin-mediated Trx inhibition is required for ASK1 activation.
FIG. 5.
Activation of ASK1 kinase activity by capsaicin is attenuable by a reducing agent and augmented by a Trx inhibitor. AsPC-1 cells were treated with (A) 50 μM β-mercaptoethanol or (C) 25 μM 1chloro 2,4-dinitro benzene (CDNB) for 30 min before treatment with capsaicin for 24 h, and whole-cell lysates were immunoblotted with Trx, p-ASK1 (Thr845), and ASK1. Each blot was stripped and reprobed with anti-actin antibody to ensure equal protein loading. Bar diagram shows the quantitation of respective western blots. These experiments were performed three times independently with similar observations made in each experiment. In another experiment, AsPC-1 cells were transiently transfected with an HA-tagged WT-ASK1 expression plasmid and treated with (B) 5 mM β-mercaptoethanol or (D) 25 μM CDNB for 30 min before treatment with 150 μM capsaicin for 24 h, and the kinase assay was performed as described in Figure 4. To determine phosphorylation of ASK1 and apoptosis in the same sample, the upper part of the membrane (>50 kDa) was cut out and immunoblotted with anti-p-ASK-1 (Thr 845) and Cl-PARP antibody. Bar diagram shows the quantitation of ASK1 kinase activity, respectively. *Statistically different compared with control or WT-ASK1 (p<0.05) and **statistically different when compared with capsaicin or capsaicin and WT-ASK1 treatment (p<0.05), as analyzed by the one-way ANOVA followed by the Bonferroni's post-hoc test.
Trx plays an essential role in capsaicin-mediated activation of ROS, ASK1, and apoptosis
To further confirm that capsaicin treatment generates ROS that leads to the depletion of Trx, resulting in the activation of ASK1 by disrupting the Trx-ASK1 complex, AsPC-1 and BxPC-3 cells were transfected with HA-tagged WT-ASK1 or HA-tagged mutant ASK1 and treated with capsaicin. As shown in Figure 6A and B, WT-ASK1 overexpression significantly increased capsaicin-induced apoptosis, whereas kinase dead mutant ASK1 overexpression suppressed capsaicin-induced apoptosis, indicating that functionally active ASK1 is required for apoptosis in both the cell lines. Next, we wanted to evaluate whether Trx overexpression could prevent capsaicin-induced apoptosis by blocking ROS generation. To test this hypothesis, cells were transfected with a Trx expression plasmid; as expected, Trx overexpression blocked capsaicin-mediated ROS generation (Fig. 6C) and apoptosis in AsPC-1 (Fig. 6D) and BxPC-3 (Fig. 6E) cells in a concentration-dependent manner. ROS were measured for H2O2 and superoxide anion generation by a flow cytometer. Since H2O2 is mainly generated by capsaicin in our model, as shown by us previously (30), the level of H2O2 in these experiments was also confirmed using a commercial kit (data not shown).
FIG. 6.
Trx plays essential role in blocking capsaicin-mediated activation of reactive oxygen species (ROS), ASK1, and apoptosis. (A) AsPC-1 and (B) BxPC-3 cells were transiently transfected with HA-tagged WT-ASK1 (2 μg) and HA-tagged mutant ASK1 (2 μg) or (C–E) Flag-tagged Trx (2 μg) for 24 h and then treated with DMSO or 150 μM capsaicin for 24 h. Apoptosis was determined using annexin-V/FITC and propidium iodide and analyzed by flow cytometry as described in the Materials and Methods section. Results are expressed as means±SD (n=3) of three independent experiments. (C) ROS generation was determined by a flow cytometer after transfected cells were treated with 150 μM capsaicin for 2 h and stained with 5 μM dihydroethidine (DHE) and 10 μM dichlorodihydrofluorescein diacetate (DCFDA) for 30 min. Right-side panel represents the flow cytometry graph of the same. *Statistically different compared with control (p<0.05) and **statistically different when compared with capsaicin treatment between WT-ASK1 and mutant-ASK1 (p<0.05), as analyzed by the one-way ANOVA followed by the Bonferroni's post-hoc test. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars).
Capsaicin suppresses tumor growth in vivo in athymic nude mice
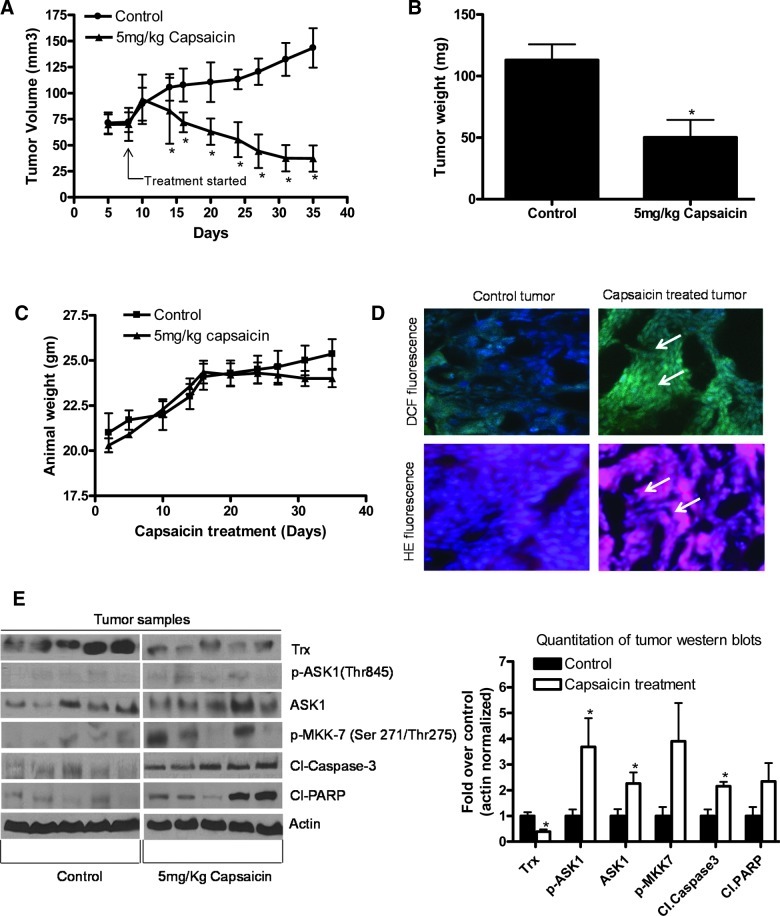
To test the possibility that capsaicin treatment would suppress pancreatic tumor growth, AsPC-1 tumor xenografts were implanted in athymic nude mice. Once each mouse had palpable tumors, mice were randomized into two groups. The treated group of mice was fed 5 mg capsaicin/kg body weight every day, and tumor growth was recorded periodically. Our results show that oral gavage of 5 mg/kg every day drastically reduced the growth of the tumors starting day 15 of the treatment and continued till the end of the experiment (Fig. 7A). At day 35 of the treatment, the tumor volume in the treated group was reduced by 73% as compared with control groups (143.44±60.30 mm3 versus 37.20±40.00 mm3, n=10; Fig. 7A). The average wet weight of the tumors dissected from capsaicin-treated mice was ∼56% less than the weight of the tumors from the control mice (Fig. 7B). The average body weight of control and capsaicin-treated mice did not change throughout the experiment, and no signs of discomfort were noticed during feeding, suggesting that capsaicin at this dose may not be toxic to the mice (Fig. 7C). We next wanted to know whether capsaicin treatment could increase ROS levels in vivo. Excised tumors were analyzed for ROS using DHE for superoxide and 6-carboxy-2, 7-dichlorodihydrofluorescein diacetate (DCFDA) for H2O2, respectively, by immunofluorescence (Fig. 7D). Our results demonstrated that the staining for DHE and DCF increased in the tumors of capsaicin-treated mice as compared to controls, indicating that capsaicin treatment leads to an increase in ROS generation in vivo in pancreatic tumor cells.
FIG. 7.
Capsaicin treatment suppresses pancreatic tumor growth by inhibiting Trx and activating ASK1. About 1×106 AsPC-1 cells were injected subcutaneously on right flanks of each athymic nude mouse in phosphate-buffered saline/Matrigel suspension. When tumors reached 70 mm3 in size, mice were randomly divided into two groups with 10 mice in each group. Mice were fed with 5 mg capsaicin/kg body weight everyday by oral gavage. Control mice received vehicle only. Tumors were measured by vernier calipers three times a week. Each mouse was weighed twice a week. Effect of capsaicin on the (A) tumor volume, (B) tumor weight and (C) body weight was evaluated. (D) Excised tumors were frozen and stained with 10 μM DHE to measure superoxide levels and 2 μM of DCFDA to measure hydrogen peroxide levels in the tumors. Results are representative of three independent experiments (E) Tumor lysates from control and capsaicin-treated nude mice were immunoblotted and analyzed for Trx, p-ASK1 (Thr845), ASK1, p-MKK-7 (Ser271/Thr275), Cl-caspase-3, and Cl-PARP. The blots were stripped and reprobed for actin to ensure equal protein loading. Right-side diagram shows the quantitation of tumor western blots. Values are means±SD of 5 samples. *p<0.05 statistically significant when compared with control. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars).
Capsaicin treatment inhibits Trx and activates ASK1 in pancreatic tumors
To confirm whether capsaicin-mediated inhibition of Trx and activation of ASK1 were associated with capsaicin-mediated tumor growth suppression in vivo, tumors from control and capsaicin-treated mice were examined by western blotting. As shown in Figure 7D, expression of Trx was drastically reduced by capsaicin treatment, whereas phosphorylation of ASK1 and MKK-7 was increased significantly. The cleavage caspase-3 and PARP were also observed in capsaicin-treated tumors, indicating apoptosis. Consistent with our in vitro results, we observed increased phosphorylation of ASK1 at Thr845 and inhibition of Trx in capsaicin-treated tumors as compared to control tumors, indicating that inhibition of Trx and activation of ASK1 were associated with the overall capsaicin-mediated tumor growth suppression in vivo.
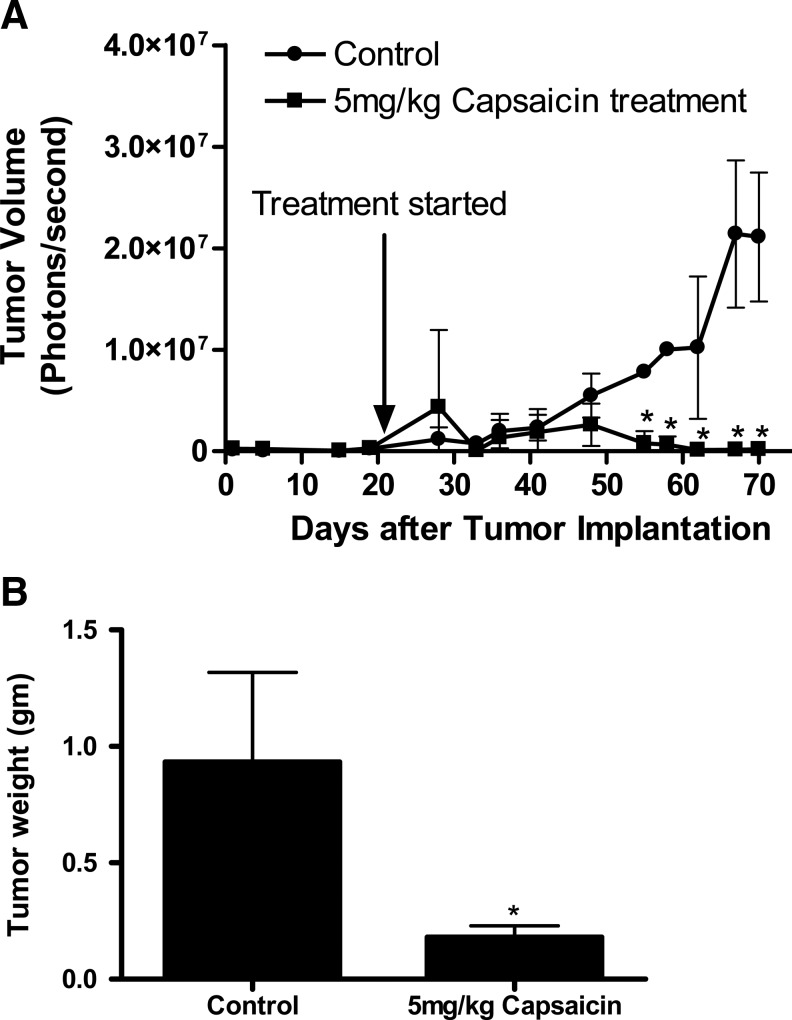
Capsaicin inhibits orthotopic pancreatic tumor growth
The xenograft assay does not truly depict the tumor microenvironment, as the tumor is implanted on the skin. To confirm the observations made in the xenograft assay, PanC-1 pancreatic cancer cells stably transfected with luciferase were orthotopically implanted in the pancreas of each mouse after performing minor survival surgery, as mentioned in the Materials and Methods section. The growth of the tumors was measured three times a week using a noninvasive IVIS Bio Luminescent Imaging system. After 3 weeks of tumor cell implantation, mice were randomly divided into two groups, and the treated group started receiving 5 mg capsaicin/kg body weight every day by oral gavage. As shown in Figure 8A, as compared to controls, capsaicin treatment drastically reduced the tumor growth (2.1×107 vs. 1.8×105 photons/s). In addition, the average wet weight of tumor from capsaicin-treated mice was about 80% less than that of tumor from control mice (Fig. 8B). Taken together, our results demonstrated the antitumor effects of capsaicin in two different mouse models and using two different pancreatic cancer cell lines.
FIG. 8.
Capsaicin inhibits orthotopic pancreatic tumor growth. Around 1×106 PanC-1-luc cells were injected into the subcellular region of the pancreas. After 3 weeks of tumor cell implantation, animals were divided into two groups with 5 mice in each group. Mice in the treatment group received 5 mg capsaicin/kg body weight every day by oral gavage, whereas control mice received vehicle only. Tumor luminescence and animal weight were measured thrice a week for 7 weeks. Effects of capsaicin on (A) tumor growth (B) tumor weight were evaluated. Values are means±SD of 5 samples. *p<0.05 statistically significant when compared with control.
Discussion
Elevated ROS that are generated in response to various stress signals play an essential role in controlling a broad range of physiological and pathological processes, such as cell proliferation, inflammation, and apoptosis. Our previous studies have demonstrated that capsaicin causes ROS generation in pancreatic cancer cells by disrupting mitochondrial electron transport chain complex III, leading to oxidative stress; however, the exact mechanism was not clear (30). Cellular redox homeostasis is maintained by a fine balance between antioxidant and pro-oxidant levels. Trx is a critical intracellular antioxidant that prevents intracellular oxidative stress and maintains redox balance. Furthermore, TrxC32S and TrxC35S constitutively associate with ASK1 and inhibit ASK1-mediated apoptotic activity in a TNF/ROS-resistant manner (47). In the present study, we demonstrated that capsaicin significantly inhibits the growth of pancreatic cancer cells by inducing apoptosis through dissociation of ASK1-Trx complex in vitro and in vivo models.
The main mechanism by which capsaicin activates ASK1 and increases apoptosis is by inhibiting intracellular antioxidant Trx. A previous study has shown that Trx is a physiological inhibitor of ASK1 and binds only to the N-terminal region of ASK1 (33). Our current results support these findings, where capsaicin significantly inhibits Trx, and Trx transfection substantially blocks capsaicin-mediated increase in ASK1 kinase activity and cleavage of PARP. Furthermore, capsaicin-mediated increase in ASK1 kinase activity and cleavage of PARP were blocked by an antioxidant tiron and PEG-catalase, confirming the involvement of pro-oxidants in Trx depletion and ASK1 activation.
The Trx-ASK1 complex interaction is highly dependent on the reducing condition. Binding of Trx with ASK1 was only observed in the presence of a strong reducing agent such as DTT (33). In agreement with these observations, our present results revealed that capsaicin-mediated phosphorylation of ASK1 was inhibited by β-ME or DTT, by sustaining a reduced Trx level. Since only the reduced form of Trx can bind to ASK1, it is possible that in our model, capsaicin-mediated ROS oxidize Trx, disrupt the complex, and consequently activate ASK1. Our redox western blots indeed showed that capsaicin treatment decreased the reduced form of Trx and increased the oxidized form of Trx. The oxidation of Trx was as early as 1 h after capsaicin treatment. Trx preferentially binds with ASK1 under reducing condition; the Trx reductase inhibitor CDNB oxidizes Trx and activates ASK1 (33). In agreement, our results demonstrated that capsaicin oxidizes Trx and inhibits Trx reductase. In addition, CDNB treatment increased capsaicin-mediated phosphorylation of ASK1 at Thr845 and hence kinase activity in pancreatic cancer cells, suggesting that oxidation of Trx resulted in the activation of ASK1. Our findings also revealed that Trx overexpression blocked capsaicin-induced ROS generation and apoptosis, indicating its critical role in capsaicin-mediated apoptosis.
Previous studies have shown that ASK1 plays a critical role in oxidative stress-mediated apoptosis through the activation of JNK- and p38-signaling pathways (42). Our observations indicated that capsaicin-mediated ROS acted as an important signaling intermediate in ASK1-dependent apoptosis. During oxidative stress, the Trx-ASK1 complex is dissociated, and liberated ASK1 gets activated by phosphorylation at Thr845. Phosphorylated ASK1 further activates MKK-4 and MKK-7 cascades, leading to apoptosis (4). Our results demonstrated that capsaicin treatment increases the phosphorylation of ASK1 at Thr845, which further phosphorylates downstream molecules MKK-4 and MKK-7. Our results are in agreement with other studies that reported that ROS generation increase ASK1 kinase activity and phosphorylation at Thr845 (4, 33). Ectopic expression of ASK1 increased the apoptosis-inducing effects of capsaicin, whereas capsaicin failed to exert any effect in the cancer cells with overexpression of kinase dead mutant of ASK1, indicating the requirement of functionally active ASK1 to induce apoptosis.
As a proof of principle, our present in vivo studies demonstrated that oral administration of 5 mg capsaicin/kg body weight substantially suppressed the growth of established AsPC-1 tumor xenografts in athymic nude mice without any noticeable side effects. The tumors from capsaicin-treated mice showed reduced levels of Trx, activation of ASK1, and cleavage of caspase-3 and PARP, indicating apoptosis. Consistent with in vitro studies, our in vivo results also demonstrate that the overall tumor growth suppression by capsaicin was associated with Trx inhibition and activation of ASK1. Although few other studies have shown that capsaicin inhibits the growth of prostate tumor xenografts in mice at similar doses, capsaicin was administered intraperitonealy in those studies (34, 48). A tumor xenograft model does not truly portray the tumor microenvironment, as the tumor is implanted on the skin. Hence, the antitumor effects of capsaicin were further confirmed in an orthotopic tumor model where PanC-1 cells were implanted in the pancreas of athymic nude mice after performing minor surgery. Consistent with the results of the xenograft assay, oral administration of 5 mg capsaicin/kg body weight drastically suppressed the growth of pancreatic tumors in an orthotopic in vivo model as well. Our current studies using two different pancreatic cancer cell lines demonstrated the antitumor effects of capsaicin in two different mouse tumor models. In addition, we have recently shown that feeding 10 ppm or 20 ppm capsaicin in the diet significantly blocks the PanIN lesions in a LSL-KrasG12D/Pdxl-Cre transgenic mouse model (1).
Capsaicin has been used clinically to suppress postoperative wound and cancer-related pain (17, 43). It was also used to treat pain associated with peripheral neuropathy and urinary incontinence (10). To the best of our knowledge, no clinical studies have been performed till date to evaluate the anticancer effects of capsaicin. Our current and previous in vivo studies strongly suggest that capsaicin has remarkable antitumor activity and provides a convincing rationale for further clinical investigation of capsaicin.
Taken together, our findings suggest that capsaicin-mediated ROS generation disrupts the Trx-ASK1 interaction and activates ASK1 by inhibiting Trx, leading to apoptosis in pancreatic tumor cells in vitro and in vivo.
Materials and Methods
Chemicals and antibodies
Capsaicin (purity > 99%), anti-actin, PEG-catalase, tiron, MBP, CDNB, β-mercaptoethanol, 6 M guanidine-HCl, iodoacetic acid (IAA), and Sephadex G-25 were obtained from Sigma. The antibodies against Cl-caspase-3, Cl-caspase-9, Cl-PARP, survivin, ASK1, p-ASK1 (Thr845), HA-tagged, Flag-tagged, Trx-1, and p-MKK-4(T261)/7(S271/T275) were purchased from Cell Signaling. Trx-Reductase was purchased from Santa Cruz Biotechnology, Inc. The specific probes dihydroethidine (DHE), DCFDA, and Goat anti-mouse IgG (H + L) were obtained from Molecular Probes, and apoptosis detection kit Annexin V- fluorescein isothiocyanate (FITC) was procured from BD Bio-Sciences, Inc. Alexa fluor 488 (green), alexa fluor 594 (red)-conjugated goat-anti-rabbit secondary antibody, and 4′, 6-diamidino-2-phenylindole (DAPI) were obtained from Invitrogen. Radioactive [γ32P]-ATP was purchased from MP Biomedical.
Cell culture
Human pancreatic cancer cell lines AsPC-1 and BxPC-3 were obtained from ATCC. Monolayer cultures of AsPC-1 and BxPC-3 cells were maintained in the RPMI medium supplemented with 10% fetal bovine serum, PSN antibiotic mixture (10 ml/l), 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, and 20% glucose. All the cultures were maintained at 37°C in a humidified chamber of 95% air and 5% CO2.
Transient transfection
The AsPC-1 cells were transiently transfected with HA-tagged WT-ASK1 or HA-tagged mutant-ASK1 or Flag-tagged Trx plasmid (a generous gift from Dr. Hidenori Ichijo, University of Tokyo, Japan) using FuGENE-6 transfection reagent (Roche Diagnostics). Briefly, 0.3×106 AsPC-1 cells were transfected with 2 μg of HA-tagged WT or HA-tagged-mutant ASK1 and 2 μg of Flag-tagged Trx using an Opti-MEM serum-free medium. Cells were incubated with a plasmid-FuGENE-6 mixture for 6 h, and after that media were replaced with fresh RPMI media. Transfected cells were treated with 150 μM of capsaicin for an indicated time period.
Immunoprecipitation assay
Immunoprecipitaion assay was performed to examine the effect of capsaicin on the interaction of ASK1 with Trx. Briefly, AsPC-1 cells were treated with DMSO or 150 μM capsaicin or first transiently transfected with HA-tagged ASK1 (2 μg) or Flag-tagged Trx (2 μg) and then treated with 150 μM capsaicin for 24 h. Cells were lysed using RIPA buffer and immonoprecipitated with anti-ASK1 or anti-Trx or anti-HA or anti-Flag tagged antibody. The samples were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunobloted with anti-ASK1, anti-p-ASK1 (Thr845) and anti-Trx antibody.
Determination of Trx mRNA transcripts
To determine the effect of capsaicin on Trx gene transcription, AsPC-1 and HPDE-6 cells were treated with various concentrations of capsaicin for 24 h. RNA was extracted from control and capsaicin-treated cells with Trizol RNA extraction reagent (Life Technologies, Inc.), and RNA samples were prepared for reverse transcriptase–polymerase chain reaction (RT-PCR) as described previously (31). The following primer sets were used: sense, 5′-ATGGTGAAGCAGATCGAGAG-3′; antisense, 5′-GTCACGCAGATGGCAACTGGTT-3′. RT-PCR was performed in 50 μL reactions using 100 ng RNA and 0.5 μM of each primer. The amplification conditions are 95°C for 4 min for denaturation of RNA/DNA hybrids; 55°C for 1 min and 72°C for 1 min for the first cycle; and 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min for 40 cycles of annealing. The last cycle includes extension at 72°C for 10 min. The PCR products were separated on a 1.5% agarose gel, stained with 0.5 mg/ml ethidium bromide, and visualized under UV light.
ASK1 kinase activity
ASK1 kinase activity was measured using MBP as a direct substrate. After transfection with HA-tagged WT-ASK1 or HA-tagged mutant-ASK1 or Flag-tagged WT-Trx for 24 h with FuGENE-6 transfection reagent (Roche Diagnostics), cells were treated with DMSO or 150 μM capsaicin for 24 h. The immunocomplex kinase assay was performed as described previously (11). Briefly, cells were lysed in a buffer containing 20 mM N-[(2-hydroxyethyl) piperazine-N-(2-ethanesulfonic acid)]–KOH (HEPES–KOH) pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA)–Na, 1 mM ethylene glycol tetraacetic acid (EGTA)–Na, 3 mM DTT containing 250 mM sucrose and a mixture of protease inhibitors. Cell extracts were prepared by centrifugation, and the supernatants were immunoprecipitated with anti-ASK1. The beads were washed twice with RIPA buffer with 2 mM DTT, protease, and phosphatase inhibitor. Immune complexes were incubated with MBP (40 μg/ml) in a buffer containing 20 mM Tris-HCL (pH 7.5), 20 mM Mgcl2, and 0.5 μCi of [γ32P] ATP. The proteins were resolved on a 6%–12.5% gradient gel and transferred onto PVDF membrane. MBP phosphorylation was detected using autoradiography. To determine the phosphorylation of ASK1 and apoptosis in the same sample, the upper portion of the membrane (>50 kDa) was cut and immunoblotted with the p-ASK-1 (Thr845) and cleaved PARP antibodies.
Apoptosis assay
Apoptosis induction by capsaicin was assessed using APOPTEST™-FITC kit according to manufacturer's instructions and analyzed by Accuri C6 flow cytometer as described by us previously (32). About 0.3×106 AsPC-1 cells were seeded in a six well plate and treated with 150 μM capsaicin for 24 h after HA-tagged WT-ASK1 or mutant-ASK1 or Flag-tagged WT-Trx transfection. Approximately 10,000 cells were evaluated for each sample.
ROS generation assay
Intracellular ROS generation was determined by measuring the levels of H2O2 and superoxide produced in the cells by flow cytometry as described by us previously (14, 32). Briefly, 0.3×106 cells were plated in six-well plates and allowed to attach overnight. After WT-Trx transfection cells were treated with DMSO or 150 μM capsaicin for 2 h and further incubated with 5 μM DCFDA and 2 μM DHE at 37°C for 30 min. Subsequently, cells were removed, washed, and resuspended in phosphate-buffered saline (PBS) and analyzed using Accuri C6 flow cytometer. Approximately 10,000 cells were evaluated for each sample.
Immunofluorescence assay
Immunofluoresence study was observed by fluorescence microscopy as described by previously with minor modification (39). Briefly, BxPC-3 cells were plated on coverslips, allowed to attach overnight, and then treated with 150 μM of capsaicin for 24 h. Treated and untreated cells were fixed with acetone:methanol (1:1) mixture and blocked with goat-serum for 1 h and incubated with anti-p-ASK1 (Thr845) antibody for overnight at 4°C. Immunofluoresence was detected by anti-rabbit immunoglobulin G (IgG) conjugated with Alexa fluor 488 (green) (1:1000 dilution), DAPI (blue) (1 μg/ml), and Alexa fluor 594 (red) (1:1000 dilution). After four washings, the coverslips were then mounted with antifade mounting reagents. Nuclei were stained with DAPI, and the immmunofluoresence was observed by a fluorescence microscope (Olympus Inc.) using oil immersion at 60×magnification.
Tumor therapy model
Tumor therapy experiment was performed as described by us previously (31) with minor modifications. The use of athymic nude mice and their treatment was approved by the Institutional Animal Care and Use Committee, Texas Tech University Health Science Center. All the experiments were carried out in strict compliance with their regulations. Exponentially growing AsPC-1 (1×106) cells were injected subcutaneously into the right flanks of 20 mice. When the tumors reached a size of ∼70mm3, mice were randomly segregated into 2 groups with 10 mice in each group. Test group of mice received 5 mg capsaicin/kg body weight in PBS by oral gavage every day for 35 days, whereas control mice received vehicle alone. Tumor volume (tumor volume={Length×(Breadth×Breadth)}/2) (mm)3 and animal weights were taken as we have described previously (31).
ROS measurement in tumor sections
In vivo ROS measurement was performed in freshly harvested control and capsaicin treated tumors. About 10 μm tumor section were immediately sliced, placed on glass slides, and kept frozen at −80°C for 24 h. After rapid thaw control and capsaicin-treated tumors were incubated with HANKS solution for 30 min at 37°C. Immediately, slides were incubated in dark with either 2 μM of DCFDA or 10 μM of DHE with DAPI at 37°C. Slides were then washed in PBS for 5 min followed by mounting with cytoseal™ 60 (Richard-Allan Scientific). Fluorescence was visualized immediately using a fluorescence microscope (Olympus Inc.).
In vivo orthotopic tumor model
Four- to 6-week-old female athymic nude mice were obtained from Charles River and kept on an antioxidant-free AIN-76A diet (TestDiet). The animal use and experiments were approved and strictly carried out according to the guidelines of Institutional Animal Care and Use Committee (IACUC). Mice were anesthetized by Ketamine–Xylazine–Acepromazine mixture (30, 6, and 1 mg/kg, respectively) and a small incision made to implant stably luciferase-expressing PanC-1 (PanC-1-luc) cells orthotopically. About 20 μl PBS suspension containing 1×106 exponentially growing PanC-1-luc cells were injected into the subcapsular region on the pancreas using a 30-gauge sterile needle without intra-peritoneum leakage, and then the peritoneum and skin incisions were closed sequentially with absorbable suture. Pain killer was given to the animals at every 8 h for initial 2 days, and animal sickness was closely monitored twice a day until the animals recovered completely from the surgery stress. On the very next day of surgery, animal were imaged for basal luminescence using IVIS Bio Luminescent System equipped with Living Image software (Caliper LifeSciences) after injecting luciferin (3 mg/mouse, ip). Three weeks after the surgical implantation of PanC-1-luc cells, mice were divided randomly into 2 groups with 5 mice in each group. Group I served as controls and received 0.1 ml vehicle every day by oral gavage. Group II received 5 mg capsaicin/kg body weight every day by oral gavage. Tumor luminescence and animal weight was measured thrice a week for 7 weeks. At the end of the experiment, mice were sacrificed; tumors and pancreas were excised from each mouse, weighed, and snap-frozen.
Western blot analysis
Cells were exposed to various concentrations or 150 μM capsaicin for 24 h and lysed on ice as described by us previously (2). In a separate experiment, cells were pretreated with PEG-catalase (500 U/ml) or tiron (10 mM) for 1 h followed by treatment with 150 μM capsaicin for 24 h. Whole-cell extracts were prepared as mentioned above. For ASK1 kinase activity, samples from control and capsaicin-treated cells after transfection with HA-tagged WT-ASK1, HA-tagged mutant-ASK1, and WT-Trx were prepared on ice in buffer containing 20 mM N-[(2-hydroxyethyl) piperazine-N-(2-ethanesulfonic acid)]–KOH (HEPES–KOH) pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA–Na, 1 mM EGTA–Na, and 1 mM DTT containing 250 mM sucrose and mixture of protease inhibitors. The tumors from control and capsaicin-treated mice were minced and lysed by the procedure described by us previously (2). The cell lysate was cleared by centrifugation at 14,000 g for 30 min. Cell lysate containing 10–80 μg protein was resolved by 6%–12.5% SDS-PAGE and the proteins were transferred onto polyvinylidene fluoride membrane. After blocking with 5% nonfat dry milk in Tris buffered saline, membrane was incubated with the desired primary antibody (1:1000 dilution) overnight. Subsequently, the membrane was incubated with appropriate secondary antibody (1:2000 dilution) and the antibody binding was detected by using an enhanced chemiluminescence kit according to the manufacturer's instructions. Each membrane was stripped and re-probed with antibody against actin (1:20000 dilutions) to ensure equal protein loading.
Redox western blotting
Redox state of Trx was evaluated as described previously with minor modification (3, 7, 37). Briefly, AsPC-1 cells were treated with 150 μM capsaicin at various time points. The cells were lysed and carboxymethylated in a buffer containing 50 mM Tris/HCl pH 8.3, 3 mM EDTA, and 5 mM IAA containing 0.5% Triton X-100 and 6 M guanidine- HCl and kept for 1 h at 37°C in the dark. After 1 h at 37°C, excess of IAA was removed using Sephadex G-25 spin columns. Trx-1 redox state was determined using a discontinuous native slab gel electrophoresis.
Statistical analysis
All statistical calculations were performed using Graph Pad Prizm 5.0. Analysis of variance was used to test the statistical significance of difference between control and treated groups followed by Bonferroni's post-hoc analysis for multiple comparisons. p-values <0.05 were considered statistically significant.
Supplementary Material
Abbreviations Used
- ANOVA
analysis of variance
- ASK1
apoptosis signal-regulating kinase 1
- β-ME
β-mercaptoethanol
- CDNB
1-chloro-2-4-dinitrobenzene
- Cl-PARP
cleaved poly (ADP-ribose) polymerase
- DAPI
4′, 6-diamidino-2-phenylindole
- DCFDA
6-carboxy-2, 7-dichlorodihydrofluorescein diacetate
- DHE
dihydroethidine
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- FITC
fluorescein isothiocyanate
- H2O2
hydrogen peroxide
- HA
hemagglutinin
- IAA
iodoacetic acid
- MBP
myelin basic protein
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- RT-PCR
reverse transcriptase–polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TNF
tumor necrosis factor
- Trx
thioredoxin
Acknowledgments
This work was supported in part by R01 Grants CA106953 and CA129038 (to S.K.S.) awarded by the National Cancer Institute, NIH. Kind gifts of Trx, ASK1, and mtASK1 expression plasmids from Dr. Hidenori Ichijo, the University of Tokyo, Japan, are greatly appreciated.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bai H. Li H. Zhang W. Matkowskyj KA. Liao J. Srivastava SK. Yang GY. Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice. Carcinogenesis. 2011;32:1689–1696. doi: 10.1093/carcin/bgr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boreddy SR. Pramanik KC. Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res. 2011;17:1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernando MR. Nanri H. Yoshitake S. Nagata-Kuno K. Minakami S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur J Biochem. 1992;209:917–922. doi: 10.1111/j.1432-1033.1992.tb17363.x. [DOI] [PubMed] [Google Scholar]
- 4.Fujino G. Noguchi T. Matsuzawa A. Yamauchi S. Saitoh M. Takeda K. Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27:8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotoh Y. Cooper JA. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 6.Govindarajan VS. Sathyanarayana MN. Capsicum—production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit Rev Food Sci Nutr. 1991;29:435–474. doi: 10.1080/10408399109527536. [DOI] [PubMed] [Google Scholar]
- 7.Halvey PJ. Watson WH. Hansen JM. Go YM. Samali A. Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 9.Hori M. Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 10.Hussain IF. Fowler CJ. Use of intravesical capsaicin for urge urinary incontinence and irritative voiding syndromes. Curr Opin Urol. 1998;8:293–296. doi: 10.1097/00042307-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Ichijo H. Nishida E. Irie K. ten Dijke P. Saitoh M. Moriguchi T. Takagi M. Matsumoto K. Miyazono K. Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 12.Ito K. Nakazato T. Yamato K. Miyakawa Y. Yamada T. Hozumi N. Segawa K. Ikeda Y. Kizaki M. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64:1071–1078. doi: 10.1158/0008-5472.can-03-1670. [DOI] [PubMed] [Google Scholar]
- 13.Jung MY. Kang HJ. Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001;165:139–145. doi: 10.1016/s0304-3835(01)00426-8. [DOI] [PubMed] [Google Scholar]
- 14.Kandala PK. Srivastava SK. Activation of checkpoint kinase 2 by 3,3′-diindolylmethane is required for causing G2/M cell cycle arrest in human ovarian cancer cells. Mol Pharmacol. 2010;78:297–309. doi: 10.1124/mol.110.063750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim AH. Khursigara G. Sun X. Franke TF. Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim CS. Park WH. Park JY. Kang JH. Kim MO. Kawada T. Yoo H. Han IS. Yu R. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J Med Food. 2004;7:267–273. doi: 10.1089/jmf.2004.7.267. [DOI] [PubMed] [Google Scholar]
- 17.Knotkova H. Pappagallo M. Szallasi A. Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin J Pain. 2008;24:142–154. doi: 10.1097/AJP.0b013e318158ed9e. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y. Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 19.Lo YC. Yang YC. Wu IC. Kuo FC. Liu CM. Wang HW. Kuo CH. Wu JY. Wu DC. Capsaicin-induced cell death in a human gastric adenocarcinoma cell line. World J Gastroenterol. 2005;11:6254–6257. doi: 10.3748/wjg.v11.i40.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Carrillo L. Hernandez Avila M. Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am J Epidemiol. 1994;139:263–271. doi: 10.1093/oxfordjournals.aje.a116993. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M. Masutani H. Nakamura H. Miyajima S. Yamauchi A. Yonehara S. Uchida A. Irimajiri K. Horiuchi A. Yodoi J. Protective activity of adult T cell leukemia-derived factor (ADF) against tumor necrosis factor-dependent cytotoxicity on U937 cells. J Immunol. 1991;147:3837–3841. [PubMed] [Google Scholar]
- 22.Matucci Cerinic M. McCarthy G. Lombardi A. Pignone A. Partsch G. Neurogenic influences in arthritis: potential modification by capsaicin. J Rheumatol. 1995;22:1447–1449. [PubMed] [Google Scholar]
- 23.Modly CE. Das M. Don PS. Marcelo CL. Mukhtar H. Bickers DR. Capsaicin as an in vitro inhibitor of benzo(a)pyrene metabolism and its DNA binding in human and murine keratinocytes. Drug Metab Dispos. 1986;14:413–416. [PubMed] [Google Scholar]
- 24.Monsereenusorn Y. Subchronic toxicity studies of capsaicin and capsicum in rats. Res Commun Chem Pathol Pharmacol. 1983;41:95–110. [PubMed] [Google Scholar]
- 25.Mori A. Lehmann S. O'Kelly J. Kumagai T. Desmond JC. Pervan M. McBride WH. Kizaki M. Koeffler HP. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006;66:3222–3229. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- 26.Morita K. Saitoh M. Tobiume K. Matsuura H. Enomoto S. Nishitoh H. Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura H. Matsuda M. Furuke K. Kitaoka Y. Iwata S. Toda K. Inamoto T. Yamaoka Y. Ozawa K. Yodoi J. Adult T cell leukemia-derived factor/human thioredoxin protects endothelial F-2 cell injury caused by activated neutrophils or hydrogen peroxide. Immunol Lett. 1994;42:75–80. doi: 10.1016/0165-2478(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi S. Nishio A. Nakamura H. Asada M. Tamaki H. Kawasaki K. Fukui T. Yodoi J. Chiba T. Overexpression of redox-active protein thioredoxin-1 prevents development of chronic pancreatitis in mice. Antioxid Redox Signal. 2006;8:1835–1845. doi: 10.1089/ars.2006.8.1835. [DOI] [PubMed] [Google Scholar]
- 29.Powis G. Mustacich D. Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med. 2000;29:312–322. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 30.Pramanik KC. Boreddy SR. Srivastava SK. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PloS One. 2011;6:e20151. doi: 10.1371/journal.pone.0020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahu RP. Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101:176–193. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahu RP. Zhang R. Batra S. Shi Y. Srivastava SK. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009;30:1744–1753. doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitoh M. Nishitoh H. Fujii M. Takeda K. Tobiume K. Sawada Y. Kawabata M. Miyazono K. Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez AM. Malagarie-Cazenave S. Olea N. Vara D. Chiloeches A. Diaz-Laviada I. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis. 2007;12:2013–2024. doi: 10.1007/s10495-007-0119-z. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez AM. Sanchez MG. Malagarie-Cazenave S. Olea N. Diaz-Laviada I. Induction of apoptosis in prostate tumor PC-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin. Apoptosis. 2006;11:89–99. doi: 10.1007/s10495-005-3275-z. [DOI] [PubMed] [Google Scholar]
- 36.Sasada T. Iwata S. Sato N. Kitaoka Y. Hirota K. Nakamura K. Nishiyama A. Taniguchi Y. Takabayashi A. Yodoi J. Redox control of resistance to cis-diamminedichloroplatinum (II) (CDDP): protective effect of human thioredoxin against CDDP-induced cytotoxicity. J Clin Invest. 1996;97:2268–2276. doi: 10.1172/JCI118668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saxena G. Chen J. Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sicuteri F. Fusco BM. Marabini S. Campagnolo V. Maggi CA. Geppetti P. Fanciullacci M. Beneficial effect of capsaicin application to the nasal mucosa in cluster headache. Clin J Pain. 1989;5:49–53. doi: 10.1097/00002508-198903000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Sunters A. Madureira PA. Pomeranz KM. Aubert M. Brosens JJ. Cook SJ. Burgering BM. Coombes RC. Lam EW. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 40.Surh YJ. Lee SS. Capsaicin, a double-edged sword: toxicity, metabolism, and chemopreventive potential. Life Sci. 1995;56:1845–1855. doi: 10.1016/0024-3205(95)00159-4. [DOI] [PubMed] [Google Scholar]
- 41.Teel RW. Effects of capsaicin on rat liver S9-mediated metabolism and DNA binding of aflatoxin. Nutr Cancer. 1991;15:27–32. doi: 10.1080/01635589109514108. [DOI] [PubMed] [Google Scholar]
- 42.Tobiume K. Matsuzawa A. Takahashi T. Nishitoh H. Morita K. Takeda K. Minowa O. Miyazono K. Noda T. Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vorobeychik Y. Gordin V. Mao J. Chen L. Combination therapy for neuropathic pain: a review of current evidence. CNS Drugs. 2011;25:1023–1034. doi: 10.2165/11596280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Watson CP. Evans RJ. Watt VR. Post-herpetic neuralgia and topical capsaicin. Pain. 1988;33:333–340. doi: 10.1016/0304-3959(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 45.Yoshitani SI. Tanaka T. Kohno H. Takashima S. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by dietary capsaicin and rotenone. Int J Oncol. 2001;19:929–939. doi: 10.3892/ijo.19.5.929. [DOI] [PubMed] [Google Scholar]
- 46.Yun TK. Update from Asia. Asian studies on cancer chemoprevention. Ann N Y Acad Sci. 1999;889:157–192. doi: 10.1111/j.1749-6632.1999.tb08734.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang R. Al-Lamki R. Bai L. Streb JW. Miano JM. Bradley J. Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 48.Zhang R. Humphreys I. Sahu RP. Shi Y. Srivastava SK. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis. 2008;13:1465–1478. doi: 10.1007/s10495-008-0278-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.