Abstract
Background & Aim
The mitogen-activated protein kinases (MAPKs) c-Jun N-terminal kinase (JNK) and p38 mediate liver ischemia/reperfusion (I/R) injury via cell death and inflammatory cytokine expression, respectively. Nilotinib is an orally available receptor tyrosine kinase inhibitor used for chronic myelogenous leukemia that also has in vitro activity against JNK and p38. In this study, we examine its therapeutic potential against hepatic I/R injury.
Methods
The effects of nilotinib on liver I/R injury were tested using a murine model of warm, segmental liver I/R. Serum ALT was measured and livers were analyzed by histology, RT-PCR, western blot, and flow cytometry. The in vitro effects of nilotinib on hepatocyte and nonparenchymal cell (NPC) MAPK activation and cytokine production were also tested.
Results
Mice receiving nilotinib had markedly lower serum ALT levels and less histologic injury and apoptosis following liver I/R. Nilotinib did not inhibit its known receptor tyrosine kinases. Nilotinib lowered intrahepatic expression of IL-1β, IL-6, MCP-1, and MIP-2 and systemic levels of IL-6, MCP-1, and TNF. Nilotinib reduced NPC activation of p38 MAPK signaling and decreased the recruitment of inflammatory monocytes and their production of TNF. Nilotinib attenuated JNK phosphorylation and hepatocellular apoptosis. In vitro, nilotinib demonstrated direct inhibition of JNK activation in isolated hepatocytes cultured under hypoxic conditions, and blocked activation of p38 MAPK and cytokine production by stimulated NPCs.
Conclusions
Nilotinib lowers both liver JNK activation and NPC p38 MAPK activation and may be useful for ameliorating liver I/R injury in humans.
Keywords: p38 MAPK, c-Jun NH2-terminal kinase, hepatocytes, nonparenchymal cells, cytokines
INTRODUCTION
Liver I/R injury is a significant clinical problem that is frequently encountered during liver transplantation, partial hepatectomy, and various forms of circulatory shock [1]. The temporary deprivation of blood flow to oxygen-dependent hepatocytes and NPCs followed by reperfusion results in a complex series of events, including the release of reactive oxygen species and danger signals, activation of signal transduction cascades, production of inflammatory cytokines and chemokines, and upregulation of adhesion molecules [2]. These events lead to hepatocyte death by both apoptosis and necrosis, and ultimately to organ dysfunction [3]. Clinically, there are no directed therapies for patients with liver I/R injury, and treatment is only supportive.
The MAPKs JNK and p38 mediate intracellular signaling in response to extracellular stimuli [4]. JNK and p38 are phosphorylated early in the response to liver I/R [5–7]. IL-1, TNF-α, and oxidative stress [8] activate the MAPK kinases (MAP2K) MKK4 and MKK7, which subsequently phosphorylate JNK [9]. Transient JNK activation in response to inflammatory cytokines promotes hepatocellular proliferation and survival by inducing the upregulation of NF-κB responsive genes, including the growth arrest DNA damage-inducible gene 45β (Gadd45β), which blocks MAP2K-induced activation of JNK [10]. Loss of hepatoprotective Gadd45β worsens liver injury in several models [10]. Prolonged JNK activation results in hepatocyte death through the modulation of pro- and anti-apoptotic protein function [11–13]. p38 MAPK signaling has a major role in post-ischemic liver damage by inducing inflammatory cytokine expression as well as the recruitment and activation of leukocytes [4, 14]. Inflammatory cytokines, oxidative stress, and MyD88-dependent danger signals activate MKK3 and MKK6, the MAP2Ks specific for p38 [4, 5]. p38 has several downstream targets including MAPK-activated protein kinase 2 (MAPKAPK2), which is important for inflammatory cytokine expression by NPCs [5].
Nilotinib (Tasigna®; Novartis Pharmaceuticals) is a second generation receptor tyrosine kinase inhibitor originally designed for patients with chronic myelogenous leukemia who developed resistance to imatinib (Gleevec®; Novartis) [15]. Overall, nilotinib is well tolerated by patients and has few side effects [16]. Nilotinib has higher affinity for BCR-ABL than does imatinib, while both drugs have similar affinity for platelet-derived growth factor receptor (PDGFR)-α/β and KIT. Both drugs inhibit the discoidin domain receptors 1 and 2, as well as the receptor for colony stimulating factor 1 (CSF-1R). In addition to its receptor tyrosine kinase targets, nilotinib inhibits JNK and p38 MAPK in vitro [15]. Therefore, we hypothesized that nilotinib may have therapeutic potential in liver I/R injury, given the central importance of MAPKs in its pathogenesis.
MATERIALS AND METHODS
Animals and procedures
Eight- to 10-week old wild-type male C57BL/6J (WT) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Nilotinib and imatinib (Novartis Pharmaceuticals, Basel, Switzerland) were resuspended in a mixture of 0.5% methycellulose and 0.05% Tween 80. Mice were administered nilotinib (30 mg/kg), imatinib (100 mg/kg), or vehicle by oral gavage 12h and 2h prior to undergoing sham or liver I/R. Animals were maintained in a pathogen-free housing facility at Memorial Sloan-Kettering Cancer Center (New York, NY). All procedures were approved by the Institutional Animal Care and Use Committee.
Model of liver I/R
A nonlethal model of segmental (70%) warm hepatic I/R was performed as previously described [17], with minor modifications. Briefly, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injection. An upper midline incision was performed and the liver was exteriorized to expose the hilum. The vessels supplying the left and median lobes of liver were carefully dissected from surrounding tissues and occluded with a microvascular clamp (Roboz Surgical Instruments, Gaithersburg, MD) for 1h. During clamping, the liver was replaced into the abdominal cavity and the incision was closed temporarily with a single skin staple to decrease evaporative loss. Body temperature was maintained by use of a warming pad and rectal temperature probe (Fine Science Tools, Foster City, CA). Sham mice underwent the same procedure without application of the microvascular clamp. At the end of the observation period, mice were euthanized by carbon dioxide inhalation.
Assessment of liver damage
Hepatocellular injury was determined by measuring serum ALT levels using an Olympus AU400 Chemistry Analyzer (Laboratory of Comparative Pathology, Sloan-Kettering Institute). Serum cytokines were measured using a cytometric bead array according to the manufacturer’s protocol (Mouse Inflammation Kit, BD Biosciences, San Jose, CA). Blood was flushed from livers by injecting sterile PBS via the portal vein, and ischemic lobes were removed and fixed in 10% formalin. Hematoxylin-eosin staining and immunohistochemistry were performed on 5 μm paraffin-embedded liver sections. A pathologist was blinded to experimental groups and assigned a score for tissue damage based on the Suzuki criteria [18]. Briefly, liver sections were scored from 0 to 4 based on the degree of sinusoidal congestion (none, minimal, mild, moderate, severe), cytoplasmic vacuolization (none, minimal, mild, moderate, severe), and necrosis of parenchymal cells (none, single cell necrosis, <30%, 30–60%, >60%). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining was performed using the ApopTag Red In Situ Apoptosis Detection Kit (Millipore, Billerica, MA) according to the manufacturer’s protocol. Immunohistochemistry was performed using a rabbit polyclonal antibody for phospho-JNK (1:50 dilution; Cell Signaling Technology, Danvers, MA), according to the manufacturer’s protocol.
Preparation of protein extracts and western blotting
Whole protein was extracted from frozen liver tissue or liver cells using ice-cold lysis buffer (10 mM Tris HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 10% glycerol, 2 mM sodium orthovanadate, and complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN)). Cytoplasmic-free nuclear protein extract was obtained using the NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Thermo Scientific, Rockford, IL), according to the manufacturer’s protocol. Protein concentrations were quantified using the Bradford method. Bulk liver NPCs were isolated as described previously [17, 19], and protein was isolated as above. Briefly, after livers were harvested, they were homogenized in 1% Type IV collagenase. Hepatocytes were separated from the supernatant using three spins at 70g for 2 minutes. The supernatant was then centrifuged at 450g for 5 minutes to pellet NPCs, which were further enriched by a 40% Optiprep (Sigma-Aldrich, St. Louis, MO) density gradient according to the manufacturer’s protocol. Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was then probed with antibodies against total and phospho-MKK3/6, MKK4, SAPK/JNK, p38 MAPK, p44/42 MAPK, MAPKAPK2, PDGFRα, PDGFRβ, CSF-1R, KIT, and ABL; cleaved PARP; GAPDH; and β-actin (Cell Signaling Technology), according to the manufacturer’s protocol. Appropriate secondary antibodies were used (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were visualized by using an enhanced chemiluminescence detection kit (GE Healthcare Biosciences, Piscataway, NJ).
Quantitative RT-PCR
Total RNA from liver tissue was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. For cDNA synthesis, 0.5 μg of total RNA was transcribed with the TaqMan Reverse Transcription Reagents Kit using random hexamers (Applied Biosystems, Foster City, CA). PCR TaqMan probes for murine Bcl-2, Bax, IL-1β, IL-6, TNF, MCP-1, MIP-2, IL-10, Gadd45β, and GAPDH (Applied Biosystems) were used. Quantitative RT-PCR was performed using the ABI 7900 system (Applied Biosystems). Relative mRNA expression levels were calculated by the 2− Ct method.
Flow cytometry
Flow cytometry and intracellular cytokine analysis was performed on a FACSAria (BD Biosciences) as described previously [20]. We defined neutrophils as CD11bhiLy6Ghi, inflammatory monocytes as CD11bintLy6Chi, and Kupffer cells as CD11bintF4/80+. Cells were stained with fluorochrome-conjugated antibodies to CD45.2 (PerCP-Cy5.5; clone 104), CD11b (APC-Cy7; clone M1/70), Ly6G (PE; clone 1A8), Ly6C (PE-Cy7; clone AL-21), TNF (PE; clone MPC-XT22) (all from BD Biosciences), and F4/80 (APC; clone BM8; Invitrogen, Carlsbad, CA).
In vitro assays
For murine hepatocyte isolation, the liver was perfused via the portal vein with calcium- and magnesium-free HBSS followed by 0.03% collagenase (Worthington Biochemical, Lakewood, NJ). Liver homogenates were filtered through a 100 m cell strainer, mixed with an equal volume of Percoll, and spun at 70g for 8 minutes. The cell pellets were washed twice with serum-free DMEM for 2 minutes at 70g and resuspended at 106 cells/ml in complete DMEM. 2 ml aliquots were dispensed on to 6-well collagen I-coated plates cultured overnight in the presence of vehicle or nilotinib under normoxia or hypoxia (1% O2, 5% CO2, 94% N2) using a modular incubator chamber (Billups-Rothenberg, Del Mar, CA). Nilotinib was dissolved in DMSO and serially diluted in media to give final working concentrations. Cell lysates were used for detecting phospho-JNK by western blot. For assessment of cytokine production, murine NPCs (106 cells/ml) were cultured in triplicate wells in 96-well plates at 37°C using standard RPMI media as described previously [17, 19]. Cells were pre-treated for 1h with vehicle or nilotinib. 10 μg/ml of the Toll-like receptor 9 (TLR9) agonist CpG (ODN M362; Invivogen, San Diego, CA) was added to some wells, and cells were cultured overnight. Supernatant cytokines were measured using a cytometric bead array (Mouse Inflammation Kit, BD Biosciences). For assessment of p38 MAPK signaling, NPCs were pretreated with vehicle or 1μM nilotinib and cultured in 6-well plates in serum-free media (X-Vivo 15, Lonza Walkersville, Inc, Walkersville, MD). Cells were then stimulated with CpG and cultured at 37°C for 2h. Protein was extracted and western blots were performed as above.
Statistical analysis
Results are expressed as mean + SEM. Statistical significance was determined by the two-tailed Student’s t test, one-way ANOVA, or two-way ANOVA as appropriate, using statistical software (Prism 5.0; GraphPad Software, Inc., La Jolla, CA). p < 0.05 was considered statistically significant.
RESULTS
Nilotinib protects against liver I/R injury
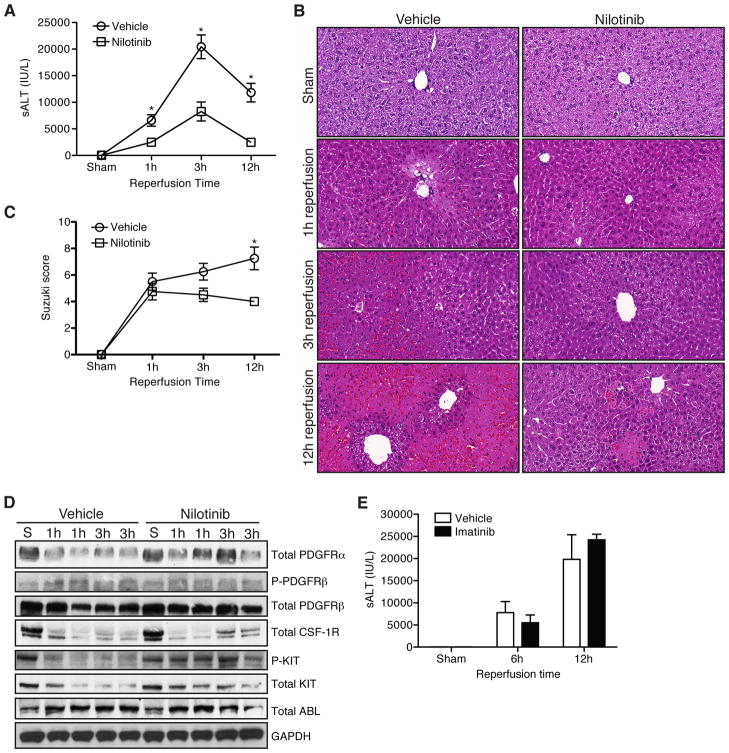
To test the therapeutic potential of nilotinib, we treated WT mice with nilotinib or vehicle prior to sham or 1h of 70% liver ischemia followed by reperfusion. Animals treated with nilotinib had significantly lower serum ALT levels 1, 3, 6, and 12h after reperfusion (Fig. 1A and Supplemental Fig. 1). Nilotinib reduced liver histologic injury qualitatively (Fig. 1B) and quantitatively by the Suzuki score [18] at 12h (Fig. 1C).
Figure 1. Nilotinib protects against liver I/R injury.
WT mice were treated with vehicle or nilotinib prior to undergoing sham or liver I/R. (A) Serum ALT at serial time points. (B) Representative H&E staining (40× magnification). (C) Histologic injury score based on Suzuki criteria. (D) Ischemic whole liver protein from the indicated time points was assessed for receptor tyrosine kinase expression and phosphorylation. Each lane represents 1 animal. (E) WT mice were treated with vehicle or imatinib prior to undergoing sham or liver I/R. Serum was analyzed for ALT at the indicated time points. Data are representative of 2 experiments with similar results. n = 2–3 mice/group (sham) and 7–8 mice/group per time point (I/R). S – sham. P – phospho. *p < 0.05.
To investigate the mechanism by which nilotinib protected against liver I/R injury, we analyzed the phosphorylation (phospho) status of its known receptor tyrosine kinase targets, which include PDGFRα, PDGFRβ, KIT, CSF-1R, and ABL (Fig. 1D). Nilotinib did not decrease phospho-KIT or phospho-PDGFR Phospho-PDGFRα, CSF-1R, and ABL were not detectable in either vehicle or nilotinib treated animals (data not shown). Nilotinib did not affect the expression of total PDGFRα, PDGFRβ, or ABL, but was associated with increased total KIT and CSF-1R. To further establish that nilotinib was not acting through its known receptor tyrosine kinase targets, we tested if imatinib, which has a similar tyrosine kinase profile [15], could mediate protection from liver I/R injury. Imatinib did not protect against liver I/R injury as measured by serum ALT at 6 and 12h (Fig. 1E). Collectively, these data suggest that nilotinib mediates protection from hepatic I/R injury by a mechanism independent of inhibiting its known receptor tyrosine kinase targets.
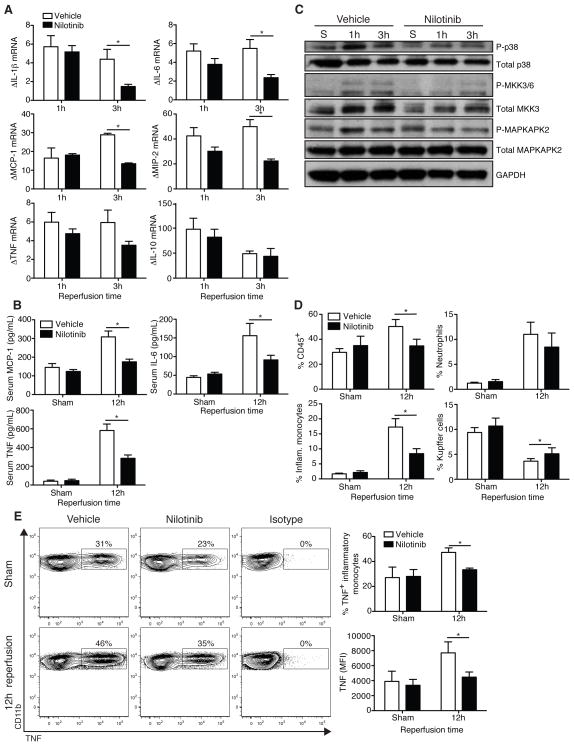
Nilotinib blocks p38 MAPK activation, decreases cytokine production, and modulates leukocyte recruitment to the liver following I/R
Cytokines and chemokines are important to the pathogenesis of liver I/R injury through the recruitment and activation of leukocytes, as well as direct local effects on hepatocytes, including activation of the JNK stress kinase pathway [6]. We analyzed whole liver by RT-PCR for expression of a panel of cytokines known to be important in hepatic I/R injury (Fig. 2A). There were no differences at 1h following reperfusion. However, at 3h, nilotinib treated mice expressed considerably less IL-1β, IL-6, MCP-1, and MIP-2. Nilotinib also decreased TNF expression, but this did not reach statistical significance. IL-10 mRNA levels were not affected. At 12h, mice treated with nilotinib expressed 2-fold lower serum levels of IL-6, MCP-1, and TNF (Fig. 2B). Hepatic NPCs constitute a small percentage of total liver protein [21], but account for the majority of cytokine expression [1, 22]. p38 MAPK plays an important role in the expression of inflammatory cytokines [5], therefore we investigated its activation in isolated NPCs after liver I/R (Fig. 2C). Nilotinib reduced p38 MAPK phosphorylation in NPCs. Nilotinib also lowered the activation of upstream MKK3/6 and downstream MAPKAPK2. In contrast, nilotinib did not affect JNK signaling or p44/42 (ERK1/2) activation in NPCs after liver I/R (data not shown).
Figure 2. Nilotinib blocks p38 MAPK activation, decreases cytokine production, and modulates leukocyte recruitment to the liver following I/R.
WT mice were treated with vehicle or nilotinib prior to undergoing sham or I/R. (A) Ischemic whole liver was analyzed by RT-PCR for cytokine expression. Values are normalized to sham animals. (B) 12h serum cytokines were measured by cytometric bead array. (C) NPCs were isolated as described from sham or ischemic lobes at 1h and 3h and analyzed by western blot for activation of the p38 MAPK pathway. (D) Intrahepatic immune infiltrate in sham animals or ischemic lobes 12h following I/R as determined by flow cytometry. (E) Representative TNF staining on inflammatory monocytes from ischemic lobes at 12h. Data are representative of 2 experiments with similar results. n = 2–3 mice/group (sham) and 7–8 mice/group per time point (I/R). S – sham. P – phospho. *p < 0.05.
The hepatocellular injury resulting from liver I/R releases a variety of danger signals and results in the recruitment of leukocytes to the damaged areas of the liver. Neutrophils [19], inflammatory monocytes [17], and CD4+ T cells [23] are detrimental, whereas dendritic cells [17] and Kupffer cells [24, 25] are protective. To investigate whether nilotinib affected the immune response to liver I/R, we analyzed hepatic leukocytes at 3 and 12h following reperfusion. Nilotinib treatment did not alter the percentage of neutrophils, inflammatory monocytes, or Kupffer cells in the livers of sham animals or in the ischemic lobes 3h after liver I/R (Supplemental Fig. 2). However, 12h following reperfusion, mice treated with nilotinib had a lower percentage of CD45+ cells, a 2-fold reduction in the recruitment of inflammatory monocytes, and diminished the loss of Kupffer cells in the ischemic lobes (Fig. 2D). We confirmed the reduction in inflammatory monocyte recruitment by immunohistochemistry, and also found that nilotinib-treated mice expressed lower levels of ICAM-1 (data not shown). TNF and inflammatory monocytes are critical mediators of liver I/R injury [8, 17]. Therefore, we analyzed inflammatory monocytes from the ischemic lobes for TNF expression. Nilotinib lowered the percentage of TNF+ inflammatory monocytes and the amount of TNF expression on a per cell basis (Fig. 2E). Kupffer cells expressed lower levels of TNF and expression was not affected by nilotinib (data not shown). Nilotinib did not alter the percentage of intrahepatic conventional dendritic cells, plasmacytoid dendritic cells, B cells, NK cells, NKT cells, CD4+ T cells, CD8+ T cells, or FoxP3+ T regulatory cells after liver I/R (data not shown). Taken together, these data suggest that nilotinib blocks p38-dependent inflammatory cytokine expression and modulates the recruitment and activation of inflammatory leukocytes in liver following I/R.
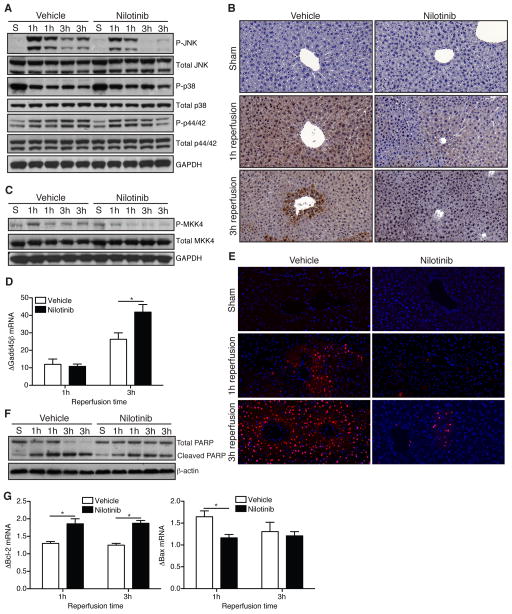
Nilotinib attenuates JNK signaling and hepatocellular apoptosis after hepatic I/R
Expression of phospho-JNK occurs early after reperfusion of the liver [26]. Others have shown that liver ischemia alone without reperfusion activates JNK signaling [7], which we found as well (data not shown). Given the importance of the JNK pathway in the pathogenesis of liver I/R injury [6, 27, 28], and because nilotinib has activity against JNK in vitro [15], we investigated the effect of nilotinib on JNK activity following liver I/R. Nilotinib markedly reduced expression of phospho-JNK in ischemic whole liver at 3h following reperfusion (Fig. 3A). In contrast, it did not alter phospho-p38 MAPK or phospho-p44/42 (ERK1/2). Immunohistochemistry confirmed the reduction in phospho-JNK in nilotinib treated animals compared to vehicle controls that we showed by western blot (Fig. 3B). Activation of MKK4, which is directly upstream of JNK, was also reduced by nilotinib (Fig. 3C). The transient activation of JNK signaling by inflammatory cytokines also induces upregulation of Gadd45β through NF-κB [10]. We analyzed ischemic liver and found that by 3h, mice receiving nilotinib expressed significantly more Gadd45β (Fig. 3D). Prolonged JNK activation results in modulation of apoptotic protein function and increased cellular apoptosis. Accordingly, nilotinib reduced the number of TUNEL-positive nuclei compared to vehicle controls at 1 and 3h (Fig. 3E). TUNEL staining does not differentiate between necrotic and apoptotic cell death [3], therefore we analyzed nuclear extracts for cleaved PARP and tissue sections for cleaved caspase-3 staining, both markers of apoptosis. Following I/R, mice treated with nilotinib had less cleaved PARP at 1 and 3h (Fig. 3F) and lower levels of cleaved caspase-3 at 6h (data not shown). Furthermore, whole livers from mice treated with nilotinib expressed more Bcl-2 (anti-apoptotic) and less Bax (apoptotic) (Fig. 3G). Overall, these data suggest that nilotinib attenuates JNK signaling following liver I/R, diminishing the downstream pro-apoptotic effects of prolonged JNK activation.
Figure 3. Nilotinib attenuates JNK signaling and hepatocellular apoptosis after hepatic I/R.
WT mice were treated with vehicle or nilotinib prior to undergoing sham or I/R. (A) Western blot of ischemic whole liver protein for total and phospho-JNK, p38 MAPK, and p44/42 (ERK1/2). Each lane represents 1 animal. (B) Representative immunostaining for phospho-JNK on liver sections (40× magnification). (C) Western blot of ischemic whole liver protein for total and phospho-MKK4. (D) Ischemic whole liver mRNA transcript level for Gadd45β as measured by RT-PCR. Values are normalized to sham animals. (E) Representative TUNEL staining of liver sections from sham livers or ischemic lobes (40× magnification). (F) Western blot of ischemic whole liver nuclear protein extract for total and cleaved PARP. Each lane represents 1 animal. (G) mRNA transcript levels for Bcl-2 and Bax from whole liver as determined by RT-PCR. Values are normalized to sham animals. Data are representative of 2 experiments with similar results. n = 2–3 mice/group (sham) and 7–8 mice/group per time point (I/R). S – sham. P – phospho. *p < 0.05.
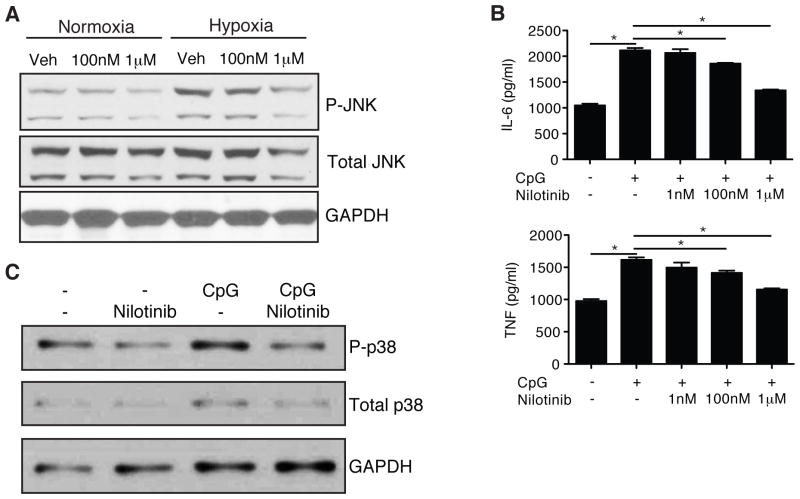
Nilotinib mediates direct effects against hepatocyte JNK and NPC p38 MAPK activation in vitro
The complex interaction between hepatocytes and NPCs during the pathogenesis of liver I/R injury makes it difficult to delineate whether the overall effects of nilotinib in vivo resulted from a direct effect on hepatocytes, NPCs, or a combination of both. To mimic I/R conditions in vitro, we cultured WT hepatocytes from unmanipulated mice under normoxic or hypoxic conditions in the presence of vehicle or nilotinib. We found markedly increased phosphorylation of JNK in hypoxic hepatocytes compared to normoxic hepatocytes (Fig. 4A). Nilotinib blocked the upregulation of phospho-JNK in hypoxic hepatocytes in a dose-dependent fashion. Nilotinib did not affect activation of p38 MAPK or p44/42 (ERK1/2) (data not shown).
Figure 4. Nilotinib mediates direct effects against hepatocyte JNK and NPC p38 MAPK activation in vitro.
(A) WT hepatocytes were isolated and pre-treated with vehicle or nilotinib, followed by overnight culture under normoxic or hypoxic conditions. Cell lysates were isolated and analyzed by western blot for phospho-JNK. (B and C) WT NPCs were treated with vehicle or nilotinib prior to stimulation with CpG. (B) Supernatant cytokines were measured by cytokine bead array after overnight culture. (C) Western blot for activation of p38 MAPK after 2h of stimulation with CpG. Data are representative of 2 experiments with similar results. Veh – vehicle. P – phospho. *p < 0.05.
To test if nilotinib could modulate NPCs directly, we cultured NPCs from unmanipulated mice in the presence of the TLR9 agonist CpG. We have previously demonstrated that TLR9 plays an important role in liver I/R, as liver I/R injury is diminished in WT mice given a TLR9 inhibitor and in TLR9−/− mice [19]. TLR9 signaling has also been shown to mediate liver injury in acetaminophen toxicity [29] and liver fibrosis from bile duct ligation [30]. TLR9 is known to activate p38 MAPK via MyD88 signaling [4]. Based on this rationale, we tested if nilotinib could inhibit CpG-mediated activation of NPCs. We found that nilotinib inhibited CpG induction of TNF and IL-6 in a dose-dependent manner (Fig. 4B). In particular, nilotinib blunted the increase in phospho-p38 MAPK by CpG (Fig. 4C), while it had no effect on phospho-JNK or phospho-p44/42 (ERK1/2) (data not shown). Therefore, nilotinib can directly block JNK activation in hepatocytes and p38 MAPK-dependent cytokine expression in NPCs.
DISCUSSION
Warm liver I/R injury is characterized by stress kinase activation, hepatocellular injury, release of danger signals, and activation of liver NPCs [1]. The soluble mediators released by NPCs result in the recruitment and activation of neutrophils and inflammatory monocytes, and also create a feedback loop that further activates hepatocellular stress kinase pathways, exacerbating injury [1, 31]. The interaction between hepatocytes and NPCs following I/R is complex, and an ideal therapeutic agent would target early events in the pathway and minimize the generation of detrimental feedback loops that amplify injury. We have found that nilotinib attenuates early JNK activation in hepatocytes and p38 MAPK activation in NPCs, significantly reducing hepatocellular injury.
It is well established that JNK is detrimental in liver I/R and that JNK inhibition can decrease injury [28, 32]. Cytotoxic signaling pathways activated during cellular stress converge on JNK, including those initiated by cytokines, death receptor signaling, and oxidative stress [6]. Transient JNK activation promotes cell survival whereas prolonged JNK activation leads to modulation of pro- and anti-apoptotic proteins and activation of cell death pathways [10–13]. Accordingly, we found that in vivo, nilotinib attenuated JNK signaling, increased expression of the JNK inhibitor Gadd45β, increased expression of anti-apoptotic Bcl-2, and decreased expression of pro-apoptotic Bax (Fig. 3A, D, and G).
Nilotinib did not affect p38 MAPK activation in bulk liver (Fig. 3A), although intrahepatic and systemic inflammatory cytokine expression was significantly decreased (Fig. 2A and B). NPCs are responsible for the majority of intrahepatic cytokine expression but constitute a small fraction of total liver protein. When we examined the status of p38 MAPK in NPCs isolated from livers after I/R, we found that nilotinib specifically diminished phospho-p38 MAPK (Fig. 2C). Our data are consistent with prior reports that specific inhibition of p38 MAPK is beneficial in liver I/R [33, 34]. Notably, although nilotinib decreased pro-inflammatory cytokine secretion, it did not alter IL-10 expression (Fig. 2A). IL-10 production is primarily controlled by ERK1/2 [35], which was not altered by nilotinib therapy (Fig. 2C and data not shown).
The inherent intricacy of events in the liver microenvironment following liver I/R obfuscates identification of the precise mechanism by which nilotinib works. Our in vitro assays indicated that nilotinib can directly inhibit JNK activation in hepatocytes and p38 activation in NPCs under conditions of cellular stress (Fig. 4). Given that nilotinib attenuated JNK activation and decreased downstream apoptotic effects (Fig. 3), but did not affect cytokine expression at 1h (Fig. 2A), we postulate that nilotinib may primarily protect hepatocytes, reducing the exposure and response of NPCs to danger signals, blunting the inflammatory response that can drive prolonged JNK expression and result in more hepatocellular injury.
It is possible that nilotinib works through other targets besides JNK and p38 MAPK. One potential target is ZAK kinase, a MAP2K kinase, which activates both p38 MAPK and JNK [36, 37]. Nilotinib binds ZAK kinase with high affinity in vitro, but it is uncertain whether it actually inhibits ZAK kinase activity [15]. Our in vivo data suggest that ZAK may be involved since nilotinib diminished the phosphorylation of the MAP2Ks MKK4 and MKK3/6, which are downstream of ZAK and upstream of JNK (Fig. 2B and 3C). The importance of ZAK kinase activity in doxorubicin-induced JNK-mediated apoptosis of the human keratinocyte cell line HaCaT has been demonstrated [38]. In the presence of siRNA knockdown of ZAK kinase or nilotinib, JNK activation and cellular apoptosis were diminished. Currently, the available reagents for ZAK are highly limited, which precludes further direct analysis in our model.
We could not demonstrate an inhibitory effect of nilotinib on KIT, CSF-1R, ABL, PDGFRα, or PDGFRβ (Fig. 1D), and imatinib, which also inhibits these targets [39], did not protect against liver I/R injury (Fig. 1E). However, it is possible that the failure of imatinib to mediate protection could result from pharmacokinetic differences between the drugs, as imatinib requires active transport into cells whereas nilotinib does not [40]. Injury from liver I/R may affect the active transport of imatinib into hepatocytes and NPCs. CSF-1R regulates key effector functions of macrophages and contributes to inflammation [41]. Within the liver, CSF-1R is expressed by Kupffer cells [42], and we observed a higher degree of total CSF-1R expression at 3h in mice treated with nilotinib (Fig. 1D). Both imatinib and nilotinib target CSF-1R with similar affinity [39]. Imatinib mediates protection against autoimmune arthritis [43] via inhibition of CSF-1R [44]. Given the potential pharmacokinetic differences between nilotinib and imatinib, we investigated the therapeutic potential of direct inhibition of CSF-1R. Administration of the CSF-1R inhibitor GW2580 [45] did not alter serum ALT or hepatocellular injury (data not shown).
While nilotinib decreased phospho-KIT expression in sham animals, we found higher phospho-KIT expression in animals treated with nilotinib following I/R (Fig. 1D). Both murine and human livers contain a subset of liver progenitor cells that express KIT [46]. It is possible that these cells are lost in vehicle treated mice given the degree of injury, whereas they are relatively spared in mice treated with nilotinib. This hypothesis is supported by the fact that the pattern of total KIT expression was similar to that of phospho-KIT expression following I/R (Fig. 1D).
Nilotinib decreases chronic liver fibrosis induced by carbon tetrachloride or bile duct ligation [47]. The proposed mechanism is multifactorial, including blockade of PDGFR signaling in hepatic stellate cells resulting in decreased collagen deposition. It has been proposed that in addition to PDGFRβ, nilotinib blocks ABL and transforming growth factor-β signaling, and causes apoptosis of hepatic stellate cells. The role of hepatic stellate cells in the acute phase of liver I/R is unclear, as it has been shown that they impair sinusoidal microcirculation [48] but may mediate protection [49]. We did not address the specific function of hepatic stellate cells in the current study.
Using a murine model of warm segmental liver I/R, we demonstrated that nilotinib mediates protection from liver I/R injury. Nilotinib reduced histopathologic injury, apoptosis, and the inflammatory response to I/R injury, thereby diminishing the recruitment and TNF expression by inflammatory monocytes. The mechanism of protection involved attenuated JNK activation in hepatocytes and reduced p38 MAPK-dependent cytokine expression by NPCs. There are currently no targeted therapies for liver I/R injury. Given its safety and tolerability, nilotinib may be useful for ameliorating liver I/R injury in humans.
Supplementary Material
WT mice were treated with vehicle or nilotinib prior to undergoing sham or I/R. Serum ALT was measured at serial time points. Data are pooled from 4 experiments with similar results, n = 7–18 mice/group. *p < 0.05.
WT mice were treated with vehicle or nilotinib prior to undergoing sham or liver I/R. NPCs were isolated from whole livers (sham) or ischemic lobes (I/R) following 3h of reperfusion. Groups were pooled and analyzed by flow cytometry. (A) Representative contour plots for neutrophils, inflammatory monocytes, and Kupffer cells at 3h in gated live, CD45+ NPCs. n = 2–3 mice/group (sham) and 7–8 mice/group per time point (I/R).
Acknowledgments
Financial support: This work was supported by National Institutes of Health grants DK068346 and T32 CA009501 (RPD).
We are grateful to Edward King in the Lab of Comparative Pathology. Dr. Jinru Shia performed the histopathologic scoring. Paul Manley (Novartis) provided technical insight. Mithat Gonen assisted with statistical analysis.
Abbreviations
- MAPK
mitogen-activated protein kinase
- JNK
c-Jun-N-terminal kinase
- I/R
ischemia/reperfusion
- ALT
alanine aminotransferase
- NPC
nonparenchymal cell
- MAP2K
MAPK kinase
- MAPKAPK2
MAPK activating protein kinase 2
- PDGFR
platelet-derived growth factor receptor
- CSF-1R
colony stimulating factor receptor 1
- WT
wild type
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- TLR9
Toll-like receptor 9
Footnotes
Conflict of interest: RPD has served as a consultant for Novartis Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90:665–677. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337–345. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 4.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 6.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCloskey CA, Kameneva MV, Uryash A, Gallo DJ, Billiar TR. Tissue hypoxia activates JNK in the liver during hemorrhagic shock. Shock. 2004;22:380–386. doi: 10.1097/01.shk.0000140660.78744.bf. [DOI] [PubMed] [Google Scholar]
- 8.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Destrument A, Tournier C. Physiological roles of MKK4 and MKK7: insights from animal models. Biochim Biophys Acta. 2007;1773:1349–1357. doi: 10.1016/j.bbamcr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090–1101. doi: 10.1016/j.bcp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 11.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, et al. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 12.Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNFalpha- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 13.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, et al. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 14.King LA, Toledo AH, Rivera-Chavez FA, Toledo-Pereyra LH. Role of p38 and JNK in liver ischemia and reperfusion. J Hepatobiliary Pancreat Surg. 2009;16:763–770. doi: 10.1007/s00534-009-0155-x. [DOI] [PubMed] [Google Scholar]
- 15.Manley PW, Drueckes P, Fendrich G, Furet P, Liebetanz J, Martiny-Baron G, et al. Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta. 2010;1804:445–453. doi: 10.1016/j.bbapap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Deininger MW. Nilotinib. Clin Cancer Res. 2008;14:4027–4031. doi: 10.1158/1078-0432.CCR-07-5015. [DOI] [PubMed] [Google Scholar]
- 17.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ocuin LM, Bamboat ZM, Balachandran VP, Cavnar MJ, Obaid H, Plitas G, et al. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J Leukoc Biol. 2011;89:423–432. doi: 10.1189/jlb.0810479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 22.Okaya T, Lentsch AB. Cytokine cascades and the hepatic inflammatory response to ischemia and reperfusion. J Invest Surg. 2003;16:141–147. [PubMed] [Google Scholar]
- 23.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devey L, Ferenbach D, Mohr E, Sangster K, Bellamy CO, Hughes J, et al. Tissue-resident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol Ther. 2009;17:65–72. doi: 10.1038/mt.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellett JD, Atkinson C, Evans ZP, Amani Z, Balish E, Schmidt MG, et al. Murine Kupffer cells are protective in total hepatic ischemia/reperfusion injury with bowel congestion through IL-10. J Immunol. 2010;184:5849–5858. doi: 10.4049/jimmunol.0902024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradham CA, Stachlewitz RF, Gao W, Qian T, Jayadev S, Jenkins G, et al. Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology. 1997;25:1128–1135. doi: 10.1002/hep.510250514. [DOI] [PubMed] [Google Scholar]
- 27.Theruvath TP, Czerny C, Ramshesh VK, Zhong Z, Chavin KD, Lemasters JJ. C-Jun N-terminal kinase 2 promotes graft injury via the mitochondrial permeability transition after mouse liver transplantation. Am J Transplant. 2008;8:1819–1828. doi: 10.1111/j.1600-6143.2008.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uehara T, Bennett B, Sakata ST, Satoh Y, Bilter GK, Westwick JK, et al. JNK mediates hepatic ischemia reperfusion injury. J Hepatol. 2005;42:850–859. doi: 10.1016/j.jhep.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabele E, Muhlbauer M, Dorn C, Weiss TS, Froh M, Schnabl B, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008;376:271–276. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks--a review. Liver Transpl. 2010;16:1016–1032. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 32.Uehara T, Xi Peng X, Bennett B, Satoh Y, Friedman G, Currin R, et al. c-Jun N-terminal kinase mediates hepatic injury after rat liver transplantation. Transplantation. 2004;78:324–332. doi: 10.1097/01.tp.0000128859.42696.28. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi M, Takeyoshi I, Yoshinari D, Matsumoto K, Morishita Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery. 2002;131:344–349. doi: 10.1067/msy.2002.121097. [DOI] [PubMed] [Google Scholar]
- 34.Yoshinari D, Takeyoshi I, Kobayashi M, Koyama T, Iijima K, Ohwada S, et al. Effects of a p38 mitogen-activated protein kinase inhibitor as an additive to university of wisconsin solution on reperfusion injury in liver transplantation. Transplantation. 2001;72:22–27. doi: 10.1097/00007890-200107150-00007. [DOI] [PubMed] [Google Scholar]
- 35.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 36.Gotoh I, Adachi M, Nishida E. Identification and characterization of a novel MAP kinase kinase kinase, MLTK. J Biol Chem. 2001;276:4276–4286. doi: 10.1074/jbc.M008595200. [DOI] [PubMed] [Google Scholar]
- 37.Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauter KA, Magun EA, Iordanov MS, Magun BE. ZAK is required for doxorubicin, a novel ribotoxic stressor, to induce SAPK activation and apoptosis in HaCaT cells. Cancer Biol Ther. 2010;10:258–266. doi: 10.4161/cbt.10.3.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manley PW, Stiefl N, Cowan-Jacob SW, Kaufman S, Mestan J, Wartmann M, et al. Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib. Bioorg Med Chem. 2010;18:6977–6986. doi: 10.1016/j.bmc.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 40.White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- 41.Irvine KM, Burns CJ, Wilks AF, Su S, Hume DA, Sweet MJ. A CSF-1 receptor kinase inhibitor targets effector functions and inhibits pro-inflammatory cytokine production from murine macrophage populations. FASEB J. 2006;20:1921–1923. doi: 10.1096/fj.06-5848fje. [DOI] [PubMed] [Google Scholar]
- 42.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 43.Paniagua RT, Sharpe O, Ho PP, Chan SM, Chang A, Higgins JP, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116:2633–2642. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paniagua RT, Chang A, Mariano MM, Stein EA, Wang Q, Lindstrom TM, et al. c-Fms-mediated differentiation and priming of monocyte lineage cells play a central role in autoimmune arthritis. Arthritis Res Ther. 2010;12:R32. doi: 10.1186/ar2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A. 2005;102:16078–16083. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knight B, Tirnitz-Parker JE, Olynyk JK. C-kit inhibition by imatinib mesylate attenuates progenitor cell expansion and inhibits liver tumor formation in mice. Gastroenterology. 2008;135:969–979. 979, e961. doi: 10.1053/j.gastro.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Wang Z, Kwong SQ, Lui EL, Friedman SL, Li FR, et al. Inhibition of PDGF, TGF-beta, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011;55:612–625. doi: 10.1016/j.jhep.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Mizunuma K, Ohdan H, Tashiro H, Fudaba Y, Ito H, Asahara T. Prevention of ischemia-reperfusion-induced hepatic microcirculatory disruption by inhibiting stellate cell contraction using rock inhibitor. Transplantation. 2003;75:579–586. doi: 10.1097/01.TP.0000052593.16876.AF. [DOI] [PubMed] [Google Scholar]
- 49.Jameel NM, Thirunavukkarasu C, Murase N, Cascio M, Prelich J, Yang S, et al. Constitutive release of powerful antioxidant-scavenging activity by hepatic stellate cells: protection of hepatocytes from ischemia/reperfusion injury. Liver Transpl. 2010;16:1400–1409. doi: 10.1002/lt.22172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT mice were treated with vehicle or nilotinib prior to undergoing sham or I/R. Serum ALT was measured at serial time points. Data are pooled from 4 experiments with similar results, n = 7–18 mice/group. *p < 0.05.
WT mice were treated with vehicle or nilotinib prior to undergoing sham or liver I/R. NPCs were isolated from whole livers (sham) or ischemic lobes (I/R) following 3h of reperfusion. Groups were pooled and analyzed by flow cytometry. (A) Representative contour plots for neutrophils, inflammatory monocytes, and Kupffer cells at 3h in gated live, CD45+ NPCs. n = 2–3 mice/group (sham) and 7–8 mice/group per time point (I/R).