Abstract
The caudomedial nidopallium (NCM) is a telencephalic area involved in auditory processing and memorization in songbirds, but the synaptic mechanisms associated with auditory processing in NCM are largely unknown. To identify potential changes in synaptic transmission induced by auditory stimulation in NCM, we used a slice preparation for path-clamp recordings of synaptic currents in the NCM of adult zebra finches (Taenopygia guttata) sacrificed after sound isolation followed by exposure to conspecific song or silence. Although postsynaptic GABAergic and glutamatergic currents in the NCM of control and song-exposed birds did not present any differences regarding their frequency, amplitude and duration after song exposure, we observed a higher probability of generation of bursting glutamatergic currents after blockade of GABAergic transmission in song-exposed birds as compared to controls. Both song-exposed males and females presented an increase in the probability of the expression of bursting glutamatergic currents, however bursting was more commonly seen in males where they appeared even without blocking GABAergic transmission. Our data show that song exposure changes the excitability of the glutamatergic neuronal network, increasing the probability of the generation of bursts of glutamatergic currents, but does not affect basic parameters of glutamatergic and GABAergic synaptic currents.
Keywords: Zebra finch, Neurotransmission, Songbird, Auditory processing, Nidopallium
Introduction
Both songbirds (Order Passeriformes, suborder Oscines) and humans share the ability of using learned vocalizations for communication. As important as vocalizing correctly is the ability to make sense of the vocalizations in accordance to the species’ use of the vocal signal. Although vocal communication in humans is complex and conveys ideas and actions in a very structured manner, most songbirds rely on species-specific song recognition for a successful reproductive behavior and territoriality. Both male and female songbirds rely on memorization of individual songs for the effective identification of sexual partners and competitors (Catchpole and Slater 1995; Kroodsma and Miller 1996). An intensively studied brain area that plays important roles in the perceptual processing of birdsong is the caudomedial nidopallium (NCM), thought to be equivalent to supragranular layers of the mammalian auditory cortex (Vates et al. 1996). NCM receives input from the primary telencephalic auditory area field L (Vates et al. 1996) and its evoked auditory responses show selectivity to conspecific song, as demonstrated by electrophysiological recordings (Chew et al. 1995) and expression analysis of the zenk (a.k.a. zif 268, krox 24, ngf1-A or egr-1) and Arc genes (Mello et al. 1995; Velho et al. 2005; reviewed in Mello 2002; Mello et al. 2004). NCM also shows marked changes in responses to repeated song presentation, in the form of a long-lasting song-specific habituation of neuronal firing (Chew et al. 1995, 1996) and zenk expression (Mello et al. 1995). Recent evidence from both electrophysiology and gene expression studies has further suggested a role for NCM in recognizing familiar from unfamiliar songs (Woolley and Doupe 2008) and in storing and recognizing the tutor song model required for vocal learning (Bolhuis and Gahr 2006; Phan et al. 2006; Gobes and Bolhuis 2007; London and Clayton 2008). Altogether, these studies indicate that NCM plays pivotal roles in conspecific song processing and recognition. They also point to NCM as an important player in higher processing of auditory signals and an attractive target area for studying synaptic plasticity phenomena related to song learning.
Despite the evidence discussed above, it is unknown whether synaptic transmission in NCM might show plasticity upon auditory stimulation. NCM is known to have a high density of GABAergic neurons, which are song-responsive, as evidenced by zenk expression (Pinaud et al. 2004; Pinaud and Mello 2007). Patch-clamp recordings of NCM neurons have demonstrated the presence of highly frequent spontaneous GABAergic synaptic currents and the appearance of strong and coordinated glutamatergic activity after blocking GABAergic transmission with bicuculline (Pinaud et al.2008a), suggesting the existence of a strong GABAergic inhibition of the glutamatergic network. Furthermore, song stimulation increases the expression of synapsyn mRNA and protein in NCM (Pinaud et al. 2008b; Velho and Mello 2008), suggesting a modulatory action on synaptic physiology. Knowing how auditory stimulation changes the synaptic dynamics of neurotransmission and the excitation/inhibition balance in NCM is crucial for understanding how NCM circuitry functions under the influence of sensory stimulation. Here we studied electrophysiologically the neurotransmission in NCM slices of adult zebra finches under resting conditions (continued exposure to silence after overnight sound isolation) or after repetitive presentation of a set of novel conspecific songs, in order to detect possible experience-induced changes in both GABAergic and glutamatergic synaptic transmission. We observed that our protocol of song exposure increases the probability of appearance of bicuculline-induced glutamatergic bursts, which are more prominent in both control and song-exposed male birds, but does not affect “conventional” spontaneous GABAergic and glutamatergic synaptic currents. We suggest that song exposure directly modulates the activity level of glutamatergic neurons in NCM or adjacent areas providing input to NCM. In addition, the prevalence of the bursting behavior in males strongly suggests a gender influence on the excitability of the glutamatergic neurons in zebra finch NCM.
Materials and methods
Animals and song exposure protocol
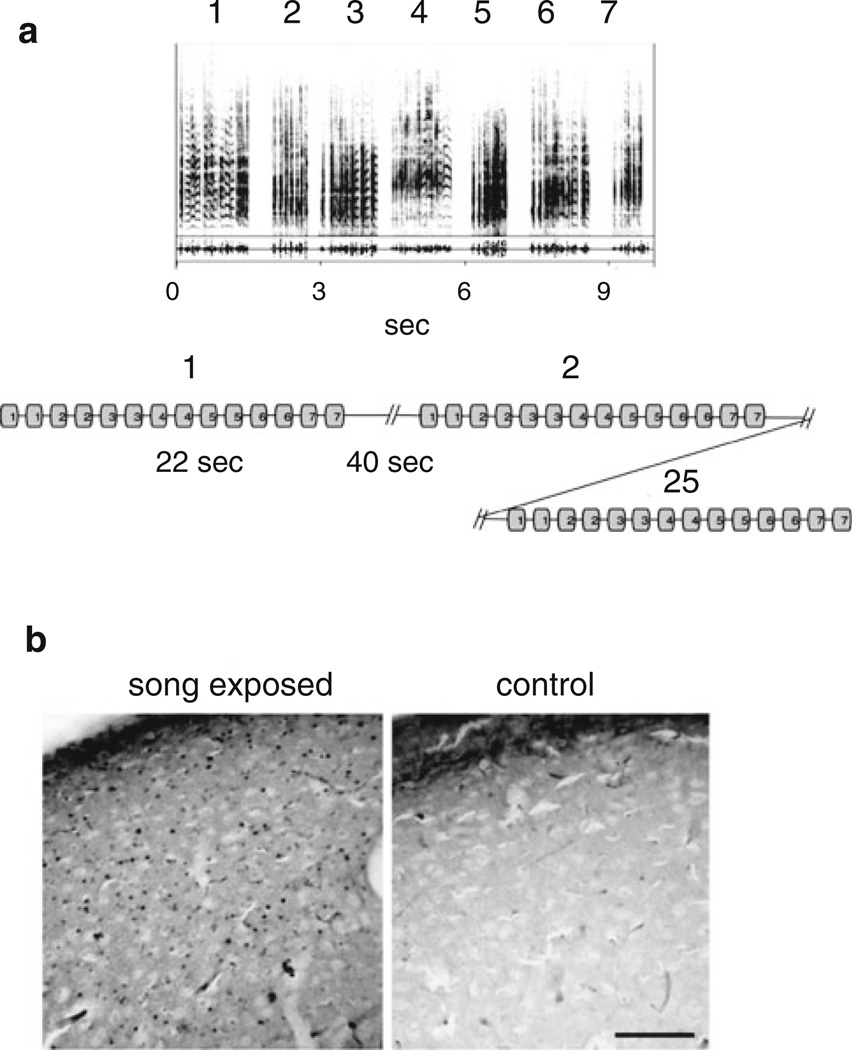
Adult zebra finches (Taeniopygia guttata) from both sexes (n = 42 males and 36 females) were purchased from local breeders and kept in captivity in common aviaries with water and food ad libitum in a temperature- and light-controlled environment (22 °C and a 12/12 h light cycle). On the day preceding the recordings, the birds were isolated overnight (about 15 h) in sound isolation chambers. On the following day, the birds were individually submitted to the song exposure protocol (modified from Mello and Ribeiro 1998) or to silence. To maximize diversity, the stimulus consisted of a sequence of seven songs of 1.5–2 s of duration each (two motif presentations per song), for a total sequence duration of about 20 s. This sequence was followed by 40 s of silence. This 1-min cycle was repeated 25 times, for total stimulus duration of 25 min (Fig. 1a). The birds were then kept further in isolation and sacrificed by decapitation 90 min after the onset of the song exposure, thus coinciding with the peak of song-induced ZENK protein expression in the NCM of zebra finches in response to song exposure (Mello and Ribeiro 1998). Controls underwent the same isolation protocol, but were not subjected to song exposure, and usually were sacrificed no longer than 3 h after the lights were turned on in the isolation chamber. The effectiveness of the isolation and/or song exposure was assessed with ZENK protein immunocytochemistry (Fig. 1b); this procedure also served to ascertain that our slice preparation protocol did not lead to spurious neuronal depolarization and consequent ZENK expression in NCM.
Fig. 1.
Protocol used for song exposure and song-induced ZENK protein expression in NCM. a Top sonograms of the adult male zebra finch songs used in the song-exposure protocol. Bottom scheme of the song-exposure protocol. The numbers in the boxes correspond to the song presented (top), see “Methods” for details. b Song-induced ZENK expression in NCM slices prepared as for electrophysiology from a song-exposed (left) and a control (right) adult male zebra finch
Slice electrophysiology
Brain slices were obtained as in Pinaud et al. (2008a). Briefly, birds were sacrificed by decapitation and their brains quickly dissected and placed in ice-cold artificial cerebrospinal fluid solution (aCSF) modified for slicing and consisting of (mM): NaCl (87), NaHCO3 (25), KCl (2.5), NaH2PO4, (1.25), CaCl2 (0.5), MgCl2 (7), glucose (25), sucrose (75), ascorbic acid (0.4), sodium pyruvate (2), myo-inositol (3), 354 mOsm/kgH2O, pH 7.4 (when bubbled with carbogen 95 %O2/5 %CO2). Serial 200-µm thick parasagittal sections (4–5 per hemisphere) containing NCM and adjacent areas were obtained on a vibratome (Vibratome Series 1000Plus, Vibratome, St. Louis, MO). Slices were stored in slicing aCSF at room temperature for up to 4–6 h. For recordings, slices were transferred to a chamber mounted on a stage of an upright microscope (Olympus BX51WI) and continuously perfused with standard aCSF, which consisted of (mM): NaCl (125), KCl (2.5), NaHCO3 (25), NaH2PO4 (1.25), glucose (25), CaCl2 (2), MgCl2 (1), ascorbic acid (0.4), sodium pyruvate (2), myo-inositol (3), 310 mOsm/kgH2O, pH 7.4 when bubbled with 95 %CO2/5 %O2. Single neurons were visualized with DIC-IR optics, approached and patched with thick-walled borosilicate glass pipettes pulled using a P-97 horizontal puller (Sutter Instruments, Novato CA). Spontaneous post-synaptic currents (sPSCs) were recorded at –70 mV in whole-cell voltage-clamp with an EPC-10 patch-clamp amplifier (HEKA Electronics, Germany) using the Pulse acquisition software. For recordings, pipettes were backfilled with filtered internal solution consisting of (in mM) CsCl (140), EGTA (5), HEPES (10), ATP-Mg (4), phosphocreatine-Na (20), pH 7.3 with CsOH. When filled with this solution, the pipette resistance varied between 3 and 7 MΩ. A low chloride internal solution (130 mM CsMeSO4/10 mM KCl) was used in some situations.
Glutamatergic and GABAergic sPSCs were pharmacologically isolated using the GABAA receptor antagonist bicuculline (10 µM) and the AMPA/kainate glutamatergic receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) (5 µM), respectively. Miniature currents were isolated using 1 µM tetrodotoxin (TTX). Drugs were applied to the bath by a gravity-driven perfusion system (3 ml/min) and recordings started 5 min after initiation of drug perfusion. Recordings were made at room temperature (23–25 °C).
Synaptic currents were acquired at 10 kHz and low-pass filtered at 2 kHz (Bessel 8-pole). Series resistance (Rs) was usually compensated (50–70 %) resulting in a mean series resistance of 9.9 ± 5.5 MΩ (n = 50). Cells with Rs larger than 30 MΩ or showing large variations during the experiment were discarded. One to three neurons were recorded from each bird. Each group analyzed was composed of neurons from at least 3 birds.
Immunohistochemistry for ZENK
Brain sections were obtained as for electrophysiology, incubated for 60 min at room temperature in slicing aCSF and fixed for 20–30 min in 0.4 % paraformaldehyde in 0.1 % phosphate buffered saline. Slices were washed in phosphate buffered saline, treated with 0.3 % hydrogen peroxide and incubated overnight at 4 °C with an anti-ZENK antibody raised in goat (anti-egr-1-sc-189, Santa Cruz Biotechnology) at a 1:1,000 dilution in blocking buffer consisting of 0.5 % bovine serum albumin (BSA) and 0.3 % Triton X-100 in 0.1 M phosphate buffered saline pH 7.4. The slices were then washed three times in phosphate buffered saline, incubated with a secondary biotinylated rabbit anti-goat antibody (Vector Laboratories) for 2 h at room temperature, washed three times in phosphate buffered saline, incubated with the ABC reagent (Vector Elite kit) for 2 h, washed with phosphate buffered saline, and developed by incubation for 4–10 min in a filtered solution containing 0.03 % diaminobenzidine and 0.15 % nickel-ammonium sulfate in 0.1 M phosphate buffered saline, to which 0.001 % hydrogen peroxide was added. After the chromogen reaction, tissue was mounted on Superfrost slides (Fischer Scientific), dehydrated in a standard series of alcohols, exposed to xylenes, and coverslipped with Entellan (Merck).
Drugs and chemicals
All chemicals used were of analytical grade and obtained from Sigma (St. Louis, MO) or Merck (São Paulo, Brazil). Bicuculline (free base) was obtained from Tocris (Ellisvile, MO) and DNQX from Sigma. Stock solutions (1,000 ×) of these drugs were prepared in dimethyl sulphoxide. Tetrodotoxin (TTX) (citrate salt) was from Tocris and a stock solution (1,000 ×) was prepared in water. All stocks were kept frozen (−20 °C) and protected from light. Dimethyl sulphoxide at the concentration of 0.001 % had no effect on the synaptic currents in NCM slices (not shown).
Data analysis
Synaptic currents were analyzed using Minianalysis software (Synaptosoft, Decatur GA). Segments of 1 min acquired in duplicate were used for sPSCs analysis. Synaptic currents were detected manually and consisted of deflections that had the typical waveform of a synaptic current (i.e. fast rise time and exponential-like decay) and were clearly distinct from baseline noise. Complex events were analyzed with Minianalysis’ multi-peak function. The control mean amplitudes and frequencies passed a Kolmogorov–Smirnoff normality test (as in Pinaud et al. 2008a) and were thus analyzed with parametric statistics. For determination of statistical significance between two conditions, we compared means using Student’s unpaired t tests. For analyzing the effect of gender on song-exposed and control birds, we used two-way analysis of variance. The proportions of neurons presenting glutamatergic bursts after bicuculline were analyzed by comparing the proportions of independent samples using the formula
where p̄s and p̄i are the probabilities of glutamatergic bursts appearing after application of bicuculline to slices from song-exposed and control birds, respectively, p̄ is the overall probability of appearance of bicuculline-induced bursts, Δ is the hypothesized difference between control and song-exposed conditions (which was set to zero) and ns and ni are the number of cells in the song-exposed and control groups, respectively. The data fulfilled the test requirements (np and nq > 5). All two-tailed significance levels were set below 0.05.
Results
Efficacy of the song exposure protocol and slicing procedures
ZENK expression was used as a marker of NCM activation, to verify the efficacy of the song exposure, and to test for possible undesirable activation of NCM neurons in response to the tissue manipulation, slicing procedure and/or incubation used for the electrophysiological recordings, which per se could potentially induce synaptic and neuronal changes, hindering the detection of song-induced effects. We performed immunohistochemical labeling of ZENK protein in slices from control and song-exposed birds and observed that ZENK expression was very low or absent in the NCM of control birds (Fig. 1b, right panel), in contrast to the very robust expression in the NCM of song–song-exposed birds (Fig. 1b, left panel). We conclude that our protocol was highly effective in inducing neuronal activation in the telencephalon of the birds included in the study and that the slicing and incubation procedures by themselves do not induce ZENK expression, likely reflecting a lack of substantive neuronal firing in NCM in the absence of song exposure in vivo.
GABAergic neurotransmission in NCM is not affected by song exposure
To study the neurotransmission in NCM, we prepared parasagittal brain slices from adult zebra finches of both sexes. The slices were from a region spanning from the midline up to 1 mm lateral and contained a large and easily identifiable NCM, as well as the more rostral field L2 and caudomedial mesopallium (CMM). Neurons were recorded in both caudal and rostral NCM, with no apparent differences in neurotransmission detected between these areas. Using this same preparation, we have previously reported that NCM neurons show a high frequency of spontaneous synaptic currents (sPSCs), which are mostly GABAergic based on their sensitivity to the GABAA receptor antagonist bicuculline (Pinaud et al. 2004, 2008a). This activity is strongly driven by action potential firing, since it is highly sensitive to TTX (Pinaud et al. 2008a), and it could derive either from the neuronal network intrinsic to NCM or from the adjacent areas field L and/or CMM. In contrast, glutamatergic sEPSCs, which can be isolated by application of bicuculline to the bath, are much less frequent and insensitive to TTX and can thus be considered miniature EPSCs (Pinaud et al. 2008a).
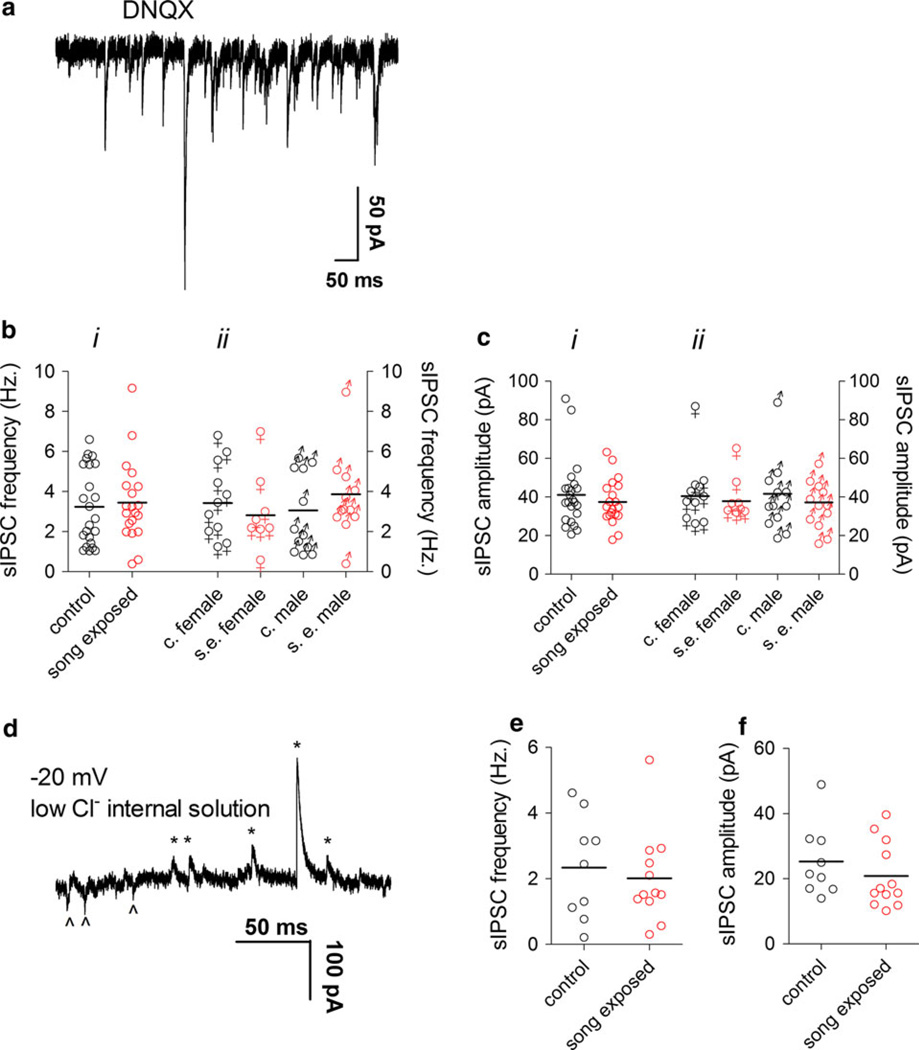
GABAergic neurons represent a significant neuronal population in NCM (Pinaud et al. 2004, 2008a) and GABAA receptors have been shown to be modulated by song exposure (Jeong et al. 2011a). To analyze the effect of song exposure on GABAergic sIPSCs in our NCM slice preparations, we first isolated sIPSCs pharmacologically by applying the AMPA/kainate glutamatergic receptor antagonist DNQX to the bath (Fig. 2a). We observed no differences in the frequency or amplitude of sIPSCs when comparing song-exposed and control birds (Fig. 2bi, ci).
Fig. 2.
Spontaneous GABAergic post-synaptic currents in the NCM are not affected by song exposure. a Representative sIPSCs recorded at −70 mV using a high-chloride internal solution in the presence of DNQX. b i sIPSC frequency in the NCM of control and song-exposed birds (control: 3.2 ± 0.4 Hz, n = 23; song-exposed: 3.4 ± 0.4 Hz, n = 20), b ii sIPSC frequency in the NCM of control and song-exposed birds segregated by gender (control females: 3.4 ± 0.5 Hz, n = 11; song-exposed females: 2.8 ± 0.7 Hz, n = 8; control males: 3.1 ± 0.6 Hz, n = 12; song-exposed males: 3.9 ± 0.6 Hz, n = 12). c i sIPSC amplitude in the NCM of control and song-exposed birds (control: 41.1 ± 3.6 pA, n = 23; song-exposed: 37.5 ± 2.6 pA, n = 20). c ii sIPSC amplitude in the NCM of control and song-exposed birds segregated by gender (control females: 40.6 ± 5 pA, n = 11; song-exposed females: 37.9 ± 4 pA, n = 8; control males: 41.7 ± 5 pA, n = 12; song-exposed males: 37.2 ± 3 pA, n = 12). d Representative sIPSCs recorded at −20 mV using a low-chloride internal solution. Outward currents are indicated by asterisks and inward currents by arrowheads. e sIPSC frequency measured at −20 mV with a low-chloride internal solution (control: 2.3 ± 0.5 Hz, n = 9, 6 males and 3 females; song-exposed: 2.0 ± 0.4 Hz, n = 12, 6 males and 6 females). f sIPSC amplitude measured at −20 mV in a low-Cl internal solution (control: 25.3 ± 3 pA, n = 9, 6 males and 3 females; song-exposed: 20.9 ± 3 pA, n = 12, 6 males and 6 females). Values above correspond to the mean ± SEM; on the graphs, each symbol corresponds to a value of a single neuron and the horizontal line represents the mean
NCM is a site of estrogen production in zebra finches (Saldanha et al. 2000; Pinaud et al. 2006 Rohmann et al. 2007; Remage-Healey et al. 2011), and estrogen can affect GABAergic neurotransmission and song auditory processing in NCM (Tremere et al. 2009; Tremere and Pinaud 2011). Thus, we compared sIPSCs from male and female zebra finches to test for possible gender differences that might be linked to a local action of estrogen in NCM, but we did not observe any differences in the basic parameters of sIPSCs related to gender (Fig. 2bii, cii).
Quinoxaline compounds, such as DNQX are known to stimulate GABAergic synapses in some preparations (Brickley et al. 2001). If this occurred in NCM, it might obscure changes in GABAergic transmission produced by song presentation. To control for that possibility, instead of applying DNQX to the NCM slices we next measured sIPSCs by using a low-chloride internal solution and keeping the membrane potential of the recorded cell at −20 mV, a condition where GABAergic sIPSCs are detected as outward currents and glutamatergic sEPCS as inward currents (Fig. 2d; also see Pinaud et al. 2008a). We did not find group differences in either the amplitude or frequency of sIPSCs (Fig. 2e, f) when recording under this configuration.
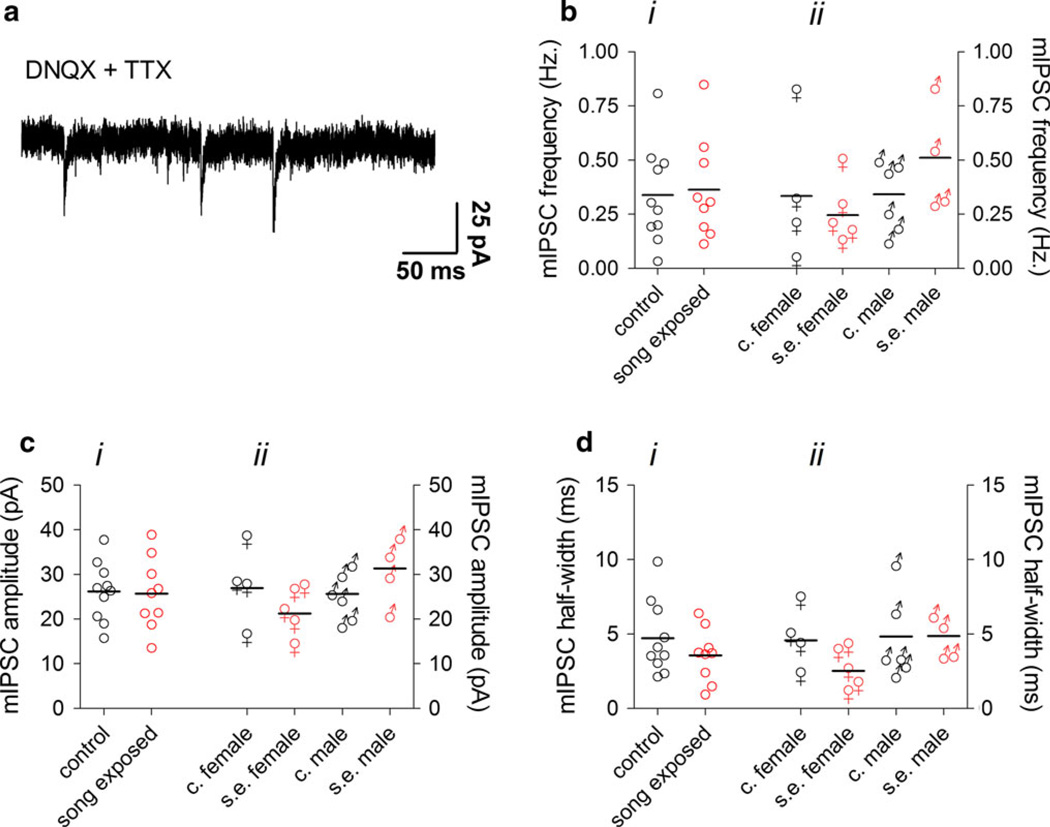
GABAergic sIPSCs in our NCM slice preparations are strongly driven by action potentials, since their frequency and amplitude are very sensitive to TTX (Pinaud et al. 2008a). Action potential-independent miniature post-synaptic currents (mPSCs) recorded in the presence of TTX can give more precise information regarding basic properties, such as synaptic vesicle release probability and post-synaptic conductances. Although the frequency of mPSCs reflects the release probability of pre-synaptic vesicles, changes in the amplitude and half width normally reflect changes in postsynaptic mechanisms at the receptor level. To isolate GABAergic mIPSCs, we applied TTX (1 µM) in the presence of DNQX (Fig. 3a), but found no differences in the frequency, amplitude or half width of mIPSCs in NCM when comparing control and song-exposed birds, even taking gender in account (Fig. 3b–d). We conclude that song exposure does not alter GABAergic post-synaptic currents in NCM.
Fig. 3.
Miniature GABAergic synaptic currents in the NCM are not affected by song exposure. a Representative recording of mIPSCs in the presence of TTX. b i mIPSC frequency in the NCM of control and song-exposed birds (control: 0.34 ± 0.07 Hz, n = 10; song-exposed: 0.37 ± 0.08 Hz, n = 9). b ii sIPSC frequency in the NCM of control and song-exposed birds segregated by gender (control females: 0.33 ± 0.2 Hz, n = 4; song-exposed females: 0.24 ± 0.06 Hz, n = 5; control males: 0.3 ± 0.2 Hz, n = 6; song-exposed males: 0.5 ± 0.1 Hz, n = 4). c i mIPSC amplitude in the NCM of control and song-exposed birds (control: 26.2 ± 2.1 pA, n = 10; song-exposed: 25.7 ± 2.7 pA, n = 9). c ii sIPSC amplitude in the NCM of control and song-exposed birds segregated by gender (control females: 27 ± 4 pA, n = 4; song-exposed females: 21.2 ± 2 pA, n = 5; control males: 25.7 ± 2 pA, n = 6; song-exposed males: 31.3 ± 4 pA, n = 4). d i mIPSC half width in the NCM of control and song-exposed birds (control: 4.7 ± 0.8 ms, n = 10; song-exposed: 3.6 ± 0.6 ms, n = 9). d ii mIPSC half width in the NCM of control and song-exposed birds segregated by gender (control females: 4.6 ± 1 ms, n = 4; song-exposed females: 2.5 ± 0.6 ms, n = 5; control males: 4.8 ± 1 ms, n = 6; song-exposed males: 4.8 ± 0.6 pA, n = 4). Values above correspond to the mean ± SEM; on the graphs, each symbol corresponds to a value of a single neuron and the horizontal line represents the mean
Song exposure does not affect glutamatergic sEPSCs, but promotes the appearance of bursting EPSCs in NCM
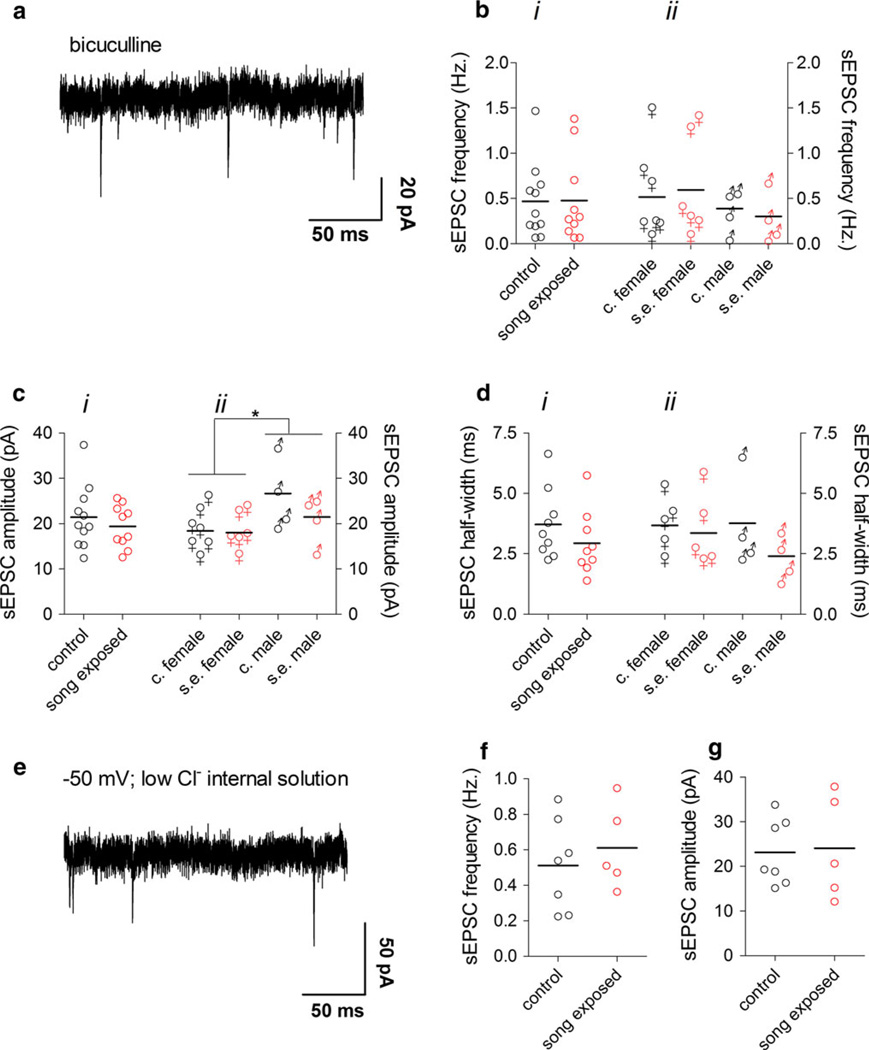
We next studied glutamatergic transmission in NCM slices of control and song–song-exposed birds. Glutamatergic sEPSCs were recorded after application of the GABAA receptor antagonist bicuculline (Fig. 4a) to the slices. We have previously shown that co-application of bicuculline and DNQX fully abolishes synaptic transmission in NCM (Pinaud et al. 2008a), thus the synaptic currents left after application of bicuculline are glutamatergic. Consistent with our previous studies (Pinaud et al. 2004, 2008a), we observed the existence of two forms of glutamatergic transmission in our NCM slice preparations under bicuculline: spontaneous excitatory post-synaptic currents (sEPSCs) of small amplitude and low frequency which are not affected by TTX (Fig. 4a), thus representing action potential-independent miniature EPSCs and, in some neurons, spontaneous bursts of glutamatergic currents (bursting EPSCs; Fig. 5a, right panel), which are likely the result of synchronous firing of a population of interconnected neurons.
Fig. 4.
Spontaneous glutamatergic synaptic currents in the NCM are not affected by song exposure. a Representative sEPSCs recorded at −70 mV using a high-chloride internal solution in the presence of bicuculline. b i sEPSC frequency in the NCM of control and song-exposed birds (control: 0.46 ± 0.12 Hz, n = 11; song-exposed: 0.48 ± 0.15 Hz, n = 10). b ii sEPSC frequency in the NCM of control and song-exposed birds segregated by gender (control females: 0.5 ± 0.2 Hz, n = 7; song-exposed females: 0.6 ± 0.2 Hz, n = 6; control males: 0.4 ± 0.2 Hz, n = 4; song-exposed males: 0.3 ± 0.1 Hz, n = 4). c i sEPSC amplitude in the NCM of control and song-exposed birds (control: 21.4 ± 2.1 pA, n = 11; song-exposed: 19.4 ± 1.5 pA, n = 10). c ii sEPSC amplitude in the NCM of control and song-exposed birds segregated by gender (control females: 18.4 ± 1.8 pA, n = 7; song-exposed females: 18.01 ± 1.7 pA, n = 6; control males: 26.7 ± 3.9 pA, n = 4; song-exposed males: 21.5 ± 3 pA, n = 4; *p < 0.05). d i sEPSC half width in the NCM of control and song-exposed birds (control: 3.7 ± 0.5 ms, n = 9; song-exposed: 2.9 ± 0.4 ms, n = 9). d ii sEPSC half width in the NCM of control and song-exposed birds segregated by gender (control females: 3.7 ± 0.5 ms, n = 5; song-exposed females: 3.3 ± 0.7 ms, n = 5; control males: 3.8 ± 1 ms, n = 4; song-exposed males: 2.4 ± 0.5 ms, n = 4). e Representative recording of sEPSCs recorded at −50 mV using a low-chloride internal solution. f sEPSC frequency measured at −50 mV in a low-chloride internal solution (control: 0.5 ± 0.1 Hz, n = 7; song-exposed: 0.6 ± 0.1 Hz, n = 5). g sEPSC amplitude measured at −50 mV in a low-chloride internal solution (control: 23.1 ± 3 pA, n = 7; song-exposed: 24.08 ± 5 pA, n = 5;). Data in e–g were from male birds. Values above correspond to the mean ± SEM; on the graphs, each symbol corresponds to a value of a single neuron and the horizontal line represents the mean
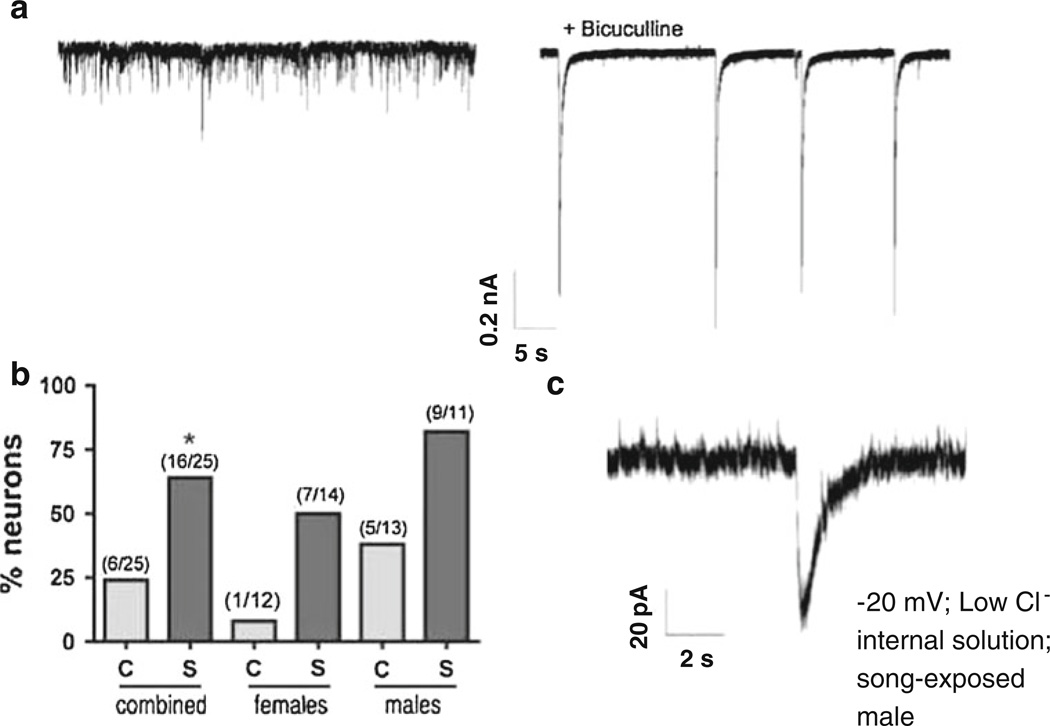
Fig. 5.
Bursting EPSCs are increased by song exposure. a Generation of bursting behavior of glutamatergic neurotransmission after bicuculline application to NCM slices. Left trace no drug, right trace after bicuculline application. b Percentage of neurons that presented bursting neurotransmission after bicuculline (Ccontrol, S song-exposed, *p < 0.05); values in parentheses indicate absolute numbers of recorded cells with bursting behavior and total number of recorded cells per group. c Example of an individual spontaneous bursting EPSC observed in an NCM neuron from a song-exposed male without blocking GABAergic transmission. The currents were measured at −20 mV using a low-chloride internal solution. Under these conditions, glutamatergic currents are downward and GABAergic currents upward
We found no differences in basic parameters of synaptic currents (frequency, amplitude and half-width) when comparing pharmacologically isolated glutamatergic sEPSCs in NCM between control and song-exposed birds (Fig. 4bi–di). Interestingly, the amplitude of the sEPSCs was affected by gender, being significantly larger in males than in females (F = 6.23, df1,17, p = 0.023, 2-way analysis of variance; Fig. 4cii). However, song exposure did not significantly affect the amplitude of the sEPSCs in either males or females (Fig. 4cii). The frequency and half width of the sEPSCs were, on the other hand, not affected by gender or condition (Fig. 4bii, dii).
Some bicuculline salts have been reported to have nonspecific effects, for example by inhibiting small conductance calcium-activated potassium channels (SK channels) (Johnson and Seutin 1997; Seutin and Johnson 1999). We used bicuculline free base, which has not been reported to inhibit SK channels (Johnson and Seutin 1997), but to further control for possible non-specific effects of this drug (Seutin and Johnson 1999), we examined the frequency of sEPSCs recorded in a low-chloride internal solution at the measured reversal potential for chloride sIPSCs (around −50 mV); under these conditions, glutamatergic sEPSCs are detected as inward currents, while GABAergic sIPSCs are undetected (Fig. 4e). Because a small but non-significant decrease in the sEPSC amplitude was observed in song-exposed males under bicuculline, we performed the recordings with low-chloride internal solution in slices from male birds. Under these conditions, however, both the frequency and amplitude of glutamatergic sEPSCs did not differ between control and song-exposed birds (Fig. 4f, g).
In contrast, the number of glutamatergic bursting EPSCs appeared to increase in the NCM of song-exposed birds as compared to controls. To quantify this effect, we analyzed the probability of appearance of the bursting EPSCs under bicuculine. If a neuron did not present bursting EPSCs within at least 5 min of bicuculline application, we considered that bicuculline did not produce glutamatergic bursts in that cell. We observed that song exposure increased the probability of appearance of EPSCs bursts from 24 to 64 % of the recorded cells in an analysis combining both sexes (Fig. 5b, left columns; z = 2.85, df = 49, p = 0.004). The increased probability of observing bursting EPSCs was seen in neurons from both females (Fig. 5b, middle columns: control 8 %, song exposed 38 %) and males (Fig. 5b, right columns: control 38 %, song exposed 82 %). In general, we observed that bursting EPSCs were more frequent in the NCM of males (58 %) as compared to females (31 %) (z = 1.96, df = 49, p = 0.05). Moreover, in 8 out of 11 neurons from song-exposed males we observed the spontaneous appearance of single bursts in the absence of bicuculline (Fig. 5c), which was not observed in NCM neurons from any control birds, nor in our previous study where birds were taken directly from the aviary (Pinaud et al. 2008a). This observation suggests that the glutamatergic neurons in the NCM of song-exposed males can sometimes overcome the strong GABAergic inhibition that keeps them from firing action potentials.
We conclude that a protocol of song exposure that generates neuronal activity in NCM, as assessed by ZENK protein expression, does not produce long-lasting changes in the properties of GABAergic and glutamatergic spontaneous post-synaptic currents in NCM, but increases the probability of generation of glutamatergic bursting EPSCs.
Discussion
NCM is a postulated site of experience-dependent plasticity associated with the formation of song auditory memories, both in the context of song perception and vocal learning (Mello et al. 2004; Bolhuis and Gahr 2006), but cellular mechanisms are currently unclear. A major goal of our present study was to determine if exposure of zebra finches to conspecific song leads to detectable changes in the properties of glutamatergic and GABAergic synaptic currents measured in NCM-containing slices. If detected, such changes could represent a form of song-induced synaptic plasticity underlying NCMs role in song memory formation. We used a song-exposure protocol that induces neuronal activation in NCM, as confirmed by ZENK protein expression, but observed no differences in the basic parameters of glutamatergic and GABAergic synaptic currents in NCM when comparing control and song-exposed birds. However, the occurrence of bursting glutamatergic EPSCs was significantly higher in song-exposed birds. This effect could reflect an adaptation of NCM to stimulus-induced activity and is potentially relevant for information processing and plasticity in song-responsive circuits.
Possible mechanisms of bursting EPSCs
We have previously reported that a fraction of the recorded neurons in NCM slices show seizure-like bursts of glutamatergic currents after blocking GABAergic transmission with bicuculline (Pinaud et al. 2004, 2008a). The occurrence of these bursts suggests that glutamatergic neurons in NCMs intrinsic circuitry, or projection neurons from field L and/or CMM that provide inputs to NCM, are highly interconnected and able to synchronize their activity, as also occurs in the CA3 region of the mammalian hippocampus (Ishizuka et al. 1990; Johnston and Amaral 2004). In addition, this glutamatergic network is under the control of local GABAergic neurons, as demonstrated by the induction of bursting EPSCs upon inhibition of GABAergic transmission. In previous studies, however, the birds were not controlled for the amount of song exposure, but were taken directly from the aviary, where they were kept in the presence of singing males. Our present data show that the expression of bursting EPSCs in NCM slices is positively modulated by prior in vivo song exposure. Thus, auditory stimulation with song appears to result in an augmented spontaneous activity of the glutamatergic neural network, increasing the likelihood of the occurrence of bursting EPSCs and such increase is long-lived enough to be detected in the slice a few hours after the end of the song exposure protocol.
The increased probability of occurrence of bursting EPSCs in the NCM of song–song-exposed birds could result from an activity-dependent plasticity of intrinsic neuronal excitability which could be manifested by an increased expression or up-regulation of ion channels involved with regenerative action potential generation like the inward nonselective cationic conductance (Ih) and/or the low-threshold calcium current (ICaT) (McCornick and Pape 1990; McCornick and Huguenard 1992; Maccaferri and McBain 1996: Gill et al. 2006; Klueva et al. 2012) Neurons that express spontaneous low-frequency action potential firing near resting are prime candidates for being song-activated neurons. Our previous (Pinaud et al. 2008a) and current studies have not yet yielded evidence of pacemaker neurons in the NCM, suggesting that they probably do not represent a sizeable fraction of the neurons in this brain area. Alternatively, these neurons could project to NCM from other locations, such as field L and/or CMM.
Correlations with in vivo neurophysiological responses to song
It is interesting to note that repetitive presentation of a given song produces a long-lasting reduction in the firing of NCM neurons in vivo (Chew et al. 1995). This habituation effect contrasts markedly with the observed increase in the frequency of bursting EPSCs in NCM slices of song-exposed birds as compared to controls. In addition, we did not observe a decrease in amplitude or frequency of glutamatergic EPSCs or an increase in the GABAergic ISPCs frequency or amplitude as would be expected in a song-induced depression of neural activity. There are two limitations in our experiments that should be taken into consideration: first, because putative changes in synaptic currents induced by song exposure are probably restricted to specific synapses activated by song, it is possible they were undetected in our measurements. Second, these synaptic changes might be restricted to specific neuronal types, which are still unknown in NCM. To reduce the contribution of these constraints and increase our chances to observe song-induced synaptic changes, we maximized neuronal activation throughout NCM by increasing song diversity in our song exposure paradigm. On the other hand, Thompson and Gentner (2010) have demonstrated that neurons in the NCM of European starlings (Sturnus vulgaris) responded more strongly to unfamiliar songs than learned ones, so our observations could be a response to the unfamiliarity of the songs which were presented to the birds, rather than related to the habituation process. At present, it seems clear that even if song exposure does not produce visible change in spontaneous synaptic currents in NCM, it results in significant changes in the excitatory network that are detectable as an increase in bursting EPSCs in NCM slice recordings. The possible consequences of such changes in terms of song perceptual processing remain to be determined.
Gender differences in NCM neurotransmission
We observed that the NCM of males have larger glutamatergic sEPSCs and is more likely to express bursts of glutamatergic currents than the NCM of females. Moreover, the NCM of song-exposed males can occasionally show spontaneous bursts of EPSCs even when GABAergic inhibition is not blocked. This effect does not seem to result from a decreased GABAergic tone in the NCM of song–song-exposed birds, since we did not observe differences in GABAergic inhibition parameters between control and song-exposed birds. These observations point to a gender influence on the glutamatergic transmission in NCM. The NCM of both male and female zebra finches shows robust electrophysiological and gene expression responses to conspecific song (Mello et al. 1995; Chew et al. 1995, 1996; Bailey and Wade 2005; Terpstra et al. 2006). While no clear sex differences have been reported in parameter-like song-induced ZENK expression in NCM, a sex difference has been observed in the number of calbindin-positive cells, which represent a sub-population of GABAergic cells in caudal NCM (Pinaud et al. 2006). In addition, NCM not only expresses high levels of aromatase (Saldanha et al. 2000; Pinaud et al. 2006), a major enzyme in the conversion of testosterone to estrogen, but it also contains significant numbers of cells that express estrogen receptors (Saldanha and Coomaralingam 2005; Tremere et al. 2009; Jeong et al. 2011b). Furthermore, estradiol is synthesized in NCM in response to song stimulation (Remage-Healey et al. 2011; Tremere and Pinaud 2011), affects NCM responses to song, and inhibits GABAergic neurotransmission in NCM (Tremere et al. 2009; Tremere and Pinaud 2011). Thus, NCM is both a site of local estrogen production and a target of estrogen action. The sex difference differences we observed could result from a differential action of circulating or locally produced estrogen in males versus females, but further studies involving hormonal manipulations would be required to clarify this relationship.
We conclude that, using slice physiology, there is no effects of song exposure on basic parameters of glutamatergic and GABAergic synaptic transmission in zebra finch NCM. However, we show that the NCM of song-exposed birds generates more bursts of glutamatergic currents as compared to controls, a phenomenon that may result from an increased coordinated firing of glutamatergic neurons. This effect is long lasting, since the increased bursting could be detected in the slices hours after the onset of song exposure. We believe our findings are of relevance for understanding how the song auditory processing circuitry in NCM operates and how it is modulated by experience. Our data also points to a significant sex difference in the glutamatergic network, an effect that could be related to the known local actions of estrogen in NCM. The functional implications of our slice observations await further studies.
Acknowledgments
The animal handling and experimentation procedures were approved by the Ethics Committee on Animal Experimentation of the Ribeirão Preto School of Medicine of the University of São Paulo and by Oregon Health and Science University’s Institutional Animal Care and Use Committee, and are consistent with the National Institute of Health guidelines. Work supported by a grant from Fundação de Pesquisa do Estado de São Paulo (03/0419-0) to RML and a National Institute of Health-Fogarty International Collaborative Research Award (TW006955) to CVM and RML. AAD was supported by a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. We thank Drs. Carlos Eduardo L. Almado for discussing the statistical analysis and Christopher Kushmerick for reviewing the manuscript.
Abbreviations
- aCSF
Artificial cerebrospinal fluid
- AMPA
2-Amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid
- CMM
Caudomedial mesopallium
- DNQX
6,7-Dinitroquinoxaline-2,3-dione
- EPSC
Excitatory post-synaptic current
- mIPSC
Miniature inhibitory post-synaptic current
- NCM
Nidopallium caudomedialis
- sEPSC
Spontaneous excitatory post-synaptic current
- sIPSC
Spontaneous inhibitory post-synaptic current
- TTX
Tetrodotoxin
Contributor Information
André A. Dagostin, Departamento de Fisiologia, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes 3900, Ribeirão Preto, SP 14049-900, Brazil
Claudio V. Mello, Department of Behavioral Neuroscience, Oregon Health and Science University, Portland, OR, USA
Ricardo M. Leão, Email: leaor@fmrp.usp.br, Departamento de Fisiologia, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes 3900, Ribeirão Preto, SP 14049-900, Brazil.
References
- Bailey DJ, Wade J. FOS and ZENK responses in 45-day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav Brain Res. 2005;162:108–115. doi: 10.1016/j.bbr.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Farrant M, Swanson GT, Cull-Candy SG. CNQX increases GABA-mediated synaptic transmission in the cerebellum by an AMPA/kainate receptor-independent mechanism. Neuropharmacology. 2001;41:730–736. doi: 10.1016/s0028-3908(01)00135-6. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird song: biological themes and variation. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci USA. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci USA. 1996;93:195–1950. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill CH, Brown JT, Shivji N, Lappin SC, Farmer C, Randall A, McNaughton NC, Cobb SR, Davies CH. Inhibition of Ih reduces epileptiform activity in rodent hippocampal slices. Synapse. 2006;59:308–316. doi: 10.1002/syn.20242. [DOI] [PubMed] [Google Scholar]
- Gobes SM, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Curr Biol. 2007;17:783–789. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Terleph TA, Burrows K, Tremere LA, Pinaud R. Expression and rapid experience-dependent regulation of type-A GABAergic receptors in the songbird auditory forebrain. Dev Neurobiol. 2011a;71:803–817. doi: 10.1002/dneu.20896. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Burrows K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. Eur J Neurosci. 2011b;34:283–291. doi: 10.1111/j.1460-9568.2011.07743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- Johnston D, Amaral DG. Hippocampus. In: Shepherd GM, editor. Synaptic organization of the brain. New York: Oxford University Press; 2004. pp. 455–498. [Google Scholar]
- Klueva J, Lima AD, Meis S, Voigt T, Munsch T. Hyperpolarization-activated cation current contributes to spontaneous network activity in developing neocortical cultures. Neurosignals. 2012;20:35–47. doi: 10.1159/000330813. [DOI] [PubMed] [Google Scholar]
- Kroodsma DE, Miller EH. Ecology and evolution of acoustic communication in birds. Ithaca: Cornell University Press; 1996. [Google Scholar]
- London S, Clayton D. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurone. J Physiol. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCornick DA, Huguenard DA. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- McCornick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV. Mapping vocal communication pathways in birds with inducible gene expression. J Comp Physiol A. 2002;188:943–959. doi: 10.1007/s00359-002-0347-1. [DOI] [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton DF. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in zebra finch telencephalon. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Velho TA, Pinaud R. Song-induced gene expression: a window on song auditory processing and perception. Ann N York Acad Sci. 2004;1016:263–281. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Mello CV. GABA immunoreactivity in auditory and song control brain areas of zebra finches. J Chem Neuroanat. 2007;34:1–21. doi: 10.1016/j.jchemneu.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Velho TA, Jeong JK, Tremere LA, Leao RM, von Gersdorff H, Mello CV. GABAergic neurons participate in the brain’s response to birdsong auditory stimulation. Eur J Neurosci. 2004;20:1318–1330. doi: 10.1111/j.1460-9568.2004.03585.x. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J Neurobiol. 2006;66:182–195. doi: 10.1002/neu.20211. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Terleph TA, Tremere LA, Phan ML, Dagostin AA, Leao RM, Mello CV, Vicario DS. Inhibitory network interactions shape the auditory processing of natural communication signals in the songbird auditory forebrain. J Neurophys. 2008a;100:441–455. doi: 10.1152/jn.01239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Osorio C, Alzate O, Jarvis ED. Profiling of experience-regulated proteins in the songbird auditory forebrain using quantitative proteomics. Eur J Neurosci. 2008b;27:1409–1422. doi: 10.1111/j.1460-9568.2008.06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Coomaralingam L. Overlap and co-expression of estrogen synthetic and responsive neurons in the songbird brain—a double-label immunocytochemical study. Gen Comp Endocrinol. 2005;14:66–75. doi: 10.1016/j.ygcen.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Schlinger AP, Arnold BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends Pharmacol Sci. 1999;20:268–270. doi: 10.1016/s0165-6147(99)01334-6. [DOI] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, Riebel K, van der Burg JM, den Boer-Visser AM. Localized brain activation specific to auditory memory in a female songbird. J Comp Neurol. 2006;494:784–791. doi: 10.1002/cne.20831. [DOI] [PubMed] [Google Scholar]
- Thompson J, Gentner T. Song recognition learning and stimulus-specific weakening of neural responses in the avian auditory forebrain. J Neurophys. 2010;103:1785–1882. doi: 10.1152/jn.00885.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Velho TA, Mello CV. Synapsins are late activity-induced genes regulated by birdsong. J Neurosci. 2008;28:11871–11882. doi: 10.1523/JNEUROSCI.2307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho TA, Pinaud R, Rodrigues PV, Mello CV. Co-induction of activity-dependent genes in songbirds. Eur J Neurosci. 2005;22:1667–1678. doi: 10.1111/j.1460-9568.2005.04369.x. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]