Abstract
The inconclusive results of past trials and recent findings of partial protection of Tenofovir 1% gel underscore the need to better understand product adherence in microbicide trials. This study aimed to identify factors predicting couples’ ability to sustain topical gel and condom use during clinical trial participation. We enrolled 100 Indian participants of a randomized, controlled safety trial of Tenofovir 1% gel (CT cohort) and 100 similar women who were ineligible or declined trial participation (NCT cohort). Compared to the NCT cohort, CT women reported higher baseline condom use, more positive attitudes towards condoms and higher levels of protection efficacy. While NCT condom use remained low, CT condom use increased dramatically during the study. Reported gel consistency was higher than condom consistency. Individual and couple-related factors predicted condom consistency and interest in future gel use, but not gel consistency. Findings could inform trial recruitment strategies and product introduction.
Keywords: Microbicides, HIV, Clinical trial research, Adherence, Couples
Introduction
For more than a decade, scientists have sought to develop a topical microbicide product to protect women from HIV [1,2]. The currently available options have some limitations in preventing the spread of HIV in women. Condoms, when used consistently and correctly, are upwards of 80% effective in the prevention of HIV transmission to women [3, 4]. However, their use is low in most parts of the world—particularly among intimate couples [5, 6]. While male circumcision has been shown to reduce the risk of female-to-male HIV transmission, possibly by 50–60% [7-9], the circumcision of HIV-infected men does not appear to reduce HIV transmission to their uninfected women partners [10].
Being married or in an intimate relationship is increasingly recognized as one of the most important HIV risk determinants for women [11-14]. Although few scientists have tried to quantify the effect on HIV transmission of being in an intimate relationship, using demographic and health survey data from Zambia and Rwanda, Dunkle et al. [15] estimated that between 60 and 94% of new HIV infections occurred within married or cohabitating serodiscordant couples. In many cultural contexts, being married increases a woman’s exposure to sex with a partner who may have multiple sexual partners, while decreasing her ability to assess risk or negotiate condom use or other protective behaviors [6]. A female-initiated product might prove particularly useful within relationships where women are unable to negotiate condom use.
Recent findings from an effectiveness trial of 1% Tenofovir gel in South Africa suggest that a female-initiated topical microbicide product may eventually become a reality [16]. In this Phase IIb trial of almost 900 sexually-active, HIV-negative women randomized to the active gel or placebo, Tenofovir 1% reduced HIV acquisition by 39% (CI, 6–60). Nevertheless, given the conclusion of partial effectiveness in the centre for the AIDS program of research in South Africa (CAPRISA) trial, and a history of first generation microbicide trials that produced no conclusive, positive evidence of effectiveness [17-21], questions remain about the potential impact of poor gel adherence and/or high condom adherence on study findings [22], underscoring the continuing need for behavioral research to better understand both gel and condom use within topical microbicide effectiveness trials. But, integration of behavioral/social science research into clinical trials can be problematic and requires careful planning and compromise [23].
In this paper, we present findings from a 6-month prospective study conducted in Pune, India that aimed to identify factors influencing couples’ ability to initiate and sustain risk reduction behaviors—specifically, condom and gel use, within the context of clinical trial participation. Two phases of formative research had been conducted prior to the prospective study to identify and develop measures for factors that might predict microbicide acceptability and adherence. This earlier research was based on the premise that women’s willingness and ability to use microbicides would be influenced by more than their attitudes towards product attributes (i.e., whether the product is drippy or easy to insert). Indeed, data from the first qualitative phase indicated that women’s interest in using a microbicide was linked to their perceived risk of HIV, which was most commonly associated with a perception of partner infidelity and a lack of marital harmony. Women’s perceptions of control and sexual power also influenced their attitudes towards microbicide use [24]. Based on these qualitative data, we developed and validated psychometric scales to measure couple harmony, HIV risk perception and protection efficacy during a second formative phase (Tolley et al. 2006, unpublished).
In this third research phase, we built on an NIAID-funded safety trial of Tenofovir 1% gel [HPTN 059 trial] to assess whether these factors (couple harmony, HIV risk perception and protection efficacy) were associated with condom and/or gel use consistency among women and their partners participating in a 6-month safety trial of Tenofovir 1% gel and a separate cohort of non-trial participants from the same communities. Our findings provide some insight into product adherence behaviors within clinical trials, as well as characteristics of those who choose to enroll in trials. They may also inform product introduction strategies that target married or intimate partnerships once an effective product is available.
The findings presented in this study focus specifically on the following study questions and hypotheses:
How do reported consistencies of gel and condom use change over the study?
How do trial participants’ condom use patterns compare to those not in a trial?
Do baseline psychosocial factors related to individual and intimate partner relationships predict consistency of reported gel/condom use, and interest in gel use outside of the clinical trial? Do these relationships vary by cohort?
We hypothesized that individuals in both the CT and non-CT cohorts with stronger perceived control over sexual behavior and condom or gel use (captured in a “protection efficacy” scale) and who had more positive attitudes towards condom and/or gel attributes would report higher condom and gel use consistency. In contrast, we assumed that relationships between couple-related factors (couple harmony and HIV risk perception variables) and product use consistency would vary by cohort. For example, we hypothesized that couple harmony would be positively associated with gel and condom consistency among CT participants, but not associated with condom consistency outside the CT. We also hypothesized that women’s perception of HIV risk would be positively associated with condom use consistency outside the trial, but not associated with condom or gel use consistency within the CT cohort. These hypotheses were based on two assumptions: (a) that women who enrolled in the trial would have obtained their husband’s approval beforehand and those whose husbands were most supportive would achieve greater consistency of product use and report higher levels of couple harmony— and that their product use behavior would be more associated with being in the trial, rather than as a reflection of their need for protection; and (b) that women reporting more consistent condom use outside of a trial setting would more likely be using condoms for disease prevention and consequently have higher HIV risk perception and more variable assessments of couple harmony.
Methods
Study Population
Data were collected in parallel to a randomized, controlled clinical trial (HPTN 059) that aimed to examine the safety and acceptability of Tenofovir 1% gel. Between August 2006 and September 2007, the HPTN 059 clinical trial enrolled a total of 200 HIV-negative, sexually active, non-menopausal women aged 18–50 year old—100 from two sites in the US and 100 in Pune, India. Eligibility criteriaincluded being willing to use condoms, as well as effective contraception during the trial, including a hormonal method, the intrauterine device (IUD) or female sterilization. Women were excluded from participation if they were pregnant or breastfeeding, reported more than an average of two sex acts per day in the last 2 weeks, had abnormal pelvic exam or laboratory readings or reported allergies to latex or were taking any chronic hepatitis B medications.
This parallel acceptability study was conducted in the Pune, India site only. Two separate cohorts of women were recruited into the acceptability study—the 100 women who were enrolled in the India site for HPTN 059 (CT cohort) and 100 women recruited from the same communities (NCT cohort). Clinical trial recruitment took place largely through community-based meetings during which potential participants were informed about the trial and pre-screened for eligibility. During these meetings, any sexually active, HIV-negative women aged 18–50 who were either ineligible for the clinical trial or unwilling to enroll were invited to join the parallel acceptability study. (Women who were considering participation in HPTN 059 were only recruited into the CT cohort of this parallel acceptability study once they had actually enrolled in HPTN 059.) In addition, 100 male partners, approximately 50% from each of the two female cohorts participated in the parallel acceptability study. Husbands were only recruited if the participating wife agreed. For the purposes of this study, we focus analysis and discussion on women’s data.
Measures
The dependent variables, “consistency of gel use” and “consistency of condom use”, were calculated from responses given by women study participants to two questions: In the past 2 months, have you ever missed using your study gel (or: a condom) during sex. Unless participants responded never, they were asked how frequently they missed using their gel or using condoms—rarely, sometimes, frequently or always. In essence, responses to these questions became responses to 5-point scales. In our final analysis, we recoded these Consistency variables into 3 point scales: 1 = never/rarely used; 2 = sometimes/frequently used; 3 = always used. A third dependent variable, “interest in gel use outside a clinical trial” was calculated as the mean score of a reverse-scored item, You would not be interested in using this gel, and two other items, You would think about using it with at least one sexual partner and You would definitely try to use it with at least one sexual partner, each measured on a six-point scale from 1 = strongly disagree to 6 = strongly agree (α = 0.85).
Information on independent or predictor variables was also collected. At baseline, women were administered sets of statements assessing their couple relationship; perception of HIV risk due to their own behavior, their partner’s behavior, or other causes; and perceived ability to protect themselves from HIV. These psychosocial predictors were the result of an earlier phase of research to develop psychometric scales that might predict initiation and sustained use of microbicides. They include the following psychometric scales: couple harmony (14 items, α = 0.91, e.g. Both your partner and you are willing to adjust to keep peace within the house), perception of partner Infidelity (3 items, α = 0.83, e.g. It is possible that your partner is having outside relations when you are not around); Pervasive HIV Risk (8 items, α = 0.65, e.g. There is nothing you can do to prevent getting AIDS); and Protection Efficacy (12 items, α = 0.68, e.g. If you were worried about AIDS, your partner and you could use condoms correctly and consistently). All psychometric scales were measured on a 6-point response scale from 1 (strongly disagree) to 6 (strongly agree). In addition, we included the single item, Your own behavior can put you at risk of getting AIDS, measured on a 6-point response scale (from strongly agree to strongly disagree) and an overall acceptability of gel (or condom) use measured on a 5-point response scale (from 1 = strongly dislike to 5 = strongly like).
Analyses
Descriptive statistics, nonparametric Mann–Whitney tests, and chi-square tests were used to evaluate differences between cohorts with respect to socio-demographic and psychosocial characteristics. We used linear mixed models to examine the simultaneous effects of our study cohort, follow-up time, and each baseline predictor variable on condom consistency (and on gel consistency for the CT cohort). Linear mixed models account for the longitudinal nature of the data by directly modeling the intra-individual correlation over time. We used an unstructured covariance matrix as this requires minimal assumptions regarding within-person correlation. Separate models were run to assess each predictor variable’s influence on either condom or gel use consistency. Condom use models controlled for baseline condom use. We used appropriately specified contrasts to compare the CT and NCT women with respect to average associations between the predictor variables and the outcomes. (These contrasts are shown in the last column of Table 2.)
Table 2.
Models of psychosocial predictors on condom and gel use consistency, and on general interest in gel use, by cohort (each psychosocial variable fit separately)
| Psychosocial variable | CT cohort |
NCT cohort |
CT vs. NCT | ||||
|---|---|---|---|---|---|---|---|
| Estimate | Lower CI | Upper CI | Estimate | Lower CI | Upper CI | P value | |
| Models of condom use consistency | |||||||
| Couple harmony | 0.13 | 0.06 | 0.21 | 0.11 | 0.01 | 0.21 | 0.73 |
| Partner infidelity | −0.10 | −0.15 | −0.04 | −0.00 | −0.06 | 0.05 | 0.03 |
| Pervasive HIV risk | −0.13 | −0.22 | −0.05 | 0.02 | −0.06 | 0.09 | 0.01 |
| HIV risk perception (due to own behavior) | 0.04 | 0.00 | 0.07 | 0.02 | −0.01 | 0.05 | 0.50 |
| Protection efficacy | 0.30 | 0.17 | 0.43 | 0.02 | −0.06 | 0.11 | <0.001 |
| Overall attitudes towards condoms | 0.15 | 0.06 | 0.24 | 0.06 | −0.03 | 0.15 | 0.19 |
| Models of gel use consistency | |||||||
| Couple harmony | −0.06 | −0.23 | 0.12 | ||||
| Partner infidelity | −0.05 | −0.14 | 0.05 | ||||
| Pervasive HIV risk | −0.06 | −0.19 | 0.07 | ||||
| HIV risk perception (due to own behavior) | 0.02 | −0.03 | 0.06 | ||||
| Protection efficacy | 0.02 | −0.16 | 0.20 | ||||
| Product attribute (Gel) | 0.04 | −0.09 | 0.16 | ||||
| Models of general interest in gel use | |||||||
| Couple harmony | 0.15 | −0.03 | 0.34 | 0.13 | −0.11 | 0.38 | 0.91 |
| Partner infidelity | −0.06 | −0.20 | 0.08 | −0.02 | −0.17 | 0.13 | 0.70 |
| Pervasive HIV risk | −0.14 | −0.35 | 0.07 | −0.01 | −0.20 | 0.18 | 0.37 |
| HIV risk perception (due to own behavior) | −0.08 | −0.16 | −0.00 | −0.01 | −0.09 | 0.07 | 0.23 |
| Protection efficacy | 0.36 | 0.07 | 0.65 | 0.56 | 0.36 | 0.76 | 0.26 |
| Overall attitudes towards gel | 0.11 | −0.04 | 0.25 | ||||
Analytic Note
The CT cohort was followed up monthly as part of HPTN 059. Additionally, in our parallel acceptability study, both CT and NCT participants were followed at baseline and then at 2, 4 and 6 months after their initial interview. Several data collection accommodations were made in order to facilitate the integration of this behavioral research into the clinical trial. First, due to concern from clinical staff about staff burden—baseline data for the parallel acceptability study were collected on or within 2 weeks of the HPTN 059 enrollment visit for our CT cohort. Second, a separate team administered parallel acceptability data collection; a community liaison worked assiduously with clinical and acceptability teams to coordinate participants’ study visits. Finally, data management and analysis were de-linked from the HPTN clinical trial—important variables (including treatment group, clinical adherence measures and clinic visit dates) for parallel study analysis were made available only after the clinical study finished.
The acceptability baseline interview did not record a participant’s date of enrollment into the clinical trial. Therefore, we only discovered during analysis that approximately half of clinical trial participants were recruited into the parallel acceptability study more than 2 weeks after their enrollment in the HPTN 059 clinical trial. In some cases, the baseline acceptability interview occurred more than a month after their trial enrollment, meaning that the final 6-month interview occurred after a participant was no longer in the trial or had access to study gel. To address this problem, we re-aligned participants’ adherence data, using actual time in the clinical trial to assign responses to the appropriate baseline or follow-up period. This led to missing data for some women. Fortunately, mixed models analysis allows for missing outcome data. When women’s baseline interviews were conducted out of range, we used the first set of responses to psychosocial variables as their baseline for our models. In addition, we ran a sensitivity analysis using data from only those women with correctly timed data collection to check the consistency of our main results with this the smaller subset of women. The trends using this sensitivity analysis were in a similar direction and magnitude as in the main analysis.
This study was reviewed and approved by FHI’s protection of human subjects committee (PHSC) and the National AIDS Research Institute’s Ethics Committee.
Results
Study participants’ socio-demographic and psychosocial characteristics at baseline are reported in Table 1. Overall, women were between 19 and 48 years old, with a mean age of 34 years. All were married with an average of two living children (range 0–6), most had some secondary school education and more than half earned their own income. Women’s socio-demographic and psychosocial characteristics did not differ significantly by cohort. Women reported similar levels of couple harmony, perception of partner infidelity and pervasive HIV risk. However, women who participated in the clinical trial were significantly different from their non-trial counterparts in terms of their attitudes towards risk reduction behaviors, reporting at baseline significantly higher Protection Efficacy (mean of 5.26 vs. 4.70, P < 0.0001), more positive attitudes towards condoms (3.29 vs. 2.80, P < 0.01) and consistent condom use (1.57 vs. 1.29, P < 0.004) and Interest in gel use outside a clinical trial (5.14 vs. 4.10, P < 0.0001), should one become available.
Table 1.
Baseline sociodemographic and psychosocial characteristics, by cohort
| CT women N = 100 |
NCT women N = 100 |
P-value | |
|---|---|---|---|
| Socio-demographic characteristics | |||
| Age (years) | 34.7 | 33.7 | 0.23 |
| Number of living children | 2.4 | 2.2 | 0.10 |
| Educational level | 0.65 | ||
| No schooling | 6.0% | 3.0% | |
| Some primary [1, 2, 7, 8] | 11.0% | 9.0% | |
| Some secondary [3–6, 9, 10] | 64.0% | 71.0% | |
| >10 years | 19.0% | 17.0% | |
| Earn income (yes) | 55.0% | 57.0% | 0.78 |
| Average income/month (Rs.) | 1526 | 1281 | 0.13 |
| Psychosocial characteristics | |||
| Couple harmony | 5.35 | 5.39 | 0.98 |
| Perception of partner infidelity | 1.59 | 1.66 | 0.53 |
| Pervasive HIV risk | 2.06 | 2.08 | 0.64 |
| Your own behaviour can put you at risk of getting AIDS |
3.00 | 2.85 | 0.65 |
| Protection efficacy | 5.26 | 4.70 | <0.0001 |
| Overall attitudes towards Condoms (range 1–5) |
3.29 | 2.80 | 0.01 |
| Perception of consistent condom use |
1.57 | 1.29 | 0.004 |
| Interest in gel use outside a clinical trial |
5.14 | 4.10 | <0.0001 |
CT clinical trial cohort; NCT non-clinical trial cohort
Data represent mean scores, except where otherwise indicated. Ranges for psychosocial characteristics were 1–6, except where otherwiseindicated
Patterns of Condom and Gel Use
We observed large differences in condom use between the CT and NCT cohorts. At baseline, 40% of NCT women had never used condoms before joining the study, compared to approximately 9% of CT women (4 of 46 women with baseline data within 14 days of enrollment). Among those who had ever used condoms previously, women inthe NCT cohort reported frequently or always having sex without using a condom in the 2 months prior to the study; their use of condoms did not change over time (Fig 1). In contrast, CT women reported higher baseline condom use, which increased almost a full point on a three-point scale (from 1.6 to 2.5) between baseline and 4 months, and then fell slightly during the last 2 months of the study. CT women reported higher consistency of gel than condom use for the period between baseline and 4 months. However, as with condom use, their reported consistency of gel use fell between 4 and 6 months.
Fig. 1.
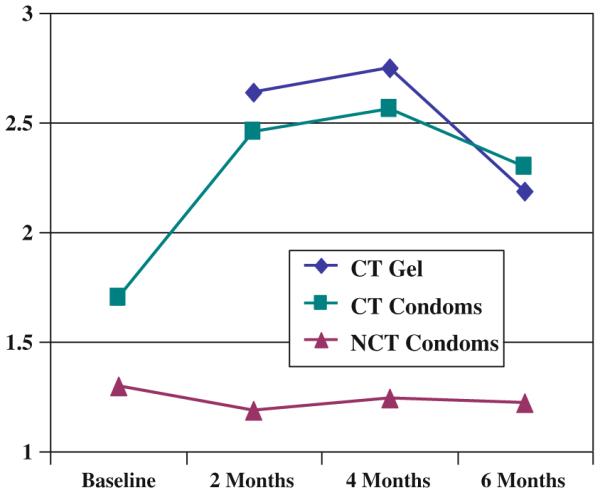
Perceived consistency of condom and gel use, by cohort. (*1 = Frequently/always miss; 2 = Sometimes/rarely miss; 3 = Never miss using condoms, last 2 months)
Predictors of Condom and Gel Use Consistency
Table 2 presents estimates and confidence intervals of separate models assessing the influence of each psychosocial predictor on consistent condom or consistent gel use. As hypothesized, among women in the CT cohort, average condom use consistency increased with each unit increase of couple harmony (by 0.13 on the three point scale), protection efficacy (by 0.30) and overall attitudes towards condoms (by 0.15). However, contrary to our hypothesis, average condom use consistency among the CT women actually decreased with each unit increase of HIV risk perception due to a partner’s perceived infidelity (by −0.10) or other pervasive sources of risk (by −0.13).
In the NCT cohort, the strongest predictor of condom consistency was baseline condom use (not shown). In contrast to our predictions, average condom consistency increased with each unit increase of couple harmony(by 0.11), but was not significantly associated with protection efficacy (0.02, CI −0.06 to 0.11), Overall attitudes towards condoms (0.06, CI −0.03 to 0.15) or other sources of HIV risk perception (Table 2). Implicit in our assumptions was the notion that the relationship between some psychosocial variables and condom consistency would vary by cohort. We found significant differences between the CT and NCT cohorts in two of the risk perception models (perception of partner infidelity and pervasive HIV risk perception) and the protection efficacy model, as shown in the last column of Table 2.
Interestingly, none of the psychosocial variables significantly predicted gel use consistency (Table 2). Nevertheless, gel use was generally more acceptable than condom use among women and their partners in the CT cohort. In addition, while approximately 30% of men and 10% of women reported at baseline that condoms made sex less pleasurable, most women and men reported that gel use had no effect on sexual pleasure; some (5% of women and 8% of men) suggested the gel enhanced sexual pleasure (data not shown).
Predictors of Interest in Gel Use Outside of the Clinical Trial Setting
Several psychosocial variables predicted interest in gel use outside a clinical trial (Table 2). In both the CT and NCT cohorts, women who scored higher on the protection efficacy scale tended to be more interested, on average, in using a gel in the future (by 0.36 and 0.56 per unit increase in protection efficacy, respectively). Among CT women, each unit increase in HIV risk perception due to one’s own behavior decreased the average interest in gel use outside a clinical trial (by −0.08). None of the associations differed between groups.
Discussion
This study sought to identify psychosocial predictors of gel adherence with the aim of better understanding how and to whom microbicides, once proven effective, should be introduced. While the study identified predictors of condom use consistency both within and outside the clinical trial, we found no evidence that any of our measures predicted gel use consistency. Overall gel acceptability was high; women found the gel easy to use and fewer women and their partners reported that gel use interrupted sex as compared to condoms. One possible explanation for the apparent lack of association between psychosocial predictors and consistent gel use is that this trial-related behavior had not become associated (as yet) with the need for protection and therefore infidelity or distrust, and so did not require the same kind of negotiation that appears to be needed to obtain consistent condom use with an intimate partner.
In contrast, higher condom use consistency was associated with increased harmony in both cohorts. In the clinical trial cohort, condom use consistency was also predicted by other psychosocial factors, including decreased perception of a partner’s infidelity, more positive attitudes toward product attributes, and women’s increased perception of protection efficacy. The relationship between condom consistency, couple harmony and protection efficacy is likely to have been reinforced over time within the CT cohort, since these women entered the trial having accepted to use condoms and received monthly risk reduction counseling and condoms as part of their trial participation, as well as having evaluated on a bimonthly basis their perception of HIV risk and aspects of their marital relationships as part of our parallel acceptability study. Indeed, Marlow, in an analysis of qualitative couples’ data collected in a subset of our CT and NCT participants, found that participation in both the clinical and the parallel study created a safe space in which couples could communicate about sexual and prevention behaviors, resulting for some in a greater sense of marital harmony [25]. The lack of evidence of an association between these other predictors and consistent condom use in the non-clinical cohort may have been due to overall low levels of condom use among married couples in our non-clinical cohort. Findings from our formative research [24] indicated that, while condoms might be used as a temporary contraceptive method, their use was largely associated with HIV prevention and therefore stigmatized. Furthermore, our models on interest in gel use outside a clinical trial suggest that women’s perceived vulnerability to HIV, as well as their ability to enact protective behaviors (protection efficacy) will be critical factors in whether or not they initiate gel use.
Past acceptability research has focused on physical aspects of the gel (i.e. cooling effect, consistency, odor/fragrance), ease of application (i.e. having a prefilled applicator), and issues of affordability and accessibility to inform product introduction. Findings from this research suggest that product introduction should also consider the psychosocial aspects affecting couples’ ability to initiate and sustain risk reduction behaviors. It remains unclear whether the kinds of sociocultural barriers to condom use will also impede use of a topical microbicide once it is made available outside a trial setting. On the one hand, participants’ higher ratings of gel acceptability compared to condom acceptability, ease of use and some reports of lubrication enhancing sexual pleasure imply that topical gel use may be easier to initiate and sustain than condoms. On the other hand, it’s quite possible that gel use in a realworld context may develop associations with HIV and/or infidelity, reducing women’s ability to initiate or sustain use. This suggests that future microbicide introduction programs must pay careful attention to crafting messages and developing strategies that avoid such connotations.
There are certain limitations to the study. The first relates to the timing of our baseline measures. Assessing psychosocial variables and baseline condom use at screening rather than enrollment would have been a more rigorous design, but one that appeared overly burdensome at the time the protocol was developed. Because women in the clinical trial were counseled to use condoms and their baseline condom use assessment could have occurred up to 14 days after enrollment, we expect that women in the clinical cohort would have been less likely to report never using condoms at baseline than women who did not join the trial. However, analyses of qualitative data from a subset of CT and NCT couples suggest it is also possible that women were less likely to join the trial in the first place unless they and their partners had previously used condoms and women were able and willing to negotiate their use for the trial [25].
A second limitation is the mistiming between clinical and acceptability follow-up; adjustment of the behavioral adherence data to align with clinic trial follow-up caused us to lose some valuable data. However, we feel these adjustments make the analysis more robust, because adherence responses corresponded to the participants’ actual clinical trial participation and gel use. A qualitative comparison of the analysis using the adjusted dataset suggests that conclusions drawn from our models are not substantially different from those using the nominal follow-up visits. An additional limitation is the study’s relatively short duration of follow-up. Some behavioral theories [26] consider 6 months to be the minimum duration for determining maintenance of complex behavior like condom (or gel) use. Finally, married women in India may differ from other women—particularly those living in high prevalence countries like South Africa. Replication of this study in some African settings would help inform the degree to which factors influencing use within Indian couples are similar to intimate partnerships in other populations.
Finally, it is clear that women who chose to join the clinical trial were different than those who did not. Additionally, these differences were not in terms of the sociodemographics most often examined by clinical researchers—but of psychosocial factors not usually examined. For example, women in the clinical trial reported higher baseline condom use and had more favorable attitudes towards condoms as compared to their non-CT counterparts. In fact, qualitative data with a subset of couples in the trial suggested that the condom use requirement itself limited trial participation [25]. Because the trial aimed to examine the safety rather than effectiveness of Tenofovir 1% gel, women’s self-selection into or out of the trial based on their ability to negotiate condoms is likely to have had little real consequence for the trial. However, such selfselection could be counter-productive in an effectiveness trial if women who cannot negotiate condoms would be less likely to participate in the trial, since these would be the women most likely to benefit from an eventual product and to contribute to the trial’s ability to determine product effectiveness. While this dilemma has been recognized— and debated in the public health literature, no clear resolution has been suggested. It remains a key challenge to conducting effectiveness trials of HIV prevention technologies [27-30].
Conclusion
More attention is needed as to who gets recruited into HIV prevention clinical trials. Women with high perceived efficacy may be better able to enact complex trial behaviors required of them. However, they may also be less at risk of acquiring HIV in the first place—perhaps one explanation for unexpectedly low event rates observed in some earlier trials [19, 30].
Findings from this study provide some evidence that microbicide products may be more acceptable than condoms for HIV prevention. However, they also suggest that widespread uptake—especially if use among more intimate relationships is a goal—is likely to depend on introduction strategies that avoid associations with infidelity, perhaps by framing introduction strategies and messages to emphasize positive product attributes and the potential to enhance couple harmony.
Acknowledgments
This study was conducted with support from the United States Agency for International Development (USAID) through Cooperative Agreement GPO-A-OO-05-00022-00; however, the views expressed do not necessarily reflect those of USAID.
Contributor Information
Elizabeth E. Tolley, Behavioral and Social Science Research Department, Health & Development Sciences, FHI 360, 2224 East Highway 54, Durham, NC 27319, USA
Sharon Tsui, Behavioral and Social Science Research Department, Health & Development Sciences, FHI 360, 2224 East Highway 54, Durham, NC 27319, USA.
Sanjay Mehendale, Indian Council for Medical Research, Chennai, India.
Mark A. Weaver, University of North Carolina, Chapel Hill, USA
Rewa Kohli, National AIDS Research Institute, Pune, India.
References
- 1.Uckun FM, D’Cruz OJ. Prophylactic contraceptives for HIV/ AIDS. Hum Reprod Update. 1999;5(5):506–14. doi: 10.1093/humupd/5.5.506. [DOI] [PubMed] [Google Scholar]
- 2.Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: where have we been? where are we going? J Wom Health Gend Base Med. 2001;10(2):163–73. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- 3.Weller S, Davis K. Condom effectiveness in reducing hetero-sexual HIV transmission. Cochrane Database Syst Rev. 2002;(1):CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]
- 4.Steiner MJ, Cates W., Jr. Condoms and sexually-transmitted infections. N Engl J Med. 2006;354(25):2642–3. doi: 10.1056/NEJMp068111. [DOI] [PubMed] [Google Scholar]
- 5.Steiner MJ, Cates W, Jr, Warner L. The real problem with male condoms is nonuse. Sex Transm Dis. 1999;26(8):459–62. doi: 10.1097/00007435-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Misovich SJ, Fisher JD, Fisher WA. Close relationships and elevated HIV risk behavior: evidence and possible underlying psychological processes. Rev Gen Psychol. 1997;1(1):72–107. [Google Scholar]
- 7.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 9.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 10.Wawer MJ, Makumbi F, Kigozi G, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374(9685):229–37. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch JS, Meneses S, Thompson B, Negroni M, Pelcastre B, del Rio C. The inevitability of infidelity: sexual reputation, social geographies, and marital HIV risk in rural Mexico. Am J Public Health. 2007;97(6):986–96. doi: 10.2105/AJPH.2006.088492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith DJ. Modern marriage, men’s extramarital sex, and HIV risk in southeastern Nigeria. Am J Public Health. 2007;97(6):997–1005. doi: 10.2105/AJPH.2006.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh SA. The political economy of marriage and HIV: the ABC approach, “safe” infidelity, and managing moral risk in Uganda. Am J Public Health. 2007;97(7):1198–208. doi: 10.2105/AJPH.2006.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangakhedkar RR, Bentley ME, Divekar AD, et al. Spread of HIV Infection in married monogamous women in India. J Am Med Assoc. 1997;278:2090–2. [PubMed] [Google Scholar]
- 15.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371(9631):2183–91. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 16.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 18.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. [Google Scholar]
- 19.Family Health International Phase 3 trial in Nigeria evaluating the effectiveness of savvy gel in preventing HIV infection in women will close. 2006 Aug 28; [Google Scholar]
- 20.Stover K. Anti-HIV gel shows promise in large-scale study in women. National Institute of Allergy and Infectious Diseases (NIAID); [Google Scholar]
- 21.Microbicides Development Programme [accessed December 29, 2009];HIV ‘prevention’ gel PRO 2000 proven ineffective. http://www.mdp.mrc.ac.uk/ archive.html.
- 22.van de Wijgert J, Jones H. Challenges in microbicide trial design and implementation. Stud Fam Plann. 2006;37(2):123–9. doi: 10.1111/j.1728-4465.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 23.Tolley EE, Severy LJ. Integrating behavioral and social science research into microbicide clinical trials: challenges and opportunities. AJPH. 2006;96(1):79–83. doi: 10.2105/AJPH.2004.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolley EE, Eng E, Kohli R, et al. Examining the context of microbicide acceptability among married women and men in India. Cult, Health Sex. 2006;8(4):351–69. doi: 10.1080/13691050600793071. [DOI] [PubMed] [Google Scholar]
- 25.Marlow HM, Tolley EE, Kohli R, Mehendale S. Exploring married couples’ sexual communication within the context of a microbicide clinical trial and acceptability study in pune, India. Cult, Health Sex. 2010;12(8):899–912. doi: 10.1080/13691058.2010.508843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catania J, Kegeles S, Coates T. Towards an understanding of risk behavior: an AIDS risk reduction model (ARRM) Health Educ Q. 1990;17:53–72. doi: 10.1177/109019819001700107. [DOI] [PubMed] [Google Scholar]
- 27.Potts M. Thinking about vaginal microbicide testing. AJPH. 2000;90(2):188–90. doi: 10.2105/ajph.90.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wittkowski K. Further thoughts about vaginal microbicide testing. AJPH. 2000;90(2):1155–6. doi: 10.2105/ajph.90.7.1155a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Zoysa I, Elias CJ, Bentley ME. Ethical challenges in efficacy trials of vaginal microbicides for HIV prevention. AJPH. 1998;88(4):571–5. doi: 10.2105/ajph.88.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham SM, Shah PS, Aesch ZC, Beyene J, Bayoumi AM. A systematic review of the quality of trials evaluating biomedical HIV prevention interventions shows that many lack power. HIV Clin Trials. 2009;10(6):413–31. doi: 10.1310/hct1006-413. [DOI] [PMC free article] [PubMed] [Google Scholar]


