Abstract
Objective:
The main objective of this study was to determine the prevalence of some potential entropathogens among primary school children.
Methodology:
This study was conducted, on a sampled population of3258 primary school going children in the age group of 6-11 years. They were investigated for the presence of some potential enteropathogens in their stools.
Results:
The overall prevalence of enteropathogens was 10.44 percent. Salmonella and Shigella species were found among 114 percent children. Multiple drug resistance was common in the isolated species of Salmonella and Shigella with ma exception of Nalidixic acid and cephalothin in Shigella. The prevalence rate of parasitic infection was 9.30%. The most common parasite found was giardia lambia, 8.16 percent, and next most common was Entamoeba histolytica 0.74%, followed by other parasites: (Hemenolepis nana, Ascaris lumbricoides, Trichuris trichuira and Enterobious vermicularis, in order of their frequencies).
Conclusion:
This study lays emphasis on the importance of asymptomatic carriers as a potential source of infection and demonstrates the emergence of resistance in salmonella and Shigella species.
Keywords: Asymptomatic Salmonella, Shigella, Intestinal Parasites, School Children
INTRODUCTION
School children form an important closed community, with chances of child to child transmission of infection. Available studies show high prevalence of enteropathogens among school going children1,2. Little is known about the prevalence of asymptomatic enteropathogens among these children in Saudi Arabia. Most of the studies available, have been carried out on food handlers and hospital patients3–6.
Enteropathogens are known to have deleterious effect on the health and nutrition of the children, leading to many complications7,8. The present study of asymptomatic salmonella, Shigella, and intestinal parasites among school going children was conducted in the Dammam area of the Eastern Province of Saudi Arabia during 1992-93. The main objective of the study was to determine the prevalence of some potential enteropathogens among these children and discuss their public health importance.
MATERIAL AND METHODS
The study population consisted of primary school children, in the age group of 6-11 years. This represented 54% of the total school population in this age group in the area. A multistage systemic random sampling method was used to select children for the study. For each child, general information was recorded in a predesigned proforma and the age was taken from school records. The main criteria for inclusion in the study were; an asymptomatic child with no history of diarrhoea/dysentery or antibiotic use within past two weeks. The schools were visited one day prior to the collection of the stool sample. One section was taken per day. A plastic container was given to each child who was advised to bring the stool sample next morning. To avoid delay the samples were taken directly after collection to the laboratory for analysis. The compliance rate was 95%. Intestinal parasites were identified by direct microscopy, using a wet mount of stool specimen with normal saline and iodine and the concentration technique used as described in medical laboratory manual.9 Direct plating of stool sample was done to detect salmonella and Shigella species as per standard method described by WHO. Sensitivity testing was done for ampicillin, cotrimoxazole, tetracyclines, chloramphanicol nalidixic acid and cephalothin.10 The chi-square test and analysis of variance were applied for statistical analysis.
RESULTS
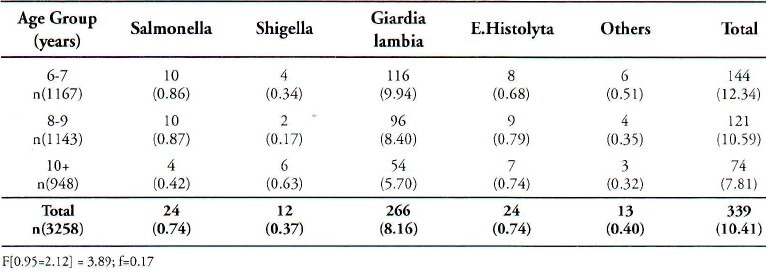
Stool specimens of 3258 children were collected and examined for the presence of intestinal parasites and cultured for salmonella and Shigella species. The percentage of boys and girls in the study group was 49.02% and 50.98% respectively. Saudi children formed 75.61% of the sample. Age and sex distribution of these children is shown in Table 1. Out of total sample, 340 (10.44 %) were positive for one of the enteropathogens. Table 2 is a summary of the isolated enteropathogens from these children. The prevalence among the boys and the girls was 10.58% and 10.30% respectively. Non typhiodal Salmonella and Shigella species (Shigella dysenteriae, Shigella flexmeri, Shigella boydii and Shigella sonnei) were isolated among 1.14% children and intestinal parasites among 9.30% children. There was only one sample with dual infection. No significant statistical relationship was observed in the prevalence of different enteropathogens, between boys and girls. Age specific isolation rates given in Table 3, which show that these isolated enteropathogens were common in all age groups. A higher percentage of Giardia lambia was found in the lower age groups. The Regression coefficient between the age groups did not show any significant relationship. Invitro antibiotic sensitivity of Salmonella and Shigella species indicates that these isolates were resistant to commonly used antibiotics except nalidixic acid and cephalothin in Shigella species.
Table 1.
Age and Sex Disribution of the Study Group
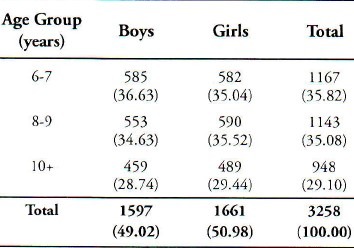
Table 2.
Sex Wise Prevalence of Enteropathogens Among Study Group
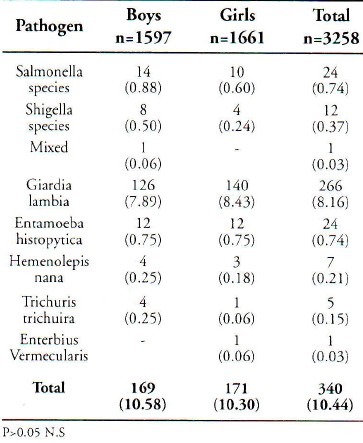
Table 3.
Age Specific Prevalence of Salmonella, Shigella and Intestinal Parasites
DISCUSSION
Three hundred and forty children carried one of the potential enteropathogens, with an overall prevalence rate of 10.44%. The prevalence rate of bacterial and parasitic infections among these children show that asymptomatic infection is common, affecting boys and girls equally. The rates of non-typhiodal Salmonella and Shigella species were 0.74% and 0.73% respectively. Perhaps the rates would have been higher if more than one sample had been collected from the same child.
Isolation of these enteropathogens from these asymptomatic children indicate that they are carriers of these microogranisms. A source of infection that may be hazardous to the health of other children and the community at large. Salmonella and Shigella species are commonly isolated microorganisms among children suffering from diarrhoea/dysentery.11 In our study, the isolated Salmonella and Shigella species were resistant to commonly used antibiotics (Ampicillin, Cotrimoxazole, Chloramphanicol, Tetracyclines). Similar reports of multidrug resistance to the standard chemotherapy are now accumulating from different countries12–15 and many outbreaks due to these resistant microorganisms16,17 have also been recorded.
Unfortunately, the option for antimicrobial therapy of Shigellosis has diminished considerably in recent years, as bacterial resistance has increased. Resistance to ampicillin and cotrimoxazole is now widespread, particularly in Shigella dysentariae type I, and in Shigella flexnari. All the children in our study who were positive for Shigella species were given a course of Nalidixic acid, to which the organism was highly sensitive. Nalidixic acid was used as backup during the treatment, but although a drug of’ choice for Shigellosis at present, there are cases of resistance to it.18
New drugs of promise for the treatment of Shigella infection include pencillin and new floroquinolines. These drugs are expensive and their safety in children have not been established.18 The children who were positive for Salmonella species did not receive any treatment, since treatment of asymptomatic Salmonellosis increases drug resistance. Their stools were cultured again and again until three consecutive samples were free of pathogenic organisms.
The parasitic infection rates were higher among these school children, with Giardia lambia predominating. Similar results have been obtained by a study conducted in Bahrain, which reported a prevalence of 14.3% and less than prevalence reported by the studies conducted in developing countries.19–22 The factors responsible for this similarity in parasitic infection found among children living in Bahrain and our study may possibly be due to common regional characteristics with Bahrain and socioeconomic and cultural differences with other developing countries may explain the difference in results between our study and of other countries. All the children who were positive for Giardia lambia and Entamoeba histolytica were given a course of metronidazole.
In practical terms, Salmonella, Shigella species, Giardia lambia, Entamoeba histolytica can be transmitted directly to other children. The transmission through swimming ponds and intact chicken eggs have also been reported by studies.23–25
In view of the better living conditions and safe water supply in Saudi Arabia the possible source of infection may be contaminated food, vegetables and fruits, which are known to play an important role in the transmission of these enteropathogens. We cannot exclude the role of workers from different endemic countries, particularly food handlers in disseminating the infection. However, the risk of transmission among these school going children may be controlled by increasing health education activities focused on improving hygienic practices, like hand washing, handling and consuming raw and uncooked food. There is also a need to upgrade the diagnostic facilities in school health care services for periodic stool examination and effective treatment. This study also points to the emerging resistance in Salmonella and Shigella species and a need to monitor growing antibiotic resistance among these species countrywide. However, in-depth studies are needed on epidemiological and other aspects of Intestinal enteropathogens.
ACKNOWLEDGMENT
We appreciate the cooperation offered by the school staff and school health services. We express our gratitude for the interest shown by our laboratory staff and also Mr. Reyaz A. Beigh for his assistance in preparing the manuscript.
REFERENCES
- 1.Olusanya O, Shonukan OO, Ogwa V A. Childhood Carriers of Salmonella and Shigella species in the rural areas of lle-lfe. Nigeria-Shop Med J. 1990;28(2):49–50. [PubMed] [Google Scholar]
- 2.Taha A Z A, Al-Amin HB, Sohaibani M, Ali M A, AI Omran K M. Prevalence and manifestations of Intestinal Parasites in Urban School Children - Eastern Province, Saudi Arabia. Journal of Tropical Medicine. 1991;5:85–88. [Google Scholar]
- 3.Abu-AI Saud AS. Faecal Parasites in Non Saudi catering and domestic staff in the Riyadh Military Hospital. Saudi Med J. 1983;4:259–262. [Google Scholar]
- 4.Khan Z A, Al-Joma A A, Medan I. Parasitic infections among food handlers in Dammam and Al-Khobar, Saudi Arabia. Annals Saudi Med. 1987;7:47–50. [Google Scholar]
- 5.Al-Fayaz S F, Khogheen Y A. A follow up study on prevalence of parasitic infections among patients attending King Abdulaziz University Hospital. Saudi Med J. 1989;10(3):193–197. [Google Scholar]
- 6.Qadri H M, Khalil S H. Intestinal parasites: Incidence and aetiology in over 1000 patients at King Faisal Specialist Hospital in Riyadh. Annals Saudi Med. 1987;7:207–211. [Google Scholar]
- 7.Korman S H, Baroz B, Mandelberg A, Matoth I. Giardiasis with protein losing enteropathy diagnosis by faecal alpha 1-antitrypsin determination. J Paediatr Gastroenterol Nutr. 1990;10(2):249–52. doi: 10.1097/00005176-199002000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Benish M L. Potentially lethal complications of shigellosis. Rev Infect Dis. 1991;(Suppl 4):319–24. doi: 10.1093/clinids/13.supplement_4.s319. [DOI] [PubMed] [Google Scholar]
- 9.Monica C. Medical Laboratory Manual for Tropical Countries. 2nd edition. Vol. 1. London: Buterworth-Heinemann Ltd; 1991. [Google Scholar]
- 10.World Health Organization. Manual for laboratory investigation of Acute Enteric Infections. Programme for Control of Diarrohoeal Diseases WHO Geneva CDD. 1983;3 [Google Scholar]
- 11.Qadri H, Mohammad A, Muhammad IH. Acute diarrhoeal diseases in children under 5 years of age in the Eastern Province of Saudi Arabia. Annal of Saudi Medicine. 1990;10(3):65–70. [Google Scholar]
- 12.Salam MA, Bennish M L. Antimicrobal Therapy for shigellosis. Rev Infect Dis. 1991;13(4):332–41. doi: 10.1093/clinids/13.supplement_4.s332. [DOI] [PubMed] [Google Scholar]
- 13.Leano FT, Saniel MC, Monzon OT. Prevalent Serogroups and antimicrobal susceptibility of Shigella strains in Metro Manila, 1982-1988. Southeast Arian. J Trop Med Public Health. 1990;21(2):207–13. [PubMed] [Google Scholar]
- 14.Bean N, Blake PA. Antimicrobal resistance of shigella isolates in the U.S.A. The importance of international travellers. J Infect Dis. 1990;162(5):1107–11. doi: 10.1093/infdis/162.5.1107. [DOI] [PubMed] [Google Scholar]
- 15.Zoukh K. Resistance to antibiotics of Salmonella other than typhi and paratyphi isolated in Algeria from 1979 to 1985. Pathol Biol (Paris) 1988;36:255–257. [PubMed] [Google Scholar]
- 16.Lee LA, Ostroff SM, McGee HB, Jhonson DR, Downess FP, Cameron DN, et al. An outbreak of shigellosis at an outdoor music festivals. Am J Epidemiol. 1991;133(6):608–15. doi: 10.1093/oxfordjournals.aje.a115933. [DOI] [PubMed] [Google Scholar]
- 17.Hernden J, Meriwether R A, Mac Cormak JN, Levine RM. A large outbreak of antibiotic resistant shigellosis at a mass gathering. J Infect Dis. 1990;162(6):1324–8. doi: 10.1093/infdis/162.6.1324. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. The management of Bloody diarrhoea in young children. Programme of diarrhoeal disease WHO/CDD 1994.49 Geniva [Google Scholar]
- 19.Musaiger AO, Gregorg WB. Changes in Parasitic infections among schoolchildren in Bahrain, 1980--1986. A preliminary study. Saudi Med J. 1990;11(2):113–115. [Google Scholar]
- 20.WHO Prevention and Control of intestinal Parasitic infection Geneva. World Health Organization, Tech. Rep.749. 1987:7–18. [PubMed] [Google Scholar]
- 21.Bolbol AS, Mahmoud AA. Laboratory and Clinical study of intestinal pathogenic parasites among the Riyadh population. Saudi Med J. 1984;5:159–166. [Google Scholar]
- 22.Abdul Hafeez MA, El-Kadi N, Noah MS, Bolbol MS, Bakinia MH. Parasitic infestations in expatriates in Riyadh, Saudi Arabia. Annals Saudi Med. 1987;7:202–206. [Google Scholar]
- 23.Pellitier AR, Finger RF, Sosin DM. Shigellosis in Kentucky, 1986-1989. South MedJ. 1991;84(7):818–21. doi: 10.1097/00007611-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Blostein J. Shigellosis from Swimming in a park pond in Michigan. J Public Health Rep. 1991;106(3):317–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Mishu B, Griffin PM, Tauxe RV, Cameron DN, Hutchesan RH, Schaffner W. Salmonella enteritidis gastoenterites transmitted by intact chicken egg. Am Intern Med. 1991;115(3):190–4. doi: 10.7326/0003-4819-115-3-190. [DOI] [PubMed] [Google Scholar]


