Abstract
Background
Children with allergic asthma have more frequent and severe human rhinovirus (HRV)-induced wheezing and asthma exacerbations through unclear mechanisms.
Objective
To determine whether increased high-affinity IgE receptor (FcεRI) expression and cross-linking impairs innate immune responses to HRV, particularly in allergic asthmatic children.
Methods
Peripheral blood mononuclear cells (PBMC) were obtained from 44 children and surface expression of FcεRI on plasmacytoid dendritic cells (pDCs), myeloid dendritic cells (mDCs), monocytes, and basophils was assessed using flow cytometry. Cells were also incubated with rabbit anti-human IgE to cross-link FcεRI, followed by stimulation with HRV-16, and interferon (IFN)-α and -λ1 were measured by Luminex. The relationships among FcεRI expression and cross-linking, HRV-induced IFN-α and -λ1 production, and childhood allergy and asthma were subsequently analyzed.
Results
FcεRIα expression on pDCs was inversely associated with HRV-induced IFN- α and IFN-λ1 production. Cross-linking FcεRI prior to HRV stimulation further reduced PBMC IFN-α (47% relative reduction, 95% confidence interval [CI], 32–62%, p<0.0001) and IFN-λ1 (81% relative reduction, 95% CI, 69–93%, p<0.0001) secretion. Allergic asthmatic children had higher surface expression of FcεRIα on pDCs and mDCs when compared to non-allergic non-asthmatic children. Further, after FcεRI cross-linking, allergic asthmatic children had significantly lower HRV-induced IFN responses than allergic non-asthmatics (IFN-α, p=0.004; IFN-λ1, p=0.02) and non-allergic non-asthmatics (IFN-α, p=0.002; IFN-λ1, p=0.01).
Conclusions
Allergic asthmatic children have impaired innate immune responses to HRV that correlate with increased FcεRI expression on pDCs and are reduced by FcεRI cross-linking. These effects likely increase susceptibility to HRV-induced wheezing and asthma exacerbations.
Keywords: asthma, allergic, rhinovirus, interferon, FcεRI, IgE receptor, plasmacytoid dendritic cells
Introduction
Aeroallergen sensitization is a pivotal risk factor for the development of human rhinovirus (HRV) wheezing illnesses.1 Children who wheeze with HRV infections during early life are at increased risk for school-age asthma,2,3 and children with concomitant aeroallergen sensitization have the greatest risk of subsequent asthma development.2,4 Once asthma is established, HRV is the most common cause of exacerbations, having been identified as a causative etiology in 50–90% of asthma exacerbations in children and adults.5–8 In a cohort of school-aged children with asthma, sensitization to aeroallergens was associated with similar numbers of viral infections, but more frequent and severe upper and lower virus-induced respiratory illnesses.9 Further, Heymann and colleagues10 have demonstrated that school-age children with asthma exacerbations severe enough to require hospitalization are likely to be both allergic and infected with HRV. However, the mechanisms underlying allergy-virus interactions in asthma inception and exacerbation are incompletely understood and represent an important area for further study.
Interferons (IFN) contribute significantly to the host response to virus infections, and impaired interferon production from mononuclear cells has been associated with both asthma and allergic sensitization.11–13 Insufficient production of IFN-λ1 by airway mononuclear cells has been linked to more severe HRV-induced illness and obstructive patterns on pulmonary function testing in allergic asthmatics compared to non-allergic controls.11 Additionally, plasmacytoid dendritic cells (pDCs) from allergic individuals produce less IFN-α upon toll-like receptor (TLR)-9 stimulation12 and pDCs from allergic asthmatics produce less IFN-α following stimulation with influenza virus.13 Interestingly, this impaired IFN-α production in pDCs has been linked to surface expression of the high-affinity IgE receptor (FcεRI). While FcεRI on mast cells and basophils is classically known to mediate the effector phase of the allergic response via Type I hypersensitivity reactions, a trimeric form of FcεRI is also expressed on pDCs, myeloid dendritic cells (mDCs) and monocytes.14 Increased expression and cross-linking of FcεRI on pDCs has been associated with diminished IFN-α production to influenza viruses.13 Therefore, we hypothesized that increased FcεRI expression on peripheral blood mononuclear cells (PBMC) would reduce HRV-induced IFN-α and IFN-λ1 production. Furthermore, HRV-induced IFN-α and IFN-λ1 production would be further reduced by cross-linking FcεRI, and would be lowest in allergic asthmatic children. To test these hypotheses, we examined PBMC responses to HRV in a subset of children enrolled in the Childhood Origins of ASThma (COAST) study.
Methods
Study population
Participants in this mechanistic study were 10–12 year old children enrolled in the COAST study, a birth cohort including children at high-risk for asthma and allergic disease based upon having at least 1 parent with asthma or allergic sensitization. Details regarding the study design and characteristics of its subjects have been previously published.2,15 Additional children with asthma were enrolled into COAST between 9 and 11 years of age to increase the study population. These subjects were born during the same time frame and met the same inclusion criteria as the children in the original cohort. For this mechanistic study, we obtained peripheral blood samples from 44 subjects as part of their annual COAST study visit. The Human Subjects Committee of the University of Wisconsin approved the study, informed consent was obtained from the parents, and assent was obtained from the children.
Definitions of Allergy and Asthma
Total IgE and allergen-specific IgE to dog, cat, cockroach, ragweed, birch, timothy grass, Alternaria alternata, Dermatophagoides farinae and Dermatophagoides pteronyssinus were measured using automated fluoroenzyme immunoassay (Unicap® 100, Pharmacia and Upjohn Diagnostics; Kalamazoo, MI) (FEIA) as previously described.1 Allergen-specific IgE values ≥ 0.35 KU/L (Class I) were considered positive, and the sensitivity for detection of total IgE was 2 KU/L. The presence of allergic sensitization was defined as having one or more positive values for allergen-specific IgE.
Current asthma was diagnosed based on previously published criteria2 of at least one of the following characteristics in the previous year: (1) physician diagnosis of asthma, (2) use of albuterol for coughing or wheezing episodes (prescribed by physician), (3) use of a daily controller medication, (4) step-up plan including use of albuterol or short-term use of inhaled corticosteroids during illness and (5) use of prednisone for asthma exacerbation.
Immunologic studies
Specimens of peripheral blood were collected and PBMCs were isolated using Ficoll® density gradient centrifugation as previously described.16 PBMCs were incubated with either rabbit IgG isotype control (1 μg/mL; Bethyl Laboratories; Montgomery, TX), rabbit anti-human IgE (0.1 or 1 μg/mL) or media alone for 2 hours. PBMCs were then incubated with either HRV-16 (2.5 × 106 plaque forming units/mL) or media alone for 24 hours. Supernatants were subsequently collected and analyzed for IFN-α and IFN-λ1 production by a multiplex assay (Millipore; Billerica, MA).
Flow cytometry
The following fluorochrome-conjugated anti-human Abs were used for identification of pDCs, mDCs, monocytes and basophils and assessment of FcεRIα expression in peripheral blood: Lineage FITC cocktail (containing CD3, CD14, CD16, CD19, CD20 and CD56), CD123-PE-Cy5, HLA-DR-PerCP, CD11c-APC, CD14-Pacific Blue (BD Biosciences; San Jose, CA) and FcεRIα-PE (eBiosciences; San Diego, CA). Data was acquired using a BD™ LSRII Flow Cytometer. Immune cell populations were pidentified as follows: pDCs: lineage-negative, HLA-DR-positive, CD11c-negative, CD123-positive cells; mDCs: lineage-negative, HLA-DR-positive, CD11c-positive, CD123-negative cells; monocytes: CD14-positive cells; basophils: lineage-negative, HLA-DR-negative, CD123-positive cells. FcεRI expression, reported as percent FcεRI positive cells, was determined on pDCs, mDCs, monocytes and basophils. Sample compensation was performed by using unstained and single stained anti-mouse IgG, κ polystyrene microparticle beads (BD Biosciences) as controls. Compensation was calculated in FACS DiVa (version 6.0; BD Biosciences) during acquisition and calculated again in FlowJo (version 9.3.2; TreeStar Inc.; Ashland, OR) for final data analysis.
Statistical analysis
Repeated measures ANOVA models were used to assess the effects of cross-linking FcεRI on HRV-induced IFN production in peripheral blood mononuclear cells incubated with isotype control IgG, anti-IgE (0.1 or 1 μg/mL) or media alone. Linear models were constructed to incorporate allergic sensitization and asthma as covariates for interferon responses and possible effect modifiers for FcεRI cross-linking. Global tests for main effects and interactions were performed, and pairwise comparisons between the asthma/allergy groups were evaluated according to Fisher’s protected least significant difference (LSD). Pairwise relationships between total IgE, HRV-induced IFNs, and percent of pDCs, mDCs, monocytes and basophils positive for surface FcεRIα expression were assessed using Pearson’s correlation coefficient after log transformation. Two-sided P-value of 0.05 was considered to be statistically significant. Analyses were performed in SAS version 9.2 (SAS Institute; Cary, NC).
RESULTS
Study Population
Of the 44 children included in this study, 14 had aeroallergen sensitization and current asthma (allergic asthma), 3 were not sensitized to aeroallergens but had current asthma (non-allergic asthma), 13 had aeroallergen sensitization but did not have asthma (allergic non-asthmatic), and 14 had neither aeroallergen sensitization nor asthma (non-allergic non-asthmatic). (Table I)
Table I.
Baseline patient characteristics of 4 groups. All values expressed as medians with 25th and 75th percentile ranges when indicated. FEV1: Forced expiratory volume in one second. Pre-bronchodilator % predicted FEV1 values expressed.
| Allergic Asthmatics | Non-Allergic Asthmatics | Allergic Non- Asthmatics | Non-Allergic Non-Asthmatics | |
|---|---|---|---|---|
| N | 14 | 3 | 13 | 14 |
| Age (years) | 11.6 | 11.8 | 11.33 | 11.3 |
| Gender (Male/Female) | 10/4 | 1/2 | 4/9 | 6/8 |
| Total IgE (KU/L) | 533 (218,884) | 21(12,109) | 60(37,333) | 20 (7,66) |
| Number of positive allergen- specific in vitro IgE | 6 (3,7) | 0 | 2 (1,5) | 0 |
| FEV1 (% predicted) | 100 (92,110) | 107 (95,107) | 104 (95,111) | 102 (95,120) |
Increased FcεRI Expression and Cross-linking Inhibits HRV-induced Interferon Production
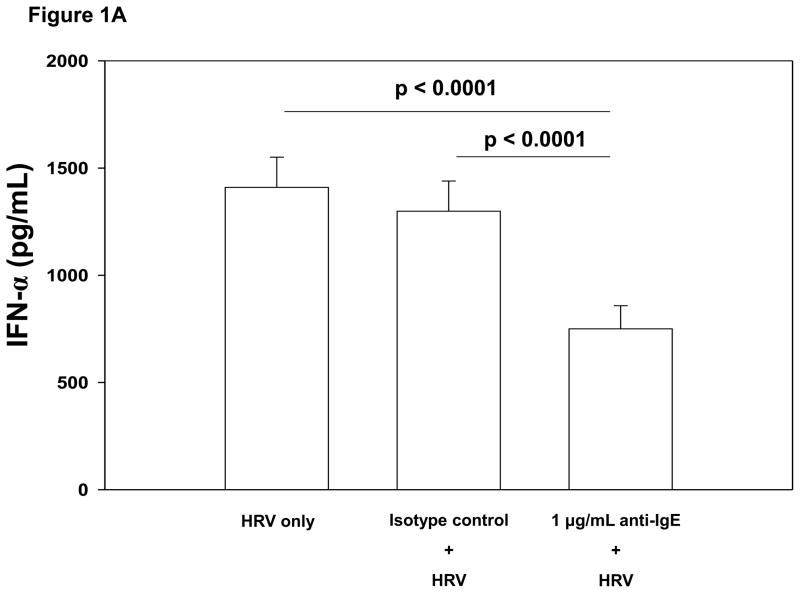
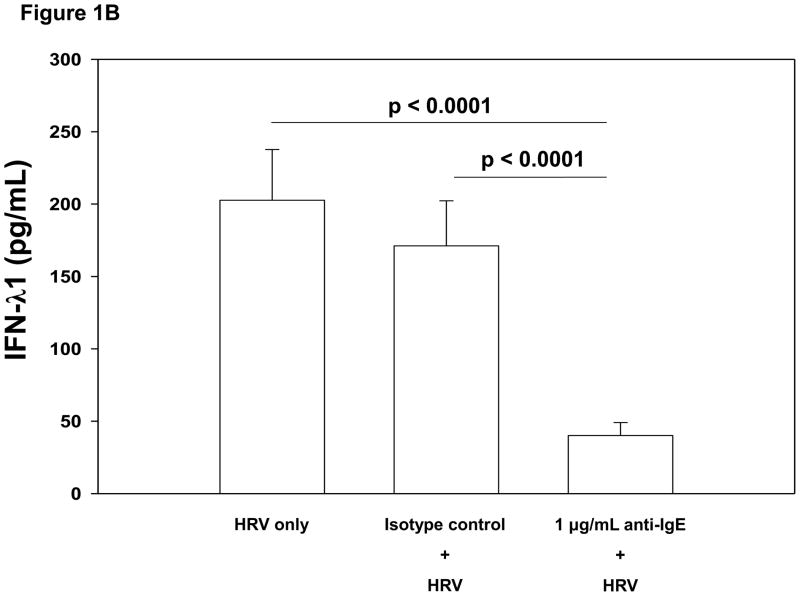
The percentage of FcεRIα positive pDCs was inversely associated with HRV-induced IFN-α and IFN-λ1 production (Table II). Cross-linking FcεRI on PBMCs prior to HRV stimulation significantly reduced HRV-induced IFN-α production. This effect was observed at both the 0.1 μg/mL and 1 μg/mL concentrations of anti-IgE when compared to no pretreatment (0.1 μg/mL: mean±SE 1192±126 versus 1410±150 pg/mL, p=0.002; 1 μg/mL: 750±108 versus 1410±150 pg/mL, p<0.0001; Figure 1A). Cross-linking FcεRI also reduced HRV-induced IFN-λ1 secretion (0.1 μg/mL: 125±20 versus 206±36 pg/mL, p<0.0001; 1 μg/mL: 39±9 versus 206±36 pg/mL, p<0.0001; Figure 1B). Similar inhibition of HRV-induced interferon production was obtained by preincubation with a monoclonal Ab specific for FcεRIα (data not shown).
Table II.
Relationships between percent FcεRI positive pDCs, mDCs, monocytes, and basophils and production of HRV-induced IFN-α and IFN-λ1. All values expressed are Pearson’s Rho estimates.
| pDC | mDC | Monocytes | basophils | |
|---|---|---|---|---|
| IFN-α | R: −0.37 (p = 0.01) | R: −0.29 (p = 0.05) | R: −0.12 (p = 0.42) | R: −0.20 (p = 0.18) |
| IFN-λ1 | R: −0.42 (p = 0.005) | R: −0.24 (p = 0.11) | R: −0.18 (p = 0.24)3 | R: −0.28 (p = 0.07) |
Figure 1.
Figure 1A. Comparison of PBMC HRV-induced mean IFN-α production when pretreated with media (no pretreatment), 1 μg/mL rabbit IgG isotype control Ab or 1 μg/mL anti-IgE for 2 hours. Pretreatment of PBMCs using 1 μg/mL anti-IgE to cross-link FcεRI resulted in significantly lower HRV-induced IFN-α (mean [±SE] 750±108 pg/mL) compared to pretreatment with 1 μg/mL rabbit IgG isotype control (1299±141 pg/mL; p<0.0001) or no pretreatment (1410±150 pg/mL; p<0.0001). N= 44.
Figure 1B. Comparison of PBMC HRV-induced mean IFN-λ1 production (pg/mL) when pretreated with media (no pretreatment), 1 μg/mL rabbit IgG isotype control Ab or 1 μg/mL anti-IgE for 2 hours. Pretreatment of PBMCs using 1 μg/mL anti-IgE to cross-link FcεRI resulted in significantly lower HRV-induced IFN-λ1 (mean [±SE] 39±9 pg/mL) compared to pretreatment with 1 μg/mL rabbit IgG isotype control (171±31 pg/mL; p<0.0001) or no pretreatment (206±36 pg/mL; p<0.0001). N = 44.
Allergic Sensitization, Asthma, and Interferon Responses
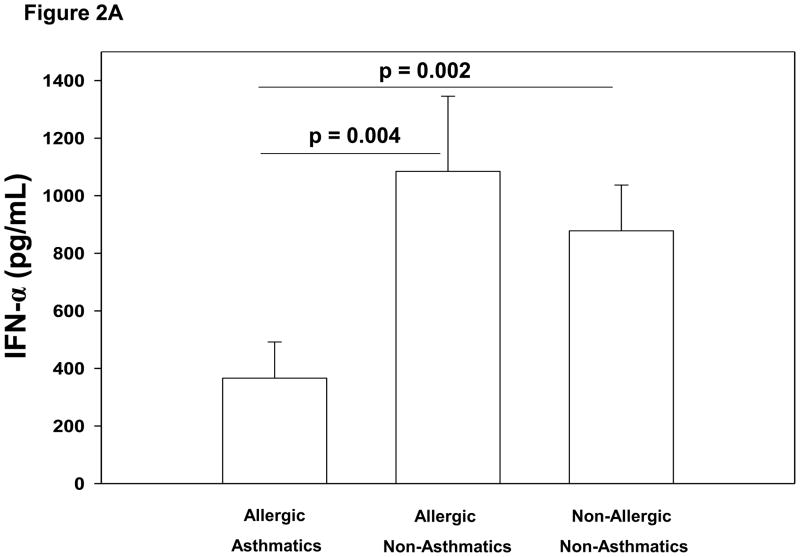
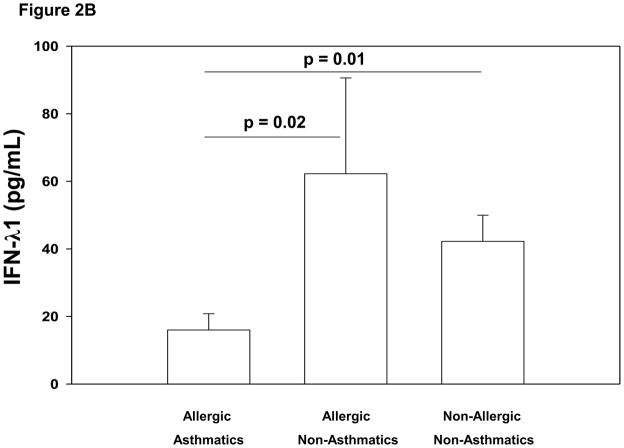
We next examined whether the observed relationships between FcεRI and HRV-induced interferon secretion from PBMCs was associated with asthma and/or allergic sensitization clinical phenotypes. In the absence of FcεRI cross-linking, HRV-induced IFN-α and IFN-λ1 responses tended to be lower in allergic asthmatics compared to other children, but these differences were not statistically significant. After FcεRI cross-linking, however, HRV-induced IFN-α production was significantly lower in allergic asthmatics (mean±SE: 366±126 pg/mL) than in both allergic non-asthmatics (1085±261 pg/mL, p=0.004) and non-allergic non-asthmatics (878±159 pg/mL, p=0.002) (Figure 2A). Similarly, IFN-λ1 production following FcεRI cross-linking was significantly lower in allergic asthmatics (16±5 pg/mL) than both allergic non-asthmatics (62±28 pg/mL, p=0.02) and non-allergic non-asthmatics (42±8 pg/mL, p=0.01) (Figure 2B). There were no significant differences between the IFN production of the allergic non-asthmatic and non-allergic non-asthmatic children (Figure 2A and 2B). The group of non-allergic asthmatics was too small (n=3) to provide adequate power to compare these children to the other 3 groups.
Figure 2.
Figure 2A. Comparison of allergic asthmatic (N = 14), allergic non-asthmatic (N = 13), and non-allergic non-asthmatic (N = 14) children’s PBMC HRV-induced IFN-α production (pg/mL) after cross-linking FcεRI with 1 μg/mL anti-IgE for 2 hours prior to HRV stimulation. Allergic asthmatic children had significantly lower HRV induced IFN-α production (mean [±SE] 366±126 pg/mL) than both allergic non-asthmatics (1085±261 pg/mL; p = 0.004) and non-allergic non-asthmatics (878±159 pg/mL; p=0.002).
Figure 2B. Comparison of allergic asthmatic (N = 14), allergic non-asthmatic (N = 13), and non-allergic non-asthmatic (N = 14) children’s PBMC HRV-induced mean IFN-λ1 production (pg/mL) after cross-linking FcεRI with 1 μg/mL anti-IgE for 2 hours prior to HRV stimulation. Allergic asthmatic children had significantly lower HRV-induced IFN-λ1 production (mean [±SE] 16±5 pg/mL) than both allergic non-asthmatics (62±28 pg/mL; p=0.02) and non-allergic non-asthmatics (42±8 pg/mL; p=0.01).
FcεRI Expression, Allergy, and Asthma
We next compared FcεRIα expression on PBMCs in children based upon allergic and asthma phenotypes. Children with allergic asthma had a higher percentage of FcεRIα positive pDCs (mean±SE 74.3±5.9 versus 44.8±7.2, adjusted p=0.009) and mDCs (83.1±2.1 versus 66.8±4.8, p=0.04) when compared to non-allergic non-asthmatic children (Table III). Allergic non-asthmatic children had significantly greater percent FcεRIα positive pDCs and mDCs, compared to non-allergic non-asthmatic children (Table III).
Table III.
Comparison of percent FcεRI positive(mean+/SE) pDCs, mDCs, monocytes and basophils of children based upon allergic and asthma phenotypes.
| % FcεRI positive cells(mean ±SE) | Allergic Asthmatics (n = 14) | Allergic Non-Asthmatics (n = 13) | Non-Allergic Non-Asthmatics (n = 14) | |
| pDCs | 74±6* | 60±6 | 45±7 | |
| mDCs | 83±2* | 83±2¥ | 67±5 | |
| monocytes | 7±2 | 7±1 | 2±0.4 | |
| basophils | 93±2 | 93±1¥ | 76±7 |
Denotes significant difference between allergic asthmatic and non-allergic non-asthmatic subjects (pDCs: p=0.009; mDCs: p=0.04).
Denotes significant difference between allergic non-asthmatic and non-allergic non-asthmatic subjects (mDCs: p=0.05). All p values adjusted for multiple comparisons.
Relationships Among Total IgE, FcεRI Expression, and HRV-induced IFN Production
Finally, we examined total IgE as a potential biomarker for FcεRI expression and the cross-linking relationships observed above. There was a significant positive correlation between total IgE and percent FcεRIα positive pDCs (R= 0.60, p<0.0001), mDCs (R= 0.48, p=0.001) and basophils (R= 0.47, p<0.001). A similar trend was observed for FcεRIα positive monocytes (R= 0.28, p=0.07). Moreover, the percentage of FcεRI positive pDCs correlated with the percentage of FcεRIα positive mDCs (R= 0.70, p<0.0001) and basophils (R= 0.73, p<0.0001). However, HRV-induced interferon production was not significantly associated with total IgE in the absence of FcεRI cross-linking (IFN-α: R= -0.18, p=0.24; IFN-λ: R= −0.12, p=0.44). When FcεRI was cross-linked prior to HRV stimulation, total IgE was inversely associated with HRV-induced IFN-α (1 μg/mL: R= −0.42, p=0.004) and IFN-λ1 production (0.1 μg/mL: R= −0.38, p=0.01; 1 μg/mL: R= −0.39, p=0.009).
DISCUSSION
In this study, we identified a mechanism that may underlie the important clinical observation that children with allergic sensitization, particularly those both sensitized and exposed, have more clinically significant lower respiratory tract illnesses and asthma exacerbations caused by human rhinoviruses (HRV).9,10,17 We have demonstrated that the frequency of pDCs expressing the high-affinity IgE receptor (FcεRI) is inversely associated with HRV-induced IFN-α and IFN-λ1 secretion. Moreover, cross-linking of this receptor prior to HRV stimulation using antibodies to either IgE or the receptor itself significantly inhibited IFN-α and IFN-λ1 production by half or more, and these effects were especially pronounced in children with allergic asthma. These findings are of particular clinical relevance because up to 90% of asthma exacerbations in children are related to HRV infection,8 they most often occur in children with concomitant aeroallergen sensitization10 and current therapies are only partially effective in preventing exacerbations.18
Notably, the inverse relationships between total IgE and diminished IFN secretion were only apparent after FcεRI cross-linking. An argument could be made that the observed lack of correlation with total IgE was due to culturing the PBMCs in IgE-free media; however, the half-life of the IgE-FcεRI complex has been found to be approximately 16 hours in suspension, 19 and our experiments were initiated within a few hours of blood sample collection. The significant correlations between total IgE levels and percentages of FcεRI-positive PBMCs identified in our patient population suggest that FcεRI crosslinking may represent a key event leading to impairment of virus-induced interferon production. More importantly, the significant inverse correlations between total IgE and HRV-induced interferon in the presence of FcεRI cross-linking provide direct evidence for the potential biologic relevance of this pathway in vivo, where cross-linking of allergen-specific IgE in the scenario of sensitization and exposure could also result in significant impairment of interferon responses to HRV.
Plasmacytoid dendritic cells (pDCs) produce up to 95% of the IFN-α20 in peripheral blood upon stimulation with viruses, despite making up only 0.2–0.8% of the PBMC population.21 In our study, HRV-induced interferon secretion was most significantly inversely associated with pDC FcεRI expression, suggesting that these cells are principally involved in this effect. The mechanism by which increased FcεRI expression on pDCs, even in the absence of FcεRI cross-linking, leads to impaired IFN production is unclear. One possibility may be related to signaling through the FcεRIγ subunit, an immunoreceptor-based tyrosine activation motif (ITAM) that recruits and regulates tyrosine kinases such as the Src and Syc families.22 Interestingly, immunoglobulin-like transcript 7 (ILT7) and blood dendritic cell antigen 2 (BDCA2) are regulatory surface receptors specific to pDCs that both signal through FcεRIγ, and cross-linking either ILT7 or BDCA2 inhibits interferon synthesis in response to viruses.22,23 Thus, it is possible that cross-linking FcεRI directly inhibits HRV-induced IFN-α and IFN-λ1 production, but additional mechanisms may contribute to impaired interferon production in allergic asthmatic children as well.
Unchecked interferon production by pDCs has been associated with autoimmune diseases such as lupus24 and Sjogren syndrome;25 therefore, tight regulation of IFN responses is critical for immune homeostasis. Thus, it is possible that effects of FcεRI on interferon secretion comprise a counter-regulatory pathway in place to control interferon responses. Consequently, in allergic asthmatic individuals, overexpression of FcεRI may lead to excessive interferon inhibition. Gill and colleagues13 recently demonstrated diminished pDC IFN-α production in response to influenza virus in allergic asthmatics compared to non-allergic controls. Taken together with our findings, this counter-regulatory pathway may be important in the antiviral response to numerous respiratory viruses.
A novel aspect of our findings was the evaluation of IFN-λ1 production, especially given the recent data linking diminished IFN-λ1 responses to asthma.11 Specifically, Contoli and colleagues11 have demonstrated that insufficient levels of IFN-λ1 in the asthmatic airway are associated with more severe HRV induced illness and obstructive patterns on pulmonary function testing in allergic asthmatic adults.11 Interestingly, IFN-λ1 mediates its actions through a receptor distinct from the receptor for Type I IFNs. The results of our study suggest allergic asthmatics children have diminished Type III IFN production to rhinovirus, which may represent an exciting opportunity to develop distinct anti-viral therapies aimed at this pathway.
One strength of our study is the inclusion of allergic non-asthmatic children, which allows separate evaluation of effects on allergy vs. asthma. Additionally, we utilized PBMCs, rather than isolated pDCs, in order to evaluate the role of FcεRI expression and cross-linking on multiple innate immune cell types and to allow for cell-cell interactions. Furthermore, the subjects studied have been well characterized including comprehensive and repeated assessments of allergic and asthma phenotypes. A study limitation is that only 3 non-allergic asthmatics were evaluated and thus we lacked power to compare these children to the other phenotypes; however, the vast majority of school-age children with asthma have concomitant allergic sensitization.18,26
Our results may have particular clinical relevance to a recent study in inner city children with allergic asthma by Busse and colleagues,27 which demonstrated the efficacy of omalizumab in preventing exacerbations during peak HRV seasons. Studying HRV-induced IFN production pre-/post-omalizumab therapy could provide further evidence to support to our hypothesis. Further, it is well known that allergic sensitization is an important risk factor for asthma inception,28 and recent evidence supports a causal role for sensitization in the development of HRV wheezing in preschool children.1 It could be postulated from our study that preschool children with aeroallergen sensitization and exposure have reduced innate immune responses that could lead to greater severity of HRV illnesses in early life and, consequently, lead to the development of persistent airway inflammation and asthma.
In summary, we have identified a mechanism that may link allergic asthma in childhood to deficient antiviral responses. Specifically, increased expression and cross-linking of the high-affinity IgE receptor, FcεRI, prior to HRV infection inhibits IFN-α and IFN-λ1 secretion from PBMCs. These findings, which were most pronounced in the context of allergic asthma, suggest that therapeutic strategies aimed at reducing FcεRI expression and cross-linking represent viable approaches to prevent HRV-induced wheezing illnesses and asthma exacerbations.
Key Messages.
Increased expression and cross-linking of the high-affinity IgE receptor, FcεRI, on plasmacytoid dendritic cells is associated with reduced human rhinovirus-induced IFN-α and IFN-λ1 secretion.
Allergic asthmatic children have significantly reduced human rhinovirus-induced IFN-α and IFN-λ1 production after cross-linking of FcεRI.
Acknowledgments
Supported by NIH grants: 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), R01 HL61879, P01 HL70831, M01 RR03186, T32 AI007635 and a AAAAI/GSK Career Development Award
Abbreviations
- HRV
Rhinovirus
- IFN
Interferon
- pDC
plasmacytoid dendritic cells
- mDC
myeloid dendritic cells
- PBMC
peripheral blood mononuclear cells
- COAST
Childhood Origins of Asthma study
- TLR
toll-like receptor
- ITAM
Immunoreceptor-based tyrosine activation motif
- ILT7
Immunoglobulin-like transcript 7
- BDCA2
Blood dendritic cell antigen 2
- FEIA
Fluoroenzyme immunoassay
- FEV1
Forced expiratory volume in 1 second
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jackson DJ, Evans MD, Gangnon RE, et al. Evidence for a Causal Relationship Between Allergic Sensitization and Rhinovirus Wheezing in Early Life. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–60. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khetsuriani N, Kazerouni NN, Erdman DD, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bizzintino J, Lee WM, Laing IA, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37:1037–42. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olenec JP, Kim WK, Lee WM, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–6. e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 12.Tversky JR, Le TV, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-alpha via Toll-like receptor 9. Clin Exp Allergy. 2008;38:781–8. doi: 10.1111/j.1365-2222.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill MA, Bajwa G, George TA, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–72. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 15.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13 (Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 16.Neaville WA, Tisler C, Bhattacharya A, et al. Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. J Allergy Clin Immunol. 2003;112:740–6. doi: 10.1016/s0091-6749(03)01868-2. [DOI] [PubMed] [Google Scholar]
- 17.Murray CS, Poletti G, Kebadze T, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–82. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–7. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonnell JM, Calvert R, Beavil RL, et al. The structure of the IgE Cepsilon2 domain and its role in stabilizing the complex with its high-affinity receptor FcepsilonRIalpha. Nature structural biology. 2001;8:437–41. doi: 10.1038/87603. [DOI] [PubMed] [Google Scholar]
- 20.Barchet W, Blasius A, Cella M, Colonna M. Plasmacytoid dendritic cells: in search of their niche in immune responses. Immunol Res. 2005;32:75–83. doi: 10.1385/IR:32:1-3:075. [DOI] [PubMed] [Google Scholar]
- 21.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 22.Cao W, Rosen DB, Ito T, et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao W, Zhang L, Rosen DB, et al. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17:394–9. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottenberg JE, Cagnard N, Lucchesi C, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc Natl Acad Sci U S A. 2006;103:2770–5. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemanske RF, Jr, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–85. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sly PD, Boner AL, Bjorksten B, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–6. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]