Abstract
The TNF family cytokine TL1A (TNFSF15) costimulates T cells through its receptor DR3 (TNFRSF25) and is required for autoimmune pathology driven by diverse T cell subsets. TL1A has been linked to human inflammatory bowel disease (IBD), but its pathogenic role is not known. We generated transgenic mice that constitutively express TL1A in T cells or dendritic cells. These mice spontaneously develop IL-13 dependent inflammatory small bowel pathology that strikingly resembles the intestinal response to nematode infections. These changes were dependent on the presence of a polyclonal TCR repertoire, suggesting that they are driven by components in the intestinal flora. FoxP3+ Treg were present in increased numbers despite the fact that TL1A suppresses the generation of inducible Treg. Finally, blocking TL1A-DR3 interactions abrogates TNBS-colitis, indicating that these interactions influence other causes of intestinal inflammation as well. These results establish a novel link between TL1A and IL-13 responses that results in small intestinal inflammation and establish that TL1A-DR3 interactions are necessary and sufficient for T cell-dependent IBD.
Introduction
Interactions between TNF family receptors and their ligands play major roles in shaping critical features of immune responses, including programmed cell death and lymphocyte co-stimulation. DR3 (TNFRSF25/TRAMP/LARD/WSL-1) is one such TNF family receptor that like TNF-R1 contains a death domain and can activate NF-κB and MAP kinases, or caspases and apoptosis depending on the cellular context (1, 2). However, unlike TNF-R1, T cells express the highest levels of DR3. TL1A, the TNF family ligand for DR3, costimulates T cell proliferation and cytokine production in vitro (3). TL1A expression is highly regulated and requires induction by TNF-α and IL-1β in endothelial cells (3) and TLR or FcR stimulation in myeloid cells (4-6). TCR stimulation can also induce expression of TL1A in T cells with slower kinetics (4). Such selective expression of TL1A may explain the fact that DR3-deficient mice are resistant to multiple mouse models of T cell-mediated autoimmune disease, but generally display normal systemic responses to the antigens used to induce these autoimmune diseases (4-6).
Recently, several reports have suggested a role for TL1A and DR3 in inflammatory bowel disease (IBD) (7, 8). Increased expression of both TL1A and DR3 was reported in the lamina propria in biopsies of patients with Ulcerative Colitis (UC) and Crohn’s Disease (CD), with the level of TL1A expression correlating with the severity of the inflammation. In addition, elevated TL1A and DR3 expression was found in two different animal models of IBD, SAMP1/YitFc and TNFΔARE (8). Single nucleotide polymorphisms in the TL1A locus have also been linked to Crohn’s disease in multiple genome-wide scans (9-11). While these data provide strong circumstantial evidence that TL1A is involved in the pathogenesis of IBD, the causal role of TL1A-DR3 interactions in IBD is not known. To address this issue, we have generated several lines of TL1A transgenic mice that constitutively express TL1A in T cells and dendritic cells at levels similar to that observed when cells are induced to express TL1A by pro-inflammatory stimuli, and studied the effects of TL1A blockade during induction of a hapten-driven model of inflammatory colitis.
Results
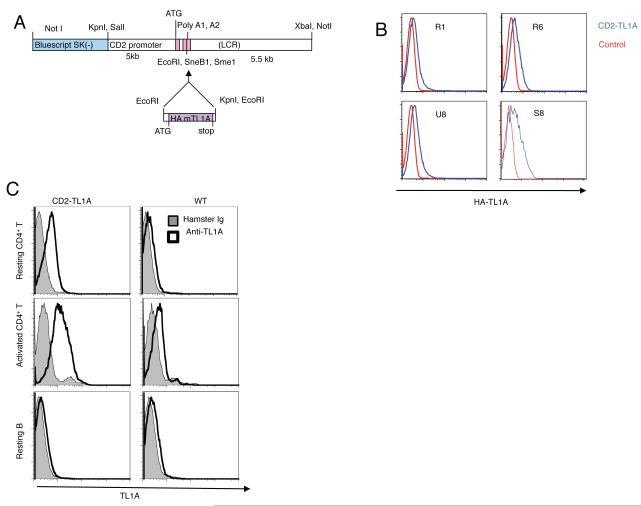
In transgenic mice with an HA epitope-tagged murine TL1A cDNA linked to the CD2 promoter enhancer cassette (12) four founder lines had detectable levels of TL1A expression in T cells assayed by flow cytometry (Figure 1A, B). Line R6 was selected for in-depth analysis. To confirm expression of surface TL1A on transgenic T cells and compare with endogenous levels, we generated monoclonal anti-mouse TL1A antibodies (Figure S1A). Staining of resting and activated T cells with mAb 5G4.6 revealed levels of surface TL1A on resting CD2-TL1A transgenic T cells that are higher than that present on wild-type T cells after activation with anti-CD3 and anti-CD28 (Figure 1C). Activation of transgenic T cells resulted in increased transgenic and endogenous surface TL1A (Figure 1C). B cells expressed only very low levels of endogenous TL1A that was not increased in transgenic cells (Figure 1C, bottom panels). Thus the mice generated using the CD2-mTL1A cassette constitutively expressed TL1A at levels at least as high as peak levels on activated T cells.
Figure 1. Generation and TL1A expression in CD2-TL1A transgenic mice.
(A) Schematic of CD2-TL1A transgenic construct illustrating placement of the mouse TL1A-HA cDNA in the CD2 promoter-enhancer cassette. (B) Comparison of transgene expression in four independent founder lines of CD2-TL1A transgenic mice assayed by intracellular flow cytometry of anti-HA on CD3-gated splenocytes from transgenic (blue) and control (red) mice. (C) Transgenic vs. endogenous cell surface TL1A expression assayed by flow cytometry with anti-TL1A mAb 5.4G6 vs control hamster Ig on resting CD4+ T cells isolated from spleen and lymph nodes (top), on CD4+ T cells 48 hours after activation with anti-CD3/anti-CD28 (middle), and on resting B cells gated on B220 (bottom) from line R6 CD2-TL1A transgenic and control C57BL/6 mice.
T cell activation and accelerated memory T cell generation in TL1A transgenic mice
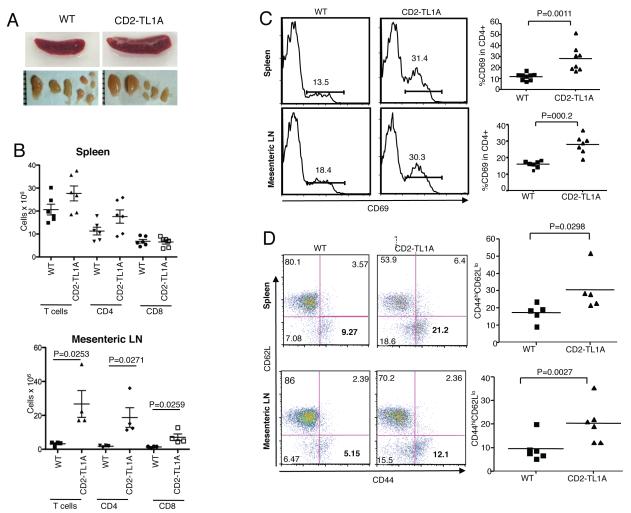
To investigate whether constitutively expressed TL1A can costimulate T cells in vivo we examined the lymphoid compartment and the state of T cell activation in CD2-TL1A transgenic mice. We found mild splenomegaly and mild to moderate mesenteric lymphadenopathy in adult mice of each of the CD2-TL1A transgenic lines (Figure 2A). Flow cytometric analysis revealed increased numbers of CD4+ T cells in mesenteric lymph nodes (mLN) and to a lesser extent in spleen (Figure 2B). CD8+ T cell yields were mildly increased in mLN and normal in the spleen. B cell and myeloid cell numbers were not affected (data not shown). Percentages of cells expressing CD69, a marker of recent activation, and also memory T cell markers were elevated in CD4+ T cells in spleen and mLN (Figure 2 C,D), and to a lesser extent in CD8+ T cells (Figure S2). Thus, TL1A can co-stimulate T cells to drive increased activation and memory cell formation in vivo without exogenous stimuli in CD2-TL1A transgenic mice.
Figure 2. Characterization of the T cell compartment in TL1A transgenic mice.
(A) Gross appearance of spleen and mesenteric lymph nodes from C57BL/6 wild-type and line R6 CD2-TL1A transgenic mice over 3 months of age. (B) Absolute number of T cells, CD4+ T cells and CD8+ T cells from spleen and mesenteric lymph nodes from line R6 CD2-TL1A transgenic mice 20-24 weeks old and age-matched controls. (C) Representative flow cytometric profiles and compilation of the percentage of CD69+ cells as a percentage of CD4+ T cells in spleen and mLN from line R6 CD2-TL1A transgenic mice 10-16 weeks of age and age-matched controls. (D) Representative flow cytometric profiles and compilation of the percentage of memory CD44hiCD62Llo cells as a percentage of CD4+T cells in spleen and mLN from line R6 CD2-TL1A transgenic mice of 10-16 weeks old and age-matched controls. Statistical analysis for comparison of percentages of the indicated subsets in transgenic and control mice was performed by unpaired two-tailed Student’s t-test.
Spontaneous intestinal inflammation in CD2-TL1A transgenic mice
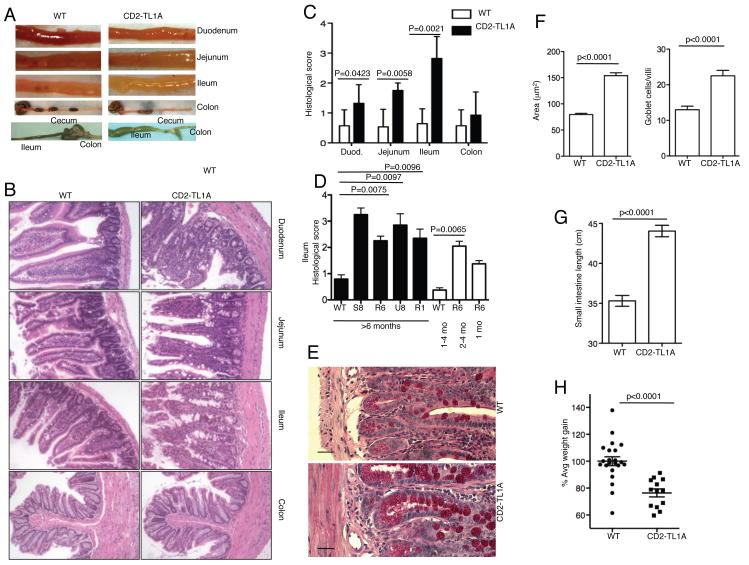
Inspection of the intestines of CD2-TL1A transgenic mice over six weeks of age revealed 100% incidence of visible small intestinal inflammatory changes including bowel wall thickening (Figure 3A), dilatation and lengthening that progressed with age and culminated in massive ileal dilatation in many animals at 6 months of age or older (Figure 3A bottom panel). Remarkably, the colons of these mice had no macroscopic evidence of inflammation. Histological examination revealed inflammatory changes in the small intestine which when fully developed included thickening of the muscularis and submucosa, lengthening and/or distorted villi accompanied by infiltration of the lamina propria with inflammatory cells including lymphocytes, macrophages and neutrophils. Finally, there was striking hyperplasia of goblet cells, particularly in the jejunum and ileum (Figure 3B). Quantitative scoring of inflammatory changes using a previously described scoring system (13) revealed significant inflammation present in the small intestine with the most severe changes in the ileum and no significant inflammation in the colon (Figure 3C). Similar severity of histological changes were found in the ileum of most founder lines when disease was fully developed at six months of age (Figure 3D). Within line R6, which was examined in more detail, pathology worsened as mice aged (Figure 3D). PAS staining of the ileum revealed remarkable increase in number and size of goblet cells (Figures 3E,F). The total length of the small intestine was significantly increased in CD2-TL1A transgenic mice compared to age-matched controls (Figure 3G), likely as a consequence of goblet cell and smooth muscle hyperplasia. At two weeks of age, T cell activation and memory markers were similar to controls and no small intestinal inflammation could be seen, whereas at four weeks of age, goblet cell hyperplasia, and increased CD69+ and memory phenotype T cells could be seen (Figure S3). CD2-TL1A transgenic mice also gained weight at a significantly lower rate in the two weeks following weaning compared to wild-type littermates (Figure 3H), indicating that the small intestinal pathology had clinical consequences.
Figure 3. TL1A transgenic develop spontaneous inflammatory bowel disease.
(A) Gross appearance of the indicated sections of intestine from 3 month old line R6 CD2-TL1A Tg mice and age-matched controls, with an example of the appearance of the grossly distended ileum from a 1.5 year old CD2-TL1A Tg mice, compared with an aged-matched control mice in the bottom panel. (B) H&E stained tissue sections of the indicated portions of intestine from a 3 month old line R6 CD2-TL1A Tg mouse compared with a littermate control. (C) Compilation of histological scores of all 4 founders over 6 months of age for each section. Each section was scored by an observer blinded to the genotype of the mouse. Statistical analysis for comparison of transgenic and control mice was performed by Mann-Whitney test. Sections from at least 4 mice per group were scored. (D) Comparison of histological scores from the ileum of mice from 4 independent founders of CD2-TL1A Tg mice, with at least 5 sections scored for each group of the indicated age (except for line S8 and 1 month old line R6, n=2). Statistical analysis for comparison of transgenic and control mice was performed by Mann-Whitney test. (E) Goblet cell hyperplasia in the Ileum of CD2-TL1A line R6 transgenic mouse and WT control. PAS staining, scale bar = 50 μM (F) Quantitation using Image J software of average goblet cell area and number of goblet cells/villus in sections of ileum from CD2-TL1A line R6 transgenic mice and age matched controls controls (n=5). (G) Small intestinal length (measured from the pylorus to ileocecal valve) of adult WT and CD2-TL1A Tg mice (n>5 each group). Statistical analysis for comparison between transgenic and control mice was performed by unpaired two-tailed Student’s t-test. (H) Comparison of weight gain for the 14 days after weaning of CD2-TL1A Tg mice (line R6). Statistical analysis for comparison between transgenic and control mice was performed by unpaired two-tailed Student’s t-test.
Dose-dependent effects of deregulated expression of TL1A in dendritic cells
In addition to being expressed on activated T cells, TL1A expression can be induced in activated dendritic cells by TLR and Fc-receptor signaling (4, 14, 15). To determine whether TL1A expressed by dendritic cells can result in pathological consequences similar to T cell-expressed TL1A, we also produced transgenic mouse lines expressing murine TL1A under the control of the CD11c promoter (16). CD11c-TL1A transgenic mouse lines exhibited a much wider range of mTL1A expression (3 – 65 fold) compared to endogenous cells than did CD2-TL1A transgenic lines (Figure S4A). TL1A expression in the lines expressing the highest levels of TL1A was similar to peak levels of TL1A expression in mouse DC stimulated with LPS or immunoglobulin crosslinking (4). However, when normalized to expression in resting T cells, TL1A mRNA in DC of CD11c transgenic mice was at least an order of magnitude lower than observed on T cells in CD2-TL1A transgenic lines (Figure S4B). In founder lines expressing TL1A on DC more than eight-fold higher than endogenous levels, we also noted inflammatory changes in the small intestine with similar pathological features to that in CD2-TL1A transgenic mice, including goblet cell hyperplasia. Pathological changes were generally milder and more restricted to the ileum than in the CD2-TL1A transgenic mice. Inflammation was accompanied by lengthening of the small intestine and failure to gain weight after weaning with the severity depending on transgene expression level (Figure S4D-F). Mice expressing less than eight-fold more TL1A than wild-type DC (TL1A Lo) had less severe histological changes and no significant increases in small intestinal length or impairment of weight gain after weaning. This suggests that the absolute level, rather than the source of TL1A is the most important factor in triggering intestinal inflammation.
Intestinal pathology in CD2-TL1A transgenic mice is dependent on IL-13 and DR3
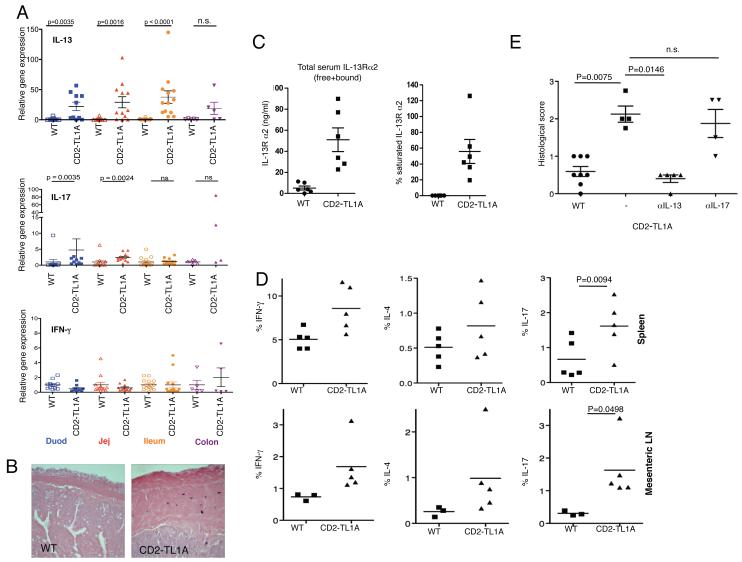
To determine which cytokines are associated with the small intestinal inflammation observed in TL1A-transgenic mice, we performed real-time quantitative RT-PCR on RNA isolated from different sections of the intestine of CD2-TL1A transgenic mice and wild-type littermates between 8 weeks and 6 months of age (Figure 4A). The most striking abnormality was 20- to 50-fold elevation of IL-13 mRNA in tissue from all sections of the small intestine. Elevation in IL-13 was accompanied by increases in IL-5 but not IL-4 mRNA and therefore did not represent a typical Th2 response (data not shown). IL-17 mRNA levels were elevated to a lesser extent than IL-13, and only in the jejunum and duodenum. Expression of IL-22, a member of the IL-10 family that is known to be co-expressed by some IL-17 secreting T cells, and the IL23 receptor, which is expressed on Th17 cells were also not increased above baseline (data not shown). This suggests that either T cells producing IL-17 but not IL-22, or another cell type may be producing IL-17 in the intestine of CD2-TL1A transgenic mice. Strikingly, Interferon-γ expression was not elevated above baseline in any areas of the intestine in TL1A transgenic mice (Figure 4A). IL-13 expression was accompanied by an age-associated accumulation of mast cells in the submucosa and serosal layers of the intestine, seen in other types of intestinal inflammation associated with Th2 responses (Figure 4B). Levels of IL-13Rα2 protein and saturation of IL-13Rα2 with IL-13, which are indicators of IL-13 activity (17), were both elevated in the serum of TL1A transgenic mice (Figure 4C). Taken together, these data identify IL-13 as a prominent cytokine induced by TL1A in biologically significant amounts.
Figure 4. Cytokine expression in CD2-TL1A transgenic mice.
(A) mRNA expression of the indicated cytokines was analyzed from the indicated sections of intestine by quantitative RT-PCR from 8-52 week old line R6 CD2-TL1A Tg mice and age-matched controls. Gene expression was normalized first to β2-microglobulin for each sample and then the average gene expression of the control mice for each region of intestine was normalized to 1.0. Statistical significance of comparisons of Tg vs. WT groups is indicated above (Mann-Whitney test). (B) Mast cell accumulation in the submucosa of tissue sections from CD2-TL1A Tg mice. Toluidine blue staining of ileal sections of a 6 months old mouse line R6 TL1A Tg mouse and an age matched control mouse is shown. (C) Soluble IL-13Rα2 and the saturation of IL13Rα2 in sera from CD2 TL1A transgenic mice (line R6) and controls. (D) Intracellular cytokine staining of T cells isolated from the indicated tissues of 8-10 week old CD2-TL1A Tg or control mice stimulated with PMA and ionomycin. The percentage of CD4+ T cells expressing the indicated cytokine is shown, with the average of each group indicated by the line and significant differences between WT and Tg samples indicated (two tailed Student’s t test). (E) Histological analysis of the ileum sections of intestine in CD2-TL1A transgenic mice treated with 10 mg/kg neutralizing antibodies against IL-13 or IL-17 intraperitoneally weekly for 6-8 weeks beginning at 2 weeks of age. Histological scores of non-transgenic littermates and littermates treated with isotype control antibody are shown (statistical analysis using Mann-Withney test). Anti IL-13 antibody data is representative of two independent experiments/
The increased numbers of T cells in the mLN suggested that there might be increased trafficking of T cells between the intestinal lamina propria and mLN. To determine if this was the case we measured the percentage of T cells expressing the chemokine receptor CCR9, a known marker of T cells that home to the lamina propria (18). CCR9 expression was markedly elevated in the mLN but not in the spleen of CD2-TL1A transgenic mice (Figure S5A). Confirming this idea, the percentage of CD4+ T cells in preparations of LPL from CD2-TL1A transgenic mice was markedly increased compared to controls (Figure S5B). Immunofluorescence confirmed the accumulation of CD4+ lymphocytes in the lamina propria, particularly in the tips of enlarged villi (Figure S5C). To determine what effector T cell subsets are promoted by chronic TL1A stimulation, we isolated T cells from spleen, mesenteric lymph nodes and the small intestinal lamina propria and then measured intracellular cytokine production after stimulation with PMA and ionomycin. We observed increases in IL-17, IL-4 and IFN-γ producing CD4+ T cells in the mLN and spleen of TL1A transgenic mice, with increases in IL-17 producing T cells being the most significant (Figure 4D). In T cells isolated from the lamina propria, the frequency of IL-17, IL-13 and IL-4 producing cells was slightly increased, and IFN-γ producing cells was decreased (Figure S5D). However, since CD4+ T cell yields were increased in all of these tissues from CD2-TL1A transgenic mice, the absolute number of effector T cells is likely to be relatively higher than in controls. TL1A overexpression can therefore promote accumulation of many types of effector T cells in the intestinal lamina propria and mesenteric lymph nodes and to a lesser extent systemically.
To determine whether IL-13 or IL-17 were pathogenic in the small intestinal inflammation driven by TL1A, we treated cohorts of CD2-TL1A transgenic mice with neutralizing antibodies against IL-13 and IL-17 beginning at 2 weeks after birth. Anti-IL-13 was highly effective in reducing inflammatory pathology while neutralizing IL-17 had little effect (Figure 4E), suggesting that IL-13 is a key pathogenic cytokine in the intestinal inflammation induced by chronic TL1A expression.
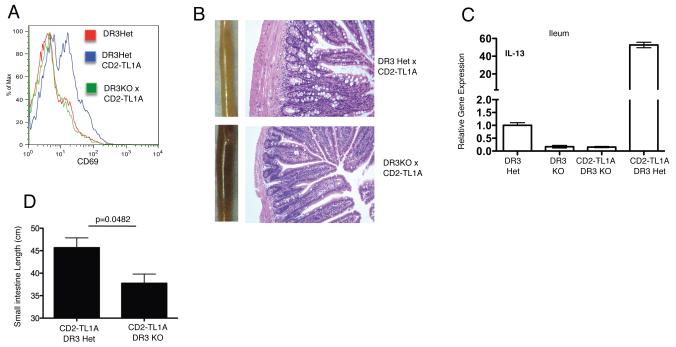
The TNF receptor DR3 is thought to mediate most, but not all, of the biological effects of TL1A (4, 6, 19). To determine which effects of constitutive TL1A expression we observed above are due to stimulation through DR3, we crossed CD2-TL1A transgenic mice to DR3-deficient mice (20). The increased CD69 expression seen in TL1A transgenic mice was ablated on a DR3 deficient background (Figure 5A). The small and large bowel had a normal appearance in TL1ATg x DR3KO mice, and as shown in figure 5B, the gross and microscopic features of the inflammatory pathology in the ileum driven by TL1A were absent on the DR3KO background. IL-13 levels were also reduced to background levels in TL1ATg x DR3KO mice (Figure 5C). In addition, the length of the small intestine from TL1ATg x DR3KO mice was significantly decreased compared to TL1ATg mice (Figure 5D). These results show that DR3 is the primary receptor mediating the effects of TL1A expressed constitutively in transgenic mice.
Figure 5. T cell activation and intestinal inflammation in TL1A transgenic mice depend on DR3.
CD2-TL1A line R6 transgenic mice were crossed to DR3 deficient mice on a B6 background to generate DR3KO-TL1ATg mice. (A) CD69 expression on the surface of TCRb+CD4+ T cells from the spleens of mice of the indicated genotypes of 20-24 week old mice. (B) Gross and histological appearance of representative ileum sections from mice of the indicated genotypes at 3 months of age. (C) Expression of IL-13 mRNA in the ileum of mice of the indicated genotypes at 8 weeks of age. Numbers are the average of triplicate measurements from 1-2 mice each. (D) Small intestine length of 8-20 week old DR3KO-TL1ATg (n=3) and DR3Het CD2-TL1A Tg mice (n=4). Statistical analysis by two-tailed unpaired Student’s t test.
NKT cell and FoxP3+ regulatory T cell function in CD2-TL1A transgenic mice
Inflammation in various models of IBD can result from dysfunction of NKT cells or FoxP3+ regulatory T cells (Treg) (21-24). We used CD1d tetramers loaded with the synthetic glycolipid PBS57 to detect invariant NKT expressing Jα18 reactive against CD1d-restricted glycosphingolipids (25). Interestingly, although in some contexts these cells have been found to be pro-inflammatory, we observed reduced percentages and absolute numbers of iNKT cells in the spleen, liver and lamina propria of TL1A-Tg mice compared to wild-type littermate controls (Figure S6A,B). This was likely due to peripheral depletion because percentages and numbers of iNKT cells in the thymus were less significantly reduced in the same mice. iNKT cells can be positive or negative regulators of immune responses depending on the context (26, 27). Although we cannot definitively rule out a pathogenic role for iNKT in this model until TL1A mice are back-crossed to Jα18 deficient mice, these results suggest that depletion of iNKT is more likely secondary to the ongoing intestinal inflammation as has been seen in human IBD and other diseases involving intestinal inflammation (28, 29).
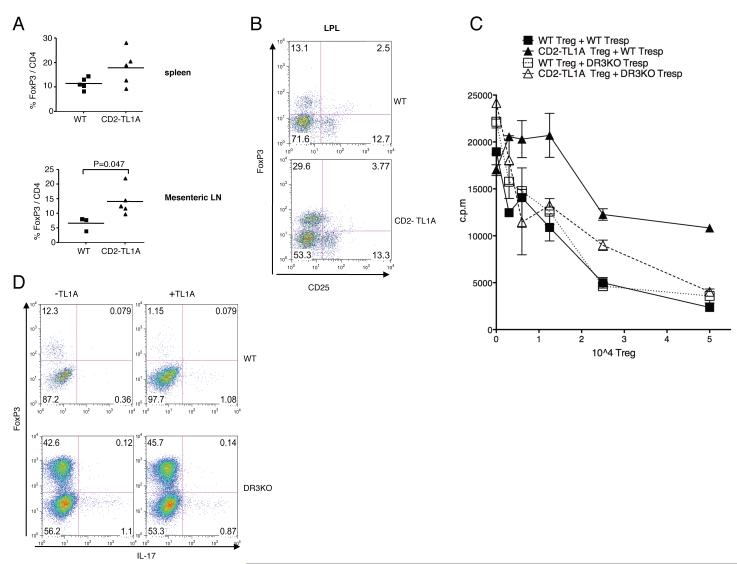
In further studies we focused on Treg numbers and function in CD2-TL1A transgenic mice. We first determined the percentage of FoxP+CD4+ T cells in various tissues of CD2-TL1A transgenic mice. We found that CD4+ T cells from mesenteric lymph nodes and spleen were enriched in FoxP3+CD4+ T cells (Figure 6A). Lamina propria T cells were also enriched in FoxP3 expressing cells, which like Treg from wild-type mice, expressed only low levels of CD25 (Figure 6B). To determine whether Tregs from CD2-TL1A transgenic mice exhibit normal regulatory function, we isolated FoxP3+ CD4+ cells from CD2-TL1A transgenic and control mice crossed to a knock-in mouse line expressing a FoxP3-GFP fusion protein (30). This enabled sorting of live FoxP3+ Treg. Highly purified FACS sorted Treg from TL1A transgenic mice were somewhat less effective than wild-type Treg at suppressing proliferation of wild-type T cells (Figure 6C). When DR3 deficient responder T cells were used in these assays, proliferation was suppressed by TL1A transgenic Treg similarly to WT Treg (Fig 6C). This suggests that TL1A expressed by transgenic Treg in these cultures counteracts the proliferative suppression through the DR3 receptor on Tresp.
Figure 6. Effects of TL1A on the frequency, function and generation of regulatory T cells.
(A) The percentage of FoxP3 positive CD4+ T cells in the indicated tissues of 8-16 week old CD2-TL1A transgenic and control mice. The line represents the average percent expression. Statistical analysis by two-tailed unpaired Student’s t test. (B) FoxP3 and CD25 expression within CD4+ T cells isolated from the small intestinal lamina propria of representative CD2-TL1A transgenic mice and controls. (C) Treg suppressive assay with 5×104 naive C57BL/6 wild-type or DR3 deficient CD4+ T cells mixed with the indicated number of FoxP3+ Treg from WT or TL1A transgenic mice. Proliferation assayed by 3H thymidine incorporation is shown for triplicate. This data is representative of two independent experiments. (D) Polarization of naive CD4+ T cells of C57BL/6 wild-type or DR3 deficient mice towards iTreg in presence of wild-type APCs. Representative of two experiments using DR3KO mice and controls.
Antigen delivered to the small intestine can be presented by specialized dendritic cells to generate antigen-specific Treg in a manner dependent on Transforming Growth factor-β (TGF-β) and retinoic acid (31, 32). Although total Treg numbers are increased in TL1A transgenic mice, TL1A might suppress the production of antigen-specific Treg, which could allow escape of autoreactive T cells from peripheral tolerance. To test this idea, we activated naive FoxP3 negative T cells in the presence of TGF-β, which promotes the generation of inducible Treg (iTreg) with a suppressive phenotype (33) with or without TL1A. TL1A strongly suppressed induction of FoxP3 expressing iTreg from naive precursors (Figure 6D). TL1A did not divert differentiation of T cells into IL-17 producers (Figure 6D). DR3 on T cells was necessary for this effect, as TL1A could not suppress iTreg generation in DR3-deficient T cells. In addition, DR3-deficient T cells differentiated into FoxP3 positive Treg with enhanced efficiency, suggesting that endogenous TL1A-DR3 interactions suppress development of iTreg. These data identify two distinct defects in Treg generation and function in TL1A transgenic mice that may result in inefficient control of the excess effector T cells produced in these mice by Treg.
Role of the T cell repertoire in pathology driven by TL1A
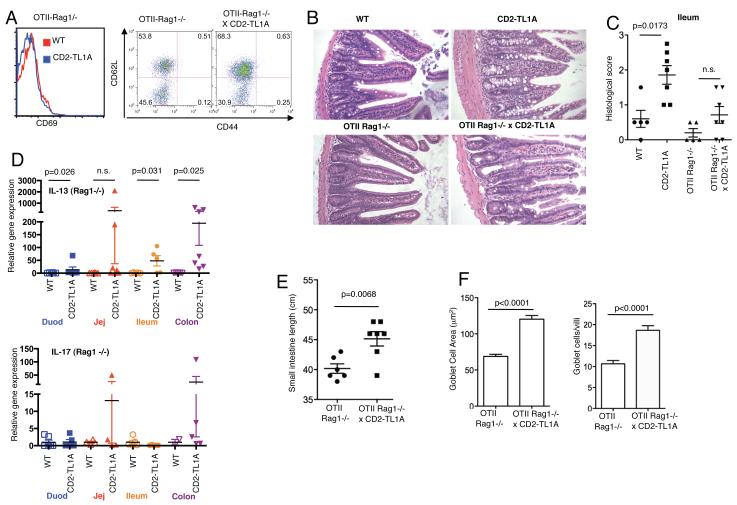
To determine whether the costimulation and intestinal inflammation triggered by TL1A is dependent on T cells capable of recognizing microflora or other environmental mucosal antigens, we restricted the T cell repertoire of TL1A transgenic mice to a monoclonal specificity by back-crossing the R6 CD2-TL1A transgenic line to the OT-II ovalbumin specific TCR transgene on a Recombination Activation Gene-1 (RAG1) deficient background. All the T cells in these mice express the OT-II TCR specific for Ovalbumin, with little or no cross-reactivity to endogenous antigens. On this background, transgenic TL1A expression was not able to increase the percentage of CD69+ T cells or memory T cells, and all T cells in either TL1A transgenic or control mice were of a CD4+ naive phenotype (Figure 7A). Inspection of the small intestine of OT-II Tg-Rag1−/− mice with or without the TL1A transgene revealed a significant amelioration of TL1A-dependent intestinal changes by the OT-II/Rag1−/− background. As shown in the examples of ileal sections in figure 7B, thickening of the submucosa and muscularis and distortion of the villi are partially reversed in OT-II Tg-Rag1−/− mice compared with parental CD2-TL1ATg samples. Analysis of inflammatory changes in ileum histology in a cohort of TL1ATgxOT-II Tg mice on the RAG1 background revealed only non-significant differences from control OT-II Tg-RAG1 deficient mice (Figure 7C). However, the small intestine was still lengthened in the TL1A transgenic mice compared to controls (Figure 7D). Analysis of cytokine mRNA in intestinal tissue from these mice revealed generally lower levels of IL-13 compared to CD2-TL1ATg mice, but remained significantly higher than in OT-II Tg-Rag1−/− controls in jejunum and colon (Figure 7E). Both IL-13 and IL-17 mRNA levels actually increased in the colon compared to TL1A transgenic mice with an intact lymphocyte compartment, which may be due to the absence of Treg in these mice. Quantitation of goblet cell number and size in the ileum of OT-II TCR transgenic mice revealed that there was still significant elevation in the TL1A transgenic samples on the OT-II RAG1−/− background. (Figure 7F). These data suggest that in the absence of recognition of endogenous antigens by the TCR, constitutive expression of TL1A is not able to costimulate T cells, abrogating the accumulation of activated T cells. However, IL-13 production and goblet cell hyperplasia is still stimulated by TL1A in a manner independent of a full TCR repertoire.
Figure 7. Restriction of the T cell repertoire abolishes peripheral T cell hyperactivation but does not prevent intestinal inflammation in the presence of transgenic TL1A.
A) CD69 expression and CD44/CD62L surface expression in splenic CD4+ T cells from 20-24 week old TL1A transgenic and control mice on an OT-II TCR, Rag1−/− background. (B) Representative tissue sections from the ileum of TL1A transgenic and control mice on an OT-II TCR Rag1−/− background, shown with CD2-TL1A Tg and wild-type controls on a C57BL/6 background. (C) Histological scores of ileal sections from a cohort of OT-II TCR Rag1 deficient mice with and without expression of the TL1A transgene and age-matched WT and TL1A transgenic controls from 3-6 months of age. Statistical analysis by Mann-Whitney test. (D) IL-13 and IL-17 mRNA expression in the indicated sections of intestine from TL1A transgenic and control mice on an OT-II TCR Rag1−/− background. Gene expression is normalized to the average of wild-type mice and means and s.e.m. is indicated by the horizontal bars. p values from significant differences using a Mann-Whitney test are given. (E) Small intestinal length from the cohort of mice analyzed in (C). Statistical analysis by two-tailed unpaired Student’s t test. (F) Average goblet cell area and goblet cells per villus calculated from analysis of 5 sections per genotype as in Figure 3. Statistical analysis by two-tailed unpaired Student’s t test.
TNBS colitis depends on TL1A-DR3 interactions
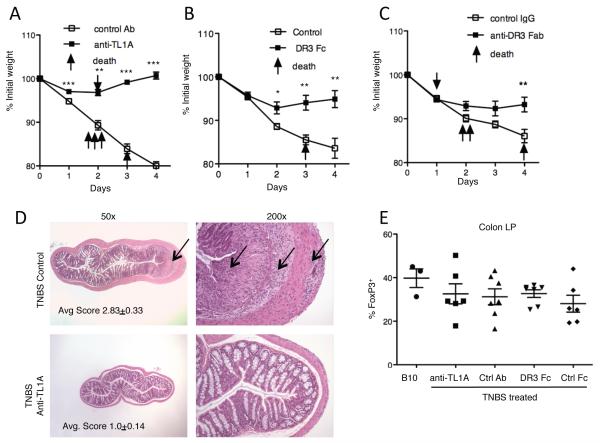
Prior studies have shown that inhibition of TL1A-DR3 interactions by administration of blocking anti-TL1A antibody has a modestly ameliorating effect on dextran sodium sulfate (DSS)-induced colonic inflammation (34). Similarly, as shown here, DR3-deficient mice are modestly protected from this model of intestinal inflammation at early time points after colitis induction (Figure S7). T cell-deficient mice remain susceptible to DSS-colitis, indicating that this model is mainly due to an innate immune response to intestinal flora (35, 36). To determine whether TL1A-DR3 interactions are necessary in an inducible T cell-dependent murine model of colitis, we turned to the TNBS model of colitis, which depends on T cells secreting interferon-γ in the acute phase (13). We induced acute TNBS-colitis in susceptible C57BL/10 mice with and without concomitant i.p. injection of the antagonistic anti-TL1A mAb 5.4G6 (Figure S1), DR3-Fc, or an antagonistic Fab mAb to DR3. Mice treated with anti-TL1A and to a lesser extent with DR3-Fc or anti-DR3 Fab were remarkably protected from the weight loss and mortality in TNBS-induced colitis (Figure 8A-C). Reduced morbidity and mortality correlated with dramatically reduced microscopic inflammation (Figure 8D). This protection from TNBS-colitis did not stem from changes in the local Treg population as the percentage of FoxP3-expressing T cells in the colon was slightly decreased in TNBS colitis but not different in any of the treatment groups (Figure 8E). Taken together these results show a critical role for TL1A-DR3 interactions in this inducible model of colitis that depends on T cells, but is associated with a different anatomical location of inflammation and a contrasting set of cytokines than that seen in the setting of chronic TL1A overexpression.
Figure 8. Dependence of TNBS-induced colitis on TL1A.
(A) Weight loss in a cohort of mice induced to develop TNBS colitis with 20 mg/kg anti-TL1A (open squares, n=10) or control hamster Ig (closed squares, n=10) injected i.p on day −1 and 0. Each point represents the average weight of the cohort. Mice that died before the end of the experiment are indicated with arrows. Anti-TL1A data is representative of two independent experiments with minimun of 8 mice per group. ** indicates p<0.01 and *** indicates p<0.0001 by unpaired two-tailed Student’s t-test. (B) TNBS colitis experiment carried out as in (A) with DR3 Fc (closed squares, n=8) and control Fc (open squares, n=8). * indicates p<0.05 and ** indicates p<0.01 by unpaired two-tailed Student’s t-test. (C) TNBS colitis experiment carried out as in (A) with DR3 Fc (closed squares, n=10) and control Fc (open squares, n=10). ** indicates p<0.01 by unpaired two-tailed Student’s t-test. (D) Representative H&E sections of the colon from mice induced to develop TNBS colitis treated with control or anti-TL1A mAb as in (A). Arrow indicates area of severe inflammation of the control Ab treated mouse. Left panels are 50x, and right panels 200x enlargements of thesame sections. Average pathology scores of the mice in (A) at day 6 after induction of colitis are indicated. (E) FoxP3+ cells within CD4+ T cells isolated from colon lamina propria of mice induced to develop TNBS colitis treated with the indicated reagents.
Discussion
These results establish that constitutive expression of the TNF family cytokine TL1A leads to dose-dependent gastrointestinal inflammation marked by continuous epithelial and goblet cell hyperplasia, thickening of the submucosal layers of the intestine, and lamina propria inflammatory infiltrates. All of these changes are confined to the small bowel and most intense in the terminal ileum. The pathology induced by TL1A differed significantly from murine models of small bowel inflammation associated with overproduction of TNF (37, 38) or LIGHT (39). In TL1A-driven inflammation, IL-13 was uniquely important for the pathogenesis of intestinal inflammation, as shown by significant amelioration of the pathology by treatment with anti-IL13 mAb. While IL-17 production was also modestly increased in mesenteric lymph node and lamina propria cells in CD2-TL1A transgenic mice, the pattern of increased IL-17 expression in various segments of the small intestine did not correlate with areas of maximum inflammation, and blocking IL-17 had little effect on the changes in the small intestine induced by TL1A, suggesting that IL-17 plays a secondary role in the pathogenesis of intestinal inflammation driven by TL1A.
Much of the intestinal pathology observed in TL1A transgenic mice may be attributed to IL-13, since this cytokine can act directly on epithelial cells to cause goblet cell hyperplasia and mucous production both in the lung and intestine (40, 41). Smooth muscle cells can also respond to IL-13 with hypertrophy and increased contractility (42), suggesting that the hypertrophy of the muscularis layer of the small intestine seen in TL1A transgenic mice may also be due to IL-13. The colon may be spared from IL-13 driven pathology because of elevated levels of non-signaling IL-13R2 receptor subunit (43). The lack of IFN-γ production we observed in TL1A transgenic mice may explain the relative longevity of these mice despite the inflammatory pathology observed, since IFN-γ is associated with enterocyte cell death that may lead to more severe clinical consequences (44). Overall, the pathology induced by constitutive TL1A expression bears a striking resemblance to the intestinal response to nematode infections, where IL-13 mediates induction of goblet cell hyperplasia, mucous production and muscular hypertrophy that aids in worm expulsion, but spares the host from lethal inflammation (41, 45, 46). The similarity between the pathology caused by the constitutive expression of TL1A and nematode infection raises the interesting possibility that TL1A might be an important inducer of IL-13 during such infections.
These data establish a novel link between TL1A, acting through its receptor DR3, to induce IL-13 and associated gastrointestinal pathology. Previous studies have shown that TL1A-DR3 interactions can enhance secretion of multiple cytokines, not just those associated with Th2 immune responses. IL-13 may be produced in CD2-TL1A transgenic mice by many cell types, including T cells, invariant NKT cells, intraepithelial intestinal NK cells, mast cells and basophils (41, 47, 48). Since DR3 is mainly expressed on lymphocytes, T cells, NKT cells and NK cells, those cells are among likely cellular sources of IL-13. However, intracellular staining of T cells in the mLN and intestinal lamina propria only revealed small increases in Th2 cells, suggesting that other cell types may contribute to IL-13 production induced by TL1A. NKT cells have been found to produce IL-13 and implicated in IBD, particularly in ulcerative colitis (22). However since these cells are relatively depleted in TL1A transgenic mice, they are less likely to be required. Intra-epithelial NK cells or recently identified novel ‘innate’ lymphocyte subsets in the mesenteric lymph nodes and fat-associated lymphoid clusters that produce IL-13 in the setting of helminth infections (48-50) are other possible sources of IL-13 produced in response to TL1A.
CD4+ T cells are activated and expanded in TL1A transgenic mice and this is abrogated when the T cell repertoire is fixed through crossing these mice to the OT-II TCR transgenic mice on a Rag deficient background. These results strongly suggest that TL1A-driven T cell activation requires a concomitant TCR signal which is provided in vivo by antigens in the bowel lumen or other environmental antigens. Autoreactive T cells appear to be directly or indirectly required for some of the intestinal pathology in TL1A transgenic mice, because of the reduced histological scores in TL1A transgenic mice crossed to the OT-II TCR transgenic mice on a RAG1 deficient background. However, intestinal IL-13 and goblet cell hyperplasia are still present in TL1A transgenic mice on the OT-II/Rag1−/− background, suggesting that the TL1A present on resting T cells is sufficient to drive these pathological changes, likely acting through another cell type present in the small intestine.
Given the important role of Tregs in other models of intestinal inflammation we examined Treg function in CD2-TL1A transgenic mice. We as well as Taraban et al. (51) noted that Tregs are numerically increased in mice constitutively expressing TL1A driven either by CD2 or CD11c promoters. Treg isolated from CD2-TL1A transgenic mice which constitutively produce TL1A are less effective at suppressing proliferation of naive T cells than wild-type Treg, and Taraban et al. (51) show that soluble recombinant TL1A can also partially reverse proliferative suppression by Treg. This suggests that costimulation through DR3 can reverse suppression by Treg, either directly, or indirectly through promoting cytokine production. This is consistent with the known costimulatory effect of TL1A on T cell proliferation which is mediated through DR3. Costimulation by DR3 is associated with increased production of multiple cytokines, including IL-2 (3, 4), and the ability of IL-2 to reverse suppression by Treg (52). Recent studies have shown that orally delivered antigen can induce de novo generation of antigen-specific Treg through antigen presentation by a specialized population of dendritic cells capable of secreting retinoic acid and TGF-β (31, 53). We found that TL1A greatly inhibited TGF-β-induced differentiation of FoxP3+ T cells in vitro from naive T cell precursors. This suggests that peripheral induction of Treg in the gastrointestinal tract of transgenic mice might be defective in the setting of TL1A overexpresion. It is likely that in TL1A transgenic mice, a combination of Treg defects and TL1A-mediated costimulation of effector T cells results in failure of immunological tolerance to mucosal antigens, spontaneous T cell activation and intestinal inflammation.
The efficacy of blocking TL1A-DR3 interactions in the TNBS colitis model suggests that these interactions are necessary for inflammatory bowel disease involving a broader range of cytokines than is seen in TL1A overexpression. Antibody blockade of DR3-TL1A interactions by anti-TL1A, DR3-Fc and an antagonistic anti-DR3 Fab all led to a striking and virtually complete inhibition of TNBS-induced colitis. In the acute phase, TNBS-induced colitis is associated with T cells secreting IFN-γ (54, 55) and can be blocked by antibodies against the p40 common subunit of IL-12 and IL-23 (56). More recently, IL-17 has also been implicated in the pathogenesis of acute TNBS colitis (57). Crohn’s disease has been associated with IL-12 and IL-23, and Ulcerative Colitis with IL-13 (58, 59), and TL1A-DR3 interactions may contribute to inflammatory bowel disease through either of these two pathways. Because no major defects in systemic immunity have thus far been found in DR3 or TL1A deficient mice, treatment of inflammatory bowel disease by blocking TL1A-DR3 interactions may have a favorable therapeutic index.
Methods
Mice
DR3-deficient mice were obtained from Eddie C-Y Wang (University of Cardiff, UK) and FoxP3-GFP mice were obtained from Yasmine Belkaid (NIAID, NIH). C57BL/6 and C57BL/10 mice were purchased from Jackson Laboratories. OT-II TCR transgenic Rag1 deficient mice were obtained from the NIAID Taconic mouse contract facility. TL1A coding sequences were amplified by PCR with primers encoding a 5′ Hemagglutin tag and cloned into the EcoR1 sites of a CD2 promoter/enhancer transgenic cassette vector (12), and a CD11c promoter/enhancer cassette vector (60). Transgenic mice were screened by southern blotting and subsequent generations screened by PCR using the following primers HA forward 5′-CCATACGACGTCCCAGACTACGC-3′ and TL1A reverse 5′-CAGGTGTCTCTCGGCTTGCC-3′. All animals were used under protocols approved by the NIAMS and NIAID ACUC.
Measurement of gene expression by Quantitative RT-PCR
Quantitative RT-PCR was performed with the use of an ABI PRISM 7700 sequence-detection system with qScript One-Step qRT-PCR Kit, Low ROX (Quanta BioSciences, Inc.). Predesigned primer/probe sets were from Applied Biosystems: IFN-γ (Mm00801778_m1), IL-13 (Mm00434204_m1), IL-17 (Mm00439619_m1), β2-microglobulin (Mm00437762_m1), and sequences designed to detect full-length TL1A were forward: 5′-CCCCGGAAAAGACTGTATGC-3′; reverse: 5′-GGTGAGTAAACTTGCTGTGGTGAA-3′; probe: 5′-TCGGGCCATAACAGAAGAGAGATCTGAGC-3′). Each measurement was normalized to expression of β2-microglobulin (delta Ct). 2^-deltaCt was then used as the level of gene expression. Gene expression levels were normalized to the average gene expression in control mice in each experiment.
Detection of free and IL-13-complexed soluble IL-13Rα2
Serum levels of the IL-13/sIL-13Rα2 complex were measured as previously described (17) by ELISA using affinity purified goat anti-mouse Ab (R&D Systems) to capture the complex onto an Immulon2-HB microtiter plate followed by detection with biotin-labeled anti-IL-13mAb (courtesy of Centocor,Inc.). Total levels (free and complexed) of sIL-13Rα2 were detected with the same assay except that rIL-13 (100ng/ml) was added to the serum before performing the assay. The percent saturation of sIL-13Rα2 with IL-13 was determined by dividing the concentration of IL-13/sIL-13Rα2 complex by the total sIL-13Rα2 concentration detected when rIL-13 was added to ‘pull down’ free (non-complexed) sIL-13Rα2.
Antibody treatments and Generation of anti-TL1A, DR3-Fc and anti-DR3-Fab
Anti-TL1A monoclonal antibodies were generated by immunizing Armenian hamsters with recombinant murine TL1A. Antibodies were screened primarily by flow cytometry against 293T cells transfected with a N-terminal GFP-TL1A fusion protein as described in figure S1. The mouse DR3-Fc protein consists of the extracellular domain of mouse DR3 (M1-Q197) that was cloned and fused with human Fc region at C-terminal generated by Human Gemome Sciences. Anti-DR3 Fab was generated by using mouse DR3-Fc to select ScFv from human naive phage display libraries (Cambridge Antibody Technology/MedImmune Cambridge). The specific binders were further screened for their ability to inhibit the binding activity of mouse TL1A to DR3-Fc. The selected clone was sequenced and subcloned into an Fab expression vector where mouse serum albumin sequence was fused to the C-terminal of CH1 to generate mDR3-Fab MSA. The fusion protein was expressed in 293 cells and purified through affinity chromatography by Human Gemome Sciences. Anti IL-17 M210 mAb (mouse IgG2a) was obtained from Dr. Joel Tocker, Amgen, Inc. and anti IL-13 mAb (ratIgG1) was obtained from Centocor/Johnson and Johnson, Inc.
T cell polarization, Flow Cytometry and Measurement of Intracellular Cytokine Expression
For polarization studies, 5×105 T cell-depleted APCs were cultured with 105 naive CD4+ T cells from C57BL/6 mice or DR3-deficient mice. iTreg cell polarization was driven with rhTGF-β (10 ng/ml) (eBioscience), rhIL-2 (100U), in presence of 5ug/ml soluble anti-CD3/anti-CD28. After 3 days of culture, intracellular cytokine staining was performed as described below. All antibodies for flow cytometry were purchased from eBioscience (San Diego, CA) unless indicated otherwise. CD1d/PBS57 tetramers that recognize Vα14 iNKT cells were prepared by the National Institute of Health (NIH) tetramer core facility. Preparation of lamina propria lymphocytes was carried out as previously described (61.) For measurement of intracellular cytokine production, T cells were restimulated either for 5h for splenocytes or mLN or for 2h for LPL with 10 nm phorbol myristate acetate and 1 uM ionomycin in presence of monensin. Cells were fixed in 3% paraformaldheyde, permeabilized in 1% Saponin, and analyzed on a FACS Calibur flow cytometer (Becton Dickinson). For detection of FoxP3 expression, fixation and permeabilization solutions provided by the manufacturer (ebioscience) were used.
Immunofluorescence
Sections of intestine were frozen in OCT solution. Samples were sectioned and stained with anti E-Cadherin-FITC, anti CD4-PECy5, and DAPI followed by examination under a fluorescence microscope.
T cell suppression assay
CD4+CD25− responder T cells (5×104) with irradiated T-depleted APC (5×104) and various concentrations of Treg (FoxP3-GFP+CD4+CD25+) were cultured in presence of 0.125ug anti-CD3. After three days 1μCi of 3H-thymidin was added for the last 16h of culture.
TNBS and DSS induced colitis
TNBS colitis was induced by intra-rectal administration of 2,4,6 trinitrobenzenesulfonic acid (TNBS) in B10 mice as previously described (13). 20 mg/kg anti-TL1A, DR3-Fc protein, anti-DR3 Fab, or control hamster IgG, control Fc protein or control mouse IgG respectively was administered intraperitoneally at day −1 and day 0. Mice were weighed every day for five days and then colon were harvested, cut into 1cm pieces and fixed in 10% neutral buffered formalin (Sigma). Mice losing more than 20% body weight were euthanized according to ACUC guidelines. Samples were embedded, sectioned and stained with H&E. Each section was scored in a blind-fashion according to previously described criteria (13). DSS colitis was induced by feeding mice with 5% (w/v) DSS in their drinking water for 5 days, followed by 2 days consumption of water. Mice were weighed every day for seven days. Inflammatory changes were scored on samples masked to treatment or genotype according to a scoring system adapted from that used in TNBS colitis (13).
Supplementary Material
Acknowledgements
We would like to thank Anthony Cruz for technical assistance, Jin-Moo Lee for providing the TL1A cDNA, Pat Korty and Ethan Shevach for assistance with generating monoclonal antibodies, Lionel Feigenbaum and the NCI transgenic facility for producing the mice, Brian Kelsall and Yasmine Belkaid for reagents and discussions, Eddie C-Y Wang for DR3-deficient mice, Anuk Das for providing the anti-mouse IL-13 mAb, Joel Tocker for providing the anti-mouse IL-17 mAb, Pascal Schneider for ACRP-TL1A fusion protein and Jim Simone and the NIAMS flow cytometry facility for cell sorting. This work was supported by NIAMS intramural research funding and a fellowship to F.M. from the Crohn’s and Colitis Foundation of America.
Footnotes
Disclosure: Thi Migone and Larry Lo are employees of Human Genome Sciences, inc., and owners of HGSI stock
References
- 1.Screaton GR, Xu XN, Olsen AL, Cowper AE, Tan R, McMichael AJ, et al. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci U S A. 1997;94(9):4615–9. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsters SA, Sheridan JP, Donahue CJ, Pitti RM, Gray CL, Goddard AD, et al. Apo-3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-kappa B. Curr Biol. 1996;6(12):1669–76. doi: 10.1016/s0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 3.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002 Mar;16(3):479–92. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 4.Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, et al. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008 Jul 18;29(1):79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappu BP, Borodovsky A, Zheng TS, Yang X, Wu P, Dong X, et al. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008 May 12;205(5):1049–62. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008 May 12;205(5):1037–48. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prehn JL, Mehdizadeh S, Landers CJ, Luo X, Cha SC, Wei P, et al. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004 Jul;112(1):66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Bamias G, Mishina M, Nyce M, Ross WG, Kollias G, Rivera-Nieves J, et al. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006 May 12; doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiebaut R, Kotti S, Jung C, Merlin F, Colombel JF, Lemann M, et al. TNFSF15 polymorphisms are associated with susceptibility to inflammatory bowel disease in a new European cohort. Am J Gastroenterol. 2009 Feb;104(2):384–91. doi: 10.1038/ajg.2008.36. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki K, McGovern D, Ragoussis J, Paolucci M, Butler H, Jewell D, et al. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn’s disease. Hum Mol Genet. 2005 Nov 15;14(22):3499–506. doi: 10.1093/hmg/ddi379. [DOI] [PubMed] [Google Scholar]
- 11.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008 Aug;40(8):955–62. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell- specific expression in transgenic mice. J Immunol Methods. 1995;185(1):133–40. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- 13.Scheiffele F, Fuss I. Induction of TNBS Colitis in Mice. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Wiley-Blackwell; Hoboken, NJ: 2002. [DOI] [PubMed] [Google Scholar]
- 14.Cassatella MA, da Silva GP, Tinazzi I, Facchetti F, Scapini P, Calzetti F, et al. Soluble TNF-like cytokine (TL1A) production by immune complexes stimulated monocytes in rheumatoid arthritis. J Immunol. 2007 Jun 1;178(11):7325–33. doi: 10.4049/jimmunol.178.11.7325. [DOI] [PubMed] [Google Scholar]
- 15.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007 Apr 1;178(7):4033–8. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 16.Brocker T, Riedinger M, Karjalainen K. Driving gene expression specifically in dendritic cells. Adv Exp Med Biol. 1997;417:55–7. doi: 10.1007/978-1-4757-9966-8_9. [DOI] [PubMed] [Google Scholar]
- 17.Khodoun M, Lewis CC, Yang JQ, Orekov T, Potter C, Wynn T, et al. Differences in expression, affinity, and function of soluble (s)IL-4Ralpha and sIL-13Ralpha2 suggest opposite effects on allergic responses. J Immunol. 2007 Nov 15;179(10):6429–38. doi: 10.4049/jimmunol.179.10.6429. [DOI] [PubMed] [Google Scholar]
- 18.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005 Oct 17;202(8):1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Lamki RS, Wang J, Tolkovsky AM, Bradley JA, Griffin JL, Thiru S, et al. TL1A both promotes and protects from renal inflammation and injury. J Am Soc Nephrol. 2008 May;19(5):953–60. doi: 10.1681/ASN.2007060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang EC, Thern A, Denzel A, Kitson J, Farrow SN, Owen MJ. DR3 regulates negative selection during thymocyte development. Mol Cell Biol. 2001 May;21(10):3451–61. doi: 10.1128/MCB.21.10.3451-3461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–38. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 22.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004 May;113(10):1490–7. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006 Aug;25(2):319–29. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Powrie F. Immune regulation in the intestine: a balancing act between effector and regulatory T cell responses. Ann N Y Acad Sci. 2004 Dec;1029:132–41. doi: 10.1196/annals.1309.030. [DOI] [PubMed] [Google Scholar]
- 25.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004 Dec 3;306(5702):1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 26.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007 Nov;28(11):491–6. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Zeissig S, Kaser A, Dougan SK, Nieuwenhuis EE, Blumberg RS. Role of NKT cells in the digestive system. III. Role of NKT cells in intestinal immunity. Am J Physiol Gastrointest Liver Physiol. 2007 Dec;293(6):G1101–5. doi: 10.1152/ajpgi.00342.2007. [DOI] [PubMed] [Google Scholar]
- 28.van der Vliet HJ, von Blomberg BM, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, et al. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001 Aug;100(2):144–8. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 29.Grose RH, Thompson FM, Baxter AG, Pellicci DG, Cummins AG. Deficiency of invariant NK T cells in Crohn’s disease and ulcerative colitis. Dig Dis Sci. 2007 Jun;52(6):1415–22. doi: 10.1007/s10620-006-9261-7. [DOI] [PubMed] [Google Scholar]
- 30.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007 Apr;13(4):423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007 Aug 6;204(8):1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007 Aug 6;204(8):1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004 Nov;4(11):841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 34.Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008 Aug;135(2):552–67. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994 Dec;107(6):1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 36.Hale LP, Cianciolo G. Treatment of experimental colitis in mice with LMP-420, an inhibitor of TNF transcription. J Inflamm (Lond) 2008;5:4. doi: 10.1186/1476-9255-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kontoyiannis D, Pasparakis M, Pizarro T, Cominelli F, Kollias G. Impaired On/Off Regulation of TNF Biosynthesis in Mice Lacking TNF AU-Rich ElementsImplications for Joint and Gut-Associated Immunopathologies. Immunity. 1999 Mar 1;10(3):387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 38.Rivera-Nieves J, Bamias G, Vidrich A, Marini M, Pizarro TT, McDuffie MJ, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003 Apr;124(4):972–82. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 39.Shaikh RB, Santee S, Granger SW, Butrovich K, Cheung T, Kronenberg M, et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001 Dec 1;167(11):6330–7. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 40.Corry DB. IL-13 in allergy: home at last. Curr Opin Immunol. 1999 Dec;11(6):610–4. doi: 10.1016/s0952-7915(99)00025-4. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998 Mar 12;8(6):339–42. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhao A, McDermott J, Urban JF, Jr., Gause W, Madden KB, Yeung KA, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003 Jul 15;171(2):948–54. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 43.Morimoto M, Zhao A, Madden KB, Dawson H, Finkelman FD, Mentink-Kane M, et al. Functional importance of regional differences in localized gene expression of receptors for IL-13 in murine gut. J Immunol. 2006 Jan 1;176(1):491–5. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest. 2008 Mar;118(3):1132–42. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urban JF, Jr., Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998 Feb;8(2):255–64. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 46.Finkelman FD, Wynn TA, Donaldson DD, Urban JF. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr Opin Immunol. 1999 Aug;11(4):420–6. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Sim TC, Alam R. IL-13 released by and localized in human basophils. J Immunol. 1996 Jun 15;156(12):4833–8. [PubMed] [Google Scholar]
- 48.Mcdermott J, Humphreys N, Forman S, Donaldson D, Grencis R. Intraepithelial NK Cell-Derived IL-13 Induces Intestinal Pathology Associated with Nematode Infection. The Journal of Immunology. 2005 Sep 1;175(5):3207. doi: 10.4049/jimmunol.175.5.3207. [DOI] [PubMed] [Google Scholar]
- 49.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010 Jan 28;463(7280):540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 50.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010 doi: 10.1038/nature08900. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taraban V. TL1A costimulates conventional and regulatory T cells and attenuates suppression by regulatory T cells. al. e. submitted.
- 52.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004 Jun 1;172(11):6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 53.Coombes JL, Maloy KJ. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin Immunol. 2007 Apr;19(2):116–26. doi: 10.1016/j.smim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Becker C, Dornhoff H, Neufert C, Fantini MC, Wirtz S, Huebner S, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006 Sep 1;177(5):2760–4. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- 55.Fichtner-Feigl S, Strober W, Geissler EK, Schlitt HJ. Cytokines mediating the induction of chronic colitis and colitis-associated fibrosis. Mucosal Immunol. 2008 Nov;1(Suppl 1):S24–7. doi: 10.1038/mi.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z, Zheng M, Bindas J, Schwarzenberger P. Critical Role of IL-17 Receptor Signaling in Acute TNBS-induced Colitis. Inflammatory Bowel Diseases. 2006 Jan 1; doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 58.Fuss IJ, Becker C, Yang Z, Groden C, Hornung RL, Heller F, et al. Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis. 2006 Jan;12(1):9–15. doi: 10.1097/01.mib.0000194183.92671.b6. [DOI] [PubMed] [Google Scholar]
- 59.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005 Aug;129(2):550–64. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997 Feb 3;185(3):541–50. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer’s patch, and lamina propria cells. Curr Protoc Immunol. 2001 May;:19. doi: 10.1002/0471142735.im0319s17. Chapter 3:Unit 3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.