Abstract
Background
ECP involves ex vivo leukocyte treatment with methoxsalen and UVA light to generate a tolerogenic response. A previous trial demonstrated that ECP permits corticosteroid withdrawal in steroid-dependent Crohn’s disease (CD) patients who were in clinical remission. We studied the effect of ECP on steroid withdrawal in steroid-dependent CD.
Methods
Patients with CD for ≥6 months, in remission at baseline while on steroids, but who had failed at ≥1 steroid withdrawal were included. Patients received 2 ECP treatments every 2 weeks for the 24-week steroid tapering period and underwent steroid-tapering. Patients completing steroid tapering could receive maintenance ECP (2 treatments/week) every month for 24 weeks.
Results
31 patients (CDAI 91; IBDQ 172.5) were enrolled (baseline corticosteroid dose − 20 mg/day). 65% were refractory to/intolerant of anti-TNF agents or immunosuppressants. After 24 weeks of ECP, 7 of 31 (22.6%) patients discontinued steroids while maintaining a CDAI of < 150. At Week 24, the steroid dose for the remaining patients on corticosteroids was 10 mg (p < 0.003 vs. baseline) with a CDAI of 110 and an IBDQ of 179. Following maintenance treatment, 3 patients remained in steroid-free remission. The 10 patients in the study and receiving ECP at Week 48 had a steroid dose of 3.5 mg with a CDAI of 40 and an IBDQ of 188.
Conclusions
ECP permitted discontinuation or reduction of steroids in a population of refractory steroid-dependent CD patients. ECP may be useful in permitting steroid withdrawal in selected steroid-dependent CD patients. Ideally, these results need to be confirmed in a “sham-controlled” clinical trial.
Keywords: Extracorporeal Photopheresis, Crohn’s Disease, Steroid Dependent
Introduction
Despite the dramatic success of corticosteroid therapy in achieving clinical remission in Crohn’s disease1 a large proportion of patients experience recurrent symptoms while the corticosteroid dose is being reduced, or shortly after being withdrawn from corticosteroids2. Steroid-dependency is reported in 28% to 36% of patients after the first course of steroids3,4 and even more frequent after previous use of steroids5. Franchimont6 noted that steroid-dependent Crohn’s disease patients may be characterized by younger age, concurrent cigarette and oral contraceptive use, and presence of colonic or perianal disease.
Corticosteroid dependence carries the risk of steroid-induced complications, such as osteopenia, hypertension, diabetes mellitus and increased susceptibility to infections. Thus, therapeutic maneuvers to reduce corticosteroid dependence have been introduced and particularly include the addition of immunomodulators or anti-TNF alpha agents. Methotrexate may be used to reduce prednisone dose in patients in remission7 but long-term tolerability and modest overall response rates (39%) compared to placebo (19%) are an issue with this approach. Azathioprine and 6-mercaptopurine are effective for maintenance of remission, but their slow onset of action limits their effectiveness in inducing clinical remission. Additionally, patients with moderate-to-severe Crohn’s disease who were treated with infliximab plus azathioprine were more likely to have a corticosteroid-free clinical remission than those receiving azathioprine or infliximab monotherapy alone, which results in the dilemma of long-term double immunosuppression and its potential sequelae on immunocompetence of patients8. Thus, approaches to reduce or discontinue steroids in patients with steroid-dependent Crohn’s disease are available, but are associated with potential toxicities or of modest efficacy only in the long-term management.
Extracorporeal photopheresis (ECP) involves the treatment of apheresed peripheral blood leukocytes with 8-methoxypsoralen (8-MOP) ex vivo. Subsequent exposure of the 8-MOP-treated leukocytes to UVA light then induces apoptosis through intercalation of deoxyribonucleic acid (DNA) and covalent binding to DNA. Apheresed leukocytes are then re-infused into the patient. Apoptotic cells are taken up by antigen presenting cells (APC) and induce regulatory T cells and a tolerogenic immune response9–12. Untreated monocytes exposed to ECP-treated leukocytes undergo a shift in monocyte cytokine-secretory pattern toward one associated with immune tolerance. Recently, a mechanism of ECP-induced immune tolerance has been linked to the stimulation of the anti-inflammatory cytokines IL-10 and TGF-beta by T regulatory cells, following the infusion of ECP-exposed CD11c(+) APCs13.
A single-center study by Reinisch and co-workers14 demonstrated a steroid-sparing effect of ECP in Crohn’s disease patients who were in clinical remission but remained steroid dependent. The objective of our 3-phase study was to assess the steroid sparing potential of ECP in CD patients who were in clinical remission while receiving corticosteroids, but who were corticosteroid-dependent and who had exhibited at least one relapse following steroid tapering.
Methods
This was a single arm, open label, 3 period, multicenter trial that was conducted at 15 study sites in North America and Europe between March of 2003 and November of 2005. The Institutional Review Board or Institutional Ethics Committee at each participating study center approved the protocol. All patients gave written informed consent.
Inclusion and Exclusion Criteria
Male or female patients age ≥ 18 years with corticosteroid-dependent CD and who may also have been refractory or intolerant to immunosuppressants and/or an anti-TNF agent were eligible to participate in the study. CD had to be of at least 6 months duration and confirmed by radiography or endoscopy. A baseline CDAI score of <220 was required to be present and all patients had to have failed at least one attempt at corticosteroid tapering within the previous six months. For the purpose of this study, “corticosteroid-dependent” was defined as: 1) relapse of CD within 60 days following completion of corticosteroid treatment, or 2) a relapse occurring during corticosteroid tapering at prednisone-equivalent doses of ≥ 10 mg/day, or 3) a relapse occurring within 3 months following corticosteroid tapering, while receiving a corticosteroid dose ≥ 10 mg/day of prednisone equivalents. Patients with a CDAI score of < 150 at baseline must have: 1) been on a stable dose of oral corticosteroids (other than oral budesonide) ≥ 10 mg/day to ≤ 40 mg/day (prednisone equivalent) for CD for at least 2 weeks prior to screening; and 2) had clinically inactive CD for at least 2 weeks prior to the study screening visit. Patients with a CDAI score of ≥ 150 to < 220 must have: 1) been on oral corticosteroids (other than oral budesonide) ≥ 10 mg/day to ≤ 40 mg/day (prednisone equivalent) for CD; and also 2) had no worse than mild disease for at least 2 weeks prior to enrolling in the study in the screening period. Patients with a CDAI score between ≥ 150 to < 220 could enter a steroid-tapering run-in phase of the screening period, as described below. Patients receiving the following concomitant medications must have been on stable doses for the following periods of time prior to the screening visit: aminosalicylates for four weeks; azathioprine, 6-mercaptopurine, or methotrexate for at least 8 weeks prior to screening. Patients not using aminosalicylates must have discontinued treatment at least 4 weeks prior to screening. Patients not using immunosuppressants such as azathioprine, 6-mercaptopurine, or methotrexate must have discontinued treatment at least 4 weeks prior to screening. Patients who had been receiving infliximab must have stopped therapy at least 8 weeks prior to screening. Patients who had been receiving adalimumab, cyclosporine, tacrolimus, or mycophenolate mofetil must have stopped therapy at least 4 weeks prior to screening. Patients who had incidental (eg, perianal) fistulae were permitted, provided 1) they had predominantly luminal Crohn’s disease, and 2) fistulae were not associated with retention. The patient’s platelet count was to have been ≥ 20,000/cmm and body weight ≥ 40 kg in order to undergo ECP. Female patients had to be postmenopausal, surgically incapable of bearing children, or practicing an acceptable method of birth control. If a female patient was of childbearing potential, she must have had a negative urine pregnancy test at screening. Patients must have been able and willing to comply with all study procedures and signed informed consent must have been obtained in advance of the study. Eligible subjects may have been either refractory or intolerant to immunosuppressants and/or an anti-TNF agent. A subject was defined as refractory to immunosuppressants or anti-TNF agents when unresponsive to doses of 1 or more of the following that did not allow for a reduction in corticosteroid use: azathioprine 2–3 mg/kg/day for 12 weeks, 6-mercaptopurine 1.5 mg/kg/day for 12 weeks, methotrexate 25 mg/wk for 8 weeks, infliximab ≥ 5 mg/kg intravenously (IV) for 4 weeks (at least 1 infusion), or adalimumab ≥ 40 mg subcutaneously (SC) for 4 weeks (at least 2 injections). A subject was defined as intolerant to immunosuppressants or anti-TNF agents if the subject experienced a related side effect to 1 of the agents listed above that limited or proscribed its use at a dose needed to adequately control Crohn’s disease activity.
Key exclusion criteria included: symptomatic intestinal strictures, local manifestations of CD such as abscesses, or disease manifestations for which surgery might be indicated, or which might preclude utilization of a CDAI to assess response to therapy (such as “short gut” syndrome), stomas, rectovaginal fistulae, patients who required antibiotics for the treatment of Crohn’s disease, use of oral budesonide, chronic diarrhea due to conditions other than inflammatory CD (eg, bacterial or parasitic gastroenteritis, bile salt diarrhea, or bacterial overgrowth), concomitant use of an anti-TNF agent, antibiotics, nonsteroidal anti-inflammatory drugs (NSAIDs), cyclosporine, tacrolimus, mycophenolate mofetil, or investigational therapies, patients unable to tolerate the extracorporeal volume shifts associated with ECP treatment, or patients receiving TPN as the sole source of nutrition within 3 weeks of screening, patients with hypersensitivity or allergy to psoralen (methoxsalen), or patients with hypersensitivity or allergy to both heparin and citrate products, and patients with active bleeding.
Study Design
This trial had three study periods – screening (which could include a run-in phase in order to permit steroid tapering prior to ECP treatment), corticosteroid tapering and maintenance.
Screening Period
During the screening period, patients were evaluated to determine if they were in clinical remission (CDAI < 150) under a stable dose of corticosteroids (≥ 10 mg/day to ≤ 40 mg/kg/day prednisone equivalent). If the patient was not in clinical remission during the screening period but had mild disease (CDAI ≥ 150 to < 220), a run-in period of up to 6 weeks was to be implemented prior to entering the corticosteroid-tapering period. During this run-in phase, the patient was to have corticosteroid dosages adjusted to a maximum of 40mg/day (prednisone-equivalent) in order to induce clinical remission (CDAI < 150). During the last 2 weeks of the run-in phase, a stable dose of corticosteroids was maintained. At the end of the run-in phase, patients whose eligibility was reconfirmed, were to enter the corticosteroid-tapering period, while a patient with a CDAI ≥ 150 or one whose dose of corticosteroids was not stable for 2 weeks were discontinued from the study.
Corticosteroid Tapering Period
Any patient who was in remission and met the criteria for “corticosteroid dependent” entered a 24-week corticosteroid-tapering period during which ECP treatment was to be initiated. ECP was administered on 2 consecutive days every 2 weeks during this period. The objective of the corticosteroid-tapering period was to reduce the daily corticosteroid dose to zero while remaining in remission.
Upon initiation of ECP treatment at baseline, the corticosteroid dose was tapered dependent upon its dose at entry. Subsequently, the clinical activity of the subject was assessed at 2-week intervals and the corticosteroid dose was reduced, kept stable or increased dependent upon absolute CDAI score, which was measured at each treatment week through Week 24 and relative change to baseline CDAI score as displayed in Figure 1. If the CDAI score was ≥ 150 and there was an increase in the CDAI score of ≥ 70 points over the baseline CDAI score, then the subject was considered to have failed corticosteroid tapering. As a result, the corticosteroid dose may have been adjusted to a level that would treat the condition. The subject could be retained in the study at the discretion of the investigator if the ECP treatments were considered beneficial to the subject.
Figure 1.
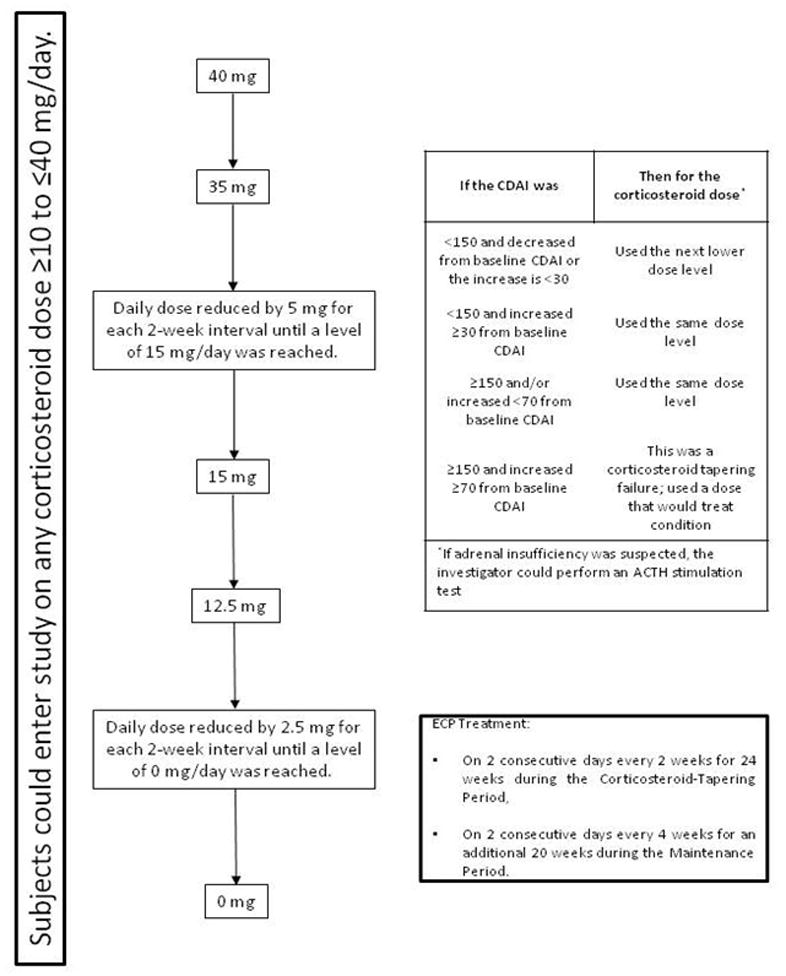
Subjects could enter study on any corticosteroid dose ≥10 to ≤40 mg/day.
If a subject reached the end of the corticosteroid-tapering regimen (0 mg/day) before the completion of 24 weeks, the subject continued to receive ECP treatments every 2 weeks until the subject completed the full 24 weeks of the corticosteroid-tapering period.
Corticosteroid Maintenance Period
Upon completion of the corticosteroid-tapering period, the investigator was to re-evaluate the condition of the patient. Patients who met the primary endpoint were to continue into the 24-week Maintenance Period during which ECP treatment was to be continued with two treatments every four weeks. Patients, who in the opinion of the investigator, showed a beneficial clinical response (partial steroid tapering) were permitted to enter the Maintenance Period as well. During the Maintenance Period patients could, at the discretion of the investigator, continue steroid tapering using the original tapering schedule. However, if the patient’s CD worsened or was shown to have an inadequate response, the patient was not to be allowed to continue into the Maintenance Period and was to be discontinued from the study.
Statistical Analysis
The Intent-to-Treat (ITT) population was defined as all enrolled subjects who have post-baseline data available. The per-protocol (PP) population was defined as all subjects in the ITT population who did not have a protocol violation that would influence the efficacy variable (either because there were no protocol violations for that subject, or any violations occurred after the efficacy variable was measured).
Partial clinical response was defined as a decrease in the baseline CDAI score > 70 points. Clinical response was defined as a decrease in the baseline CDAI score ≥100 points and/or a CDAI < 150 points. Remission was defined as a CDAI < 150 points. The primary endpoint was steroid-free remission at the end of corticosteroid tapering period, i.e. Week 24. All other patients and those who were withdrawn due to an adverse event related to ECP treatment or prior to efficacy evaluations at Week 24 were considered to be non-responders.
Patient baseline and demographic characteristics were summarized using descriptive statistics. The CDAI and IBDQ scores, change from baseline in CDAI and IBDQ scores, and the individual integral components of these scores were summarized using descriptive statistics. The mean daily doses at baseline and at week 24 were compared based on paired t test. The planned sample size for the study was 30 patients. Because this was an exploratory hypothesis-generating trial, no power calculation was performed.
Results
Baseline and Demographic Characteristics and Disposition of the Patients
A total of 32 patients were enrolled into the study (Table 1). In one patient, only baseline data were available, leaving 31 patients for the ITT efficacy analysis population. The disposition of the enrolled patients is shown in Figure 2. Twenty-five of the 32 patients (78.1%) participated in the optional steroid-tapering run-in phase of the study. Ten patients (31.3%) completed the study and 22 patients (68.7%) were withdrawn.
Table 1.
| Parameter | Study Population (n = 32) |
|---|---|
| Age, mean (SD) | 32 (10.7) |
| Males, n (%) | 16 (50) |
| Caucasian, n (%) | 30 (97) |
| Duration of Disease (yr) (mean) | 11.8 |
| Current Tobacco Use, n (%) | 13 (41) |
| Involved Gastrointestinal Area(s), n (%) | |
| - Ileum | 26 (81) |
| - Colon | 26 (81) |
| - Gastroduodenum | 5 (17) |
| - Jejunum | 4 (13) |
| Prior Intestinal Resection, n (%) | 10 (31) |
| Enterocutaneous Fistula, n (%) | 1 (3) |
| Baseline CDAI, mean (median, range) | 85.8 (91, 11 to 167) |
| Baseline IBDQ, mean (median, range) | 171 (124, 95 to 219) |
| Baseline Corticosteroid Dose, (mg/d) mean (median, range) | 21.2 (31.2, 8 to 40.0) |
| Baseline Corticosteroid Status, n (%) | |
| - Corticosteroid Dependent | 7 (22) |
| - Corticosteroid Dependent and Refractory or Intolerant to immunosuppressants and/or anti-TNF Agents | 25 (78) |
| Baseline Anti-TNF and Immunosuppressant Status, n (%) | |
| - Refractory to Immunosuppressants | 9 (28) |
| - Intolerant of Immunosuppressants | 10 (31) |
| - Refractory to Anti-TNF Agents | 9 (28) |
| - Intolerant of Anti-TNF Agents | 2 (6) |
Figure 2.
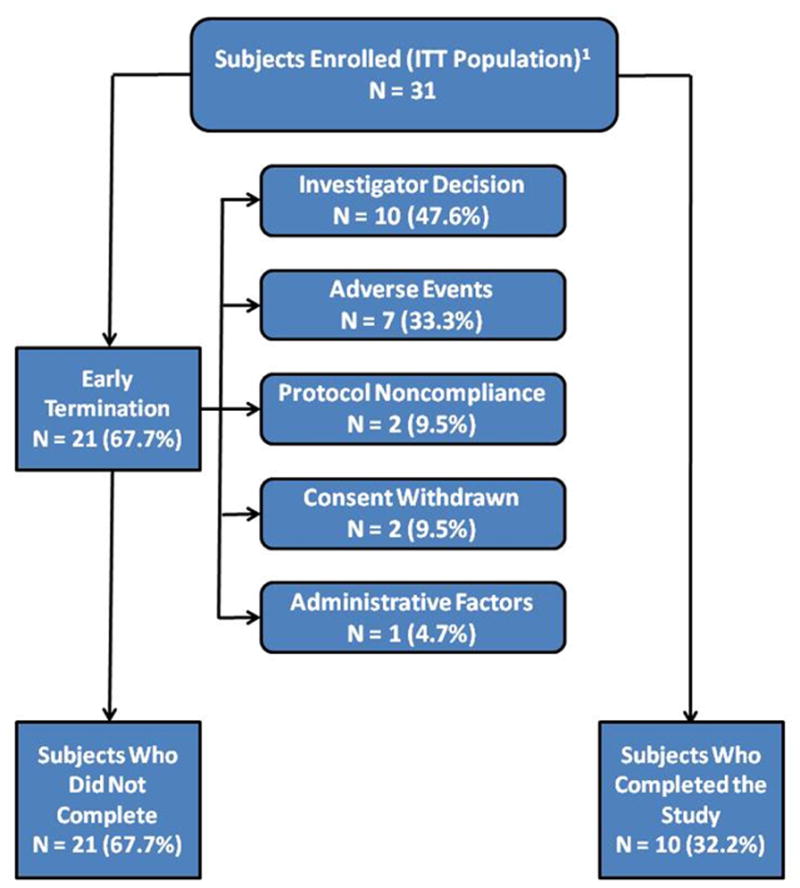
132 subjects were enrolled. However, one subject never received any ECP treatments.
Twelve of 31 patients (38.7%) withdrew from the study during the corticosteroid tapering period and 10 of 31 patients (32.2%) withdrew from the study in the Maintenance Period. The most common reasons for withdrawal during the corticosteroid tapering period included adverse events (5 of 12; 41%), investigator decision (3 of 12; 25%) and protocol non-compliance (2 of 12; 16%). Withdrawal during the Maintenance Period included investigator decision (7 of 10; 70%), adverse events (2 of 10; 20%), and withdrawn consent (1 of 10; 10%).
Safety
Seven patients (21.9%) experienced treatment-emergent adverse events that led to discontinuation from study therapy. Events in this category included 2 instances of worsening CD, and 1 instance each of anemia, catheter-related complication, mental disorder, colonic stenosis, and hypogammaglobulinemia. These latter two events were considered to be serious adverse events. No patient died during the study.
Clinical Efficacy
Primary Study Endpoint
Seven patients in the ITT population (22.6%) achieved the primary endpoint of complete remission at Week 24 without oral corticosteroids, while 6 of 15 patients (40.0%) in the per-protocol population achieved complete tapering of corticosteroid therapy and remained in complete remission (Figure 3). Twenty protocol violations occurred in 15 patients who were excluded from the intent-to-treat analysis. The majority (10 of the 20 protocol violations; 50%) were due to non-compliance with the corticosteroid-tapering regimen outlined in the protocol. Twenty-five of the 32 patients (78.1%) participated in an optional steroid-tapering run-in phase of the study. Of those patients who participated in the run-in phase, seven patients (28%) in the ITT set and six of 13 patients (46%) in the per-protocol set maintained a complete remission following full steroid tapering during the ECP treatment phase.
Figure 3.
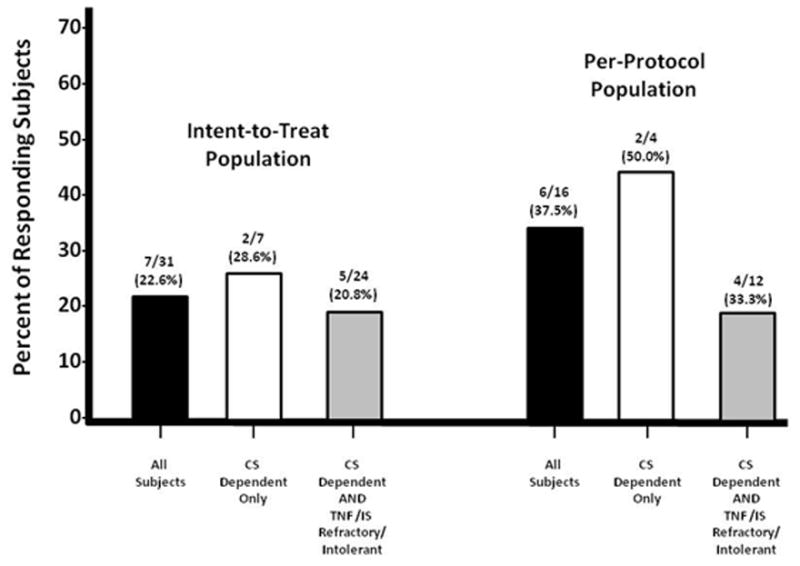
CAPTION: Number and percent of study subjects who achieved completed steroid Tapering without flare at the end of the Corticosteroid-Treatment Period (CS = Corticosteroid; IS = immunosupressant; TNF = tumor necrosis factor)
Secondary Study Endpoints
At the end of the steroid-tapering period 20 patients (64.5%) had a > 50% reduction in corticosteroid dosage compared to baseline while maintaining a remission (CDAI <150). The median daily steroid dose for the remaining patients who could not discontinue corticosteroids was 10.0 mg (range: 1 – 50 mg; p < 0.003 compared to baseline) with a median CDAI of 110 (range: 32 – 413). The median IBDQ for patients who could not discontinue corticosteroids at Week 24 was 179 (range: 118 – 222).
Three ITT patients (9.7%) maintained a steroid-free remission after 48 weeks of ECP therapy (Figure 4). The corresponding rate for the PP population was 3/15 patients (20.0%). Three other ITT patients (9.7%) also achieved a complete discontinuation of corticosteroids without clinical relapse and were with no more than mild (CDAI <220) disease after receiving up to 48 weeks of ECP therapy.
Figure 4.
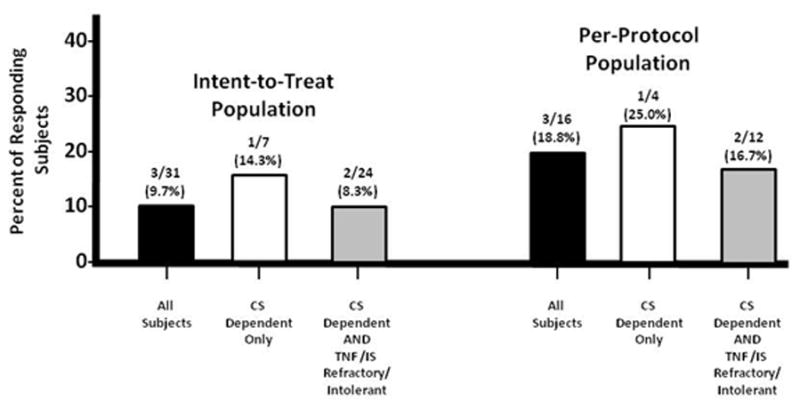
CAPTION: Number and percent of study subjects who achieved completed steroid tapering without flare at the end of the maintenance Period (CS = corticosteroid; IS = immunosupressant; TNF = tumor necrosis factor)
Discussion
In this clinical trial, patients with established Crohn’s disease of long duration and who were corticosteroid-dependent underwent 24 weeks of ECP therapy in order to taper or discontinue steroids. Seven patients (22.6%) were able to completely discontinue corticosteroid therapy – two of seven patients (28.0%) who were corticosteroid dependent and five of 24 patients (21%) who were both corticosteroid dependent and refractory to or intolerant of anti-TNF agents or conventional immunosuppressants. In 65% of patients, the steroid dose could be at least halved. Three patients (9.7%) were able to maintain a clinical remission without relapse after 48 weeks of ECP treatment.
We reported an open-label experience with ECP in steroid-dependent CD patients who had undergone a prospective 24-week forced steroid-tapering phase14. Ten steroid-dependent CD patients who could not have prednisolone tapered below 10 mg/d underwent an additional 24 weeks of treatment with ECP (two treatments on consecutive days every two weeks). Four patients (40%) achieved steroid-free remission. The results of our previous study vary from those in the current trial, since 7 of 31 patients (22.6%) have achieved steroid-free remission. ECP treatment intensity, which was two treatments on consecutive days every two weeks for 24 weeks, was identical for both studies. Also most of the other baseline demographic and disease-specific variables were concordant. The baseline CDAI scores of both cohorts were similar (previous study: 123 ± 31; current study: 85.8 ± 52) as were baseline IBDQ scores (previous study: 185 ± 8; current study 171.3 ± 32.6). The mean age of patients at baseline was 32 years in the previous and 35 years in the current study, with a slight preponderance for males in the former. The median disease duration of 9 years in the first study as compared to the 11.8 years reported herein is unlikely as well to explain the difference in steroid-free remission. As the pilot study was conducted prior to the availability of anti-TNF agents, patients could only be receiving conventional immunosuppressants as a concomitant therapy or had failed those before which in summary was the case in 70% of treated subjects. In the current study, however, 19 of 31 patients (61%) and 11 of 31 patients (35%) failed treatment with conventional immunosuppressants and anti-TNF agents, respectively. Therefore, we suggest that the presence of refractoriness or intolerance to multiple CD therapies may have contributed to the differing results between the two trials. In the intent-to-treat population corticosteroid-dependent patients only achieved numerically higher rates of steroid-free remission compared to patients who had failed multiple therapies. This disparity was even more pronounced in the per-protocol population. However, no firm conclusions from any of those comparisons should be deduced due to the very small numbers of subjects in the subgroups.
The differential use of cigarettes in the two populations may have influenced the outcome in each of the studies, as cigarette smoking exacerbates Crohn’s disease and cessation of cigarette smoking may induce a clinical remission in patients with active CD. In the previous study 3 of 10 patients (30%) smoked cigarettes, whereas 40.6% of patients in the current study smoked tobacco products. Abrupt cessation of cigarette smoking may induce a remission after approximately three months of abstinence in Crohn’s disease, which is approximately half of the duration of the initial extracorporeal photopheresis phase of both studies. Neither of these two studies tracked cigarette smoking cessation in patients who participated in the trials and it is possible that frequent health care provider contact in the studies may have provided a motivating factor for some participating patients to cease cigarette smoking, as has been previously reported.
The previous study was a single center trial whereas 15 study centers contributed 32 patients to the current trial. The selection of steroid-dependant patients who qualified for the early trial was meticulously determined during a 24 weeks lasting prospective forced steroid-tapering protocol. Among the 24 subjects who consented to the steroid-tapering phase only 10 became eligible for treatment with ECP. Six patients were prematurely withdrawn due to non-adherence to the standardized steroid reduction protocol and six other patients could successfully taper prednisolone below a maintenance dose of 10 mg despite previously having failed this benchmark in clinical routine. For the current study the prospective steroid-tapering run-in phase was employed in the majority of study patients, which might have contributed to the heterogeneity of patients included. Furthermore, in about half of the study population protocol violations were noted related to the projected steroid-tapering protocol leaving a per-protocol population of 15 patients. In the latter a steroid-free remission rate of 37.5% was observed, which much more resembles our previous finding on the steroid-sparing potential of ECP.
Our study has some limitations. It might have been useful to measure objective signs of inflammation such as C-reactive protein or fecal calprotectin prior to inducing remission with steroids. However, the fact that only patients were included in this study who were in need of steroids to suppress their symptoms rebuts the possibility that patients suffering from functional complaints only were enrolled. The open label study design employed in this trial raises obvious questions regarding the potential placebo response rate, since it could be possible that the placebo effect might be even more pronounced than with intravenous therapies. There is lack of placebo-controlled studies targeting a similar patient population, i.e. steroid-dependent patients in clinical remission at baseline who were subjected to an ensuing forced steroid-tapering protocol. Feagan conducted a randomized, double-blind, placebo-controlled evaluation of the effect of 18 months of low-dose cyclosporine treatment on the course of Crohn’s disease15. Eligible were also patients in clinical remission. Among the 94 patients with a baseline CDAI <150 who were randomized to placebo 50 subjects were on concomitant steroids with a mean prednisolone dose of 14 mg/d. Those patients could have posed as template to estimate the rate of steroid-free remission of a virtual placebo group in our current study, however the corresponding rate was not reported by the authors. In another double blind multi-center trial16 120 steroid dependent patients with Crohn’s disease affecting the ileum and/or ascending colon only and a CDAI <200 were randomly assigned to receive budesonide 6 mg once daily or placebo. Thirteen weeks after prednisolone was tapered to zero relapse rate defined as a CDAI >200 and an increase of 60 points from baseline was 65% in the placebo group. In that study steroids were to be tapered within 4 to 10 weeks and the exact rate of steroid-free remission at week 24 was not reported. The protocol also allowed the inclusion of patients with low disease activity whereas in our study patients had to be in remission at baseline, which might have impacted the rate of relapsing patients as relapse was defined by a relative increase of CDAI from baseline. Actually, the mean baseline CDAI of our patients was about 20 points lower than in the budesonide study. Furthermore, less than 10% of patients were on concurrent treatment with azathioprine in that study. Thus, extension and refractoriness of Crohn’s disease was more pronounced in our study.
In summary, two studies have now investigated the steroid sparing potential of ECP in steroid dependent CD patients. Analysis of the safety data in this open label trial revealed no new trends and ECP was well-tolerated in this population. The rates of steroid discontinuation in the two trials differ slightly which may be due to study population-based differences. A prospective, randomized, balanced “sham-controlled” clinical trial design would be an important next step in the evaluation of the steroid-sparing effect of ECP in patients with Crohn’s disease.
Footnotes
Conflict-of-Interest Disclosure:
This work was supported by a grant from Therakos, Inc., Raritan, NJ
References
- 1.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;12;369(9573):1641–57. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Yang YX, Lichtenstein GR. Corticosteroids in Crohn’s disease. Am J Gastroenterol. 2002;97:803–23. doi: 10.1111/j.1572-0241.2002.05596.x. [DOI] [PubMed] [Google Scholar]
- 3.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–60. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 4.Munkholm P, Langholz E, Davidsen M, et al. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut. 1994;35:360–2. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinisch W, Gasché C, Wyatt J, et al. Steroid dependency in Crohn’s disease. Lancet. 1995;1:345(8953):859. doi: 10.1016/s0140-6736(95)92995-9. [DOI] [PubMed] [Google Scholar]
- 6.Franchimont DP, Louis E, Croes F, et al. Clinical pattern of corticosteroid dependent Crohn’s disease. Eur J Gastroenterol Hepatol. 1998;10:821–5. doi: 10.1097/00042737-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Charpignon C, Beau P. Methotrexate as single therapy in Crohn’s disease: is its long-term efficacy limited? Gastroenterol Clin Biol. 2008;32:153–7. doi: 10.1016/j.gcb.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 9.Savill J, Dransfield I, Gregory C, et al. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 10.Stuart L, Hughes J. Apoptosis and autoimmunity. Nephrol Dial Transplant. 2002;17:697–700. doi: 10.1093/ndt/17.5.697. [DOI] [PubMed] [Google Scholar]
- 11.Aubin F, Mousson C. Ultraviolet light-induced regulatory (suppressor) T cells: an approach for promoting induction of operational allograft tolerance? Transplantation. 2004;77(1 Suppl):S29–31. doi: 10.1097/01.TP.0000112969.24120.64. [DOI] [PubMed] [Google Scholar]
- 12.Maeda A, Schwarz A, Kernebeck K, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174:5968–76. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 13.McKenna KE, Whittaker S, Rhodes LE, et al. Evidence-based practice of photopheresis a report of a workshop of the British Photodermatology Group and the UK Skin Lymphoma Group. Brit J Dermatol. 2006;154:7–20. doi: 10.1111/j.1365-2133.2005.06857.x. [DOI] [PubMed] [Google Scholar]
- 14.Reinisch W, Nahavandi H, Santella R, et al. Extracorporeal photochemotherapy in patients with steroid-dependent Crohn’s disease: a prospective pilot study. Aliment Pharmacol Ther. 2001;15:1313–22. doi: 10.1046/j.1365-2036.2001.01054.x. [DOI] [PubMed] [Google Scholar]
- 15.Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292–7. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 16.Cortot A, Colombel JF, Rutgeerts P, et al. Switch from systemic steroids to budesonide in steroid dependent patients with inactive Crohn’s disease. Gut. 2001;48:186–90. doi: 10.1136/gut.48.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]


