Abstract
Objective:
To determine the possibility of any association between HBV, HCV, elevated aminotransferase enzymes and Oral Lichen Planus (OLP)patients in Eastern Saudi Arabia.
Design:
Sera were collected from OLP patients, to be tested for HbsAg, anti-HCV and ALT/AST levels.
Settings:
All the patients who were diagnosed clinically in periodontal section, Dammam Central Hospital were from Eastern Saudi Arabia. The histopathological diagnosis was done in Histopathology Section, Dammam Regional Labs, the virological studies in the Virus Diagnosis Lab of Dammam Regional Labs and Blood Bank, and the aminotransferase tests done in the Dammam Central Hospital Labs.
Subjects:
34 serum specimens were collected from OLP patients, and 32 other samples from healthy populations of the same age and sex as the controls of the study.
Results:
Incidence of HBsAg, anti-HCV, HBsAg+HCV, and elevated aminotransferase enzymes among OLP patients were 8.8%, 14.7%, 2.9%, and 47.05% respectively and the results from the control subjects were 6.25%, 3.12%, 0%, and 3.12% respectively.
Conclusion:
There is a significant association between OLP and HCV infection. No clear evidence of this relationship appeared with HBV. All the aminotransferase elevated samples were positive to HCV, giving a clear evidence of the association of chronic HCV infection with the OLP. Aminotransferase elevated results could be used as a clue to clinical signs of asymptomatic hepatopathies, and as a marker to check the OLP cases for the relevant Hepatic Viruses. Despite the limited number of OLP patients in this study, the results could highlight the problem in this geographical area of the world. We recommend a comprehensive study to be carried out using this current study as a preliminary one.
Keywords: Oral Lichen Planus (OLP), Hepatitis C Virus (HCV), Hepatitis B Virus (HBV), Aminotransferase, Saudi Arabia
INTRODUCTION
Lichen Planus (LP) was first described in 1869 by Eramous Wilson.1 It is a pruritic eruption of violaceous polygonal papules topped by characteristic white lines called Wickham's striae.1–3 LP is known for its sub-epidermal lymphocytic infiltration that involves skin, hair, nails, oral and/or genital mucosa,2–4 such infiltration gives a saw-tooth appearance at the dermo-epidermal junction.2,3 However, oral LP is diagnosed by the presence of Wickham's striae which are more common on the buccal mucosa, tongue, gingiva, lips, floor of the mouth and the palate. About 30% of oral LP patients have extra-oral lesions, while about 10-50% of all the LP patients has oral lesions.2,4,5 Nevertheless, LP is generally a benign disease. However, erosive or atrophic LP may progress to malignancy.2–4 Despite many studies on LP, its cause is still unknown, though viral, and/or autoimmune pathogenesis has been proposed and there is some association with hepatitis viruses, especially with Hepatitis C Virus (HCV), as a good percentage of cases are associated with HCV infection.6–18 There is an elevation of the liver enzymes (aspartate amino transferase AST/GOT), and (alanine amino transferase ALT/GPT) in many cases of LP,12,13 and it is well known that the LP patients with normal hepatic enzyme levels are usually HCV negative.2–4,9
The aim of this study is to show the incidence of HCV/HBV among histopathologically diagnosed Oral Lichen Planus (OLP) and to study the levels of the hepatic enzymes in these cases. Such a study could explain the association of the hepatitis virus with the oral LP disease in this part of the world. We used hepatitis B surface antigen (HBsAg), and antibody to hepatitis C virus (anti-HCV) as our indices for Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) infection respectively. However, the basis of our OLP histopathological diagnosis was the presence of acanthosis and hyperkeratosis of the lining of the stratified squamous epithelium with dense chronic inflammatory cell infiltration in association with the destruction of the basal cell layer.
SUBJECTS AND METHODS
Subjects
Between January 1995 and March 1997, 34 specimens were collected from 21 female and 13 male patients with a mean age of 40 years (range 30-50 years) and histologically diagnosed as Oral Lichen Planus (OLP). The subjects were from the main cities of the Eastern Province of Saudi Arabia. Thirty-two serum samples were collected from healthy individuals of the same age and sex distribution as control subjects. The samples collected were either run the same day, or kept at –70°C till processing.
Methods
Anti-HCV screening: Antibodies to HCV proteins expressed by C-100-C and C-22-3 clone regions of the HCV genome were detected by Enzyme Linkage Immuno-Sorbent-Assay (ELISA) kits with sensitivity of 55.64% - 81.62% and specificity of 94.04%-100% Abbott's HCV EIA 2nd generation screening test kits (Abbott Diagnostics, Germany).
Confirmatory HCV tests: The specimens that were repeatedly reactive (RR) by the screening test were re-evaluated by Recombinant Immuno-Blotting Assay Riba IV with sensitivity, and specificity around 100%. RIBA IV kits HCV Blot 3.0 kits were used (Genelabs Diagnostics Pte Ltd, Singapore – under license from: Ortho Diagnostics Systems Inc., and Chiron Corporation, USA). The reactive positive specimens from RIBA IV were considered positive to anti-HCV.
HBsAg screening tests: ELISA technique was used to detect HBsAg (Ayzyme Monoclonal Kits from Abbott's Diagnostics, USA). The RR specimens were reevaluated by the confirmatory test for HBsAg.
HBsAg confirmatory assays: We used confirmatory Abbott's Diagnostics for HBsAg, from Abbott's USA. The confirmed HBsAg by this method was considered as positive for HBsAg.
Hepatic enzymes assay: The Hitachi 717 autoanalyser was used to evaluate the following enzymes: serum aspartate amino-transferase (AST)/Glutamic Oxaloacetic Transaminase (GOT), serum alanine amino- transferase (ALT)/Glutamic pyruvic transaminase (GPT). The normal range for this technique was 0-40 IU/L for both enzymes.
The techniques
All the methodology used in this study followed the instructions of the kit manufacturers.
RESULTS
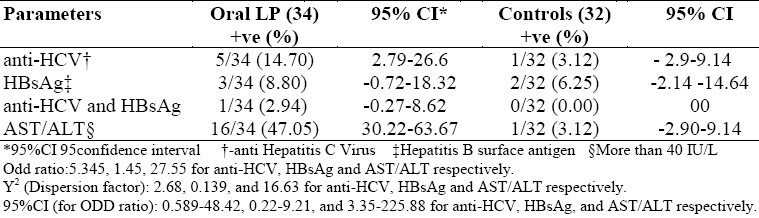
The outcome of our study was tabulated (Table 1) as follows: 14.7% (5 of 34) patients with OLP were positive with HCV antibodies, while in the controls onlyone out of 32 (3.12%) was diagnosed with HCV: HBsAg positive cases represented 8.8% (3 of 34), and 6.25% (2 of 32) of the OLP cases and the controls respectively; the double-infected cases (HBsAg and anti-HCV) represented 2.94% (1 of 34), and 0% (none of 32) among the OLP cases and the controls; aspartate amino transferase (AST/GOT) and Alanine amino transferase (ALT/GPT) elevation was found in 47.05% (16 of 34) and 3.12% (1 of 32) among the OLP cases and the controls respectively.
Table 1.
Incidence of HCV, HBV, elevated hepatic enzymes among Oral LP patients and controls
DISCUSSION
Although the exact causes of LP are still unknown, some evidence suggests that it could be a multifactorial disease or a syndrome caused by more than one etiological agent.
Hepatitis B and C viruses and their relationship to OLP have been a subject to extensive studies6–18 and this paper is reporting a comparative study of HBV and HCV and their supposed relationship to OLP. HBsAg incidence among healthy populations was previously recorded as 8.5%,19 while the incidence among OLP patients was 6.25% (not statistically significant difference) suggesting that HBV is less likely to be a direct cause of OLP, at least in this limited study.
On the other hand, there was 14.7% incidence of Hepatitis C virus among the Oral Lichen Planus (OLP) patients, while the incidence among healthy population in the same geographical area was 2.8%.20,21
The double infections (HBV+HCV) among the OLP cases were 2.94% and 0% among the controls.
In this study, elevated serum aminotransferase enzymes (ALT&AST) were found in 47.05% of the OLP cases, but in only 3.12% of the control subjects. This finding suggests a relationship between ALT/AST and OLP. However, some authors have reported a direct relationship between the aminotransferases and the OLP, especially in errosive OLP patients,9,10,17 and that oral lesions become more aggressive as the liver enzymes increase.9,10 However, OLP patients with normal hepatic enzymes were mostly negative to HCV infection. This finding needs to be confirmed in a next study.
The cause of OLP is not restricted to hepatic C virus infection alone, or to the elevation of aminotransferase enzymes (as a sign of chronic active/chronic persistent hepatitis. Other factors such as cell mediated immune reactions could possibly play a role. Just as an association between OLP and some systemic diseases of immunological origin such as ulcerative colitis, alopecia areata, diabetes mellitus has been suggested by some authors.
To the best of our knowledge, this current study is the first from Eastern Saudi Arabia, or perhaps from the whole Kingdom.
CONCLUSION
In conclusion, our results suggest an association between OLP and HCV infection, especially in patients with high amino transferase enzyme levels. However, HBV does not appear to play a major role in the causation of OLP. However, this is yet to be confirmed. We also conclude that OLP as a disease could be used as a clinical sign of the usually symptomless hepatopathies, and may play a role in the diagnosis of asymptomatic liver diseases.
ACKNOWLEDGMENT
The authors thank Mrs. Paz Duplon F BS (Statistics) of Iba, Zambales, The Philippines, for the statistical analysis of this study. We also extend our thanks to the staff of Virology Diagnostic Labs of Dammam Regional Labs & Blood Bank for their technical help during making this research.
REFERENCES
- 1.Burkhart NW, Burker EJ, Burkes J, Wolfe L. Assessing the characteristics of patients with oral lichen planus. JADA. 1996;127:648–62. doi: 10.14219/jada.archive.1996.0277. [DOI] [PubMed] [Google Scholar]
- 2.Van ML, Parks ET. Prevalence of Oral Lichen Planus in patients with Diabetes Mellitus. Oral Surg Oral Med Oral Radiol Endod. 1995;79:696–700. doi: 10.1016/s1079-2104(05)80302-6. [DOI] [PubMed] [Google Scholar]
- 3.Bunker CB, Doiod PM. John Axford Medicine. Oxford, London, UK: Blackwell; 1996. Skin Disease; pp. 14.14–14.68. [Google Scholar]
- 4.Lamey PJ, McCartan BS, MacDonald DG, Mackie RM. Basal cell cytoplasmic autoantibodies in Oral Lichenoid reaction. Oral Surg Oral Med Oral Path Oral Radiol Endod. 1995;79:44–9. doi: 10.1016/s1079-2104(05)80072-1. [DOI] [PubMed] [Google Scholar]
- 5.Jabrt C, Pawlotsky TM, Puget F, Andre C, De-Forges L, Bretagne S, et al. Lichen Planus and Hepatitis C Virus (HCV) – related chronic active hepatitis. ARCH Dermatol. 1994;130:73–6. [PubMed] [Google Scholar]
- 6.Boisnic S, Ouhayoun JP, Branchet MC, Francis C, Beranger JY, Le-Charpentier Y, Szpirglas H. Alteration of Cytokeratin expression in OLP. Oral Surg Oral Med Oral Path Oral Radiol Endod. 1995;79:207–15. doi: 10.1016/s1079-2104(05)80283-5. [DOI] [PubMed] [Google Scholar]
- 7.Porter S. OLP and chronic liver disease. Oral Surg Oral Med Oral Pathol. 1995;79:267–8. doi: 10.1016/s1079-2104(05)80217-3. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Perez J, De-Castro M, Buezo GF, Fernandez-Herrer J, Borque MJ, Garcia-Diaz A. Lichen Planus and HCV: Prevalence and clinical presentation with LP and HCV infection. Br Jour Derm. 1996;134:715–9. doi: 10.1111/j.1365-2133.1996.tb06977.x. [DOI] [PubMed] [Google Scholar]
- 9.Bagan JV, Aguirre JM, Del-Olmo JA, Milian A, Penarrocha M, Rodrigo JM, et al. Oral Lichen Planus and chronic liver disease: A clinical and morphometric study of the oral lesions in relation to transaminase elevation. Oral Surg Oral Med Oral Path. 1994;78:337–42. doi: 10.1016/0030-4220(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 10.Amichai B, Lazarov A, Halevy S. HCV infection and oral erosive LP. J Dermatol. 1994;21(10):783–4. doi: 10.1111/j.1346-8138.1994.tb03289.x. [DOI] [PubMed] [Google Scholar]
- 11.Gondolfo S, Carbone M, Carrozzo M, Gallo V. OLP and HCV infection: is there a relationship? J Pathol Med. 1994;23(3):119–22. doi: 10.1111/j.1600-0714.1994.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagao Y, Sata M, Tanikawa K, Kemyama T. A case of OLP with chronic HCV successfully treated by glycyrrhizin. Sans Enshogaku-Zasshi. 1995;69(8):940–1. doi: 10.11150/kansenshogakuzasshi1970.69.940. [DOI] [PubMed] [Google Scholar]
- 13.Jorge JJ, Lopez MA, De-Almeida OP, Scully C. Oral LP and chronic hepatitis B. Dental Update. 1994;21(8):335–7. [PubMed] [Google Scholar]
- 14.Nagao Y, Sata M, Tanikawa K, Itoh K, Kameyama T. LP and HCV in the Northern Kyushu Region of Japan. Eur J Clin Invest. 1995;25(12):910–14. doi: 10.1111/j.1365-2362.1995.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 15.Nagao Y, Sata M, Fukuizumi K, Harda H, Kameyama T. Oral cancer and HCV: Can HCV alone cause oral cancer? Kurume Med J. 1996;43(1):97–100. doi: 10.2739/kurumemedj.43.97. [DOI] [PubMed] [Google Scholar]
- 16.Carrozzo M, Broccoletti R, Carbone M, Gandolfo S, Garzino P, Cascio G. Phenotypic analysis of peripheral blood cell immunity in Italian patients with different varieties of Oral Lichen Planus. Bull Group Int Rech Sci Stomatol Odontol. 1996;39(12):33–8. [PubMed] [Google Scholar]
- 17.Papini M, Bruni PL, Bettacchi A, Liberati F. Sudden onset of oral ulcerative lichen in a patient with chronic hepatitis C on treatment with alfa-interferon. In J Dermaol. 1994;33(3):221–2. [PubMed] [Google Scholar]
- 18.Seoane L, Sanchez-Lopez M, Romero-Mendez MA, Gomez-Duaso A, Esparza-Gomez GC, Cerero-Lapiedra R. Lichen Planus of the oral mucosa in the clinical course of chronic active hepatitis. Odontoestomatol. 1991;7:109–12. [PubMed] [Google Scholar]
- 19.Fathalla SE, Namnyak SS, Al-Jama AA, Rabaria-Bautista MM. The prevalence of Hepatitis B Surface Antigen (HBsAg) in healthy subjects residing in the Eastern Province of Saudi Arabia. Saudi Med Jour. 1985;6:236–41. [Google Scholar]
- 20.Fathalla SE, Al-Jama AA. Prevalence of Hepatitis C Virus (HCV) antibodies in blood donors, pregnant women, and haemodialysis patients in the Eastern Province of Saudi Arabia. Saudi Medical Journal. 1993;14:265. [Google Scholar]
- 21.Fathalla SE, Al-Jama A, Badawy MS, Sabry HS, Awad OA, Abdulaziz FM, El-Najar MKM. Prevalence of HCV infection in the Eastern Province of Saudi Arabia by re-DNA second generation and supplemental EIA tests. Saudi Medical Journal. 1994;15(4):281–5. [Google Scholar]


