Abstract
Objective
We sought to identify risk factors for mortality in a large clinical cohort of children with abusive head trauma.
Study design
Bivariate analysis and multivariable logistic regression models identified demographic, physical examination and radiologic findings associated with in-hospital mortality of children with abusive head trauma at four pediatric centers. An initial Glasgow Coma Scale (GCS) ≤ 8 defined severe abusive head trauma. Data are shown as OR (95% CI).
Results
Analysis included 386 children with abusive head trauma. Multivariable analysis showed children with initial GCS either 3 or 4 – 5 had increased mortality versus children with GCS 12 – 15 (OR 57.8 [12.1 – 277.6] and 15.6 [2.6 – 95.1], respectively, p < 0.001). Additionally, retinal hemorrhage (RH), intraparenchymal hemorrhage and cerebral edema were independently associated with mortality. In the subgroup with severe abusive head trauma and RH (n = 117), cerebral edema and initial GCS of 3 or 4 – 5 were independently associated with mortality. Chronic subdural hematoma was independently associated with survival.
Conclusions
Low initial GCS score, RH, intraparenchymal hemorrhage and cerebral edema are independently associated with mortality in abusive head trauma. Knowledge of these risk factors may enable researchers and clinicians to improve the care of these vulnerable children.
Keywords: child physical abuse, Glasgow Coma Scale, retinal hemorrhage, subdural hematoma, pediatric, traumatic brain injury
Recent studies suggest that more than 120,000 children are victims of physical child abuse annually in the United States[1]. More than 600 children died in 2009 in the US as a result of physical abuse[1]. Survivors may have life-long physical, developmental and emotional sequelae.
Abusive head trauma is responsible for the vast majority of fatalities from physical child abuse[2] and is a unique subset of traumatic brain injury (TBI). Abusive head trauma is pathophysiologically distinct from non-abusive (accidental) TBI, as evidenced by differences between abusive head trauma and accidental TBI in neuroimaging findings[3] and the incidence of seizures[3], certain extra-cranial fractures (i.e. rib fractures)[3], retinal hemorrhage (RH)[3, 4] and acute cardiopulmonary compromise[5]. Abusive head trauma has a reported incidence of 29.7 per 100,000 person-years during the first year of life [6]. The mortality rate for children with abusive head trauma ranges up to 35.7% [6–13]. Among survivors, 42% – 96% suffer long-term neurologic morbidity [8–11, 14–18]. Both mortality and survivor neurologic outcome are worse in abusive head trauma compared with accidental TBI [3, 5, 11, 16].
Risk factors for adverse outcome and death for the entire population of children with TBI include cerebral edema [19–23], intracranial bleeding [20, 22–26], age [23, 26–28], low Glasgow Coma Scale (GCS) score [19, 20, 22, 24–35] and various physiologic variables (e.g. hypotension, intracranial hypertension) [21, 22, 24, 25, 29–39]. However, despite a distinct pathophysiology and greater morbidity and mortality in abusive head trauma, risk factors for adverse outcome for infants with abusive head trauma have been less well described, possibly because a large enough series of children with abusive head trauma has not been studied. In the current study, we analyzed a large pre-existing dataset of children with abusive head trauma of varying severity (GCS 3 – 15) from multiple centers to determine factors associated with in hospital mortality. With this unique dataset, we were able to test for clinical characteristics that might influence the relationship between the variables and in-hospital mortality. We hypothesized that physical, neurologic, radiologic and demographic factors are associated with mortality in this population.
Methods
All procedures were approved by the Institutional Review Boards of the institutions involved. A database of children who had been diagnosed with unequivocal abusive head trauma by the Child Protection Team at one of four pediatric institutions (Nationwide Children’s Medical Center [Columbus, OH], Seattle Children’s Hospital/Harborview Medical Center [Seattle, WA], Cincinnati Children’s Hospital Medical Center [Cincinnati, OH] and Children’s Hospital of Pittsburgh of UPMC [Pittsburgh, PA]) was developed with the intent of determining the relationship of socioeconomic factors on the prevalence of abusive head trauma[40]. For this database, each institution included cases that occurred between January 1, 2004 and June 30, 2009. Cases were eligible if the child was diagnosed with unequivocal abusive head trauma by the Child Protection Team at the participating hospital. Defining whether an injury is the result of abuse based on the conclusion of a Child Protection Team is frequently used in studies of abusive head trauma [6, 41–43]. Severe abusive head trauma was defined as initial GCS ≤ 8. GCS scores were further stratified into mild injury (GCS 12 – 15), moderate injury (GCS 9 – 11) and multiple levels of severe injury (GCS 3, 4 – 5 and 6 – 8).
For this secondary analysis, a number of data elements were extracted from the medical record including demographic data (age, sex, race and treatment center), physical examination findings (RH [categorized as present, absent or not evaluated] and the initial GCS score assigned), radiologic findings (skull fractures, epidural hematomas [EDH], acute subdural hematomas [SDH], chronic SDH, acute subarachnoid hemorrhage [SAH], intraparenchymal hemorrhage, ischemia/infarction and cerebral edema) and outcome (death prior to hospital discharge or survival to discharge). Routine care at all institutions included a head CT after initial stabilization; additional imaging (e.g. MRI, repeat CT scan) was case specific. Radiologic findings of initial CT and initial MRI studies were obtained from original attending Neuroradiologist reports and were dichotomized as present or absent. When the database was constructed, acute SAH and acute SDH were combined into a single variable. GCS scores are routinely assigned in the Emergency Department at all participating centers. Children without a documented GCS score were excluded.
Bivariate analysis was completed using a χ2 test to test if particular variables were associated with death before hospital discharge. A multivariable stepwise logistic regression model was then used to identify factors independently associated with mortality and odds ratios were calculated (OR [95% confidence interval]). Children who were missing any data points were excluded from the regression model. Bivariate and multivariable analyses were separately performed on children with severe abusive head trauma. For all analyses, p < 0.05 was considered significant. All calculations were performed using SAS software. Data are reported as mean ± SEM unless otherwise specified.
Results
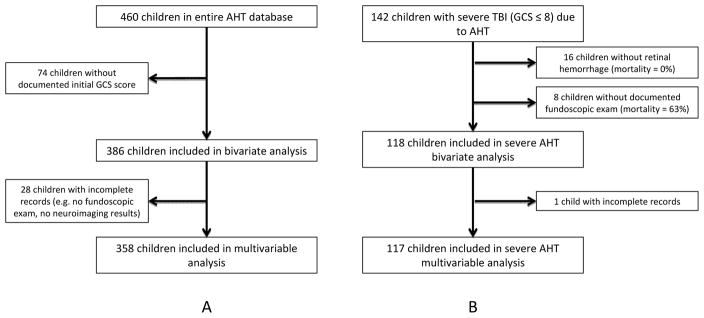
The inclusion and exclusion of children into this study is described in the Figure. The overall database contained 460 children. Exclusion of 74 children with no GCS score reported yielded a total of 386 children for bivariate analysis. An additional 28 children were excluded from the multivariable analysis because of individual missing data points. The mean age of all 386 included children was 9.6 ± 0.6 months and the mean initial GCS score was 10.6 ± 0.3 (Table I). Overall, 69 children died prior to hospital discharge for an in-hospital mortality rate of 17.9%
Figure 1.
Flow diagrams of selection of final cohort of (A) all children with Abusive Head Trauma (AHT) and (B) the severe AHT subgroup (GCS ≤8 and retinal hemorrhage)
Table 1.
Characteristics and bivariate analysis of total cohort of children with AHT (n = 386)
| n (% of cohort) | Mortality Rate (%) | p | ||
|---|---|---|---|---|
| Race (3 cases missing) | White | 254 (66.3) | 16.9 | 0.836 |
| Black | 68 (17.8) | 19.1 | ||
| Other | 61 (15.9) | 19.7 | ||
| Sex (0 cases missing) | Male | 216 (56.0) | 17.1 | 0.666 |
| Female | 170 (44.0) | 18.8 | ||
| Age (in months) (0 cases missing) | Mean (SEM) | 9.6 (0.6) | 0.001 | |
| <12mo | 293 (75.9) | 14.3 | ||
| ≥12mo | 93 (24.1) | 29.0 | ||
| Initial GCS Score (0 cases missing) | Median (IQR) | 13.5 (4 – 15) | ||
| 12 –15 | 223 (57.8) | 1.4 | <0.001 | |
| 9 – 11 | 21 (5.4) | 9.5 | ||
| 6 – 8 | 37 (9.6) | 13.5 | ||
| 4 – 5 | 21 (5.4) | 38.1 | ||
| 3 | 84 (21.7) | 60.7 | ||
| Retinal Hemorrhage (23 cases missing) | Present | 229 (59.3) | 27.1 | <0.001 |
| Not present | 134 (34.7) | 0.8 | ||
| Hospital (0 cases missing) | Center 1 | 63 (16.3) | 19.1 | 0.982 |
| Center 2 | 127 (32.9) | 18.1 | ||
| Center 3 | 125 (32.3) | 16.8 | ||
| Center 4 | 71 (18.4) | 18.3 | ||
| Skull Fracture (0 cases missing) | Present | 145 (37.6) | 15.2 | 0.282 |
| Not present | 241 (62.4) | 19.5 | ||
| Acute SDH/SAH (1 case missing) | Present | 310 (80.3) | 20.3 | 0.005 |
| Not present | 75 (19.4) | 6.7 | ||
| Chronic SDH (0 cases missing) | Present | 96 (24.9) | 5.2 | <0.001 |
| Not present | 290 (75.1) | 22.1 | ||
| Intraparenchymal | Present | 47 (12.2) | 27.7 | 0.056 |
| Hemorrhage (2 cases missing) | Not present | 337 (87.3) | 16.3 | |
| Ischemia/Infarction (1 case missing) | Present | 103 (26.7) | 37.9 | <0.001 |
| Not present | 282 (73.1) | 10.3 | ||
| Cerebral Edema (1 case missing) | Present | 141 (36.5) | 42.6 | <0.001 |
| Not present | 244 (63.2) | 3.3 | ||
| EDH (2 cases missing) | Present | 11 (2.8) | 0.0 | 0.119 |
| Not present | 373 (96.6) | 18.2 | ||
| Overall mortality | 69 (17.9) |
GCS, Glasgow Coma Scale; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; EDH, epidural hematoma
Table I shows the results of the bivariate analysis for all children with abusive head trauma. There were no associations between mortality and either sex, race or institution. Decreased GCS score was associated with mortality (p < 0.001) and the mortality increased sequentially with more severe TBI (1.4% for GCS 12 – 15 to 60.7% for GCS 3). Age (≥ 12 months old), presence of RH, and three radiological findings (acute SAH/SDH, ischemia/infarction and cerebral edema) were associated with increased mortality in the bivariate analysis. The presence of a chronic SDH was associated with decreased mortality. Bivariate analysis of children with complete records (n = 358) had similar results (Table II, available at www.jpeds.com), though intraparenchymal hemorrhage was significantly associated with mortality in this subgroup (p = 0.030). In the multivariable analysis, children with an initial GCS of either 3 or 4 – 5 had increased mortality compared with children with an initial GCS of 12 – 15 (OR 57.8 [12.1 – 277.6] and 15.6 [2.6 – 95.1], respectively, p < 0.001) (Table III). Additionally, presence of RH (OR 20.3 [2.4 – 169.8], p = 0.006), intraparenchymal hemorrhage (OR 5.0 [1.4 – 17.5], p = 0.012) and cerebral edema (OR 6.0 [2.2 – 15.9], p < 0.001) were all independently associated with mortality. The area under the curve (AUC) for the multivariate model including these variables was 0.941. EDH was not included as a variable in this model because of the absence of any deaths in children with EDH (n = 11).
Table II.
Characteristics and bivariate analysis of total cohort of children with AHT who were included in multivariate analysis (n = 358, 296 survived, 62 died)
| n (% of cohort) | Mortality Rate (%) | p | ||
|---|---|---|---|---|
| Race | White | 236 (66.0) | 16.5 | 0.935 |
| Black | 62 (17.3) | 16.1 | ||
| Other | 60 (16.7) | 18.3 | ||
| Sex | Male | 199 (55.6) | 16.1 | 0.581 |
| Female | 159 (44.4) | 18.8 | ||
| Age (in months) | Mean (SEM) | 9.6 (0.6) | 0.011 | |
| <12mo | 273 (76.3) | 14.3 | ||
| ≥12mo | 85 (23.7) | 27.1 | ||
| Initial GCS Score | Median (IQR) | 13 (4 – 15) | ||
| 12 –15 | 204 (60.0) | 1.0 | <0.001 | |
| 9 – 11 | 21 (5.9) | 9.5 | ||
| 6 – 8 | 36 (10.0) | 11.1 | ||
| 4 – 5 | 19 (5.3) | 36.8 | ||
| 3 | 78 (21.8) | 60.2 | ||
| Retinal Hemorrhage | Present | 227 (63.4) | 26.9 | <0.001 |
| Not present | 131 (36.6) | 0.8 | ||
| Hospital | Center 1 | 62 (17.3) | 19.4 | 0.867 |
| Center 2 | 109 (30.4) | 18.3 | ||
| Center 3 | 120 (33.5) | 15.0 | ||
| Center 4 | 67 (18.7) | 17.9 | ||
| Skull Fracture | Present | 131 (36.6) | 15.3 | 0.756 |
| Not present | 227 (63.4) | 18.5 | ||
| Acute SDH/SAH | Present | 290 (81.0) | 20.0 | 0.010 |
| Not present | 68 (19.0) | 5.8 | ||
| Chronic SDH | Present | 94 (26.3) | 5.3 | <0.001 |
| Not present | 264 (73.7) | 21.6 | ||
| Intraparenchymal Hemorrhage | Present | 43 (12.0) | 30.2 | 0.030 |
| Not present | 315 (88.0) | 15.6 | ||
| Ischemia/Infarction | Present | 94 (26.3) | 36.2 | <0.001 |
| Not present | 264 (73.7) | 10.6 | ||
| Cerebral Edema | Present | 131 (36.6) | 42.0 | <0.001 |
| Not present | 227 (63.4) | 3.1 | ||
| EDH | Present | 11 (3.1) | 0.0 | 0.255 |
| Not present | 347 (96.9) | 17.9 | ||
| Overall mortality | 62 (17.3) |
GCS, Glasgow Coma Scale; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; EDH, epidural hematoma
Table III.
Risk factors independently and significantly associated with in-hospital mortality from AHT in multivariable stepwise logistic regression
| Total Cohort (n=358) | Severe AHTb Cohort (n=117) | |||
|---|---|---|---|---|
| Finding | OR (95% CI) | p | OR (95% CI) | p |
| GCS 3 | 57.8a (12.1 – 277.6) | <0.001 | 15.5c (4.4 – 53.8) | <0.001 |
| GCS 4–5 | 15.6a (2.6 – 95.1) | 4.9c (1.1 – 22.3) | ||
| Intraparenchymal Hemorrhage | 5.0 (1.4 – 17.5) | 0.012 | not significant | |
| Cerebral Edema | 6.0 (2.2 – 15.9) | <0.001 | 6.5 (1.99 – 21.3) | 0.002 |
| Retinal Hemorrhage | 20.3 (2.4 – 169.8) | 0.006 | ||
| Chronic SDH | not significant | 0.19 (0.04 – 0.81) | 0.026 | |
compared with GCS 12 – 15
GCS ≤ 8 and retinal hemorrhage
compared with GCS 6 – 8
Severe abusive head trauma was diagnosed in 142 children (Figure, B). Fundoscopic exam revealed absence of RH in 16 of these children, all of whom survived. Because of this co-linearity, the severely injured subgroup was defined as severe abusive head trauma and the documented presence of RH. The demographic information for these 118 children is shown in Table IV (8 children did not have a documented fundoscopic exam). Bivariate analysis of these cases demonstrated that GCS score and cerebral edema were associated with mortality (p < 0.001). The presence of a chronic SDH was associated with decreased mortality. In the multivariable model (n = 117, 1 child with incomplete records), the findings of the bivariate analyses were confirmed (Table III). Specifically, initial GCS scores of 3 and 4 – 5 were both independently associated with increased mortality compared with GCS 6 – 8 (OR 15.5 [4.4 – 53.8]) and 4.9 [1.1 – 22.3], respectively, p < 0.001) as was the finding of cerebral edema (OR 6.5 [2.0 – 21.3], p = 0.002). Chronic SDH was independently associated with decreased mortality (OR 0.19 [0.04 – 0.81], p = 0.026). The AUC for multivariate model including these variables was 0.830. Again, EDH was not included as a variable in the multivariable analysis due to 100% survival.
Table IV.
Characteristics and bivariate analysis of children with severe AHT (GCS ≤ 8 and retinal hemorrhage) (n = 118)
| n (% of cohort) | Mortality Rate (%) | p | ||
|---|---|---|---|---|
| Race | White | 71 (60.1) | 49.3 | 0.997 |
| Black | 22 (18.6) | 50.0 | ||
| Other | 24 (20.3) | 50.0 | ||
| Sex | Male | 61 (51.7) | 50.8 | 0.854 |
| Female | 57 (48.3) | 49.1 | ||
| Age (in months) | Mean (SEM) | 13.4 (1.4) | 0.701 | |
| <12mo | 76 (64.4) | 48.7 | ||
| ≥12mo | 42 (35.6) | 52.4 | ||
| Initial GCS Score | Mean (SEM) | 4.2 (0.2) | <0.001 | |
| 6 – 8 | 30 (25.4) | 13.3 | ||
| 4 – 5 | 17 (14.4) | 41.2 | ||
| 3 | 71 (60.1) | 67.6 | ||
| Hospital | Center 1 | 20 (16.9) | 60.0 | 0.799 |
| Center 2 | 39 (33.1) | 48.7 | ||
| Center 3 | 33 (28.0) | 48.5 | ||
| Center 4 | 26 (22.0) | 46.2 | ||
| Skull Fracture | Present | 28 (23.7) | 60.7 | 0.194 |
| Not present | 90 (76.3) | 46.7 | ||
| Acute SDH/SAH | Present | 111 (94.1) | 50.5 | 0.697 |
| Not present | 7 (5.9) | 42.9 | ||
| Chronic SDH | Present | 17 (14.4) | 17.7 | 0.004 |
| Not present | 101 (85.6) | 55.5 | ||
| Intraparenchymal Hemorrhage | Present | 17 (14.4) | 64.7 | 0.19 |
| Not present | 101 (85.6) | 47.5 | ||
| Ischemia/Infarction | Present | 60 (50.9) | 55.0 | 0.269 |
| Not present | 58 (49.2) | 44.8 | ||
| Cerebral Edema | Present | 89 (75.4) | 60.7 | <0.001 |
| Not present | 29 (24.6) | 17.2 | ||
| EDH | Present | 1 (0.9) | 0.0 | 0.315 |
| Not present | 117 (99.2) | 50.4 | ||
| Overall mortality | 59 (50.0) |
GCS, Glasgow Coma Scale; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; EDH, epidural hematoma
DISCUSSION
We found that incrementally decreased GCS scores, RH, intraparenchymal hemorrhage and cerebral edema are independently associated with death in the hospital. In the subgroup of children with severe abusive head trauma and RH, mortality was associated with decreased initial GCS score and cerebral edema. Interestingly, chronic SDH was significantly associated with decreased mortality in this subgroup.
Knowledge of risk factors for mortality can aid clinicians and researchers in improving the care of these extremely vulnerable children, whose mortality rate is among the highest of any pediatric patients. In our series, the mortality rate for abusive head trauma was 17.9%, which is similar to both the control (12.0%) and experimental (21.3%) arms in the most recently completed study of hypothermia for severe TBI in children[44], despite our study including mild, moderate and severe injuries. The mortality in our cohort is greater than rates for children with severe sepsis (10.3%)[45] and acute lymphoblastic leukemia (5 year mortality = 14.3%)[46]. Children with severe abusive head trauma and RH in our cohort had a 50% mortality, equivalent to that observed for children with complete cardiopulmonary failure requiring extracorporeal membrane oxygenation (ECMO)[47].
Several researchers have published analyses of smaller cohorts of children with abusive head trauma. Barlow and Minns demonstrated that lower GCS score was associated with poor outcome in 25 children with abusive head trauma[18]. Kemp found that a Bender score of 4 (“comatose”), apnea and particular neuroimaging findings (diffuse brain swelling or hypoxic ischemia) were associated with a poor outcome in 65 children with abusive head trauma [15]. Bonnier showed that GCS score ≤ 8, retinal detachment and skull fractures were risk factors associated with poor outcome in 23 children with abusive head trauma[14]. More recently, Scavarda showed that the Pediatric Risk of Mortality score was associated with poor outcome in multivariable analysis [13] and Ilves et al showed that GCS score ≤ 8 and several neuroimaging findings were associated with poor neurodevelopmental outcome [17]. Collectively, these findings are similar to ours. However, our much larger, multi-center cohort was robust enough to control for several potential covariates through multivariable stepwise logistic regression.
King studied 364 children with abusive head trauma [8] and showed that respiratory difficulty, bruising and SDH were each associated with an increased odds ratio for mortality [8]. As in our study, cerebral edema and “decreased level of consciousness” were associated with mortality, though only 24% of their subjects had a GCS score recorded on admission compared with 84% in our study. Falcone reported 443 patients from a single center that showed that African-American race and lower respiratory rate on admission were significantly associated with mortality[7]. Similar to our findings, a GCS score ≤ 8 was associated with mortality. It was not included in their multivariable model due to an excessive amount of patients without a GCS score. Their cohort was their hospital’s entire trauma registry, with only 73% of the subjects being “considered to be probable or definite abuse cases,” an important distinction from our inclusion criteria. These studies’ results are again similar to ours.
Parks recently used ICD-10 codes to study fatal abusive head trauma[48]. They showed that most children who have fatal abusive head trauma are <12 months of age and are males. The majority of children in our cohort who died from abusive head trauma were in their first year of life and most were males. However, as our multivariate analysis was not limited to only fatal cases, we were able to show that neither age nor sex is significantly associated with mortality in children with abusive head trauma.
There is surprisingly little evidence showing an association between GCS score and mortality in pediatric TBI[49]. Several early reports demonstrated a loose association after TBI [24–26, 50–52]. An association between GCS score and Glasgow Outcome Scale score has been widely reported [20, 28–31, 53–55], though mortality per se was not analyzed in these studies as it was in ours. GCS score has been associated with mortality in general pediatric trauma [56–59] and in bivariate analysis of pediatric TBI [19, 27, 32]. Our database was large enough to perform multivariable analysis to reduce effects of confounding variables. The importance of this additional analysis is evidenced by prior work by Michaud [22] and Hackbarth [34] that showed that GCS score was associated with mortality in bivariate analysis of pediatric TBI, but not when multivariable models were created.
Our study shows a significant association between multiple GCS categories and mortality in children with TBI. This is an important distinction from Ducrocq et al who showed an association between dichotomized GCS score (> 5 and ≤ 5) and mortality in a multivariable analysis of a cohort of 585 pediatric severe TBI patients [33], 17 of whom had abusive head trauma. Although these results may be more applicable to accidentally injured children, our finding of incrementally worsening mortality for lower GCS score categories indicates that GCS depression is not an all-or-none phenomenon.
Though not significant in multivariable analysis, it is also notable that children with only moderately depressed GCS scores of 9 – 11 had a six-fold higher mortality than children with GCS scores of 12 – 15 (9.5% vs. 1.4%, p <0.001 in bivariate analysis). This suggests that children with abusive head trauma and moderately depressed GCS scores have a mortality rate similar to children with severe TBI who were accidentally injured[30, 35, 60]. Given this alarming mortality risk, close monitoring and earlier interventions may be justified in children with abusive head trauma and moderately depressed GCS scores.
In our database, 16% of children did not have a GCS score recorded. Although this is a lower rate than in other studies[8, 18], it still shows that many children with abusive head trauma do not receive an initial GCS classification, potentially related to uncertainty about a traumatic etiology or pre-arrival interventions (i.e. paralysis for intubation). As GCS score is related to mortality, assigning a GCS score could have important implications on patient management.
Levin et al showed that RH was more common in children who died from abusive head trauma than in neurologically intact survivors[61]. RH severity is associated with injury severity[4] and in our cohort of children with severe abusive head trauma, absence of RH was uniformly associated with survival. Though this co-linearity prevented a statistical analysis of the relationship between RH and outcome in children with severe TBI, we feel our data support the hypothesis that RH may be a marker of life-threatening injury in children with abusive head trauma. Although further study is needed to fully explore this theory, our findings suggest that early examination for RH may be warranted in children with abusive head trauma, though the risk of masking clinically important mydriasis or anisocoria must be weighed. Children with abusive head trauma and RH may warrant close monitoring.
Surprisingly, chronic SDH was independently associated with decreased mortality in our severely injured cohort. There are a number of possible mechanisms. Animal models have shown that brains exposed to a non-injurious stimulus (such as hypoxia/ischemia) can exhibit neurological protection from a second larger injury – so called preconditioning[62, 63]. It is theoretically possible that children with chronic SDH have experienced such a pattern of injury. The preexisting chronic SDH may have altered the compliance of the cranial vault (i.e. by causing sutures to open), allowing the brain to swell after a subsequent injury without causing intracranial hypertension. Alternatively, the brain of a child that has suffered an injury causing a chronic SDH may be more sensitive to a subsequent minor injury. In this case, symptoms may be of sufficient severity that caregivers seek medical attention for this non-fatally injured child, leading to our observation of decreased mortality with this chronic finding. Lastly, the presence of an abnormal finding on neuroimaging may lead to medical providers altering their clinical decisions. This treatment bias could lead to a better outcome through closer monitoring for signs of neurologic deterioration and better prevention of secondary insults (e.g. hypoxia and hypotension). As our sample size of children with severe abusive head trauma, RH and chronic SDH was small, further research is needed to elucidate the true magnitude and mechanism of this association with survival as it may lead to improved care of children with abusive head trauma.
Our analysis had several limitations. Because the database was designed to test the relationship between economic factors and the incidence of abusive head trauma, a number of clinical and physiologic characteristics that may be associated with outcome (e.g. blood pressure, pulse oximetry, intracranial pressure, seizures, medication use, pupil exam, repeated GCS scores, systemic injuries, etc.) were not collected. Details regarding GCS assignment (e.g. location, concurrent medication usage) and death (e.g. timing, proximate cause) were also not collected. Moreover, detailed neuropsychological assessments at relevant times after injury (3, 6 or 12 months) were not performed. Because of these limitations, it is impossible to interrogate if traditional secondary injury factors (e.g. hypotension, hypoxia and intracranial hypertension) had important effects on outcomes. Similarly, it was impossible to test for risk factors for morbidity (i.e. neurologic impairment) using this database. We were also unable to stratify neuroimaging diagnoses by imaging modality. Lastly, approximately one-fourth of the original abusive head trauma database had to be excluded, due to either missing data or to allow for creation of statistical models (i.e. those children with severe TBI and no RH), and additional data points were missing for some children included in the bivariate analysis (i.e. race not documented in 3 cases). Nevertheless, we still had sufficient statistical power to detect important associations between mortality and several variables.
Acknowledgments
Supported by the Matty Eappen Foundation, which had no role in study design; collection, analysis and interpretation of data; the writing of the report; and the decision to submit for publication.
Abbreviations
- EDH
epidural hematoma
- GCS
Glasgow Coma Scale
- SAH
subarachnoid hemorrhage
- SDH
subdural hematoma
- TBI
traumatic brain injury
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven L. Shein, Department of Critical Care Medicine, Children’s Hospital of Pittsburgh of UPMC, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA.
Michael J. Bell, Departments of Critical Care Medicine and Neurological Surgery, Children’s Hospital of Pittsburgh of UPMC, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA.
Patrick M. Kochanek, Department of Critical Care Medicine, Children’s Hospital of Pittsburgh of UPMC, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA.
Elizabeth C. Tyler-Kabara, Departments of Neurological Surgery and Physical Medicine and Rehabilitation, Children’s Hospital of Pittsburgh of UPMC, Department of Bioengineering and McGowan Institute for Regenerative Medicine, University of Pittsburgh, Pittsburgh, PA.
Stephen R. Wisniewski, Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA.
Kenneth Feldman, Seattle Children’s Hospital/Harborview Medical Center, Seattle, WA.
Kathi Makoroff, Cincinnati Children’s Hospital Medical Center. Cincinnati, OH.
Philip V. Scribano, Children’s Hospital of Philadelphia, Philadelphia, PA.
Rachel P. Berger, Department of Pediatrics, Children’s Hospital of Pittsburgh of UPMC, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.U.S. Department of Health and Human Services AoC, Youth and Families. Child Maltreatment 2009. 2011. [Google Scholar]
- 2.Graupman P, Winston KR. Nonaccidental head trauma as a cause of childhood death. Journal of neurosurgery. 2006;104:245–50. doi: 10.3171/ped.2006.104.4.245. [DOI] [PubMed] [Google Scholar]
- 3.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114:633–9. doi: 10.1542/peds.2003-1020-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin AV. Retinal hemorrhage in abusive head trauma. Pediatrics. 2010;126:961–70. doi: 10.1542/peds.2010-1220. [DOI] [PubMed] [Google Scholar]
- 5.Hymel KP, Makoroff KL, Laskey AL, Conaway MR, Blackman JA. Mechanisms, clinical presentations, injuries, and outcomes from inflicted versus noninflicted head trauma during infancy: results of a prospective, multicentered, comparative study. Pediatrics. 2007;119:922–9. doi: 10.1542/peds.2006-3111. [DOI] [PubMed] [Google Scholar]
- 6.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population based study of inflicted traumatic brain injury in young children. JAMA. 2003;290:621–6. doi: 10.1001/jama.290.5.621. [DOI] [PubMed] [Google Scholar]
- 7.Falcone RA, Jr, Brown RL, Garcia VF. Disparities in child abuse mortality are not explained by injury severity. J Pediatr Surg. 2007;42:1031–6. doi: 10.1016/j.jpedsurg.2007.01.038. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 8.King WJ, MacKay M, Sirnick A. Shaken baby syndrome in Canada: clinical characteristics and outcomes of hospital cases. CMAJ. 2003;168:155–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Haviland J, Russell RI. Outcome after severe non-accidental head injury. Arch Dis Child. 1997;77:504–7. doi: 10.1136/adc.77.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duhaime AC, Christian C, Moss E, Seidl T. Long-term outcome in infants with the shaking-impact syndrome. Pediatr Neurosurg. 1996;24:292–8. doi: 10.1159/000121058. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein B, Kelly MM, Bruton D, Cox C. Inflicted versus accidental head injury in critically injured children. Critical care medicine. 1993;21:1328–32. doi: 10.1097/00003246-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Duhaime AC, Gennarelli TA, Thibault LE, Bruce DA, Margulies SS, Wiser R. The shaken baby syndrome. A clinical, pathological, and biomechanical study. Journal of neurosurgery. 1987;66:409–15. doi: 10.3171/jns.1987.66.3.0409. [DOI] [PubMed] [Google Scholar]
- 13.Scavarda D, Gabaudan C, Ughetto F, Lamy F, Imada V, Lena G, et al. Initial predictive factors of outcome in severe non-accidental head trauma in children. Childs Nerv Syst. 2010 doi: 10.1007/s00381-010-1150-x. [DOI] [PubMed] [Google Scholar]
- 14.Bonnier C, Nassogne MC, Saint-Martin C, Mesples B, Kadhim H, Sebire G. Neuroimaging of intraparenchymal lesions predicts outcome in shaken baby syndrome. Pediatrics. 2003;112:808–14. doi: 10.1542/peds.112.4.808. [DOI] [PubMed] [Google Scholar]
- 15.Kemp AM, Stoodley N, Cobley C, Coles L, Kemp KW. Apnoea and brain swelling in nonaccidental head injury. Arch Dis Child. 2003;88:472–6. doi: 10.1136/adc.88.6.472. discussion -6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewing-Cobbs L, Kramer L, Prasad M, Canales DN, Louis PT, Fletcher JM, et al. Neuroimaging, physical, and developmental findings after inflicted and noninflicted traumatic brain injury in young children. Pediatrics. 1998;102:300–7. doi: 10.1542/peds.102.2.300. [DOI] [PubMed] [Google Scholar]
- 17.Ilves P, Lintrop M, Talvik I, Sisko A, Talvik T. Predictive value of clinical and radiological findings in inflicted traumatic brain injury. Acta Paediatr. 2010 doi: 10.1111/j.1651-2227.2010.01820.x. [DOI] [PubMed] [Google Scholar]
- 18.Barlow KM, Thomson E, Johnson D, Minns RA. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005;116:e174–85. doi: 10.1542/peds.2004-2739. [DOI] [PubMed] [Google Scholar]
- 19.Feickert HJ, Drommer S, Heyer R. Severe head injury in children: impact of risk factors on outcome. J Trauma. 1999;47:33–8. doi: 10.1097/00005373-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Chung CY, Chen CL, Cheng PT, See LC, Tang SF, Wong AM. Critical score of Glasgow Coma Scale for pediatric traumatic brain injury. Pediatric neurology. 2006;34:379–87. doi: 10.1016/j.pediatrneurol.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Ong L, Selladurai BM, Dhillon MK, Atan M, Lye MS. The prognostic value of the Glasgow Coma Scale, hypoxia and computerised tomography in outcome prediction of pediatric head injury. Pediatr Neurosurg. 1996;24:285–91. doi: 10.1159/000121057. [DOI] [PubMed] [Google Scholar]
- 22.Michaud LJ, Rivara FP, Grady MS, Reay DT. Predictors of survival and severity of disability after severe brain injury in children. Neurosurgery. 1992;31:254–64. doi: 10.1227/00006123-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Levin HS, Aldrich EF, Saydjari C, Eisenberg HM, Foulkes MA, Bellefleur M, et al. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery. 1992;31:435–43. doi: 10.1227/00006123-199209000-00008. discussion 43–4. [DOI] [PubMed] [Google Scholar]
- 24.Elias-Jones AC, Punt JA, Turnbull AE, Jaspan T. Management and outcome of severe head injuries in the Trent region 1985–90. Arch Dis Child. 1992;67:1430–5. doi: 10.1136/adc.67.12.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieh-Lai MW, Theodorou AA, Sarnaik AP, Meert KL, Moylan PM, Canady AI. Limitations of the Glasgow Coma Scale in predicting outcome in children with traumatic brain injury. J Pediatr. 1992;120:195–9. doi: 10.1016/s0022-3476(05)80426-3. [DOI] [PubMed] [Google Scholar]
- 26.Levi L, Guilburd JN, Linn S, Feinsod M. The association between skull fracture, intracranial pathology and outcome in pediatric head injury. Br J Neurosurg. 1991;5:617– 25. doi: 10.3109/02688699109002885. [DOI] [PubMed] [Google Scholar]
- 27.Campbell CG, Kuehn SM, Richards PM, Ventureyra E, Hutchison JS. Medical and cognitive outcome in children with traumatic brain injury. Can J Neurol Sci. 2004;31:213–9. doi: 10.1017/s0317167100053853. [DOI] [PubMed] [Google Scholar]
- 28.Thakker JC, Splaingard M, Zhu J, Babel K, Bresnahan J, Havens PL. Survival and functional outcome of children requiring endotracheal intubation during therapy for severe traumatic brain injury. Critical care medicine. 1997;25:1396–401. doi: 10.1097/00003246-199708000-00030. [DOI] [PubMed] [Google Scholar]
- 29.Kapapa T, Konig K, Pfister U, Sasse M, Woischneck D, Heissler H, et al. Head trauma in children, part 1: admission, diagnostics, and findings. Journal of child neurology. 2010;25:146–56. doi: 10.1177/0883073809332698. [DOI] [PubMed] [Google Scholar]
- 30.Chiaretti A, Piastra M, Pulitano S, Pietrini D, De Rosa G, Barbaro R, et al. Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst. 2002;18:129–36. doi: 10.1007/s00381-002-0558-3. [DOI] [PubMed] [Google Scholar]
- 31.Barzilay Z, Augarten A, Sagy M, Shahar E, Yahav Y, Boichis H. Variables affecting outcome from severe brain injury in children. Intensive Care Med. 1988;14:417–21. doi: 10.1007/BF00262899. [DOI] [PubMed] [Google Scholar]
- 32.Downard C, Hulka F, Mullins RJ, Piatt J, Chesnut R, Quint P, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49:654–8. doi: 10.1097/00005373-200010000-00012. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 33.Ducrocq SC, Meyer PG, Orliaguet GA, Blanot S, Laurent-Vannier A, Renier D, et al. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7:461–7. doi: 10.1097/01.PCC.0000235245.49129.27. [DOI] [PubMed] [Google Scholar]
- 34.Hackbarth RM, Rzeszutko KM, Sturm G, Donders J, Kuldanek AS, Sanfilippo DJ. Survival and functional outcome in pediatric traumatic brain injury: a retrospective review and analysis of predictive factors. Critical care medicine. 2002;30:1630–5. doi: 10.1097/00003246-200207000-00038. [DOI] [PubMed] [Google Scholar]
- 35.White JR, Farukhi Z, Bull C, Christensen J, Gordon T, Paidas C, et al. Predictors of outcome in severely head-injured children. Critical care medicine. 2001;29:534–40. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Chiaretti A, De Benedictis R, Della Corte F, Piastra M, Viola L, Polidori G, et al. The impact of initial management on the outcome of children with severe head injury. Childs Nerv Syst. 2002;18:54–60. doi: 10.1007/s00381-001-0533-4. [DOI] [PubMed] [Google Scholar]
- 37.Kokoska ER, Smith GS, Pittman T, Weber TR. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. J Pediatr Surg. 1998;33:333–8. doi: 10.1016/s0022-3468(98)90457-2. [DOI] [PubMed] [Google Scholar]
- 38.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28:310–4. doi: 10.1016/0022-3468(93)90223-8. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 39.Jagannathan J, Okonkwo DO, Yeoh HK, Dumont AS, Saulle D, Haizlip J, et al. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J Neurosurg Pediatr. 2008;2:240–9. doi: 10.3171/PED.2008.2.10.240. [DOI] [PubMed] [Google Scholar]
- 40.Berger RP, Fromkin JB, Stutz H, Makoroff K, Scribano PV, Feldman K, et al. Abusive head trauma during a time of increased unemployment: a multicenter analysis. Pediatrics. 2011;128:637–43. doi: 10.1542/peds.2010-2185. [DOI] [PubMed] [Google Scholar]
- 41.Hymel KP, Abshire TC, Luckey DW, Jenny C. Coagulopathy in pediatric abusive head trauma. Pediatrics. 1997;99:371–5. doi: 10.1542/peds.99.3.371. [DOI] [PubMed] [Google Scholar]
- 42.Jenny C, Hymel KP, Ritzen A, Reinert SE, Hay TC. Analysis of missed cases of abusive head trauma. JAMA. 1999;281:621–6. doi: 10.1001/jama.281.7.621. [DOI] [PubMed] [Google Scholar]
- 43.Berger RP, Ta’asan S, Rand A, Lokshin A, Kochanek P. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr Res. 2009;65:97–102. doi: 10.1203/PDR.0b013e31818c7e27. [DOI] [PubMed] [Google Scholar]
- 44.Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–56. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 45.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 46.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood. 2004;104:2690–6. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 47.Jen HC, Shew SB. Hospital readmissions and survival after nonneonatal pediatric ECMO. Pediatrics. 2010;125:1217–23. doi: 10.1542/peds.2009-0696. [DOI] [PubMed] [Google Scholar]
- 48.Parks SE, Kegler SR, Annest JL, Mercy JA. Characteristics of fatal abusive head trauma among children in the USA: 2003–2007: an application of the CDC operational case definition to national vital statistics data. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention. 2011 doi: 10.1136/injuryprev-2011-040128. [DOI] [PubMed] [Google Scholar]
- 49.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 50.Bruce DA, Schut L, Bruno LA, Wood JH, Sutton LN. Outcome following severe head injuries in children. Journal of neurosurgery. 1978;48:679–88. doi: 10.3171/jns.1978.48.5.0679. [DOI] [PubMed] [Google Scholar]
- 51.Esparza J, JMP, Sarabia M, Yuste JA, Roger R, Lamas E. Outcome in children with severe head injuries. Childs Nerv Syst. 1985;1:109–14. doi: 10.1007/BF00706691. [DOI] [PubMed] [Google Scholar]
- 52.Kraus JF, Fife D, Conroy C. Pediatric brain injuries: the nature, clinical course, and early outcomes in a defined United States’ population. Pediatrics. 1987;79:501–7. [PubMed] [Google Scholar]
- 53.Chiaretti A, Pezzotti P, Mestrovic J, Piastra M, Polidori G, Storti S, et al. The influence of hemocoagulative disorders on the outcome of children with head injury. Pediatr Neurosurg. 2001;34:131–7. doi: 10.1159/000056008. [DOI] [PubMed] [Google Scholar]
- 54.Song SH, Kim SH, Kim KT, Kim Y. Outcome of pediatric patients with severe brain injury in Korea: a comparison with reports in the west. Childs Nerv Syst. 1997;13:82–6. doi: 10.1007/s003810050048. [DOI] [PubMed] [Google Scholar]
- 55.Grewal M, Sutcliffe AJ. Early prediction of outcome following head injury in children: an assessment of the value of Glasgow Coma Scale score trend and abnormal plantar and pupillary light reflexes. J Pediatr Surg. 1991;26:1161–3. doi: 10.1016/0022-3468(91)90323-l. [DOI] [PubMed] [Google Scholar]
- 56.Orliaguet GA, Meyer PG, Blanot S, Jarreau MM, Charron B, Buisson C, et al. Predictive factors of outcome in severely traumatized children. Anesth Analg. 1998;87:537–42. doi: 10.1097/00000539-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Matos RI, Holcomb JB, Callahan C, Spinella PC. Increased mortality rates of young children with traumatic injuries at a US army combat support hospital in Baghdad, Iraq, 2004. Pediatrics. 2008;122:e959–66. doi: 10.1542/peds.2008-1244. [DOI] [PubMed] [Google Scholar]
- 58.Cantais E, Paut O, Giorgi R, Viard L, Camboulives J. Evaluating the prognosis of multiple, severely traumatized children in the intensive care unit. Intensive Care Med. 2001;27:1511–7. doi: 10.1007/s001340101039. [DOI] [PubMed] [Google Scholar]
- 59.Bayreuther J, Wagener S, Woodford M, Edwards A, Lecky F, Bouamra O, et al. Paediatric trauma: injury pattern and mortality in the UK. Arch Dis Child Educ Pract Ed. 2009;94:37–41. doi: 10.1136/adc.2007.132787. [DOI] [PubMed] [Google Scholar]
- 60.Exo J. Intracranial pressure-monitoring systems in children with traumatic brain injury: Combining therapeutic and diagnostic tools. Pediatr Crit Care Med. 2011 doi: 10.1097/PCC.0b013e3181e8b3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levin A. Retinal hemorrhage and child abuse. Recent Advances in Paediatics. 2000;18:151–219. [Google Scholar]
- 62.Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994;168:221–4. doi: 10.1016/0304-3940(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 63.Perez-Pinzon MA, Alonso O, Kraydieh S, Dietrich WD. Induction of tolerance against traumatic brain injury by ischemic preconditioning. Neuroreport. 1999;10:2951–4. doi: 10.1097/00001756-199909290-00014. [DOI] [PubMed] [Google Scholar]