Abstract
Medicinal chemical approaches have been applied to all four of the adenosine receptor (AR) subtypes (A1, A2A, A2B, and A3) to create selective agonists and antagonists for each. The most recent class of selective AR ligands to be reported is the class of A2BAR agonists. The availability of these selective ligands has facilitated research on therapeutic applications of modulating the ARs and in some cases has provided clinical candidates. Prodrug approaches have been developed which improve the bioavailability of the drugs, reduce side-effects, and/or may lead to site-selective effects. The A2A agonist regadenoson (Lexiscan®), a diagnostic drug for myocardial perfusion imaging, is the first selective AR agonist to be approved. Other selective agonists and antagonists are or were undergoing clinical trials for a broad range of indications, including capadenoson and tecadenoson (A1 agonists) for atrial fibrillation, or paroxysmal supraventricular tachycardia, respectively, apadenoson and binodenoson (A2A agonists) for myocardial perfusion imaging, preladenant (A2A antagonist) for the treatment of Parkinson’s disease, and CF101 and CF102 (A3 agonists) for inflammatory diseases and cancer, respectively. This article is part of a Special Issue entitled: “Adenosine Receptors”.
Keywords: Adenosine receptor, Agonist, Antagonist, Clinical trial, Medicinal chemistry, G protein-coupled receptor
1. Introduction
Extracellular adenosine acts on a family of four cell surface receptors termed adenosine receptors (ARs) of which there exist four subtypes: A1, A2A, A2B, and A3 [1,2]. The ARs are G protein-coupled receptors (GPCRs) and consist of a single polypeptide chain that transverses the membrane from the extracellular side beginning at the N terminus to form seven transmembrane helices (TMs). The A1 and A3 receptors preferentially couple to Gi protein to inhibit adenylate cyclase and consequently the production of cyclic AMP (cAMP), and the A2A and A2B subtypes stimulate the production of cAMP by coupling to Gs or Go. These two subtype pairs also share higher sequence identity: the human A1 and A3ARs are 49% identical, and the human A2A and A2BARs are 59% identical.
The human A2AAR became the first non-rhodopsin, non-adrenergic GPCR for which an X-ray crystallographic structure was reported [3]. The availability of this physically determined structure has aided in recent drug discovery efforts [4,5], as did theoretical homology models previously. The initial structure of the A2AAR contained ZM241385 (53), only one of the many known potent antagonists of nanomolar affinity. There is now an effort to crystallize the receptor with other ligands and to crystallize other AR subtypes. Another structural issue to be considered in drug discovery is the phenomenon of GPCR dimerization, which can have a major effect on the pharmacological behavior. The ARs have been proposed to participate in both homo- and heterodimerization or even oligomerization [6,7]. For example, a well-established A2AAR/D2 dopamine receptor heterodimer occurs in the striatum and is the target of drug discovery for antagonists to treat Parkinson’s disease [8–10].
The effects of activation of ARs tend to be cytoprotective in many organs and tissues under a wide variety of physiological conditions. The levels of extracellular adenosine can rise substantially in response to stress, such as hypoxic stress, and the resultant activation of ARs acts to adapt to the stress [1,2]. Extracellular adenosine concentrations may rise as a result of the release of the breakdown of extracellular ATP or from intracellular sources, which leads to the activation of ARs in the vicinity. These protective responses may take the form of decreased energy demand (e.g. bradycardia), increased energy supply (e.g. vasodilation or angiogenesis), ischemic preconditioning (e.g. in the heart or brain), inhibition of the release of excitotoxic neurotransmitters, suppression of cytokine-induced apoptosis, or reduced inflammatory response [1,2].
Medicinal chemical approaches have been applied to all four of the AR subtypes to create selective agonists and antagonists for each (see Figs. 1–9 and Tables 1–3). The most recent class of selective AR ligands to be reported is the class of A2BAR agonists [11–13]. The availability of these selective ligands has facilitated research on therapeutic applications of modulating the ARs and in some cases has provided clinical candidates. It must be kept in mind that there is a marked species dependency of ligand affinity at the ARs, and that the same ligand (especially antagonists of the A3AR) could be selective for a given subtype in one species (e.g. human) and lose or reverse that selectivity in another species (e.g. rat) [14,15,17–20]. Therefore, caution must be used when characterizing new ligands and when using them in pharmacological experiments. In addition to receptor subtype selectivity, major considerations in the design of new ligands have been bioavailability and metabolic stability.
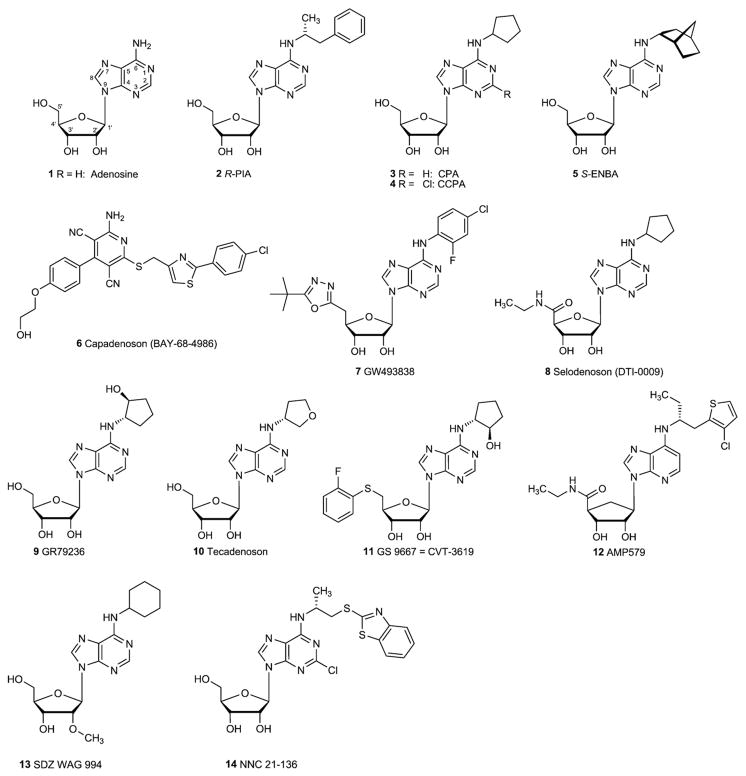
Fig. 1.
A1 adenosine receptor agonists.
Fig. 9.
Radioligands for positron emission tomography (PET) studies.
Table 1.
Adenosine receptor affinities of agonists.
| Ki (nM)a
|
|||||
|---|---|---|---|---|---|
| A1 | A2A | A2Bb | A3 | ||
| 1 | Adenosinec [35] | ca. 100 (h) | 310 (h) | 15,000 (h) | 290 (h) |
| 73 (r) | 150 (r) | 5100 (r) | 6500 (r) | ||
| A1-selective agonists | |||||
| 2 | R-PIA | 2.04 (h) [128] | 220 (r) [129] | 150,000 (h) [130] | 33 (h) [132] |
| 1.2 (r) [129] | 158 (r) [133] | ||||
| 19,000 (m) [131] | |||||
| 3 | CPA [109] | 2.3 (h) | 794 (h) | 18,600 (h) | 72 (h) |
| 4 | CCPA | 0.83 (h) [109] | 2270 (h) [109] | 18,800 (h) [109] | 38 (h) [109] |
| 1.3 (r) [134] | 950 (r) [134] | 237 (r) [134] | |||
| 0.1 (rb) [134] | 37.7 (rb) [134] | ||||
| 5 | (S)-ENBA [19] | 0.34 (r) | 477 (r) | ndd | 282 (h) |
| 915 (r) | |||||
| 6 | Capadenoson (BAY68-4986) | nd | nd | nd | nd |
| 7 | GW493838 | nd | nd | nd | nd |
| 8 | Selodenoson (DTI-0009) | nd | nd | nd | nd |
| 9 | GR79236 [109] | 3.1 (r) | 1300 (h) | nd | nd |
| 10 | Tecadenoson [109] | 6.5 (p) | 2315 (h) | nd | nd |
| 11 | CVT-3619 (GS 9667) [135] | 55 (h) | > 10,000 (h) | >50,000 (h) | > 1000 (h) |
| 12 | AMP579 [136] | 5.0 (r) | 56 (r) | nd | nd |
| 13 | SDZ WAG 994 [137] | 23 (p) | 25,000 (p) | Inactive (p) | nd |
| 14 | NNC 21-0136 [93] | 10 (r) | 630 (r) | nd | nd |
| A2A-selective agonists | |||||
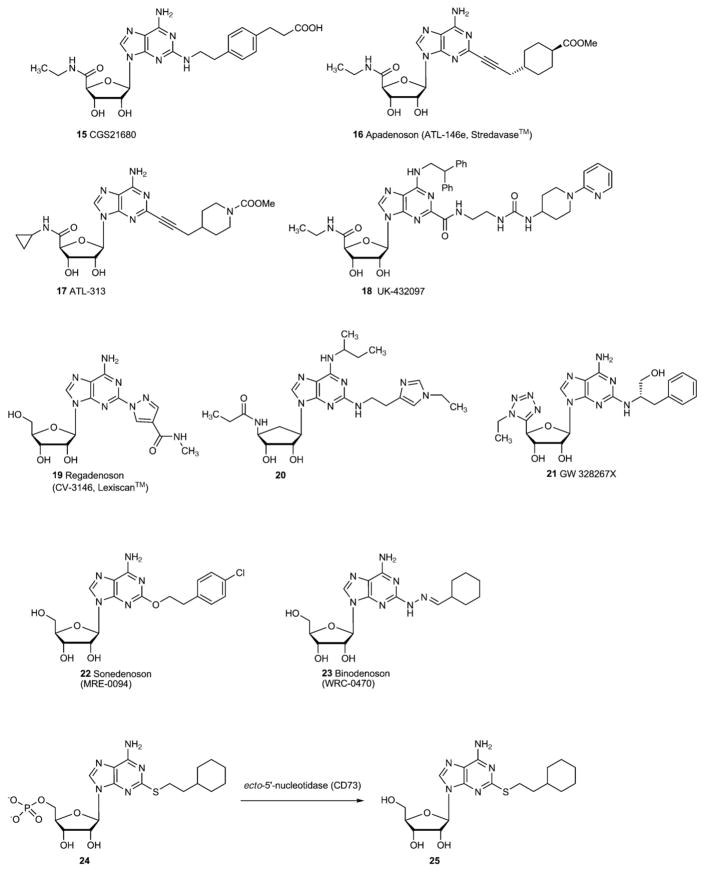
| 15 | CGS21680 | 289 (h) [109] | 27 (h) [109] | >10,000 (h) [109] | 67 (h) [109] |
| 1800 (r) [134] | 19 (r) [134] | ||||
| 120 (rb) [134] | 584 (r) [134] | ||||
| >10,000 (r) [134] | 673 (rb) [134] | ||||
| 16 | Apadenoson (ATL-146e) [109] | 77 (h) | 0.5 (h) | nd | 45 (h) |
| 17 | ATL-313 | nd | nd | nd | nd |
| 18 | UK-432097 [44] | nd | 4 (h) | nd | nd |
| 19 | Regadenoson (CV-3146) [109] | >10,000 (h) | 290 (h) | >10,000 (h) | > 10,000 (h) |
| 20 | [138] | >10,000 (h) | 5.4 (h) | 9866 (h) | 1640 (h) |
| 21 | GW328267X [138] | 882 (h) | 2.3 (h) | 51 (h) | 4.2 (h) (antagonist) |
| 22 | Sonedenoson (MRE-0094) [139] | >10,000 (h) | 490 (h) | >10,000 (h) | nd |
| 23 | Binodenoson (WRC-0470) [45] | 48,000 (h) | 270 (h) | 430,000 (h) | 903 (h) |
| 25 | [45] | 400 (r) | 372 (r) | nd | 3640 (h) |
| 50 (m) | |||||
| A2B-selective agonists | |||||
| 26 | [46] | 1050 (h) | 1550 (h) | 82 (h) | > 5000 (h) |
| 27 | MRS3997 [47] | 253 (h) | 150 (h) | 128 (h) | 90 (h) |
| 28 | BAY 60-6583 | >10,000 (h)c, [140] | > 10,000 (h)c, [140] | 3–10 (h) [140] | > 10,000 (h)c, [140] |
| 330 (m)e, [141] | |||||
| 750 (d)e, [141] | |||||
| 340 (rb)e, [141] | |||||
| A3-selective agonist | |||||
| 29 | IB-MECA (CF101) [109] | 51 (h) | 2900 (h) | 11,000 (h) | 1.8 (h) |
| 30 | Cl-IB-MECA (CF102) | 220 (h) [109] | 5360 (h) [109] | >10,000 (h) [134] | 1.4 (h) [109] |
| 280 (r) [134] | 470 (r) [134] | 0.33 (r) [134] | |||
| 35 (m) [134] | ~10,000 (m) [134] | >10,000 (m) [134] | 0.18 (m) [134] | ||
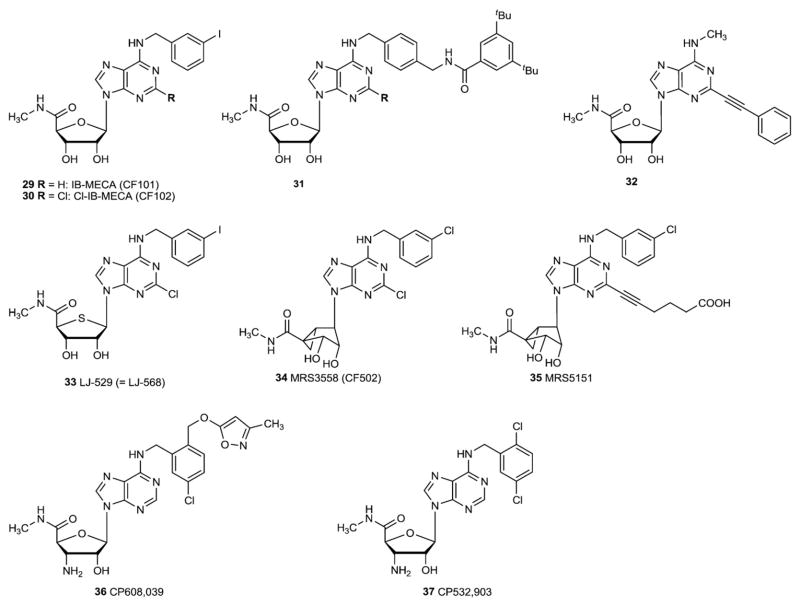
| 31 | [51] | 245 (h) | > 10,000 (h) | nd | 2.25 (h) |
| 32 | [52] | 32,800 (h) | 41,700 (h) | >30,000 (h) | 0.44 (h) |
| 33 | LJ529 [142] | 193 (h) | 223 (h) | nd | 0.38 (h) |
| 34 | MRS3558 (CF502) | 260 (h) [109] | 2330 (h) [109] | >10,000 (h) [109] | 0.29 (h) [109] |
| 105 (r) [134] | 1080 (r) [134] | 1.0 (r) [134] | |||
| 15.8 (m) [134] | 10,400 (m) [134] | 1.49 (m) [134] | |||
| 35 | MRS5151 [143] | 14,900 (h) | ~10,000 (h) | nd | 2.38 (h) |
| 10,500 (m) | > 10,000 (m) | 24.4 (m) | |||
| 36 | CP608,039 [144] | 7300 (h) | nd | nd | 5.8 (h) |
| 1750 (rb) | 83 (rb) | ||||
| 37 | CP532,903 [103] | 898 (m) | > 10,000 (m) | >10,000 (m) | 9.0 (m) |
h = human; d = dog; m = mouse; p = pig; r = rat; rb = rabbit.
Most data are from functional studies.
Data from functional studies.
nd = no data available.
Data from radioligand binding studies versus the antagonist radioligand [3H]MRS1754.
Table 3.
Adenosine receptor affinities of ligands used for positron emission tomography.
| Ki (nM)a
|
|||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
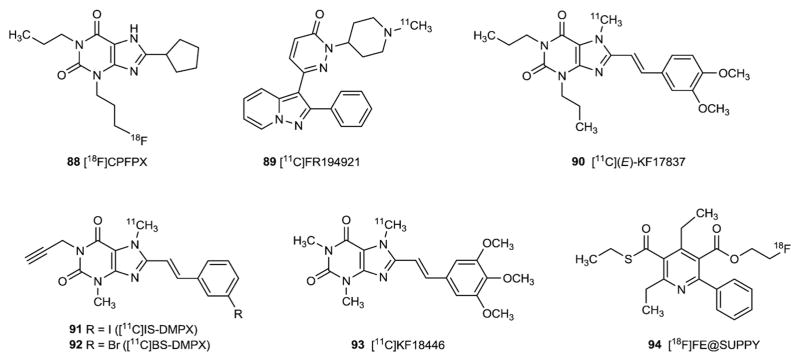
| 88 | CPFPX | 1.26 (h) [29] | 940 (h) [29] | ndb | nd |
| 0.63 (r) [29] | 812 (r) [29] | ||||
| 1.37(p) [29] | |||||
| 0.18 (c) [29] | |||||
| 89 | FR194921 | 2.91 (h) [185] | > 100 (h,r,m) [185] | nd | > 100 (h) [185] |
| 4.96 (r) [185] | |||||
| 6.49 (m) [185] | |||||
| 90 | (E)-KF17837 | 390 (r) [186] | 7.9 (r) [186] (E/Z) | 1500 (h) [186] | nd |
| 1.0 (r) [186] (E) | |||||
| 91 | IS-DMPX | 8.9 (r) [123] | > 8000 (r) [123] | nd | nd |
| 92 | BS-DMPX | 1200 (r) [187] | 8.2 (r) [187] | >10,000 (h) [188] | > 10,000 (h) [188] |
| 93 | KF18446 | nd | 5.9 (r) [124] | nd | nd |
| 94 | FE@SUPPY | 6050 (r) [189] | 9670 (r) [189] | nd | 9.67 (h) [189] |
h = human; pig; m = mouse; p = pig; r = rat; a few A2B data are from functional (cAMP) studies.
nd = no data available.
Synthetic adenosine agonists are under development as therapeutic agents. The half-life of adenosine in circulation is very short (~1 s), due to the action of enzymes that convert it to inosine (adenosine deaminase) or phosporylate it to 5′-AMP (adenosine kinase), or due to its uptake through nucleoside transporters (such as the equilibrative transporter ENT1) [1,2,21]. Therefore, analogues of adenosine for selective activation of ARs tend to prevent these processes and thereby lengthen the half-life. For example, the A3-selective agonist IB-MECA (29) has a half-life of 8–9 h in man [22]. Adenosine itself is in use as an AR agonist for the treatment of paroxysmal supraventricular tachycardia (through the A1 receptor) and in radionuclide myocardial perfusion imaging (through the A2A receptor). For those applications, the short half-life of adenosine is advantageous. There are many selective and potent synthetic AR agonists that have been introduced as research tools and for consideration to be used in humans. So far, only one synthetic adenosine agonist (A2AAR agonist regadenoson, 19, Lexiscan™) is in clinical use, and that, so far, is for a diagnostic purpose rather than therapeutic use. One major consideration in the development of AR agonists is that desensitization of the receptor can occur after agonist binding, resulting in downregulation of the receptor. Thus, AR responses can desensitize rapidly, typically on the scale of less than one hour [23].
Synthetic adenosine antagonists have also been explored for potential therapeutic applications. Various early analogues of the xanthines, which greatly increased the AR subtype-selectivity over the naturally occurring alkylxanthines, tended to be hydrophobic and poorly water-soluble and consequently of low bioavailability [24–26]. More recently introduced AR antagonists and prodrug approaches have overcome some of these issues [e.g. 25–28].
The introduction of numerous radioligands for the ARs has aided in the drug discovery process. Thus, the primary screen of newly synthesized compounds in many AR drug discovery efforts has consisted of convenient radioligand binding assays [24]. Furthermore, both agonist and antagonist ligands containing positron-emitting radioisotopes have been introduced for 3-dimensional in vivo imaging of the receptors [29]. Such ligands for positron emission tomography (PET) might prove useful for diagnostic as well as research purposes. Now fluorescent ligands have been introduced for characterization of the ARs [30–32]. Some of these spectroscopic probes are suitable for compound screening and avoid the use of radioisotopes.
2. Adenosine receptor agonists
The structure–activity relationship (SAR) of adenosine derivatives as AR agonists has been exhaustively probed. Nearly all of the known AR agonists are derivatives of purine nucleosides, either adenosine (1) or xanthosine (Figs. 1–4 and Table 1). Therefore, in screens of structurally diverse chemical libraries, most of the hits will typically provide antagonists, rather than agonists. One exception to that generalization is the class of 2-aminopyridine-3,5-dicarbonitrile derivatives that act as agonists at ARs with varied degrees of subtype selectivity [33–35].
Fig. 4.
A3 adenosine receptor agonists.
2.1. A1-selective agonists
The SAR of adenosine ligands at the A1AR was recently reviewed [36]. The earliest synthetic analogues of adenosine, such as N6-[(R)-phenylisopropyl]adenosine (2, R-PIA), to be characterized at the ARs tended to be selective for the A1AR (Fig. 1). In general, substitution of adenosine at the N6-position with a wide range of alkyl, cycloalkyl, and arylalkyl groups increases selectivity for the A1AR. In addition, any modification at the N6-position precludes the action of adenosine deaminase, which rapidly degrades adenosine itself, in vivo.
N6-Cycloalkyl substitution has been the most successful and general means of achieving selectivity for the A1AR. N6-Cyclopentyladenosine (3, CPA) and its 2-chloro analogue (4, CCPA) are among the most potent and selective A1AR in wide use as pharmacological agents. As with many other N6-substituted adenosine analogues, these two derivatives display considerable affinity at the A3AR. In fact, CCPA was shown to act as an antagonist of the human A3AR with a Ki value of 35 nM [37]. It was noted that the bicyclic analogue S-ENBA (5) has subnanomolar affinity at the A1AR and has less residual affinity than CPA (3) or CCPA (4) for other AR subtypes [19]. Bayer Co. (Germany) discovered 2-amino-3,5-dicyanopyridine derivatives, e.g. capadenoson (6), as non-nucleoside-derived adenosine receptor agonists [33,36]. Besides 6 several A1-selective adenosine derivatives, including GW493838 (7), selodenoson (8), GR79236 (9), tecadenoson (10), and CVT-3619 (GS9667, 11) have been evaluated in clinical trials for various indications (see below).
2.2. A2A-selective agonists
The SARs of ligands at the A2AAR have been reviewed recently [38,39]. Substitution of adenosine at the 2-position, especially with (thio)ethers, secondary amines, and alkynes, has resulted in many synthetic analogues selective for the A2AAR. The presence of a 5′-N-alkyluronamide modification, as found in the potent nonselective agonist NECA, a 5′-N-ethyluronamide, tends to maintain or enhance the selectivity for the A2AAR. These two modifications are present in the widely used A2AAR agonist CGS21680 (15) (Fig. 2). The 2-(2-phenylethyl)amino modification of adenosine was particularly conducive to enhanced affinity at the A2AAR and is present in an extended chain in CGS21680 (15). The carboxylate group at the terminal position of CGS21680 was found to act as a general site for chain extension and derivatization with bulky groups, including fluorescent groups and dendrimeric polymers [40,41], without losing high affinity of binding to the receptor. In receptor docking of agonist structures [42,43], this chain is pointing toward the extracellular face of the receptor, which has relaxed structural constraints relative to the main TM binding site. The 2-(2-cyclohexylethyl)amino modification of adenosine also favored high affinity at the A2AAR. Extended substituents are also present at the 2-position of the more recently introduced A2AAR, such as apadenoson (ALT-146e, 16) and ATL-313 (17), which are 5′-uronamide modified analogues.
Fig. 2.
A2A adenosine receptor agonists.
Certain N6-position substitutions have also been found to increase the affinity at the A2AAR. An example of this is the class of N6-(2,2-diphenylethyl)adenosine analogues, such as UK-432097 (18). Regadenoson (Lexiscan™, 19) [191] has been introduced as a diagnostic for stress testing due to its vasodilatatory effects, and apadenoson (16) is developed for the same application.
An inverse amide structure in the 4′-position as in the C2,N6-substituted adenosine analogue 20, which is additionally lacking the oxygen atom in the ribose-analogous cyclopentane ring, is also well tolerated by the A2AAR. Furthermore, the 4′-hydroxymethylene group in adenosine derivatives can be exchanged for a tetrazolyl residue as in GW328267X (21) (Fig. 2).
Several A2A-selective agonists including UK-432097 (18), sonedenoson (22), and binodenoson (23) have been clinically evaluated (see below). A major problem with the systemic application of A2A agonists as anti-inflammatory therapeutics has been their potent hypotensive effects. Recently, efforts have been undertaken to obtain A2A agonists which show site-specific action. A2A agonists, such as 18 have been developed for the treatment of bronchial inflammation (constructive pulmonary disease, COPD) by inhalation with limited systemic exposure [44]. In another approach 5′-phosphate prodrugs of A2A agonists have been prepared (e.g. 24) which are to be preferably cleaved releasing the A2A agonist 25 at sites of inflammation where ecto-5′-nucleotidase (CD73) is highly expressed [45].
2.3. A2B-selective agonists
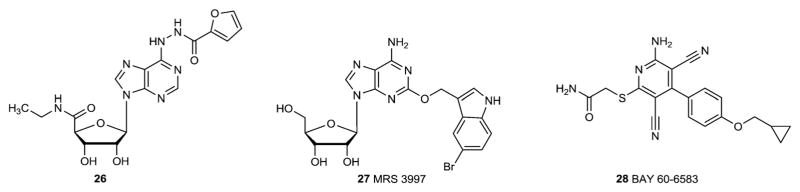
The SAR of adenosine agonists at the A2BAR was recently reviewed [11,13]. Substitution of adenosine at the N6-position with a narrow range of aryl groups increases affinity at the A2BAR. Also, very specific modification of the 5′- and C2-positions complements this increased affinity at the A2BAR (e.g. compound 26) (Fig. 3). Thus, combinations of narrowly defined modifications have resulted in compounds that interact selectively with A2BAR [11,46] or that activate the A2BAR along with the A2AAR (MRS3997, 27) [47].
Fig. 3.
A2B adenosine receptor agonists.
BAY 60-6583 (28) is one of the 2-aminopyridine-3,5-dicarbonitrile derivatives found to activate the ARs [33]. This compound appears to be an A2BAR-selective agonist [48,49].
2.4. A3-selective agonists
The SAR of ligands at the A3AR was recently reviewed [50]. Substitution with an N6-benzyl group or substituted benzyl group increases selectivity for the A3AR in both human and rat (e.g. 29, 33) (Fig. 4). Even bulky substituents as in compound 31 are well tolerated [51]. The N6-methyl (e.g. compound 32) and ethyl groups also favor A3AR in human [52]. As with A2AAR agonists, the NECA-like 5′-uronamide modification has also been found to be conducive to selectivity in A3AR agonists. IB-MECA (29) and its 2-chloro analogue Cl-IB-MECA (30) are prototypical and widely used agonists of the A3AR. Cl-IB-MECA (30) is more A3AR selective (~2000-fold compared to the A1AR) than IB-MECA (~50-fold compared to the A1AR). The 4′-thioadenosine derivative LJ-529 (33), which is otherwise equivalent to Cl-IB-MECA, acts as a highly potent and selective A3AR agonist with a subnanomolar affinity [53].
The ribose ring is normally freely twisting in solution and can adopt a range of conformations. The North conformation of the ribose ring was found to be the preferred conformation for binding to the A3AR. It is possible to chemically freeze this preferred conformation in analogues containing a [3.1.0]bicyclohexane ring system in place of the ribose 5-membered ring. This observation was utilized in the design of more potent and selective analogues such as MRS3558 (34), which displays nanomolar affinity at the A3AR [54]. The selectivity of MRS3558 for the A3AR is evident in a comparison of human ARs and rat ARs, but at the mouse ARs only 10-fold selectivity for the A3AR vs. the A1AR was observed. A third (alkynyl) substituent at the 2 position in MRS5151 (35) remedied this issue of species-dependent selectivity [55].
Recently, a PAMAM dendrimer conjugate of a chemically functionalized AR agonist was reported to bind to and activate the A3AR selectively with nanomolar affinity [56]. Such macromolecular receptor ligands can display pharmacological properties that are qualitatively different in comparison to the monomeric agonists.
3. Adenosine receptor antagonists
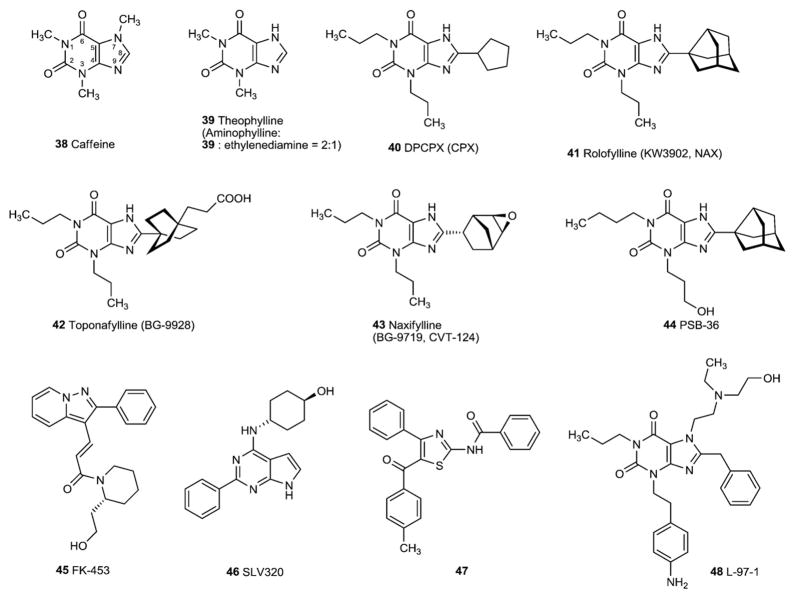
The prototypical AR antagonists were alkylxanthine derivatives. The stimulants caffeine (38) and theophylline (39) are natural products that behave as weak and nonselective AR antagonists (Fig. 5). The structure–activity relationship (SAR) of xanthine derivatives as AR antagonists has been exhaustively probed. The effects of receptor subtype selectivity of substitution at the 1-, 3-, 7-, and 8-positions have been explored in detail [15]. However, many newer, highly selective AR antagonists are more chemically diverse than the xanthines and contain nonpurine heterocyclic core structures (Figs. 5–8 and Table 2). Various classes of AR antagonists and their synthetic methods have been reviewed [21,24,57,58].
Fig. 5.
A1 adenosine receptor antagonists.
Fig. 8.
A3 adenosine receptor antagonists.
Table 2.
Adenosine receptor affinities of antagonists.
| Ki (nM)a
|
|||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| Non-selective antagonists | |||||
| 38 | Caffeine | 10,700 (h) [145] | 23,400 (h) [67] | 33,800 (h) [14] | 13,300 (h) [145] |
| 44,900 (h) [67] | 9560 (h) [145] | 10,400 (h) [149] | >100,000 (r) [133] | ||
| 41,000 (r) [146] | 45,000 (r) [147] | 20,500 (h) [150] | |||
| 44,000 (r) [147] | 32,500 (r) [148] | 30,000 (r) [131] | |||
| 47,000 (gp) [20] | 48,000 (r) [145] | 13,000 (m) [131] | |||
| 44,000 (c) [20] | |||||
| 39 | Theophylline | 6770 (h) [127] | 1710 (h) [127] | 9070 (h) [149] | 22,300 (h) [145] |
| 14,000 (r) [151] | 6700 (h) [145] | 74,000 (h) [150] | 86,400 (h) [127] | ||
| 8740 (r) [145] | 22,000 (r) [151] | 15,100 (r) [149] | >100,000 (r) [133] | ||
| 7060 (gp) [152] | 25,300 (r) [145] | 5630 (m) [141] | 85,000 (r) [154] | ||
| 4710 (rb) [152] | 11,000 (gp) [153] | >100,000 (d) [155] | |||
| 9050 (s) [152] | 17,700 (rb) [141] | ||||
| 6330 (c) [152] | 38,700 (d) [141] | ||||
| A1-selective antagonists | |||||
| 40 | DPCPX (CPX) | 3.0 (h) [25] | 129 (h) [127] | 51 (h) [25] | 795 (h) [156] |
| 0.50 (r) [25] | 60 (h) [25] | 63.8 (h) [149] | 243 (h) [25] | ||
| 1.0 (r) [149] | 157 (r) [148] | 186 (r) [149] | 509 (h) [155] | ||
| 0.18 (r) [152] | 500 (r) [149] | 200 (r) [153] | 3960 (h) [127] | ||
| 1.06 (gp) [152] | 86.2 (m) [141] | >10,000 (r) [25] | |||
| 3.9 (gp) [20] | 145 (gp) [153] | 43,000 (r) [155] | |||
| 0.21 (rb) [152] | 96.0 (rb) [141] | 708 (rb) [155] | |||
| 0.10 (s) [152] | 147 (d) [141] | 115 (d) [155] | |||
| 0.05 (c) [152] | 132 (d) [153] | ||||
| 0.29 (c) [20] | |||||
| 11.4 (d) [155] | |||||
| 41 | Rolofylline (KW3902, NAX) | 0.72 (h) [61] | 108 (h) [61] | 296 (h) [157] | 4390 (h) [157] |
| 8.0 (h) [157] | 673 (h) [157] | ||||
| 0.19 (r) [158] | 380 (r) [158] | ||||
| 12.6 (r) [61] | 510 (r) [61] | ||||
| 42 | Toponafylline (BG-9928) | 7.4 (h) [157] | 6410 (h) [157] | 90 (h) [157] | >10,000 (h) [157] |
| 3.9 (mk) [159] | 943 (mk) [159] | ||||
| 1.3 (r) [157] | 2440 (r) [157] | ||||
| 29 (d) [159] | 4307 (d) [159] | ||||
| 43 | Naxifylline (BG9719, CVT-124) | 0.45 (h) [61] | 1100 (h) [61] | 611 (h) [158] | 4810 (h) [158] |
| 12 (h) [158] | 1660 (h) [158] | 1010 (m) [141] | |||
| 0.67 (r) [61] | 1250 (r) [61] | 470 (rb) [141] | |||
| 742 (d) [141] | |||||
| 44 | PSB-36 | 0.7 (h) [25] | 980 (h) [25] | 187 (h) [25] | 2300 (h) [25] |
| 0.124 (r) [25] | 552 (r) [25] | 6500 (r) [25] | |||
| 45 | FK-453 | 18 (h) [109] | 1300 (h) [109] | 980 (h) [109] | >10,000 (h) [109] |
| 46 | SLV320 | 1.00 (h) [160] | 398 (h) [160] | 3981 (h) [160] | 200 (h) [160] |
| 2.51 (r) [160] | 501 (r) [160] | ||||
| 47 | Thiazole derivative | 57.4 (h) [63] | 6250 (h) [63] | >1000 (r) [63] | 2160 (h) [63] |
| 4.83 (r) [63] | > 1000 (r) [63] | ||||
| 48 | L-97-1 | 580 (h) [161] | > 100,000 (h) [161] | >100,000 (h) [161] | ndb |
| A2A-selective antagonists | |||||
| 49 | Istradefylline (KW6002) | 841 (h)c | 12 (h) [162] | >10,000 (h)c | 4470 (h)c |
| 230 (r)c | 91.2 (h)c | ||||
| 2.2 (r) [163] | |||||
| 4.46 (r) [164] | |||||
| 50 | CSC (Ki MAO-B=80.6 nM) [164] | 28,000 (r) [165] | 54 (r) [165] | 8200 [165] | >10,000 (r) [133] |
| 51 | MSX-2 | 900 (r) [26] | 8.04 (r) [26,148] | >10,000 (h) [26] | >10,000 (h) [26] |
| 2500 (h) [26] | 5.38 (h)d, [26] | ||||
| 14.5 (h)e, [26] | |||||
| 52 | CGS15943 | 3.5 (h) [18] | 1.2 (h) [18] | 32.4 (h) [141] | 35 (h) [18] |
| 6.4 (r) [18] | 9.07 (m) [141] | ||||
| 53 | ZM-241385 | 774 (h) [109] | 1.6 (h) [109] | 75 (h) [109] | 743 (h) [109] |
| 54 | Vipadenant (BIIB014, V2006) | 68 (h) [166] | 1.3 (h) [166] | 63 (h) [166] | 1005 (h) [166] |
| 55 | SCH-442416 | 1110 (h) [109] | 4.1 (h) [32] | >10,000 (h) [109] | >10,000 (h) [109] |
| 56 | 2720 (r) [72] | 18.3 (r) [72] | 3420 (h) [72] | 489 (h) [72] | |
| 57 | SCH-58261 | 725 (h) [109] | 5.0 (h) [109] | 1110 (h) [109] | 1200 (h) [109] |
| 58 | Preladenant (SCH-420814) | >1000 (h) [65] | 0.9 (h) [65]0 | >1000 (h) [65] | >1000 (h) [65] |
| 59 | ST-1535 | 71.8 (h) [167] | 6.6 (h) [167]9 | 352.3 (h) [167] | >1000 (h) [167] |
| 60 | SYN-115 | nd | nd | nd | nd |
| 61 | 2.8 (h) [75] | 0.0038 (h) [75] | nd | nd | |
| 0.14 (h) cAMP [75] | |||||
| 62 | 228.4 (h) [76] | 0.38 (h) [76] | nd | nd | |
| A2B-selective antagonists | |||||
| 63 | MRS1754 | 403 (h) [168] | 503 (h) [168] | 1.97 (h) [168] | 570 (h) [168] |
| 16.8 (r) [168] | 612 (r) [168] | 12.8 (r) [168] | |||
| 16.6 (r) [153] | |||||
| 3.39 (m) [141] | |||||
| 9.12 (gp) [153] | |||||
| 1.79 (rb) [141] | |||||
| 12.8 (d) [141] | |||||
| 12.3 (d) [153] | |||||
| 66 | MRE-2029-F20 | 200 (h) [169] | > 1000 (h) [169] | 5.5 (h) [169] | >1000 (h) [169] |
| 65 | PSB-603 | >10,000 (h) [14] | > 10,000 (h) [14] | 0.553 (h) [14] | >10,000 (h) [14] |
| >10,000 (r) [14] | > 10,000 (r) [14] | KD 0.403 (h) [14] | |||
| KD 0.351 (m) [14] | |||||
| 67 | GS 6201 (CVT-6883) | 1940 (h) [170] | 3280 (h) [170] | 22 (h) [170] | 1070 (h) [170] |
| 64 | PSB-1115 | >10,000 (h) [156] | 24,000 (r) [151] | 53.4 (h) [156] | >10,000 (h) [156] |
| 2200 (r) [151] | |||||
| 68 | ATL 802 | 369 (h) [168] | 654 (h) [168] | 2.36 (h) [168] | >1000 (h) [168] |
| 9583 (m) [168]1 | 8393 (m) [168] | 8.58 (m) [168] | >10,000 (m) [168] | ||
| 69 | LAS38096 | 2821 (h) [171,172] | > 1000 (h) [171,172] | 17 (h) [171,172] | 1043 (h) [171,172] |
| 70 | nd | 965 (h) [173] | 3.5 (h) [173] | nd | |
| 71 | 100 (h) [174] | 51 (h) [174] | 8 (h) [174] | nd | |
| 21 (h) cAMP [174] | |||||
| 72 | 931 (h) [174] | 239 (h) [174] | 4 (h) [174] | 3754 (h) [174] | |
| 73 | 2444 (h) [175] | 2126 (h) [175] | 11 (h) [175] | >1000 (h) [175] | |
| 74 | OSIP | 37 (h) [176] | 328 (h) [176] | 0.41 (h) [176] | 450 (h) [176] |
| 75 | QAF805 | 186 (h) [177] | 1775 (h) [177] | 3.4 (h) [177] | 10.2 (h) [177] |
| A3-selective antagonists | |||||
| 76 | MRS1523 | >10,000 (h) [134] | 3660 (h) [134] | >10,000 (h) [134] | 18.9 (h) [109] |
| 15,600 (r) [134] | 2050 (r) [134] | 113 (r) [134] | |||
| >10,000 (m) [134] | 731 (m) [134] | ||||
| 77 | MRE3008-F20 | 1200 (h) [109] | 141 (h) [109] | 2100 (h) [109] | 0.82 (h) [109] |
| 78 | 562 (h) [178] | 778 (h) [178] | >10,000 (h) [178] | 0.108 (h) [178] | |
| 79 | VUF-5574 | ≥10,000 (r) [179] | ≥10,000 (r) [179] | nd | 4.03 (h) [179] |
| 80 | KF26777 | 1800 (h) [180] | 470 (h) [180] | 620 (h) [180] | 0.20 (h) [180] |
| 81 | PSB-10 | 1700 (h) [181] | 2700 (h) [181] | nd | 0.441 (h) [28] |
| 805 (r) [28] | 6040 (r) [28] | ||||
| 82 | PSB-11 | 1640 (h) [181] | 1280 (h) [181] | 2100 (m) [28] | 2.34 (h) [181] |
| 440 (r) [181] | 2100 (r) [181] | KD 4.9 (h) [182] | |||
| 83 | >1000 (h) [183] | > 1000 (h) [183] | >1000 (h) [183] | 1.2 (h) [183] | |
| 84 | MRS5147 | 1760 (h) [55] | 1600 (h) [55] | nd | 0.73 (h) [55] |
| 85 | MRS5127 | 3040 (h) [55] | 1080 (h) [55] | nd | 1.44 (h) [55] |
| 86 | LJ1251 | 2490 (h) [184] | 341 (h) [184] | nd | 4.16 (h) [184] |
| 87 | OT7999 | >10,000 (h) [50] | > 10,000 (h) [50] | >10,000 (h) [50] | 0.95 (h) [50] |
h = human; c = cow; d = dog; gp = guinea pig; m = mouse; r = rat; rb = rabbit; s = sheep; a few A2B data are from functional (cAMP) studies.
nd = no data available.
Unpublished data (Müller et al.).
Recombinant receptors (expressed in CHO cells).
Native receptors (post-mortem brain).
3.1. A1-selective antagonists
A1-selective AR antagonists have recently been reviewed [36,59]. In general, modifications of the xanthine core structure at the 8-position with aryl or cycloalkyl groups have led to high affinity and selectivity for the A1AR. Highly selective xanthine antagonists of the A1AR have been reported. Many contain a cycloalkyl substitution at the 8-position. For example, the 8-cyclopentyl derivative DPCPX or alternately abbreviated CPX (40, 8-cyclopentyl-1,3-dipropylxanthine) (Fig. 5) is highly selective and of nanomolar affinity at the rat A1AR and is still selective, to a lesser degree, at the human A1AR. A bicycloalkyl group is present in the 8-(3-noradamantyl) group of rolofylline (41, KW-3902, MK-7418) [60]. Another 8-bicycloalkyl xanthine analogue naxifylline (BG9719, 42) was even more selective for the A1AR, with a Ki ratio human A2A/A1 of 2400 compared with a ratio of 150 for KW-3902 (41). While BG9719 is highly selective for A1AR compared to the human A2BAR, the selectivity of the related A1AR antagonist BG 9928 (42) is only ~10-fold. The 3-(3-hydroxypropyl)-substituted 1-butyl-8-noradamantylxanthine (44, PSB-36) shows a particularly high affinity and A1-selectivity [25]. Phosphate prodrugs of 3-(3-hydroxypropyl)xanthine derivatives show greatly improved water-solubility [25,62].
A variety of A1-selective antagonists with a non-xanthine structure has been developed [18,36,59], including the pyrazolopyridine derivative FK-453 (45) and the 7-deazaadenine derivative SLV320 (46), both of which have been evaluated in clinical trials. Recently, a 2-aminothiazole derivative (47) showing high A1 affinity and selectivity has been developed [63].
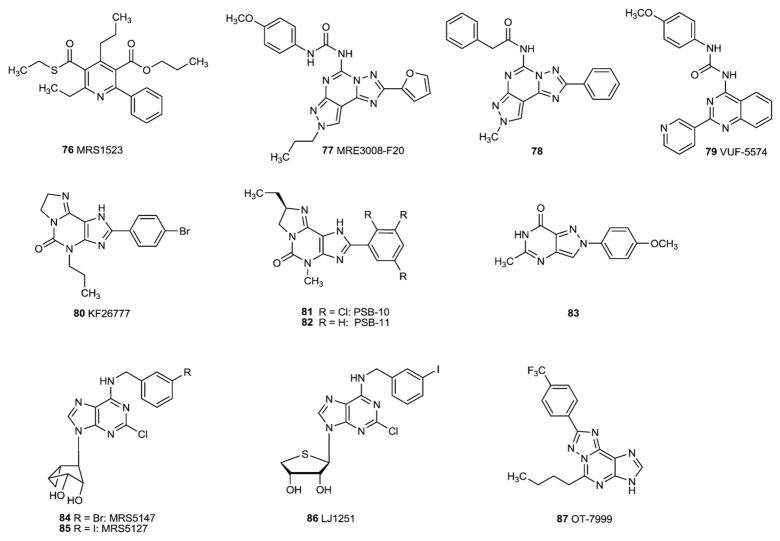
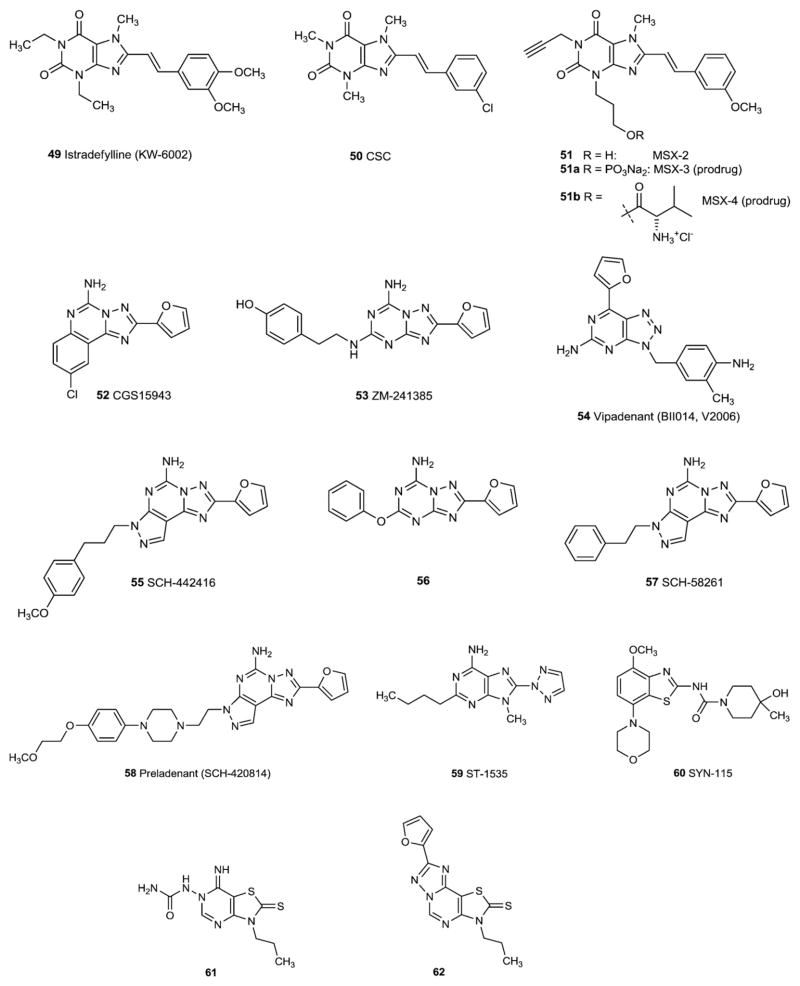
3.2. A2A-selective antagonists
Recent developments in the field of A2A antagonists have been described [64–66]. Modification of xanthines at the 8-position with alkenes (notably styryl groups) has led to selectivity for the A2AAR. The 8-styrylxanthine istradefylline (49, KW6002) was among the first A2AAR antagonists reported (Fig. 6). Some 8-styrylxanthine derivatives, such as CSC (50, 8-(3-chlorostyryl)caffeine), were later found to inhibit monoamine oxidase-B in addition to the A2AAR [67]. The phosphate prodrug MSX-3 (51a) and the L-valine ester prodrug MSX-4 (51b) have been prepared as water-soluble prodrugs of the potent and selective A2A antagonist MSX-2 (51) [26,27]. Both are now broadly used as pharmacological tools in particular for in vivo studies [e.g. 67–70].
Fig. 6.
A2A adenosine receptor antagonists.
Substituting various heterocyclic ring systems in place of the xanthine core has led to exceptionally high affinity and selectivity at the A2AAR. An early example of a heterocyclic structure proposed as an A2AAR antagonist was the triazoloquinazoline CGS15943 (52), which was later demonstrated to be only slightly selective. Later refinement of the triazoloquinazoline by addition of a third ring or alteration of the pattern of N inclusion in the heterocyclic system greatly improved the A2AAR selectivity. The triazolotriazine ZM241385 (53), the triazolopyrimidine vipadenant (54, BII014, V2006), and the pyrazolotriazolopyrimidine SCH442416 (55) are examples of highly potent A2AAR antagonists of later generation. ZM241385 (53) also binds to the human A2BAR with moderate affinity, and in both tritiated and iodinated form has been used as a radioligand at that receptor [71]. A recently described analogue (56) shows somewhat higher selectivity [72].
The affinity of SCH 442416 (55) at the human A2AAR was originally reported as Ki 0.048 nM [73], however later reports of binding assays have placed it in the low nanomolar range (4.1 nM in [32]). Related compounds include SCH 58261 (57) and preladenant (SCH 420814, 58). The latter is undergoing clinical trials for the treatment of Parkinson’s disease (see below).
Examples for further non-xanthine A2A antagonists are the adenine derivative ST-1535 (59) and the benzothiazole derivative SYN-115 (60), both of which are being clinically evaluated. Very recently, benzofurans [74], 7-imino-2-thioxo-thiazolo[4,5-d]pyrimidines [75] (e.g. 61) and the related thiazolotriazolopyrimidinethiones [76] (e.g. 62) have been described as new potent A2A-selective AR antagonists.
3.3. A2B-selective antagonists
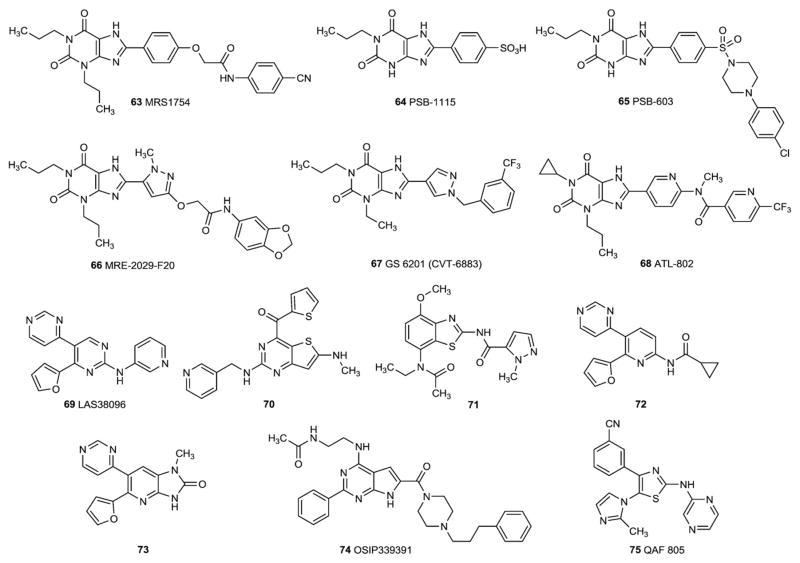
A2B AR antagonists have recently been reviewed [12,13]. Certain modifications of the xanthine core structure at the 8-position with aryl groups have been found to result in selectivity for the A2BAR [15]. The first antagonist to be reported was MRS1754 (63) (Fig. 7). Later, groups at Univ. of Bonn, Germany (PSB-1115 (64), PSB-603 (65)), Univ. of Ferrara, Italy (MRE-2029-F20, 66), at CV Therapeutics (now Gilead Sciences, CVT-6883, GS6201, 67) and at Adenosine Therapeutics (now Clinical Data Inc., ATL802, 68) improved on the degree of selectivity and/or the water solubility of the xanthines as A2BAR antagonists. For example, GS-6201 (CVT-6883, 67) has a selectivity of 88-fold vs. A1, 149-fold vs. A2A, and 49-fold vs. A3ARs (human species).
Fig. 7.
A2B adenosine receptor antagonists.
PSB-603 (65) shows a particularly high affinity and selectivity, not only in humans, but also in rodents. PSB-1115 (64) exhibits high water-solubility and is therefore useful for in vivo studies, however its A2B affinity and selectivity is lower than that for other A2B antagonists. Besides xanthines, 2-aminopyrimidine derivatives, such as LAS38096 (69), 2-aminothiazolopyrimidines (e.g. 70), benzothiazoles (e.g. 71) [174], pyridine derivatives (e.g. 72, 73) [171,175], have been developed as A2B antagonists. Compound 72 was shown to be metabolically unstable.
3.4. A3-selective antagonists
Review articles on A3AR antagonists have been previously published [16,17,77]. After it was recognized that the xanthines tended to be less potent as antagonists at the A3AR in comparison to the other AR subtypes, chemically diverse sources were examined for possible interactions with the A3AR. In an initial broad screen, several classes of nonxanthine antagonists were identified for the A3AR: 1,4-dihydropyridines, pyridines, and flavones. The pyridine derivative MRS1523 (76) (Fig. 8) has become a useful tool since it shows relatively high affinity not only for the human but also for the rat A3 receptor. Later it was noted that the potent nonselective AR antagonist CGS15943 (52) could be modified to produce A3AR selectivity, two of the most potent and selective compounds at human A3 receptors being MRE3008-F20 (77) and 78. The urea-substituted quinazoline derivative VUF5574 (79) also possesses high A2B affinity and selectivity. Some tricyclic xanthines (80–82) have been found to be very potent and selective antagonists at human A3 receptors showing increased water-solubility due to a basic nitrogen atom in the additional imidazole ring. Recently described A3-selective antagonists include pyrazolopyrimidinones (e.g. 83). Many A3 antagonists are much more potent at human as compared to rat A3 ARs.
The principles for converting selective A3AR agonists into selective A3AR antagonists are based on either a conformationally constrained ribose-like ring or one that is truncated at the 4′-position (i.e., missing the ribose CH2OH group entirely). Thus, the nucleoside derivatives MRS5147 (84) and its 3-iodo analogue MRS5127 (85) are highly selective A3AR ligands generally across species. MRS5127 (84) was recently reported as a radioligand selective for the A3AR [78]. The truncated 4′-thioadenosine derivative LJ-1251 (86), which acts as a A3AR antagonist across species, was shown to lower intraocular pressure when applied topically [79,80].
4. Adenosine receptor ligands for diagnostic and therapeutic use
A selection of clinically used or evaluated AR ligands is collected in Table 4.
Table 4.
Therapeutic drugs, imaging agents, and clinical candidates that act through adenosine receptors.
| Compound | Selectivity | Company | Indication or use (phase)a |
|---|---|---|---|
| Agonists | |||
| Adenosine (1) (Adenocard, Adenoscan) | A1, A2A | Astellas | Paroxysmal supraventricular tachycardia (approved), myocardial perfusion imaging (approved), other uses in testing |
| AMP579 (12) | A1, A2 | Aventis | Myocardial infarction (discontinued) |
| Apadenoson (16, Stedivaze, BMS068645, ATL146e) | A2A | Clinical Data | Myocardial perfusion imaging (III) |
| ATL-1222 | A2A | Clinical Data | Acute inflammatory conditions (preclinical) |
| ATL-313 (17) | A2A | Clinical Data | Ophthalmic disease (preclinical) |
| BAY 60-6583 (28) | A2B | Bayer | Atherosclerosis (preclinical) |
| Binodenoson (23, WRC-0470, MRE-0470) | A2A | Aderis, King | Myocardial perfusion imaging (III) |
| BVT.115959 | A2A | Biovitrum | Diabetic neuropathic pain (II) |
| Capadenoson (6, BAY68-4986, nonnucleoside) | A1 | Bayer Schering | Atrial fibrillation, chronic treatment (II) |
| Cl-IB-MECA (30, CF102) | A3 | Can-Fite | Liver cancer (I–II) |
| CP608,039 (36) | A3 | Pfizer | Cardiac ischemia (discontinued) |
| GS 9667 (11, CVT-3619) | A1 | Gilead | Hypertriglyceridemia associated with diabetes (I) |
| GR79236 (9) | A1 | GlaxoSmithKline | Pain, hyperlipidemia (I, discontinued) |
| GW493838 (7) | A1 | GlaxoSmithKline | Peripheral neuropathic pain (II, discontinued) |
| IB-MECA (29, CF101) | A1 | Can-Fite | Rheumatoid arthritis, psoriasis, dry eye, and other autoimmune inflammatory diseases (II), glaucoma (II) |
| MRS3558 (34, CF502) | A3 | Can-Fite | Autoimmune inflammatory diseases (preclinical) |
| NNC-21-0136 (14) | A1 | Novo Nordisk | Stroke, neurodegeneration (discontinued) |
| INO 8875 (PJ-875) | A1 | Inotek Pharmaceuticals | Glaucoma (II); atrial fibrillation (discontinued) |
| Regadenoson (19, Lexiscan, CV-3146) | A2A | Gilead and Astellas | Myocardial perfusion imaging (approved) |
| RPR749 | A1 | Aventis | Hyperlipidemia (I) |
| SDZ WAG 94 (13) | A1 | Sandoz/Novartis | Diabetes |
| Selodenoson (8) | A1 | Aderis | Atrial fibrillation (II) |
| Sonedenoson (22, MRE-0094) | A2A | King | Diabetic foot ulcers, wound healing (II) |
| Tecadenoson (10, CVT-510) | A1 | Gilead | Paroxysmal supraventricular tachycardia (III) |
| UK 432097 (18) | A2A | Pfizer | COPD (II) |
| Antagonists | |||
| ATL 844 | A2B | Clinical Data and Novartis | Asthma and/or diabetes |
| Naxifylline (43, BG-9719) | A1 | Biogen-Idec | Heart failure (renal function) (discontinued) |
| Caffeine (38) | McMaster University | Apnea | |
| CPFPX (88) | A1 | Research Center Jülich, Germany | PET imaging (18F) |
| FK-453 (45) | A1 | Astellas | Acute renal failure |
| GS 6201 (67, CVT-6883) | A2B | Gilead | Asthma (I) |
| Istradefylline (49, KW6002) | A2A | Kyowa-Hakko Kogyo | Parkinson’s disease (III, discontinued) |
| KF26777 (80) | A3 | Kyowa-Hakko Kogyo | Asthma (preclinical) |
| LAS38096 (69) | A2B | Almirall | Anti-inflammatory (preclinical) |
| LAS101057 [192] | A2B | Almirall | Antiasthmatic (I) |
| L-97-1 (48) | A1 | Endacea | Sepsis (preclinical) |
| MRE2029-F20 (66) | A2B | King | Anti-inflammatory (preclinical) |
| OSIP339391 (74) | A2B | OSI | Asthma (preclinical) |
| OT-7999 (87) | A3 | Otsuka | Glaucoma (preclinical) |
| Preladenant (58, SCH-420814) | A2A | Schering-Plough | Parkinson’s disease (III) |
| SCH-442416 (55) | A2A | Schering-Plough | PET imaging (11C) |
| QAF805 (75) | A2B, A3 | Novartis | Asthma (I) |
| Rolofylline (41, KW3902, NAX) | A1 | NovaCardia and Merck | Heart failure (renal function) (discontinued) |
| SLV320 (46) | A1 | Solvay | Heart failure (renal function) |
| ST-1535 (59) | A2A | Sigma-Tau | Parkinson’s disease (I) |
| SYN-115 (60) | A2A | Synosia Therapeutics/UCB (after Synosia Therapeutics) | Parkinson’s disease (II), addiction |
| Theophylline/aminophylline (39) | A1 | King Faisal University | Recovery after anaesthesia |
| Toponafylline (42, BG-9928) | A1 | Biogen-Idec | Heart failure (renal function) (IIb) |
| Vipadenant (54, BIIB014, V2006) | A2A | Vernalis and Biogen-Idec | Parkinson’s disease (II) |
Many of the clinical trials indicated are no longer current.
4.1. Agonists
Adenosine (1) itself for a long time was the only adenosine agonist to be used in humans. It is in widespread use in the treatment of paroxysmal supraventricular tachycardia (Adenocard®) due to its activation of A1 receptors, and as a diagnostic for myocardial perfusion imaging (Adenoscan®, Astellas Pharma, Inc.) utilizing its A2A-activating effects resulting in vasodilation. In addition, adenosine is being evaluated in several clinical trials for the treatment of inflammation, neuropathic and perioperative pain, and cardioprotection.
AMP579 (12) is a mixed agonist at A1 and A2AARs. It was in clinical trials for myocardial ischemic preconditioning and reperfusion injury. It was tolerated in patients with end-stage renal disease, but in a placebo-controlled trial of patients undergoing primary percutaneous transluminal coronary angioplasty it failed to reduce infarct size [81,82]. Recently it was also shown to activate the A2BAR, which may account for its cardioprotective properties in the rabbit heart [83].
A1AR agonists are useful in preclinical models of cardiac arrythmia and ischemia and in pain. Adenosine agonists are also of interest for the treatment of sleep disorders [84,85]. A2A agonists exhibit anti-inflammatory and immunosuppressive effects [86]. Activation of the A2B AR protects against vascular injury [87]. A3AR agonists have been proposed for the treatment of a wide range of autoimmune inflammatory conditions, such as rheumatoid arthritis, inflammatory bowel diseases, psoriasis, etc. [88–90], and also for cardiac and brain ischemia.
4.1.1. A1-selective agonists
A1-selective (partial) agonists have been clinically evaluated for the treatment of paroxysmal supraventricular tachycardia, atrial fibrillation, or angina pectoris (capadenoson (6), selodenoson (8), tecadenoson (10), and PJ-875), hypertriglyceridemia and type II diabetes (GR79236 (9), RPR-749, and GS9667/CVT-3619 (11)) and neuropathic pain (GW493838 (7), GR79236 (9)). Partial agonists are usually preferred to avoid receptor desensitization and to possibly achieve a certain tissue selectivity of the effects. The A1AR agonist SDZ WAG94 (13) was one of the first agonists of this subtype to progress to clinical trials, i.e. for consideration for treatment of diabetes [91].
A1AR agonists have antiischemic effects in the heart and brain. Recently, A1AR activation was shown to mediate neuroprotective effects through microglial cells [92]. Various A1AR agonists have been shown to be neuroprotective in ischemic and seizure models. However, the peripheral side effects of A1AR agonists could be severe. The A1AR agonist NNC-21-0136 (14) was previously in clinical development for the treatment of stroke and other neurodegenerative conditions [93]. It was found empirically to provide some degree of in vivo selectivity for the CNS in comparison to peripheral cardiovascular actions of adenosine that was not based on subtype selectivity.
Other A1AR-selective agonists are intended for activation of the receptor at peripheral locations. The A1AR-selective adenosine derivative GR79236 (9) has analgesic and anti-inflammatory actions in humans and animals [94]. The A1AR-selective agonist GW493838 (7) was also under evaluation for pain management. RPR749 (Aventis) and its methylated metabolite are orally active and selective adenosine A1AR agonists that inhibit lipolysis in adipocytes and lower plasma triglyceride levels [95]. GS-9667 (11, CVT-3619), a partial agonist of the A1AR, is in development as an antilipolytic agent. It acts as a full agonist of the A1AR in the inhibition of adenylate cyclase in adipocytes, which have a large receptor reserve and/or higher efficacy of coupling of the receptor to Gi. However, it is a partial agonist in the cardiovascular system and therefore lacks cardiovascular side effects.
A1AR agonists are of interest for use in treating cardiac arrhythmias, and it recently was suggested that a partial agonist of this subtype would have advantages over a full agonist for this use [96]. The A1AR-selective agonist selodenoson (formerly DTI-0009, 8) has been in clinic trials for treatment of acute and chronic control of tachycardia and topical treatment of diabetic foot ulcers (Aderis Pharmaceuticals). It was formulated for intravenous administration to control heart rate during acute attacks and for oral administration in the chronic management of atrial fibrillation. The nonnucleoside AR agonist BAY 68-4986 (capadenoson, 6) is under investigation for atrial fibrillation and for the treatment of angina.
4.1.2. A2A-selective agonists
The 2-substituted A2AAR agonists apadenoson (16, ATL-146e), binodenoson (23, MRE-0470 or WRC-0470), and sonedenoson (22, MRE0094) have been cardiovascular clinical candidates [97–99]. Such agonists are of interest for use as vasodilatory agents in cardiac imaging (like adenosine itself, marketed as Adenoscan®) and in suppressing inflammation [100]. Regadenoson (19, CVT-3146, Lexiscan®) is already approved for diagnostic imaging [101]. The A2A-agonist BVT.115959 (Biovitrum; structure not disclosed) has been claimed to show higher A2A affinity at pH 7.0 as compared to pH 7.4 and is currently in phase II for the treatment of diabetic neuropathic pain. Since decreased pH values are found in pathological, e.g. inflamed tissues, this would result in tissue-selective effects and reduced side-effects. Two selective A2A agonists developed by Adenosine Therapeutics (now Clinical Data) are in preclinical development for acute inflammatory conditions (ATL-1222, structure not disclosed) and ophthalmic disease (ATL-313, 17).
4.1.3. A3-selective agonists
The two A3AR agonists that are currently in clinical trials contain the 5′-N-methyluronamide modification and have nanomolar affinity at the receptor. Thus CF101 (29, Can-Fite Biopharma) and Cl-IB-MECA (30, CF102) are in trials for autoimmune inflammatory disorders and for liver cancer, respectively. CF101 (29) was recently demonstrated to be efficacious in clinical trials of rheumatoid arthritis, psoriasis, and dry eye disease [102]. Further clinical trials are planned for glaucoma and osteoarthritis. Two other A3AR agonists CP-608,039 (36) and its N6-(2,5-dichlorobenzyl) analogue CP-532,903 (37) [103] were previously under development for cardioprotection. MRS3558 (34, CF502) is in preclinical development for the treatment of autoimmune diseases.
4.2. Antagonists
The non-selective AR antagonists caffeine (38) and theophylline (39) have been used as drugs for various indications. Caffeine (38) is mainly applied for CNS stimulation to restore alertness and to counteract fatigue, for the treatment of pain (e.g. headache, migraine) typically in combination with analgesics such as acetylsalicylic acid and/or paracetamol/acetaminophen, and for the treatment of apnoea in premature babies [104]. Theophylline or its salt aminophylline (39) are mainly applied for the treatment of bronchial asthma and COPD as a second-line treatment [105], although their use is now limited as a result of side effects on the central nervous system and the renal system. Theophylline may also be used for the prevention of sleep apnea in adults and for apnea of prematurity as a substitute for caffeine. A clinical study is currently performed using aminophylline for recovery after sevoflurane anaesthesia, since sevoflurane indirectly leads to an activation of A1ARs.
A large number of synthetic AR antagonists that are much more potent and selective than the prototypical alkylxanthines have been introduced, although none have yet been approved for clinical use. Potent and selective AR antagonists display therapeutic potential as kidney protective (A1), antifibrotic (A2A), neuroprotective, antiasthmatic (A2B), and antiglaucoma (A3) agents [105–108].
4.2.1. A1 receptor antagonists
Various A1AR antagonists, xanthines and non-xanthines, have been or are currently being explored for clinical applications [109] for heart failure, and for improving renal function and treatment of acute renal failure. The xanthine derivative BG9719 (43), containing an epoxide ring, is highly selective, while the selectivity of the more water-soluble, metabolically more stable toponafylline (42) for the human A1AR compared to the human A2BAR is roughly 10. The 8-cyclopentyl derivative DPCPX (40), also known as CPX, which is selective for the A1AR in the rat with nanomolar affinity but less selective at the human AR subtypes, has been in clinical trials for cystic fibrosis through a non-AR related mechanism [110]. The highly selective A1AR antagonist L-97-1 (48, Endacea Inc.) is relatively well water-soluble and in late preclinical development for the treatment of asthma and sepsis [111]. As in the cases of DPCPX (40), rolofylline (41), naxifylline (BG 9719, 42), and others a persistent problem in the development of A1AR antagonists has been low water-solubility and low bioavailability [18,112]; thus, A1AR antagonists, e.g. toponafylline (43) and L-97-1 (48), with good water solubility are preferable clinical candidates.
Nonxanthine antagonists of the A1AR have also been shown to have high receptor subtype selectivity, e.g. FK453 (45) [113] and SLV 320 (46, Solvay Pharmaceuticals) [114]. For example, SLV 320 is in clinical trials as an intravenous treatment for acute decompensated heart failure with renal impairment.
4.2.2. A2A receptor antagonists
Several selective A2A antagonists have been evaluated in clinical trials for the treatment of Parkinson’s disease. The first one has been istradefylline (49, KW6002), which did not reach the endpoint of phase III clinical trials, but additional trials are planned [115]. The non-xanthine derivatives preladenant (58, SCH420814; phase III), vipadenant (54, BII014, V2006; phase II), ST-1535 (59; phase I), as well as SYN-115 (60, phase II). Further potential indications include other neurodegenerative diseases, such as Alzheimer’s disease, restless legs syndrome, depression, and addiction.
4.2.3. A2B receptor antagonists
Modification of xanthines at the 8 position with certain aryl groups has given rise to preclinical candidates that are selective for the A2BAR (e.g. 67, GS-6201, CVT-6883, Gilead Sciences). GS-6201 is the first selective A2B antagonist to be clinically evaluated for the treatment of asthma. Other A2B-selective xanthine and nonxanthine derivatives include ATL844 (structure not disclosed), MRE2029-F20 (66), LAS38096 (69), LAS101057, and OSIP339391 (74), which are intended for treatment of asthma and/or inflammatory diseases. The aminothiazole derivative QAF 805 (75), a mixed A2B/A3-antagonist, has failed to attenuate bronchial hyperresponsiveness to inhaled AMP in a phase 1b clinical trial in asthmatics [116], but has also been investigated for other indications.
4.2.4. A3 adenosine receptor antagonists
Cyclized derivatives of xanthines, such as PSB-11 (82), are A3AR-selective, and similar compounds have been explored by Kyowa Hakko (e.g. 80). Selective A3AR antagonists, such as the heterocyclic derivatives OT-7999 (87), are being studied for the treatment of glaucoma [117], and other such antagonists are under consideration for treatment of cancer, stroke, and inflammation [10,118]. No A3AR antagonists have yet reached human trials.
5. Radioligands for in vivo PET imaging of adenosine receptors
With the established relevance of ARs to human disease states, it has been deemed useful to develop high affinity imaging ligands for these receptors, for eventual diagnostic use in the CNS and in the periphery. Ligands for in vivo positron emission tomographic (PET) imaging of A1, A2A, and A3ARs have been developed (Fig. 9 and Table 3). For example, the xanthine [18F]CPFPX (88), similar in structure to DPCPX and the nonxanthine [11C]FR194921 (89) have been developed as centrally-active PET tracers for imaging of the A1AR in the brain [119].
11C-labeled (E)-KF17837 (90) was proposed as a potential PET radioligand for mapping the adenosine A2A receptors in the heart and brain [120,121]. 11C labeled (E)-8-(3-chlorostyryl)-1,3-dimethyl-7-[11C]methylxanthine ([11C]CSC, 50) proved to accumulate in the striatum, and PET studies on rabbits showed a fast brain uptake of [11C]CSC, reaching a maximum in less than 2 min [122]. Further styrylxanthine derivatives labeled with 11C were tested as in vivo probes [123]. [7-Methyl-11C]-(E)-3,7-dimethyl-8-(3-iodostyryl)-1-pro-pargylxanthine ([11C]IS-DMPX, 91) and [7-methyl-11C]-(E)-8-(3-bromostyryl)-3,7-dimethyl-1-propargylxanthine ([11C]BS-DMPX, 92) showed Ki affinities of 8.9 and 7.7 nM respectively, and high A2A/A1 selectivity values. Unfortunately, biological studies proved that the two ligands were only slightly concentrated in the striatum, and that the two compounds were not suitable as in vivo ligands because of low selectivity for the striatal A2A receptors and a high degree of nonspecific binding [123]. A useful A2A PET ligand for in vivo imaging proved to be [11C]KF18446 (93), also named (11C)TMSX [124]. Ex vivo autoradiography for this molecule showed a high striatal uptake and a high uptake ratio of the striatum to the other brain regions. In 2001 the synthesis and evaluation of [11C]KW-6002 (49) was reported. This molecule showed high retention in the striatum but it bound also to extra-striatal regions [190]. [11C]SCH442416 (55) has recently been explored as a PET agent in the non-invasive in vivo imaging of the human A2AAR [73,125].
Recently, an A3AR PET ligand, [18F]FE@SUPPY (94), based on a series of pyridine A3AR antagonists, was introduced [126]. Several nucleoside derivatives that bind with nanomolar affinity at the A3AR and that contain 76Br for PET imaging were recently reported, including the antagonist MRS5147 (84) [127].
6. Concluding remarks
In conclusion, selective agonists and antagonists for all four adenosine receptor subtypes have been developed and diagnostic and therapeutic applications are being explored. The first selective AR agonist, the A2A agonist regadenoson (Lexiscan®), has been approved as a diagnostic drug for myocardial perfusion imaging. Many other selective agonists and antagonists for the various receptor subtypes are undergoing clinical trials for a broad range of indications. Although some trials of the selective ligands have been discontinued, the most advanced drugs so far have been capadenoson and tecadenoson (A1 agonists for atrial fibrillation, or paroxysmal supraventricular tachycardia, respectively), apadenoson and binode-noson (A2A agonists for myocardial perfusion imaging), preladenant (A2A antagonist) for the treatment of Parkinson’s disease, and CF101 and CF102 (A3 agonists for inflammatory diseases and cancer, respectively).
Acknowledgments
KAJ acknowledges support from the Intramural Research Program of the NIH, National Institute of Diabetes & Digestive & Kidney Diseases. CEM is grateful for support by BMBF (BioPharma -Neuroallianz), DFG, DAAD, European Commission (ERANET Neuron), and the State of North-Rhine Westfalia (NRW International Research Graduate Schools BIOTECH-PHARMA and Chemical Biology).
Footnotes
This article is part of a Special Issue entitled: “Adenosine Receptors”.
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. Nomenclature and classification of adenosine receptors — an update. Pharmacol Rev. (in press) [PMC free article] [PubMed] [Google Scholar]
- 2.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 3.Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, IJzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katritch V, Jaakola V-P, Lane JR, Lin J, IJzerman AP, Yeager M, Kufareva I, Stevens RC, Abagyan R. Structure-based discovery of novel chemotypes for adenosine A2A receptor antagonists. J Med Chem. 2010;53:1799–1809. doi: 10.1021/jm901647p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson J, Yoo L, Gao ZG, Irwin J, Shoichet B, Jacobson KA. Structure-based discovery of adenosine A2A receptor ligands. J Med Chem. 2010;53:3748–3755. doi: 10.1021/jm100240h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco R, Casadó V, Cortés A, Pérez-Capote K, Mallol J, Canela E, Ferré S, Lluis C. Novel pharmacological targets based on receptor heteromers. Brain Res Rev. 2008;58:475–482. doi: 10.1016/j.brainresrev.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods AS, Ferré S, Lluis C, Bouvier M, Franco R. Adenosine A2A-dopamine D2 receptor–receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- 8.Carriba P, Navarro G, Ciruela F, Ferré S, Casadó V, Agnati L, Cortés A, Mallol J, Fuxe K, Canela EI, Lluis C, Franco R. Detection of heteromerization of more than two proteins by sequential BRET–FRET. Nat Meth. 2008;5:727–733. doi: 10.1038/nmeth.1229. [DOI] [PubMed] [Google Scholar]
- 9.Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, Agnati LF, Verniera P, Lluis C, Ferré S, Fuxe K, Franco R. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baraldi PG, Tabrizi MA, Frutarollo F, Romagnoli R, Preti D. Recent improvements in the development of A2B adenosine receptor agonists. Purinergic Signal. 2009;5:3–19. doi: 10.1007/s11302-009-9140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalla RV, Zablocki J. Progress in the discovery of selective, high affinity A2B adenosine receptor antagonists as clinical candidates. Purinergic Signal. 2009;5:21–29. doi: 10.1007/s11302-008-9119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortore G, Martinello A. A2B receptor ligands: past, present and future trends. Curr Top Med Chem. 2010;10:923–940. doi: 10.2174/156802610791268747. [DOI] [PubMed] [Google Scholar]
- 14.Borrmann T, Hinz S, Bertarelli DCG, Li W, Florin NC, Scheiff AB, Müller CE. 1-Alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity. J Med Chem. 2009;52:3994–4006. doi: 10.1021/jm900413e. [DOI] [PubMed] [Google Scholar]
- 15.Müller CE, Jacobson KA. Xanthines as adenosine receptor antagonists. In Methylxanthines. In: Fredholm BB, editor. Handbook of Experimental Pharmacology. Vol. 200. Springer; 2011. pp. 151–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller CE. A3 adenosine receptor antagonists. Mini-Rev Med Chem. 2001;1:417–427. doi: 10.2174/1389557510101040417. [DOI] [PubMed] [Google Scholar]
- 17.Müller CE. Medicinal chemistry of adenosine A3 receptor ligands. Curr Top Med Chem. 2003;3:445–462. doi: 10.2174/1568026033392174. [DOI] [PubMed] [Google Scholar]
- 18.Müller CE. A1-adenosine receptor antagonists. Exp Opin Ther Pat. 1997;7:419–440. [Google Scholar]
- 19.Gao ZG, Blaustein J, Gross AS, Melman N, Jacobson KA. N6-Substituted adenosine derivatives: selectivity, efficacy, and species differences at A3 adenosine receptors. Biochem Pharmacol. 2003;65:1675–1684. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ukena D, Jacobson KA, Padgett WL, Ayala C, Shamim MT, Kirk KL, Olsson RA, Daly JW. Species differences in structure–activity relationships of adenosine agonists and xanthine antagonists at brain A1 adenosine receptors. FEBS Lett. 1986;209:122–128. doi: 10.1016/0014-5793(86)81096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller CE, Scior T. Adenosine receptors and their modulators. Pharm Acta Helv. 1993;68:77–111. doi: 10.1016/0031-6865(93)90012-u. [DOI] [PubMed] [Google Scholar]
- 22.van Troostenburg AR, Clark EV, Carey WD, Warrington SJ, Kerns WD, Cohn I, Silverman MH, Bar-Yehuda S, Fong KL, Fishman P. Tolerability, pharmacokinetics, and concentration-dependent hemodynamic effects of oral CF101, an A3 adenosine receptor agonist, in healthy young men. Int J Clin Pharmacol Ther. 2004;42:534–542. doi: 10.5414/cpp42534. [DOI] [PubMed] [Google Scholar]
- 23.Klaasse EC, IJzerman AP, de Grip WJ, Beukers MW. Internalization and desensitization of adenosine receptors. Purinergic Signal. 2008;4:21–37. doi: 10.1007/s11302-007-9086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller CE, Stein B. Adenosine receptor antagonists: structures and potential therapeutic applications. Curr Pharm Des. 1996;2:501–530. [Google Scholar]
- 25.Weyler S, Fülle F, Diekmann M, Schumacher B, Hinz S, Klotz KN, Müller CE. Improving potency, selectivity, and water-solubility of adenosine A1 receptor antagonists: xanthines modified at position 3 and related pyrimido[1,2,3-cd] purinediones. ChemMedChem. 2006;1:891–902. doi: 10.1002/cmdc.200600066. [DOI] [PubMed] [Google Scholar]
- 26.Sauer R, Maurinsh J, Reith U, Fülle F, Klotz KN, Müller CE. Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A2A-selective adenosine receptor antagonists. J Med Chem. 2000;43:440–448. doi: 10.1021/jm9911480. [DOI] [PubMed] [Google Scholar]
- 27.Vollmann K, Qurishi R, Hockemeyer J, Müller CE. Synthesis and properties of a new water-soluble prodrug of the adenosine A2A receptor antagonist MSX-2. Molecules. 2008;13:348–359. doi: 10.3390/molecules13020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller CE, Thorand M, Qurishi R, Diekmann M, Jacobson KA, Padgett WL, Daly JW. Imidazo[2,1-i]purin-5-ones and related tricyclic water-soluble purine derivatives: potent A2A- and A3-adenosine receptor antagonists. J Med Chem. 2002;45:3440–3450. doi: 10.1021/jm011093d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holschbach MH, Olsson RA. Applications of adenosine receptor ligands in medical imaging by positron emission tomography. Curr Pharm Des. 2002;8:2345–2352. doi: 10.2174/1381612023392955. [DOI] [PubMed] [Google Scholar]
- 30.Cordeaux Y, Briddon SJ, Alexander SPH, Kellam B, Hill SJ. Agonist-occupied A3 adenosine receptors exist within heterogeneous complexes in membrane microdomains of individual living cells. FASEB J. 2008;22:850–886. doi: 10.1096/fj.07-8180com. [DOI] [PubMed] [Google Scholar]
- 31.Middleton RC, Briddon SJ, Cordeaux Y, Yates AS, Dale CL, George MW, Baker JG, Hill SJ, Kellam B. New fluorescent adenosine A1 receptor agonists that allow quantification of single living cells. J Med Chem. 2007;50:782–793. doi: 10.1021/jm061279i. [DOI] [PubMed] [Google Scholar]
- 32.Kecskés M, Kumar TS, Yoo L, Gao ZG, Jacobson KA. Novel Alexa Fluor-488 labeled antagonist of the A2A adenosine receptor: application to a fluorescence polarization-based receptor binding assay. Biochem Pharmacol. 2010;80:506–511. doi: 10.1016/j.bcp.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosentreter U, Henning R, Bauser M, Kramer T, Vaupel A, Hubsch W, Dembowsky K, Salcher-Schrauf-Stetter O, Stasch JP, Krahn T, Petzborn E. WO Pat. 2001/025210 Substituted 2-thio-3,5-dicyano-4-aryl-6-aminopyridines and the use thereof.
- 34.Beukers MW, Chang LC, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Spanjersberg RF, Brussee J, IJzerman AP. New, non-adenosine, high-potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J Med Chem. 2004;47:3707–3709. doi: 10.1021/jm049947s. [DOI] [PubMed] [Google Scholar]
- 35.Yan L, Burbiel JC, Maaß A, Müller CE. Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Exp Opin Emerg Drugs. 2003;8:537–576. doi: 10.1517/14728214.8.2.537. [DOI] [PubMed] [Google Scholar]
- 36.Kiesman WF, Elzein E, Zablocki J. A1 adenosine receptor antagonists, agonists, and allosteric enhancers. Handb Exp Pharmacol. 2009;193:25–58. doi: 10.1007/978-3-540-89615-9_2. Review. [DOI] [PubMed] [Google Scholar]
- 37.Gao ZG, Jacobson KA. 2-Chloro-N6-cyclopentyladenosine, adenosine A1 receptor agonist, antagonizes the adenosine A3 receptor. Eur J Pharmacol. 2002;443:39–42. doi: 10.1016/s0014-2999(02)01552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cristalli G, Müller CE, Volpini R. Recent developments in adenosine A2A receptor ligands. Handb Exp Pharmacol. 2009;193:59–98. doi: 10.1007/978-3-540-89615-9_3. [DOI] [PubMed] [Google Scholar]
- 39.Clementina M, Giuseppe S. A2A receptor ligands: past, present and future trends. Curr Top Med Chem. 2010;10:902–922. doi: 10.2174/156802610791268765. [DOI] [PubMed] [Google Scholar]
- 40.Brand F, Klutz A, Jacobson KA, Fredholm BB, Schulte G. Adenosine A2A receptor dynamics studied with the novel fluorescent agonist Alexa488-APEC. Eur J Pharmacol. 2008;590:36–42. doi: 10.1016/j.ejphar.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Hechler B, Gao ZG, Gachet C, Jacobson KA. PEGylated dendritic unimolecular micelles as versatile carriers for ligands of G protein-coupled receptors. Bioconjug Chem. 2009;20:1888–1898. doi: 10.1021/bc9001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanov AA, Barak D, Jacobson KA. Evaluation of homology modeling of G protein-coupled receptors in light of the A2A adenosine receptor crystallographic structure. J Med Chem. 2009;52:3284–3292. doi: 10.1021/jm801533x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherbiny FF, Schiedel AC, Maaß A, Müller CE. Homology modelling of the human adenosine A2B receptor based on X-ray structures of bovine rhodopsin, the β2-adrenergic receptor and the human adenosine A2A receptor. J Comput Aided Mol Des. 2009;23:807–828. doi: 10.1007/s10822-009-9299-7. [DOI] [PubMed] [Google Scholar]
- 44.Mantell SJ, Stephenson PT, Monaghan SM, Maw GN, Trevethick MA, Yeadon M, Walker DK, Selby MD, Batchelor DV, Rozze S, Chavaroche H, Lemaitre A, Wright KN, Whitlock L, Stuart EF, Wright PA, Macintyre F. SAR of a series of inhaled A2A agonists and comparison of inhaled pharmacokinetics in a preclinical model with clinical pharmacokinetic data. Bioorg Med Chem Lett. 2009;19:4471–4475. doi: 10.1016/j.bmcl.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 45.El-Tayeb A, Iqbal J, Behrenswert A, Romio M, Schneider M, Zimmermann H, Schrader J, Müller CE. Nucleoside-5′-monophosphates as prodrugs of adenosine A2A receptor agonists activated by ecto-5′-nucleotidase. J Med Chem. 2009;52:7669–7677. doi: 10.1021/jm900538v. [DOI] [PubMed] [Google Scholar]
- 46.Baraldi PG, Preti D, Tabrizi MA, Fruttarolo F, Romagnoli R, Carrion MD, Lopez Cara LC, Moormann AR, Varani K, Borea PA. Synthesis and biological evaluation of novel 1-deoxy-1-[6-[((hetero)arylcarbonyl)hydrazine]-9H-purin-9-yl]-N-ethyl-β-D-ribofuranuronamide derivatives as useful templates for the development of A2B adenosine receptor agonists. J Med Chem. 2007;50:374–380. doi: 10.1021/jm061170a. [DOI] [PubMed] [Google Scholar]
- 47.Adachi H, Palaniappan KK, Ivanov AA, Bergman N, Gao ZG, Jacobson KA. Structure–activity relationships of 2,N6,5′-substituted adenosine derivatives with potent activity at the A2B adenosine receptor. J Med Chem. 2007;50:1810–1827. doi: 10.1021/jm061278q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. 2008;103:203–215. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 49.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson KA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson KA, Klutz AM, Tosh DK, Ivanov AA, Preti D, Baraldi PG. Medicinal chemistry of the A3 adenosine receptor: agonists, antagonists, and receptor engineering, HEP Adenosine Receptors in Health and Disease. In: Wilson C, Mustafa J, editors. Handb Exp Pharmacol. Vol. 193. Springer; 2009. pp. 123–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devine SM, Gregg A, Figler H, McIntosh K, Urmaliya V, Linden J, Pouton CW, White PJ, Bottle SE, Scammels PJ. Synthesis and evaluation of new N6-substituted adenosine-5′-N-methylcarboxamides as A3 adenosine receptor agonists. Bioorg Med Chem. 2010;18:3078–3087. doi: 10.1016/j.bmc.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 52.Volpini R, Buccioni M, Dal Ben D, Lambertucci C, Lammi C, Marucci G, Ramadori AT, Klotz KN, Cristalli G. Synthesis and biological evaluation of 2-alkynyl-N6-methyl-5′-N-methylcarboxamidoadenosine derivatives as potent and highly selective agonists for the human adenosine A3 receptor. J Med Chem. 2009;52:7897–7900. doi: 10.1021/jm900754g. [DOI] [PubMed] [Google Scholar]
- 53.Jeong LS, Jin DZ, Kim HO, Shin DH, Moon HR, Gunaga P, Chun MW, Kim YC, Melman N, Gao Z-G, Jacobson KA. N6-Substituted D-4′-thioadenosine-5′-methyluronamides: potent and selective agonists at the human A3 adenosine receptor. J Med Chem. 2003;46:3775–3777. doi: 10.1021/jm034098e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tchilibon S, Joshi BV, Kim SK, Duong HT, Gao ZG, Jacobson KA. (N)-Methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists. J Med Chem. 2005;48:1745–1758. doi: 10.1021/jm049580r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melman A, Wang B, Joshi BV, Gao ZG, de Castro S, Heller CL, Kim SK, Jeong LS, Jacobson KA. Selective A3 adenosine receptor antagonists derived from nucleosides containing a bicyclo[3.1.0]hexane ring system. Bioorg Med Chem. 2008;16:8546–8556. doi: 10.1016/j.bmc.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klutz AM, Gao ZG, Lloyd J, Shainberg A, Jacobson KA. Enhanced A3 adenosine receptor selectivity of multivalent nucleoside–dendrimer conjugates. J Nanobiotechnol. 2008;6:12. doi: 10.1186/1477-3155-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baraldi PG, Tabrizi MA, Gessi S, Borea PA. Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem Rev. 2008;108:238–263. doi: 10.1021/cr0682195. [DOI] [PubMed] [Google Scholar]
- 58.Moro S, Gao ZG, Jacobson KA, Spalluto G. Progress in the pursuit of therapeutic adenosine receptor antagonists. Med Res Rev. 2006;26:131–159. doi: 10.1002/med.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schenone S, Brullo C, Musumeci F, Bruno O, Botta M. A1 receptors ligands: past, present, and future trends. Curr Top Med Chem. 2010;10:878–901. doi: 10.2174/156802610791268729. [DOI] [PubMed] [Google Scholar]
- 60.Slawski MT, Givertz MM. Rolofylline: a selective adenosine 1 receptor antagonist for the treatment of heart failure. Exp Opin Pharmacother. 2009;10:311–322. doi: 10.1517/14656560802682213. [DOI] [PubMed] [Google Scholar]
- 61.Pfister JR, Belardinelli L, Lee G, Lum RT, Milner P, Stanley WC, Linden J, Baker SP, Schreiner G. Synthesis and biological evaluation of the enantiomers of the potent and selective A1-adenosine antagonist 1,3-dipropyl-8-[2-(5,6-epoxynorbornyl)]xanthin. J Med Chem. 1997;40:1773–1778. doi: 10.1021/jm970013w. [DOI] [PubMed] [Google Scholar]
- 62.Müller CE. Prodrug approaches for enhancing the bioavailability of drugs with low solubility. Chem Biodivers. 2009;6:2071–2083. doi: 10.1002/cbdv.200900114. [DOI] [PubMed] [Google Scholar]
- 63.Scheiff A, Yerande SG, El-Tayeb A, Li W, Inamdar GS, Vasu KK, Sudarsanam V, Müller CE. 2-Amino-5-benzoyl-4-phenylthiazoles: development of potent and selective adenosine A1 receptor antagonists. Bioorg Med Chem. 2010;18:2195–2203. doi: 10.1016/j.bmc.2010.01.072. [DOI] [PubMed] [Google Scholar]
- 64.Müller CE, Ferré S. Blocking striatal adenosine A2A receptors: a new strategy for basal ganglia disorders. Recent Pat CNS Drug Discov. 2007;2:1–21. doi: 10.2174/157488907779561772. [DOI] [PubMed] [Google Scholar]
- 65.Müller CE, Ferré S. Blocking striatal adenosine A2A receptors: a new strategy for basal ganglia disorders. Frontiers in CNS Drug Discov. 2010;1:304–341. doi: 10.2174/157488907779561772. [DOI] [PubMed] [Google Scholar]
- 66.Shah U, Hogson R. Recent progress in the discovery of adenosine A2A receptor antagonists for the treatment of Parkinson’s disease. Curr Opin Drug Discov Devel. 2010;13:466–480. [PubMed] [Google Scholar]
- 67.Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Müller CE. Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther. 2004;308:358–366. doi: 10.1124/jpet.103.056036. [DOI] [PubMed] [Google Scholar]
- 68.Bilkei-Gorzo A, Abo-Salem OM, Hayallah AM, Michel K, Müller CE, Zimmer A. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:65–76. doi: 10.1007/s00210-007-0252-9. [DOI] [PubMed] [Google Scholar]
- 69.Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a T-maze cost/benefit procedure. Psychopharmacology. 2009;204:103–112. doi: 10.1007/s00213-008-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Worden LT, Shahriari M, Farrar AM, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: differential interaction with D1 and D2 family antagonists. Psychopharmacology. 2009;203:489–499. doi: 10.1007/s00213-008-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alexander SP, Millns PJ. [3H]ZM241385 — an antagonist radioligand for adenosine A2A receptors in brain. Eur J Pharmacol. 2001;411:205–210. doi: 10.1016/s0014-2999(00)00899-2. [DOI] [PubMed] [Google Scholar]
- 72.Pastorin G, Federico S, Paoletta S, Corradino M, Cateni F, Cacciari B, Klotz K-N, Gao Z-G, Jacobson KA, Spalluto G, Moro S. Synthesis and pharmacological characterization of a new series of 5,7-disubstituted-[1,2,4]triazolo[1,5-a][1,3,5] triazine derivatives as adenosine receptor antagonists: a preliminary inspection of ligand–receptor recognition process. Bioorg Med Chem. 2010;18:2524–2536. doi: 10.1016/j.bmc.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Todde S, Moresco RM, Simonelli P, Baraldi PG, Cacciari B, Spalluto G, Varani K, Monopoli A, Matarrese M, Carpinelli A, Magni F, Kienle MG, Fazio F. Design, radiosynthesis, and biodistribution of a new potent and selective ligand for in vivo imaging of the adenosine A2A receptor system using positron emission tomography. J Med Chem. 2000;43:4359–4362. doi: 10.1021/jm0009843. [DOI] [PubMed] [Google Scholar]
- 74.Saku O, Saki M, Kurokawa M, Ikeda K, Uchida S-i, Takizawa T, Uesaka N. Synthetic studies on selective adenosine A2A receptor antagonists. Part II: synthesis and structure–activity relationships of novel benzofuran derivatives. Bioorg Med Chem Lett. 2010;20:3768–3771. doi: 10.1016/j.bmcl.2010.04.058. [DOI] [PubMed] [Google Scholar]
- 75.Luthra PM, Mishra CB, Jha PK, Barodia SK. Synthesis of novel 7-imino-2-thioxo-3,7-dihydro-2H-thiazolo[4,5-d]pyrimidine derivatives as adenosine A2A receptor antagonists. Bioorg Med Chem. 2010;20:1214–1218. doi: 10.1016/j.bmcl.2009.11.133. [DOI] [PubMed] [Google Scholar]
- 76.Mishra CB, Barodia SK, Prakash A, Kumar JBS, Luthra PM. Novel 8-(furan-2-yl)-3-substituted thiazolo[5,4-e][1,2,4]triazolo[1,5-c]pyrimidine-2(3H)-thione derivatives as potential adenosine A2A receptor antagonists. Bioorg Med Chem. 2010;18:2491–2500. doi: 10.1016/j.bmc.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 77.Moro S, Deflorian F, Bacilieri M, Spalluto G. Ligand-based homology modeling as attractive tool to inspect GPCR structural plasticity. Curr Pharm Des. 2006;12:2175–2185. doi: 10.2174/138161206777585265. [DOI] [PubMed] [Google Scholar]
- 78.Auchampach JA, Gizewski E, Wan TC, de Castro S, Brown GG, Jacobson KA. Synthesis and pharmacological characterization of [125I]MRS5127, a high affinity, selective agonist radioligand for the A3 adenosine receptor. Biochem Pharmacol. 2010;79:967–973. doi: 10.1016/j.bcp.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeong LS, Pal S, Choe SA, Choi WJ, Jacobson KA, Gao ZG, Klutz AM, Hou X, Kim HO, Lee HW, Lee SK, Tosh DK, Moon HR. Structure activity relationships of truncated D- and L-4′-thioadenosine derivatives as species-independent A3 adenosine receptor antagonists. J Med Chem. 2008;51:6609–6613. doi: 10.1021/jm8008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z, Do CW, Avila MY, Peterson-Yatorno K, Stone RA, Gao ZG, Joshi BV, Besada P, Jeong LS, Jacobson KA, Civan MM. Nucleoside-derived antagonists to A3 adenosine receptors lower mouse intraocular pressure and act across species. Exp Eye Res. 2010;90:146–154. doi: 10.1016/j.exer.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zannikos PN, Jensen BK, Boutouyrie BX, Tripp L, Yongyi L, McGowan T, Waldman SA, Greenberg HE. Pharmacokinetics and safety of single intravenous infusions of the adenosine agonist, AMP 579, in patients with end-stage renal insufficiency. J Clin Pharmacol. 2000;40:745–751. doi: 10.1177/00912700022009503. [DOI] [PubMed] [Google Scholar]
- 82.Kopecky SL, Aviles RJ, Bell MR, Lobl JK, Tipping D, Frommell G, Ramsey K, Holland AE, Midei M, Jain A, Kellett M, Gibbons RJ. A randomized, double-blinded, placebo-controlled, dose-ranging study measuring the effect of an adenosine agonist on infarct size reduction in patients undergoing primary percutaneous transluminal coronary angioplasty: the ADMIRE (AmP579 Delivery for Myocardial Infarction REduction) study. Am Heart J. 2003;146:146–152. doi: 10.1016/S0002-8703(03)00172-8. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y, Yang X, Yang X-M, Walker S, Förster K, Cohen MV, Krieg T, Downey JM. AMP579 is revealed to be a potent A2b-adenosine receptor agonist in human 293 cells and rabbit hearts. Basic Res Cardiol. 2010;105:129–137. doi: 10.1007/s00395-009-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porkka-Heiskanen T, Alanko L, Kalinchuk A, Stenberg D. Adenosine and sleep. Sleep Med Rev. 2002;6:321–332. doi: 10.1053/smrv.2001.0201. [DOI] [PubMed] [Google Scholar]
- 85.Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, Basheer R, Haas HL, Zilles K, Bauer A. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmer TM, Trevethick MA. Suppression of inflammatory and immune responses by the A2A adenosine receptor: an introduction. Br J Pharmacol. 2008;153:S27–S34. doi: 10.1038/sj.bjp.0707524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci USA. 2008;105:792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, Auer H, Palatini J, Hassanain HH, Cardounel AJ, Javed A, Grants I, Wunderlich JE, Christofi FL. Inflamm Bowel Dis. 2006;12:766–789. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 89.Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G401–G410. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 90.Madi L, Cohen S, Ochayin A, Bar-Yehuda S, Barer FR, Fishman P. Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: involvement of nuclear factor-kappaB in mediating receptor level. J Rheumatol. 2007;34:20–26. [PubMed] [Google Scholar]
- 91.Ishikawa J, Mitani H, Bandoh T, Kimura M, Totsuka T, Hayashi S. Hypoglycemic and hypotensive effects of 6-cyclohexyl-2′-O-methyl-adenosine, an adenosine A1 receptor agonist, in spontaneously hypertensive rat complicated with hyperglycemia. Diab Res Clin Pract. 1998;39:3–9. doi: 10.1016/s0168-8227(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 92.Lauro C, Cipriani R, Catalano M, Trettel F, Chece G, Brusadin V, Antonilli L, van Rooijen N, Eusebi F, Bredholm BB, Limatola C. Adenosine A1 receptors and microglial cells mediate CX3CL1-induced protection of hippocampal neurons against Glu-induced death. Neuropsychopharmacology. 2010;35:1550–1559. doi: 10.1038/npp.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knutsen LJ, Lau J, Petersen H, Thomsen C, Weis JU, Shalmi M, Judge ME, Hansen AJ, Sheardown MJ. N-Substituted adenosines as novel neuroprotective A1 agonists with diminished hypotensive effects. J Med Chem. 1999;42:3463–3477. doi: 10.1021/jm960682u. [DOI] [PubMed] [Google Scholar]
- 94.Giffin NJ, Kowacs F, Libri V, Williams P, Goadsby PJ, Kaube H. Effect of the adenosine A1 receptor agonist GR79236 on trigeminal nociception with blink reflex recordings in healthy human subjects. Cephalalgia. 2003;23:287–292. doi: 10.1046/j.1468-2982.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 95.Shah B, Rohatagi S, Natarajan C, Kirkesseli S, Baybutt R, Jensen BKI. Pharmacokinetics, pharmacodynamics, and safety of a lipid-lowering adenosine A1 agonist, RPR749, in healthy subjects. Am J Ther. 2004;11:175–189. doi: 10.1097/00045391-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Zablocki JA, Wu L, Shryock J, Belardinelli L. Partial A1 adenosine receptor agonists from a molecular perspective and their potential use as chronic ventricular rate control agents during atrial fibrillation (AF) Curr Top Med Chem. 2004;4:839–854. doi: 10.2174/1568026043450998. [DOI] [PubMed] [Google Scholar]
- 97.Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 98.Desai A, Victor-Vega C, Gadangi S, Montesinos MC, Chu CC, Cronstein BN. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- 99.Udelson JE, Heller GV, Wackers FJ, Chai A, Hinchman D, Coleman PS, Dilsizian V, DiCarli M, Hachamovitch R, Johnson JR, Barrett RJ, Gibbons RJ. Randomized, controlled dose-ranging study of the selective adenosine A2A receptor agonist binodenoson for pharmacological stress as an adjunct to myocardial perfusion imaging. Circulation. 2004;109:457–464. doi: 10.1161/01.CIR.0000114523.03312.7D. [DOI] [PubMed] [Google Scholar]
- 100.Cerqueira MD. Advances in pharmacologic agents in imaging: new A2A receptor agonists. Curr Cardiol Rep. 2006;8:119–122. doi: 10.1007/s11886-006-0022-1. [DOI] [PubMed] [Google Scholar]
- 101.Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, Lieu H, Mahmarian JJ, Olmsted A, Underwood SR, Vitola J, Wang W. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 102.Avni I, Garzozi HJ, Barequet IS, Segev F, Varssano D, Sartani G, Chetrit N, Bakshi E, Zadok D, Tomkins O, Litvin G, Jacobson KA, Fishman S, Harpaz Z, Farbstein M, Bar-Yehuda S, Silverman MH, Kerns WD, Cohn I, Fishman P. Treatment of dry eye syndrome with orally-administered CF101: data from a phase 2 clinical trial. Ophthalmology. 2010;117:1287–1293. doi: 10.1016/j.ophtha.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008;324:234–243. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Skouroliakou M, Bacopoulou F, Markantonis SL. Caffeine versus theophylline for apnea of prematurity: a randomized controlled trial. J Paediatr Child Health. 2009;45:587–592. doi: 10.1111/j.1440-1754.2009.01570.x. [DOI] [PubMed] [Google Scholar]
- 105.Polosa R, Holgate ST. Adenosine receptors as promising therapeutic targets for drug development in chronic airway inflammation. Curr Drug Targets. 2006;7:699–706. doi: 10.2174/138945006777435236. [DOI] [PubMed] [Google Scholar]
- 106.Gottlieb SS. Adenosine A1 antagonists and the cardiorenal syndrome. Curr Heart Fail Rep. 2008;5:105–109. doi: 10.1007/s11897-008-0017-x. [DOI] [PubMed] [Google Scholar]
- 107.Che J, Chan ES, Cronstein BN. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol Pharmacol. 2007;72:1626–1636. doi: 10.1124/mol.107.038760. [DOI] [PubMed] [Google Scholar]
- 108.Yang H, Avila MY, Peterson-Yantorno K, Coca-Prados M, Stone RA, Jacobson KA, Civan MM. The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure. Curr Eye Res. 2005;30:747–754. doi: 10.1080/02713680590953147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arispe N, Ma J, Jacobson KA, Pollard HB. Direct activation of cystic fibrosis transmembrane conductance regulator channels by 8-cyclopentyl-1,3-dipropylxanthine (CPX) and 1,3-diallyl-8-cyclohexylxanthine (DAX) J Biol Chem. 1998;273:5727–5734. doi: 10.1074/jbc.273.10.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilson CN. Adenosine receptors and asthma in humans. Br J Pharmacol. 2008;155:475–486. doi: 10.1038/bjp.2008.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hess S. Recent advances in adenosine receptor antagonist research. Exp Opin Ther Pat. 2001;11:1533–1561. [Google Scholar]
- 113.Terai T, Kita Y, Kusonoki T, Shimazaki T, Ando T, Horiai H, Akahane A, Shiokawa Y, Yoshida K. A novel non-xanthine adenosine A1 receptor antagonist. Eur J Pharmacol. 1995;279:217–225. doi: 10.1016/0014-2999(95)00165-h. [DOI] [PubMed] [Google Scholar]
- 114.Hocher B. Adenosine A1 receptor antagonists in clinical research and development. Kidney Int. 2010;78:438–445. doi: 10.1038/ki.2010.204. [DOI] [PubMed] [Google Scholar]
- 115.LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM. Adenosine A2A receptor antagonist istradefylline (KW6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005) Ann Neurol. 2008;63:295–302. doi: 10.1002/ana.21315. [DOI] [PubMed] [Google Scholar]
- 116.Pascoe SJ, Knight H, Woessner R. QAF805, an A2B/A3 antagonist, does not attenuate AMP challenge in subjects with asthma. Proc Am Thorac Soc. 2007:A682. [Google Scholar]
- 117.Okamura T, Kurogi Y, Hashimoto K, Sato S, Nishikawa H, Kiryu K, Nagao Y. Structure–activity relationships of adenosine A3 receptor ligands: new potential therapy for the treatment of glaucoma. Bioorg Med Chem Lett. 2004;14:3775–3779. doi: 10.1016/j.bmcl.2004.04.099. [DOI] [PubMed] [Google Scholar]
- 118.Borea PA, Gessi S, Bar-Yehuda S, Fishman P. A3 adenosine receptor: pharmacology and role in disease. Handb Exp Pharmacol. 2009;193:297–327. doi: 10.1007/978-3-540-89615-9_10. [DOI] [PubMed] [Google Scholar]
- 119.Bauer A, Ishiwata K. Adenosine receptor ligands and PET imaging in the CNS. Handb Exp Pharmacol. 2009:617–642. doi: 10.1007/978-3-540-89615-9_19. [DOI] [PubMed] [Google Scholar]
- 120.Ishiwata K, Noguchi J, Toyama H, Sakiyama Y, Koike N, Ishii S, Oda K, Endo K, Suzuki F, Senda M. Synthesis and preliminary evaluation of [11C]KF17837, a selective adenosine A2A antagonist. Appl Radiat Isot. 1996;47:507–511. doi: 10.1016/0969-8043(95)00295-2. [DOI] [PubMed] [Google Scholar]
- 121.Noguchi J, Ishiwata K, Wakabayashi S, Nariai T, Shumiya S, Ishii S, Toyama H, Endo K, Suzuki F, Senda M. Evaluation of carbon-11-labeled KF17837: a potential CNS adenosine A2a receptor ligand. J Nucl Med. 1998;39:498–503. [PubMed] [Google Scholar]
- 122.Márián T, Boros I, Lengyel Z, Balkay L, Horváth G, Emri M, Sarkadi E, Szentmiklósi AJ, Fekete I, Trón L. Preparation and primary evaluation of [11C]CSC as a possible tracer for mapping adenosine A2A receptors by PET. App Radiat Isot. 1999;50:887–893. doi: 10.1016/s0969-8043(98)00162-6. [DOI] [PubMed] [Google Scholar]
- 123.Ishiwata K, Shimada J, Wang WF, Harakawa H, Ishii S, Kiyosawa M, Suzki F, Senda M. Evaluation of iodinated and brominated [11C]styrylxanthine derivatives as in vivo radioligands mapping adenosine A2A receptor in the central nervous system. Ann Nucl Med. 2000;14:247–253. doi: 10.1007/BF02988206. [DOI] [PubMed] [Google Scholar]
- 124.Ishiwata K, Noguchi J, Wakabayashi S, Shimada J, Ogi N, Nariai T, Tanaka A, Endo K, Suzuki F, Senda M. 11C-labeled KF18446: a potential central nervous system adenosine A2A receptor ligand. J Nucl Med. 2000;41:345–354. [PubMed] [Google Scholar]
- 125.Moresco RM, Todde S, Belloli S, Simonelli P, Panzacchi A, Rigamonti M, Galli-Kienle M, Fazio F. In vivo imaging of adenosine A2A receptors in rat and primate brain using [11C]SCH442416. Eur J Nucl Med Mol Imaging. 2005;32:405–413. doi: 10.1007/s00259-004-1688-5. [DOI] [PubMed] [Google Scholar]
- 126.Wadsak W, Mien LK, Shanab K, Ettlinger DE, Haeusler D, Sindelar K, Lanzenberger RR, Spreitzer H, Viernstein H, Keppler BK, Dudczak R, Letter K, Mitterhauser M. Preparation and first evaluation of [18F]FEQSUPPY: a new PET tracer for the adenosine A3 receptor. Nucl Med Biol. 2008;35:61–66. doi: 10.1016/j.nucmedbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 127.Kiesewetter DO, Lang L, Ma Y, Bhattacharjee AK, Gao ZG, Joshi BV, Melman A, Castro S, Jacobson KA. Synthesis and characterization of [76Br]-labeled high affinity A3 adenosine receptor ligands for positron emission tomography. Nucl Med Biol. 2009;36:3–10. doi: 10.1016/j.nucmedbio.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan L, Burbiel JC, Maaß A, Müller CE. Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Exp Opin Emerg Drugs. 2003;8:537–576. doi: 10.1517/14728214.8.2.537. [DOI] [PubMed] [Google Scholar]
- 129.Daly JW, Padgett WL, Secunda SI, Thompson RD, Olsson RA. Structure–activity relationships for 2-substituted adenosines at A1 and A2 adenosine receptors. Pharmacology. 1993;46:91–100. doi: 10.1159/000139033. [DOI] [PubMed] [Google Scholar]
- 130.Bruns RF. Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can J Physiol Pharmacol. 1980;58:673–691. doi: 10.1139/y80-110. [DOI] [PubMed] [Google Scholar]
- 131.Brackett LE, Daly JW. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem Pharmacol. 1994;47:801–814. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- 132.Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Galen PJ, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA. A binding site model and structure–activity relationships for the rat A3 adenosine binding site. Mol Pharmacol. 1994;45:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- 134.Liang, et al. In: A3 Adenosine Receptors From Cell Biology to Pharmacology. Borea, editor. 2010. pp. 257–280. [Google Scholar]
- 135.Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs. 2008;17:1901–1910. doi: 10.1517/13543780802497284. [DOI] [PubMed] [Google Scholar]
- 136.Smits GJ, McVey M, Cox BF, Perrone MH, Clark KL. Cardioprotective effects of the novel adenosine A1/A2 receptor agonist AMP 579 in a porcine model of myocardial infarction. J Pharmacol Exp Ther. 1998;286:611–618. [PubMed] [Google Scholar]
- 137.Prashad M, Prasad K, Repie O. A practical synthesis of SDZ WAG 994 by selective methylation of N6-cyclohexyladenosine. Synth Commun. 1996;26:3967–3977. [Google Scholar]
- 138.Beattie D, Brearley A, Brown Z, Charlton SJ, Cox B, Fairhurst RA, Fozard JR, Gedeck P, Kirkham P, Meja K, Nanson L, Neef J, Oakman H, Spooner G, Taylor RJ, Turner RJ, West R, Woodward H. Synthesis and evaluation of two series of 4′-aza-carbocyclic nucleosides as adenosine A2A receptor agonists. Bioorg Med Chem Lett. 2010;20:1219–1224. doi: 10.1016/j.bmcl.2009.11.131. [DOI] [PubMed] [Google Scholar]
- 139.Valls MD, Cronstein BN, Montesinos MC. Adenosine receptor agonists for promotion of dermal wound healing. Biochem Pharmacol. 2009;77(8):1117–1124. doi: 10.1016/j.bcp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–271. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Auchampach JA, Kreckler LM, Wan TC, Maas JE, van der Hoeven D, Gizewski E, Narayanan J, Maas GE. Characterization of the A2B adenosine receptor from mouse, rabbit, and dog. J Pharmacol Exp Ther. 2009;329:2–13. doi: 10.1124/jpet.108.148270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jeong LS, Lee HW, Jacobson KA, Kim HO, Shin DH, Lee JA, Gao ZG, Lu C, Duong HT, Gunaga P, Lee SK, Jin DZ, Chun MW, Moon HR. Structure–activity relationships of 2-chloro-N6-substituted-4′-thioadenosine-5′-uronamides as highly potent and selective agonists at the human A3 adenosine receptor. J Med Chem. 2006;49:273–281. doi: 10.1021/jm050595e. [DOI] [PubMed] [Google Scholar]
- 143.Melman A, Gao ZG, Kumar D, Wan TC, Gizewski E, Auchampach JA, Jacobson KA. Design of (N)-methanocarba adenosine 5′-uronamides as species-independent A3 receptor-selective agonists. Bioorg Med Chem Lett. 2008;18:2813–2819. doi: 10.1016/j.bmcl.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tracey WR, Magee WP, Oleynek JJ, Hill RJ, Smith AH, Flynn DM, Knight DR. Novel N6-substituted adenosine 5′-N-methyluronamides with high selectivity for human adenosine A3 receptors reduce ischemic myocardial injury. Am J Physiol Heart Circ Physiol. 2003;285:H2780–H2787. doi: 10.1152/ajpheart.00411.2003. [DOI] [PubMed] [Google Scholar]
- 145.Jacobson KA, IJzerman AP, Linden J. 1,3-Dialkylxanthine derivatives having high potency as antagonists at human A2B adenosine receptors. Drug Dev Res. 1999;47:45–53. doi: 10.1002/(sici)1098-2299(199905)47:1<45::aid-ddr6>3.0.co;2-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grahner B, Winiwarter S, Lanzner W, Müller CECE. Synthesis and structure–activity relationships of deazaxanthines: analogs of potent A1- and A2-adenosine receptor antagonists. J Med Chem. 1994;37:1526–1534. doi: 10.1021/jm00036a019. [DOI] [PubMed] [Google Scholar]
- 147.Daly JW, Hide I, Müller CE, Shamim M. Caffeine analogs: structure–activity relationships at adenosine receptors. Pharmacology. 1991;42:309–321. doi: 10.1159/000138813. [DOI] [PubMed] [Google Scholar]
- 148.Müller CE, Maurinsh J, Sauer R. Binding of [3H]MSX-2 (3-(3-hydroxypropyl)-7-methyl-8-(m-methoxystyryl)-1-propargylxanthine) to rat striatal membranes — a new, selective antagonist radioligand for A2A adenosine receptors. Eur J Pharm Sci. 2000;10:259–265. doi: 10.1016/s0928-0987(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 149.Kim S-A, Marschall MA, Melman N, Kim HS, Müller CE, Linden J, Jacobson KA. Structure–activity relationships at human and rat A2B adenosine receptors of xanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions. J Med Chem. 2002;45:2131–2138. doi: 10.1021/jm0104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bertarelli DCG, Diekmann M, Hayallah AM, Rüsing D, Iqbal J, Preiss B, Verspohl EJ, Müller CE. Characterization of human and rodent native and recombinant adenosine A2B receptors by radioligand binding studies. Purinergic Signal. 2006;2:559–571. doi: 10.1007/s11302-006-9012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Müller CE, Shi D, Manning M, Jr, Daly JW. Synthesis of paraxanthine analogs (1,7-disubstituted xanthines) and other xanthines unsubstituted at the 3-position: structure–activity relationships at adenosine receptors. J Med Chem. 1993;36:3341–3349. doi: 10.1021/jm00074a015. [DOI] [PubMed] [Google Scholar]
- 152.Klotz KN, Vogt H, Tawfik-Schlieper H. Comparison of adenosine receptors in brain from different species by radioligand binding and photoaffinity labelling. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:196–201. doi: 10.1007/BF00168610. [DOI] [PubMed] [Google Scholar]
- 153.Fozard JR, Baur F, Wolber C. Antagonist pharmacology of adenosine A2B receptors from rat, guinea pig and dog. Eur J Pharmacol. 2003;475:79–84. doi: 10.1016/s0014-2999(03)02078-8. [DOI] [PubMed] [Google Scholar]
- 154.Jacobson KA, Fischer B, Ji XD. A “cleavable trifunctional” approach to receptor affinity labeling: regeneration of binding to A1-adenosine receptors. Bioconjug Chem. 1995;6:255–263. doi: 10.1021/bc00033a004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol. 1997;52:846–860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- 156.Hayallah AM, Sandoval-Ramírez J, Reith U, Schobert U, Preiss B, Schumacher B, Daly JW, Müller CE. 1,8-Disubstituted xanthine derivatives: synthesis of potent A2B-selective adenosine receptor antagonists. J Med Chem. 2002;45:1500–1510. doi: 10.1021/jm011049y. [DOI] [PubMed] [Google Scholar]
- 157.Kiesman WF, Zhao J, Conlon PR, Petter RC, Jin X, Smits G, Lutterodt F, Sullivan GW, Linden J. Norbornyllactone-substituted xanthines as adenosine A1 receptor antagonists. Bioorg Med Chem. 2006;14:3654–3661. doi: 10.1016/j.bmc.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 158.Kiesman WF, Zhao J, Conlon PR, Dowling JE, Petter RC, Lutterodt F, Jin X, Smits G, Fure M, Jayaraj A, Kim J, Sullivan G, Linden J. Potent and orally bioavailable 8-bicyclo[2.2.2]octylxanthines as adenosine A1 receptor antagonists. J Med Chem. 2006;49:7119–7131. doi: 10.1021/jm0605381. [DOI] [PubMed] [Google Scholar]
- 159.Doggrell SA. BG-9928 (Biogen Idec) Curr Opin Investig Drugs. 2005;6:962–968. [PubMed] [Google Scholar]
- 160.Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg GW, Hocher B. The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol. 2007;151:1025–1032. doi: 10.1038/sj.bjp.0707319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Obiefuna PC, Batra VK, Nadeem A, Borron P, Wilson CNH, Mustafa SJ. A novel A1 adenosine receptor antagonist, L-97-1 [3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxyethyl)-amino]-ethyl}-1-propyl-3,7-dihydropurine-2,6-dione], reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J Pharmacol Exp Ther. 2005;315:329–336. doi: 10.1124/jpet.105.088179. [DOI] [PubMed] [Google Scholar]
- 162.Kase H. The adenosine A2A receptor selective antagonist KW6002: research toward a novel nondopaminergic therapy for Parkinson’s disease. Neurology. 2003;61:S97–S100. doi: 10.1212/01.wnl.0000095219.22086.31. [DOI] [PubMed] [Google Scholar]
- 163.Shimada J, Koike N, Nonaka H, Shiozaki S, Yanagawa K, Kanda T, Kobayashi H, Ichimura M, Nakamura J, Kase H, Suzuki F. Adenosine A2A antagonists with potent anti-cataleptic activity. Bioorg Med Chem Lett. 1997;7:2349–2352. [Google Scholar]
- 164.Pretorius J, Malan SF, Castagnoli N, Jr, Bergh JJ, Petzer JP. Dual inhibition of monoamine oxidase B and antagonism of the adenosine A2A receptor by (E,E)-8-(4-phenylbutadien-1-yl)caffeine analogues. Bioorg Med Chem. 2008;16:8676–8684. doi: 10.1016/j.bmc.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 165.Jacobson KA, Gallo-Rodriguez C, Melman N, Fischer B, Maillard M, van Bergen A, van Galen PJ, Karton Y. Structure–activity relationships of 8-styrylxanthines as A2-selective adenosine antagonists. J Med Chem. 1993;36:1333–1342. doi: 10.1021/jm00062a005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gillespie RJ, Bamford SJ, Botting R, Comer M, Denny S, Gaur S, Griffin M, Jordan AM, Knight AR, Lerpiniere J, Leonardi S, Lightowler S, McAteer S, Merrett A, Misra A, Padfield A, Reece M, Saadi M, Selwood DL, Stratton GC, Surry D, Todd R, Tong X, Ruston V, Upton R, Weiss SM. Antagonists of the human A2A receptor. 4. Design, synthesis, and preclinical evaluation of 7-aryltriazolo [4,5-d]pyrimidines. J Med Chem. 2009;52:33–47. doi: 10.1021/jm800961g. [DOI] [PubMed] [Google Scholar]
- 167.Minetti P, Tinti MO, Carminati P, Castorina M, Di Cesare MA, Di Serio S, Gallo G, Ghirardi O, Giorgi F, Giorgi L, Piersanti G, Bartoccini F, Tarzia G. 2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and analogues as A2A adenosine receptor antagonists. Design, synthesis, and pharmacological characterization. J Med Chem. 2005;48:6887–6896. doi: 10.1021/jm058018d. [DOI] [PubMed] [Google Scholar]
- 168.Kim Y-S, Ji X-d, Melman N, Linden J, Jacobson KA. Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A2B adenosine receptors. J Med Chem. 2000;43:1165–1172. doi: 10.1021/jm990421v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Baraldi PG, Tabrizi MA, Preti D, Bovero A, Romagnoli R, Fruttarolo F, Zaid NA, Moorman AR, Varani K, Gessi S, Merighi S, Borea PA. Design, synthesis, and biological evaluation of new 8-heterocyclic xanthine derivatives as highly potent and selective human A2B adenosine receptor antagonists. J Med Chem. 2004;47:1434–1447. doi: 10.1021/jm0309654. [DOI] [PubMed] [Google Scholar]
- 170.Elzein E, Kalla RV, Li X, Perry T, Gimbel A, Zeng D, Lustig D, Leung K, Zablocki J. Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J Med Chem. 2008;51:2267–2278. doi: 10.1021/jm7014815. [DOI] [PubMed] [Google Scholar]
- 171.Eastwood P, Gonzalez J, Paredes S, Nueda A, Domenech T, Alberti J, Vidal B. Discovery of N-(5,6-diarylpyridin-2-yl)amide derivatives as potent and selective A2B adenosine receptor antagonists. Bioorg Med Chem Lett. 2010;20:1697–1700. doi: 10.1016/j.bmcl.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 172.Vidal B, Nueda A, Esteve C, Domenech T, Benito S, Reinoso RF, Pont M, Clabet M, Lopez R, Cadavid MI, Loza MI, Cardenas A, Godessart N, Beleta J, Varrellow G, Ryder H. Discovery and characterization of 4′-(2-furyl)-N-pyridin-3-yl-4,5′-bipyridmidin-2′-amine (LAS38096), a potent, selective, and efficacious A2B adenosine receptor antagonist. J Med Chem. 2007;50:2732–2736. doi: 10.1021/jm061333v. [DOI] [PubMed] [Google Scholar]
- 173.Bedford ST, Benwell KR, Brooks T, Chen I, Comer M, Dugdale S, Haymes T, Jordan AM, Kennett GA, Knight AR, Klenke B, LeStrat L, Merrett A, Misra A, Lightowler S, Padfield A, Poullennec K, Reece M, Simmonite H, Wong M, Yule IA. Discovery and optimization of potent and selective functional antagonists of the human adenosine A2B receptor. Bioorg Med Chem Lett. 2009;19:5945–5949. doi: 10.1016/j.bmcl.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 174.Cheung AW-H, Brinkman J, Firooznia F, Flohr A, Grimsby J, Gubler ML, Guertin K, Hamid R, Marcopulos N, Norcross R, Qi L, Ramsey G, Tan J, Wen Y, Sarabu R. 4-Substituted-7-N-alkyl-N-acetyl 2-aminobenzothiazole amides: drug-like and non-xanthine based A2B adenosine receptor antagonists. Bioorg Med Chem Lett. 2010;20:4140–4146. doi: 10.1016/j.bmcl.2010.05.056. [DOI] [PubMed] [Google Scholar]
- 175.Eastwood P, Gonzales J, Paredes S, Fonquerna S, Cardús A, Alonso JA, Nueda A, Domenech T, Reinoso RF, Vidal B. Discovery of potent and selective bicyclic A2B adenosine receptor antagonists via bioisosteric amide replacement. Bioorg Med Chem Lett. 2010;20:1634–1637. doi: 10.1016/j.bmcl.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 176.Stewart M, Steinig AG, Ma C, Song JP, McKibben B, Castelhano AL, MacLennan SJ. [3H]OSIP339391, a selective, novel, and high affinity antagonist radioligand for adenosine A2B receptors. Biochem Pharmacol. 2004;68:305–312. doi: 10.1016/j.bcp.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 177.Fozard JR, et al. In: A3 Adenosine Receptors From Cell Biology to Pharmacology. Borea, editor. Chapter 1 2010. [Google Scholar]
- 178.Cheong SL, Dolzhenko A, Kachler S, Paoletta S, Federico S, Cacciari B, Dolzhenko A, Klotz K-N, Moro S, Spalluto G, Pastorin G. The significance of 2-furyl ring substitution with a 2-(para-substituted) aryl group in a new series of pyrazolotriazolo-pyrimidines as potent and highly selective hA3 adenosine receptor antagonists: new insights into structure–affinity relationship and receptor-antagonist recognition. J Med Chem. 2010;53:3361–3375. doi: 10.1021/jm100049f. [DOI] [PubMed] [Google Scholar]
- 179.van Muijlwijk-Koezen JE, Timmerman H, van der Goot H, Menge WM, Freijtag von Drabbe Künzel J, IJzerman AP. Isoquinoline and quinazoline urea analogues as antagonists for the human adenosine A3 receptor. J Med Chem. 2000;43:2227–2238. doi: 10.1021/jm000002u. [DOI] [PubMed] [Google Scholar]
- 180.Saki M, Tsumuki H, Nonaka H, Shimada J, Ichimura M. KF26777 (2-(4-bromophenyl)-7,8-dihydro-4-propyl-1H-imidazo[2,1-i]purin-5(4H)-one dihydrochloride), a new potent and selective adenosine A3 receptor antagonist. Eur J Pharmacol. 2002;444:133–141. doi: 10.1016/s0014-2999(02)01662-x. [DOI] [PubMed] [Google Scholar]
- 181.Ozola V, Thorand M, Diekmann M, Qurishi R, Schumacher B, Jacobson KA, Müller CE. 2-Phenylimidazo[2,1-i]purin-5-ones: structure–activity relationships and characterization of potent and selective inverse agonists at human A3 adenosine receptors. Bioorg Med Chem. 2003;11:347–356. doi: 10.1016/s0968-0896(02)00456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Müller CE, Diekmann M, Thorand M, Ozola V. [3H]8-Ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]purin-5-one ([3H]PSB-11), a novel high-affinity antagonist radioligand for human A3 adenosine receptors. Bioorg Med Chem Lett. 2002;12:501–503. doi: 10.1016/s0960-894x(01)00785-5. [DOI] [PubMed] [Google Scholar]
- 183.Lenzi O, Colotta V, Catarzi D, Varano F, Poli D, Filacchioni G, Varani K, Vicenzi F, Borea PA, Paoletta S, Morizzo E, Moro S. 2-Phenylpyrazolo[4,3-d] pyrimidin-7-one as a new scaffold to obtain potent and selective human A3 adenosine receptor antagonists: new insights into the receptor-antagonist recognition. J Med Chem. 2009;52:7640–7652. doi: 10.1021/jm900718w. [DOI] [PubMed] [Google Scholar]
- 184.Pal S, Choi WJ, Choe SA, Heller CL, Gao ZG, Chinn M, Jacobson KA, Hou X, Lee SK, Kim HO, Jeong LS. Structure–activity relationships of truncated adenosine derivatives as highly potent and selective human A3 adenosine receptor antagonists. Bioorg Med Chem. 2009;17:3733–3738. doi: 10.1016/j.bmc.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maemoto T, Tada M, Mihara T, Ueyama N, Matsuoka H, Harada K, Yamaji T, Shirakawa K, Kuroda S, Akahane A, Iwashita A, Matsuoka N, Mutoh S. Pharmacological characterization of FR194921, a new potent, selective, and orally active antagonist for central adenosine A1 receptors. J Pharmacol Sci. 2004;96:42–52. doi: 10.1254/jphs.fp0040359. [DOI] [PubMed] [Google Scholar]
- 186.Nonaka H, Mori A, Ichimura M, Shindou T, Yanagawa K, Shimada J, Kase H. Binding of [3H]KF17837S, a selective adenosine A2 receptor antagonist, to rat brain membranes. Mol Pharmacol. 1994;46:817–822. [PubMed] [Google Scholar]
- 187.Müller CE, Hipp J, Schobert U, Frobenius W, Pawlowski M, Suzuki F, Sandoval-Ramírez J. Synthesis and structure–activity relationships of 3,7-dimethyl-1-propargylxanthine derivatives, A2A-selective adenosine receptor antagonists. J Med Chem. 1997;40:4396–4405. doi: 10.1021/jm970515+. [DOI] [PubMed] [Google Scholar]
- 188.Müller CE. A2A Adenosine receptor antagonists — future drugs for Parkinson’s disease? Drugs Fut. 2000;25:1043–1052. [Google Scholar]
- 189.Li A-H, Moro S, Forsyth N, Melman N, Ji X-D, Jacobson KA. Synthesis, CoMFA analysis, and receptor docking of 3,5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J Med Chem. 1999;42:706–721. doi: 10.1021/jm980550w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hirani E, Gillies J, Karasawa A, Shimad J, Kase H, Opacka-Juffry J, Osman S, Kuthra SK, Hume SP, Brooks DJ. Evaluation of [4-O-methyl-11C]KW-6002 as a potential PET ligand for mapping central adenosine A2A receptors in rats. Synapse. 2001;42:164–176. doi: 10.1002/syn.1110. [DOI] [PubMed] [Google Scholar]
- 191.Al Jaroudi W, Iskandrian AE. Regadenoson: a new myocardial stress agent. J Am Coll Cardiol. 2009;54:1123–1130. doi: 10.1016/j.jacc.2009.04.089. [DOI] [PubMed] [Google Scholar]
- 192.Eastwood P, Esteve C, González J, Fonquerna S, Aiguadé J, Carranco I, Doménech T, Aparici M, Miralpeix M, Albertí J, Córdoba M, Fernández R, Pont M, Godessart N, Prats N, Loza MI, Cadavid MI, Nueda A, Vidal B. Discovery of LAS101057: a potent, selective, and orally efficacious A2B adenosine receptor antagonist. ACS Med Chem Lett. 1 doi: 10.1021/ml100249e. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]