Abstract
The term synapse applies to cellular specializations that articulate the processing of information within neural circuits by providing a mechanism for the transfer of information between two different neurons. There are two main modalities of synaptic transmission: chemical and electrical. While most efforts have been dedicated to the understanding of the properties and modifiability of chemical transmission, less is still known regarding the plastic properties of electrical synapses, whose structural correlate is the gap junction. A wealth of data indicates that, rather than passive intercellular channels, electrical synapses are more dynamic and modifiable than was generally perceived. This article will discuss the factors determining the strength of electrical transmission and review current evidence demonstrating its dynamic properties. Like their chemical counterparts, electrical synapses can also be plastic and modifiable.
Keywords: Electrical synapse, Connexin 36, Synaptic plasticity, Electrical coupling, Auditory, Synchronization, CaMKII
1. Introduction
In the nervous system, the term synapse applies to cellular specializations that provide a mechanism for communication between two neurons therefore facilitating the processing of information within neural circuits. This concept also applies to connections between a neuron and a muscle cell, gland cell or a cell of epithelial origin. It is accepted that there exist two main modalities of synaptic transmission: chemical and electrical. In chemical synapses, presynaptic electrical currents trigger the release of a transmitter molecule (neurotransmitter) that diffuses across the intercellular space to activate specific receptors located in the postsynaptic cell, which in turn generate a postsynaptic response. In the case of electrical synapses, gap junctions provide a direct pathway of low resistance for the spread of presynaptic electrical currents to the postsynaptic site. Most efforts have been dedicated to the understanding of the properties and modifiability of chemical transmission. In contrast, less is still known regarding the plastic properties of electrical synapses. Because of perhaps the relative simplicity of their underlying mechanism, it was generally perceived that electrical synapses lack plastic properties. Rather than passive intercellular channels, electrical synapses proved to be dynamic and modifiable forms of interneuronal communication. The present report does not attempt to be comprehensive review on the dynamic aspects of neuronal gap junctions, as we will not discuss regulation or their expression during development or as a result of brain injury. Rather, we will focus on some mechanisms that regulate coupling during some physiological processes. In this article we will first discuss the factors determining the strength of electrical transmission then review current evidence indicating that, as their chemical counterpart, electrical synaptic communication is highly dynamic and modifiable by various mechanisms.
1.1. Gap junctions as electrical synapses
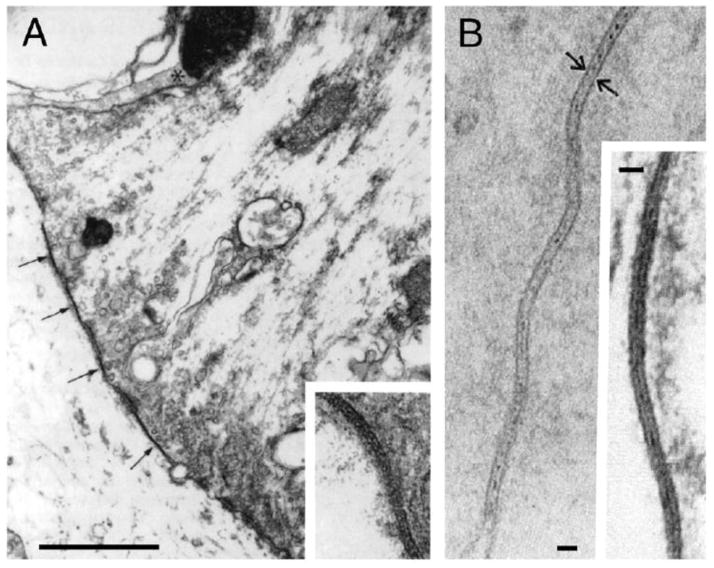
In their classical 1959 paper David Potter and Ed Furshpan provided elegant evidence for the existence of electrical transmission between cells of the crayfish nervous system [1]. The search for the basis of electrical transmission greatly contributed to identifying the cellular structures that we know today as gap junctions. Gap junctions are clusters (“plaques”) of hydrophilic intercellular channels formed by the docking of two ion channels or connexons, each contributed by a neighboring cell, allowing the direct transfer of signaling molecules and providing a pathway of low resistance for the spread of electrical currents between cells. The relationship of gap junctions with electrical transmission was established in seminal observations at identifiable synaptic contacts in teleost fish, where it was possible to correlate electrophysiological recordings with ultrastructural analysis. Together with specializations for chemical transmission, Robertson [2] found at auditory contacts on the goldfish Mauthner cell, known as Large Myelinated Club Endings [3], areas of close membrane apposition that he described as “synaptic discs” (Fig. 1A) [the Mauthner cells are a pair of colossal reticulospinal neurons that mediates tail-flip response in fishes] [4,5]. Parallel investigations involving intracellular recordings by Furshpan [6] showed that, following stimulation of these terminals, the chemically mediated synaptic potential was preceded by a short-delayed brief depolarization, with properties which were not consistent with the criteria established for chemical transmission. Furthermore, Furshpan also showed that transmission at these contacts was bi-directional, as depolarizations originated postsynaptically could be detected with brief synaptic delays at presynaptic afferents [6]. Similar electrophysiological responses where simultaneously obtained by Bennett and colleagues from neurons of the electromotor system of electric fishes [7], where ultrastructural analysis revealed the presence of identical membrane appositions (Fig. 1B). We know now that those areas of close apposition, or “synaptic discs”, correspond to gap junction plaques.
FIGURE 1. Neuronal gap junctions constitute the basis for electrical synaptic transmission.
A, Early electron micrograph of a Club ending in goldfish revealing zones of close membrane apposition (arrows, inset), or “synaptic discs”, later shown to be gap junction plaques. Calibration is 1 μm. Modified from Robertson et al. (1963) [2]. B, High magnification of junctions between dendrites of spinal electromotor neurons in mormyrid fish. The plane of section is nearly perpendicular to the membranes and the overall thickness is about 140 A. Calibrations are 200 A. Adapted from Bennett et al. (1967) [151].
Connexons are hexameric ion channels formed by proteins that represent the products of two multigene families. While connexins (Cx) are unique to chordates, innexins and pannexins encode gap junction proteins in invertebrates, prechordates and chordates [8,9,10]. In mammals, only a small number of connexins were shown to be expressed in neurons: Cx36 [11], Cx45 [12], Cx50 [13,14]. Cx57 [14] and possibly Cx30.2 [15] and Cx31.1 [16]. The number of neuronal connexins is higher in teleost fishes, which as a result of genome duplication have several homolog genes [17]. While some neuronal connexins are widely expressed others have a more restricted distribution [18]. Because of its widespread distribution and preferred neuronal expression [19], Cx36 could be considered the main “synaptic” connexin (see below). In contrast, Cx57 has been found restricted to horizontal cells in the retina [14]. Although pannexins 1 and 2 were found to be expressed in the vertebrate brain [20] and are likely to play important functional roles as hemichannels [21,22], there is no evidence at the present time indicating that these proteins form intercellular junctions between neurons. Innexins mediate electrical transmission in invertebrates and several members of this family have been identified in both drosophila [23] and C. elegans [24]. Thus, the first electrical synapse reported by Furshpan and Potter [1] is mediated by innexin-containing junctions. Interestingly, and in contrast with connexins, some innexin genes are capable of giving rise to several partially identical transcripts that in turn translate into three distinct proteins [25], presumably increasing the functional diversity of electrical transmission.
1.2. Distribution and functional roles
Electrical synapses are ubiquitous in both invertebrates and cold-blooded vertebrates. Because of their reliability and lack of synaptic delay (relative to chemical synapses), electrical synapses are essential features in escape networks [5,26,27]. As a consequence of their bi-directionality, which allows sharing variations in the membrane potential between cells, they promote the coordinated activity of networks of extensively coupled neurons [28] and are also known to underlie mechanisms of lateral excitation in sensory systems [29,30,31]. Although most electrical synapses are bi-directional, rectification of electrical transmission has been observed in both in invertebrates [1] and vertebrates [32]. It is believed that rectification might result from differential properties of the pre- and postsynaptic elements at structurally asymmetric junctions [33].
As we mentioned earlier, initial evidence for the presence of electrical transmission was clearly established in invertebrates and cold-blooded vertebrates. In addition, following the classical description of electrical coupling in the chick ciliary ganglion [34,35], substantial evidence for the presence of electrical coupling and gap junctions was reported in the avian nervous system [36–38]. On the other hand, it was generally believed that electrical of transmission was uncommon in mammals. The cloning of Cx36 led to great expansion of the known distribution of electrical transmission in the mammalian CNS [11]. Cx36 expression was identified by in situ hybridization in many mammalian CNS structures such as retina, hippocampus, cerebellum, neo-cortex, inferior olive and spinal cord, among others [39,40]. Cx36 specific antibodies and mice with markers replacing the Cx36 coding sequence are demonstrating the distributions more definitively. To date immunolabeling combined with freeze fracture supports a purely neuronal distribution of Cx36 in the CNS [19,41] [but see 42]. Electrical coupling between cortical and thalamic interneurons and in inferior olive [43–46] is nearly absent in Cx36 knockout mice [45–48]. Electrical synapses have been shown to play important functional roles in retina [49], olfactory bulb [50], suprachiasmatic nucleus [51] and inferior olive [52], hippocampus [53] and cerebellum [54], amongst others, and Cx36-containing neuronal gap junctions have been reported in most of those regions [55–58]. Also, cortical gamma oscillations (30–80 Hz), which are thought to be essential for cognitive processing, are impaired in Cx36 knockout mice [47,48]. While in most cases electrical synapses act to promote coordinated neural activity [59], their presence was also shown to promote desynchronization in a cerebellar network [54]. Thus, rather specific or prevalent for a particular tissue or function, mammalian electrical synapses have proven to be almost as ubiquitous and functionally diverse as chemical synapses.
2. Factors that determine the strength of electrical transmission
2.1. Interplay between gap junctional conductance and membrane properties
Neurons operate by computing variations of the membrane potential evoked by synaptic currents and active processes. The change in the membrane potential observed by the spread of presynaptic currents through gap junctions to a postsynaptic neuron is usually referred to as a “coupling potential”. The amplitude of this “coupling potential” does not solely depend on the conductance of the gap junction channels but also on the passive properties determined by the resistance and capacitance of the coupled neurons (Fig 2A) [60]. In other words, the impact of gap junctional currents on a neuron’s excitability, or its “synaptic strength”, is dramatically influenced by non-junctional factors. Although changes in the membrane resistance and capacitance of the postsynaptic neuron would also affect chemical synaptic transmission, their influence seems to be critical in the case of electrical synapses, which unlike chemical synapses, lack postsynaptic mechanisms of amplification of presynaptic signals. Finally, the terms “electrical coupling” and “electrical synapses” are not interchangeable because neuronal gap junctions could also provide a conduit for the passage of small regulatory molecules to activate intracellular signaling cascades at the postsynaptic cell, as proposed to occur during development [61].
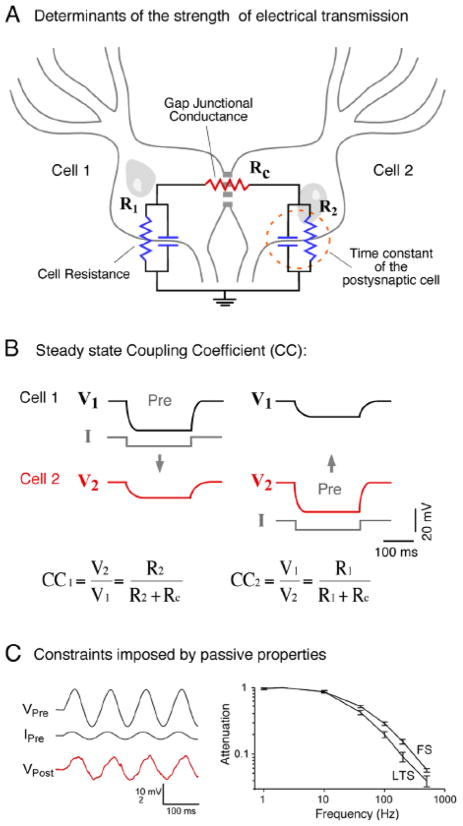
FIGURE 2. Factors that determine the strength of electrical transmission.
A, The cartoon represents a pair of coupled neurons on which an equivalent circuit of the key elements that determines strength of coupling was superimposed. The junctional conductance (Gap Junctional Conductance) is represented by a lumped resistance (Rc), whereas the passive properties of coupled cells are represented by a resistor (R1, R2) in parallel with a capacitor, which together determine the input resistance and the time constant of the neuron. B, The steady state coupling coefficient. The strength of electrical synapses can be assessed by the injection of long polarizing current pulses in one of a pair of coupled neurons. Current injection evokes a voltage drop in the presynaptic cell (Pre), which is typically accompanied by a change of the membrane potential in the coupled cell of lower amplitude, slower temporal course and similar polarity. The duration of the current pulses is usually long enough to overcome the initial attenuation of the membrane potential produced by the filtering properties imposed by the passive properties of the postsynaptic membrane, so voltage changes can be measured at “steady state”. The strength of coupling is quantified by calculating the Coupling Coefficient (CC), defined as the ratio between the voltage deflections in the post- and presynaptic cells. As it can be observed, at steady state conditions this coefficient critically depends on the resistance of the postsynaptic cell. C, Constraints imposed by the time constant of the postsynaptic cell. Due to low-pass filtering properties of the coupled neurons, time varying signals are attenuated according to their frequency content. Left, Injection of sine wave current in the presynaptic cell (IPre) evokes a sinusoidal variation of the membrane potential of the presynaptic cell (VPre) that can be recorded as a coupling potential in the postsynaptic cell (VPost). Right, Frequency transfer characteristics determined for pairs of electrically coupled fast-spiking (FS) and low-threshold spiking (LTS) inhibitory interneurons of neocortical layer 4 in the rat. Magnitude of transfer represents the ratio of membrane potential amplitude in the postsynaptic cell over that of the presynaptic cell, during sine wave injections of various frequencies at the presynaptic side, indicates that electrical contacts between pairs of FS and LTS interneurons behaves as low-pass filters. These results indicate that the strength of electrical coupling is stronger for presynaptic signals of lower frequency content. Modified from Gibson et al. (2005) [152].
With the purpose of their characterization, electrical synapses are generally explored by injecting a depolarizing or hyperpolarizing current into one of the coupled cells while recording variations in the membrane potential of both the presynaptic and postsynaptic cells (Fig. 2B). [Given that most electrical synapses are bi-directional the use of pre- and postsynaptic terminology is arbitrary and only applies to the cell in which signals were generated. Furthermore, and more importantly, when a “presynaptic” action potential propagates to the “postsynaptic” cell, the membrane resting potential of the “postsynaptic” cell simultaneously propagates to the “presynaptic” cell.] The duration of the current pulses is usually long enough to overcome the initial attenuation of the membrane potential produced by the filtering properties imposed by the passive properties of the postsynaptic membrane, so voltage changes can be measured at steady state (Fig. 2B). The steady-state “coupling coefficient” is a measure of the strength of the electrical synapse and is defined as the ratio between the voltage deflections in the post- and presynaptic cells, both measured at steady-state, that is, once membrane capacitance is charged and therefore current flows only through the resistor (Fig. 2A). As can be inferred from Fig. 2B, the steady-state coupling coefficient is influenced not only by the resistance of the gap junction (Rc) but also by the resistance of the postsynaptic cell (R). Thus, two gap junctions with identical conductance will have dramatically different coupling coefficients if the resistances of the postsynaptic cells are substantially different. The cell resistance is determined by a combination of factors including membrane resistivity and cellular geometry. This resistance can be different from the experimentally estimated input resistance, which is also affected by the gap junctional resistance and the resistance of the coupled cell. Indirect measurements of junctional conductance can be derived from estimates of steady-state coupling coefficients and the cell’s resistance [60]. These estimates of junctional conductance assume that the values of the input resistance of the coupled cells measured experimentally at the cell’s soma represent the resistance influencing electrical coupling. This is not a trivial consideration in most mammalian and invertebrate neurons where gap junctions may be located at remote dendro-dendritic compartments.
Because most naturally occurring neuronal signals are brief, the strength of electrical transmission is generally affected by the time constant of the postsynaptic cell (Fig. 2B) of usually of relatively longer duration, which determines the speed at which voltage changes in response to a particular current. The properties of an electrical synapse in the frequency domain can be explored by applying sinusoidal currents of various frequencies in the presynaptic cell while comparing changes in the membrane potential at both the pre- and postsynaptic cells. The coupling coefficient for low frequency sinusoids equals or approximate to that measured at steady state, whereas that obtained using sinusoids of higher frequencies diminishes progressively (Fig. 2C). Thus, in most mammalian neurons, which have membrane time constants (estimated with the whole cell technique) in the order of tens of milliseconds, electrical synapses behave as low-pass filters [28]. It is important to emphasize that this property does not rely on the properties of the gap junction itself but on the passive properties of the coupled cell [60]. Interestingly, likely as a result of the increased background synaptic activity, estimates of time constant obtained in vivo were shown to be much faster than those obtained in vitro [62], suggesting that transmission of fast signals might be stronger under in vivo conditions. Finally, low-pass filtering properties of electrical synapses are virtually inexistent in cells with unusually brief time constants such as the Mauthner cell (see below).
2.2. Are electrical synapses excitatory or inhibitory?
With the exception of the unusual strongly rectifying synapses [1], the sign of the electrical synaptic potentials critically depends on the characteristics of the presynaptic signal that occurs under physiological conditions. The coupling produced by naturally-occurring changes in the membrane potential of the presynaptic cell, such as action potentials [28,45,59] and synaptic potentials [63], represents the physiologically relevant signals and therefore deserves the name of “electrical postsynaptic potential” (see below). Because of the slow time constant of most neurons, presynaptic signals with higher frequency content (such as action potentials) are greatly attenuated and thus the coupling coefficient estimated from an electrical synaptic potential, generally referred to it as “spikelet”, is usually of smaller value than that estimated from steady-state coupling coefficients. In contrast, although of substantially smaller amplitude at the presynaptic cell, longer-lasting signals with lower frequency content such as the afterhyperpolarization (AHP) which follows some action potentials or synaptic potentials, are less attenuated and therefore, paradoxically, more likely to influence the postsynaptic cell [28,59,64]. This property is critical when presynaptic neurons fire trains or “bursts” of action potentials. Spikelets evoked by a burst of presynaptic action potentials associated with less pronounced AHPs can temporally summate to evoke a sizable depolarization in the postsynaptic cell [45,65]. In contrast, presynaptic action potentials with pronounced and long-lasting AHPs will produce a hyperpolarization of the postsynaptic cell [28,64]. Because the action potential is significantly more filtered than the much longer lasting AHP, this last hyperpolarizing presynaptic signal predominates and the result is a brief depolarization followed by a much larger and longer hyperpolarization, which can be long during sustained presynaptic repetitive discharges [64]. This hyperpolarization has been shown to play an important functional role promoting desynchronization in cerebellar networks [54]. Thus, depending on the characteristics of the AHP currents in the presynaptic cell, electrical synapses can either excite or inhibit the postsynaptic cell. The definition of excitatory or inhibitory action based on the predominance of depolarization or hyperpolarization is in any case arbitrary, as inhibitory hyperpolarizing conductances were also shown to trigger rebound spikes in some cell types [66]. Finally, beyond the fact that electrical coupling can in adequate contexts produce excitatory or inhibitory effects (effects that are relative to the spike threshold of the neuron), electrical synapses were postulated to be “synchronizing” [59], a denomination that emphasizes the virtues of their bi-directional properties.
3. Regulation of electrical coupling by the non-junctional membrane
We discussed in previous sections how the strength of an electrical synapse could be influenced by gap junctional and non-junctional factors. We will review now some examples indicating that, indeed, the strength of electrical transmission can be modified by regulation both gap junctions and the non-junctional membrane.
3.1. Regulation of electrical coupling by the non-junctional membrane
3.1.1. Regulation of electrical coupling by neighboring inhibitory chemical synapses
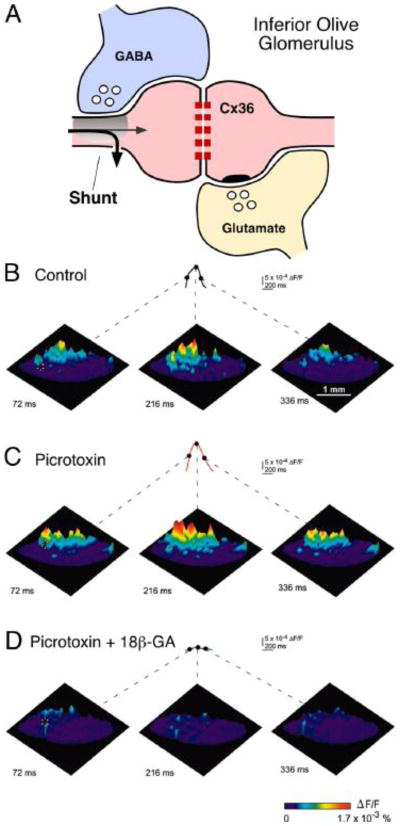
Because the electrical coupling critically depends on the resistance of the postsynaptic cell, changes in this resistance will result in increases or decreases of the amplitude of the coupling potential. Chemical inhibitory transmission often works by producing a “shunting” effect, that is, the inhibitory synaptic conductance short-circuits the currents that are generated at adjacent excitatory synapses. While shunting would reduce both chemically and electrically mediated synaptic potentials, there exist a few examples in which the anatomical arrangement clearly suggests that its action is intended to regulate the strength of electrical coupling between two neurons. The most notorious example is the inferior olive, a structure where electrical transmission constitutes the only form of synaptic communication between its principal cells [67], and where clusters of synchronized neurons are dynamically sculpted as a result of transient regulation of electrical coupling [68,69]. This regulation takes place at the glomerulus, an anatomical structure where coupled dendritic processes of inferior olivary neurons and afferent chemical synaptic contacts co-exist [67]. Here, release of GABA from terminals of axons originating in the deep cerebellar nuclei has been proposed to indirectly regulate coupling by shunting depolarizing currents in dendritic processes of inferior olivary neurons [67,68] (Fig. 3A). The time window of this “uncoupling” is determined by the duration of the shunting synaptic conductances, which while unusually long due to de-synchronized transmitter release [70], lasts only tens of milliseconds. By promoting synchronous complex spikes that influence sets of Purkinje cells in the cerebellar cortex, these quickly and transiently-formed compartments (whose size is sensitive to picrotoxin and gap junction blockers) (Fig. 3B–D) are thought to encode important functional parameters that influence the cerebellar cortex [69], suggesting that the formation of groups of functionally interconnected neurons underlies essential aspects of the inferior olive function. Similarly, inhibitory synapses were shown to regulate the strength of electrical coupling between the expansion motoneurons of the mollusk Navanax [71].
FIGURE 3. Regulation of the electrical coupling y changes of the non-junctional membrane.
A, The Inferior Olive glomerulus: Inhibitory (GABA, blue) and excitatory (Glutamate, yellow) synapses terminate in the vicinity of gap-junctions between dendrodendritic processes (spine) of inferior olivary neurons (pink). Inhibitory synaptic conductances are thought to briefly shunt excitatory currents, temporarily reducing effective electrotonic coupling between IO neurons. B – D, Optical recordings with high speed voltage-sensitive dye imaging, in vitro, describe patterns of ensemble oscillatory activity in the Inferior Olive. B, Optical recordings were cut into fragments of two or three oscillatory cycles and averaged based on the temporal profile of intracellular recordings (see Ref. 69 for details). C, Application of the GABAA receptor blocker picrotoxin enhances oscillatory clusters. D, These clusters are absent in slices treated with the gap-junction blocker 18β-glycyrrhetinic acid (18β-GA). The three representative images show the location and spatial spread of 1 oscillatory cycle. The amplitude and time-course of the optical responses for 1 pixel are also shown (asterisk in each left image). The time points for each image are labeled on the pixel trace; the start of the oscillatory cycle was defined as 0 ms. Modified from Leznik and Llinas (2005) [69].
3.1.2. Interaction with intrinsic membrane properties
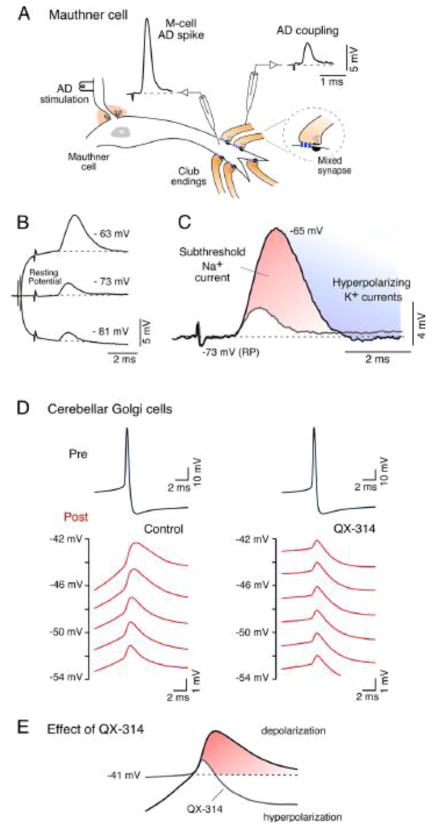
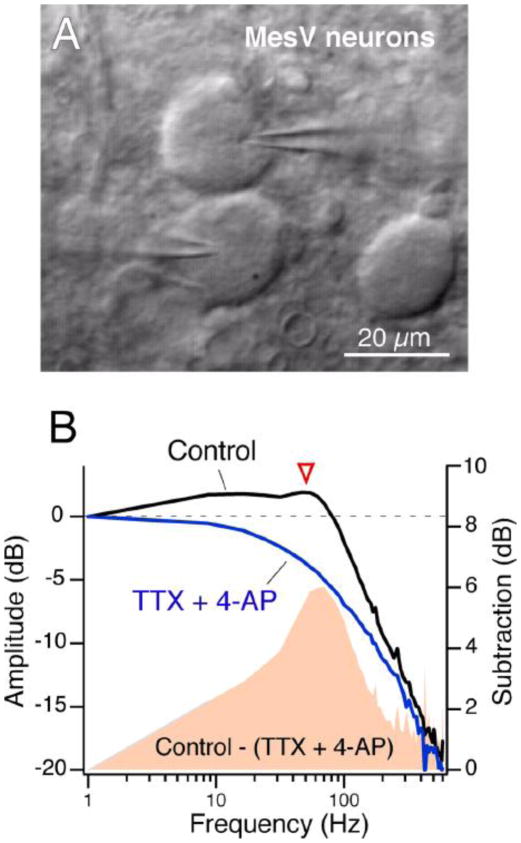
Most neurons are endowed with specific complements of intrinsic membrane properties, which determines their individual electrical phenotypes. Most of these properties represent voltage-dependent conductances that impart non-linear behaviors to membrane responses. Thus, electrical coupling could be enhanced or reduced by the closing or opening of potassium (K+) conductances in a voltage-dependent manner, which affect the input resistance of the coupled cells. In other cases, voltage-dependent conductances act to amplify electrical coupling, by adding an additional depolarization to that produced by the coupling itself. This is the case of the subthreshold sodium (Na+) currents, which were shown to amplify electrical coupling in several cell types [31,72–75]. One example is the voltage-dependence of coupling observed at the previously mentioned goldfish mixed synapses known as Club endings [31], which provided one of the first examples of electrical transmission in vertebrates (Fig. 1A). At these afferents, the amplitude of the coupling potential produced by the retrograde spread of signals from the postsynaptic Mauthner cell (Fig. 4A) is dramatically enhanced by depolarization of the presynaptic terminal (Fig. 4B). The membrane time constants of these afferents and the coupled Mauthner cells are very fast (estimated of ~400 μs in the Mauthner cell) and therefore the coupling of action potentials does not result attenuated as in most mammalian neurons [31]. This voltage-dependent enhancement of electrical coupling does not represent a property of the junctions themselves but the activation of a subthreshold Na+ current present at presynaptic terminals that acts to amplify the synaptic response and can be blocked by both extracellular TTX and intracellular application of QX-314 [31]. Interestingly, the interplay of this current with low threshold repolarizing K+ conductances reproduces closely the waveform of the coupling recorded at resting potential (Fig. 4C) [31]. Retrograde electrical communication at these afferents is thought to play an important functional role as a mechanism of lateral excitation, promoting cooperativity between afferents. Like in the Mauthner cell, amplification of electrical coupling by intrinsic membrane properties can improve communication between neuronal processes of dissimilar size and shape, which are unfavorable for bi-directional communication. Similar amplification of electrical coupling by subthreshold Na+ current has been reported in the mammalian cerebellum [72,73] and in the mesencephalic nucleus of the Trigeminus (MesV) [74], and thalamic relay nucleus [75] where electrical coupling of presynaptic spikes was shown to be amplified in a voltage-dependent manner by postsynaptic cells (Fig. 4D, left). Such amplification was no longer observed when QX-314 was added to the recording electrode solution (Fig. 4D, right). Interestingly, due to the pronounced afterhyperpolarization of the presynaptic action potential in cerebellar Golgi cells, electrical coupling was predominantly hyperpolarizing at hyperpolarized membrane potentials, whereas it became depolarizing as a result of the action of the subthreshold Na+ current, at more depolarized potentials [72]. Electrical coupling in these cells could be excitatory or inhibitory, depending on the resting potential of the cell, and this sign reversal was blocked by QX-314 (Fig. 4E). Furthermore, electrical coupling between MesV neurons is enhanced in a frequency- dependent fashion by cellular properties that endow these cells with electrical resonance and which include a persistent Na+ current and a subthreshold K+ current [74]. Remarkably, the amplitude of the transfer was higher at frequencies near 50 Hz than that obtained at steady-state conditions (Fig. 5B), indicating that transmission of electrical signals between MesV neurons exhibit some degree of frequency preference, and therefore do not behave as simple “low-pass” filter. Thus, the efficacy of an electrical synapse can be dynamically shaped in a voltage-dependent manner by properties of the non-junctional membrane.
FIGURE 4. Enhancement of electrical coupling by intrinsic membrane properties.
A, Auditory afferents (Club Endings) terminate as mixed synapses (Mixed synapse) on the lateral dendrite of the goldfish Mauthner cell. The action potential produced by the antidromic stimulation of the Mauthner cell axon in the spinal cord (M-cell AD spike) can be recorded as a coupling potential in the presynaptic afferents (AD coupling). B, The amplitude of the AD coupling is voltage-dependent; it increases with presynaptic membrane depolarization and decreases with membrane hyperpolarization (current pulses of ± 0.9 nA). C, Superimposed traces illustrate the AD potential obtained at resting potential (RP, −72 mV) and at a depolarized potential (−65 mV). Enhancement of AD coupling results from the interplay between a subthreshold Na+ current (red), that adds to the depolarization produced by the coupling, and a delayed repolarizing K+ conductance (blue), which by terminating the action of the subthreshold Na+ current, reproduces the waverform of the AD coupling obtained at resting potential. Modified from Curti and Pereda (2004) [31]. D, Voltage-dependent amplification of coupling between pairs of electrically coupled Golgi cells in the rat cerebellar cortex. Depolarizing coupling potentials recorded in the postsynaptic cell (Post) in response to action potentials generated in the presynaptic cell (Pre). Recordings were obtained in control conditions (left), and when the Na+ channel blocker QX-314 was included in the recording electrode of the postsynaptic cell (right). In control conditions, depolarizing coupling potentials were progressively bigger with postsynaptic cell depolarization. Such amplification was absent when QX-314 was added to the postsynaptic recording electrode, suggesting the participation of a subthreshold Na+ current whose activation at subthreshold membrane potentials enhances coupling potential amplitude. Because of the pronounced afterhyperpolarization of the presynaptic action potential, electrical coupling was predominantly hyperpolarizing at hyperpolarized membrane potentials, whereas it became depolarizing as a result of the action of the subthreshold Na+ current, at more depolarized potentials. This sign reversal was blocked by QX-314, indicating the critical functional role of the subthreshold Na+ current (Fig. 4E). Modified from Dugue et al. (2009) [72].
FIGURE 5.
A, Intrinsic membrane properties enhance the transfer of relatively high-frequency signals IR-DIC image of a pair of contiguous MesV neurons during a simultaneous whole-cell recording. B, Frequency transfer properties in a pair of electrically coupled MesV neurons using a sinusoidal current of increasing frequency (zap). Magnitude of the transfer under control conditions and after the extracellular application of TTX and 4-AP. The amplitude of the frequency-transfer characteristics was calculated as the ratio of the Fast Fourier Transform (FFT) of the postsynaptic cell over the FFT of the presynaptic cell. The solid pink area represents the difference in transfer in the two conditions, illustrating the contribution of active mechanisms to frequency-transfer characteristics, particularly at ~50 Hz (arrowhead). Modified from Curti el al., 2012 [74].
3.2. Gap junctional conductance is dynamically regulated
We have considered so far how non-junctional factors affect the degree of electrical coupling between neurons, as a mechanism to regulate the strength of electrical transmission. In addition to these factors, electrical synapses have been proven to be extremely dynamic as a result of modification of the gap junction channels themselves.
3.2.1. Modulation by neurotransmitter modulators
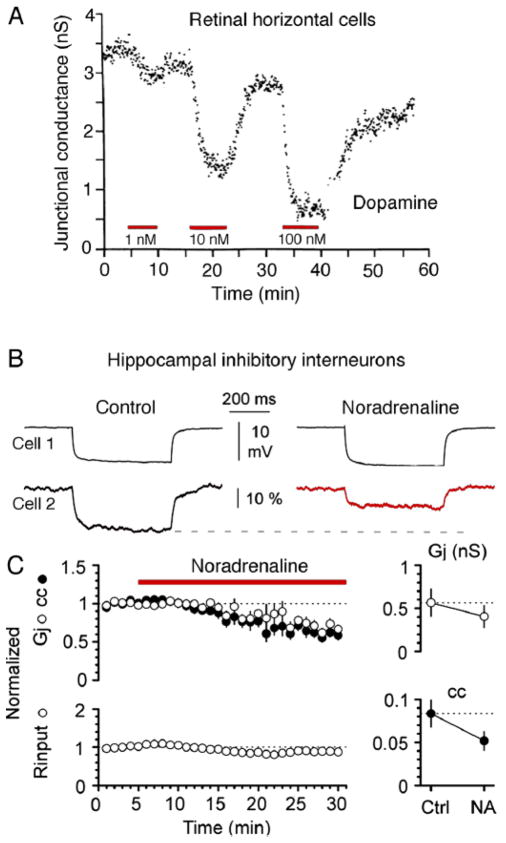
The retina provided on of the first examples for the existence of regulation of gap junctional conductance. That is, dopamine was shown to promote reduction of tracer and electrical coupling (Fig. 6A) between horizontal cells [76–81]. Dopamine was shown to mediate this action via activation of D1 receptors, which leading to an elevation of cAMP activate cAMP-dependent protein kinase A (PKA) [76–82]. The effects of dopamine in retina are not to be restricted to this cell type, and this neurotransmitter modulator was also shown to regulate electrical coupling in AII amacrine cells [83–85]. In contrast to horizontal cells, at which electrical coupling is mediated by Cx57 (Cx55.5 in teleost), coupling between Amacrine type II cells is primarily mediated by Cx36. Two PKA phosphorylation sites were identified in Cx36 and its fish homolog: Ser110 and Ser293 (Ser276 in teleost) [86,87], which are essential for PKA-mediated regulation of coupling. These two sites were shown to undergo dynamic changes in the phosphorylation state in retina [49,88] and to underlie important functional roles in both AII amacrine cells [88] and photoreceptors [89]. The retina also provided some of the clearest examples of the functional role of the regulation of electrical synapses. Because dopamine release is modulated by light, coupling between AII amacrine cells, as well between horizontal cells, changes with different degrees of luminance. Remarkably, AII amacrine cells show small receptive fields under darkness or daylight but larger receptive fields and enhanced coupling at intermediate levels, such as dim background light or twilight. In other words, light acts to reconfigure networks of electrically coupled neurons through the action of dopamine. [49] [For a complete review on regulation of electrical coupling in retina see ref. 49] The regulatory role of dopamine at electrical synapses was shown to be widespread and observed at both vertebrate [76–85, 90–92] and invertebrate [93,94] nervous systems. Further, the regulatory effects of PKA on electrical conductance were also observed to occur following the activation of noradrenergic receptors (Fig. 6B), which dynamically regulate the degree of coupling between inhibitory interneurons in hippocampus [95]. Other neurotransmitters such histamine [49,96] acting via different second messenger pathways such cGMP [83,96,97] and nitric oxide [97,98] were reported to regulate the degree of coupling by targeting junctional conductance. In summary, neurotransmitter modulators can regulate the degree of coupling between neurons by targeting gap junctional conductance. These regulatory molecules lead in most cases to state-dependent reversible effects on the strength of electrical synapses.
FIGURE 6. Neuromodulators regulate electrical transmission.
A, Dopamine reduces junctional conductance between pairs of catfish horizontal cells in a dose-dependent fashion. Dopamine (1, 10 and 100 nM) was added to the extracellular solution during the intervals indicated by the red bars. Junctional conductance (nS) is plotted as a function of time. Modified from DeVries and Schwartz (1988) [80]. B, Electrical coupling between stratum lacunosum-moleculare interneurons is modulated by α-adrenergic receptors. Application of noradrenaline (20 μM) reduces the junctional conductance and the coupling coefficient. C, Time course of the effects of noradrenaline on the normalized junctional conductance and the coupling coefficient and input resistance. Modified from Zsiros and Maccaferri (2008) [95].
3.2.2. Activity-dependent regulation of gap junctional conductance
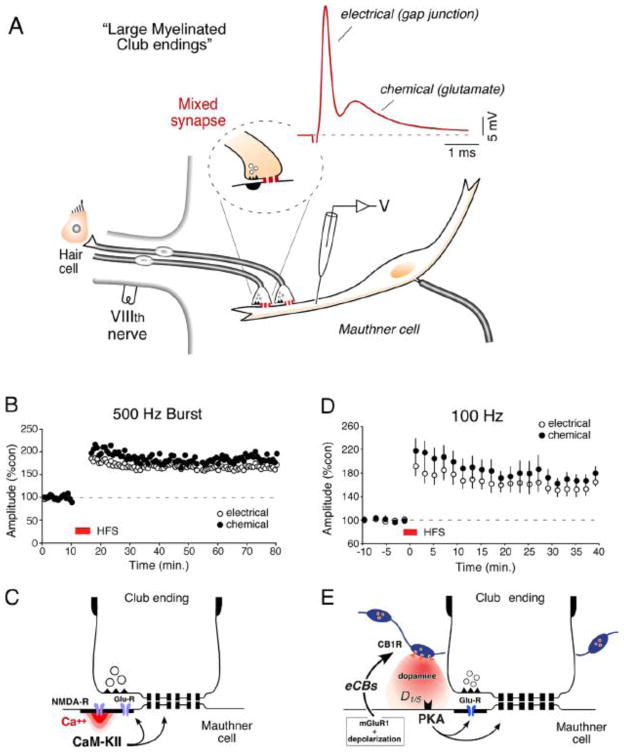
The strength of electrical synapses was also shown to be modified by the activity of glutamatergic synapses [99–104]. Experiments at Club endings (mixed synapses on the goldfish Mauthner cell; Figs. 4A and 7A) demonstrated that gap junctional conductance at these endings (as well as efficacy of chemical transmission) can be modified by afferent activity in the form of both short- [100] and long-term potentiation [99,100] and long-term depression [101,104]. Club endings are advantageous for correlations of their structural and biochemical features with their in vivo physiological properties and provide a valuable model for the study of vertebrate electrical transmission [105]. These terminals are unusually large (5–10 μm in diameter), and each has ~100 GJ plaques and up to ~100,000 channels [106], containing Cx35 [107], a fish homolog of the mammalian neuronal connexin Cx36 [108]. Recent data indicate that a second homolog of Cx36, Cx34.7, is also present at these terminals [109]. The induction of activity-dependent long-term potentiation (Fig. 7B) requires high frequency stimulation of these synapses (brief trains of 2 to 8 stimuli at 500 Hz; “500 Hz burst” protocol) and was blocked by postsynaptic injections of BAPTA and by superfusion of the medulla with NMDA receptor antagonists [99,100]. Activation of NMDA receptors lead to a localized increase in the intracellular concentration of Ca++, which in turn activates the kinase Ca++-calmodulin-dependent kinase II (CaMKII) [110], whose activity is necessary for the potentiation (Fig. 7C). Simultaneous pre and postsynaptic recordings at these single terminals demonstrated that such functional interaction takes place in the same ending, within a few micrometers [101]. Accordingly, confocal and freeze fracture immunolabeling (FRIL) of these terminals showed that the NR1 subunit of the NMDA glutamate receptor, proposed to be a key regulatory element, is present at PSDs closely associated with gap junction plaques containing Cx35 [105,107]. Thus, activity-dependent modulation of electrical coupling results form an interaction with nearby glutamatergic synapses, which in addition to modifying their own strength, also target nearby gap junctions. It was recently reported that, in similarity with the Club endings, presynaptic activity of glutamatergic ON bipolar cells increased phosphorylation of Cx36 in AII amacrine cells and this phosphorylation was dependent on activation of NMDA receptors and mediated by CaMKII [111]. In contrast with the Mauthner cell, NMDA receptors were in this case located extra-synaptically and co-localized with Cx36 on AII amacrine cells [111].
FIGURE 7. Activity-dependent plasticity of electrical transmission a primary auditory afferents on the goldfish Mauthner cell.
A, Club endings exhibit mixed synaptic transmission. Typical experimental arrangement showing VIIIth nerve auditory primary afferents (which contact saccular hair cells; “hair cell”) terminating as Club endings on the ipsilateral Mauthner cell lateral dendrite. VIIIth nerve and postsynaptic electrodes are indicated. Inset: cartoon represents a Club ending, at which both mechanisms of synaptic transmission, electrical (gap junction) and chemical, coexist. VIIIth nerve stimulation evokes mixed (electrical and chemical) EPSPs (red trace). Here and elsewhere, unless otherwise indicated, each trace represents the average of at least 20 individual responses. B, Discontinuous tetanic stimulation (trains of six pulses at 500 Hz, every 2 s for 4 min; “500 Hz protocol”) of the VIIIth nerve can evoke persistent potentiation of both components of the EPSP. Plots here and in subsequent panels illustrate the amplitudes of the electrical and chemical components vs. time (each point represents the average of 20 traces) for a single experiment. C, Schematic representation of the proposed potentiating pathway. Ca++ entering through NMDA receptors activates CaMKII that phosphorylates either glutamate receptors and connexins or regulatory molecules. Modified from Pereda et al. (1998) [110]. D, Repetitive stimulation of the posterior VIIIth nerve (100 Hz during 1 sec.; “100 Hz protocol”) evoked robust potentiation of both components of the mixed EPSP (n = 5). E, Model for endocannabinoid-mediated potentiation of electrical and chemical synaptic transmission at Club endings. Synaptic activity leads to mGluR activation paired with postsynaptic membrane depolarization, triggering endocannabinoid (eCB) release from the postsynaptic Mauthner cell dendrite, which activates CB1Rs on dopaminergic fibers. CB1R activation leads to dopamine release that, by activating postsynaptic D1/5 receptors, increases PKA activity responsible for simultaneous potentiation of electrical and glutamatergic (GluR) synaptic transmission. Modified from Cachope et al. (2007) [122].
CaMKII has been implicated in mechanisms of activity-dependent plasticity in chemical synapses [112–115]. This kinase is an essential and abundant component of glutamatergic postsynaptic densities (PSDs) [116] in which it associates with other proteins, such as NMDA receptors [116,117]. Interestingly, recent data indicate that this kinase can also molecularly interact with Cx36 [119] and electrical transmission at Club endings is mediated by fish homologs of the Cx36. That is, CaMKII was shown to bind to two cytoplasmic domains of Cx36. Both domains reveal striking similarities with segments of the regulatory subunit of CaM-KII such as the pseudosubstrate and pseudotarget sites of the kinase [119]. Furthermore, similar to the NR2B subunit of the NMDA receptor, both Cx36 binding sites exhibit phosphorylation-dependent interaction and autonomous activation of CaMKII [119]. This report also revealed the existence of multiple phosphorylation sites for αCaMKII in Cx36 [119]. In silico analysis showed that Cx36 shares most of the identified residues with its fish homologs Cx35 and Cx34.7 [120]. This analysis also showed that residues S315/S298/S300 in Cx36, Cx35 and Cx34.7, respectively, constitute exclusive phosphorylation sites for CaM-KII that are not shared with other kinases. On the other hand, residues S110 and S293 in Cx36, S110 and S276 in Cx35, and S110 and S277 in Cx34.7, are shared with PKA [82,86,108,121].
A second form of activity-dependent potentiation, which follows a different induction protocol and signaling mechanisms, was more recently reported at Club endings [122]. In this case brief continuous stimulation of these afferents at 100 Hz (100 Hz protocol) (Fig. 7D), which leads to release of endocannabinoids via activation of mGluR receptors and dendritic depolarization, triggered a long-term enhancement of both components of the synaptic response [122]. This phenomenon requires the activation of CB1 receptors and is indirectly mediated via the release of dopamine from nearby varicosities, which in turn led to potentiation of the synaptic response via a PKA postsynaptic mechanism [122] (Fig. 7E). Thus, dopamine availability can be regulated by the activity of nearby glutamatergic synapses. Two aspects of the action of dopamine on these terminals are unusual: 1) in contrast to most examples dopamine produces enhancement of gap junctional conductance, and 2) rather than a modulator dopamine acts a trigger, initiating a long-term modification of electrical transmission [91,92,122]. That is, a local brief application of dopamine in the vicinity of these terminals (puff) was sufficient to trigger a lasting potentiation of both components of the synaptic response [91,92].
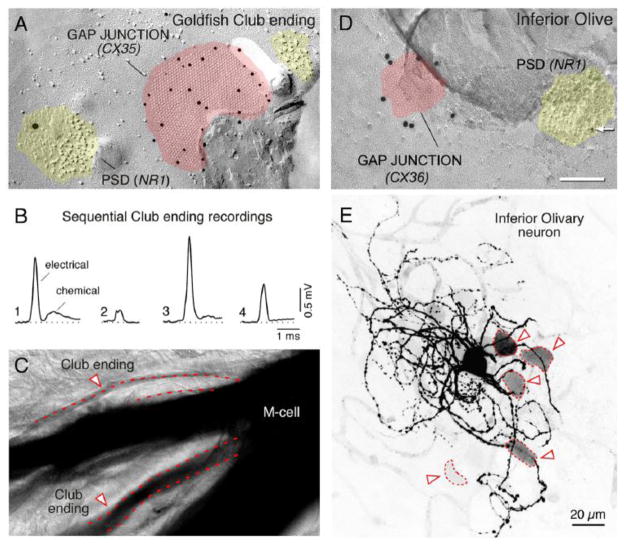
Interestingly, the activity of the glutamatergic synapses localized within the same contact at Club endings (Fig. 8A), creates a wide diversity of coupling between each of these terminals and the lateral dendrite of the Mauthner cell (Figs. 8B,C) [101]. Because induction of modulation of electrical coupling at goldfish mixed synapses (500Hz burst protocol) occurs postsynaptically [110], short-range functional interactions may not be limited only to mixed synapses but may be found where glutamatergic synapses are close to gap junctions formed by other presynaptic elements. Consistent with this possibility, FRIL analysis revealed the proximity of NR1-containing PSDs to Cx36-containing gap junctions in the inferior olive [123], at distances comparable to separations between the chemical transmitting domains and gap junctions in goldfish mixed synapses (Fig. 8D), suggesting that similar interactions between electrical and glutamatergic synapses might occur in the inferior olive. Accordingly, as with the Mauthner cell with Club endings, single olivary neurons were found differentially coupled with each of its partners (Fig. 8E) presumably as a result of local, glomerulus-specific, regulation of coupling [123]. Several lines of evidence support this possibility: 1) Cx36 and Cx35 are highly homologous and share regulatory sequences for CaMKII [120]; 2) CaMKII was shown to co-localize with both Cx36 in the inferior olive [119] and Cx35 in goldfish mixed synapses [120]; 3) CaMKII associates with both Cx36 [119] and Cx35 [120], and 4) the association of CaMKII with Cx35 is believed to be indicative of the degree of potentiation of electrical transmission at goldfish mixed synapses [120]. Also supporting this possibility, a recent report suggests that the degree of Cx36 phosphorylation can vary locally [88]. Interestingly, a recent report suggests that the drug Modafinil, an antinarcoleptic and mood-enhancing drug, might enhance electrical coupling between inferior olivary neurons through a mechanism that requires the activation of CaMKII [124]. Thus, while GABAergic terminals originating in the deep cerebellar nuclei promote transient decoupling by shunting the non-junctional membrane at the inferior olive glomeruli [68], this work raises the possibility that excitatory terminals of mesodiencephalic origin [67] could act to promote more lasting modifications of electrical coupling via activation of glutamate receptors (Fig. 3A). Short-term regulation of electrical coupling by nearby glutamatergic synapses contributes to coupling heterogeneity, which may represent a general property of networks of extensively coupled neurons and underlie important functions in various mammalian brain structures.
FIGURE 8. Neuronal gap-junctions are in close proximity to glutamatergic synapses and show variability in coupling strength.
A, Freeze-fracture immunogold labeling (FRIL) from goldfish Club endings shows Cx35 (10-nm gold beads) in a gap-junction plaque (pink). A nearby aggregate of E-face particles (yellow) represents a post-synaptic density for a glutamatergic synapse and shows labeling for the NR1 subunit (18-nm gold bead), indicating the presence of an NMDA receptor. B, Unitary EPSPs measured in the same Mauthner cell dendrite, but evoked from different club endings, show variability in the electrical conductance of the synaptic transmission, even though the pre-synaptic action potentials were highly invariable (not shown). Only one of the synaptic potentials shows a clear chemical component. Thus, electrical synapses from neighboring Club endings coexist at different degrees of conductance. C, Tracer coupling between the Mauthner cell (M-cell) and neighboring Club endings shows a similar diversity of coupling strength. The image shows that Neurobiotin injected to the Mauthner cell transferred to neighboring club endings (arrowheads) with different degrees of staining intensity, indicating that the junctions differ in their permeability. Thus, Club ending synapses on the goldfish Mauthner cell coexist and different degrees of conductance (panel B) and permeability (panel C), likely because of the regulation from nearby glutamatergic synapses (panel A, Fig. 6). A similar arrangement and coexistence of variable gap-junction strengths occurs in mammals, suggesting mechanisms similar to those in goldfish may function to modulate junctional conductance in mammals. Modified from Pereda et al. (2004) [105]. D, FRIL double labeling of Cx36 and NR1 in a rat inferior olivary neuron. The image shows a PSD (yellow) of a glutamatergic synapse with labeling for the NR1 subunit of an NMDA receptor (10nm-gold bead). The gap-junction plaque (pink) shows labeling for Cx36 (20nm-gold bead). This close arrangement of a Cx36-containing gap junction and an NR1-containing PSD is very similar to the close arrangement found in goldfish club endings (panel A). E, In the rat inferior olive, the labeling intensity of the somata of coupled cells (arrowheads) is highly variable. The image corresponds to a confocal projection (average of 17 z-sections) illustrating a neurobiotin-injected Inferior Olive neuron with multiple indirectly labeled neurons. Darker silhouettes represent more intense neurobiotin labeling; the variable labeling in the inferior olive is similar to the variable labeling of goldfish Club endings (panel C). Modified from Hoge et al. (2010) [123].
Supporting the notion that activity-dependent regulation of coupling is widespread, just as in goldfish brain and inferior olive, similar E-face particles identifying active zones were close to gap junctions in rat spinal cord and retina [125], and double labeling revealed NR1 containing e-face particles and Cx36-containing gap junctions [125]. Thus, interactions may be heterosynaptic and not limited to mixed synapses, and found where glutamatergic synapses are close to gap junctions formed by a presynaptic element. Accordingly, glutamatergic transmission was also shown to promote activity-dependent long-term depression of electrical coupling between the inhibitory neurons of the rat thalamic reticular nucleus (Fig. 9). High-frequency activation of cortico-thalamic inputs triggered a long-term depression of electrical transmission between thalamic relay neurons, which required the activation of metabotropic glutamate receptors [103]. Furthermore, the activity of glutamatergic synapses was also shown to induce increase in dye coupling between hypothalamic neurons [102]. Finally, activity-dependent depression of electrical coupling was recently observed as a result of coordinated burst firing in pairs of coupled thalamic relay neurons [126], although its mechanisms remain unknown.
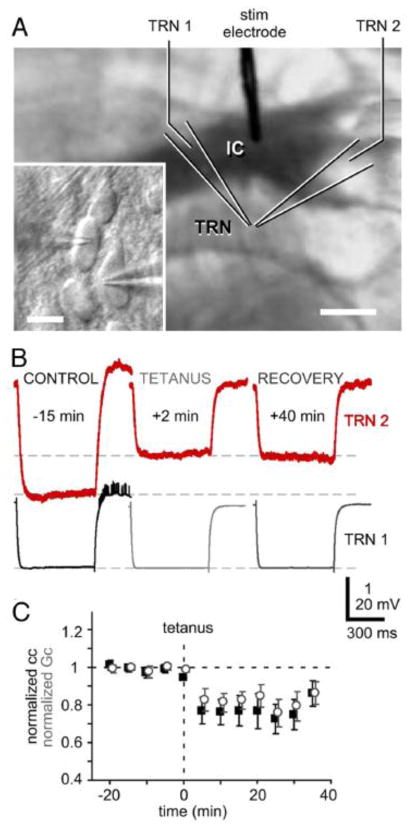
FIGURE 9. Effects of high-frequency stimulation on gap junctional strength at thalamic neurons.
A, IR-DIC image of a thalamocortical slice with recording arrangement. Inset: High-magnification view of recorded neurons in TRN. Scale bars indicate 1 mm and 20 μm, respectively. B, Voltage responses to hyperpolarizing current pulses in the presynaptic cell (TRN 1) and coupling responses in cell 2 (TRN 2) before and after high-frequency stimulation of cortico-thalamic glutamatergic afferents. C, Time-course of coupling coefficients (black squares) and estimated junctional conductance (gray open circles) for nine pairs of cells before and after high-frequency stimulation. The induction of long-term depression was prevented by superfusing with MCPG, an antagonist of mGLUR receptors. Modified from Landisman and Connors, 2003 [103].
3.2.3. Connexin-associated proteins and electrical synapses
In contrast to the extensive information on proteins associated with chemical synapses [127] involved in channel insertion, anchoring or removal from the plasma membrane [128–132], very few proteins have been shown to associate to the neuronal gap junctions. It is currently accepted that gap junctions are not only comprised of the defining intercellular channel proteins, but also associated scaffold and regulatory proteins [133]. Neuronal gap junctions in particular characteristically exhibit a PSD-like structure in electron microscopy, described as a “semi-dense cytoplasmatic matrix” [134], and which likely represents (similar to glutamatergic PSDs) proteins associated with these intercellular channels. Cx36, the most prevalent neuronal connexin that is widely distributed throughout the mammalian brain, was shown to directly interact with two relevant proteins: zonula occludens -1 (ZO-1) [135] and CaMKII [119].
ZO-1 is a member of the MAGUK family of proteins that was reported to interact with several other connexins [133]. Recent work also indicates that ZO-1 also interacts with Cx35 in goldfish Club endings [137]. Because its co-localization with Cx35 is extensive (Fig. 10A) [137], ZO-1 is expected to play a structural role, and the properties of the ZO-1/Cx35 association (lower affinity and faster kinetics when compared with Cx43 binding) [137] suggest the existence of a dynamic relation between these two proteins, possibly including a role of ZO-1 in regulating gap junctional conductance at these highly modifiable electrical synapses [137]. Accordingly, most recent indicates that the domain that connexin CT-domain containing the essential binding motif for interaction of Cx36 and its fish homologs is required for the surface expression of these connexins at teleost [138] and mammalian electrical synapses [139]. These interactions might contribute to regulate the turnover of gap junction channels at electrical synapses, which with an estimated half-life of 1–3 hours [138], is likely to contribute to the regulation of the strength of electrical transmission [138].
FIGURE 10. Connexin-associated proteins in electrical synapses.
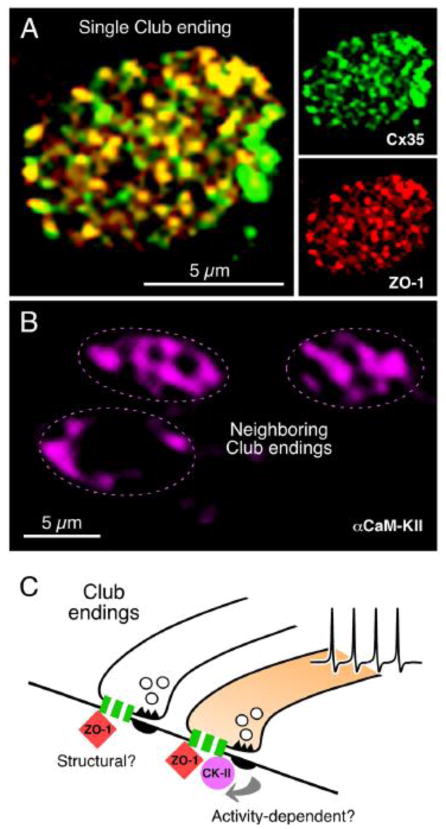
A, Cx35 and ZO-1 co-localize at Club Endings. Laser scanning confocal projection of an individual Club ending after double immunolabeling showing intense punctate labeling for Cx35 and ZO-1 (right panels) and high levels of co-localization in yellow (left panel). C, In contrast, αCaMKII labeling is more diffuse and highly variable between contiguous Club endings. While αCaMKII labeling was always observed at the periphery, where PSDs are located, it is also found at the center of the Club endings contact area in some cases (compare top Club endings with the bottom one). D, The extensive co-localization of Cx35 with ZO-1 suggests that this scaffold protein could constitute a structural component of gap junctions at these terminals. Activity of neighboring chemically transmitting regions within the terminal trigger changes in junctional conductance, via a PSD-mediated mechanism (arrows) promoting the association of CaMKII to Cx35 and ZO-1. The association of CaMKII to electrical synapses would be thus non-obligatory and driven by synaptic activity. For convenience, as simplified gap junction is illustrated; the cartoon does not indicate that association is exclusively pre- or postsynaptic. Modified from Flores et al., 2010 [120].
Although it directly associates with Cx36 and its homologs [119,120], CaMKII might not be an obligatory component of electrical synapses. As shown in the inferior olive [119], CaMKII also associates with Cx35 at Club endings [120]. Interesting, unlike other proteins, both CaMKII labeling and distribution were highly variable between contiguous Club endings (Fig. 10B), and it was not restricted to the periphery of the terminals, where glutamatergic synapses are located, but was also present at the center where gap junctions predominate. Because CaMKII characteristically undergoes activity-dependent translocation, the observed variability of labeling likely reflects physiological differences between electrical synapses of contiguous Club endings, which remarkably co-exist with differing degrees of conductance [120]. That is, its presence is highly variable, even between gap junction plaques within the same contact, and likely related to differences in the degree of potentiation between individual terminals (see above). In comparison with ZO-1, which would play a more permanent or structural role, the presence of CaMKII seems to be non-obligatory and possibly regulated by neural activity (Fig. 10C). This conclusion is consistent with the dynamic translocation properties of CaMKII, whose presence is thought to represent the history of synaptic activation [120].
Thus, evidence from goldfish Club endings suggest that ZO-1 and CaMKII belong to the same macromolecular complex [120] and together with other identified proteins such as zonula occludens proteins ZO-2 [140] and ZO-3 [141] and calmodulin [142], as well as those that might be identified in the future, are likely components of the “semi-dense cytoplasmatic matrix”, associated with intercellular channels in neuronal gap junctions which is likely to play a central regulatory role in electrical transmission.
4. Concluding remarks
It was generally perceived amongst neuroscientists that, in contrast to chemical transmission, electrical synapses lacked dynamic properties. Electrical synapses are considered now to be dynamic and modifiable forms of interneuronal communication. While gap junctions can also provide a conduit for small metabolites, the role of neuronal gap junctions is more often associated to providing a pathway of low resistance for the spread of ionic currents between neurons, generally referred as electrical coupling. The strength of electrical coupling is affected by gap junctional factors (gap junctional conductance) and non-junctional factors (passive and active neuronal properties). A wealth of evidence, some of which was reviewed in this article, indicates that both are targeted for regulation under various physiological situations. Gap junctional conductance was shown to be dynamically regulated by neurotransmitter modulators and the by activity of nearby glutamatergic synapses. The involvement of kinases such as PKA and CaMKII in regulating electrical transmission, which are also known to regulate the strength of chemical synapses, suggests a general role for these molecules in regulating interneuronal communication. Because gap junction regulation necessarily involves the interaction of gap junction channels with scaffold and regulatory proteins electrical synapses, as proposed for other gap junctions [133,143], should no longer be viewed as simple clusters of intercellular channels, but as complex structures in which many molecular elements participate in both short-term and long-term regulation. Plasticity of electrical transmission has also been observed at invertebrate electrical synapses [144,145], although the ultimate mechanisms underlying changes in electrical transmission remain unknown and might or might not involve changes in junctional conductance [145].
In summary, gap junctional communication between neurons has proven to be highly dynamic, a distinctive characteristic that is further emphasized by the dynamic regulation of their expression during development [146–148] and following brain injury [149–150].
HIGHLIGHTS.
Electrical synapses are dynamic and modifiable forms of interneuronal communication.
The strength of electrical coupling is affected by gap junctional and non-junctional factors.
Gap junctional conductance is dynamically regulated by nearby glutamatergic synapses.
PKA and CaMKII, which regulate chemical synapses, also regulate electrical synapses.
Gap junction regulation necessarily involves the interaction of gap junction channels with scaffold and regulatory proteins.
Acknowledgments
This research was supported by National Institutes of Health grants DC03186, DC011099, R21NS055726 and NS0552827 (to A.E.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furshpan EJ, Potter DD. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959;145:289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson JD, Bodenheimer TS, Stage DE. The ultrastructure of Mauthner cell synapses and nodes in goldfish brains. J Cell Biol. 1963;19:159–99. doi: 10.1083/jcb.19.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartelmez GW, Hoerr NL. The vestibular club endings in Ameiurus. Further evidence on the morphology of the synapse. J Comp Neurol. 1933;67:401–428. [Google Scholar]
- 4.Pereda A, Faber DS. Physiology of the Mauthner Cell: Discovery and Properties. In: Farrell AP, editor. Encyclopedia of Fish Physiology: From Genome to Environment. San Diego: Academic Press; 2011. [Google Scholar]
- 5.Faber DS, Pereda A. Physiology of the Mauthner cell: Functions. In: Farrell AP, editor. Encyclopedia of Fish Physiology: From Genome to Environment. San Diego: Academic Press; 2011. pp. 73–79. [Google Scholar]
- 6.Furshpan EJ. “Electrical transmission” at an excitatory synapse in a vertebrate brain. Science. 1964;144:878–880. doi: 10.1126/science.144.3620.878. [DOI] [PubMed] [Google Scholar]
- 7.Bennett MVL, Aljure E, Nakajima Y, Pappas GD. Electrotonic junctions between teleost spinal neurons: Electrophysiology and ultrastructure. Science. 1963;141:262–264. doi: 10.1126/science.141.3577.262. [DOI] [PubMed] [Google Scholar]
- 8.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–37. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 9.Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–45. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 10.White TW, Wang H, Mui R, Litteral J, Brink PR. Cloning and functional expression of invertebrate connexins from Halocynthia pyriformis. FEBS Lett. 2004;577:42–8. doi: 10.1016/j.febslet.2004.09.071. [DOI] [PubMed] [Google Scholar]
- 11.Condorelli DF, Parenti R, Spinella F, Trovato Salinaro A, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–8. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 12.Maxeiner S, Krüger O, Schilling K, Traub O, Urschel S, Willecke K. Spatiotemporal transcription of connexin45 during brain development results in neuronal expression in adult mice. Neuroscience. 2003;119:689–700. doi: 10.1016/s0306-4522(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien JJ, Li W, Pan F, Keung J, O’Brien J, Massey SC. Coupling between Atype horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. J Neurosci. 2006;26:11624–36. doi: 10.1523/JNEUROSCI.2296-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Li H, He SG. Identification of connexin 50 and 57 mRNA in Atype horizontal cells of the rabbit retina. Cell Res. 2005;15:207–11. doi: 10.1038/sj.cr.7290288. [DOI] [PubMed] [Google Scholar]
- 15.Müller LP, Dedek K, Janssen-Bienhold U, Meyer A, Kreuzberg MM, Lorenz S, Willecke K, Weiler R. Expression and modulation of connexin 30.2, a novel gap junction protein in the mouse retina. Vis Neurosci. 2010;27:91–101. doi: 10.1017/S0952523810000131. [DOI] [PubMed] [Google Scholar]
- 16.Dere E, Zheng-Fischhöfer Q, Viggiano D, Gironi Carnevale UA, Ruocco LA, Zlomuzica A, Schnichels M, Willecke K, Huston JP, Sadile AG. Connexin 31.1 deficiency in the mouse impairs object memory and modulates open-field exploration, acetylcholine esterase levels in the striatum, and cAMP response element-binding protein levels in the striatum and piriform cortex. Neuroscience. 2008;153:396–405. doi: 10.1016/j.neuroscience.2008.01.077. [DOI] [PubMed] [Google Scholar]
- 17.Eastman SD, Chen TH, Falk MM, Mendelson TC, Iovine MK. Phylogenetic analysis of three complete gap junction gene families reveals lineage-specific duplications and highly supported gene classes. Genomics. 2006;87:265–74. doi: 10.1016/j.ygeno.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Söhl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 19.Rash JE, Staines WA, Yasumura T, Patel D, Furman CS, Stelmack GL, Nagy JI. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin36 but not connexin32 or connexin43. Proc Natl Acad Sci U S A. 2000;97:7573–8. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–9. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 22.MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Stebbings LA, Todman MG, Phillips R, Greer CE, Tam J, Phelan P, Jacobs K, Bacon JP, Davies JA. Gap junctions in Drosophila: developmental expression of the entire innexin gene family. Mech Dev. 2002;113:197–205. doi: 10.1016/s0925-4773(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 24.Starich T, Sheehan M, Jadrich J, Shaw J. Innexins in C. elegans. Cell Commun Adhes. 2001;8:311–4. doi: 10.3109/15419060109080744. [DOI] [PubMed] [Google Scholar]
- 25.Phelan P, Goulding LA, Tam JL, Allen MJ, Dawber RJ, Davies JA, Bacon JP. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Curr Biol. 2008;18:1955–60. doi: 10.1016/j.cub.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelan P, Nakagawa M, Wilkin MB, Moffat KG, O’Kane CJ, Davies JA, Bacon JP. Mutations in shaking-B prevent electrical synapse formation in the Drosophila giant fiber system. J Neurosci. 1996;16:1101–13. doi: 10.1523/JNEUROSCI.16-03-01101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonsen BL, Edwards DH. Differential dye coupling reveals lateral giant escape circuit in crayfish. J Comp Neurol. 2003;466:1–13. doi: 10.1002/cne.10802. [DOI] [PubMed] [Google Scholar]
- 28.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 29.Pereda A, Bell T, Faber DS. Retrograde synaptic communication via gap junctions coupling auditory afferents to the Mauthner cell. J Neurosc. 1995;15:5943–5955. doi: 10.1523/JNEUROSCI.15-09-05943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herberholz J, Antonsen BL, Edwards DH. A lateral excitatory network in the escape circuit of crayfish. J Neurosci. 2002;22:9078–85. doi: 10.1523/JNEUROSCI.22-20-09078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curti S, Pereda A. Voltage-dependent enhancement of electrical coupling by a sub-threshold sodium current. J Neurosc. 2004;24:3999–4010. doi: 10.1523/JNEUROSCI.0077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auerbach AA, Bennett MV. A rectifying electrotonic synapse in the central nervous system of a vertebrate. J Gen Physiol. 1969;53:211–37. doi: 10.1085/jgp.53.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–51. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 34.Martin AR, Pilar G. Dual mode of synaptic transmission in the avian ciliary ganglion. J Physiol. 1963;68:443–63. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AR, Pilar G. An analysis of electrical coupling at synapses in the avian ciliary ganglion. J Physiol. 1964;171:454–75. doi: 10.1113/jphysiol.1964.sp007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao M, Gottesman-Davis A, Popratiloff A, Peusner KD. Dye coupling in developing vestibular nuclei. J Neurosci Res. 2008;86:832–44. doi: 10.1002/jnr.21541. [DOI] [PubMed] [Google Scholar]
- 37.Kihara AH, Paschon V, Cardoso CM, Higa GS, Castro LM, Hamassaki DE, Britto LR. Connexin36, an essential element in the rod pathway, is highly expressed in the essentially rodless retina of Gallus gallus. J Comp Neurol. 2009;512:651–63. doi: 10.1002/cne.21920. [DOI] [PubMed] [Google Scholar]
- 38.Kihara AH, Paschon V, Akamine PS, Saito KC, Leonelli M, Jiang JX, Hamassaki DE, Britto LR. Differential expression of connexins during histogenesis of the chick retina. Dev Neurobiol. 2008;68:1287–302. doi: 10.1002/dneu.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudo G. Expression of Cx36 in mammalian neurons. Brain Res Rev. 2000;32:72–85. doi: 10.1016/s0165-0173(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 40.Al-Ubaidi MR, White TW, Ripps H, Poras I, Avner P, Gomes D, Bruzzone R. Functional properties, developmental regulation, and chromosomal localization of murine connexin36, a gap-junctional protein expressed preferentially in retina and brain. J Neurosci Res. 2000;59:813–26. doi: 10.1002/(SICI)1097-4547(20000315)59:6<813::AID-JNR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobrenis K, Chang HY, Pina-Benabou MH, Woodroffe A, Lee SC, Rozental R, Spray DC, Scemes E. Human and mouse microglia express connexin36, and functional gap junctions are formed between rodent microglia and neurons. J Neurosci Res. 2005;82:306–15. doi: 10.1002/jnr.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–5. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 44.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–9. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 45.Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–9. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci. 2002;22:10898–905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–85. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 48.Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin36-deficient mice. Neuron. 2001;31:487–95. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 49.Bloomfield SA, Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron. 2005;46:761–72. doi: 10.1016/j.neuron.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 51.Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci. 2005;8:61–6. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- 52.Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, Dortland B, Wellershaus K, Degen J, Deuchars J, Fuchs EC, Monyer H, Willecke K, De Jeu MT, De Zeeuw CI. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58:599–612. doi: 10.1016/j.neuron.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Allen K, Fuchs EC, Jaschonek H, Bannerman DM, Monyer H. Gap junctions between interneurons are required for normal spatial coding in the hippocampus and short-term spatial memory. J Neurosci. 2011;31:6542–52. doi: 10.1523/JNEUROSCI.6512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vervaeke K, Lorincz A, Gleeson P, Farinella M, Nusser Z, Silver RA. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron. 2010;67:435–51. doi: 10.1016/j.neuron.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KG, Yasumura T, Shigemoto R, Rash JE, Nagy JI. Connexin45-containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques, forming bihomotypic gap junctions, with scaffolding contributed by zonula occludens-1. J Neurosci. 2008;28:9769–89. doi: 10.1523/JNEUROSCI.2137-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rash JE, Davidson KG, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen OP, Olson CO, Nagy JI. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J Neurocytol. 2005;34:307–41. doi: 10.1007/s11068-005-8360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rash JE, Olson CO, Davidson KG, Yasumura T, Kamasawa N, Nagy JI. Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus. Neuroscience. 2007;147:938–56. doi: 10.1016/j.neuroscience.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rash JE, Olson CO, Pouliot WA, Davidson KG, Yasumura T, Furman CS, Royer S, Kamasawa N, Nagy JI, Dudek FE. Connexin36 vs. connexin32, “miniature” neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus. Neuroscience. 2007;149:350–71. doi: 10.1016/j.neuroscience.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 60.Bennett MVL. Physiology of electrotonic junctions. Ann NY Acad Sci. 1966;137:509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- 61.Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18:1419–27. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paré D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons In vivo. J Neurophysiol. 1998;79:1450–60. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- 63.Zsiros V, Aradi I, Maccaferri G. Propagation of postsynaptic currents and potentials via gap junctions in GABAergic networks of the rat hippocampus. J Physiol. 2007;578:527–44. doi: 10.1113/jphysiol.2006.123463. Epub 2006 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci. 2001;2:425–33. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- 65.Bennett MVL, Pappas GD. The electromotor system of the stargazer: a model for integrative actions at electrotonic synapses. Journal of Neuroscience. 1983;3:748–61. doi: 10.1523/JNEUROSCI.03-04-00748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–64. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 67.De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SK, Ruigrok TJ. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- 68.Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 69.Leznik E, Llinas R. Role of gap junctions in synchronized neuronal oscillations in the inferior olive. J Neurophysiol. 2005;94:2447–2456. doi: 10.1152/jn.00353.2005. [DOI] [PubMed] [Google Scholar]
- 70.Best AR, Regehr WG. Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA. Neuron. 2009;62:555–565. doi: 10.1016/j.neuron.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spira ME, Spray DC, Bennett MV. Electrotonic coupling: effective sign reversal by inhibitory neurons. Science. 1976;94:1065–7. doi: 10.1126/science.185698. [DOI] [PubMed] [Google Scholar]
- 72.Dugué GP, Brunel N, Hakim V, Schwartz E, Chat M, Lévesque M, Courtemanche R, Léna C, Dieudonné S. Electrical coupling mediates tunable low-frequency oscillations and resonance in the cerebellar Golgi cell network. Neuron. 2009;61:126–139. doi: 10.1016/j.neuron.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 73.Mann-Metzer P, Yarom Y. Electrotonic coupling interacts with intrinsic properties to generate synchronized activity in cerebellar networks of inhibitory interneurons. J Neurosci. 1999;19:3298–3306. doi: 10.1523/JNEUROSCI.19-09-03298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Curti S, Hoge G, Nagy JI, Pereda A. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012;32:4341–59. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haas JS, Landisman CE. State-dependent modulation of gap junction signaling by the persistent sodium current. Front Cell Neurosci. 2011;5:31. doi: 10.3389/fncel.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piccolino M, Neyton J, Gerschenfeld H. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′:5′-monophosphate in horizontal cells of turtle retina. J Neurosci. 1982;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA. 1985;82:3025–9. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lasater EM. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA. 1987;84:7319–23. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMahon DG, Knapp AG, Dowling JE. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci USA. 1989;86:7639–43. doi: 10.1073/pnas.86.19.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414:351–75. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992;445:201–30. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitropoulou G, Bruzzone R. Modulation of perch connexin35 hemi-channels by cyclic AMP requires a protein kinase A phosphorylation site. J Neurosci Res. 2003;72:147–57. doi: 10.1002/jnr.10572. [DOI] [PubMed] [Google Scholar]
- 83.Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992;12:4911–22. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–7. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- 85.Mills SL, Xia XB, Hoshi H, Firth SI, Rice ME, Frishman LJ, Marshak DW. Dopaminergic modulation of tracer coupling in a ganglion-amacrine cell network. Vis Neurosci. 2007;24:593–608. doi: 10.1017/S0952523807070575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Urschel S, Höher T, Schubert T, Alev C, Söhl G, Wörsdörfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- 87.Kothmann WW, Li X, Burr GS, O’Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–11. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li H, Chuang AZ, O’Brien J. Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. J Neurosci. 2009;29:15178–86. doi: 10.1523/JNEUROSCI.3517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci. 1993;13(8):3456–71. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc Natl Acad Sci USA. 1992;89:12088–12092. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pereda A, Nairn A, Wolzson L, Faber DS. Postsynaptic modulation of synaptic efficacy at mixed synapses on the Mauthner cell. J Neurosc. 1994;14:3704–3712. doi: 10.1523/JNEUROSCI.14-06-03704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson BR, Peck JH, Harris-Warrick RM. Dopamine induces sign reversal at mixed chemical-electrical synapses. Brain Res. 1993;625:159–64. doi: 10.1016/0006-8993(93)90149-h. [DOI] [PubMed] [Google Scholar]
- 94.Johnson BR, Peck JH, Harris-Warrick RM. Amine modulation of electrical coupling in the pyloric network of the lobster stomatogastric ganglion. J Comp Physiol A. 1993;172(6):715–32. doi: 10.1007/BF00195397. [DOI] [PubMed] [Google Scholar]
- 95.Zsiros V, Maccaferri G. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci. 2008;28:1804–15. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hatton GI, Yang QZ. Synaptically released histamine increases dye coupling among vasopressinergic neurons of the supraoptic nucleus: mediation by H1 receptors and cyclic nucleotides. J Neurosci. 1996;16:123–129. doi: 10.1523/JNEUROSCI.16-01-00123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rorig B, Sutor B. Nitric oxide-stimulated increase in intracellular cGMP modulates gap junction coupling in ratneocortex. Neuro Report. 1996;7:569–572. doi: 10.1097/00001756-199601310-00046. [DOI] [PubMed] [Google Scholar]
- 98.O’Donnell P, Grace AA. Cortical afferents modulate striatal gap junction permeability via nitric oxide. Neuroscience. 1997;76:1–5. doi: 10.1016/s0306-4522(96)00433-2. [DOI] [PubMed] [Google Scholar]
- 99.Yang XD, Korn H, Faber DS. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990;348:542–545. doi: 10.1038/348542a0. [DOI] [PubMed] [Google Scholar]
- 100.Pereda AE, Faber DS. Activity dependent short-term plasticity of intercellular coupling. J Neurosci. 1996;16:983–992. doi: 10.1523/JNEUROSCI.16-03-00983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith M, Pereda AE. Chemical synaptic activity modulates nearby electrical synapses. Proc Natl Acad Sci USA. 2003;100:4849–4854. doi: 10.1073/pnas.0734299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hatton GI, Yang QZ. Activation of excitatory amino acid inputs to supraoptic neurons: I. Induced increases in dye-coupling in lactating, but not virgin or male rats. Brain Res. 1990;513:264–269. doi: 10.1016/0006-8993(90)90465-n. [DOI] [PubMed] [Google Scholar]
- 103.Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 104.Yang XD, Faber DS. Initial synaptic efficacy influences induction and expression of long-term changes in transmission. Proc Natl Acad Sci USA. 1991;88:4299–4303. doi: 10.1073/pnas.88.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pereda AE, Rash JE, Nagy JI, Bennett MVL. Dynamics of electrical transmission at club endings on the Mauthner cells. Brain Res Rev. 2004;47:227–244. doi: 10.1016/j.brainresrev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 106.Tuttle R, Masuko S, Nakajima Y. Freeze fracture study of the Large Myelinated Club Ending synapse on the goldfish Mauthner cell: special reference to the quantitative analysis of gap junctions. J Comp Neurol. 1986;246:202–211. doi: 10.1002/cne.902460206. [DOI] [PubMed] [Google Scholar]
- 107.Pereda A, O’Brien J, Nagy JI, Bukauskas F, Davidson KG, Kamasawa N, Yasumura T, Rash JE. Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells. J Neurosci. 2003;23:7489–503. doi: 10.1523/JNEUROSCI.23-20-07489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Brien J, Bruzzone R, White TW, Al-Ubaidi MR, Ripps H. Cloning and expression of two related connexins from the perch retina define a distinct subgroup of the connexin family. J Neurosci. 1998;18:7625–7637. doi: 10.1523/JNEUROSCI.18-19-07625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rash JE, et al. Neuronal gap junctions in goldfish hindbrain are primarily or exclusively at glutamatergic mixed synapses and are heterotypic, with Cx35 in axon terminals and Cx34.7 in somata and dendrites. Society for Neurosciences Abstracts, 2010. 2010 Abstract Viewer/Itinerary Planner (online) [Google Scholar]
- 110.Pereda A, Bell T, Chang B, Czernik A, Nairn A, Soderling T, Faber DS. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap junctional conductance and glutamatergic transmission. Proc Natl Acad Sci USA. 1998;95:3272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kothmann W, Trexler EB, Whitaker CM, Li W, Massey SC, O’Brien JO. Nonsynaptic NMDA Receptors Mediate Activity-Dependent Plasticity of Gap Junctional Coupling in the AII Amacrine Cell Network. J Neurosci. 2012;32:6747–6759. doi: 10.1523/JNEUROSCI.5087-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12:293–299. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- 113.Schulman H. Activity-dependent regulation of calcium/calmodulin-dependent protein kinase II localization. J Neurosci. 2004;24:8399–8403. doi: 10.1523/JNEUROSCI.3606-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26:645–53. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 115.Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–a754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- 117.Yang E, Schulman H. Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1999;274:26199–26208. doi: 10.1074/jbc.274.37.26199. [DOI] [PubMed] [Google Scholar]
- 118.Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 119.Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Höher T, Matsubara M, Willecke K, Spray DC, Dermietzel R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci USA. 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flores CE, Cachope R, Nannapaneni S, Ene S, Nairn AC, Pereda AE, Flores CE, et al. Variability of distribution of Ca(2+)/calmodulin-dependent kinase II at mixed synapses on the mauthner cell: colocalization and association with connexin 35. J Neurosci. 2010;30:9488–9499. doi: 10.1523/JNEUROSCI.4466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ouyang X, Winbow VM, Patel LS, Burr GS, Mitchell CK, O’Brien J. Protein kinase A mediates regulation of gap junctions containing connexin35 through a complex pathway. Brain Res Mol Brain Res. 2005;135:1–11. doi: 10.1016/j.molbrainres.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cachope R, Mackie K, Triller A, O’Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–1047. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoge G, Davidson K, Yasumura T, Castillo P, Rash JR, Pereda A. The extent and strength of electrical coupling between inferior olivary neurons is heterogeneous. J Neurophysiol. 2011;105:1089–101. doi: 10.1152/jn.00789.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Urbano FJ, Leznik E, Llinas RR. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci U S A. 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rash JE, Pereda A, Kamasawa N, Furman CS, Yasumura T, Davidson KGV, Magnie A, Dudek FE, Olson C, Li X, Nagy JI. High-resolution proteomic mapping in the vertebrate central nervous system: Close proximity of connexin35 to NMDA glutamate receptor clusters and co-localization of connexin36 with immunoreactivity for zonula occludens protein-1 (ZO-1) J Neurocytol. 2004;33:131–151. doi: 10.1023/B:NEUR.0000029653.34094.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haas JS, Zavala B, Landisman CE. Activity-dependent long-term depression of electrical synapses. Science. 2011;334:389–93. doi: 10.1126/science.1207502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 128.Lin Y, Skeberdis VA, Francesconi A, Bennett MVL, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 130.Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 131.Mori H, Manabe T, Watanabe M, Satoh Y, Suzuki N, Toki S, Nakamura K, Yagi T, Kushiya E, Takahashi T, Inoue Y, Sakimura K, Mishina M. Role of the carboxy-terminal region of the GluR epsilon2 subunit in synaptic localization of the NMDA receptor channel. Neuron. 1998;21:571–580. doi: 10.1016/s0896-6273(00)80567-x. [DOI] [PubMed] [Google Scholar]
- 132.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 133.Herve JC, Bourmeyster N, Sarrouilhe D. Diversity in protein-protein interactions of connexins: emerging roles. Biochim Biophys Acta. 2004;1662:22–41. doi: 10.1016/j.bbamem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 134.Sotelo C, Korn H. Morphological correlates of electrical and other interactions through low-resistance pathways between neurons of the vertebrate central nervous system. Internat Rev Cytol. 1978;55:67–107. doi: 10.1016/s0074-7696(08)61887-2. [DOI] [PubMed] [Google Scholar]
- 135.Li X, Olson C, Lu S, Kamasawa N, Yasumura T, Rash JE, Nagy JI. Neuronal connexin36 association with zonula occludens-1 protein (ZO-1) in mouse brain and interaction with the first PDZ domain of ZO-1. Eur J Neurosci. 2004;19:2132–2146. doi: 10.1111/j.l460-9568.2004.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Flores C, Li X, Bennett MVL, Nagy JI, Pereda A. Interaction between connexin 35 and zonula occludens 1 and its potential role in regulation of electrical synapses. Proc Natl Acad Sci (USA) 2008;105:12545–12550. doi: 10.1073/pnas.0804793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flores CE, Nannapaneni S, Davidson KG, Yasumura T, Bennett MV, Rash JE, Pereda AE. Trafficking of gap junction channels at a vertebrate electrical synapse in vivo. Proc Natl Acad Sci USA. 2012;109:E573–82. doi: 10.1073/pnas.1121557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Helbig I, Sammler E, Eliava M, Bolshakov AP, Rozov A, Bruzzone R, Monyer H, Hormuzdi SG. In vivo evidence for the involvement of the carboxy terminal domain in assembling connexin 36 at the electrical synapse. Mol Cell Neurosci. 2010;45:47–58. doi: 10.1016/j.mcn.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ciolofan C, Li XB, Olson C, Kamasawa N, Gebhardt BR, Yasumura T, Morita M, Rash JE, Nagy JI. Association of connexin36 and zonula occludens-1 with zonula occludens-2 and the transcription factor zonula occludens-1-associated nucleic acid-binding protein at neuronal gap junctions in rodent retina. Neuroscience. 2006;140:433–451. doi: 10.1016/j.neuroscience.2006.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li X, Lu S, Nagy JI. Direct association of connexin36 with zonula occludens-2 and zonula occludens-3. Neurochem Int. 2009;54:393–402. doi: 10.1016/j.neuint.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Burr GS, Mitchell CK, Keflemariam YJ, Heidelberger R, O’Brien J. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem Biophys Res Commun. 2005;335:1191–1198. doi: 10.1016/j.bbrc.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spray DC, Duffy HS, Scemes E. Gap junctions in glia. Types, roles, and plasticity. Adv Exp Med Biol. 1999;468:339–59. [PubMed] [Google Scholar]
- 144.Tsai LY, Tseng SH, Yeh SR. Long-lasting potentiation of excitatory synaptic signaling to the crayfish lateral giant neuron. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:347–54. doi: 10.1007/s00359-004-0589-1. [DOI] [PubMed] [Google Scholar]
- 145.Kazama H, Yaksi E, Wilson RI. Cell death triggers olfactory circuit plasticity via glial signaling in Drosophila. J Neurosci. 2011;31:7619–30. doi: 10.1523/JNEUROSCI.5984-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci. 2005;8:1720–6. doi: 10.1038/nn1588. [DOI] [PubMed] [Google Scholar]
- 147.Park WM, Wang Y, Park S, Denisova JV, Fontes JD, Belousov AB. Interplay of chemical neurotransmitters regulates developmental increase in electrical synapses. J Neurosci. 2011;31:5909–20. doi: 10.1523/JNEUROSCI.6787-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Belousov AB. The regulation and role of neuronal gap junctions during development. Commun Integr Biol. 2011;4:579–81. doi: 10.4161/cib.4.5.16380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y, Denisova JV, Kang KS, Fontes JD, Zhu BT, Belousov AB. Neuronal gap junctions are required for NMDA receptor-mediated excitotoxicity: implications in ischemic stroke. J Neurophysiol. 2010;104:3551–6. doi: 10.1152/jn.00656.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y, Song JH, Denisova JV, Park WM, Fontes JD, Belousov AB. Neuronal gap junction coupling is regulated by glutamate and plays critical role in cell death during neuronal injury. J Neurosci. 2012;32:713–25. doi: 10.1523/JNEUROSCI.3872-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bennett MV, Pappas GD, Aljure E, Nakajima Y. Physiology and ultrastructure of electrotonic junctions. II. Spinal and medullary electromotor nuclei in mormyrid fish. J Neurophysiol. 1967;30:180–208. doi: 10.1152/jn.1967.30.2.180. [DOI] [PubMed] [Google Scholar]
- 152.Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 2005;93:467–480. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]