Abstract
Striatal degeneration may contribute to cognitive impairment in older people. Here, we examine the relation of degeneration of the striatum and substructures to cognitive decline and dementia in subjects with a wide range of cognitive function. Data are from the prospective community-based Honolulu Asia Aging Study of Japanese American men born 1900–1919. Brain MRI (1.5T) was acquired on a stratified sub-sample (n=477) that included four groups defined by cognitive status relative to the scan date: subjects without dementia (n=347), subjects identified as demented 2–3 years prior to brain scanning (n=30), at the time of scanning (n=58), and 3–5 years after scanning (n=42). Volumes of the striatum, including the accumbens, putamen, and caudate nucleus were automatically estimated from T1 MR images. Global cognitive function was measured with the CASI, at four exams spanning an 8 year interval. Trajectories of cognitive decline were estimated for each quartile of striatal volume using mixed models, controlling for demographic variables, measures of cerebro-vascular damage, global brain atrophy, and hippocampal volume. Diagnosis of dementia before, during, and after brain scanning was associated with smaller volumes of n. accumbens and putamen, but not with caudate nucleus volume. Subjects in the lowest quartile of n. accumbens, both in the total sample and in the subjects not diagnosed with dementia during the study, had a significantly (p < 0.0001) steeper decline in cognitive performance compared to those in the highest quartile. In conclusion, volumes of the n. accumbens and putamen are closely associated with the occurrence of dementia and n. accumbens volume predicts cognitive decline in older people. These associations were found independent of the magnitude of other pivotal markers of cognitive decline, i.e. cerebro-vascular damage and hippocampal volume. The present study suggests a role for the ventral striatum in the development of clinical dementia.
Keywords: MRI, volumetry, striatum, ventral striatum, nucleus accumbens, putamen, caudate nucleus, dementia
1. Introduction
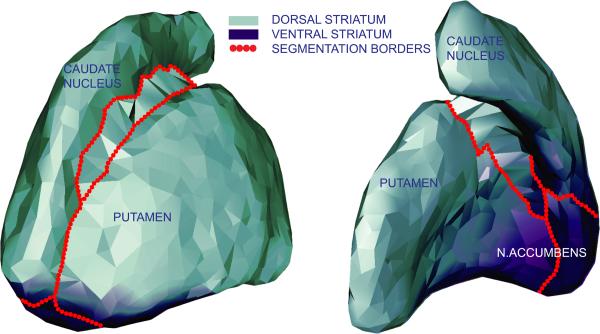
The effects on cognition of degenerative changes in the medial temporal lobe have been widely studied. However, other structures atrophy with age as well and may also contribute significantly to late-life cognitive impairment. The striatum is of particular interest because it is part of two systems prone to degeneration in older people, the limbic and the fronto-striatal system. The striatum, depicted in figure 1, is anatomically divided by the capsula interna into the caudate nucleus, putamen, and nucleus accumbens. The caudate nucleus and putamen are histologically similar and their functions are thought to be congruous with their somato-topographical connections to the neo-cortex. The caudate nucleus is part of circuits to the dorsolateral prefrontal cortex, lateral orbital prefrontal cortex, and posterior parietal cortex. The putamen is part of circuits with the motorcortex and the somato-sensory cortex (Utter and Basso, 2008). The nucleus accumbens, located ventro-anterior, differs histologically and functionally from the caudate nucleus and putamen. Its cells have smaller dimensions and are organised into subnuclei (Brockhaus, 1942). The n. accumbens projects to, and receives input from, several limbic regions including the medial temporal lobe and anterior cingulate cortex. Functionally, the ventral striatum (n. accumbens and fundi of the caudate and putamen) participates in processing limbic information and the dorsal striatum (caudate nucleus and putamen) in sensorimotor information (Voorn et al., 2004).
Figure 1. Anatomical and functional division of striatum.
Presented is a 3D model of the striatum, based on the segmentation of those voxels that were labeled striatum in > 20% of the segmentation masks. Model was created with GAMEs (Ferrarini et al., 2007) and displayed here for explanational purposes.
The role of the striatum in cognitive processes has been studied in specific basal ganglia disorders and as part of basal forebrain atrophy in Alzheimer's disease (AD). In Huntington's disease (HD), atrophy of the caudate nucleus is associated with impaired executive functioning (Peinemann et al., 2005), bicaudate ratio with impaired language learning (De Diego-Balaguer et al., 2008), and smaller volumes of the putamen with worse psychomotor function (Jurgens et al., 2008). Apart from classical basal ganglia diseases, in a recent volumetric study it was observed that AD cases had significantly decreased volumes of the putamen compared to memory complainers (de Jong et al., 2008). Also, basal forebrain atrophy, including parts of the ventral striatum, was observed as long as 4.5 years before the development of clinical symptoms (Hall et al., 2008; Teipel et al., 2005). Despite the data on striatal volumes in dementia and basal ganglia diseases, little is known on the relation between striatal volume and cognitive decline in older people, varying from cognitively “normal” to impaired. Also not known is, whether other predictors of cognitive impairment, such as cerebro-vascular damage, global brain atrophy, or hippocampal volume, mediate this relationship or whether striatal volumes can improve our ability to predict cognitive decline in older people.
Here, we examine the relation of striatal volume to dementia and global cognitive function and decline, in the entire spectrum from cognitively healthy to demented older subjects. We account for the presence and extent of several pivotal cerebro-vascular damage parameters, hippocampal volume, and global brain atrophy. Subjects are from the well-characterized population based cohort of the Honolulu-Asia Aging Study (HAAS), who participated in an MRI sub-study.
2. Methods
2.1 Subjects and study design
Study subjects were older Japanese-American men, born between 1900–1919, who participated in the HAAS, an expansion of the Honolulu-Heart Program. A detailed description of the HAAS can be found elsewhere (White et al., 1996). In short, subjects were examined in 1991–1993 (baseline exam 4), and in three follow-up exams in 1994–1996 (exam 5), 1997–1999 (exam 6), and 1999–2000 (exam 7). The study was approved by the IRB of the Kuakini Hospital and all respondents signed informed consent forms, except those who were demented, for whom an informed caretaker signed the consent
Assessment of cognitive function and dementia
During each exam all subjects were evaluated on cognitive performance and dementia cases were ascertained using a multi-step procedure described elsewhere (White et al., 1996). Briefly, all subjects were screened with the Cognitive Ability Screening Instrument (CASI), which ranges in score from 0–100 (Teng et al., 1994). If subjects were screened positive, they were further evaluated with neuro-psychological tests based on the CERAD battery (Morris et al., 1989), a neurologic exam, a proxy interview, and a diagnostic brain scan. Diagnoses were made in a consensus meeting in which the DSM-IIIR was applied for dementia, the NINDS-ADRDA-criteria (McKhann et al., 1984) for AD, and the CADDTC-criteria were applied for vascular dementia (VaD) (Chui et al., 1992). Depressive symptomology was measured in the first exam, using the Center for Epidemiologic Studies-Depression scale (CES-D) (Radloff LS, 1977).
During the first follow-up examination (exam 5 1994–1996), whole brain Magnetic Resonance Imaging (MRI) was obtained on a stratified sub-sample of the total cohort. This sub-sample included a random sample of approximately 10 percent of the cognitively unimpaired participants and a selected over-sample of subjects with prevalent dementia (exam 4), subjects who scored poorly on cognitive testing but did not meet the criteria for clinical dementia, subjects who possessed the apoliprotein E type ε4 (ApoE ε4 positive), and subjects with clinical stroke (Scher et al., 2007). Successful brain MR images were obtained from 575 subjects.
2.2 MRI acquisition and readings
Magnetic Resonance Imaging (MRI) was performed using a 1.5 Tesla MRI system (GE Signa Advantage) in the Kuakini Medical Center in Honolulu. The protocol has been described (Scher et al., 2007). Standardized MR readings were performed by readers at the John Hopkins Reading Center, blinded to the subject's medical history or health status at the time of scanning. The number of cerebral infarcts, lacunes, subcortical infarcts, and white matter lesions was evaluated according to Cardiovascular Health Study protocols (Longstreth et al., 1998). Lacunar infarcts were defined having a maximal diameter of 3.0 – 20 mm. For this analysis, subjects were grouped according to the number of lacune identified (0= no lacunar infarcts, 1=1–2, and 2=3–5 lacunes). White matter hyperintensities (WMH) on proton density images were scored on a scale from 0–9 (0 = no white matter hyperintensities and 9 = all white matter involved), and for the analyses grouped as follows: 1=1–3, 2=4–6, and 3=7–9. As an indicator of global brain athrophy, we measured the bi-frontal distance, on defined on T1 weighted axial slices, as the largest right-left distance between the lateral borders of the right and left frontal horns. Inner table distance, the right-left dimension between the inner tables of the skull, was measured at the same level of the bi-frontal distance, and used to correct the bi-frontal distance for intra cranial volume (Korf et al., 2004). Manually measured intra cranial volume (ICV) and hippocampal volume were acquired using a protocol described previously (Korf et al., 2004).
2.3 Segmentation of cortical and deep grey matter structures
The algorithm FIRST (FMRIB's Integrated Registration and Segmentation Tool), of the FSL package (Smith et al., 2004) was used for automated segmentation of left and right nucleus accumbens, caudate nucleus, and putamen (Patenaude, 2007). This software package has been evaluated relative to manual tracing methods and other automated segmentation tools and an average Dice coefficient for the striatum of 0.81 was estimated (Babalola et al., 2009; Morey et al., 2009). We used the run_first_all-script with default options, followed by a boundary correction with a Z-score of 3. The output of FIRST consisted of non-normalized striatal volumes. Total striatal volume was calculated by adding up the volumes of the caudate nucleus, putamen, and n. accumbens.
2.4 Statistical analysis
Analytical sample; demented and not demented cases
There were 549 scanned subjects with successfully processed ICV and hippocampal volume (Korf et al., 2006). For the present study we excluded subjects whose scans could not be successfully processed by FIRST (n=18), those with subcortical infarcts > 20 mm (n=20), which may cause large errors in the computed volumes, and those who were diagnosed with or developed types of dementia other than AD, VaD, or mixed type of AD/VaD dementia (n=34). Our final study sample included 477 subjects. In general, the sample of prevalent and incident dementia cases in this analysis tended to be of mild severity and to have a slower rate of functional decline than cases who were not scanned. Of those who were not diagnosed with dementia during the study about 30% did not survive the total 8 year follow-up time.
Comparison striatal volumes by time of dementia diagnosis relative to brain scan
Each striatal structure volume was normally distributed across the total study sample of 477 subjects. To assess whether the striatal volumes are smaller in subjects with dementia or will be diagnosed with dementia in the future, we assigned each subject to one of 4 exhaustive and mutually exclusive categories; (1) those who had been identified as demented at the first HAAS examination 4 (baseline prevalent dementia cases: n=30), (2) those who were identified as demented at the first follow up examination 5 (incident dementia cases when scanned n=59), (3) those who were identified as demented in follow up exams 6 or 7 (n=42), and (4) all others (n=347). For descriptive purposes, we will refer to these groups as: prevalent dementia, incident dementia, future dementia, and no dementia. The groups were compared on age, ICV, years of education, CASI-score, CES-D score, bi-frontal distance (corrected for inner table distance), hippocampal volume, and striatal volumes by one-way ANOVA, and compared on presence of cerebral and lacunar infarcts, WMH grade, and presence of ApoE 4 allele (yes = 34 and 44, no=all other genotypes) (Hixson and Powers, 1991) by a Pearson's chi square test. Tests for linear association and pairwise comparison of striatal volumes between the study groups were performed.
Assessment of the association of striatal volume to cognitive decline
For the longitudinal analyses the total sample was divided by quartile of volume of the striatum and sub-structures. Slopes from quartiles 1 – 3 were, separately, compared to the slope of the 4th quartile (highest volume quartile). For this, a mixed model approach was used, with random intercepts and age at each of the four HAAS exams as the time line. The mixed model accounted for varying time intervals between the exams of different subjects, differences in the number of measurements per subject (unbalanced data), and differences in age at baseline exam. Whether or not slopes differed across quartiles and over time, was tested with an interaction term of age (the time scale) and quartile of volume, compared to the 4th quartile. We adjusted for age at baseline exam, educational level, ICV, CES-D, lacune grade, presence of cerebral infarcts, WMH grade, bifrontal distance, presence of ApoE 4 allele, and quartiles of hippocampal volume. To check whether potential associations were not due to overrepresentation of demented subjects in the lower quartiles of volume, we re-ran the mixed model analysis on the no dementia group only (n=347). Furthermore, we tested non-linearity in the cognitive trajectories by adding a quadratic term into the model. This term was not significant, and the linear model was more parsimonious without sacrificing the model fit (supplementary table 1), so we proceeded with the linear model.
3. Results
3.1 Dementia and smaller striatal volumes
The dementia study groups differ in age (p = 0.0083), CASI-score (p < 0.0001), volumes of the striatum (p = 0.0004), putamen and accumbens volume (p < 0.0001), all three indicators of cerebro-vascular damage, i.e. the presence of cerebral and lacunar infarcts and WMH grade (p < 0.0001), bi-frontal distance (p < 0.0001), and hippocampal volume (p < 0.0001) (Table 1). Pairwise comparison between no dementia group and each of the three dementia groups shows significantly smaller volumes of the total striatum, n. accumbens, and putamen in the prevalent (all p < 0.001) and incident dementia (p = 0.002 for total striatum, p < 0.001 for n. accumbens, p = 0.002 for putamen) groups, and also smaller volumes of the accumbens in the future dementia group (p = 0.003). Comparison of prevalent dementia with future dementia shows larger volumes of the striatum (p = 0.032), putamen (p = 0.012), and accumbens (p = 0.001) in future dementia and comparison of prevalent dementia with incident dementia shows larger a volume of the accumbens (p = 0.014) in incident dementia.
Table 1.
Group characteristics according to identification of dementia relative to MRIa : HAAS MRI sub-sample
| No dementia (n=347) | Future dementia (n=42) | Incident dementia (n=58) | Prevalent dementia (n=30) | p-value b | |
|---|---|---|---|---|---|
| Age [years] | 81.3 (5.0) | 82.7 (5.1) | 82.2 (4.9) | 84.2 (5.1) | 0.0082 |
| ICV [cm3] | 1436 (110) | 1429 (116) | 1440 (107) | 1442 (100) | 0.9525 |
| Education [years] | 10.4 (3.0) | 10.2 (3.1) | 9.9 (3.2) | 9.4 (2.6) | 0.2293 |
| CASI-score | 79.8 (9.8) | 72.5 (8.5) | 60.3 (16.2) | 44.8 (19.8) | < 0.0001 |
| CES_D score | 3.7 (3.5) | 4.5 (4.5) | 4.3 (2.2) | 3.0 (2.2) | 0.3172 |
| Bi-frontal distance | 0.34 (0.03) | 0.34 (0.04) | 0.36 (0.03) | 0.36 (0.03) | < 0.0001 |
| Striatum [cm3] | 20.0 (2.3) | 19.7 (2.6) | 18.9 (3.3) | 18.5 (2.0) | < 0.0004 |
| Accumbens [cm3] | 1.38 (0.24) | 1.26 (0.23) | 1.21 (0.28) | 1.08 (0.18) | < 0.0001 |
| Putamen [cm3] | 9.7 (1.2) | 9.4 (1.4) | 9.1 (1.7) | 8.7 (1.1) | < 0.0001 |
| Caudate nucleus[cm3] | 8.9 (1.3) | 9.0 (1.3) | 8.6 (1.7) | 8.7 (1.6) | 0.2972 |
| Hippocampus [cm3] | 5.6 (0.8) | 5.2 (0.7) | 4.9 (0.9) | 4.3 (0.9) | < 0.0001 |
| Apoliprotein E ε4 genotype [%] | 37 | 29 | 26 | 30 | < 0.0001 |
| Cerebral infarcts [%] | |||||
| 0 infarcts | 93 | 93 | 88 | 81 | |
| 1 infarcts | 6 | 7 | 7 | 15 | } < 0.0001 |
| ≥ 2 infarcts | 1 | 0 | 5 | 4 | |
| Lacune grade [%] | |||||
| 0 lacunes | 58 | 61 | 47 | 46 | |
| 1–2 lacunes | 31 | 34 | 32 | 35 | } < 0.0001 |
| 3–5 lacunes | 10 | 5 | 21 | 19 | |
| WMH grade [%] | |||||
| 0–3 | 75 | 71 | 60 | 46 | |
| 4–6 | 20 | 24 | 29 | 35 | } <0.0001 |
| 7–9 | 4 | 5 | 10 | 7 |
Abbreviations: MRI = Magnetic Resonance Imaging; ICV = Intra Cranial Volume; CASI-score = Cognitive Ability Screening Instrument - score; CES-D score = Center for Epidemiologic Studies Depression score; WMH grade = White Matter Hyperintensity grade.
All values are means with standard deviations in parenthesis, unless otherwise noted.
p-values are for the overall association of the characteristics to study group.
3.2 Striatal volume and prediction of cognitive decline
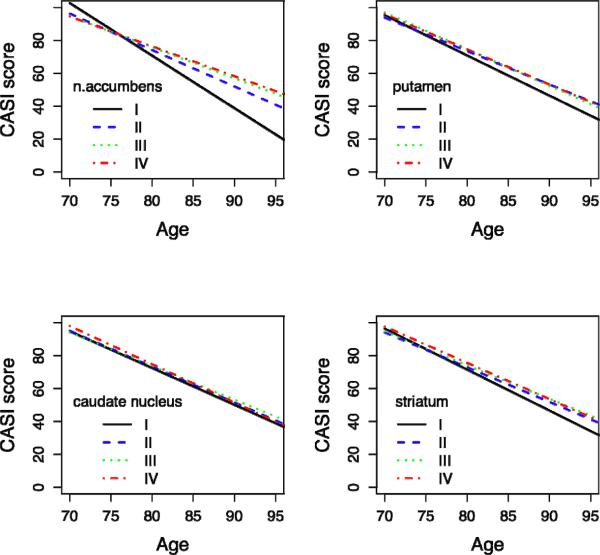
Figure 2 summarizes the results of the longitudinal analysis, showing the predicted decline in CASI scores over time per quartile of striatal volume; spaghetti plots of a random sample of 10 subjects of each quartile are also included in the figure. There is a significant difference in decline of CASI score in quartile IV compared to quartile I for the n. accumbens (slope Δ ± SE = −1.39 ± 0.21, p-value <0.0001). There are no significant differences between quartile IV and I for the putamen (slope Δ ± SE = −0.33 ± 0.22, p-value = 0.13), caudate nucleus (slope Δ ± SE = −0.01 ± 0.22, p-value = 0.98), or total striatal volume (slope Δ ± SE = −0.34 ± 0.21, p-value = 0.11).
Figure 2. Predicted CASI-score over time per quartile of striatal volume.
X-axis: Age = Age of observation
Y-axis: CASI Score = Predicted mean CASI (Cognitive Ability Screening Instrument) score over 8-year interval, adjusted for age, age at baseline, educational level, ICV, CES-D score, lacunes grade, presence of cerebral infarcts, WMH grade, bi-frontal distance/innertable distance, ApoE 4 allele, hippocampal volume quartiles.
Spaghetti plots of a random sample of 10 subjects per striatal volume quartile are displayed.
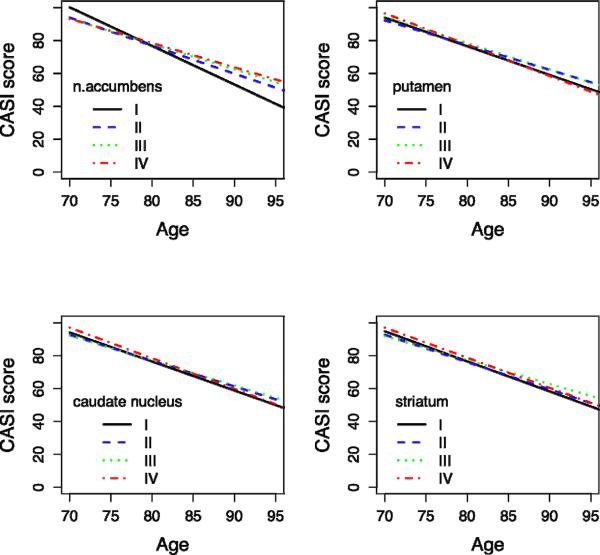
A summary of the longitudinal analysis with the no-dementia group only (n=347) is shown in Figure 3. Similar to the analysis on the total sample, a significant slope difference between quartile IV and I for the n. accumbens is seen (slope Δ ± SE = −1.39 ± 0.21, p-value <0.0001), but not for the putamen (slope Δ ± SE = 0.14 ± 0.15, p-value = 0.47), caudate nucleus (slope Δ ± SE = 0.07 ± 0.19, p-value = 0.71), or striatum (slope Δ ± SE = −0.03 ± 0.19, p-value = 0.89).
Figure 3. Predicted CASI-score over time per quartile of striatal volume (non-demented people only).
X-axis: Age = Age of observation
Y-axis: CASI Score = Predicted mean CASI (Cognitive Ability Screening Instrument) score over 8-year interval, adjusted for age, age at baseline, educational level, ICV, CES-D score, lacunes grade, presence of cerebral infarcts, WMH grade, bi-frontal distance/innertable distance, ApoE 4 allele, hippocampal volume quartiles.
Spaghetti plots of a random sample of 10 subjects per striatal volume quartile are displayed.
The overall significance of the models estimating the cognitive change slope by quartile of striatal volume are shown in Table 2; results are adjusted for age, education in years, gender, ICV, CES_D score, presence of cerebral infarcts, lacune grade, WMH grade, bi-frontal distance, and the presence of ApoE 4 allele. For comparison we show similar analyses for the hippocampus. We find overall the slope of the cognitive decline to significantly differ among the quartiles of the hippocampus volume. Adjusting for the hippocampus quartiles, as well as other markers of brain pathology and CVD risk factors, we find there is still a significant difference among quartiles of the n. accumbens (p < 0.0001). As shown in Figures 2 and 3, this largely reflects the steeper slope in decline of the 4th quartile relative to the first quartiles. Volumes of the total striatum, caudate nucleus, and putamen, are not associated with cognitive decline.
Table 2.
Effects of volume of striatum and hippocampus in model for prediction of cognitive decline
| Full sample (n=477) | No dementia sample (n=347) | ||||
|---|---|---|---|---|---|
| Model: | Effects a: | F-value | p-value | F-value | p-value |
| Hippocampus | HIPP quartiles | 9.8 | < 0.0001 | 5.4 | 0.0010 |
| Age * HIPP quartiles | 12.8 | < 0.0001 | 6.4 | 0.0003 | |
| N. accumbens | NACC quartiles | 14.9 | <0.0001 | 6.5 | 0.0002 |
| HIPP quartiles | 12.7 | < 0.0001 | 2.9 | 0.0352 | |
| Age * NACC quartiles | 17.1 | < 0.0001 | 7.3 | <0.0001 | |
| Caudate nucleus | CN quartiles | 0.6 | 0.6460 | 1.5 | 0.2186 |
| Hipp quartiles | 19.5 | < 0.0001 | 3.8 | 0.0101 | |
| Age * CN quartiles | 0.5 | 0.6512 | 1.5 | 0.2228 | |
| Putamen | PUT quartiles | 1.1 | 0.3644 | 1.6 | 0.1858 |
| HIPP quartiles | 15.8 | < 0.0001 | 3.7 | 0.0123 | |
| Age * PUT quartiles | 1.4 | 0.2540 | 1.7 | 0.1631 | |
| Striatum | STR quartiles | 1.4 | 0.2308 | 1.7 | 0.1607 |
| HIPP quartiles | 16.2 | < 0.0001 | 3.2 | 0.0227 | |
| Age * STR quartiles | 1.7 | 0.1577 | 1.7 | 0.1587 | |
Abbreviations: HIPP = hippocampus; NACC = nucleus accumbens; CN = caudate nucleus; PUT = putamen; STR = striatum.
Separate effects of age, education in years, gender, ICV, CES_D score, presence of cerebral infarcts, lacune grade, WMH grade, bi-frontal distance, and presence of ApoE 4 allele are not displayed.
4. Discussion
The present study assessed the relation of the volume of the striatum and its sub-structures and global cognitive performance in the entire spectrum of cognitively healthy to demented older people. We found volumes of the n. accumbens (as they were at the time of scanning) to be lower in subjects diagnosed with dementia 2–3 years prior to, at the time of, and 3–5 years after, the brain MR-scan was acquired. Total volumes of the striatum, and separately the putamen were smaller in subjects diagnosed with dementia prior to and at the time of scanning. Furthermore, we found that quartiles of n. accumbens volume significantly differed in the rate of cognitive decline both in the total sample and the sample of subjects who were not identified with dementia during the course of the study. Specifically, subjects in the lowest quartile of accumbens volume had a significantly steeper slope of cognitive decline measured over an 8-year period, independent of global brain atrophy, hippocampal volume, presence of ApoE 4 allele, or the amount of cerebrovascular damage. Thus, our findings suggest, the ventro-anterior striatal sub-structure, the n. accumbens, is a significant indicator for cognitive decline. Prospective studies of people who are not demented at baseline are needed to investigate whether atrophy in the accumbens is a marker for some specific cognitive trajectories, such as a fast or steep decline.
These findings contribute to our understanding of the stages of cognitive decline. The striatum is the largest structure of the basal ganglia, both hemispheres together measuring approximately 20 cm3, and is regarded as an input nucleus for cortical projections (Utter and Basso, 2008). Several studies have described the topographical arrangement of the human striatum in distinct, sometimes partially overlapping, circuits serving motor and cognitive functions (Alexander et al., 1986; Draganski et al., 2008; Leh et al., 2007; Middleton and Strick, 2000). Of interest here is the anterior cingulate loop described by Alexander (Grahn et al., 2008). This loop includes the ventral striatum, which receives input from the anterior cingulate cortex, hippocampal cortex, entorhinal cortex, and the superior and inferior temporal gyri. The ventral striatum consists of the n. accumbens, fundi of the caudate and putamen, and olfactory stria (Brockhaus, 1942). Since the accumbens is part of the limbic circuit, we had postulated that it shared the limbic circuits' vulnerability to degenerate during the dementing process. In our study the n. accumbens was significantly smaller in subjects with dementia and subjects who were going to become demented. Volume of the accumbens contributed independently to the model explaining cognitive decline in older people. This contribution was independent of more generally used indicators of cognitive decline hippocampal volume, global brain atrophy, and cerebro-vascular damage parameters. The results were similar in the sample not diagnosed with dementia during the 8 year follow-up period.
Pathological studies of the striatum in AD have shown that in particular, the cholinergic interneurons contain neurofibrillary tangles and are lost in the ventral striatum, which may be a potential explanation for our findings (Lehericy et al., 1989; Selden et al., 1994). Studies are needed to determine the processes leading to smaller accumbens volumes in older (demented) subjects, as well as the temporal relationship with other neurodegenerative changes in the brain. It has been postulated that the n. accumbens plays a pivotal role in memory and learning processes (Goldenberg et al., 1999; Gonzalez-Burgos and Feria-Velasco, 2008; Graybiel, 2008), possibly explaining the association of smaller volumes with more rapid cognitive decline. However, the n. accumbens is part of the intricate basal forebrain system and detailed knowledge of how this system facilitates cognitive functioning is still lacking, as is our understanding of the precise role of the n. accumbens (Alheid and Heimer, 1988).
We also found associations between the volume of the putamen and diagnosis of dementia, although not with cognitive decline. Previously, smaller volumes of the putamen were observed in people with dementia (de Jong et al., 2008). It is possible that decrease in the volume of the putamen becomes evident in more progressed stages of dementia and not in preclinical stages, which may explain the association with dementia but not with prediction of cognitive decline. Contrary to expectation, the caudate nucleus was not associated with dementia or cognitive decline in our study, whereas, other studies have pointed out the occurrence of degeneration of the head of the caudate nucleus in AD (Frisoni et al., 2002; Karas et al., 2003; Rombouts et al., 2000). Possibly, this inconsistency reflects differences in where the borders were placed between the n. accumbens and the caudate nucleus, but it may also be that the volume differences for the caudate nucleus are too small to detect by our method.
A major advantage of our study was the highly reproducible separate segmentation of caudate, putamen, and n. accumbens, and that we were able to study these sub-structures in association with longitudinal changes in cognition and dementia status. However, the limitations in the extent to which the “borders” between the striatal sub-structures can be identified, need to be taken into account when interpreting the data. The borders of these structures are better viewed as transition zones with overlapping functions. Currently, it is not possible to delineate sub-structures based on a functional division, because of overlapping functional zones and low contrast within the neo-striatal structure on MR. Finally, the striatal volume calculations were based on the delineation of the surface or boundary voxels, which does not account for within structure changes, including lacunar infarcts, iron accumulation, and enlarged Virchow Robin spaces in the deep grey matter. This may lead to a potential over-estimation of striatal volume. To minimize this effect we excluded subjects with large infarcts (>20mm) in the deep grey matter region, and controlled for the presence of lacunar infarcts.
5 Conclusion
The present study shows that the volume of the n. accumbens is closely associated with cognitive performance in older subjects, independent of other common brain changes in older persons. Additional studies are needed to further determine the clinical significance of atrophy in the striatum of community-based individuals
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health, Bethesda, Maryland (National Institute on Aging [NIA] Contract NO1-AG-4-2149, Cooperative Agreements 5U01AG017155-09 and 5U01AG019349-08, and the Intramural Research Program at NIA) and by ZonMW (AGIKO grant number 92003536).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors report no conflicts of interest.
8 References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Babalola KO, Patenaude B, Aljabar P, Schnabel J, Kennedy D, Crum W, Smith S, Cootes T, Jenkinson M, Rueckert D. An evaluation of four automatic methods of segmenting the subcortical structures in the brain. Neuroimage. 2009;47:1435–1447. doi: 10.1016/j.neuroimage.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Brockhaus H. The finer anatomy of the septum and of the striatum. J Psychol Neurol. 1942;51:1–56. [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- De Diego-Balaguer R, Couette M, Dolbeau G, Durr A, Youssov K, Bachoud-Levi AC. Striatal degeneration impairs language learning: evidence from Huntington's disease. Brain. 2008;131:2870–2881. doi: 10.1093/brain/awn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RG, Bollen EL, de Bruin PW, Middelkoop HA, van Buchem MA, van der Grond J. Strongly reduced volumes of putamen and thalamus in Alzheimer's disease: an MRI study. Brain. 2008;131:3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini L, Olofsen H, Palm WM, van Buchem MA, Reiber JH, Admiraal-Behloul F. GAMEs: growing and adaptive meshes for fully automatic shape modeling and analysis. Med Image Anal. 2007;11:302–314. doi: 10.1016/j.media.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G, Schuri U, Gromminger O, Arnold U. Basal forebrain amnesia: does the nucleus accumbens contribute to human memory? J Neurol Neurosurg Psychiatry. 1999;67:163–168. doi: 10.1136/jnnp.67.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Feria-Velasco A. Serotonin/dopamine interaction in memory formation. Prog Brain Res. 2008;172:603–623. doi: 10.1016/S0079-6123(08)00928-X. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hall AM, Moore RY, Lopez OL, Kuller L, Becker JT. Basal forebrain atrophy is a presymptomatic marker for Alzheimer's disease. Alzheimers Dement. 2008;4:271–279. doi: 10.1016/j.jalz.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson JE, Powers PK. Restriction isotyping of human apolipoprotein A-IV: rapid typing of known isoforms and detection of a new isoform that deletes a conserved repeat. J Lipid Res. 1991;32:1529–1535. [PubMed] [Google Scholar]
- Jurgens CK, van de Wiel L, van Es AC, Grimbergen YM, Witjes-Ane MN, van der Grond J, Middelkoop HA, Roos RA. Basal ganglia volume and clinical correlates in 'preclinical' Huntington's disease. J Neurol. 2008;255:1785–1791. doi: 10.1007/s00415-008-0050-4. [DOI] [PubMed] [Google Scholar]
- Karas GB, Burton EJ, Rombouts SA, van Schijndel RA, O'Brien JT, Scheltens P, McKeith IG, Williams D, Ballard C, Barkhof F. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage. 2003;18:895–907. doi: 10.1016/s1053-8119(03)00041-7. [DOI] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Brain aging in very old men with type 2 diabetes: the Honolulu-Asia Aging Study. Diabetes Care. 2006;29:2268–2274. doi: 10.2337/dc06-0243. [DOI] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Hirsch EC, Cervera P, Hersh LB, Hauw JJ, Ruberg M, Agid Y. Selective loss of cholinergic neurons in the ventral striatum of patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:8580–8584. doi: 10.1073/pnas.86.21.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WT, Jr., Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Patenaude B. Bayesian Statistical Models of Shape and Appearance for Subcortical Brain Segmentation. University of Oxford; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J. Executive dysfunction in early stages of Huntington's disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J Neurol Sci. 2005;239:11–19. doi: 10.1016/j.jns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Witter MP, Scheltens P. Unbiased whole-brain analysis of gray matter loss in Alzheimer's disease. Neurosci Lett. 2000;285:231–233. doi: 10.1016/s0304-3940(00)01067-3. [DOI] [PubMed] [Google Scholar]
- Scher AI, Xu Y, Korf ES, White LR, Scheltens P, Toga AW, Thompson PM, Hartley SW, Witter MP, Valentino DJ, Launer LJ. Hippocampal shape analysis in Alzheimer's disease: a population-based study. Neuroimage. 2007;36:8–18. doi: 10.1016/j.neuroimage.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Selden N, Mesulam MM, Geula C. Human striatum: the distribution of neurofibrillary tangles in Alzheimer's disease. Brain Res. 1994;648:327–331. doi: 10.1016/0006-8993(94)91136-3. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Flatz WH, Heinsen H, Bokde AL, Schoenberg SO, Stockel S, Dietrich O, Reiser MF, Moller HJ, Hampel H. Measurement of basal forebrain atrophy in Alzheimer's disease using MRI. Brain. 2005;128:2626–2644. doi: 10.1093/brain/awh589. [DOI] [PubMed] [Google Scholar]
- Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- Utter AA, Basso MA. The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev. 2008;32:333–342. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb JD. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.