Abstract
Serum resistin was initially hypothesized as a link between obesity and insulin resistance in mice. The latest evidence suggests that serum resistin is proinflammatory cytokines. Inflammation plays a key role in the pathogenesis of type 2 diabetes mellitus (T2DM). Many reports have previously identified changed serum resistin levels in patients with T2DM, but little is known of the levels of resistin in saliva. In our study, saliva and serum samples were collected from 38 patients with newly diagnosed T2DM at each time point of OGTT and 35 nondiabetic controls at fasting state. Resistin concentrations were measured using ELISA. We have demonstrated the presence of resistin in saliva of T2DM and nondiabetic subjects. Saliva resistin levels of T2DM are significantly higher than those of nondiabetic controls. Resistin levels in saliva are not affected by eating activity and correlated with serum resistin levels at any time points of OGTT. A positive correlation of serum and salivary resistin with BMI and HOMA-IR existed in T2DM. Measurement of resistin in saliva is a simple, noninvasive and may be an acceptable alternative to blood sampling for evaluatinginflammation/obesity/insulin resistance state.
1. Introduction
Resistin is peptide hormone produced by adipocytes and macrophages. It was originally proposed as the link between obesity and diabetes in mice [1]. In addition, resistin was found to be an in vitro antagonist of insulin on human preadipocytes [2]. Human hepatic cells overexpressing resistin had impaired glucose uptake and glycogen synthesis [3]. The latest evidence suggests that resistin is proinflammatory cytokines [4]. It was positively correlated with proinflammatory factors in adults with pathophysiological conditions such as atherosclerosis, renal disease, inflammation of respiratory tracts, and type 2 diabetes mellitus [5–8].
Human saliva mirrors the body's health and can be collected noninvasively, does not require specialized skills and is suitable for large population-based screening programs [9]. Cortisol levels in saliva at 0:00 are detected to diagnose Cushing's syndrome. Salivary endothelin concentrations are assessed chronic heart failure [10, 11]. Currently, it is reported that adipokines, such as adiponectin, leptin, resistin, and visfatin, can be detected in saliva of healthy subjects [12–14]. To the best of our knowledge, no data on saliva resistin levels in T2DM patients are available at present. The aim of this study was to measure and compare the saliva resistin and serum resistin levels in newly diagnostic T2DM patients and to evaluate whether the saliva and serum levels are correlated. Salivary resistin may be used as a tool to evaluate insulin resistance and inflammatory state for T2DM patients.
2. Materials and Methods
2.1. Subjects
From June to November 2011, 38 patients (18 males/20 females) with newly diagnosed T2DM and 35 cases (18 males/17 females) of non-diabetes mellitus, who underwent a medical examination at the First Hospital of Shanxi Medical University, Health Screening Center, enrolled in this study. Our local Research Ethics Committee approved the studies, and written informed consent was received from all participants. T2DM group and non-diabetes mellitus control group were matched in male/female ratio and mean age.
It is noted that, before our medical examination, none of these participants suffered from endocrine malignant or chronic inflammatory diseases or severe systemic illnesses or any recent weight change. None were taking medications including oral contraceptives. In the medical examination process, T2DM was defined according to American Diabetes Association set in 2010. That is, A diagnostic cut point of ≥126 mg/dL (7.0 mmol/L) for FPG and ≥200 mg/dL (11.1 mmol/L) for 2 h PG [15].
2.2. Measurements
2.2.1. Anthropometric Measurements
We designated a person to anthropometric measurements. Body weight was measured to an accuracy of ±0.2 kg with a standard scale, dressed only with very light clothing, and height was measured to an accuracy of ±0.5 cm using a height bar fixed on the wall, with subjects standing with back, buttocks, and heels pressed together against the wall. Waist circumference was measured to the nearest 0.1 cm at the narrowest part of the torso as seen from the anterior aspect. Hip circumference was measured to the nearest 0.1 cm at the point of maximum extension of the buttocks. Blood pressure was measured twice using a standard mercury manometer, with the participants seated. BMI was computed as weight (kg) divided by squared height (m2). Waist-hip ratio (WHR) was computed as waist circumference (cm) divided by hip circumference (cm).
2.2.2. Samples Collection
Whole blood and saliva were obtained before and one, two, and three hours after intake of 75 g glucose in T2DM patients. Only fasting serum and saliva samples were obtained in non-diabetes mellitus. Blood sugar was timely detected at each time point. We also detected HbA1c and liver and kidney function at the fasting state. For serum true insulin and resistin assay, the extracted blood samples were then centrifuged at 1100 g for 10 min. Serum was separated immediately and stored at −80°C until assay was performed. All saliva samples collected by using S-Monovette (Sarstedt, Germany) in 10min were used for this study after being dentally assessed and rinsing their mouths with water thoroughly. Immediately after collection, saliva samples were centrifuged for 10 min at 1100 g, and the supernatant serum was stored at −80°C until analysis of resistin level. Blood and saliva sampling were taken simultaneously but saliva sampling took 5 min much time to collect. Salivary flow rate [16] was computed as volume (mL) divided by time (min). Blood and saliva sampling were measured in the same experimental set.
2.2.3. Sample Detection Methods
Insulin was measured by monoclonal antibody-based sandwich enzyme-linked immunosorbent assay (ELISA), which was developed in the Key Laboratory of Endocrinology, Peking Union Medical College Hospital. The assay had a detection limit of 0.8 mIU/L, covered a working range of 1.5~160.0 mIU/L, and did not cross-react with high dosage of human proinsulin (2000 pmol/L) and C peptide (5000 pmol/L). The mean recovery was 101.7% and the intraassay coefficients of variation and interassay coefficients of variation were below 4.1% and 7.0%, respectively [17].
Glucose concentrations were determined by using the glucose-oxidase-derived technique. Insulin resistance index was calculated by homeostasis model assessment of insulin resistance (HOMA-IR) as (fasting insulin mIU/L) × (fasting glucose mmol/L)/22.5 [18].
Resistin was determined by enzyme immunoassay (EK-028-36, Phoenix Pharmaceuticals Inc., USA). The standards used contained 0, 0.016, 0.031, 0.062, 0.125, 0.25, 0.5, or 1 ng/mL. Intra- and interassay coefficients of variation were below 3.0% and 10%, respectively. Serum samples were diluted with assay buffer at 1 : 20 and salivary samples at 1 : 5 [19].
2.3. Statistical Analysis
Descriptive statistics and analysis were performed in SPSS 13.0 for Windows (SPSS Inc. IL). Before statistical analysis, normal distribution was tested. Results are displayed as mean values ± standard deviation. Data of nonnormal distribution were described using the median (M) and quartile (Q1~Q4). The relationships between variables were analyzed by unpaired t-test, repeated measures ANOVA, and simple correlation (Pearson's test). Correlations with a critical value of P < 0.05 were considered significantly.
3. Results
3.1. Comparative Analysis
The difference of age, sex distribution, blood pressure, and fasting salivary flow rate between newly diagnosed T2DM group and control group was not statistically significant (P > 0.05). BMI, WHR, HbA1c, fasting glucose, fasting insulin levels, HOMA-IR, serum and salivary resistin levels of T2DM patients were higher than those of nondiabetes mellitus (P < 0.05) (Table 1, Figure 1).
Table 1.
The general information and laboratory data of the new diagnosed type 2 diabetic patients and non-diabetes mellitus.
| Control | T2DM | |
|---|---|---|
| Participants (M/F) | 35 (18/17) | 38 (18/20) |
| Age (years) | 43 ± 13 | 45 ± 10 |
| Body mass index (kg/m2) | 23.9 ± 3.3 | 25.5 ± 4.9 |
| Waist circumference (cm) | 83.1 ± 6.0 | 92.2 ± 5.1 |
| Systolic pressure (mmHg) | 125.1 ± 12.4 | 138.0 ± 18.3 |
| Diastolic pressure (mmHg) | 78.3 ± 8.2 | 86.1 ± 9.3 |
| Fasting plasma glucose (mM) | 6.1 ± 0.9 | 7.4 ± 1.7* |
| HbA1c (%) | 6.0 ± 0.6 | 7.1 ± 0.6* |
| Fasting insulin (mIU/L) | 5.7 ± 2.8 | 9.2 ± 6.2* |
| HOMA-IR | 3.3 (2.5~4.6) | 5.5 (3.6~6.7)* |
| Fasting serum resistin (ng/mL) | 6.1 ± 0.6 | 12.3 ± 2.7* |
| Fasting saliva resistin (ng/mL) | 1.5 ± 0.3# | 3.4 ± 0.4∗# |
| Fasting saliva flow rate (mL/min) | 2.2 ± 0.8 | 2.4 ± 1.1 |
Results are displayed as mean values ± standard deviation. Data of nonnormal distribution were described using the median (M) and quartile (Q1~Q4).
*P < 0.05: comparison of resistin level between T2DM group and non-diabetes mellitus group.
# P < 0.01: comparison between serum resistin level and saliva resistin level.
Figure 1.
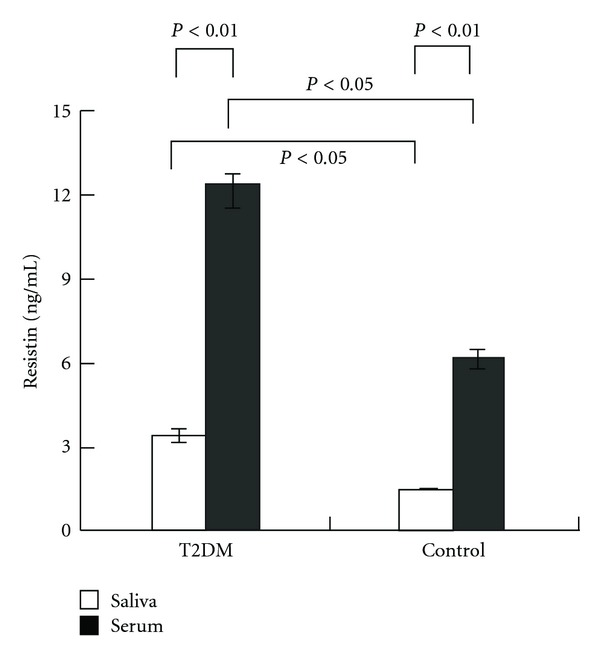
Fasting serum and salivary resistin levels in patients with T2DM and the control.
Either in diabetic patients or nondiabetes mellitus, resistin can be detected in saliva. Both in control group and T2DM group, resistin levels in serum were significantly higher than those in saliva (P < 0.01) (Table 1, Figure 1).
In newly diagnosed T2DM group, salivary flow rate, resistin levels in serum and saliva were no significant difference at time points of OGTT. The results suggest that resistin levels in serum and saliva are not affected by eating activity. However, resistin levels in serum were significantly higher than those in saliva (P < 0.01) at each time point of OGTT (Table 2).
Table 2.
The general information and laboratory data of the new diagnosed diabetic patients at each time point of OGTT.
| OGTT time (hours) | 0 | 1 | 2 | 3 | P value |
|---|---|---|---|---|---|
| Participants (M/F) | 38 (18/20) | 38 (18/20) | 38 (18/20) | 38 (18/20) | |
| Plasma glucose (mM) | 7.4 ± 1.7 | 11.8 ± 3.7 | 17.5 ± 4.6 | 10.3 ± 2.7 | <0.01 |
| Insulin levels (mIU/L) | 9.2 ± 6.2 | 17.3 ± 10.4 | 40.2 ± 26.1 | 27.5 ± 16.7 | <0.01 |
| Saliva flow rate (mL/min) | 2.4 ± 1.1 | 2.5 ± 0.9 | 2.6 ± 1.0 | 2.5 ± 1.2 | >0.05 |
| Serum resistin (ng/mL) | 12.3 ± 2.7 | 11.9 ± 4.6 | 12.4 ± 5.1 | 11.4 ± 4.1 | >0.05 |
| Saliva resistin (ng/mL) | 3.4 ± 0.4# | 3.1 ± 0.7# | 3.8 ± 1.1# | 3.9 ± 0.8# | >0.05 |
Results are displayed as mean values ± standard deviation.
# P < 0.01: comparison between serum resistin level and saliva resistin level at each time point of OGTT.
3.2. Correlation Analysis
In newly diagnosed T2DM group, salivary and serum resistin levels were positively correlated at each time point of OGTT. In control group, salivary and serum resistin levels were positively correlated too. When we merged two sets of data for correlation analysis, this conclusion still holds (Table 3).
Table 3.
Correlation analysis between serum resistin and salivary resistin levels at each time point of OGTT in T2DM group or fasting state in control group and merged group.
| OGTT 0 hour (T2DM) |
OGTT 1 hour (T2DM) |
OGTT 2 hours (T2DM) |
OGTT 3 hours (T2DM) |
Fasting state (control) |
Fasting state (all enrolled people) |
|
|---|---|---|---|---|---|---|
| r | 0.407 | 0.222 | 0.487 | 0.338 | 0.233 | 0.198 |
| P | 0.000 | 0.039 | 0.000 | 0.015 | 0.024 | 0.032 |
We also observed a positive correlation of serum and salivary resistin with BMI and HOMA-IR in T2DM group or control group or merged group (Table 4).
Table 4.
Correlation coefficients between serum and salivary resistin levels and anthropometric features values.
| Number | Age | Body mass index | Waist circumference | Systolic pressure | Diastolic pressure | Fasting plasma glucose | Fasting insulin | HbA1c | HOMA-IR | |
|---|---|---|---|---|---|---|---|---|---|---|
| T2DM | 38 | |||||||||
| Serum resistin | 0.11 | 0.48* | 0.01 | 0.41* | 0.18 | 0.08 | 0.13 | 0.22* | 0.09 | |
| Salivary resistin | 0.08 | 0.39* | 0.13 | 0.12 | 0.07 | 0.14 | 0.09 | 0.31* | 0.20* | |
| Control | 35 | |||||||||
| Serum resistin | 0.09 | 0.38* | 0.09 | 0.18 | 0.35* | 0.37* | 0.21 | 0.13 | 0.34* | |
| Salivary resistin | 0.12 | 0.17 | 0.07 | 0.01 | 0.21 | 0.28* | 0.13 | 0.17 | 0.19 | |
| Merged group | 73 | |||||||||
| Serum resistin | 0.07 | 0.29* | 0.11 | 0.24* | 0.30* | 0.22 | 0.41* | 0.13 | 0.35* | |
| Salivary resistin | 0.07 | 0.33* | 0.08 | 0.22* | 0.19 | 0.30* | 0.02 | 0.06 | 0.29* |
*P < 0.05.
4. Discussion
Recently, more and more articles report the detection of biomarkers in saliva [20, 21]. Toda et al. suggested salivary adiponectin as a marker of increased risk of noninsulin-dependent diabetes mellitus or cardiovascular disease [22]. Mirco et al. found that salivary leptin is a candidate diagnostic marker in salivary gland tumors [23]. Mamali et al. introduced a method of determining resistin in saliva along with a significant association with serum resistin levels in healthy people [14]. For the first time, we have demonstrated the level of resistin in saliva of newly diagnostic T2DM patients. Saliva resistin levels of T2DM patients are significantly higher than those of non-diabetes mellitus. Resistin levels in saliva are not affected by eating activity (i.e., oral glucose load) and correlated with serum resistin levels at any time point of OGTT. Measurement of resistin in saliva is noninvasive, simple, and easy to multipoint dynamic observation and thus may be an acceptable alternative to serum sampling [24]. Therefore, it could contribute to the elucidation of the physiology and pathological role of resistin in T2DM.
Adipocytokine resistin is a member of the newly discovered family of cysteine-rich protein. Recent data suggest that macrophages are a major source of human resistin. Given the obesity-insulin resistance-inflammation link and convergence of adipocyte and macrophage function, resistin may provide unique insight into links between obesity, inflammation, and metabolic syndrome risk in humans. However, available data regarding the relationship of serum resistin with BMI and insulin resistance are conflicting. Specifically, AI-Sari et al. confirmed a positive correlation of serum resistin levels with BMI [25]. Tokuyama et al. [26] and Fujinami et al. [27] reported that serum resistin levels in diabetic patients were significantly higher than control, and this change was negatively correlated with insulin sensitivity. Our study observed a positive correlation of serum and salivary resistin with BMI and HOMA-IR in T2DM group or control group or merged group (considered T2DM group and control together). This is consistent with the previous findings. Therefore, resistin levels in saliva may be used as a tool to evaluate inflammation/obesity/insulin resistance state for T2DM patients. However, in Mamali et al. research [14], no association of serum and salivary resistin levels with either BMI or insulin sensitivity was reported. They also elaborated the possible reasons of discrepancy, such as the sample size of each study, different hormonal determination methods, and lack of adjustment to the effect of additional variables. Further studies should be pursued in future investigations.
Source of resistin in saliva is not clear to date. Marchetti et al. [28] found that diabetes increased salivary gland basement membrane permeability, allowing serum proteins to saliva by ultrafiltration. However, Carda et al. [29] found that the parotid acinar and interstitial tissue of T2DM patients were rich in lipids, which suggested adipokines in saliva of these people may be secreted by fat cells in the salivary glands. Boström et al. [30] found that the levels of resistin were upregulated locally in the salivary glands and corresponded to the intensity of lymphocytic inflammation in patients with Sjögren's syndrome, which suggested resistin is expressed in the salivary glands of those patients and may be a driving factor of local inflammation. In our study, we found that serum resistin levels were significantly higher than salivary resistin levels of T2DM patients, and the saliva and serum levels are correlated. Furthermore, the fluctuating trend of resistin levels in saliva and blood during OGTT is consistent. Therefore, the source of saliva resistin in newly diagnostic T2DM is mainly derived from blood resistin by ultrafiltration.
5. Conclusions
To sum up, this is the first study to show the analysis of salivary resistin in T2DM. Resistin levels in saliva may be used as a tool to evaluate inflammation/obesity/insulin resistance state for T2DM patients.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
This paper was supported by grants from Key Laboratory of Endocrinology Ministry of Health and Peking Union Medical College Hospital, New Star Project of Beijing Science and Technology (2004A027), National Natural Science Foundation of China (30872165), and National Clinical Key Specialty Construction Projects.
References
- 1.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. Journal of Biological Chemistry. 2001;276(14):11252–11256. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 3.Sheng CH, Di J, Jin Y, et al. Resistin is expressed in human hepatocytes and induces insulin resistance. Endocrine. 2008;33(2):135–143. doi: 10.1007/s12020-008-9065-y. [DOI] [PubMed] [Google Scholar]
- 4.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. Journal of Immunology. 2005;174(9):5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 5.Harsch IA, Koebnick C, Wallaschofski H, et al. Resistin levels in patients with obstructive sleep apnoea syndrome—the link to subclinical inflammation? Medical Science Monitor. 2004;10(9):CR510–CR515. [PubMed] [Google Scholar]
- 6.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111(7):932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 7.Axelsson J, Bergsten A, Qureshi AR, et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney International. 2006;69(3):596–604. doi: 10.1038/sj.ki.5000089. [DOI] [PubMed] [Google Scholar]
- 8.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27(10):2450–2457. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 9.Topkas E, Keith P, Dimeski G, Cooper-White J, Punyadeera C. Evaluation of saliva collection devices for the analysis of proteins. Clinica Chimica Acta. 2012;413(13-14):1066–1070. doi: 10.1016/j.cca.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Rachel D, Alex TA, Paul M, et al. Salivery endothelin concentrations in theassessment of chronic heart failure. The Lancet. 2000;5:468–469. [Google Scholar]
- 11.Whitson PA, Chen YM. Saliveryendothelin and its response to postural changes inhumans. Acta Physiologica Scandinavica. 1997;160:291–292. doi: 10.1111/j.1365-201X.1997.00999.x. [DOI] [PubMed] [Google Scholar]
- 12.Randeva HS, Karteris E, Lewandowski KC, Sailesh S, O’Hare P, Hillhouse EW. Circadian rhythmicity of salivary leptin in healthy subjects. Molecular Genetics and Metabolism. 2003;78(3):229–235. doi: 10.1016/s1096-7192(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 13.Toda M, Tsukinoki R, Morimoto K. Measurement of salivary adiponectin levels. Acta Diabetologica. 2007;44(1):20–22. doi: 10.1007/s00592-007-0236-8. [DOI] [PubMed] [Google Scholar]
- 14.Mamali I, Roupas ND, Armeni AK, Theodoropouloua A, Markoua KB, Georgopoulos NA. Measurement of salivary resistin, visfatin and adiponectin levels. Peptides. 2011;33(1):120–124. doi: 10.1016/j.peptides.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Gavin JR, III, Alberti KGMM, Davidson MB, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 16.Fontana M, Zunt S, Eckert GJ, Zero D. A screening test for unstimulated salivary flow measurement. Operative Dentistry. 2005;30(1):3–8. [PubMed] [Google Scholar]
- 17.Li M, Wu CY, Song AL, et al. Development and preliminary application of enzyme-linked immunosorbent assay for human net insulin in serum. Chinese Journal of Endocrinology and Metabolism. 1997;13:215–217. [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Fisette A, Zhao XY, Deng JY, Mi J, Cianflone K. Serum resistincorrelates with central obesity but weakly with insulin resistance in Chinese children and adolescents. International Journal of Obesity. 2009;33(4):424–439. doi: 10.1038/ijo.2009.44. [DOI] [PubMed] [Google Scholar]
- 20.Frank B, Sabine W, Robert P, et al. Weight loss and changes in salivary ghrelin and adiponectin: comparison between sleeve gastrectomy and Roux-en-Y gastric bypass and gastric banding. Obesity Surgery. 2011;21(5):616–624. doi: 10.1007/s11695-011-0374-5. [DOI] [PubMed] [Google Scholar]
- 21.Suleyman A, Ihsan H, Ibrahim H, et al. A comparison of leptin and ghrelin levels in plasma and saliva of young healthy subjects. Peptides. 2005;26(4):647–652. doi: 10.1016/j.peptides.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Toda M, Tsukinoki R, Morimoto K. Measurement of salivary adiponectin levels. Acta Diabetologica. 2007;44(1):20–22. doi: 10.1007/s00592-007-0236-8. [DOI] [PubMed] [Google Scholar]
- 23.Mirco S, Olaf W, Michael G, Renate S, Heinrich I, Johannes Z. Salivary leptin as a candidate diagnostic marker in salivary gland tumors. Clinical Chemistry. 2009;55(5):914–922. doi: 10.1373/clinchem.2008.116939. [DOI] [PubMed] [Google Scholar]
- 24.Gröschl M. The physiological role of hormones in saliva. BioEssays. 2009;31(8):843–852. doi: 10.1002/bies.200900013. [DOI] [PubMed] [Google Scholar]
- 25.Al-Sari IE, Al-Quobaili FA, Kabalan YM. Serum resistin levels in Syrian obese patients with diabetes mellitus type II. Saudi Medical Journal. 2007;28(12):1890–1894. [PubMed] [Google Scholar]
- 26.Tokuyama Y, Osawa H, Ishizuka T, et al. Serum resistin level is associated with insulin sensitivity in Japanese patients with type 2 diabetes mellitus. Metabolism. 2007;56(5):693–698. doi: 10.1016/j.metabol.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Fujinami A, Obayashi H, Ohta K, et al. Enzyme-linked immunosorbent assay for circulating human resistin: resistin concentrations in normal subjects and patients with type 2 diabetes. Clinica Chimica Acta. 2004;339(1-2):57–63. doi: 10.1016/j.cccn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti P, Giannarelli R, Masoni A, Cecchetti P, Di Carlo A, Navalesi R. Salivary immunoreactive insulin concentrations are related to plasma free-insulin levels in insulin-treated diabetic patients. Diabete et Metabolisme. 1990;16(1):16–20. [PubMed] [Google Scholar]
- 29.Carda C, Mosquera-LIoreda N, Salom L, et al. Structeral and functional salivary disorders in type 2 diabetic patients. Medicina Oral, Patologia Oral Y Cirugia Bucal. 2006;11(4):E309–E314. [PubMed] [Google Scholar]
- 30.Boström E, D’Elia HF, Dahlgren ULF, et al. Salivary resistin reflects local inflammation in Sjögren’s syndrome. Journal of Rheumatology. 2008;35(10):2005–2011. [PubMed] [Google Scholar]


