Abstract
Psychological stress, an evolutionary adaptation to the fight-or-flight response, triggers a number of physiological responses that can be deleterious under some circumstances. Stress signals activate the hypothalamus-pituitary-adrenal (HPA) axis and the sympathetic nervous system. Elements derived from those systems (e.g., cortisol, catecholamines and neuropeptides) can impact the immune system and possible disease states. Skin provides a first line of defense against many environmental insults. A number of investigations have indicated that the skin is especially sensitive to psychological stress, and experimental evidence shows that the cutaneous innate and adaptive immune systems are affected by stressors. For example, psychological stress has been shown to reduce recovery time of the stratum corneum barrier after its removal (innate immunity) and alters antigen presentation by epidermal Langerhans cells (adaptive immunity). Moreover, psychological stress may trigger or exacerbate immune mediated dermatological disorders. Understanding how the activity of the psyche-nervous -immune system axis impinges on skin diseases may facilitate coordinated treatment strategies between dermatologists and psychiatrists. Herein, we will review the roles of the HPA axis and the sympathetic nervous system on the cutaneous immune response. We will selectively highlight how the interplay between psychological stress and the immune system affects atopic dermatitis and psoriasis.
1. Introduction
Psychological stress can trigger the activation of numerous physiological responses, including the endocrine, nervous, and immune systems [1–7]. Nearly 100 years ago, Cannon hypothesized that the release of substances (adrenalin, epinephrine, etc.) by the adrenal medulla during “pain and the major emotions” (fear, rage, and asphyxia) was an evolutionary adaptation for survival [8]. For example, an encounter with a predator induces an acute psychological stress which in turn activates the release of substances from the adrenal medulla. Substances released by the adrenal medulla induce profound physiological changes (increased circulation to the lungs, heart and limbs; increased cardiac vigor and increased sugar content in the blood; cessation of the activities of the alimentary canal) that endow the intended prey to flee or to fight. However, the connotation of emotional distress as an adaptation for survival has dramatically changed for most modern humans. Today, for example, there may be psychological stress due to divorce or unemployment, with the peripheral physiological responses associated with stress being unwanted.
The concept that psychological stress impacts the health of an individual has long been postulated. Accumulating experimental evidence is beginning to delineate how stress can induce or exasperate disease processes. A comprehensive understanding of the mechanisms whereby psychological stress contributes to disease processes may deepen our understanding of the mind-body connection and may provide novel approaches to patient treatment.
The skin constitutes the largest bodily organ and is bombarded daily with environmental insults including infectious and toxic agents, allergens, ultraviolet light, and mechanical damage. Therefore, the skin is equipped with innate and adaptive properties to respond to the myriad of environmental factors encountered. In addition to environmental factors, skin also appears especially responsive to psychological stressors. Indeed, a number of psychodermatologic disorders associated with stress have been reported, including (1) psoriasis, (2) atopic dermatitis, (3) pruritus, (4) alopecia areata, (5) lichen planus, and (6) rosacea [9]. A plausible interprofessional arena between dermatology and psychiatry is elucidated by studies on outpatients in dermatology clinics showing psychiatric morbidity [10, 11]. In fact, cooccurring psychiatric disorders in patients with skin disorders show a prevalence of around 30% [12]. The purpose of this paper is to review the impact of psychological stress on the cutaneous immune response and highlight the potential role of psychological stress in two skin diseases commonly encountered in the clinic: atopic dermatitis and psoriasis.
2. Skin and the Neuroendocrine System
The central hypothalamic-pituitary-adrenal (HPA) axis is activated following stress signals such as 5-hydroxytryptamine [13, 14], acetylcholine [15], and inflammatory cytokines [16, 17]. Stress signals also activate the locus coeruleus (LC) of the brain stem eliciting a sympathetic nervous system response. There exists a positive, reverberatory feedback loop between these two major systems [18]. When the HPA axis is activated, stress hormones are released including corticotropin-releasing hormone (CRH) and arginine vasopressin [19] from the hypothalamus, which induces adrenocorticotropic hormone (ACTH) release from the anterior pituitary [20]. CRH also activates the LC-noradrenergic pathways resulting in norepinephrine secretion by the peripheral sympathetic nervous system and norepinephrine and epinephrine secretion from the adrenal medulla [21]. ACTH regulates secretion of glucocorticoids including cortisol from the adrenal gland [22]. Cortisol negatively regulates CRH production in a feedback loop mechanism [23]. Norepinephrine is a major neurotransmitter released by sympathetic fibers to innervated tissues, including the skin [24–26]. Activation of the sympathetic nervous system also leads to increased production of other factors including catecholamines [27]. A highly schematic overview of the central HPA axis and locus coeruleus/norepinephrine (LC-NE) sympathetic response to stress signals including the downstream effects on the cutaneous immune response is shown in Figure 1.
Figure 1.
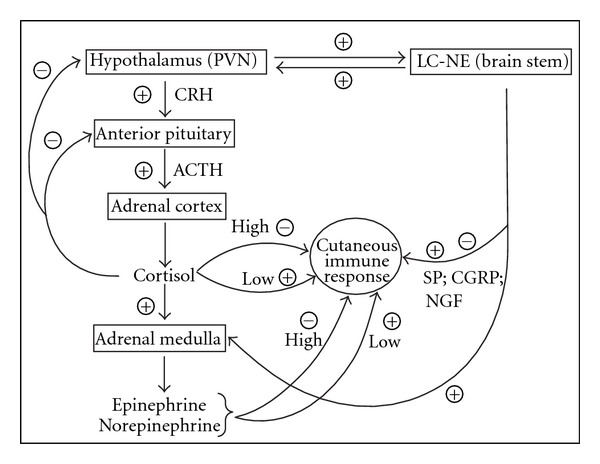
A schematic representation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous interaction with the cutaneous immune system. Stress signals induce release of hormones, including corticotropin-releasing hormone (CRH) from the paraventricular nucleus (PVN) of the hypothalamus. CRH induces adrenocorticotropic hormone (ACTH) release from the anterior pituitary [20]. In turn, ACTH regulates glucocorticoid secretion from the adrenal cortex [22]. Cortisol has several functions including negative feedback of the hypothalamus and anterior pituitary and induces epinephrine and norepinephrine from the adrenal medulla [23]. Glucocorticoids, such as cortisol, as well as epinephrine and norepinephrine may enhance cutaneous immune responses at low concentrations and suppress immune responses at high concentrations [5, 28]. Stress signals also stimulate the locus coeruleus (LC) norepinephrine cells (NE) of the sympathetic nervous system [18]. Neuropeptide products of the sympathetic response (substance P (SP), calcitonin gene-related peptide (CGRP), and cutaneous nerve growth factor (NGF)) have been shown to be proinflammatory and anti-inflammatory dependent on the immune cell type [29–34]. There also exists a positive, reverberatory feedback loop between the HPA axis and LC-NE [18, 21]. Results show that HPA and sympathetic stress responses both modify the cutaneous immune response.
Investigations have shown that human skin expresses CRH as well as CRH receptors (CRH-R). The CRH-R1α isoform is the predominant CRH receptor in skin and is expressed in all major cell populations of epidermis, dermis, and subcutis. By contrast, CRH-R2 is expressed predominately in hair follicles, sebaceous and eccrine glands, muscle and blood vessels [35]. CRH protein is also present in murine skin although CRH mRNA has not been detected [35]. However, both mRNA and protein products for CRH-R1 and 2 have been detected in murine skin [36]. In addition to CRH, human skin also expresses urocortin I [37] and urocortin II mRNA [35]. CRH-R1 binds to urocortin I, but not to urocortin II; while CRH-R2 binds to urocortin II, but not urocortin I [38, 39] leading us to belief that the skin has a depth of responsiveness and interaction to the environment that is little understood. Finally, skin produces the precursor protein, proopiomelanocortin protein (POMC) and POMC derived peptides that give rise to ACTH and other polypeptide products [40, 41].
Ito et al. have shown that human hair follicles can synthesize cortisol and that cortisol synthesis is regulated by endogenous feedback controls [42]. Thus, the skin apparently has a peripheral equivalent of the HPA axis that is fully functional. The peripheral skin HPA axis may coordinate or fine tune peripheral stress responses with the central HPA axis. In addition to expressing components of the HPA axis, skin also produces a number of other neuroendocrine signals including prolactin [43–45], melatonin [46], and catecholamines [47, 48].
In addition to the HPA axis, the skin is highly innervated with sensory nerves that produce neurotrophins and neuropeptides. Sensory nerves derive from the dorsal root ganglion in the skin and C-fibers form the cutaneous sensory nervous system. Psychological stress leads to increased concentrations of cutaneous nerve growth factor (NGF) [29]. NGF has a number of biological activities including (1) axon sprouting of peptidergic and sympathetic neurons, (2) promoting cross-talk between neural cells, glia, and immune cells, and (3) facilitating monocyte/macrophage migration through vascular endothelium [30]. NGF upregulates SP+ nerve fibers in the dermis of stressed mice. Calcitonin gene-related peptide (CGRP), a potent vasodilator, is also upregulated in response to NGF [29]. SP and CGRP have different distributions within the skin with SP nerve fibers detected in the dermis and subcutis and CGRP nerve fibers are in the epidermis around the distal hair follicle and the arrector pili muscle [31].
3. Impact of Psychological Stress on Innate and Adaptive Immunity in the Skin
The innate immune response consists of elements that contribute to the immediate and generic defense of the skin; immunological memory does not develop. By contrast, the adaptive immune response requires time for the development of a specific defense and can create immunological memory. Psychological stress has been shown to impact both innate and adaptive immune responses.
3.1. Innate Immune Responses to Stress
The stratum corneum is terminally differentiated epidermis that forms the outer most layer of the skin. The corneocytes forming the stratum corneum arise from the underlying keratinocytes although unlike their predecessors, corneocytes, lack nuclei and most cell organelles. The intercorneocyte spaces contain high concentrations of nonpolar lipids contributing to the water impermeability of the stratum corneum. The stratum corneum plays an integral role in maintaining tissue hydration, and its mechanical or chemical disruption results in transepidermal water loss. In addition to its role in hydration, the stratum corneum is normally sloughed off, potentially removing skin microorganisms such as potential pathogens. Finally, the stratum corneum contains melanocyte-derived melanin that protects the skin from ultraviolet radiation [49]. Denda et al. evaluated the impact of immobilization stress and crowding stress on the barrier function of the stratum corneum as measured by barrier recovery after its removal by tape stripping or sodium dodecyl sulfate treatment in rats. In that study, immobilization induced stress and crowding stress both significantly delayed barrier recovery for up to 7 days in both male and female mice. Interestingly, the tranquillizers diazepam and chlorpromazine resulted in an increased rate of barrier recovery. Thus, pharmacological reduction of psychological stress promoted stratum corneum formation [50].
Garg et al. evaluated the impact of psychological stress on barrier recovery in humans [51]. Individuals with high levels of perceived psychological stress had significantly delayed barrier recovery rates as compared with those reporting low perceived stress levels. These investigators concluded that stress-induced changes in epidermal function may serve as precipitators of dermatoses. Using SKH-1 mice and stress induced by continuous light and radio noise, Choi et al. found that impaired stratum corneum barrier function could be linked to decreased synthesis of epidermal lipids [52]. Choi et al. hypothesized that increased concentrations of glucocorticoids could result in the epidermal abnormalities observed during psychological stress, including the delay in the stratum corneum barrier recovery [53]. Subsequent treatment of psychologically stressed mice with RU-486 (a glucocorticoid receptor antagonist) or antalarmin (a CRH antagonist that blocks increased glucocorticoid production) returned stratum corneum recovery to normal rates. These results highlight the importance of glucocorticoids induced during psychological stress and stratum corneum homeostasis.
Skin also synthesizes and secretes antimicrobial peptides encapsulated in lamellar bodies. Aberg et al. evaluated the impact of cutaneous Streptococcus pyogenes infections on psychologically stressed mice [54]. Animals stressed by continuous light and radio noise downregulated the antimicrobial peptides (cathelin-related peptide and β-defensin) and developed correspondingly more severe S. pyogenes cutaneous infections as compared with nonstressed control mice. Pharmacological blockade of CRH or glucocorticoid production returned antimicrobial peptides to normal levels and reduced the infection severity. Thus, psychological stress appears to be directly linked to the innate immunity conferred by antimicrobial peptides via the central or peripheral HPA axis.
Mast cells are found throughout connective tissues, including the dermis [55–57]. ACTH and CRH activate mast cells, and human mast cells express CRH receptors [58]. Recent work by Asadi et al. has shown that SP can induce the expression of functional CRH receptor-1 in human mast cells [32]. Acute psychological stress is linked with mast cell activation and the release of IL-6. The finding that serum levels of IL-6 are abrogated in mast cell-deficient mice following restraint stress as compared with their wildtype counterparts underscores the importance of mast cells in the production of systemic IL-6 [59]. Importantly, IL-6 can cross the blood/brain barrier [60] and activate the HPA axis [61]. IL-6 can also induce immune reactions including lymphocyte activation [62, 63] and increased antibody production via CD4+ T-cell help [64]. Systemic effects of IL-6 include induction of fever [65] and acute phase protein production [66, 67].
Mast cells also play a role in neurogenic inflammation. Singh et al. reported that restraint-induced stress resulted in significantly enhanced degranulation of mast cells in mice as compared with their nonstressed counterparts. Pretreatment of mice before stress with CRH antiserum, the neurotensin receptor antagonist SR48692 and capsaicin to deplete sensory neurons were all found to inhibit mast cell degranulation. These results suggested a role for neurogenic inflammation in psychological stress that is in addition to the HPA axis [68]. In fact, a number of investigators have shown that psychological stress activates the mast cell/nerve fiber interface leading to neurogenic inflammation [69, 70]. Shimoda et al. reported that administration of an antipsychotic drug (chlorpromazine) and anxiolytic reagents (tandospirone and CRA1000) significantly reduced degranulation of dermal mast cells in mice stressed by electric foot shock [71]. These results may suggest that antipsychotic and anxiolytic agents may be effective treatments for stress-aggravated inflammatory skin diseases by inhibition of mast-cell degranulation [71].
3.2. Adaptive Immune Responses to Stress
The adaptive immune response requires the interaction of antigen-presenting cells (i.e., dendritic cell) with antigen-specific lymphocytes (i.e., T cells). Activation of lymphocytes requires their complex interplay with antigen presenting cells and co-stimulatory molecules on the surfaces of both cell types as well as the production of cytokines.
Dhabhar and Mcewen investigated the impact of acute stress on contact hypersensitivity (CHS) reactions in rats using stress induced by a 2-hour confinement in a plexiglass box [4]. Briefly, animals were sensitized using 2,4-dinitrofluorobenzene (DNFB), stressed on day 5 following sensitization and challenged on the pinna of the ear on day 6 with ear swelling used as the read-out after the DNFB challenge. Acute stress markedly increased the ear swelling response in stressed rats as compared with the control animals. Elimination of glucocorticoid and epinephrine by adrenalectomy eliminated the stress-induced enhancement, underscoring the importance of these hormones for immunomodulation. Moreover, administration of corticosterone or epinephrine at low doses enhanced stress-induced ear swelling suggesting that these hormones play a role in immunoenhancement. On the other hand, high doses of corticosterone or epinephrine had the opposite effect, that is, ear swelling was reduced. Therefore, the outcome of corticosterone and epinephrine depends on their concentrations. Using a different contact sensitizing reagent (trinitrochlorobenzene) and isolation stress, Nakano also found that stress enhanced the cutaneous immune response as evaluated by ear swelling [72]. However, stress alone did not enhance the ear swelling response of mice treated with the contact irritant, sodium dodecyl sulfate. These results suggested that elements of the adaptive immune response were required for acute stress-induced immune enhancement, as irritants do not develop immunological memory.
In contrast to the results described above, Flint et al. showed that restraint stress prior to DNFB sensitization resulted in suppression of the immune response [73]. Thus, experimental studies have provided seemingly contradictory results. It is, therefore, tempting to speculate that the nature of the sensitizing agent, the dose of the contact sensitizing agent and the timing of the stressor are all variables that are important for the ensuing immune response.
Different strains of mice may have different skin sensitivities to psychological stressors. Flint et al. reported that C57BL/6 mice had blunted ear swelling responses to restraint stress as compared with BALB/c mice [74]. Importantly, ear swelling responses in stressed C57BL/6 strain could not be enhanced even after exogenous corticosterone. The nature of the stressor may also impact the magnitude of the CHS response in animals. Bowers et al. compared CHS responses in mice acutely or chronically stressed by restraint, forced swim, isolation, handling, and low temperature [7]. Restraint stress and forced swim stress resulted in the most dramatic increase in the CHS response as assessed by the ear swelling assay. Taken all together, experimental studies have shown a correlation between acute stress and contact hypersensitivity responses in rodents. However, further investigations are required to delineate conditions under which acute stress suppresses immune responses and under which conditions acute stress enhances immune responses. Findings that the outcomes of acute psychological stress are related to mouse strain also suggest that genetic background may impact the interplay between stress and skin inflammation.
The impact of chronic stress on skin immune responses has also been investigated. Chronic stress has been reported to lead to immunosuppression in a number of systems, including skin graft rejection [75]. In a model of chronic restraint-induced stress, the CHS response was markedly suppressed [3]. By contrast, other studies using chronic restraint-induced stress resulted in enhanced CHS responses [7]. The thyroid axis may also modulate immune responses during chronic stress [76].
Among the factors that may account for psychological stress-induced changes in the adaptive immune response are changes in the numbers, proportions, and distributions of immune cells. Previous studies found that psychological stress markedly decreased the percentages of leukocytes in the blood [1, 2]. Interestingly, administration of corticosterone to adrenalectomized mice closely mirrored the decrease in blood leukocytes observed in stressed animals [2].
In addition to differences in cell numbers and distributions, psychological stress may modulate the activities of immunological cells. For example, stress has been shown to impair lymphocyte function [77]. Stress has also been shown to decrease the density of Langerhans cells in the epidermis in both mice and humans [78, 79]. Langerhans cells are epidermal members of the dendritic cell family of antigen-presenting cells. Conventionally, Langerhans cells have been considered pivotal for the generation of adaptive immunity although current studies suggest that their immunological activities may be considerably more complex (reviewed in [80]). A number of stress related molecules have been shown to impact Langerhans cells and dendritic cells. Hoetzenecker et al. have shown that corticosteroids induce the apoptosis of Langerhans cells and impair their expression of costimulatory molecules [81]. Studies in vitro have shown that epinephrine inhibits antigen presentation in epidermal cell preparations as well as in purified Langerhans cells [82]. Glucocorticoids inhibit dendritic cell production of IL-12 [83, 84]; and IL-12 suppression may skew the TH1/TH2 balance toward TH2 and thus impact the nature of the immune response [85]. Importantly, blockade of β2-AR with the antagonist ICI188, 551 impaired the migration of Langerhans cells to the lymph nodes and blunted the subsequent CHS response when mice were sensitized with the fluorescein isothiocyanate contact sensitizing reagent [82]. In contrast to impaired dendritic cell activities, other stress-related molecules appear to enhance dendritic cell functions. For example, Yanagawa et al. showed that noradrenaline enhanced phosphatidylinositol 3-kinase-induced antigen endocytosis by dendritic cells in vitro [86].
Neuropeptides can also impact the biological activities of antigen-presenting cells. Hosoi et al. showed that CGRP impinged on Langerhans cells in the epidermis and CGRP was directly detected on the surfaces of some Langerhans cells. Moreover, CGRP was found to inhibit the ability of Langerhans cells to present antigen in vitro [33]. Recently, Ding et al. showed that treatment of Langerhans cells with CGRP decreased antigen presentation to a TH1 T-cell clone but increased antigen presentation to a TH2 T-cell clone. Those researchers suggested that exposure of Langerhans cells to nerve-derived CGRP may polarize the immune response to a TH2 type of immunity [87]. Other neuropeptides may also modulate the ability of Langerhans cells to effectively present antigen. Staniek et al. found that SP can bind to human Langerhans cells and impair T-cell proliferative responses in the mixed epidermal-cell lymphocyte reaction. Based on those results the investigators concluded that SP can impair antigen presentation [34].
In summary, psychological stressors and stress-related molecules (e.g., epinephrine, glucocorticoids, and noradrenaline) have been shown to impact various cell behaviors, costimulatory molecule expression and cytokine profiles of immune cells in skin adaptive immune responses, including dendritic cells and lymphocyte immune cell subsets.
4. Psychological Stress and Human Skin Diseases
A number of skin diseases may be preceded or exacerbated by psychological stress. In the following section, we review what is known about the impact of psychological stress on atopic dermatitis and psoriasis. Our focus is on these two skin diseases because they are relatively common skin disorders.
4.1. Atopic Dermatitis
Atopic dermatitis is a chronic inflammatory skin disorder characterized by eczematous lesions and pruritus. It is a common disorder affecting 6% of the population in the USA [88]. Atopic dermatitis may be the result of genetic predisposition and environmental conditions, and no single etiologic agent is known. Analyses of sequential patch-testing skin biopsies have suggested that atopic dermatitis has a biphasic TH1/TH2 T-cell response. Acute inflammation is primarily TH2 with a shift toward TH1 chronification [89, 90]. Psychological stress is known to aggravate atopic dermatitis and a psychological profile that includes anxiety, depression, and excitability has been linked to this disease [91]. Traumatic events, including natural disasters, may increase psychological stress in the population at large, exasperating the incidence of atopic dermatitis symptoms. For example, after the Great Hanshin earthquake in January 1995, subjective distress was found to be the root cause for the enhanced symptoms of atopic dermatitis in the populations of the affected geographic areas [92].
Buske-Kirschbaum et al. analyzed leukocyte subsets, serum IgE levels, and cytokine concentrations in atopic dermatitis patients and nonatopic controls stressed in front of an audience using the Trier Social Stress Test (TSST) [93]. Both groups showed significant elevations in the numbers of serum lymphocytes, monocytes, neutrophils, and basophils with no differences between the two groups. However, eosinophil numbers were significantly higher in atopic dermatitis patients as compared with nonatopic controls. Similarly, IgE levels were significantly greater in the atopic dermatitis patients than their nonatopic counterparts. In both groups, TSST resulted in increased concentrations of IFNγ and a reduction in IL-4 concentrations with no significant differences between the two groups. These studies showed that immunological similarities and differences exist between atopic dermatitis patients and nonatopic individuals subjected to psychological stressors.
Interestingly, patients with atopic dermatitis have been shown to have reduced production of cortisol and ACTH due to experimental TSST stressors as compared with nonatopic controls. By contrast, catecholamine levels were significantly higher in atopic patients as compared with nonatopic controls. Thus, atopic dermatitis patients have blunted HPA axis reactivity as assessed by cortisol and ACTH measurements, but an overactive sympathetic adrenomedullary system as suggested by the high concentrations of catecholamine [94]. Both the HPA axis and the SAM system suppress TH1 activity potentially via IL-12, thus skewing the TH1/TH2 balance toward TH2. Thus, flares in atopic dermatitis following psychological stress may reflect TH2 skewing to acute disease symptoms.
Concentrations of NGF and SP are elevated in in the sera of atopic dermatitis patients. Moreover, NGF and SP concentrations have been positively correlated with disease severity [95, 96]. Recently, Lonne-Rahm et al. compared skin biopsies from patients with atopic dermatitis and chronically stressed atopic dermatitis patients [97]. Cortisol concentrations were used to define which patients were psychologically stressed. In both groups, the CD3+ cell infiltrates expressed the 5-hydroxytryptamine 2A receptor and the serotonin transporter protein. Furthermore, the numbers of mast cells were significantly greater in the skin lesions as compared with uninvolved skin. Likewise, nerve fibers were found in the epidermis and papillary dermis of involved skin as compared to uninvolved skin. In contrast, the number of SP and CGRP positive nerve fibers was not significantly different between involved and noninvolved skin. Nonetheless, chronic stress was correlated with greater numbers of 5-hydroxytryptamine 2A receptor positive cells in the papillary dermis of involved skin. These results showed that atopic dermatitis results in differences in skin innervation and modulation of the serotonin system that also occurs in atopic dermatitis patients during chronic stress.
In summary, atopic dermatitis is a dermatological disorder that is characterized initially as an acute TH2-mediated disease that becomes TH1 polarized with chronicity. Atopic dermatitis seems to worsen in patients that are psychologically stressed, and adult atopic dermatitis patients have a constellation of psychological conditions that may place them at risk for this dermatitis. Finally, it has been reported that atopic dermatitis patients have a blunted HPA response and an overactive sympathetic adrenomedullary system that may exacerbate disease.
4.2. Psoriasis
Approximately 2% of the population in the USA is diagnosed with psoriasis [98]. Most newly diagnosed psoriasis patients are under the age of 30. Psoriatic arthritis, which is potentially debilitating, develops in 10–40% of psoriatics [99, 100].
Psoriasis is a multifactorial disease shaped by genetics and environmental factors that include psychological stress [101]. Currently, it is believed that T cells play a significant role in disease pathogenesis, particularly T cells expressing IL-17. Kryczek et al. have proposed that activated TH1 cells are recruited into the skin and secrete IFNγ. In turn, the IFNγ induces local antigen-presenting cells to secrete IL-1 and IL-23 that promote the expansion and survival of IL-17 expressing CD4+ and CD8+ T cells. Trafficking of IL-17 expressing CD8+ T cells into the epidermis then promotes epidermal hyperplasia [102]. The T-cell-activating antigen(s) remain unknown.
Psychological stressors have been reported to precede the onset of psoriasis in 44% of patients and to initiate recurrent skin flares in 88% of psoriatics [103, 104]. Buske-Kirschbaum et al. have reported psoriatics exposed to the TSST stressor had greater numbers of CD4+ T cells and monocytes in their blood as compared with a nonpsoriatic control group. On the other hand, numbers of CD3+/CD25+ T cells were decreased in psoriatics as compared with nonpsoriatic controls. Psychological stress increased the numbers of CD3+, CD8+, CD16+/CD56+ (i.e., NK cells), and CD3+/HLA-DR leukocytes in the blood, although the differences between psoriatics and nonpsoriatics were insignificant [105]. Schmid-Ott et al. evaluated circulating levels of T cells and NK cells in psoriatics and nonpsoriatic following experimental psychological stress [106]. In contrast to results obtained by Buske-Kirschbaum et al., levels of CD3+ T cells increased significantly only in the blood of psoriatics following psychological stress. Importantly, increased T-cell counts were due to increased numbers of CD8+ and CD3+CLA+ T cells (CLA, cutaneous lymphocyte-associated antigens). Similarly, CLA+ NK cells were increased significantly only in the circulation of psoriatics following psychological stress. Importantly, the CLA molecule is required for the trafficking of the cells to skin.
Interestingly, psoriasis patients who reported stress-exacerbated flares were found to have decreased levels of cortisol and epinephrine [107]. Thus, similar to atopic dermatitis patients, it would appear that the response of the HPA axis is blunted in psoriatics sensitive to psychological stressors. By contrast, Karanikas et al. recently reported that HPA axis reactivity was not correlated with psychopathological and immune parameters in psoriatics [108].
Psychological stress may also enhance neurogenic inflammation in psoriatics. Harvima et al. evaluated involved and uninvolved skin from stressed and nonstressed patients through immunohistochemistry [109]. CGRP and vasoactive intestinal peptide nerve fibers were detected in the papillary dermis of the skin in stressed patients, whereas these nerve fibers were only weakly detected in nonstressed individuals. Moreover, concentrations of neuropeptide degrading enzymes (i.e., chymase) were decreased in stressed patients as compared with the nonstressed psoriatic controls.
In summary, psoriasis is a multifactorial disease with a strong T-cell component. Psychological stress has been shown to trigger disease and exacerbate skin flares in some patients. Experimental psychological stressors have been shown to increase circulating levels of T cells, including T cells expressing the requisite proteins for skin homing (e.g., CLA). Second, the response of the HPA axis may be blunted in psoriasis patients with stress sensitivity. However, discordant results suggest that more studies are needed to determine the role of the HPA axis in psoriasis. Finally, psychological stress may enhance neurogenic inflammation in psoriatics.
5. Patient Treatment at the Intersection of Dermatology and Psychiatry
Psychotropic medications (antipsychotics, lithium, antidepressants, and anticonvulsants) can lead to skin rash and skin allergy as well as severe skin reactions (Stevens-Johnson syndrome for treatment with anticonvulsants). On the other hand, adverse psychiatric effects to dermatological medications and treatments can include depression (e.g., isotretinoin and IFNα treatment) and psychosis (dapsone treatment). Locala has recently reviewed skin diseases caused or exacerbated by psychotropic medications as well as psychiatric adverse effects of dermatologic medications [110].
The apparent psychophysiologic responses of many dermatoses may suggest that treatment programs structured at the dermatology/psychiatry interface may be useful for patient treatment, including programs that incorporate (1) psychotherapy, (2) biofeedback, (3) hypnosis, and (4) cognitive behavioral methods [111–113].
Hypnosis is just one example of psychiatric treatment augmenting dermatological treatments for dermatoses. Shenefelt performed a MEDLINE search that covered the years 1966–1998 using search terms related to hypnosis and skin disease [111]. Results from MEDLINE showed that a wide range of dermatological disorders could be improved using hypnosis as an alternative or complementary therapy for skin disease treatment, including (1) atopic dermatitis, (2) psoriasis, (3) alopecia areata, (4) rosacea, (5) vitiligo, (6) hyperhidrosis, and (7) ichthyosis vulgaris [111]. Other psychiatric treatments may also benefit dermatology patients. For example, one study showed that patients who chose to participate in a cognitive behavioral therapy program reported reduced frequencies and numbers of psoriasis symptoms as long as 6 months after the program ended [114].
6. Summary and Conclusions
The response to psychological stress is hypothesized to be an evolutionary adaptation for the fight-or-flight response. In contrast, for contemporary humans, activation of the HPA axis as a result of psychological stress can result in a number of undesirable physiological responses including the exacerbation of skin diseases. It has been shown that elements of the HPA axis as well as the sympathetic nervous system can modulate the innate and adaptive cutaneous immune responses, and a number of experiments have suggested that psychological stress can impact disease development and progression.
Recent studies have shown that skin has its own HPA axis that may “fine tune” the response of the central HPA axis. The skin is especially sensitive to psychological stressors. Indeed, cooccurring psychiatric disorders are prevalent in patients with skin disorders. Both the innate and adaptive cutaneous immune responses are impacted by psychological stress as demonstrated in a number of experimental studies in both laboratory rodents and humans. Mouse models of contact hypersensitivity strongly suggest that the nature of the sensitizing agent, the dose of the contact sensitizing agent, and the timing of the stressor are all variables that are important for the ensuing immune response. Modulation of the cutaneous immune system by psychological stress most likely affects the course of skin diseases, including atopic dermatitis and psoriasis. Future investigations that explore the interconnections between psychological stress and the cutaneous innate and adaptive immune responses will enhance our understanding of skin immunology and immunological mediated skin diseases, provide unique insight into the mind and body connection, and may lead to new treatment programs that will improve patient care.
Acknowledgment
This work was supported by NIH Grant RO1 AR48840.
References
- 1.Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain, Behavior, and Immunity. 1994;8(1):66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- 2.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution: dynamics and hormonal mechanisms. Journal of Immunology. 1995;154(10):5511–5527. [PubMed] [Google Scholar]
- 3.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain, Behavior, and Immunity. 1997;11(4):286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 4.Dhabhar FS, Mcewen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(3):1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity: the role of stress hormones and leukocyte trafficking. Annals of the New York Academy of Sciences. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- 6.Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. Journal of Investigative Dermatology. 2001;117(2):309–317. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 7.Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain, Behavior, and Immunity. 2008;22(1):105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon WB. The emergency function of the adrenal medulla in pain and the major emotions. American Journal of Physiology. 1914;33(2):356–372. [Google Scholar]
- 9.Jafferany M. Psychodermatology: a guide to understanding common psychocutaneous disorders. Primary Care Companion to the Journal of Clinical Psychiatry. 2007;9(3):203–213. doi: 10.4088/pcc.v09n0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aktan S, Özmen E, Şanli B. Psychiatric disorders in patients attending a dermatology outpatient clinic. Dermatology. 1998;197(3):230–234. doi: 10.1159/000018002. [DOI] [PubMed] [Google Scholar]
- 11.Picardi A, Abeni D, Melchi CF, Puddu P, Pasquini P. Psychiatric morbidity in dermatological outpatients: an issue to be recognized. British Journal of Dermatology. 2000;143(5):983–991. doi: 10.1046/j.1365-2133.2000.03831.x. [DOI] [PubMed] [Google Scholar]
- 12.Shenefelt PD. Psychodermatological disorders: recognition and treatment. International Journal of Dermatology. 2011;50(11):1309–1322. doi: 10.1111/j.1365-4632.2011.05096.x. [DOI] [PubMed] [Google Scholar]
- 13.Tsagarakis S, Navara P, Rees LH, Besser M, Grossman A. Morphine directly modulates the release of stimulated corticotrophin-releasing factor-41 from rat hypothalamus in vitro. Endocrinology. 1989;124(5):2330–2335. doi: 10.1210/endo-124-5-2330. [DOI] [PubMed] [Google Scholar]
- 14.Calogero AE, Bernardini R, Margioris AN, et al. Effects of serotonergic agonists and antagonists on corticotropin-releasing hormone secretion by explanted rat hypothalami. Peptides. 1989;10(1):189–200. doi: 10.1016/0196-9781(89)90096-x. [DOI] [PubMed] [Google Scholar]
- 15.Wei R, Phillips TM, Sternberg EM. Specific up-regulation of CRH or AVP secretion by acetylcholine or lipopolysaccharide in inflammatory susceptible Lewis rat fetal hypothalamic cells. Journal of Neuroimmunology. 2002;131(1-2):31–40. doi: 10.1016/s0165-5728(02)00251-5. [DOI] [PubMed] [Google Scholar]
- 16.Raber J, Sorg O, Horn TFW, et al. Inflammatory cytokines: putative regulators of neuronal and neuro- endocrine function. Brain Research Reviews. 1998;26(2-3):320–326. doi: 10.1016/s0165-0173(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 17.Kariagina A, Romanenko D, Ren SG, Chesnokova V. Hypothalamic-pituitary cytokine network. Endocrinology. 2004;145(1):104–112. doi: 10.1210/en.2003-0669. [DOI] [PubMed] [Google Scholar]
- 18.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacological Reviews. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 19.Bernardini R, Chiarenza A, Kamilaris TC, et al. In vivo and in vitro effects of arginine-vasopressin receptor antagonists on the hypothalamic-pituitary-adrenal axis in the rat. Neuroendocrinology. 1994;60(5):503–508. doi: 10.1159/000126787. [DOI] [PubMed] [Google Scholar]
- 20.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Progress in Neurobiology. 1993;40(5):573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 21.Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatologic Therapy. 2008;21(1):22–31. doi: 10.1111/j.1529-8019.2008.00166.x. [DOI] [PubMed] [Google Scholar]
- 22.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki Y, Yokozeki H, Awad S, et al. Glucocorticoids augment the chemically induced production and gene expression of interleukin-1α through NF-κB and AP-1 activation in murine epidermal cells. Journal of Investigative Dermatology. 2000;115(4):746–752. doi: 10.1046/j.1523-1747.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman BG, Whitmore L. Transmitter release in skin and muscle blood vessels during sympathetic stimulation. The American Journal of Physiology. 1967;212(5):1043–1054. doi: 10.1152/ajplegacy.1967.212.5.1043. [DOI] [PubMed] [Google Scholar]
- 25.Pergola PE, Kellogg DL, Jr., Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. American Journal of Physiology. 1993;265(3):H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg DL., Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. Journal of Applied Physiology. 2006;100(5):1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiological Reviews. 2009;89(2):535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 28.Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain, Behavior, and Immunity. 2009;23(8):1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Joachim RA, Kuhlmei A, Dinh QT, et al. Neuronal plasticity of the “brain-skin connection”: stress-triggered up-regulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathways. Journal of Molecular Medicine. 2007;85(12):1369–1378. doi: 10.1007/s00109-007-0236-8. [DOI] [PubMed] [Google Scholar]
- 30.Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends in Neurosciences. 1996;19(11):514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- 31.Peters EMJ, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R. Hair-cycle-associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. Journal of Investigative Dermatology. 2001;116(2):236–245. doi: 10.1046/j.1523-1747.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 32.Asadi S, Alysandratos KD, Angelidou A, Miniati A, Sismanopoulos N, Vasiadi M. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. Journal of Investigative Dermatology. 2012;132(2):324–329. doi: 10.1038/jid.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosoi J, Murphy GF, Egan CL, et al. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363(6425):159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 34.Staniek V, Misery L, Péguet-Navarro J, et al. Binding and in vitro modulation of human epidermal Langerhans cell functions by substance P. Archives of Dermatological Research. 1997;289(5):285–291. doi: 10.1007/s004030050194. [DOI] [PubMed] [Google Scholar]
- 35.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145(2):941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slominski A, Wortsman J, Pisarchik A, et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB Journal. 2001;15(10):1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 37.Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. Journal of Clinical Endocrinology and Metabolism. 2000;85(2):815–823. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 38.Hsu SY, Hsueh AJW. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nature Medicine. 2001;7(5):605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 39.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends in Endocrinology and Metabolism. 2002;13(10):436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 40.Slominski A, Szczesniewski A, Wortsman J. Liquid chromatography-mass spectrometry detection of corticotropin-releasing hormone and proopiomelanocortin-derived peptides in human skin. Journal of Clinical Endocrinology and Metabolism. 2000;85(10):3582–3588. doi: 10.1210/jcem.85.10.6863. [DOI] [PubMed] [Google Scholar]
- 41.Kono M, Nagata H, Umemura S, Kawana S, Osamura RY. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. The FASEB Journal. 2001;15(12):2297–2299. doi: 10.1096/fj.01-0254fje. [DOI] [PubMed] [Google Scholar]
- 42.Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB Journal. 2005;19(10):1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 43.Foitzik K, Krause K, Nixon AJ, et al. Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen. American Journal of Pathology. 2003;162(5):1611–1621. doi: 10.1016/S0002-9440(10)64295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langan EA, Foitzik-Lau K, Goffin V, Ramot Y, Paus R. Prolactin: an emerging force along the cutaneous-endocrine axis. Trends in Endocrinology and Metabolism. 2010;21(9):569–577. doi: 10.1016/j.tem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Ramot Y, Bíró T, Tiede S, et al. Prolactin—a novel neuroendocrine regulator of human keratin expression in situ. FASEB Journal. 2010;24(6):1768–1779. doi: 10.1096/fj.09-146415. [DOI] [PubMed] [Google Scholar]
- 46.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends in Endocrinology and Metabolism. 2008;19(1):17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Weihe E, Schütz B, Hartschuh W, Anlauf M, Schäfer MK, Eiden LE. Coexpression of cholinergic and noradrenergic phenotypes in human and nonhuman autonomic nervous system. Journal of Comparative Neurology. 2005;492(3):370–379. doi: 10.1002/cne.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schütz B, von Engelhardt J, Gördes M, et al. Sweat gland innervation is pioneered by sympathetic neurons expressing a cholinergic/noradrenergic co-phenotype in the mouse. Neuroscience. 2008;156(2):310–318. doi: 10.1016/j.neuroscience.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks R. The stratum corneum barrier: the final frontier. Journal of Nutrition. 2004;134(8, aupplement):2017S–2021S. doi: 10.1093/jn/134.8.2017S. [DOI] [PubMed] [Google Scholar]
- 50.Denda M, Tsuchiya T, Hosoi J, Koyama J. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. British Journal of Dermatology. 1998;138(5):780–785. doi: 10.1046/j.1365-2133.1998.02213.x. [DOI] [PubMed] [Google Scholar]
- 51.Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Archives of Dermatology. 2001;137(1):53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- 52.Choi EH, Brown BE, Crumrine D, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. Journal of Investigative Dermatology. 2005;124(3):587–595. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- 53.Choi EH, Demerjian M, Crumrine D, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. American Journal of Physiology. 2006;291(6):R1657–R1662. doi: 10.1152/ajpregu.00010.2006. [DOI] [PubMed] [Google Scholar]
- 54.Aberg KM, Radek KA, Choi EH, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. Journal of Clinical Investigation. 2007;117(11):3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graham HT, Lowry OH, Wahl N, Priebat MK. Mast cells as sources of tissue histamine. The Journal of experimental medicine. 1955;102(3):307–318. doi: 10.1084/jem.102.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cowen T, Trigg P, Eady RAJ. Distribution of mast cells in human dermis: development of a mapping technique. British Journal of Dermatology. 1979;100(6):635–640. doi: 10.1111/j.1365-2133.1979.tb08066.x. [DOI] [PubMed] [Google Scholar]
- 57.Eady RAJ, Cowen T, Marshall TF. Mast cell population density, blood vessel density and histamine content in normal human skin. British Journal of Dermatology. 1979;100(6):623–633. doi: 10.1111/j.1365-2133.1979.tb08065.x. [DOI] [PubMed] [Google Scholar]
- 58.Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. Journal of Immunology. 2005;174(12):7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 59.Huang M, Berry J, Kandere K, Lytinas M, Karalis K, Theoharides TC. Mast cell deficient W/WV mice lack stress-induced increase in serum IL-6 levels, as well as in peripheral CRH and vascular permeability, a model of rheumatoid arthritis. International Journal of Immunopathology and Pharmacology. 2002;15(3):249–254. doi: 10.1177/039463200201500314. [DOI] [PubMed] [Google Scholar]
- 60.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neuroscience Letters. 1994;179(1-2):53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 61.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. Journal of Clinical Endocrinology and Metabolism. 1993;77(6):1690–1694. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- 62.Kitani A, Hara M, Hirose T, et al. Autostimulatory effects of IL-6 on excessive B cell differentiation in patients with systemic lupus erythematosus: analysis of IL-6 production and IL-6R expression. Clinical and Experimental Immunology. 1992;88(1):75–83. doi: 10.1111/j.1365-2249.1992.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell- specific stat3-deficient mice. Journal of Immunology. 1998;161(9):4652–4660. [PubMed] [Google Scholar]
- 64.Dienz O, Eaton SM, Bond JP, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. Journal of Experimental Medicine. 2009;206(1):69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kagiwada K, Chida D, Sakatani T, et al. Interleukin (IL)-6, but not IL-1, induction in the brain downstream of cyclooxygenase-2 is essential for the induction of febrile response against peripheral IL-1α . Endocrinology. 2004;145(11):5044–5048. doi: 10.1210/en.2004-0054. [DOI] [PubMed] [Google Scholar]
- 66.Castell JV, Gomez-Lechon MJ, David M, et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Letters. 1989;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 67.Castell JV, Andus T, Kunz D, Heinrich PC. Interleukin-6: the major regulator of acute-phase protein synthesis in man and rat. Annals of the New York Academy of Sciences. 1989;557:87–101. [PubMed] [Google Scholar]
- 68.Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: a link to neurogenic skin disorders. Brain, Behavior, and Immunity. 1999;13(3):225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- 69.Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a “brain-hair follicle axis (BHA)”: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. The FASEB Journal. 2001;15(13):2536–2538. doi: 10.1096/fj.00-0699fje. [DOI] [PubMed] [Google Scholar]
- 70.Pavlovic S, Daniltchenko M, Tobin DJ, et al. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. Journal of Investigative Dermatology. 2008;128(2):434–446. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- 71.Shimoda T, Liang Z, Suzuki H, Kawana S. Inhibitory effects of antipsychotic and anxiolytic agents on stress-induced degranulation of mouse dermal mast cells: experimental dermatology. Clinical and Experimental Dermatology. 2010;35(5):531–536. doi: 10.1111/j.1365-2230.2009.03650.x. [DOI] [PubMed] [Google Scholar]
- 72.Nakano Y. Effect of chronic topical exposure to low-dose noxious chemicals and stress on skin sensitivity in mice. Journal of Occupational Health. 2007;49(6):431–442. doi: 10.1539/joh.49.431. [DOI] [PubMed] [Google Scholar]
- 73.Flint MS, Miller DB, Tinkle SS. Restraint-induced modulation of allergic and irritant contact dermatitis in male and female B6.129 Mice. Brain, Behavior, and Immunity. 2000;14(4):256–269. doi: 10.1006/brbi.2000.0604. [DOI] [PubMed] [Google Scholar]
- 74.Flint MS, Tinkle SS. C57BL/6 mice are resistant to acute restraint modulation of cutaneous hypersensitivity. Toxicological Sciences. 2001;62(2):250–256. doi: 10.1093/toxsci/62.2.250. [DOI] [PubMed] [Google Scholar]
- 75.Wistar R, Jr., Hildemann WH. Effect of stress on skin transplantation immunity in mice. Science. 1960;131(3394):159–160. doi: 10.1126/science.131.3394.159. [DOI] [PubMed] [Google Scholar]
- 76.Cremaschi GA, Gorelik G, Klecha AJ, Lysionek AE, Genaro AM. Chronic stress influences the immune system through the thyroid axis. Life Sciences. 2000;67(26):3171–3179. doi: 10.1016/s0024-3205(00)00909-7. [DOI] [PubMed] [Google Scholar]
- 77.Bartrop RW, Luckhurst E, Lazarus L. Depressed lymphocyte function after bereavement. Lancet. 1977;1(8016):834–836. doi: 10.1016/s0140-6736(77)92780-5. [DOI] [PubMed] [Google Scholar]
- 78.Hosoi J, Tsuchiya T, Denda M, et al. Modification of LC phenotype and suppression of contact hypersensitivity response by stress. Journal of Cutaneous Medicine and Surgery. 1998;3(2):79–84. doi: 10.1177/120347549800300205. [DOI] [PubMed] [Google Scholar]
- 79.Kleyn CE, Schneider L, Saraceno R, et al. The effects of acute social stress on epidermal Langerhans’ cell frequency and expression of cutaneous neuropeptides. Journal of Investigative Dermatology. 2008;128(5):1273–1279. doi: 10.1038/sj.jid.5701144. [DOI] [PubMed] [Google Scholar]
- 80.Clausen BE, Kel JM. Langerhans cells: critical regulators of skin immunity. Immunology and Cell Biology. 2010;88(4):351–360. doi: 10.1038/icb.2010.40. [DOI] [PubMed] [Google Scholar]
- 81.Hoetzenecker W, Meingassner JG, Ecker R, Stingl G, Stuetz A, Elbe-Bürger A. Corticosteroids but not pimecrolimus affect viability, maturation and immune function of murine epidermal langerhans cells. Journal of Investigative Dermatology. 2004;122(3):673–684. doi: 10.1111/j.0022-202X.2004.22324.x. [DOI] [PubMed] [Google Scholar]
- 82.Seiffert K, Hosoi J, Torii H, et al. Catecholamines inhibit the antigen-presenting capability of epidermal langerhans cells. Journal of Immunology. 2002;168(12):6128–6135. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- 83.Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proceedings of the Association of American Physicians. 1996;108(5):374–381. [PubMed] [Google Scholar]
- 84.Panina-Bordignon P, Mazzeo D, Di Lucia P, et al. β2-agonists prevent Th1 development by selective inhibition of interleukin 12. Journal of Clinical Investigation. 1997;100(6):1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seiffert K, Granstein RD. Neuroendocrine regulation of skin dendritic cells. Annals of the New York Academy of Sciences. 2006;1088:195–206. doi: 10.1196/annals.1366.011. [DOI] [PubMed] [Google Scholar]
- 86.Yanagawa Y, Matsumoto M, Togashi H. Enhanced dendritic cell antigen uptake via α2 adrenoceptor-mediated PI3K activation following brief exposure to noradrenaline. Journal of Immunology. 2010;185(10):5762–5768. doi: 10.4049/jimmunol.1001899. [DOI] [PubMed] [Google Scholar]
- 87.Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases langerhans cells toward Th2-type immunity. Journal of Immunology. 2008;181(9):6020–6026. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanifin JM, Reed ML, Drake LA, et al. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18(2):82–91. doi: 10.2310/6620.2007.06034. [DOI] [PubMed] [Google Scholar]
- 89.Thepen T, Langeveld-Wildschut EG, Bihari IC, et al. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial T(H2) response to a T(H1) response in situ: an immunocytochemical study. Journal of Allergy and Clinical Immunology. 1996;97(3):828–837. doi: 10.1016/s0091-6749(96)80161-8. [DOI] [PubMed] [Google Scholar]
- 90.Grewe M, Bruijnzeel-Koomen CAFM, Schöpf E, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunology Today. 1998;19(8):359–361. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 91.Hashizume H, Takigawa M. Anxiety in allergy and atopic dermatitis. Current Opinion in Allergy and Clinical Immunology. 2006;6(5):335–339. doi: 10.1097/01.all.0000244793.03239.40. [DOI] [PubMed] [Google Scholar]
- 92.Kodama A, Horikawa T, Suzuki T, et al. Effect of stress on atopic dermatitis: investigation in patients after the Great Hanshin Earthquake. Journal of Allergy and Clinical Immunology. 1999;104(1):173–176. doi: 10.1016/s0091-6749(99)70130-2. [DOI] [PubMed] [Google Scholar]
- 93.Buske-Kirschbaum A, Gierens A, Höllig H, Hellhammer DH. Stress-induced immunomodulation is altered in patients with atopic dermatitis. Journal of Neuroimmunology. 2002;129(1-2):161–167. doi: 10.1016/s0165-5728(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 94.Buske-Kirschbaum A, Geiben A, Höllig H, Morschhäuser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. Journal of Clinical Endocrinology and Metabolism. 2002;87(9):4245–4251. doi: 10.1210/jc.2001-010872. [DOI] [PubMed] [Google Scholar]
- 95.Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. British Journal of Dermatology. 2002;147(1):71–79. doi: 10.1046/j.1365-2133.2002.04803.x. [DOI] [PubMed] [Google Scholar]
- 96.Wang IJ, Hsieh WS, Guo YL, et al. Neuro-mediators as predictors of paediatric atopic dermatitis. Clinical and Experimental Allergy. 2008;38(8):1302–1308. doi: 10.1111/j.1365-2222.2008.03026.x. [DOI] [PubMed] [Google Scholar]
- 97.Lonne-Rahm SB, Rickberg H, El-Nour H, Mårin P, Azmitia EC, Nordlind K. Neuroimmune mechanisms in patients with atopic dermatitis during chronic stress. Journal of the European Academy of Dermatology and Venereology. 2008;22(1):11–18. doi: 10.1111/j.1468-3083.2007.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sander HM, Morris LF, Phillips CM, Harrison PE, Menter A. The annual cost of psoriasis. Journal of the American Academy of Dermatology. 1993;28(3):422–425. doi: 10.1016/0190-9622(93)70062-x. [DOI] [PubMed] [Google Scholar]
- 99.Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. Journal of the American Academy of Dermatology. 2005;53(4):573–577. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 100.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Archives of Dermatology. 2005;141(12):1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 101.Elder JT, Bruce AT, Gudjonsson JE, et al. Molecular dissection of psoriasis: integrating genetics and biology. Journal of Investigative Dermatology. 2010;130(5):1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 102.Kryczek I, Bruce AT, Gudjonsson JE, et al. Induction of IL-17+ T cell trafficking and development by IFN-γ: mechanism and pathological relevance in psoriasis. Journal of Immunology. 2008;181(7):4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al’Abadie MS, Kent GG, Gawkrodger DJ. The relationship between stress and the onset and exacerbation of psoriasis and other skin conditions. British Journal of Dermatology. 1994;130(2):199–203. doi: 10.1111/j.1365-2133.1994.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 104.Griffiths CEM, Richards HL. Psychological influences in psoriasis. Clinical and Experimental Dermatology. 2001;26(4):338–342. doi: 10.1046/j.1365-2230.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- 105.Buske-Kirschbaum A, Kern S, Ebrecht M, Hellhammer DH. Altered distribution of leukocyte subsets and cytokine production in response to acute psychosocial stress in patients with psoriasis vulgaris. Brain, Behavior, and Immunity. 2007;21(1):92–99. doi: 10.1016/j.bbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 106.Schmid-Ott G, Jaeger B, Boehm T, et al. Immunological effects of stress in psoriasis. British Journal of Dermatology. 2009;160(4):782–785. doi: 10.1111/j.1365-2133.2008.09013.x. [DOI] [PubMed] [Google Scholar]
- 107.Buske-Kirschbaum A, Ebrecht M, Kern S, Hellhammer DH. Endocrine stress responses in TH1-mediated chronic inflammatory skin disease (psoriasis vulgaris)—do they parallel stress-induced endocrine changes in TH2-mediated inflammatory dermatoses (atopic dermatitis)? Psychoneuroendocrinology. 2006;31(4):439–446. doi: 10.1016/j.psyneuen.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 108.Karanikas E, Harsoulis F, Giouzepas I, Griveas I, Chrisomallis F. Neuroendocrine stimulatory tests of hypothalamus-pituitary-adrenal axis in psoriasis and correlative implications with psychopathological and immune parameters. Journal of Dermatology. 2009;36(1):35–44. doi: 10.1111/j.1346-8138.2008.00583.x. [DOI] [PubMed] [Google Scholar]
- 109.Harvima IT, Viinamaki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychotherapy and Psychosomatics. 1993;60(3-4):168–176. doi: 10.1159/000288690. [DOI] [PubMed] [Google Scholar]
- 110.Locala JA. Current concepts in psychodermatology. Current Psychiatry Reports. 2009;11(3):211–218. doi: 10.1007/s11920-009-0033-x. [DOI] [PubMed] [Google Scholar]
- 111.Shenefelt PD. Hypnosis in dermatology. Archives of Dermatology. 2000;136(3):393–399. doi: 10.1001/archderm.136.3.393. [DOI] [PubMed] [Google Scholar]
- 112.Shenefelt PD. Biofeedback, cognitive-behavioral methods, and hypnosis in dermatology: is it all in your mind? Dermatologic Therapy. 2003;16(2):114–122. doi: 10.1046/j.1529-8019.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- 113.Heller MM, Lee ES, Koo JY. Stress as an influencing factor in psoriasis. Skin Therapy Letter. 2011;16(5):1–4. [PubMed] [Google Scholar]
- 114.Fortune DG, Richards HL, Griffiths CEM, Main CJ. Targeting cognitive-behaviour therapy to patient’s implicit model of psoriasis: results from a patient preference controlled trial. British Journal of Clinical Psychology. 2004;43(1):65–82. doi: 10.1348/014466504772812977. [DOI] [PubMed] [Google Scholar]


