Summary
Experiments employing guinea pig heart Langendorff preparations compared the coronary vasoactivity of a functionalized congener of adenosine, 2-[(2-amino-ethylaminocarbonylethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine, APEC, with the vasoactivity of the product of the reaction of APEC with 1,4-phenylenediisothiocyanate, 4-isothiocyanatophenylaminothiocarbonyl-APEC (DITC-APEC). Previous experiments showed that whereas APEC binds reversibly to the A2A adenosine receptor of brain striatum, DITC-APEC binds irreversibly. APEC caused concentration-dependent coronary vasodilation that persisted unchanged when agonist administration continued for up to 165 min, but promptly faded when the agent was withdrawn. The unselective adenosine receptor antagonist 8-(4-sulfophenyl)theophyline (8-SPT) antagonized the vasoactivity of APEC. By contrast, DITC-APEC (0.125 – 1.0 nM) caused progressive, concentration-independent vasodilation that persisted unchanged for as long as 120 min after the agent was stopped and that was insensitive to antagonism by subsequently applied 8-SPT. However, perfusion of the heart with buffer containing 0.1 mM 8-SPT strongly antagonized the coronary vasodilatory action of DITC-APEC given subsequently. Such observations indicate that the covalent binding of DITC-APEC causes irreversible activation of the guinea pig coronary artery A2A adenosine receptor. Neither APEC nor DITC-APEC appeared to desensitize the coronary adenosine receptor during two or more hours of exposure to either agonist.
Keywords: A2-adenosine receptor, Irreversible agonist, Coronary vasodilation, Functionalized congener
Introduction
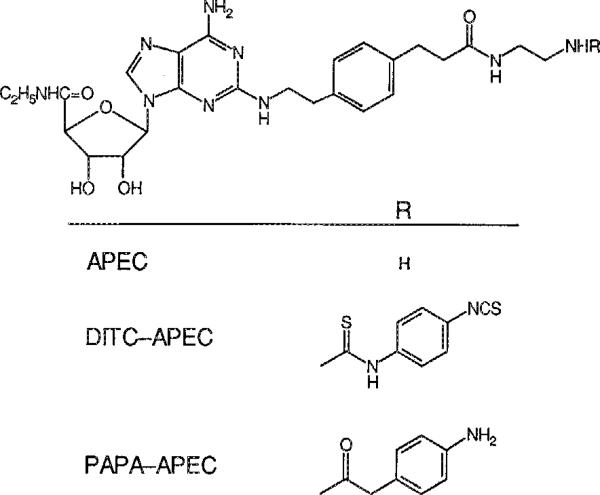
The heart contains at least four types of adenosine receptors. Studies employing adenosine analogues highly selective for A1 and A2A receptors (A1AR and A2AAR respectively) show that an A1AR adenosine receptor coupled to a muscarinic K+ channel mediates the negative chronotropic, dromotropic and atrial inotropic actions of adenosine and an A1AR coupled to adenylate cyclase mediates the anti-adrenergic action of this nucleoside (Belardinelli et al. 1989). The pharmacologic profile of receptor-selective analogues suggests that an A2AAR adenosine receptor mediates coronary vasodilation (Kusachi et al. 1983; Hamilton et al. 1987; Mustafa and Askar 1985). The heart also contains the messenger RNA of the recently described A3AR (Zhou et al 1992); the physiological role of this receptor is unknown. Pig coronary artery smooth muscle contains an adenosine receptor coupled to a K+ channel (Cornfield et al. 1992). A distinctive pharmacological profile is the basis for designating that receptor the A4AR. All of the analogues selective for the A1AR are N6-substituted adenosines, and most, but not all, of the analogues selective for the A2AAR are 2-substituted adenosines (Trivedi et al. 1990). The latter category includes 2-[(2-aminoethylaminocarbonylethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine, APEC, a very potent and highly selective agonist at the A2AAR in rat brain striatum membranes (Jacobsen et al. 1989). The reaction of APEC with the bifunctional reagent 1,4-phenylenediisothiocyanate, present in excess to minimize its reaction with two molecules of APEC, results in the attachment of a 4-isothiocyanatophenylthioureido residue at the terminus of the C-2 substituent (Fig. 1) (Jacobson et al. 1992). That product, DITC-APEC, contains a highly reactive arylisothiocyanato group that binds irreversibly to the A2AR in rabbit striatum membranes.
Fig. 1.
Structural formulae of APEC, DITC-APEC and PAPA-APEC, adenosine analogues mentioned in the text
Although the covalent binding of an agonist to a receptor will abolish the ability of that receptor to subsequently bind radioligands, the loss of binding activity does not necessarily imply a loss of biological activity. For example, photoaffinity labeling of the rat adipocyte A1AR with 2-azido-N6-[1-(4-hydroxyphenyl)-2R-propyl]adenosine, 2-AHPIA, fully and irreversibly activated the receptor, as shown by persistent suppression of agonist-induced activation of adenylate cyclase (Lohse et al. 1986). However, the binding of 2-AHPIA to the A2AR on platelets inactivated that receptor (Lohse et al. 1991). Photoaffinity labeling of 90% of the receptors caused a 50% reduction of the stimulation of adenylate cyclase by 5′-N-ethylcarboxamidoadenosine (NECA) without affecting stimulation by agonists such as PGE1.
Because the azido group is immediately adjacent to the purine base of 2-AHPIA and the isothiocyanato group of DITC-APEC is at the terminus of a long C-2 substituent, the two functional groups probably react with different parts of the A2AR that are at some distance from each other. Accordingly, one cannot use the outcome of experiments with 2-AHPIA to predict whether DITC-APEC will activate or inactivate an A2AR. Here we present evidence that DITC-APEC irreversibly activates an A2AR that mediates coronary vasodilation.
Materials and methods
Bioassays employed a guinea pig heart Langendorff preparation perfused with a buffer composed of (mM): NaCl 120, NaHCO3 27, KCl 3.7, KH2PO4 1.3, MgSO4 0.64, CaCl2 1.3, Na pyruvate 2 and glucose 5. The buffer was gassed with 5% CO2 – 95% O2, equilibrated at 37°C in a heat exchanger and delivered at a perfusion pressure equivalent to 55 mmHg. Ligation of the venae cavae and cannulation of the pulmonary artery permitted timed collections of total coronary venous drainage, a measure of coronary flow. Two measures attempted to keep cardiac work low and constant, thereby minimizing changes in the “metabolic” component of coronary resistance. Those were (a) left atrial pacing at a constant rate of 250/min and (b) the drainage of buffer that entered the cavity of the left ventricle via Thebesian veins by means of a catheter inserted across the mitral valve. A 30-min stabilization period preceded experimental observations.
APEC (Jacobson et al. 1989), DITC-APEC (Jacobson et al. 1992) and 8-SPT (Daly et al. 1984) were synthesized as described. APEC was used as the free amine, 8-SPT was the potassium salt. A stock solution of APEC in DMSO was diluted immediately prior to use with perfusion buffer to give concentrations of 0.1 and 10 μM. Immediately before use a stock solution of DITC-APEC in anhydrous DMSO was diluted in perfusion buffer to a concentration of 0.1 μM. The agonists and antagonists were infused into ports in the coronary perfusion line at rates that did not exceed 600 μL/min. For studies of antagonism the coronary perfusate contained 0.1 mM 8-SPT.
Experiments in four preparations assayed the coronary vasoactivity of APEC. After a 30-min period to allow the preparation to stabilize, solutions of APEC in perfusion buffer (initially 0.1 μM and, for higher perfusate concentrations, 10 μM) were infused via a port in the aortic cannula at rates that increased stepwise, but did not exceed 600 mL/min; that infusion protocol ultimately caused maximum coronary vasodilation. Each assay examined at least 15 concentrations of agonist. We made coronary flow measurements during the stable phase of the response to each rate of agonist administration. The quotient of the rate of APEC administration (mole/min) divided by coronary flow rate (L/min) provided an estimate of the concentration of APEC (M) in the coronary perfusate. Logit transformation of the coronary flow data and solution of the regression of logit (flow) on log [APEC] for logit = 0 calculated the EC50 of APEC.
Experiments employing two additional preparations assessed the persistence of the coronary vasodilation produced by APEC. Agonist infusion at a rate calculated to give a concentration of 1 nM in the coronary perfusate continued until flow stabilized at a near-maximum rate, whereupon APEC infusion was stopped. Coronary flow measurements in the absence of agonist administration continued over the subsequent 45 or 60 min.
Experiments assessing the possibility of agonist-induced desensitization by APEC employed two additional hearts and consisted of coronary flow measurements during an infusion of 1.0 μM APEC at 0.1 mL/min for 135 or 165 min.
Experiments assessing the vasoactivity of DITC-APEC employed continuous infusions at rates calculated, on the basis of control coronary flow rates, to give initial concentrations of 0.125, 0.25, 0.5 or 1 nM (2 preparations each). When coronary flow approached maximum, the administration of agonist was stopped and an infusion of 10 mM 8-SPT commenced at a rate (~150 μL/min) adjusted to give a concentration of 0.1 mM in the coronary perfusate. In a search for evidence of agonist-induced desensitization, the measurements of coronary flow in some hearts continued for up to an additional 2 h. Three additional experiments assessed the effect of the prior equilibration of the heart with 0.1 mM 8-SPT on the coronary vasoactivity of 0.5 nM DITC-APEC administered subsequently. In those experiments, perfusion with buffer containing 8-SPT began 20 min before and continued throughout the administration of DITC-APEC.
Results
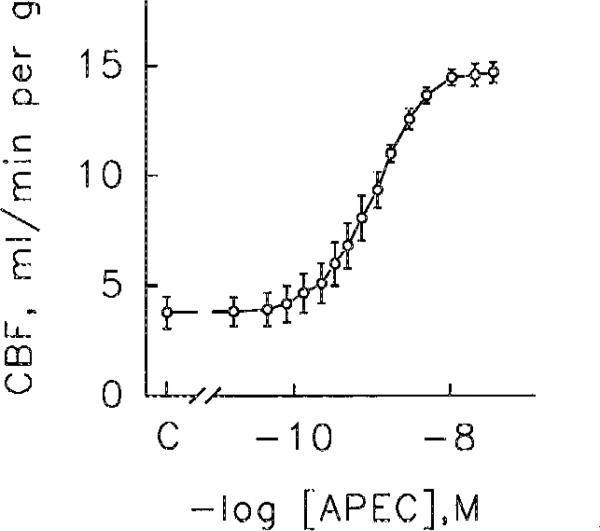
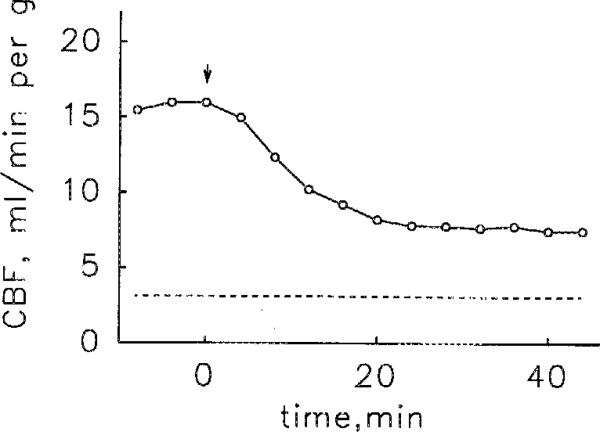
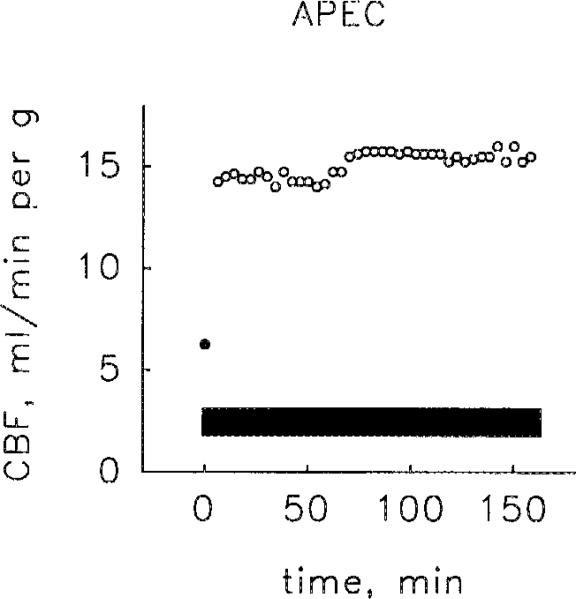
APEC produced concentration-dependent coronary vasodilation; the geometric mean of the EC50s was 0.6 nM (range 0.4–1.3 nM, 4 hearts). When data points from the four hearts were combined, the resulting concentration-response curve (Fig. 2) had an EC50 of 0.8 nM. APEC appeared to be a full agonist, causing coronary vasodilation equal to that produced by a supramaximum dose of adenosine. Stopping the administration of APEC caused coronary flow to fall, rapidly for about 20 min and more slowly thereafter (Fig. 3). Perfusion with 0.1 mM 8-SPT antagonized the vasoactivity APEC (data not shown). The sustained administration APEC for 135 or 165 min caused stable coronary vasodilation (Fig. 4).
Fig. 2.
Coronary flow (CBF) response to graded doses of APEC. Each datum of the composite dose-response curve represents the average of observations on 4 hearts. The EC50 of this composite curve is 0.8 nM. Vertical bars represent±1 SEM. For clarity the horizontal bars representing the SEM of each average concentration have been omitted
Fig. 3.
Reversibility of the coronary flow (CBF) response to APEC infused at a rate giving a concentration in the coronary perfusate of 1 nM. The data are from one heart but are representative of the results in a second heart. The arrow indicates the end of APEC infusion and the dashed horizontal line the control coronary flow
Fig. 4.
Sustained coronary vasodilation produced by APEC infused at a rate giving a concentration of 0.7 nM. Note that coronary flow (CBF) is stable throughout the entire period of agonist administration (solid bar), which lasted for 2 h and 45 min. This experiment is typical of two such experiments. The closed circle (●) represents control flow rate
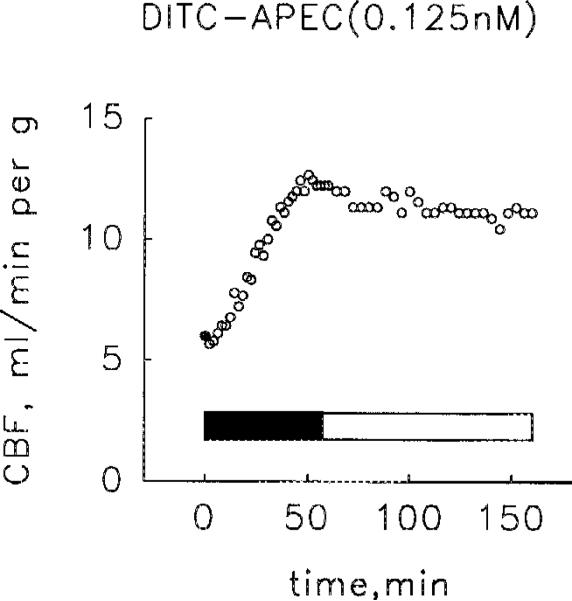
Intracoronary infusions of DITC-APEC at rates (≤ 50 μL/min), calculated on the basis of basal flow rate to give agonist concentrations of 0.125, 0.25, 0.5 or 1.0 nM, caused progressive coronary vasodilation that reached maximum flow rates in 30–60 min. Thus, DITC-APEC, too, appeared to be a full agonist. Coronary flow did not change when the administration of DITC-APEC was stopped and the hearts were perfused for up to 2 h with 0.1 mM 8-SPT (Fig. 5).
Fig. 5.
Coronary vasoactivity of DITC-APEC. During the administration of DITC-APEC (solid bar), at a rate calculated on the basis of basal flow rate to give a concentration of 0.125 nM, coronary flow (CBF) rose progressively to a level near maximum. Stopping the infusion of DITC-APEC and perfusing the heart with 0.1 mM 8-SPT (open bar) did not reduce coronary flow. This result is typical of experiments in 4 pairs of hearts perfused with DITC-APEC at initial concentrations of either 0.125, 0.25, 0.5 or 1.0 nM. The closed circle (●) represents control flow rate
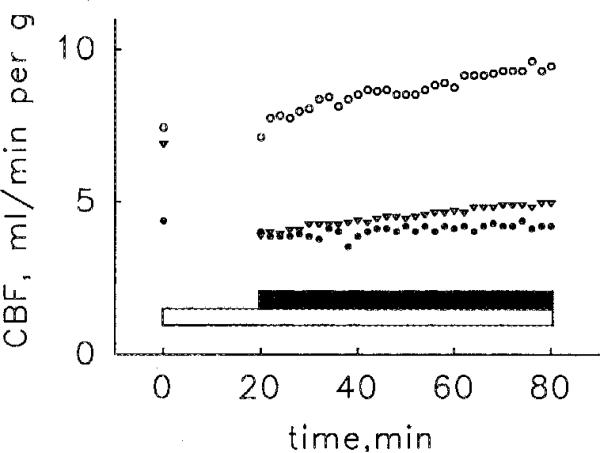
Perfusion with 0.1 mM 8-SPT for 20 min prior to and then during the administration of 0.5 nM DITC-APEC either completely blocked or markedly curtailed the coronary vasoactivity of the agonist (Fig. 6).
Fig. 6.
Antagonism of the coronary vasoactivity of DITC-APEC (solid bar) by the administration of 0.1 mM 8-SPT (open bar). begun 20 min before and continuing throughout the administration of DITC-APEC. Each symbol represents observations in a different heart. In one heart (∇) 8-SPT lowered coronary flow (CBF) from the control level by 40%, evidence that the concentration of adenosine in the interstitial space exceeded the threshold of vasoactivity, possibly because the initial period of equilibration was not long enough. However, even in this heart 8-SPT strongly antagonized the vasoactivity of DITC-APEC
Discussion
Radioligand binding studies show that DITC-APEC binds irreversibly to the A2AAR in rabbit striatum membranes (Jacobson et al. 1989), but provide no information about the functional consequences of irreversible occupancy of the receptor. The major finding of the present study is that DITC-APEC produced progressive, concentration-independent coronary vasodilation that, once developed, persisted despite replacing the administration of the agonist with the administration of 8-SPT, an unselective adenosine receptor antagonist (Daly et al. 1985). 8-SPT begun before and continued during the administration of DITC-APEC greatly curtailed or abolished the progressive coronary vasodilation, evidence that (a) 0.1 mM 8-SPT is a dose that effectively blocks the coronary receptor and, consequently, (b) the inability of 8-SPT to antagonize the coronary vasoactivity of DITC-APEC, once established, is evidence that activation of the coronary A2AR is irreversible, not simply that the antagonist is ineffective.
The observations on the response to APEC provide a useful basis for comparing the effects of DITC-APEC. As one would expect of a reversible agonist, APEC caused concentration-dependent vasodilation that faded when infusion of the agonist was discontinued. That coronary flow only partially returned to control during the 45 – 55-min period of washout probably is due to a combination of factors; (a) the affinity of this agonist is high, which means that dissociation rate, the major determinant of the affinity of a ligand for a receptor, is low; (b) APEC is quite hydrophobic and so will persist in tissue, where it remains available for binding to receptors; and (c) since clearance depends partly on perfusion rate, the decline in flow rate will, of itself, tend to diminish clearance of the agonist.
Although it would have been desirable to use a selective A2AR antagonist in these studies, such agents only became available (Shimada et al. 1992) after this study was completed. Whereas 8-SPT shows only modest potency and essentially no selectivity for receptor type (Daly et al. 1985), those attributes are offset by its inability to penetrate into cells (Heller and Olsson 1985), which is a major advantage because it confines the actions of this antagonist to cell surface receptors and reduces the likelihood of the confounding receptor-independent actions of dialkylxanthines, for example, the inhibition of cyclic nucleotide phosphodiesterase. In addition, its restricted tissue distribution and polarity mean that it is cleared rapidly.
Recent evidence suggests that coronary vasodilation may involve the activation of more than one kind of A2AR, which in turn raises the possibility that such receptors occur in more than one kind of cell in the arterial wall. The evidence for vasodilation mediated by an A2AAR is indirect, consisting of a strong correlation between vasodilator activity and agonist potency at the A2AAR in striatum membranes. Several classes of analogues show such a correlation, N6-substituted adenosines (Hamilton et al. 1987), 2-alkynyladenosines (Matsuda et al. 1992), 2-aminoadenosines (Francis et al. 1991), 2-oxoadenosines (Ueeda et al. 1991a), and 2-thioadenosines (Wiley et al. 1991). The A2AAR in striatum activates adenylate cyclase (Daly et al. 1983), and presumably the coronary artery receptor does so also. Evidence for a second receptor consists of the observation that glibenclamide, an antagonist of opening of the KATP channel, antagonizes the coronary vasoactivity of adenosine (Daut et al. 1990). Patch-clamp studies of porcine coronary smooth muscle cells demonstrate that 2-phenylaminoadenosine and a structurally similar analogue, CGS 22988, which is N-ethyl-1R-(2-phenylaminoaden-9-yl)-2,3S-dihydroxy-cyclopentane-4S-carboxamide, promote the opening of K+ channels but CGS 21680, the prototypical A2AAR agonist (Watson and Abbott 1992), does not (Cornfield et al. 1992). That study did not determine whether the K+ channel was sensitive to glibenclamide. The distinctive profile of agonist activity and coupling to a K+ channel are the basis for designating the receptor as the A4AR. In the present study, the high activity of APEC, a selective A2AAR agonist chemically derived from CGS 21680, excludes an action at an A4AR.
Because DITC-APEC is vasoactive at concentrations in the sub-nanomolar range, it might be a useful reagent for testing the hypothesis that the A2AAR-mediating coronary vasoactivity is located on endothelial cells. The model of coronary vasodilation proposed by Nees (1989) is the rationale behind such a test. That model envisions endogenous adenosine acting through an A2AR on smooth muscle and exogenous adenosine acting through an A2AR on endothelial cells. The experimental support for that model includes autoradiographic studies showing that intracoronary infusions of [3H]-adenosine at concentrations < 1 μM result in selective sequestration of the nucleoside in endothelial cells (Nees et al. 1985a). Since such concentrations are well within the vasoactive range of adenosine – in the guinea pig the EC50 of the coronary vasoactivity of adenosine is ~ 50 nM (Ueeda et al 1991b) – and the coronary endothelium is an effective barrier to the passage of adenosine (Nees et al. 1985b), Nees (1989) concluded that A2ARs in endothelial cells mediate the coronary vasoactivity of exogenous adenosine.
The same line of reasoning that implicates endothelial cells in the vasoactivity of low doses of exogenous adenosine suggests that DITC-APEC, which was vasodilatory when administered in concentrations between 0.125 and 1 nM, might have acted only at an A2AR on endothelial cells. However, studies of monolayers of coronary microvascular endothelial cells in vitro (Watanabe et al. 1992) somewhat weaken that interpretation. Those studies showed that NECA, isoproterenol and forskolin raised cellular cAMP levels and also increased the permeability of the monolayers to albumin. Accordingly, the possibility exists that DITC-APEC, through stimulation of endothelial cell adenylate cyclase, might increase endothelial permeability, allowing the agonist to diffuse between coronary endothelial cells and thereby gain access to receptors on the underlying smooth muscle cells.
The present experiments provided an opportunity to look for evidence of agonist-induced desensitization of the coronary A2AAR. The exposure of DDT1 MF2 smooth muscle cells to the selective A2AAR agonist 4-aminophenylmethylcarbonyl-APEC (PAPA-APEC, Fig. 1) caused a rapid (t1/2 45 min) loss of the ability of that agonist to stimulate adenylate cyclase (Ramkumar et al. 1991). Desensitization neither reduced the number of receptors, the affinity of the functioning receptors for [125I]PAPA-APEC, nor the sensitivity of adenylate cyclase to stimulation by other kinds of receptors, for example, the β-adrenoceptor. Although that study did not identify the mechanism of desensitization, the results tend to exclude (a) internalization of the A2AAR, (b) a decrease in the tight functional coupling to Gs that is characteristic of the A2AAR or (c) a decrease in the catalytic activity of adenylate cyclase. The possibility that endothelial cell receptors may have mediated the coronary vasoactivity of APEC and DITC-APEC makes relevant a study of the desensitization of the A2AR on bovine endothelial cells in vitro (Luty et al. 1989). Exposing cultured cells to 0.5 μM NECA for 3 h lowered NECA-stimulated adenylate cyclase to a stable level 70% lower than control, evidence that desensitization had a t1/2 on the order of 1 h.
Either APEC or DITC-APEC administered to guinea pig hearts for periods approaching 3 h caused stable, sustained coronary vasodilation, evidence against agonist-induced desensitization of the coronary A2AAR. There is precedent for the insensitivity of the coronary A2AAR to agonist-induced desensitization. The exposure of rat striatum slices to either 10 μM N6-cyclopentyladenosine or 10 μM NECA leads to, after l5 – 30 min, enhanced stimulation of adenylate cyclase by these agonists (Abbracchio et al. 1992). Stimulation reflects the preservation of the A2AAR under conditions that desensitize the A1AR. The present experiments do not explain why the rapid desensitization of the A2AAR in cultured cells fails to predict the resistance to desensitization of the coronary A2AAR in beating hearts. It is possible that the coronary receptor is indeed susceptible to desensitization, but at a rate too slow to detect in the limited period of observation available in these experiments. Alternatively, in vitro culture may alter the A2AAR in some way that increases its susceptibility to desensitization. The fact that neither the reversible nor the irreversible agonist caused desensitization tends to exclude the possibility that irreversible agonists uniquely protect against desensitization. Our observations underscore the need for caution when transferring observations on cultured cells to more physiological preparations.
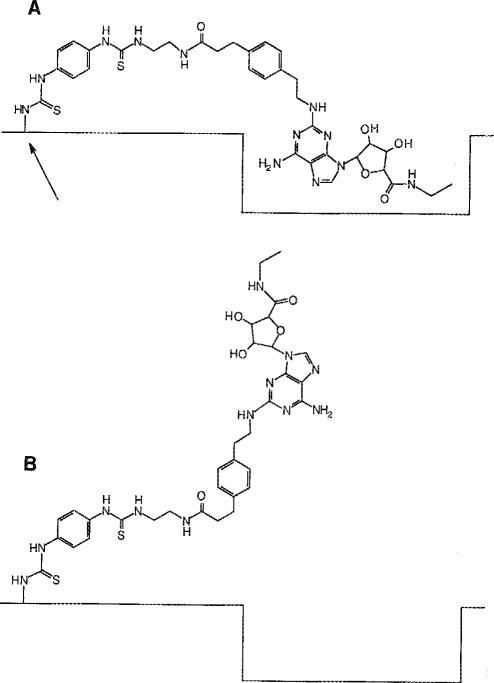
Lohse et al. (1991) have used the irreversible agonist 2-AHPIA to obtain quantitative evidence that the activation of the platelet A2AR is due to occupancy of the receptor rather than to the rate of reversible binding of agonist to the receptor (Paton 1961). The persistent, antagonist-insensitive coronary vasodilation produced by DITC-APEC provides qualitative support for the occupancy model. However, the shape of the DITC-APEC molecule qualifies that conclusion somewhat. Because the azido group of 2-AHPIA is attached directly to the adenine base, the photolysis product is linked intimately to the receptor, through a single nitrogen atom. Linkage through such a short tether insures that the receptor is occupied at all times. By contrast, the reactive isothiocyanato group of DITC-APEC is at the end of a very long side chain that retains considerable flexibility despite containing two benzene rings, an amido group and, after covalent linkage has occurred, two thioureido groups. Studies of molecular models show that when the side chain is fully extended, the point at which DITC-APEC attaches to the receptor could be nearly 30 Å away from the region that accommodates the adenosine pharmacophore. Consequently, it is possible that even though the DITC-APEC molecule is irreversibly bound to the receptor, the adenosine moiety is still able to dissociate from its binding site (Fig. 7). However, that possibility is highly unlikely, for if such were the case, 8-SPT in the concentration employed should have competed effectively with the adenosine moiety of DITC-APEC. Thus, the persistent activation of the coronary receptor appears to be due to persistent occupation by agonist.
Fig. 7.
Hypothetical model of the irreversible binding of DITC-APEC. The flexibility of the long side chain linking the adenosine pharmacophore to the point of covalent attachment (arrow) allows ligand bound to the receptor (A) to reversibly dissociate (B). However, the lack of antagonism by 8-SPT argues against such a model. See text for additional discussion
In summary, DITC-APEC, an irreversible A2AR agonist, causes activation of a coronary A2AR, resulting in vasodilation that cannot be antagonized by an effective dose of 8-SPT. Such a result suggests that the covalent binding of the 2-substituent of DITC-APEC to the ligand-binding peptide of the coronary receptor irreversibly activates the A2AR in guinea pig coronary arteries. Whether the same is true for the A2AR in other tissues and species is unknown. Likewise, whether covalent linkage through substituents on other parts of the adenosine molecule will also activate the coronary A2AR is unknown. The administration of APEC or DITC-APEC caused coronary vasodilation that did not fade during observations lasting nearly 3 h, evidence against agonist-induced desensitization of the coronary artery A2AAR.
Acknowledgement
The authors wish to thank Martha Petrous for technical assistance and Patricia Botero for preparation of the manuscript. The Ed C. Wright Chair in Cardiovascular Research, University of South Florida, and NIH HL 30391 supported this research.
References
- Abbracchio MP, Fogliatto G, Paoletti AM, Rovati GE, Cattabeni F. Prolonged in vitro exposure to rat brain slices to adenosine analogues: selective desensitization of adenosine A1 but not A2 receptor. Eur J Pharmacol. 1992;227:317–324. doi: 10.1016/0922-4106(92)90010-s. [DOI] [PubMed] [Google Scholar]
- Belardinelli L, Linden J, Berne RM. The cardiac effects of adenosine. Prog Cardiovasc Dis. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- Cornfield LJ, Hu S, Hurt SD, Sills MA. [3H]2-phenylaminoadenosine ([3H]CV 1808) labels a novel adenosine receptor in rat brain. J Pharmacol Exp Ther. 1992;263:552–561. [PubMed] [Google Scholar]
- Daly JW, Butts-Lamb P, Padgett W. Subclasses of adenosine receptors in the central nervous system: Interactions with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983;3:69–80. doi: 10.1007/BF00734999. [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett W, Shamin MT, Butts-Lamb P, Waters J. 1,3-Dialkyl-8-(p-sulophenyl)xanthines: Potent water-soluble antagonists for A1- and A2-adenosine receptors. J Med Chem. 1985;28:487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Günther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Francis JE, Webb RL, Ghai GR, Hutchison AJ, Moskal MA, deJesus R, Yokoyama R, Rovinski SL, Contardo N, Dotson R, Barclay B, Stone GA, Jarvis MF. Highly selective adenosine A1 receptor agonists in a series of N-alkylated 2-aminoadenosines. J Med Chem. 1991;34:2570–2579. doi: 10.1021/jm00112a035. [DOI] [PubMed] [Google Scholar]
- Hamilton HW, Taylor MD, Steffen RP, Haleen SJ, Bruns RF. Correlation of adenosine receptor affinities and cardiovascular activity. Life Sci. 1987;41:2295–2302. doi: 10.1016/0024-3205(87)90542-x. [DOI] [PubMed] [Google Scholar]
- Heller LJ, Olsson RA. Inhibition of rat ventricular automaticity by adenosine. Am J Physiol. 1985;248:H907–H913. doi: 10.1152/ajpheart.1985.248.6.H907. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Barrington WW, Pannell LK, Jarvis MF, Ji X-D, Williams M, Hutchison AJ, Stiles GL. Agonist derived molecular probes for A2 adenosine receptors. J Mol Recognition. 1989;2:170–178. doi: 10.1002/jmr.300020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Stiles GL, Ji X-D. Chemical modification and irreversible inhibition of striatal A2a adenosine receptors. Mol Pharmacol. 1992;42:123–133. [PMC free article] [PubMed] [Google Scholar]
- Kusachi S, Thompson RD, Olsson RA. Ligand selectivity of dog coronary adenosine receptor resembles that of adenylate cyclase stimulatory (Ra) receptors. J Pharmacol Exp Ther. 1983;227:316–321. [PubMed] [Google Scholar]
- Lohse MJ, Klotz K-N, Schwabe U. Agonist photoaffinity labeling of A1 adenosine receptors: Persistent activation reveals spare receptors. Mol Pharmacol. 1986;30:403–409. [PubMed] [Google Scholar]
- Lohse MJ, Klotz K-N, Schwabe U. Mechanism of A2 adenosine receptor activation. I. Blockade of A2 adenosine receptors by photoaffinity labeling. Mol Pharmacol. 1991;39:517–523. [PubMed] [Google Scholar]
- Londos C, Cooper DMF, Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci USA. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luty J, Hunt JA, Nobbs PK, Kelly E, Keen M, MacDermot J. Expression and desensitization of A2 purinoceptors on cultured bovine aortic endothelial cells. Cardiovasc Res. 1989;23:303–307. doi: 10.1093/cvr/23.4.303. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Shinozaki M, Yamaguchi T, Homma H, Nomoto R, Miyasaka T, Watanabe Y, Abiru T. Nucleosides and nucleotides. 103. 2-Alkynyladenosines: A novel class of selective adenosine A2 receptor agonists with potent antihypertensive effects. J Med Chem. 1992;35:241–252. doi: 10.1021/jm00080a007. [DOI] [PubMed] [Google Scholar]
- Mustafa SJ, Askar AO. Evidence suggesting an Ra-type adenosine receptor in bovine coronary arteries. J Pharmacol Exp Ther. 1985;232:49–56. [PubMed] [Google Scholar]
- Nees S. The adenosine hypothesis of metabolic regulation of coronary flow in the light of newly recognized properties of the coronary endothelium. Z Kardiol. 1989;78([Suppl] 6):42–49. [PubMed] [Google Scholar]
- Nees S, Böck M, Herzog V, Becker BF, Des Rosiers C, Gerlach E. The adenine nucleotide metabolism of the coronary endothelium: Implications for the regulation of coronary flow by adenosine. In: Stefanovich N, Rudulphi K, Schubert P, editors. Adenosine receptors and modulation of cell function. IRL; Oxford, UK: 1985a. pp. 419–436. [Google Scholar]
- Nees S, Herzog V, Becker BF, Böck M, Des Rosiers C, Gerlach E. The coronary endothelium: A highly active metabolic barrier for adenosine. Basic Res Cardiol. 1985b;80:515–529. doi: 10.1007/BF01907915. [DOI] [PubMed] [Google Scholar]
- Paton WDMA. Theory of drug action based on the rate of drug-receptor combination. Proc R Soc Lond Biol Sci. 1961;154:21–69. [Google Scholar]
- Ramkumar V, Olah ME, Jacobson KA, Stiles GL. Distinct pathways of desensitization of A1- and A2-adenosine receptors in DDT1 MF2 cells. Mol Pharmacol. 1991;40:639–647. [PMC free article] [PubMed] [Google Scholar]
- Shimada J, Suzuki F, Nonaka H, Ishii A, Ichikawa S. (E)-1,3-Dialkyl-7-methyl-8-(3,4,5-trimethoxystyryl)xanthines: Potent and selective adenosine A2 antagonists. J Med Chem. 1992;35:2342–2345. doi: 10.1021/jm00090a027. [DOI] [PubMed] [Google Scholar]
- Trivedi BK, Bridges AJ, Bruns RF. Structure-activity relationships of adenosine A1 and A2 receptors. In: Williams M, editor. Adenosine and adenosine receptors. Humana; Clifton, NJ: 1990. pp. 57–103. [Google Scholar]
- Ueeda M, Thompson RD, Padgett WL, Secunda S, Daly JW, Olsson RA. Cardiovascular actions of adenosines, but not adenosine receptors, differ in rat and guinea pig. Life Sci. 1991a;49:1351–1358. doi: 10.1016/0024-3205(91)90199-l. [DOI] [PubMed] [Google Scholar]
- Ueeda M, Thompson RD, Arroyo LH, Olsson RA. 2-Alkoxy-adenosines: Potent and selective agonists at the coronary artery A2 adenosine receptor. J Med Chem. 1991b;34:1334–1339. doi: 10.1021/jm00108a014. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kuhne W, Schwartz P, Piper M. A2-adenosine receptor stimulation increases macromolecule permeability of coronary endothelial cells. Am J Physiol. 1992;262:H1174–H1181. doi: 10.1152/ajpheart.1992.262.4.H1174. [DOI] [PubMed] [Google Scholar]
- Watson S, Abbott A. Adenosine receptors. Trends Pharmacol Sci Receptor. 1992;([Suppl] 3) [Google Scholar]
- Wiley DM, Szabo I, Maguire MH. Inhibition of [3H]CGS21680 binding to the adenosine-A2 receptor by 2-substituted adenosine analogs. FASEB J. 1991;5:A1768. [Google Scholar]
- Zhou Q-Y, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: The A3 adenosine receptor. Proc Natl Acad Sci USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]