Abstract
Inflammatory periodontal diseases are a leading cause of tooth loss and are linked to multiple systemic conditions, such as cardiovascular disease and stroke. Reconstruction of the support and function of affected tooth-supporting tissues represents an important therapeutic endpoint for periodontal regenerative medicine. An improved understanding of periodontal biology coupled with current advances in scaffolding matrices has introduced novel treatments that use cell and gene therapy to enhance periodontal tissue reconstruction and its biomechanical integration. Cell and gene delivery technologies have the potential to overcome limitations associated with existing periodontal therapies, and may provide a new direction in sustainable inflammation control and more predictable tissue regeneration of supporting alveolar bone, periodontal ligament, and cementum. This review provides clinicians with the current status of these early-stage and emerging cell- and gene-based therapeutics in periodontal regenerative medicine, and introduces their future application in clinical periodontal treatment. The paper concludes with prospects on the application of cell and gene tissue engineering technologies for reconstructive periodontology.
Tissue engineering and regenerative medicine are part of an emerging multidisciplinary and interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biologic substitutes; they have the potential to revolutionize the way health and quality of life are improved for millions of people worldwide by restoring, maintaining, or enhancing tissue and organ function.1 Applied to periodontal biology, tissue engineering has the potential to improve the regeneration of lost tooth-supporting structures in a more predictable manner than conventional periodontal treatments (Fig. 1). Periodontal engineering uses advanced engineering and life science technologies to restore the structure and function of alveolar bone, periodontal ligament (PDL), cementum, and gingival tissue, recreating the proper periodontal interface complex characterized by multitissue integration and proper tissue cell orientation.
Figure 1.
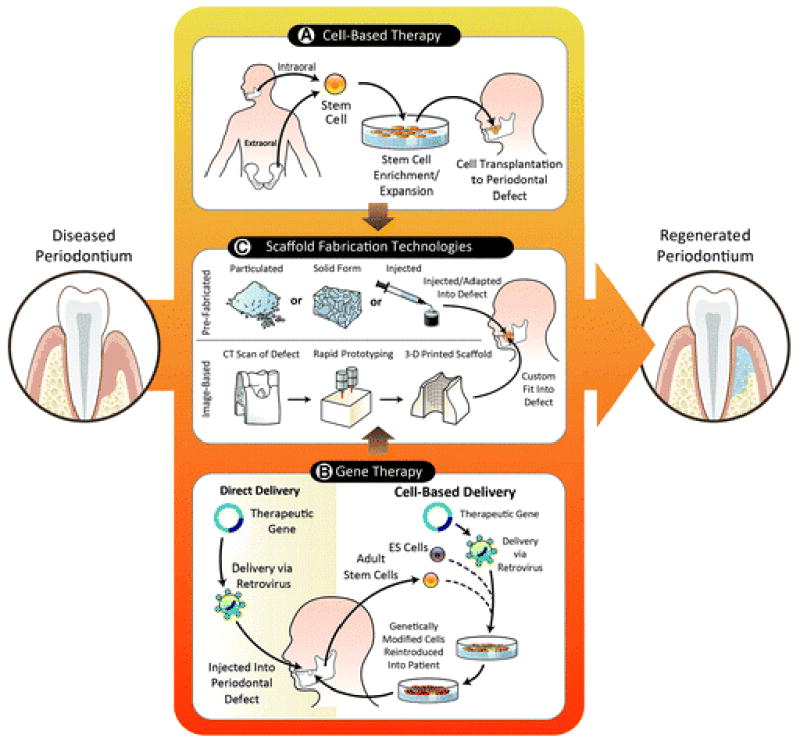
Cell- and gene-based technologies using scaffolding matrices for periodontal tissue engineering. A) Extraoral and intraoral stem cells represent a viable and accessible alternative source to harvest and expand multipotent colonies. Adequate cell density could be reached in vitro under a controlled environment and made readily available for reimplantation into a periodontal defect site. B) The available direct and cell-based delivery of a therapeutic gene has been shown to increase the regenerative potential and enhance the availability of important factors. The gene of interest is either injected directly into the periodontal defect via a retrovirus or alternatively could be incorporated into an embryonic stem cell (ES) or adult stem cell that is subsequently expanded and delivered into the area of interest. C) Prefabricated and image-based scaffolds are becoming an essential component in regenerative medicine. A defined supporting structure allows the localization and guidance of the appropriate cells and proteins and the establishment of a mechanically competent environment. Currently, scaffolds for periodontal regeneration are available in particulated, solid, and injectable forms. New developing technology has allowed the customization of scaffolds that fit into the periodontal defect and include an external and internal architecture that enhances tissue orientation and regeneration. This figure highlights the potential of integrating the available tissue engineering strategies to enhance the outcome of periodontal regenerative therapy.
A successful outcome of periodontal tissue engineering requires the following essential factors: appropriate cells, signals, scaffolds, blood supply, mechanical loading, and pathogen control. Cells provide the machinery for new tissue growth and differentiation, whereas growth factors and other molecules modulate the cellular activity and provide stimuli for cells to differentiate and support tissue neogenesis. A three-dimensional template structure is provided by scaffolds to support and facilitate these processes that are critical for tissue regeneration.2 New vascular networks promoted by angiogenic signals provide the nutritional base for tissue growth and homeostasis, whereas appropriate mechanical loading is essential for the development of highly organized, functional PDL fibers. Finally, because of the microbial load at the periodontal lesion, strategies to control infection and host response are required to optimize periodontal regeneration.
This paper reviews the concepts and available information in tissue engineering concerning early-stage cell and gene delivery for periodontal therapy. The discussion focuses primarily on three important aspects: 1) cell-based therapy, 2) gene therapy, and 3) scaffold fabrication technologies for delivering cells and genes to periodontal osseous defects.
CELLS FOR PERIODONTAL REGENERATION
In general, cell therapy involves the treatment of disease or disorder by transferring new cells into a tissue. Cells are the center of new tissue growth and differentiation. In cell-based regenerative medicine, cells are delivered to a defect site with the goal of improving the regeneration process.3 Cell delivery approaches are used to accelerate periodontal regeneration through two primary mechanisms: to use cells as carriers to deliver growth or cellular signals, and to provide cells that are able to differentiate to multiple cell types to promote regeneration. The use of cells as vehicles to deliver growth factors can stimulate an endogenous regeneration process.1 This strategy has been intensively investigated in both soft and hard periodontal tissue regeneration. Stem cell research has soared in the past few years and the effects on healing and regenerative potential have been extensively studied.
Mesenchymal stem cells (MSCs) are self-renewable and can differentiate into a variety of cell types that form mesenchymal and connective tissues.3,4 Bone marrow stromal cells are the most widely investigated MSCs because they are easily accessible. Bone marrow stromal cells were initially isolated and described nearly 50 years ago based on their ability to adhere to plastic substrates of cell culture plates.5 Since then, this simple protocol has been widely used to isolate MSCs from many tissues, such as adipose tissue, muscle, liver, pancreas, and cartilage.6 MSCs have a tremendous potential in regenerative medicine because of their multipotency and capability to form a variety of tissues, including the periodontium (Table 1).7–21 Regarding periodontal tissue engineering, both extraoral and intraoral stem cells can be harvested and then subjected to enrichment and expansion techniques (Fig. 1). Within this context, multiple sources of stem cells have been evaluated for the treatment and regeneration of the periodontium.22
Table 1.
Preclinical Applications of Cell Therapies for Periodontal Tissue Engineering
| Vector | Type | Advantages | Disadvantages |
|---|---|---|---|
| Retrovirus | Viral | Non-immunogenic | Infects only dividing cells |
| Constitutive transgene expression | Insertional mutagenesis | ||
| Lentivirus | Viral | Infects dividing and non-dividing cells | Insertional mutagenesis |
| Infects wide range of cell types | Potential pathogenicity | ||
| Low immune response | Complex large-scale preparation | ||
| Adenovirus | Viral | Infects dividing and non-dividing cells | Potential immunogenicity |
| Does not integrate into target cell genome | Transient expression | ||
| Adeno-associated virus | Viral | Infects dividing and non-dividing cells | Difficult to produce at high titers |
| Low immunogenicity | Small transgenes | ||
| Non-pathogenic in human | |||
| Plasmid | Non-viral | Non-immunogenic | Low transduction efficiency |
| Non-pathogenic | |||
| DNA polymer complexes | Non-viral | Infects dividing and non-dividing cells | Low transduction efficiency |
| Cell-specific targeting |
Extraoral MSCs for Oral and Periodontal Engineering
There is strong potential for the use of MSC sources from outside the oral cavity for transplantation to the oral, periodontal, and craniofacial complex.6,9,23 Kawaguchi et al.7 showed that bone marrow MSC transplantation promoted periodontal regeneration in experimental Class III defects in dogs. The treatment promoted up to 20% of cementum and bone regeneration. Using a cell-labeling technique, it was shown that these cells differentiate into cementoblasts, PDL fibroblasts, and alveolar bone osteoblasts in vivo.8 In a subsequent small clinical trial, autologous, expanded bone-marrow stromal cells mixed with atelocollagen were transplanted into periodontal osseous defects at the time of periodontal surgery, and positive clinical results were obtained. Furthermore, the use of MSCs and platelet-rich plasma (PRP) demonstrated a reduction in intrabony defect depth and resolution of bleeding and tooth mobility.24
Bone marrow stromal cells have also been shown to promote bone healing and dental implant osseointegration.25 In a series of studies, Yamada et al.10–12 used PRP as an autologous scaffold with in vitro expanded bone-marrow stromal cells to increase osteogenesis in dental implant surgery. This “autogenous injectable bone treatment” resulted in higher marginal bone levels, better bone implant contact, and increased bone density compared to PRP alone and particulated cancellous bone and marrow control groups. Recently, it was shown that cells harvested from the bone marrow can be driven down MSC pathways via a single-pass perfusion process to promote bone regeneration in tooth extraction socket and sinus floor augmentation procedures.26
Adipose tissue is another extraoral and non-craniofacial source of MSCs for oral and periodontal tissue engineering. Its greatest advantage is ease of access. Tobita et al.13 demonstrated in rats that, mixed with PRP, adipose-derived stromal cells could promote periodontal regeneration.
Oral and Craniofacial MSCs for Periodontal Engineering
Just as PDL is essential for the osteogenesis and cementogenesis during development and remodeling, cells derived from this tissue are supportive for the healing response to injury.27 Transplantation of PDL cells has shown the potential to regenerate periodontal attachment apparatus in vivo.14,28 Akizuki et al.15 developed a PDL cell sheet using temperature-responsive cell culture dish technique and hyaluronic acid (HA) carrier. After the transplantation of PDL cell sheets in small and large preclinical animal models, significant cementum formation and anchoring PDL fibers were observed together with new alveolar bone formation. Using specific labeling techniques, Lekic et al.16 showed that transplanted PDL cells integrate and differentiate into newly formed periodontal tissues.
Specific cell types derived from the periodontium have also been examined for their potential and roles in periodontal regeneration. Cementoblasts have been shown to induce mineralization in an ex vivo model29 and in vivo in periodontal wounds.21 By contrast, periodontal healing was inhibited when less-differentiated dental follicle cells were delivered in a similar fashion.21 Similarly, progenitor cells isolated from dental follicle failed to form dentin, cementum, or bone in vivo, although they expressed high levels of bone sialoprotein, osteocalcin, and alkaline phosphatase.30 Such results are suggestive of selective behaviors of different cell types in periodontal regeneration. PDL stem cells (PDLSCs) have recently been isolated and they express several mesenchymal stem cell markers, such as STRO-1 and CD44, and exhibit osteogenic, adipogenic, and chondrogenic characteristics under defined culture conditions.3,22 Implanted PDLSCs generate cementum and PDL-like structures similar to native periodontal apparatus.17 In a porcine periodontal defect model, PDLSCs were shown to regenerate new bone, cementum, and PDL, and the height of the new alveolar bone was significantly greater than that of a ceramic carrier group.28 Combining PDLSCs with another stem cell population from the root apical papilla of human teeth (stem cells from apical papilla), Sonoyama et al.31 generated a “bio-root” structure encircled with PDL tissue. Most recently, Gault et al.32 have taken autologous PDLSCs from extracted teeth, grown them in tissue bioreactors, and delivered them onto titanium implants as a cell transplantation approach. These implants form ligamentous attachments anchoring bone-to-implant surface and are termed “ligaplants.” These ligaplants demonstrated functional loading for extended periods of time as shown in a case series of human participants.33 The use of cell therapy approaches as hybrid living, implantable biomaterials offers another avenue for oral tissue-engineering strategies.33
Besides stem cell delivery, other strategies have been developed based on the concept that transplanted cells promote regeneration by secreting growth factors via autocrine and paracrine pathways. Allogenic foreskin fibroblasts have been shown to be safe and are able to promote keratinized gingiva formation at gingival recession defects.34 A tissue-engineered living cellular construct composed of viable neonatal keratinocytes and fibroblasts was reported to achieve comparable clinical outcome as gingival graft35 with strong potential to promote tissue neogenesis through the stimulation of angiogenic signals.36
Supporting the significant potential of cell-based therapy to form a variety of periodontal tissues, these investigations support the use of this approach to deliver important cues that drive the regenerative process.
GENE THERAPY FOR PERIODONTAL ENGINEERING
Gene therapy is defined as the treatment of disease or disorder by transferring genetic materials to introduce, suppress, or manipulate specific genes that direct an individual’s own cells to produce a therapeutic agent.37 This therapeutic concept has emerged as a promising strategy for the modulation of the host response triggered by periodontal microbe and the regeneration of periodontium during diseases. Gene therapy has several advantages over traditional treatments, such as those involving compounds and proteins: 1) greater sustainability than that of a single protein or compound application; whereas the half-lives of pharmaceutical compounds or recombinant protein usually range from several hours to several days, viral vector genes can be expressed in vivo from weeks to years; 2) gene delivery reduces technical challenges associated with ex vivo protein expression and purification, such as palmitoylation and glycosylation; 3) transient and controlled delivery of genetic sequences encoding a combinatorial group of regenerative factors could mimic the natural biologic healing response; and 4) coupled with tissue-engineering strategies, delivery of different genes in a spatially controlled and bioavailable fashion offers strong potential in regenerating tissues in three dimensions at the tooth-ligament–bone interface.
Applied to periodontal tissue engineering, a therapeutic gene can be introduced into a patient by either direct delivery through cell-based delivery method with or without a scaffolding matrix or indirectly through ex vivo approaches (Fig. 1). In general, the indirect or cell-based delivery approach is considered safer and a more controlled approach because the targeted cells are isolated and the therapeutic gene is delivered ex vivo under controlled conditions.
Gene Delivery Methods
Specific conditions are an important consideration for gene transfer applications:38 1) the required duration of protein release (transient versus long-term expression); 2) target cells (dividing and non-dividing cells, targeted locale of cells); 3) host immune response to vectors in viral-based approaches; 4) route of gene delivery (ex vivo or in vivo); and 5) the anatomic constraints of the target site. For example, a one- or two-walled defect may require the use of a supportive carrier, such as a scaffold. Other, more contained defect sites, such as three- and four-wall defects, may be conducive to the use of an adenoviral vector embedded in a collagen matrix. A wide variety of viral and non-viral vectors have been developed for gene delivery. Examples of viral vectors are retroviruses, lentiviruses, adenoviruses (Ad), and adeno-associated viruses (AAVs). Non-viral vectors include plasmids, DNA polymer complexes, nanobubbles and microbubbles, and ultrasound (Table 2).39
Table 2.
Viral and Non-Viral Gene Therapy Vectors Used in Tissue Engineering
| Cell Type | Autograft/Allograft | Defect Type | Reference(s) |
|---|---|---|---|
| Bone marrow stromal cells | Auto | Class III defects | 8,9 |
| Auto | Periodontal fenestration | 10 | |
| Auto | Osteotomy | 11–13 | |
| Adipose stromal cells | Auto | Periodontal palatal defects | 14 |
| Periodontal ligament cells | Auto | Class II defects | 15 |
| Auto | Periodontal fenestration | 16 | |
| Allo/Xeno | Periodontal fenestration | 17 | |
| Periodontal ligament stem cells | Allo | Ectopic | 18 |
| Allo | Periodontal fenestration | 15,19 | |
| Auto | Periodontal defects | 20 | |
| Cementoblasts | Allo | Ectopic | 21 |
| Allo | Periodontal fenestration | 22 | |
| Dental follicle cells | Allo | Ectopic | 21,22 |
| Allo | Periodontal fenestration | 22 |
Xeno = xenograft; Allo = allograft; Auto = autograft.
Retroviral vectors are single-stranded RNA viruses that are replicated in a host cell through the enzyme reverse transcription to produce DNA from its RNA genome, and the resulting reverse-transcribed viral DNA is incorporated into the host cell’s DNA strand by an integrase enzyme. When the genetically altered host cell divides, its descendants contain the viral DNA copy. These vectors have significant advantages for sustained and efficient transgene expression that is ideal for the treatment of life-threatening hereditary disorders, although most retroviruses can only infect dividing cells. Because the integrase enzyme may insert the DNA copy into an arbitrary position of the target cell DNA, endogenous gene expression may be disrupted by insertional mutagenesis of a proto-oncogene or tumor suppressor, and carcinogenesis may occur.40
Lentiviruses, such as the human immunodeficiency virus, are a specialized class of the retrovirus family and are characterized by a long incubation period. Lentiviral vectors are one of the most efficient methods in gene delivery, being able to transfect both dividing and non-dividing cells. These vectors are also integrated into the host cell genome. Despite the evidence that the insertion sites of lentivirus are more restricted than other retroviruses, the carcinogenesis induced by insertional mutation is still a hurdle for clinical application. Additionally, their human immunodeficiency virus origin raises many concerns regarding the possibility that recombination events will lead to replication-competent viruses.41
Ad are non-enveloped icosahedral viruses composed of a nucleocapsid and a double-stranded linear DNA genome.42 In contrast to lentiviruses, adenoviral vectors are attractive gene delivery vehicles because of a number of features: 1) Ad have high transduction efficiency in both dividing and non-dividing cells, 2) Ad do not induce apparent phenotypic changes in transduced cells, and 3) Ad fail to integrate into the host genome and remain episomal. These vectors may be advantageous in periodontal tissue engineering because the transient expression of growth factors may prevent the overgrowth of newly formed tissue. However, in large-size craniofacial defects, the short-term gene expression may be insufficient to induce complete tissue regeneration.43 One major concern regarding Ad gene delivery is the strong host immune response to viral capsid proteins, and viral backbone modification for reduction of immunogenicity has been investigated.43 Recently, several studies have provided supporting evidence indicating that local therapeutic Ad seem safe and efficient in diabetic foot ulcer treatment and periodontal regeneration.44,45
AAVs derive from the parvovirus family and are small viruses with a single-stranded DNA genome.39 AAV has attracted considerable interest from gene therapy researchers because of its several significant advantages: 1) AAV is currently not related to any human disease, 2) AAV presents very low immunogenicity, and 3) AAV infects both dividing and non-dividing cells. It has the ability to integrate its genetic material into the host cell genome at a specific site in the human chromosome 19, which makes it more predictable than retrovirus.46 However, random integration of AAV DNA into the host genome is low but detectable. A recent report raised concerns about the clinical use of AAV vectors when mice developed hepatocellular carcinoma after neonatal injection of an AAV vector, which is associated with the insertion in a 6-kb region of chromosome 12.47 Types of recombinant AAV have been developed either to remain extrachromosomal or integrate into non-specific chromosomal sites.39 One disadvantage of the AAV is that it is small and it can only carry target DNA usually <5 kb.43
Non-viral alternatives can also deliver genetic material into a host’s cell.42 They include naked plasmid, cationic lipids, polymers, peptides, and physical methods (electroporation and ultrasound). A major disadvantage for non-viral delivery methods is that non-viral gene carriers consistently exhibit significantly reduced transfection efficiency. However, because of their low immunogenicity, lack of DNA insert size limitation, and potential for large-scale production, non-viral vectors will be given more consideration in the future, especially in the field of siRNA gene therapy.48 In the past decade, a significant amount of research has focused on designing cationic compounds that can form complexes with DNA and can avoid both in vitro and in vivo barriers for gene delivery.49 Because of the anatomic advantage, some non-invasive physical methods may have a great opportunity in delivering DNA to the periodontium. Chen et al.50 reported that a gene transfer approach using ultrasound and nanobubbles and microbubbles leads to high gene expression in gingival tissue.
Target Genes
Platelet-derived growth factor
Initially identified as a platelet-derived mitogen specific for cells of mesenchymal origin, platelet-derived growth factor (PDGF) is a member of a family of multifunctional polypeptide growth factors.51 PDGF is considered a critical switch to initiate tissue repair process.
Initial studies showed that Ad-PDGF can effectively transduce cells derived from the periodontium, including osteoblasts, PDL fibroblasts, gingival fibroblasts, and cementoblasts, which led to enhancing mitogenic effect on these cells.52,53 Further studies demonstrated that Ad-PDGF treatment prolonged the PDGF signaling effect.54,55 Using in vivo optical imaging, sustained and localized gene expression in periodontal lesions for up to 21 to 35 days after direct gene targeting has been demonstrated.44,56 Regarding the safety profile of Ad gene therapy, Chang et al.44 demonstrated that Ad–PDGF-B delivered in a collagen matrix exhibits acceptable safety parameters for possible use in human clinical studies. Ad–PDGF-B was well contained within the localized osseous defect area without viremia or distant organ involvement. No significant histopathologic changes were observed. Although minor alterations in specific hematologic and blood chemistries were seen, most measures were within normal limits.44
It has been shown that Ad–PDGF-B transduction was able to enhance soft tissue defect fill by induction of human gingival fibroblast migration and proliferation in an ex vivo model. Jin et al.56 demonstrated that by using collagen as carrier, direct in vivo gene transfer of Ad–PDGF-B stimulated tissue regeneration in large periodontal defects. Ad–PDGF-B treatment resulted in more cell proliferation, a four-fold increase in bridging bone, and a six-fold increase in cementum repair above vector alone, whereas bone fill was significantly less in Ad–PDGF-1308 (a loss-of-function mutant of PDGF) treated defects.56 Similarly, in a dental implant model, Chang et al.57 reported that Ad PDGF-B shows regenerative capabilities for bone tissue engineering and osseointegration in alveolar bone defects comparable to rhPDGF-BB protein delivery in vivo.
Bone morphogenetic proteins
In dentistry, bone morphogenetic proteins (BMPs) have been shown to be potent growth factors stimulating alveolar bone formation. Jin et al.20 used an ex vivo strategy, where dermal fibroblast were transduced with BMP7 and transplanted into large mandibular alveolar bone defects. Ex vivo BMP2 gene delivery using autologous bone marrow MSC has been evaluated using animal models for periodontal regeneration. This approach has been shown to regenerate not only cementum with Sharpey fiber insertion, but also statistically significant quantities of bone, reestablishing a more normal relationship among the components of the regenerated periodontal attachment apparatus.58 Gene delivery by BMP7 resulted in rapid chondrogenesis, with subsequent osteogenesis, cementogenesis, and predictable bridging of periodontal bone defects,20 whereas BMP signaling knockdown by Ad-Noggin tended to inhibit bone repair and cementogenesis.20,59 In a direct gene therapy approach, Ad-BMP7 delivery with a collagen matrix significantly enhanced alveolar bone defect fill, new bone formation, and bone implant contact in a dental implant model.60 BMPs have been recently studied for a variety of other periodontal gene-delivery applications.58,61
Wingless (WNTs)
Wnts are a family of 19 secreted glycoproteins that are crucial for embryonic development and post-developmental physiology through regulation of cell proliferation, differentiation, and apoptosis.62 In the last several years, the role of Wnt signaling in bone development, postnatal maintenance of bone mass, and tooth morphogenesis has been investigated, and several pharmaceutical targets in Wnt signaling pathway for skeletal diseases have been identified, such as leucine-responsive regulatory protien 5 and sclerostin.63 The role of Wnts in periodontal homeostasis and regeneration remains largely unknown, although recently two studies highlighted the promising future of regulating Wnt signaling pathway. Nemoto et al.64 showed that canonical Wnt/β-catenin signaling has been shown to inhibit murine cementoblasts differentiation and enhance cell proliferation. In the other study, Chang et al.18 transduced human periodontal mesenchymal cells with retroviral Wnt-4, a non-canonical Wnt, and transplanted them into the experimental periodontal defects. The results demonstrated that Wnt-4 gene delivery promotes healing of alveolar bone wounds in vivo.
Transcription factors and regulators
In addition to growth factors, other genes that are critical transcription factors and regulators of osteogenesis, such asRunx2, Osterix (Osx), and LIM domain mineralization protein (LMP), may hold promise in periodontal tissue engineering, especially in alveolar bone augmentation. Runx2 is a master transcription activator of osteoblast differentiation. Ex vivoRunx2 gene delivery is able to induce bone marrow stromal cells and dermal fibroblasts to form mineral tissue in vivo.65–67 Osx is a zinc-finger–containing transcription factor that works downstream of Runx2 in osteoblast differentiation. Osxgene knockout results in impaired osteoblast differentiation and absence of bone formation. Tu et al.68 reported that overexpression of Osx in bone marrow MSCs by retroviral vectors stimulates healing of critical-sized defects. Using the same ex vivo strategy, the same group further showed Osx gene therapy increases bone density and elevates bone–implant contact in a titanium implant model.69 LMP-1 is an intracellular protein that is highly upregulated at the early stage of osteoblast differentiation. It has been shown that gene delivery of LMP-1 or a truncated version LMP-3 induces efficient bone formation in vivo in heterotopic and orthotopic (spine fusion and bone fracture healing) sites.70–73 The value of LMPs shows potential in the modulation of periodontal progenitor cells but still requires further investigation for periodontal tissue engineering.74
Gene Delivery for Host Modulation of Periodontal Disease
Acknowledging that tissue regeneration alone is not the only answer to predictably securing a long-term stable treatment for the patient with a history of periodontal disease is very important. Host modulation therapies are therefore rising as an important aspect in the control of periodontal diseases and tissue reengineering.75 Future approaches for tissue engineering may benefit from dual delivery of tissue regenerative molecules with either antimicrobial or host modulatory factors. Based on the understanding that the host response against pathogenic bacteria is a major cause of periodontal tissue destruction, new strategies have been developed to target these factors, such as MMPs, cathepsins, and other osteoclast-derived mediators of bone resorption.75 Gene therapy has also been investigated for the possibility of long-term maintenance of therapeutic proteins. Cirelli et al.76 used AAV to deliver the tumor necrosis factor receptor-immunoglobulin Fc (TNFR:Fc) fusion gene to experimental Porphyromonas gingivalis–lipopolysaccharide-mediated bone loss. Gene therapy resulted in sustained therapeutic levels of serum TNFR protein for ≥3 months, and P. gingivalis–lipopolysaccharide-mediated bone loss volume and density was inhibited after AAV2/1-TNFR:Fc administration. Tristetraprolin, a key cytokine-regulating RNA-binding protein, downregulates inflammatory cytokines by transferring mRNA transcripts to degradation machinery.77 Patil et al.77 showed that tristetraprolin overexpression by an adenoviral vector significantly reduces the expression of interleukin-6, TNF-α, and prostaglandin E2 in vitro and protects inflammation-induced bone loss and inflammatory infiltrate in an experimental periodontitis model. Most recently, Yu et al.78 demonstrated the potential of mitogen-activated protein kinase phosphatase 1 to prevent alveolar bone loss in vivo. These findings suggest the possible application of gene delivery strategies in the modulation of periodontal disease progression.
The potential of transferring genetic materials to modulate specific genes that direct an individual’s own cells to produce a therapeutic effect is the idea behind treating a disease by gene therapy. Dictating the local spatial and temporal distribution of the gene-therapy effect has been enhanced by their incorporation into natural and synthetic scaffolds.
SCAFFOLDING MATRICES USED TO DELIVER CELLS AND GENES FOR PERIODONTAL REGENERATION
Scaffolding matrices are used in tissue engineering to provide an environment where space is created and maintained during a period of time for cellular growth and tissue in-growth. These matrices serve as three-dimensional template structures to physically support and facilitate periodontal tissue regeneration when combined with cell- or gene-based tissue engineering (Fig. 1). During the past two decades, scaffolds have been extensively developed, studied, and used. Several fundamental requirements for scaffold design have been proposed.79 In their application to tissue engineering, they should 1) provide a three-dimensional architecture that supports a desired volume, shape, and mechanical strength; 2) consist of a high porosity and surface-to-volume ratio with a well-interconnected open pore structure to promote high seeding density and embrace bioactive molecules; 3) be biocompatible; and 4) degrade at a controlled rate and pattern that allows sufficient support until tissue defects are fully regrown. Scaffolds can also be engineered to serve as supportive carriers that conduct a sustained release of bioactive factors, thereby inducing stimuli for tissue formation. Transplantation of cells can be carried via tissue-engineered scaffolds79,80 that provide adhesion and anchorage for interacting stem cells to control the presentation of adhesion sites, thereby improving cell survival and participation.81,82 By furthering the pattern of tissue structure formed by stem cells, a new mandible was formed in a patient by using a metal and polymer scaffold seeded with stem cells and BMPs.83 Bioactive molecules, such as growth factors, may also be encapsulated into nanoparticles and microparticles embedded into the matrices to aid in their sustained release from scaffolds. Other approaches include mimicking stem cell niches to regulate daughter cell proliferation, differentiation, and dispersion into surrounding tissue or by attracting useful cells to a desired anatomic site.1
Understanding that proper periodontal regeneration is supported by important factors that include multiple tissue integration and cell–tissue directionality is critical for the optimization and further development of emerging scaffold technologies.84,85 Today, the feasibility to establish a three-dimensional polarity and a customizable microscaffold and macroscaffold architecture is one step forward to the ability to create biomimetic scaffold surfaces that are amenable for gene- and cell-therapy strategies.86 Several scaffold fabrication technologies as applied to periodontal tissue engineering have developed during the past 10 years including conventional prefabricated scaffolds, such as particulated, solid form, and injectable scaffolds that are adapted or administered into a periodontal defect, and novel image-based designs that result in a three-dimensional printed scaffold that is custom fit to a defect (Fig. 1).
Prefabricated Scaffolding Matrices
Conventional scaffolds used to regenerate tissue in vivo are prefabricated, and many techniques have been described that produce both natural and synthetic polymeric scaffolds. Naturally derived scaffolds include autografts, allografts, and xenografts. Other naturally derived scaffolds that have been adapted for tissue regeneration but remain unexplored as carriers in gene and cell therapy approaches for periodontal engineering include the coral-derived matrices and the hydroxyapatite and chitosan carriers.87,88 Alloplasts and other polymers are synthetically engineered materials consisting of bioactive molecules serving a purpose similar to that of natural scaffolds (Table 3).7,15,16,29,32,44,80,89–97
Table 3.
Scaffolding Matrices for Delivery of Cells and Genes for Periodontal Engineering
| Scaffold Origin | Biomaterial | Components | Application |
|---|---|---|---|
| Naturally Derived | Allografts | Calcified freeze-dried bone, decalcified freeze-dried bone | Non-viral vector (PDGF-BB)89 |
| Xenografts | Bovine mineral matrix, bovine-derived HA, bovine inorganic bone mineral | Non-viral vector (TGF-β1)90 | |
| Collagen (bovine/porcine xenograft) | Sponge | Oral/craniofacial MSCs (PDL cells)91 | |
| Membrane | Non-viral vector (b-FGF)92 | ||
| Gel/Gelatin | Oral/craniofacial MSCs (PDL cells)17 | ||
| Extraoral MSCs (bone marrow MSC)8 | |||
| Adenovirus vector (PDGF-B)44 | |||
| Synthetic/Alloplasts | Polymers | PLLA | Oral MSCs (PDL fibroblasts)93 |
| PGA | Retroviral vector (BMP7); periosteal cells94 | ||
| PLGA (copolymer of PLLA and PGA) | Oral/craniofacial MSCs (cementoblasts)29 | ||
| Retroviral vector (BMP2); W-20 cell line95 | |||
| Ca-P-based ceramics | β-TCP, Ca-P cement | Non-viral vector (TGF-β1)90 | |
| Hydroxyapatite-based scaffolds | Hydroxyapatite dense HA, porous HA, resorbable HA, non-porous non-resorbable granular HA | Oral/craniofacial MSCs (PDL cells)32 | |
| Hydrogels | HA ester | Oral/craniofacial MSCs (PDL cells)16 | |
| Methylcellulose | Non-viral vector (PDGF-BB; IGF-I)96 | ||
| CaCO3 | Coralline calcium carbonate ester | Non-viral vector (TGF-β1)97 |
PGA = polyglycolic acid; PLLA = polylactic acid; PLGA = poly(lactic-co-glycolic acid); TCP = tricalcium phosphate; TGF = transforming growth factor; FGF = fibroblast growth factor; IGF = insulin-like growth factor; Ca-P = calcium phosphate; bFGF = basic fibroblast growth factor.
Synthetic polymers have been studied extensively as gene therapy delivery systems because they provide greater freedom for property modification, such as control of macrostructure and degradation time, compared to naturally derived scaffolds.98 Furthermore, the release mechanism and exposure duration of bioactive molecules, such as growth factors, can be controlled.39 By acting as a localized gene depot, synthetic polymer scaffolds have the ability to maintain the therapeutic level of encoded proteins that limit unwanted immune response and potential side effects.99 Such polymers as poly(lactic-co-glycolic acid) have drawn much attention for its excellent properties for encapsulation of genes.100 Poly(lactic-co-glycolic acid) microspheres have been used previously to deliver antibiotics, as an occlusive membrane for guided tissue regeneration, as growth factor carrier for periodontal regeneration, and for cementum and complex tooth structure engineering.29,101–105 Microsphere systems have demonstrated promising results in the past; however, more novel approaches to microtechnologies today are focusing on nanosized particles.106 Nanotechnology has been attracting much attention for therapeutic agent and gene delivery, and a number of studies and reviews have delineated its contribution and capability to meet challenges of current regeneration therapy.100,106,107 Nanoscaled fibrillar structure of collagen shows promising effects on cellular biologic activities, and therefore, the potential of a synthetic polymer scaffold that mimics the nanofibrous structure of collagen.108 Furthermore, a recent study has developed macroporous polymer scaffolds with varying pore wall architecture to enhance the environment for induction of cellular activity and provide guidance for three-dimensional regeneration.109 Therefore, a delivery scaffold can provide a suitable environment for targeted cells and tissues and controlling the dynamic release of entrapped biologics. Periodontal therapy based on these systems, however, remains in its infancy.
The use of HA in the dental field has been demonstrated to restore periodontal defects and to carry and deliver growth factors, such as BMPs and fibroblast growth factor-2.110 Although no clinical or in vivo studies have used HA for gene and cell therapy strategies for periodontal engineering purposes, a recent in vitro study has shown an HA and collagen combination scaffold to be a suitable environment for the growth of human PDL cells, therefore indicating its potential for periodontal tissue engineering.111
Inorganic calcium-phosphate–based materials have also been used as delivery systems. Such materials as β-tricalcium phosphate are synthetic scaffolds that can be used to repair osseous defects around teeth or dental implants by acting as a bone substitute or as a carrier for growth factor delivery and cells.90 Gene-and cell-therapy tissue-engineering methods have used β-tricalcium phosphate as a carrier for bone reengineering approaches but its value for periodontal regeneration remains to be explored.112,113
Hydrogels, formed by the cross-linking or self-assembly of a variety of natural or synthetic hydrophilic polymers to produce structures that contain over 90% water, are obtained from natural materials, such as collagen chitosan, dextran, alginate, or fibrin. They are favorable for tissue engineering because of their innate ability to interact with and mediate degradation by cells.106,114–116 Vector release from hydrogels is dependent on the physical structure and degradation of the hydrogel and its interactions with the vector.116
Computer-Based Applications in Scaffold Design and Fabrication
Computer-based applications in tissue engineering are some of the more recent developments in scaffold design and fabrication for cell and gene delivery.117 This type of technology, image-based design, has been used in recent years to define virtual three-dimensional models for surgical planning by using data from computed tomography and magnetic resonance imaging. Specifically, in tissue engineering, computed tomography or magnetic resonance imaging data are used to define the three-dimensional anatomic geometry of a defect that can be used to create a template for a scaffold on a global anatomic level. This three-dimensional printed scaffold, because it is produced from the three-dimensional model, precisely fills the defect space (Fig. 2). Furthermore, the architecture of the scaffold can be defined to design the heterogeneous internal structure in a way to create region-specific variations in porous microstructures and scaffold surface topography, thereby altering material and biologic properties in specific regions of the scaffold, such as modulus, permeability, and cell orientation.118 This new generation of scaffolds is addressing specific periodontal functional requirements, such as PDL fiber orientation and tissue integration of cell- and gene-based technologies (Fig. 3).86
Figure 2.
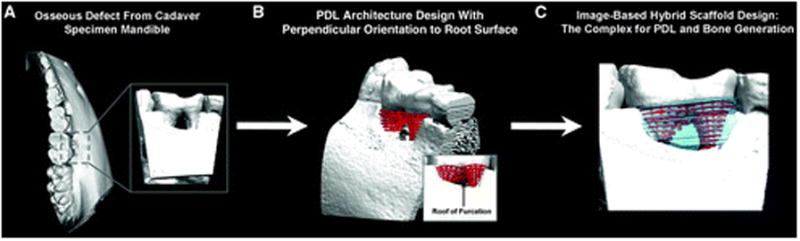
Hybrid scaffolds for periodontal cell and gene delivery. A through C) Surgically created Class III furcation defect is observed in this figure. The image-based scaffold aims at generating a three-dimensional polarity and patterning within the defect geometry to guide and establish cell tissue integration and directionality.
Figure 3.
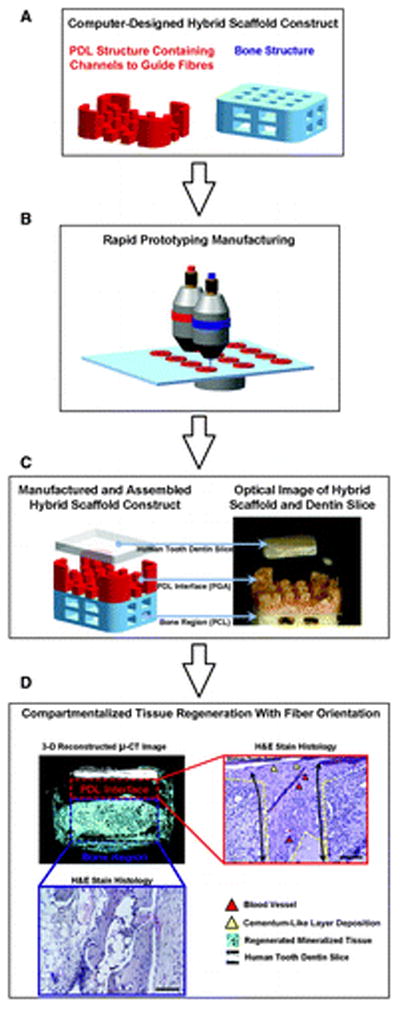
The hybrid scaffold concept has proved effective at establishing an adequate periodontal tissue interface that integrates the newly formed cementum, bone, and properly oriented PDL fibers. A) The main two compartments of the scaffold are depicted as PDL region (red) and bone region (blue). B) A rapid prototyping technique is used to generate the three-dimensional geometry. C) Wax molds for PDL and bone architectures are manufactured and fabricated to cast polymeric materials, poly-glycolide (PGA) for PDL and poly-ε-caprolactone (PCL) for bone of the hybrid scaffold. D) Microcomputed tomography (μ-CT) showed that the designed hybrid scaffold guides multiple tissue formation with the specific dimension in PDL interface (red dash-lined box) and bone region (blue dash-lined box). The hematoxylin and eosin staining was used to analyze mineralized tissue formation and fibrous connective tissue formation (scale bar = 50 μm original magnification ×10). In PDL interface, fibrous tissue orientation along the PDL topography (yellow dash-lined border) was found with blood vessel formations (red triangles) and limited cementum-like layer formation on the tooth dentin surface (yellow triangles).86
Various novel delivery scaffolding systems are being extensively studied and fabricated and are demonstrating capabilities to meet the challenges of current regeneration therapy. There are several techniques and technologies that have been developed and applied to fabrication of scaffold matrices. Only through further research and development in this area, along with cell-based and gene therapy, can tissue engineering continue to advance.
FUTURE DIRECTIONS IN PERIODONTAL REGENERATION
Tissue engineering is making an important impact on periodontal therapy. The use of cell and gene therapy to enhance and direct periodontal wound healing into a more predictable regenerative path is being exploited in bioengineering efforts that aim at developing a therapeutic system to promote periodontal repair. However, numerous challenges remain. As discussed within the content of this review, there are a number of developing systems that have the potential to optimize tissue-healing biology. A major obstacle that remains today is how to maximize the use of cells and genes delivered to a passive or permissive environment where there is context for the type of cell needed, but in which some biologic signals are given to encourage normal cell function. The tissue-engineering field still needs to confront other hurdles, such as identifying cell sources and clinically relevant cell numbers, the integration of new cells into existing tissue matrices, and the achievement of functional properties of tissue equivalents using an expanded repertoire of biomaterials. Major constraints to the cell- and gene-transfer fields remain in the practical and regulatory requirements to apply these technologies to the clinical arena.
CONCLUSIONS
Today, the available cell-based therapy strategies founded on tissue-engineering approaches have the most solid background for clinical application in human periodontal defects. Gene-based therapy is at the preclinical level at this point, and as such it may be several years before it enters the clinical arena. However, the cell-based, scaffold, and gene-therapy methods collectively interface and complement each other to enhance the potential to restore tissue function and structure in a predictable manner. The success and the future of periodontal regenerative therapy will thereby be supported by our understanding and ability to recognize those clinical situations that will benefit from one or more of these new emerging technologies.
Acknowledgments
The authors thank Chris Jung, the Department of Periodontics and Oral Medicine, University of Michigan, Ann Arbor, Michigan, for assisting with the preparation of the figures. This work was supported by National Institutes of Health Grants DE13397 (WVG), 1K23DE019872 (HFR), and the ITI Foundation (WVG).
Footnotes
The authors report no conflicts of interest related to this review.
References
- 1.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taba M, Jr, Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthod Craniofac Res. 2005;8:292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao JJ, Giannobile WV, Helms JA, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 6.Ward BB, Brown SE, Krebsbach PH. Bioengineering strategies for regeneration of craniofacial bone: A review of emerging technologies. Oral Dis. 2010;16:709–716. doi: 10.1111/j.1601-0825.2010.01682.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi H, Hirachi A, Hasegawa N, et al. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol. 2004;75:1281–1287. doi: 10.1902/jop.2004.75.9.1281. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa N, Kawaguchi H, Hirachi A, et al. Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. J Periodontol. 2006;77:1003–1007. doi: 10.1902/jop.2006.050341. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Yan F, Lei L, Li Y, Xiao Y. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs. 2009;190:94–101. doi: 10.1159/000166547. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Ueda M, Hibi H, Nagasaka T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: From basic research to clinical case study. Cell Transplant. 2004;13:343–355. doi: 10.3727/000000004783983909. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Ueda M, Naiki T, Nagasaka T. Tissue-engineered injectable bone regeneration for osseointegrated dental implants. Clin Oral Implants Res. 2004;15:589–597. doi: 10.1111/j.1600-0501.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: Tissue-engineered bone regeneration. Tissue Eng. 2004;10:955–964. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 13.Tobita M, Uysal AC, Ogawa R, Hyakusoku H, Mizuno H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A. 2008;14:945–953. doi: 10.1089/ten.tea.2007.0048. [DOI] [PubMed] [Google Scholar]
- 14.Doğan A, Ozdemir A, Kubar A, Oygür T. Healing of artificial fenestration defects by seeding of fibroblast-like cells derived from regenerated periodontal ligament in a dog: A preliminary study. Tissue Eng. 2003;9:1189–1196. doi: 10.1089/10763270360728099. [DOI] [PubMed] [Google Scholar]
- 15.Akizuki T, Oda S, Komaki M, et al. Application of periodontal ligament cell sheet for periodontal regeneration: A pilot study in beagle dogs. J Periodontal Res. 2005;40:245–251. doi: 10.1111/j.1600-0765.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 16.Lekic PC, Rajshankar D, Chen H, Tenenbaum H, McCulloch CA. Transplantation of labeled periodontal ligament cells promotes regeneration of alveolar bone. Anat Rec. 2001;262:193–202. doi: 10.1002/1097-0185(20010201)262:2<193::AID-AR1028>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 18.Chang J, Sonoyama W, Wang Z, et al. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- 19.Majewski M, Betz O, Ochsner PE, Liu F, Porter RM, Evans CH. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008;15:1139–1146. doi: 10.1038/gt.2008.48. [DOI] [PubMed] [Google Scholar]
- 20.Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 2003;74:202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao M, Jin Q, Berry JE, Nociti FH, Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75:154–161. doi: 10.1902/jop.2004.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nöth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev. 2010;62:765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, Ueda M, Hibi H, Baba S. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: A clinical case report. Int J Periodontics Restorative Dent. 2006;26:363–369. [PubMed] [Google Scholar]
- 25.Bueno EM, Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nat Rev Rheumatol. 2009;5:685–697. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- 26.Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A. 2010;16:2809–2820. doi: 10.1089/ten.tea.2010.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimono M, Ishikawa T, Ishikawa H, et al. Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003;60:491–502. doi: 10.1002/jemt.10290. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Zheng Y, Ding G, et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin QM, Zhao M, Webb SA, Berry JE, Somerman MJ, Giannobile WV. Cementum engineering with three-dimensional polymer scaffolds. J Biomed Mater Res A. 2003;67:54–60. doi: 10.1002/jbm.a.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morsczeck C, Götz W, Schierholz J, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gault P, Black A, Romette JL, et al. Tissue-engineered ligament: Implant constructs for tooth replacement. J Clin Periodontol. 2010;37:750–758. doi: 10.1111/j.1600-051X.2010.01588.x. [DOI] [PubMed] [Google Scholar]
- 33.Giannobile WV. Getting to the root of dental implant tissue engineering. J Clin Periodontol. 2010;37:747–749. doi: 10.1111/j.1600-051X.2010.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuire MK, Nunn ME. Evaluation of the safety and efficacy of periodontal applications of a living tissue-engineered human fibroblast-derived dermal substitute. I. Comparison to the gingival autograft: a randomized controlled pilot study. J Periodontol. 2005;76:867–880. doi: 10.1902/jop.2005.76.6.867. [DOI] [PubMed] [Google Scholar]
- 35.McGuire MK, Scheyer ET, Nunn ME, Lavin PT. A pilot study to evaluate a tissue-engineered bilayered cell therapy as an alternative to tissue from the palate. J Periodontol. 2008;79:1847–1856. doi: 10.1902/jop.2008.080017. [DOI] [PubMed] [Google Scholar]
- 36.Morelli T, Neiva R, Nevins ML, et al. Angiogenic biomarkers and healing of living cellular constructs. J Dent Res. 2011;90:456–462. doi: 10.1177/0022034510389334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ditto AJ, Shah PN, Yun YH. Non-viral gene delivery using nanoparticles. Expert Opin Drug Deliv. 2009;6:1149–1160. doi: 10.1517/17425240903241796. [DOI] [PubMed] [Google Scholar]
- 38.Elangovan S, Karimbux N. Review paper: DNA delivery strategies to promote periodontal regeneration. J Biomater Appl. 2010;25:3–18. doi: 10.1177/0885328210366490. [DOI] [PubMed] [Google Scholar]
- 39.Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dent Clin North Am. 2006;50:245–263. doi: 10.1016/j.cden.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waheed AA, Freed EO. The role of lipids in retrovirus replication. Viruses. 2010;2:1146–1180. doi: 10.3390/v2051146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telenti A, McLaren P. Genomic approaches to the study of HIV-1 acquisition. J Infect Dis. 2010;202(Suppl 3):S382–S386. doi: 10.1086/655969. [DOI] [PubMed] [Google Scholar]
- 42.Partridge KA, Oreffo RO. Gene delivery in bone tissue engineering: Progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10:295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- 43.Phillips JE, Gersbach CA, García AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–229. doi: 10.1016/j.biomaterials.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 44.Chang PC, Cirelli JA, Jin Q, et al. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Hum Gene Ther. 2009;20:486–496. doi: 10.1089/hum.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu DL, Nguyen T, Gonzalez AM, et al. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol Ther. 2004;9:699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Surosky RT, Urabe M, Godwin SG, et al. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J Virol. 1997;71:7951–7959. doi: 10.1128/jvi.71.10.7951-7959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi Y, Nishikawa M, Takakura Y. Nonviral vector-mediated RNA interference: Its gene silencing characteristics and important factors to achieve RNAi-based gene therapy. Adv Drug Deliv Rev. 2009;61:760–766. doi: 10.1016/j.addr.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 50.Chen R, Chiba M, Mori S, Fukumoto M, Kodama T. Periodontal gene transfer by ultrasound and nano/microbubbles. J Dent Res. 2009;88:1008–1013. doi: 10.1177/0022034509346119. [DOI] [PubMed] [Google Scholar]
- 51.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45:319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 52.Giannobile WV, Lee CS, Tomala MP, Tejeda KM, Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J Periodontol. 2001;72:815–823. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Z, Lee CS, Tejeda KM, Giannobile WV. Gene transfer and expression of platelet-derived growth factors modulate periodontal cellular activity. J Dent Res. 2001;80:892–897. doi: 10.1177/00220345010800030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen QP, Giannobile WV. Adenoviral gene transfer of PDGF downregulates gas gene product PDGFalphaR and prolongs ERK and Akt/PKB activation. Am J Physiol Cell Physiol. 2002;282:C538–C544. doi: 10.1152/ajpcell.00419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Z, Sugai JV, Jin Q, Chandler LA, Giannobile WV. Platelet-derived growth factor-B gene delivery sustains gingival fibroblast signal transduction. J Periodontal Res. 2008;43:440–449. doi: 10.1111/j.1600-0765.2008.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang PC, Seol YJ, Cirelli JA, et al. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration. Gene Ther. 2010;17:95–104. doi: 10.1038/gt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen YL, Chen PK, Jeng LB, et al. Periodontal regeneration using ex vivo autologous stem cells engineered to express the BMP-2 gene: An alternative to alveolaplasty. Gene Ther. 2008;15:1469–1477. doi: 10.1038/gt.2008.131. [DOI] [PubMed] [Google Scholar]
- 59.Jin QM, Zhao M, Economides AN, Somerman MJ, Giannobile WV. Noggin gene delivery inhibits cementoblast-induced mineralization. Connect Tissue Res. 2004;45:50–59. doi: 10.1080/03008200490278142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunn CA, Jin Q, Taba M, Jr, Franceschi RT, Rutherford RB, Giannobile WV. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol Ther. 2005;11:294–299. doi: 10.1016/j.ymthe.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L, Zhang Y, Dong R, et al. Effects of adenoviral-mediated coexpression of bone morphogenetic protein-7 and insulin-like growth factor-1 on human periodontal ligament cells. J Periodontal Res. 2010;45:532–540. doi: 10.1111/j.1600-0765.2009.01268.x. [DOI] [PubMed] [Google Scholar]
- 62.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 63.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 64.Nemoto E, Koshikawa Y, Kanaya S, et al. Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone. 2009;44:805–812. doi: 10.1016/j.bone.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 65.Phillips JE, Guldberg RE, García AJ. Dermal fibroblasts genetically modified to express Runx2/Cbfa1 as a mineralizing cell source for bone tissue engineering. Tissue Eng. 2007;13:2029–2040. doi: 10.1089/ten.2006.0041. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Z, Wang Z, Ge C, Krebsbach P, Franceschi RT. Healing cranial defects with AdRunx2-transduced marrow stromal cells. J Dent Res. 2007;86:1207–1211. doi: 10.1177/154405910708601213. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12:247–253. doi: 10.1016/j.ymthe.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Tu Q, Valverde P, Li S, Zhang J, Yang P, Chen J. Osterix overexpression in mesenchymal stem cells stimulates healing of critical-sized defects in murine calvarial bone. Tissue Eng. 2007;13:2431–2440. doi: 10.1089/ten.2006.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu B, Zhang J, Brewer E, et al. Osterix enhances BMSC-associated osseointegration of implants. J Dent Res. 2009;88:1003–1007. doi: 10.1177/0022034509346928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pola E, Gao W, Zhou Y, et al. Efficient bone formation by gene transfer of human LIM mineralization protein-3. Gene Ther. 2004;11:683–693. doi: 10.1038/sj.gt.3302207. [DOI] [PubMed] [Google Scholar]
- 71.Boden SD, Liu Y, Hair GA, et al. LMP-1, a LIM-domain protein, mediates BMP-6 effects on bone formation. Endocrinology. 1998;139:5125–5134. doi: 10.1210/endo.139.12.6392. [DOI] [PubMed] [Google Scholar]
- 72.Boden SD, Titus L, Hair G, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1) Spine (Phila Pa 1976) 1998;23:2486–2492. doi: 10.1097/00007632-199812010-00003. [DOI] [PubMed] [Google Scholar]
- 73.Lattanzi W, Parrilla C, Fetoni A, et al. Ex vivo-transduced autologous skin fibroblasts expressing human Lim mineralization protein-3 efficiently form new bone in animal models. Gene Ther. 2008;15:1330–1343. doi: 10.1038/gt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin Z, Navarro VP, Kempeinen KM, et al. LMP1 regulates periodontal ligament progenitor cell proliferation and differentiation. Bone. 2010;47:55–64. doi: 10.1016/j.bone.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giannobile WV. Host-response therapeutics for periodontal diseases. J Periodontol. 2008;79(Suppl 8):1592–1600. doi: 10.1902/jop.2008.080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cirelli JA, Park CH, MacKool K, et al. AAV2/1-TNFR:Fc gene delivery prevents periodontal disease progression. Gene Ther. 2009;16:426–436. doi: 10.1038/gt.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patil CS, Liu M, Zhao W, et al. Targeting mRNA stability arrests inflammatory bone loss. Mol Ther. 2008;16:1657–1664. doi: 10.1038/mt.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu H, Li Q, Herbert B, et al. Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther. 2011;18:344–353. doi: 10.1038/gt.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy WL, Mooney DJ. Controlled delivery of inductive proteins, plasmid DNA and cells from tissue engineering matrices. J Periodontal Res. 1999;34:413–419. doi: 10.1111/j.1600-0765.1999.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 80.Anusaksathien O, Jin QM, Ma PX, Giannobile WV. Scaffolding in periodontal engineering. In: Ma PX, Elisseeff JH, editors. Scaffolding in Tissue Engineering. Boca Raton: Taylor & Francis; 2005. pp. 437–454. [Google Scholar]
- 81.Alsberg E, Kong HJ, Hirano Y, Smith MK, Albeiruti A, Mooney DJ. Regulating bone formation via controlled scaffold degradation. J Dent Res. 2003;82:903–908. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 82.Davis ME, Motion JP, Narmoneva DA, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 84.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 86.Park CH, Rios HF, Jin Q, et al. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31:5945–5952. doi: 10.1016/j.biomaterials.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran CT, Gargiulo C, Thao HD, Tuan HM, Filgueira L, Michael Strong D. Culture and differentiation of osteoblasts on coral scaffold from human bone marrow mesenchymal stem cells [published online ahead of print August 12, 2010] Cell Tissue Bank. 2010 doi: 10.1007/s10561-010-9208-2. [DOI] [PubMed] [Google Scholar]
- 88.Oliveira JM, Rodrigues MT, Silva SS, et al. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials. 2006;27:6123–6137. doi: 10.1016/j.biomaterials.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 89.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74:1282–1292. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 90.Gille J, Dorn B, Kekow J, Bruns J, Behrens P. Bone substitutes as carriers for transforming growth factor-beta(1) (TGF-beta(1)) Int Orthop. 2002;26:203–206. doi: 10.1007/s00264-002-0353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakahara T, Nakamura T, Kobayashi E, et al. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue Eng. 2004;10:537–544. doi: 10.1089/107632704323061898. [DOI] [PubMed] [Google Scholar]
- 92.Rossa C, Jr, Marcantonio E, Jr, Cirelli JA, Marcantonio RA, Spolidorio LC, Fogo JC. Regeneration of Class III furcation defects with basic fibroblast growth factor (b-FGF) associated with GTR. A descriptive and histometric study in dogs. J Periodontol. 2000;71:775–784. doi: 10.1902/jop.2000.71.5.775. [DOI] [PubMed] [Google Scholar]
- 93.Mei F, Zhong J, Yang X, et al. Improved biological characteristics of poly(L-lactic acid) electrospun membrane by incorporation of multiwalled carbon nanotubes/hydroxyapatite nanoparticles. Biomacromolecules. 2007;8:3729–3735. doi: 10.1021/bm7006295. [DOI] [PubMed] [Google Scholar]
- 94.Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: Applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999;42:488–495. [PubMed] [Google Scholar]
- 95.Laurencin CT, Attawia MA, Lu LQ, et al. Poly(lactide-co-glycolide)/hydroxyapatite delivery of BMP-2-producing cells: A regional gene therapy approach to bone regeneration. Biomaterials. 2001;22:1271–1277. doi: 10.1016/s0142-9612(00)00279-9. [DOI] [PubMed] [Google Scholar]
- 96.Giannobile WV, Hernandez RA, Finkelman RD, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 97.Tatakis DN, Wikesjö UM, Razi SS, et al. Periodontal repair in dogs: Effect of transforming growth factor-beta 1 on alveolar bone and cementum regeneration. J Clin Periodontol. 2000;27:698–704. doi: 10.1034/j.1600-051x.2000.027009698.x. [DOI] [PubMed] [Google Scholar]
- 98.Jang JH, Houchin TL, Shea LD. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev Med Devices. 2004;1:127–138. doi: 10.1586/17434440.1.1.127. [DOI] [PubMed] [Google Scholar]
- 99.Ghali S, Dempsey MP, Jones DM, Grogan RH, Butler PE, Gurtner GC. Plastic surgical delivery systems for targeted gene therapy. Ann Plast Surg. 2008;60:323–332. doi: 10.1097/SAP.0b013e31806917b0. [DOI] [PubMed] [Google Scholar]
- 100.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 101.Williams RC, Paquette DW, Offenbacher S, et al. Treatment of periodontitis by local administration of minocycline microspheres: A controlled trial. J Periodontol. 2001;72:1535–1544. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- 102.Cetiner D, Unsal B, Parlar A, Gültekin E, Kurtiş B. Evaluation of periodontal healing in class II furcation defects following guided tissue regeneration with two different types of polylactic acid membranes. Chin Med J (Engl) 2004;117:270–274. [PubMed] [Google Scholar]
- 103.Kurtiş B, Unsal B, Cetiner D, et al. Effect of polylactide/glycolide (PLGA) membranes loaded with metronidazole on periodontal regeneration following guided tissue regeneration in dogs. J Periodontol. 2002;73:694–700. doi: 10.1902/jop.2002.73.7.694. [DOI] [PubMed] [Google Scholar]
- 104.Moioli EK, Hong L, Guardado J, Clark PA, Mao JJ. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12:537–546. doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 106.Agarwal A, Mallapragada SK. Synthetic sustained gene delivery systems. Curr Top Med Chem. 2008;8:311–320. [PubMed] [Google Scholar]
- 107.Sanvicens N, Marco MP. Multifunctional nanoparticles — Properties and prospects for their use in human medicine. Trends Biotechnol. 2008;26:425–433. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Woo KM, Jun JH, Chen VJ, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28:335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 109.Wei G, Ma PX. Partially nanofibrous architecture of 3D tissue engineering scaffolds. Biomaterials. 2009;30:6426–6434. doi: 10.1016/j.biomaterials.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wikesjö UM, Lim WH, Thomson RC, Cook AD, Wozney JM, Hardwick WR. Periodontal repair in dogs: Evaluation of a bioabsorbable space-providing macroporous membrane with recombinant human bone morphogenetic protein-2. J Periodontol. 2003;74:635–647. doi: 10.1902/jop.2003.74.5.635. [DOI] [PubMed] [Google Scholar]
- 111.Wang LX, Zhao H, Jiang B, Ding Y. Adhesion and growth of human periodontal ligament cells on hyaluronic acid/collagen scaffold. Hua Xi Kou Qiang Yi Xue Za Zhi. 2009;27:220–223. [PubMed] [Google Scholar]
- 112.Jiang XQ, Sun XJ, Lai HC, Zhao J, Wang SY, Zhang ZY. Maxillary sinus floor elevation using a tissue-engineered bone complex with beta-TCP and BMP-2 gene-modified bMSCs in rabbits. Clin Oral Implants Res. 2009;20:1333–1340. doi: 10.1111/j.1600-0501.2009.01755.x. [DOI] [PubMed] [Google Scholar]
- 113.Zhao J, Hu J, Wang S, et al. Combination of beta-TCP and BMP-2 gene-modified bMSCs to heal critical size mandibular defects in rats. Oral Dis. 2010;16:46–54. doi: 10.1111/j.1601-0825.2009.01602.x. [DOI] [PubMed] [Google Scholar]
- 114.Chen FM, Shelton RM, Jin Y, Chapple IL. Localized delivery of growth factors for periodontal tissue regeneration: Role, strategies, and perspectives. Med Res Rev. 2009;29:472–513. doi: 10.1002/med.20144. [DOI] [PubMed] [Google Scholar]
- 115.Moioli EK, Clark PA, Xin X, Lal S, Mao JJ. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv Drug Deliv Rev. 2007;59:308–324. doi: 10.1016/j.addr.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He H, Cao J, Wang D, Gu B, Guo H, Liu H. Gene-modified stem cells combined with rapid prototyping techniques: A novel strategy for periodontal regeneration. Stem Cell Rev. 2010;6:137–141. doi: 10.1007/s12015-009-9110-0. [DOI] [PubMed] [Google Scholar]
- 118.Hollister SJ, Maddox RD, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095–4103. doi: 10.1016/s0142-9612(02)00148-5. [DOI] [PubMed] [Google Scholar]


