Abstract
Three experiments examined the processes mediating rat serial pattern learning for rule-consistent versus rule-violating pattern elements (“violation elements”). In all three experiments, rats were trained to press retractable levers in a circular array in a specific sequence for brain stimulation reward (BSR). Experiment 1 examined the role of lever location (L) and element serial position (SP) cues in rats’ ability to learn to anticipate a violation element positioned at the end of a 24-element serial pattern. Rats with L cues either alone or in combination with SP cues learned to anticipate the violation element, whereas those with SP cues alone did not. Rats in groups L and L+SP underwent a series of transfers designed to remove various cues that might have controlled their performance on the violation element. Results indicated that intra-chamber lever location cues mediated performance on the violation element whereas performance on rule-consistent elements within pattern chunks was mediated by an internal mnemonic representation that was insensitive to changes in lever location cues. Experiment 2 examined whether rats could learn to use SP cues alone to anticipate a violation element if it was positioned earlier in a serial pattern. Rats learned to anticipate the violation element based on SP cues alone when it was located in SP6 in a 24-element pattern, but not when it was in SP12. Experiment 3 examined whether or not rats spontaneously encode information about chunk length and the serial position of phrasing cues in serial patterns. Rats were trained to a high criterion on the serial pattern used in Experiment 1, then were challenged with three probe patterns that manipulated both chunk length and overall pattern length. Results indicated that rats spontaneously encoded information regarding the serial position of phrasing cues in relation to chunk length. Thus, rats appear to use at least three cognitive processes concurrently in serial pattern learning tasks, namely, item memory involving external discriminative cues, counting- or timing-like processes for encoding serial position, and rule abstraction for encoding an internal representation of pattern structure.
In the 1970s, Stewart H. Hulse and his students conducted research on rats’ ability to learn to anticipate a series of different food quantities presented in a runway (Hulse, 1973; Hulse & Campbell, 1975; Hulse & Dorsky, 1977; 1979). This seemingly simple paradigm belied a sophisticated approach to answering a central question in animal learning theory. The question Hulse posed was whether or not nonhuman animals such as rats could learn about the structure of a sequence, that is, the abstract organizational properties of the sequence. Hulse’s conclusion that rats could in fact go “beyond the information given” (cf. Bruner, 1957), that is, that they could abstract and encode a representation of the formal rule structure of the sequences they experienced, aroused heated debate within the field and helped propel the notion of cognitive processes in animal behavior into mainstream learning theory.
Hulse’s rule-learning theory, laid out in some detail in his chapter in the influential 1978 volume, Cognitive Processes in Animal Behavior, which he co-edited with Harry Fowler and Vern Honig, proposed that rats are able to learn a representation of the abstract rules that describe organized sequences they experience (Hulse, 1978; Hulse et al., 1977; 1979). The implication was that rats did not have to rely on chaining or remote associations alone to master sequences (e.g., Hull, 1931; Skinner, 1934). In contrast, Capaldi and colleagues proposed a competing view that serial pattern learning in rats could be accounted for by more traditional associative mechanisms alone (Capaldi & Molina, 1979; Capaldi, Verry, & Davidson, 1980a; 1980b; Capaldi, Nawrocki, & Verry, 1982; Capaldi, Nawrocki, Miller, & Verry, 1985; e.g., Capaldi, 1986). Capaldi likened sequential learning to other discrimination learning problems where items in sequences and other valid cues other than sequential elements themselves served as cues for forthcoming events. Adding to the complexity of the debate, the rule-learning view frequently failed to predict outcomes for shorter patterns, and Hulse (1980) conceded that Capaldi’s item memory theory was a better account in that domain. According to Hulse, short patterns approximate the paradigm of paired-associate learning rather than that of sequential learning. Roitblat, Pologe, and Scopatz (1983) proposed yet another view, namely, that learning about the serial position of events played an important role in rat serial-pattern learning. Serial position models assume that sequence elements become associated with their position in the sequence, not with other sequence items (Roitblat, Pologe, & Scopatz, 1983; cf. Chen, Swartz, & Terrace, 1997; Burns & Gordon, 1988; Burns, Hulbert, & Cribb, 1990). Although serial position models may have much in common with Capaldi’s item memory view, the critical difference is that sensitivity to serial position seems to imply the additional cognitive ability to count or time serial events. Evidence for counting- or timing-like processes in rat sequential learning has since been obtained (Capaldi, 1993; Capaldi & Miller, 1988a; 1988b; 1988c; 1988d; Capaldi, Miller, & Alptekin, 1988).
Although the foregoing models were originally proposed as mutually exclusive accounts of sequential learning and memory, recent behavioral and psychobiological work has produced evidence to support all of these positions, sometimes suggesting that rats may employ more than one strategy or type of information to encode and reproduce a single complex behavioral sequence (Fountain et al., in press; Fountain & Rowan, 1995a; 1995b; 2000b; Fountain & Benson, Jr., 2006). The failure to account for such evidence via a single general process could be due to weaknesses in the available theories, but we believe the evidence implicates multiple, concurrent behavioral and brain processes in serial pattern learning.
We report three experiments designed to examine the nature of potential concurrent processes mediating rat serial pattern learning for rule-consistent versus rule-violating pattern elements (“violation elements”). In all three experiments, rats were trained to press retractable levers in a circular array in a specific sequence for brain stimulation reward (BSR). Experiment 1 examined the role of lever location (L) and element serial position (SP) cues in rats’ ability to learn to anticipate a violation element positioned at the end of a 24-element serial pattern, that is, in SP24. Experiment 2 examined whether rats could learn to use SP cues alone to anticipate a violation element if it was positioned earlier in a serial pattern. Experiment 3 examined whether or not rats spontaneously encode information about chunk length and the serial position of phrasing cues in serial patterns. The goal was to examine whether rats may employ more than one strategy or type of information concurrently to encode and reproduce a single complex behavioral sequence (Fountain et al., 1995a; 1995b; 2000b).
2 Experiment 1
How nonhuman animals and humans respond to violations of pattern structure in serial patterns has been considered particularly revealing with regard to how they represent serial patterns (Fountain et al., 1995a; 1995b; Fountain, Krauchunas, & Rowan, 1999; Fountain & Rowan, 2000b; Restle & Burnside, 1972). When rats and humans learn a highly periodic and repetitive pattern of movements, responses that are exceptions to the implicit pattern are considerably more difficult to learn than other elements that conform to the implicit pattern. In addition, on the exceptions, termed "violation elements" because they violate pattern structure, errors are not random; rather, they tend to be responses consistent with pattern structure. For example, in one experiment, rats learned serial patterns in a “serial multiple choice task” (Fountain et al., 1995a). In an octagonal chamber with a lever on each wall, rats learned to choose from the circular array of 8 levers in the proper sequential order on successive trials to obtain reinforcement. The levers were designated Levers 1 through 8 in clockwise fashion with Lever 8 adjacent to Lever 1. All of the levers were presented at the beginning of each trial and the rat could press any of the 8 levers. If the correct lever was pressed, then the rats received hypothalamic brain-stimulation reward (BSR). If an incorrect lever was pressed, then all of the levers except the correct lever were removed from the box and the animal was not reinforced until the correct response was emitted. Rats were required to learn patterns made up of “runs” or “trills”. Two groups of rats learned run sequences (one perfect pattern and one pattern with a violation element) and two groups learned trill sequences (also perfect versus violation patterns). The perfect run sequence was 123-234-345-456-567-678-781-812. The perfect trill sequence was 121-232-343-454-565-676-787-818. One group from each set was required to learn the pattern with a violation element in the last chunk. A violation element is defined here as an element that is not predicted by the overall structure of the pattern. The run pattern with the violation element (underelined) was 123-234-345-456-567-678-781-818. The trill pattern with the violation element (underlined) was 121-232-343-454-565-676-787-812. The violation sequences differed from the perfect sequences only in the last element. Learning the violation element proved to be a very difficult task for rats. In addition, for the runs violation group, the most common error for the violation element was "2", which fit with the overall structure of the pattern. Similarly, the most common error for the violation element of the trills violation pattern was "8", the response predicted by the pattern structure (Fountain et al., 1995a). Results with mice in an analogous task paralleled those with rats (Fountain et al., 1999). These results have been interpreted as supporting the notion that different processes may mediate learning about structured and violation elements in serial patterns (Fountain, 2006; Fountain & Rowan, 1995b; 1995c; Fountain, Wallace, & Rowan, 2002).
Experiment 1 examined the role of lever location (L) and element serial position (SP) cues in rats’ ability to learn to anticipate a violation element positioned at the end of the 24-element serial pattern of runs used in the foregoing study and in a number of more recent experiments in our lab (Fountain et al., 1995a; Fountain et al., 1999; Fountain et al., 2000b; Fountain, Rowan, Kelley, Willey, & Nolley, 2008; Kundey & Fountain, 2010). The violation pattern used in Experiment 1, 123-234-345-456-567-678-781-818, was structurally composed of eight 3-element chunks, each signaled by distinctive temporal breaks that served as “phrasing cues,” and a violation element at the end of the pattern in SP 24. Thus, the serial pattern was made up of several types of elements, namely, chunk-boundary elements (the first element of each chunk), within-chunk elements, and a single violation element. In Experiment 1, we manipulated various internal and external cues to determine if rats’ sequential behavior was controlled by discriminative cues or if their behavior was guided by rules.
Experiment 1 was designed to answer three questions. First, how do animals learn where to make the violation response? Second, is learning about the violation element of the pattern different from learning about the rest of the pattern? And finally, is there one process that governs serial pattern learning, or are there multiple processes (i.e., discrimination learning and rule learning)? Answering the first two questions is a necessary step in answering the third. These questions were addressed by examining rats’ performance on within-chunk elements, chunk-boundary elements, and violation elements that made up the sequence rats learned in this study. To answer these questions, 4 groups of rats in Experiment 1 were required to learn to anticipate the violation element in a 24-item sequence with different types of potential cues at their disposal. One group had no relevant cues for predicting when the violation element would next occur in the sequence, one group had relevant item cues (lever location cues within the chamber), one group had relevant serial position cues, and one group had both relevant location and serial position cues. Afterwards, rats that succeeded in learning their pattern experienced a series of transfers where various relevant cues were systematically manipulated. Capaldi et al.’s (1979) item memory view predicts that animals will use item cues (i.e., location cues) to guide behavior. If this is the case, when the pattern is well-learned, performance on the violation element should be good when location cues remain the same in transfer, and performance should be poor when location cues are altered in transfer. The serial position hypothesis predicts that animals track the violation element by knowing its serial position. If rats use serial position to anticipate the violation element, implying a timing or counting mechanism, when its serial position remains constant then performance should remain good in transfer, but should be poor when serial position cues are altered. If it could be shown that location and serial position cues alone control rats’ behavior in this task, then the rule-learning explanation would be unparsimonious.
2.1 Method
Subjects
Fourteen naive male hooded rats (Rattus norvegicus) served as subjects. All were implanted with bipolar electrodes (MS301, Plastic Products, Roanoke, VA) for hypothalamic brain-stimulation reward (coordinates, skull level: 4.5 mm posterior, 1.5 mm lateral, 8.5 mm below the surface of the skull) and were at least 90 days of age at the time of surgery. Rats were deeply anesthetized by 35.56 mg/kg ketamine and 3.56 mg/kg xylazine i.p. injection before surgery and received antibiotics (60,000 units of penicillin intramuscularly) to reduce the chance of infection after surgery. They were also carefully monitored for infection after surgery and were provided at least 1 week for recovery from surgery. Rats were housed in individual cages with food and water freely available on a 15:09 hr light:dark cycle and were tested during the light portion of the cycle. Both food and water were freely available in the home cage.
Apparatus
Shaping chambers (30 × 30 × 30 cm) equipped with a lever and a commutating device centered in the ceiling were used to shape rats to lever press for brain stimulation. The walls and ceiling of the chambers were constructed of clear Plexiglas with a floor of stainless steel rods. The shaping chambers were enclosed in a sound attenuating chamber made of particle board (20 × 60 × 65 cm) and were housed in a room separate from the testing rooms.
The test chambers were octagonal and measured approximately 40 cm between parallel walls. The walls and ceiling were made of clear Plexiglas with a floor of hardware cloth. A commutating device was located in the center of the ceiling of the apparatus. A retractable response lever was centered on each wall 5.0 cm above the floor (Fountain et al., 1995a; 1995b). The octagonal chambers were located in separate rooms (approximately 2 × 2.6 m) illuminated throughout testing by fluorescent lighting.
Levers in both the shaping and testing chambers required approximately 0.15 Newton (N) force for activation. Rats were connected to the stimulator by way of a flexible cord (Plastic Products, MS304) and a commutating device. The experiment was controlled from an adjoining room using a microcomputer and interface (Interface and Med-State Software: Med Associated Inc., Fairfield, VT).
Procedure
Following at least one week’s recovery from surgery, rats were shaped to lever press for brain stimulation reward (BSR) in a shaping chamber containing one lever. Reinforcement consisted of a single 200-ms BSR “pulse” of a 60-Hz sinusoidal pulse train from a constant current source of 20–100µA. In all procedures rats received one such pulse for each correct response. Rats were required to make at least 1000 bar press responses within a 30-min session and received up to three sessions to meet criterion. Rats that failed to meet the criterion were excluded from the study. Once the rats were trained to lever press for brain stimulation, they were divided into four groups and trained six days a week in the octagonal chamber until they reached criterion.
The rats were required to learn a 24-item sequence containing a violation element with the use of a correction procedure. Violation element is defined in this paper as an element that is not predicted from the structure of the pattern. The sequence was: 123 234 345 456 567 678 781 818. The sequence was made up of a series of eight 3-element groups called chunks. Between each element within chunks there was a 1-s interval. Between each chunk was a 3-s interval that served as a phrasing cue (Stempowski, Carman, & Fountain, 1999). Each sequence began with one lever inserted into the chamber. Rats were required to press that lever to move on to the next element in the series. Once the first lever was pressed, it was retracted and a pulse of BSR was delivered. After a one-second pause, all the levers were inserted into the chamber and the animal had to press the correct one to receive the BSR. If the correct lever was not pressed, all the levers except the correct one were retracted and the animal had to press the correct lever to receive BSR and to move on to the next element in the pattern. This procedure was repeated for successive elements of the sequence. The rats received 24 repetitions of the sequence, six days a week.
Rats were randomly assigned to four groups. Rats in the Location Cues (L) only group started each sequence on a different randomly chosen lever, but the violation was always at the same location in the chamber. For example, on one day rats in L might receive the following sequences, among others:
123 234 345 456 567 678 787 812
567 678 787 812 123 234 345 456
787 812 123 234 345 456 567 678
234 345 456 567 678 787 812 123
Rats in the Serial Position Cues only (SP) group started on different randomly chosen levers but the violation was always at Serial Position 24 (SP24) in the sequence:
123 234 345 456 567 678 781 818
234 345 456 567 678 781 812 121
345 456 567 678 781 812 123 232
456 567 678 781 812 123 234 343
Rats in the Location and Serial Position Cues (L+SP) group started on the same lever and the violation was always at SP24:
123 234 345 456 567 678 781 818
123 234 345 456 567 678 781 818
123 234 345 456 567 678 781 818
123 234 345 456 567 678 781 818
Finally, rats in the No Cues (N) control group started each sequence on a different randomly chosen lever, and the violation could be in any location in the chamber:
123 234 345 456 567 676 781 812
234 343 456 567 678 781 812 123
345 456 565 678 781 812 123 234
456 567 678 781 812 123 232 345
The rats were required to learn the patterns to a criterion of two or less violation errors per day for two consecutive days. Once the animals met this criterion, they experienced a series of cue-removal transfers. In the first transfer all rats were transferred from their original group to the SP condition for a single session. After each transfer, the rats were returned to their original group until they met the original criterion once again. In the second transfer, rats were tested with the octagonal chamber rotated 180 degrees, but the rats remained in their original boxes and training groups. In the third transfer, rats remained in their original groups, but were tested in a different box in a different testing room. Finally, Group L+SP only was transferred one more time into the L condition (that is, SP cues were removed).
2.2 Results
Acquisition Phase
Error rates for three categories of pattern elements were examined for the acquisition phase of the experiment: chunk-boundary errors, within-chunk errors, and violation errors. Chunk-boundary errors are errors made on the first element of each chunk, except the first element of the first chunk since that is a given. Within-chunk errors are errors made on the second and third items of each chunk. Finally, violation errors are errors made on the violation element of the sequence. Total number of errors for each group was also investigated. A repeated measures analysis of variance (ANOVA) was conducted on the total number of errors for each group. In all reported analyses, main effects and interactions were considered significant if p < .05. The analysis indicated a significant main effect for days. The group main effect and the Days × Group interaction were not found to be significant.
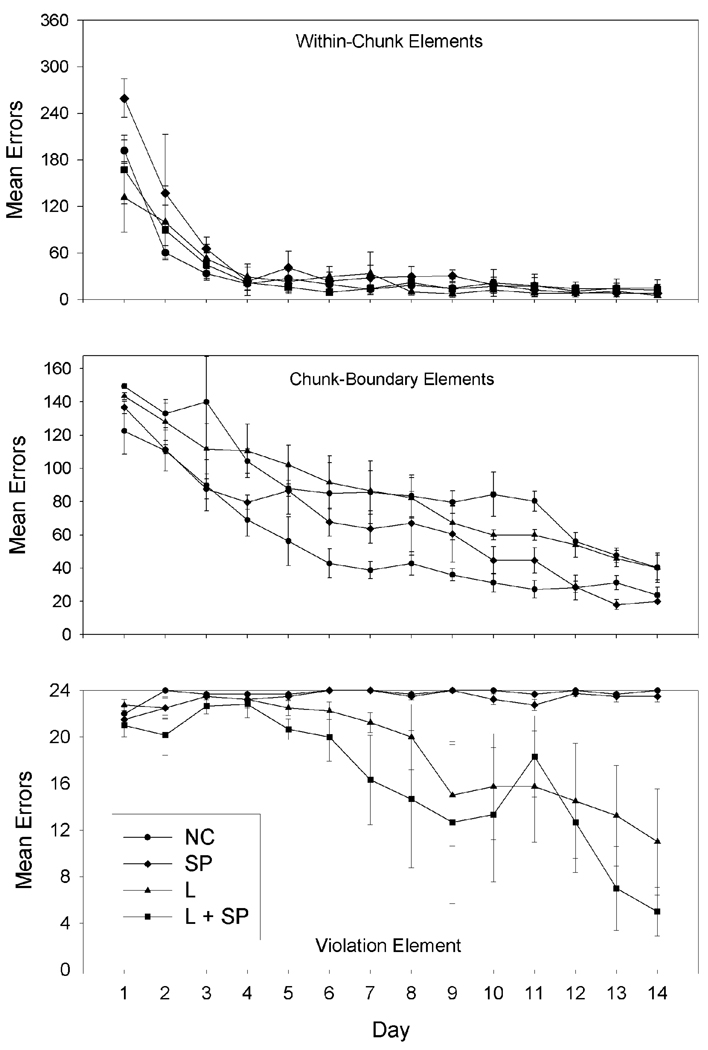
Acquisition curves for each element of the pattern are shown in Figure 1. The top, middle, and bottom panels of Figure 1 show the acquisition curves for within-chunk errors, chunk-boundary errors, and violation errors, respectively. Repeated measures ANOVAs were conducted on acquisition of the within-chunk, chunk-boundary, and violation elements of the sequence by each group over the first 14 days of the experiment. Starting on Day 15, at least one of the animals began the transfer series. This limited the number of days (to the first 14) that could be analyzed for the acquisition phase of Experiment 1. A repeated measures ANOVA conducted on within-chunk errors showed a significant main effect for days, F(13, 130) = 29.005, but not for group, and no significant interactions were found. A repeated measures ANOVA conducted on chunk-boundary errors indicated main effects for days, F(13, 130) = 50.34, and group, F(3, 10) = 9.114. Planned comparisons based on the appropriate error term from the ANOVA revealed that none of the differences between group means was significant. The repeated measures ANOVA conducted on violation errors revealed significant main effects for days, F(13, 130) = 7.176, and group, F(3, 10) = 26.119, and a significant interaction effect for Days × Group, F(39, 130) = 2.958. Planned comparisons showed significant differences between NC and L on Days 16–25 and 26–40; NC and L+SP on Days 18, 20,23,25, 28–29,31,33–34; SP and L on Days 14–37. SP and L+SP were never different.
Figure 1.
Acquisition curves for within-chunk elements (top panel), chunk-boundary elements (middle panel), and the violation element (bottom panel), respectively, for the first 14 days of Experiment 1 shown as day-by-day group mean errors for the Location (L), Serial Position (SP), Location and Serial Position (L+SP), and No Cues (NC) groups. Error bars: ± SEM.
Visual inspection of the acquisition curves in Figure 1 suggests that errors on within-chunk elements decreased more rapidly than errors at the chunk-boundary and violation elements. Errors on violation elements decreased at the slowest rate. Generally for all groups, within-chunk errors were learned most quickly with chunk-boundary errors being learned almost as quickly. The L and L+SP cues groups were able to anticipate the violation element, whereas the N and SP cues groups’ error rates never decreased.
The animals in each of the four groups continued training beyond Day 14 for different lengths depending on how long it took them to reach criterion. Animals in N and SP never reached criterion within 70 days of training, so they never underwent any transfers. Animals in the L and L+SP conditions reached criterion on Days 14–22.
Cue removal phase
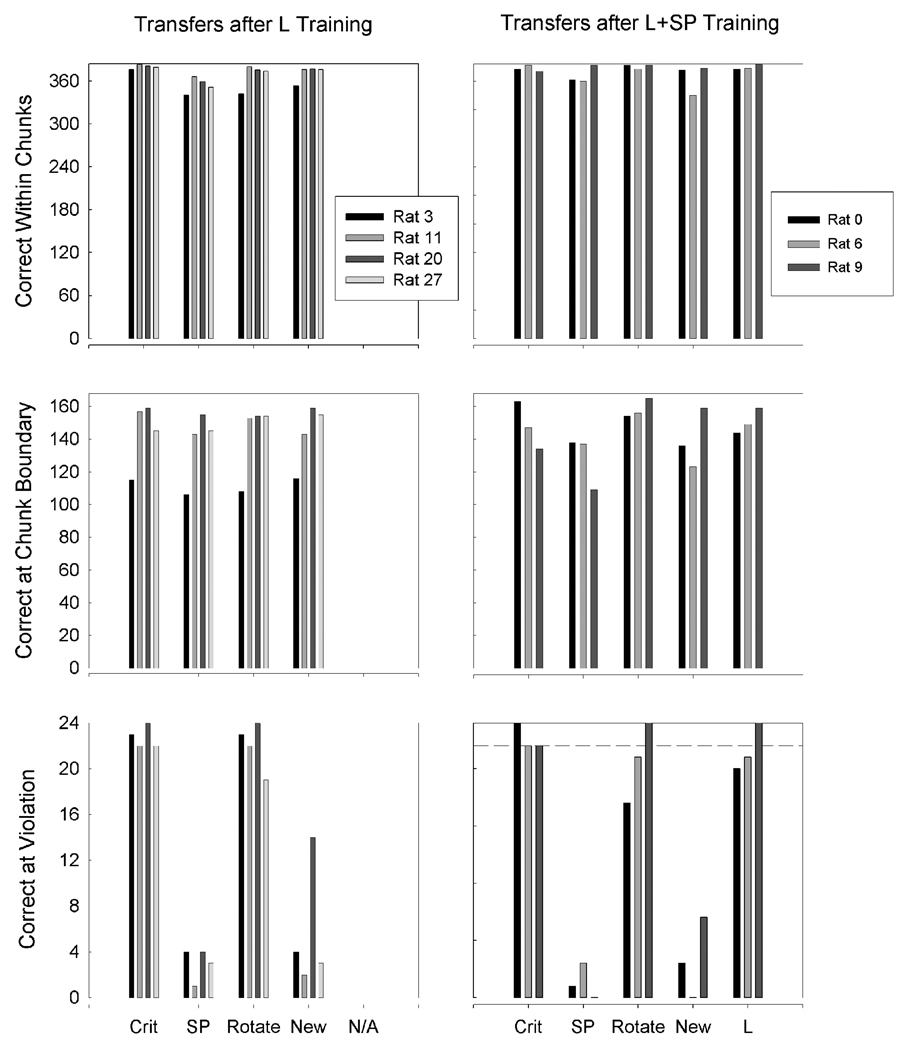
Figure 2 shows L and L+SP’s performance for the successive transfers for the different element categories of the sequence. Note that the transfer phase results are presented in terms of correct responses. The top panel of Figure 2 shows groups L and L+SP’s correct response rates by individual subjects across transfers on the within-chunk elements of the sequence for each transfer. A repeated measures ANOVA conducted on these data showed a significant main effect for cue removal, F(3,15) = 5.062. The Group × Transfer interaction was not significant (p > .05). Multiple t-test analyses showed no differences between groups L and L+SP in performance on the within-chunk elements during the transfers.
Figure 2.
Location (L) and Location and Serial Position (L+SP) groups’ (left and right columns of figures, respectively) correct response rates in the successive transfers for within-chunk elements (top panels), chunk-boundary elements (middle panels), and the violation element (bottom panels) in Experiment 1. Data are shown for the day rats met criterion (Crit) and daily transfer sessions with serial position cues only (SP), the chamber rotated 180° (Rotate), transfer to a new octagonal operant chamber in a different room (New), and—for the L+SP group only—training involving SP cue removal with location cues only (L). Transfers that removed location cues caused severe disruptions in anticipation of the violation element. The dashed line in the bottom panels indicates the criterion level of performance on violation elements required before transfers were initiated.
The middle panel of Figure 2 shows groups L and L+SP’s correct response rates by individual subjects across transfers on the chunk-boundary elements of the sequence. A repeated measures ANOVA conducted on these data showed no significant effects. There were no significant differences between groups L and L+SP in performance on the chunk-boundary elements during the transfers.
The bottom panel of Figure 2 shows groups L and L+SP’s correct response rates by individual subjects across transfers on the violation element of the sequence. A repeated measures ANOVA conducted on these data showed a significant main effect for cue removal, F(3,15) = 117.908, but no significant interaction effect. Multiple t tests using a Bonferroni adjustment revealed significant differences in performance from criterion on violation elements during the SP Cues Only transfer and the New Box transfer compared to criterion day. There was no significant difference in performance between groups L and L+SP during any of the transfers. Data for Rat 20 appear to show improved performance on the violation element when it was moved to a new box. It should be noted that during pilot testing for this study it was discovered that Rat 20’s original training box and the new box to which it was transferred were found to be very similar in terms of intra-maze cues.
2.3 Discussion
Four groups of rats were required to learn a 24-item sequence containing a violation element. Different groups were provided different cues that were relevant to predicting when to make the correct response on the violation element of the pattern. All four groups learned to respond correctly to within-chunks elements faster than to chunk-boundary and violation elements. Chunk boundaries were also learned fairly quickly, as Figure 2 shows. L and L+SP learned to anticipate the violation element at about the same rate. The N and SP groups, having no location cues, never learned to predict where to make the correct response for the violation element within 40 days of training.
The L and L+SP groups experienced a series of transfers once criterion was met. These transfers consisted of transferring the animals to a serial position cues only condition (i.e., location cues removed), rotating the octagonal chamber 180 degrees, and transferring the rats to a new chamber. Additionally, the L+SP group was transferred to a location cues only condition (i.e., serial position cues removed). The transfer data indicate that within-chunk elements and chunk-boundary elements were unaffected by these manipulations. Earlier work showed that responding to chunk-boundary elements seems to be mediated by phrasing cues (Fountain, Benson, & Wallace, 2000a; Stempowski et al., 1999). That work showed that removal of phrasing cues produced deficits in performance on the next trial (the first element of each chunk in that case). Consistent with the idea that phrasing cues control chunk-boundary responses, performance on chunk-boundary elements was unaffected by the foregoing manipulations because phrasing cues were present throughout the study.
Violation errors increased when rats were transferred to the serial position cues only condition and when they were tested in a new chamber, but not when their original chamber was rotated. This suggests that responding to the violation element was governed by intra-maze cues. That is, rats were using information from within the chamber to predict where to make the violation response. If they had been using extra-maze cues, rotating the box should have increased errors in positions in the chamber corresponding to the 180° shift, but this was not observed. In addition, when location cues were removed from the L+SP group, performance on the violation element was profoundly impaired, implying that the serial position cues played little role in controlling responses on the violation element.
The results of this study suggest three things. First, learning to anticipate violation elements depended on discriminative cues for location within the chamber and, perhaps, different cognitive processes from those mediating performance on the rest of the pattern (viz., on chunk-boundary and within-chunk elements). This claim is supported by the transfer data. The violation element was the only element impaired by the SP cues only transfer and the new box transfer. This evidence favors the view that rats used different cues and encoding processes for learning about violation elements.
Second, from the within-chunk data it can be concluded that there is possibly more than one process governing serial pattern learning. None of the manipulations made in this experiment had any effect on the within-chunk element performance. This suggests that while performance on other parts of the sequence are mediated by associative mechanisms involving external cues, performance on within-chunk elements is controlled by some sort of internal representation, perhaps by a representation of pattern structure. Another interpretation of the within-chunk data is that internal cues serve as discriminative stimuli. For example the animals could be using proprioceptive or motor cues to guide responding on within-chunk elements, rather than representations of abstract rules.
Third, animals did not show evidence of using serial position as a cue for anticipating the violation element. This is an interesting finding considering other literature that suggests rats do use serial position as a cue (Burns, Dunkman, Jr., & Detloff, 1999; Burns, Johnson, Harris, Kinney, & Wright, 2004; Burns, Kinney, & Criddle, 2000). Burns et al. (2000) trained two groups of rats on 3-trial series in which the first two trials consisted of rewards while the third trial was not rewarded. On the test day all groups were transferred to a 3-trial nonreward series. During training the animals should have learned to predict when they would receive a reward and when they would not. Running speeds in the runway were used to indicate whether the rats learned to predict the reward outcomes. If position cues were important in predicting reward outcomes, then on the test day the animals should have maintained their running pattern (i.e., fast-fast-slow). This is indeed what occurred. The researchers concluded that while reward memories played a role in approach times, results could not be understood without the position cues theory. This report (Burns et al., 2000) and others similar to it (Burns et al., 1999; Chen et al., 1997) prompted Experiment 2, which was designed to explore the conditions under which rats might be able to use serial position cues in learning longer sequences than those used in the foregoing studies.
3 Experiment 2
The data from Experiment 1 showed no evidence that rats used serial position as a predictor for when to make the correct response on the violation element. This is an interesting finding considering that there is evidence in the literature that supports the view that nonhuman animals can use serial position cues to control responding in sequential tasks (i.e., Chen et al., 1997; Burns et al., 1999, 2000). An explanation for why the rats showed no sensitivity to the serial position cue when it was a valid predictor in Experiment 1 may be that the pattern contained 24 items and the violation occurred on the last item. Scalar timing theory states that as the interval or number to be counted increases, it becomes more difficult to discriminate its relative length or number. If rats were using timing to track the violation element, SP24 may have been too far into the sequence for them to discriminate accurately the amount of time that had passed. Similarly, if the rats were counting the number of elements that were in each pattern it may have been that 24 was too high for them to count. In addition, if they were counting chunks, 8 may also have been too high for them to count.
The purpose of Experiment 2 was to determine if there is a situation in which rats will use serial position cues to track the violation element of a sequence. To do this, serial position of the violation element was manipulated while sequence length was kept constant at 24 elements.
3.1 Method
Subjects
The rats that were run in groups N and SP during Experiment 1 served as subjects in Experiment 2.
Apparatus
The apparatus was the same as in Experiment 1.
Procedure
The procedure was the same as in Experiment 1 except for the following changes. The length of the pattern remained 24 items long but the serial position of the violation was positioned at SP6 for one group, and at SP12 for another. In both cases, the only valid predictor of the violation element was serial position. Rats that had been in groups N and SP in Experiment 1 were randomly assigned to one of two groups. Both groups received twenty-four 24-item patterns seven days per week for 70 days. On two days during the experiment, one animal in group SP6 was run by mistake under slightly different procedures. On Day 3, phrasing cues were 2 s rather than 3 s for this one rat. On Day 42, the same rat was run on the correct program in the wrong box. Due to these errors, data from those days were dropped from the analysis.
3.2 Results
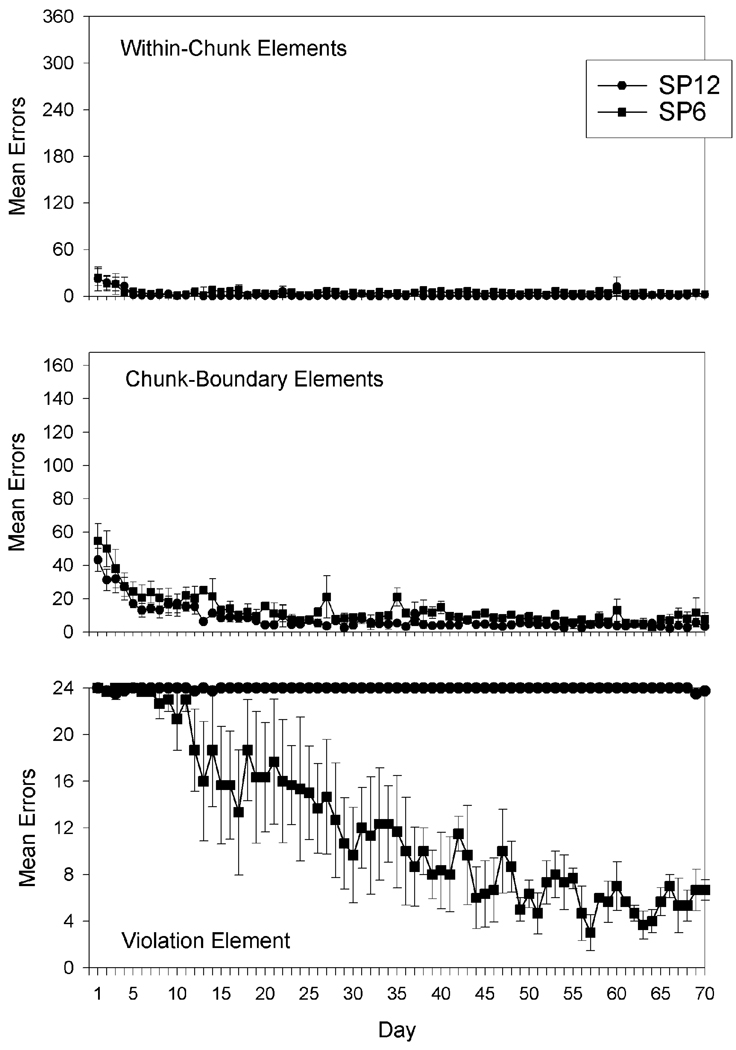
As in Experiment 1, error rates for three main element error categories were examined: chunk-boundary errors, within-chunk errors, and violation errors. Total number of errors for each group was also analyzed. An ANOVA was conducted on the total number of errors for each group. In all reported analyses, main effects and interactions were considered significant if p < .05. The analysis indicated significant main effects for days, F (2.705, 10.820) = 6.670, and group, F (1,4) = 8.845. However, the Days × Group interaction was not significant. The results indicated that rats in SP6 performed better overall than rats in SP12.
Figure 3 shows both groups’ mean error rates on the within, chunk-boundary, and violation elements through day 70. A repeated measures ANOVA was conducted on the acquisition of each of the three element categories by both groups over 70 days of the experiment. The top panel shows daily mean error rates on within-chunk elements for groups SP6 and SP12. There were no significant differences between SP6 and SP12 on acquisition of within-chunk elements, showing that both groups learned to anticipate the within-chunk elements. The middle panel of Figure 3 shows both groups’ mean error rates on chunk-boundary elements. A repeated measures ANOVA conducted on the acquisition of the chunk-boundary elements indicated a main effect for days, F (2.810, 11.239) = 7.434, but not for group, indicating that the groups learned to anticipate chunk boundaries at the same rate. The bottom panel shows mean error rates on the violation element of the sequence for both groups. Regarding the violation element, the repeated measures ANOVA revealed significant main effects for days, F (1.026, 4.104) = 31.063, and group, F (1,4) = 128.967, and a significant interaction effect for Days × Group, F (1.026, 4.104) = 31.386. Planned comparisons also showed that group SP6 produced fewer errors on the violation element than SP12 on days 37, 39 – 41, 44–46, and 48 – 70. Planned comparisons also showed that SP12 never showed improvement in anticipating the violation element.
Figure 3.
Acquisition curves for within-chunk elements (top panel), chunk-boundary elements (middle panel), and the violation element (bottom panel) through Day 70 of Experiment 2 shown as day-by-day group mean errors for the groups with a violation element positioned at Serial Position 6 (SP6) and Serial Position 12 (SP12) of their 24-element patterns. Error bars: ± SEM.
3.3 Discussion
The results from Experiment 1 suggested that rats did not use serial position to predict the violation element positioned at SP24 within the 40 days of the experiment. Experiment 2 was designed to determine whether this was true due to the nature of the task, or because rats cannot use serial position as a cue in any case. The results from Experiment 2 show that it is possible for rats to use SP cues discriminatively. One problem in Experiment 1 that might have prevented rats from using SP cues could have been the position of the violation element in the sequence. When all other cues were removed, and the violation element was in SP6, rats in Experiment 2 used serial position to predict when to make the correct response on the violation element. However, rats in group SP12 never learned to make the correct response to the violation element, suggesting that the twelfth position in a 24-item pattern may be too far into the pattern to learn to use SP as a cue. It could be that the difficulty of learning where to make the correct response increases as the serial position of the violation element increases, as suggested by Scalar Timing Theory and other similar theories, and that SP12 could be learned with further training.
4 Experiment 3
If rats are capable of using timing or counting to determine when a violation will occur, as Experiment 2 suggests, then it is reasonable to ask whether they use these processes to predict when other elements of the pattern will occur as well. Although the manipulations made in Experiment 1 did not affect performance on chunk boundaries or within-chunk elements, we wondered how changing chunk length might affect performance on elements throughout the pattern. In Experiment 3, chunk length was increased and decreased from the standard 3 elements on which rats were trained in a series of three transfer patterns. Chunk lengths varied from1 to 5 elements and the overall length of the pattern varied from 18 to 30 elements. Stempowski et al. (1999) showed that phrasing cues act as discriminative cues and Fountain, Benson, and Wallace (2000) showed that phrasing cues can come to control performance at chunk boundaries even when the cues varied in serial position within the pattern. Therefore, any evidence of encoding phrasing cues in relation to serial position would be evidence of at least two learning processes at work concurrently, namely, discrimination learning and serial position learning presumably involving timing or counting processes. Thus, if in Experiment 3 errors on chunk boundaries and within-chunk elements increase as a result of manipulating the serial position of phrasing cues, such findings would support a multiple-process theory of serial pattern learning.
Method
Subjects
The rats that were run in groups L and L+SP during Experiment 1 served as subjects in Experiment 3.
Apparatus
The apparatus used was the same as in Experiments 1 and 2.
Procedure
Rats from the L and L+SP groups were retrained on the original 24-element sequence containing a violation element: 123-234 345-456-567-678-781-818. Rats were retrained on 20 patterns per day for 14 days. During retraining, all procedures were the same as before except that intervals between elements within chunks were 2 s whereas intervals between chunks that served as phrasing cues were a 0.5 s. A 9-s inter-pattern interval was inserted between patterns. By the end of retraining, performance on the violation element was 2 or fewer errors throughout the pattern for all rats. Beginning the next day, rats experienced three probe patterns inserted into daily training each day. The probe patterns manipulated both chunk length and overall pattern length. An 18-element probe pattern was made up of chunks of lengths varying from 2 to 5 elements: 1234-34-34567-6781-818. A 24-element probe pattern was the same length as the training pattern, but contained chunks ranging in length from 1- to 5 elements: 12-123-23456-56-5678-7-6781-818. A 30-element probe pattern was made up of chunks of lengths varying from 1 to 5 elements: 123-23-2345-4-34567-67-678-78-78-781-818. One of each probe pattern was randomly chosen to be presented after the fifth, tenth, and fifteenth training pattern in a normal day’s testing for 10 days.
Results
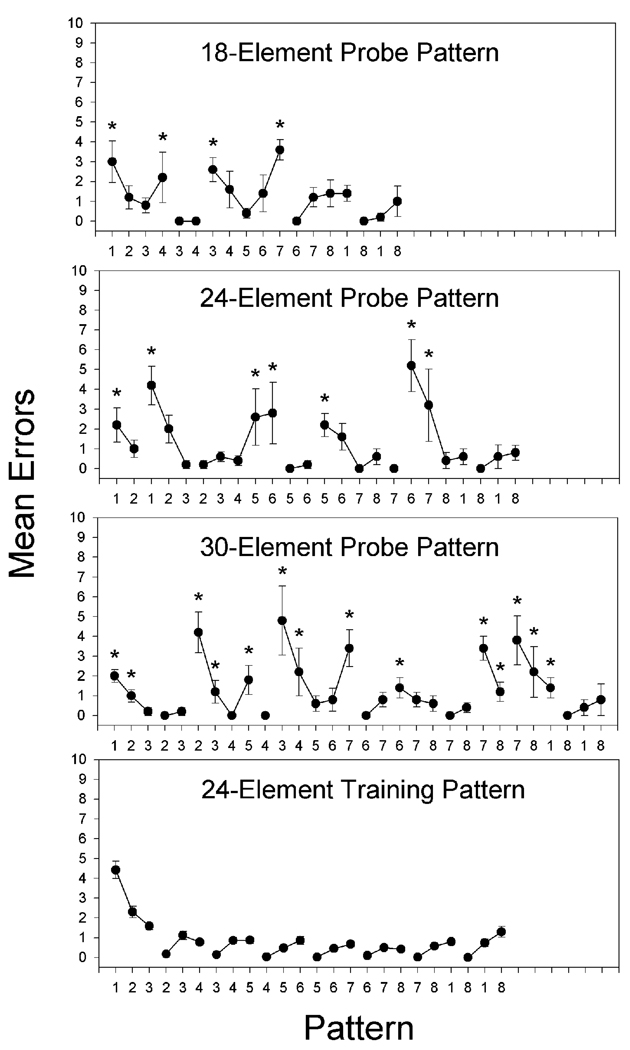
Three repeated measures ANOVAs were conducted to determine differences in error rates for pattern elements in the three probe patterns when errors were counted across the 10 days of probe testing. A repeated measures ANOVA on data from 18-element probe patterns revealed a significant main effect for elements, F(17, 68) = 3.337, p < .01. As shown in the top panel of Figure 4 depicting results of 18-element probe patterns, planned comparisons based on the appropriate error term from the ANOVA showed that errors on elements at positions 1, 4, 7, and 11 differed significantly from at least one within-chunk element in that pattern, as indicated by asterisks. A repeated measures ANOVA on data from 24-element probe patterns also revealed a significant main effect for elements, F(23, 92) = 3.949, p < .01. As shown in the middle panel of Figure 4 depicting results of 24-element probe patterns, planned comparisons showed that errors on elements at positions 1, 3, 9, 10,13, 18, and 19 differed significantly from at least one within-chunk element in that pattern. A repeated measures ANOVA on data from 30-element probe patterns also revealed a significant main effect for elements, F(29, 116) = 4.304, p < .01. As shown in the bottom panel of Figure 4 depicting results of 30-element probe patterns, planned comparisons showed that errors on elements at positions 1, 2, 6, 7, 9, 11, 12, 15, 18, 23, 24, 25, 26, and 27 differed significantly from at least one within-chunk element in that pattern.
Figure 4.
Rats' mean number of pattern tracking errors for the successive elements of the 18-, 24-, and 30-element probe patterns and the 24-element training pattern experienced in the probe pattern phase of Experiment 3. Error bars: ± SEM. Asterisks: p <.05.
Discussion
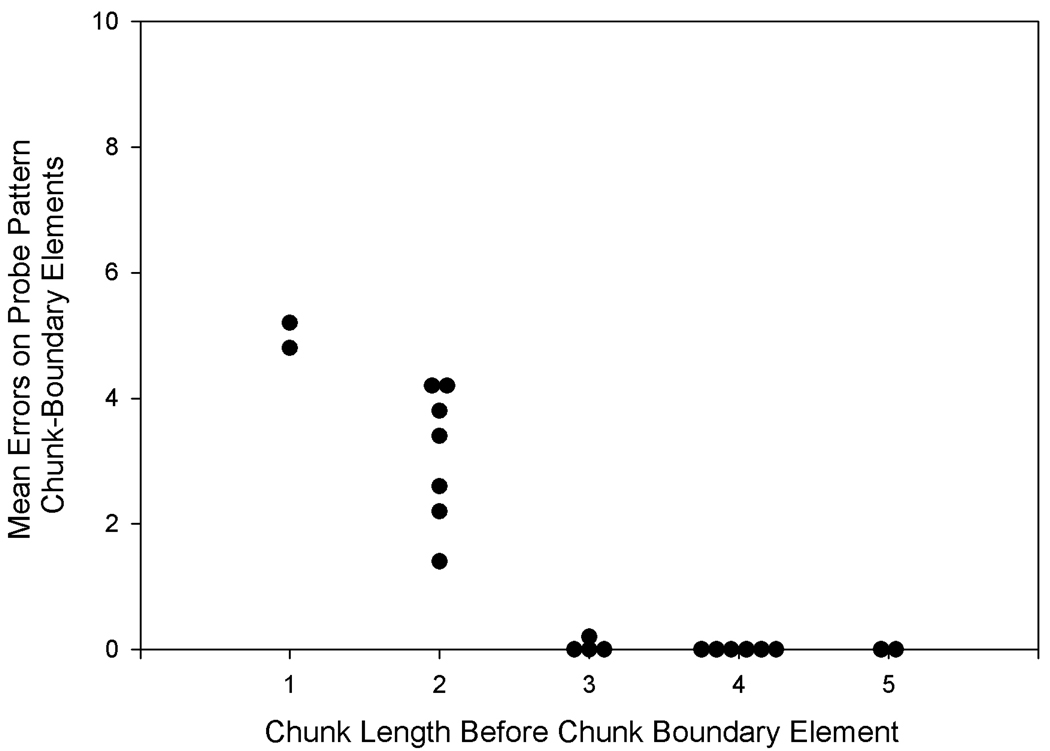
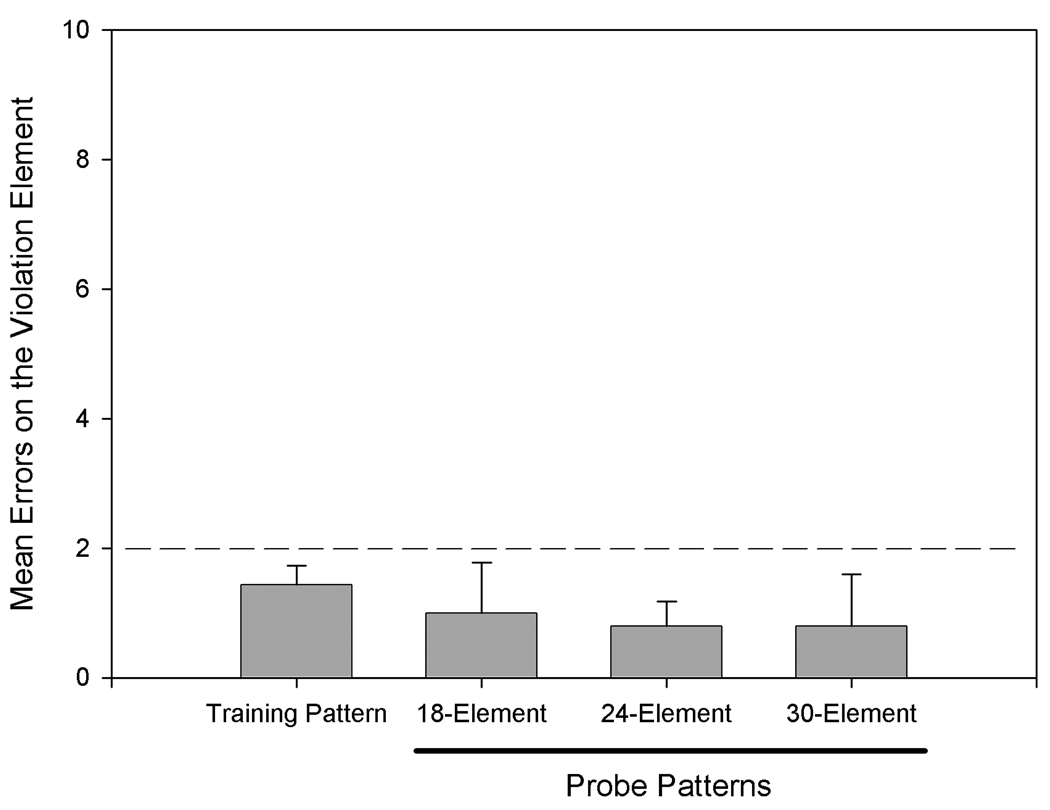
Results from this experiment showed that when probe pattern chunks were longer than 3 elements, error rates generally increased on elements beyond the third. Thus, rats showed some evidence of anticipating a chunk boundary after 3 elements even on trials that did not follow phrasing cues. Figure 5 demonstrates that when phrasing cues followed chunks shorter than 3 elements, errors were high on the following chunk-boundary element, and when phrasing cues followed chunks that were 3 elements or longer, errors were low on the following chunk-boundary element. Thus, phrasing cues and serial position cues formed compound or configural cues. Figure 6 shows that none of the probe patterns resulted in poor performance on the violation element despite numerous element and chunk changes in the probe pattern leading up to the violation chunk and serial position changes for the violation element of 6 positions in two of the probe patterns.
Figure 5.
Rats’ mean probe pattern errors on elements following phrasing cues (i.e., chunk-boundary errors) as a function of the length of the chunk before the phrasing cue in Experiment 3 (data re-plotted from Figure 4). When chunks before phrasing cues were shorter than 3 elements in length, that is, shorter than the chunk length experienced in the training phase, error rates were inflated on the chunk-boundary element after the phrasing cue.
Figure 6.
Rats’ mean errors on the violation element for the 24-element training pattern and for the 18-, 24-, and 30-element probe patterns in Experiment 3 (data re-plotted from Figure 4). Despite dramatic changes in the length and organization of probe patterns, performance on violation elements was unaffected because the location of the violation element within the chamber in probe patterns was consistent with earlier training. The dashed line indicates the criterion level of performance required on the training pattern before probe pattern testing was initiated. Error bars: ± SEM.
Based on the results of Experiment 3, some general principles guiding rats’ performance in the training violation pattern have been identified. First, for within-chunk responding the data suggest that rats turn right until a phrasing cue is encountered unless chunk length exceeds 3 elements. Secondly, rats consistently turn left after a phrasing cue if the phrasing cue occurs after 3 or more chunk elements. The results indicate that rats are sensitive to the serial position of phrasing cues and chunk boundaries, implicating internal timing or counting processes and a sensitivity to serial position at the level of chunks. Thirdly, responding on the violation element appears to be controlled by location cues alone. Together with Experiments 1 and 2, these results suggest that rats’ performance in hierarchically organized patterns with violations can be accounted for by location cues for anticipating the violation element, phrasing cues occasioned by serial position cues for performing responses at chunk boundaries, and proprioceptive cues or internal rules guiding within-chunk element responses.
5 General Discussion
Recent evidence indicates that both human and nonhuman animals process sequential information via multiple concurrent cognitive processes. For example, Fountain and Benson (2006) described evidence that rats learning a sequence of patterned responses in a circular array employed both discrimination-learning and rule-learning processes concurrently to learn to anticipate elements of elaborate “interleaved” serial patterns.
There were three main objectives of the three experiments in this study. First, we wanted to determine the extent to which identifiable cues control responding for different elements of a structured sequence containing a violation element. To do this we used a 24-item sequence made up of eight chunks (separated by longer or shorter pauses) and containing a violation element somewhere in the pattern. Performance for three types of elements (chunk boundaries, within-chunk elements, and violation elements) was examined closely in acquisition in Experiments 1 and 2 and after systematically removing or modifying location and serial position cues in Experiments 1 and 3. We found that within-chunk element responding was controlled by internal cues or cognitive structure, perhaps consisting of a system of rules. Earlier work by Stempowski et al. (1999) showed that responding to elements at chunk boundaries was controlled by phrasing cues, but Experiment 3 showed that phrasing cues are coded along with serial position information about chunk length as compound or configural cues. The fact that rats show a tendency to produce chunk-boundary responses after the third element of chunks even without phrasing cues suggests that phrasing and serial position cues may be coded as compounds rather than bound together as configural cues. Finally, Experiments 1 and 3 in our study showed that violation element responding was mediated by location cues independent of serial position cues.
A second objective was to determine if rats are capable of using serial position as a cue when it was the only predictor for a violation element. Work by Burns et al. (1999; 2000) has provided support that rats are capable of using serial position when it is a valid cue. However, results of the experiments reported here indicate that rats’ ability to use serial position cues discriminatively may be quite constrained. Experiment 2 showed that rats can with difficulty use serial position cues to anticipate violation elements if the violation is no more than 6–12 positions into the pattern. This may suggest that rats have a limited capacity to use serial position as a cue.
A third objective was to determine whether rats use more than one process concurrently in serial pattern learning. The results demonstrated that anticipating the violation element depended on cues different from those required for anticipating within-chunk and chunk-boundary elements. Violation element performance depended on external “intramaze” location cues. Chunk-boundary performance depended on discriminable temporal intervals that served as phrasing cues together with element position within the chunk. Within-chunk performance depended on unidentified internal processes not affected by changes in external cues or serial position. Taken together, these results fit well with the idea that more than one process is involved in serial pattern learning. Responding to the different elements was controlled by different mechanisms that are not readily explained by a single mechanism such as discrimination learning or rule learning.
5.1 Rule Abstraction in Serial Pattern Learning
The rule-learning hypothesis states that animals have the ability to abstract a rule or set of rules to guide behavior in a given task. Studies have shown that humans and other animals learn highly organized sequences faster than patterns that are poorly organized (Restle & Brown, 1970a, 1970b; Restle, 1972; Restle & Burnside, 1972; Restle, 1973; Hulse & Dorsky, 1977; Fountain & Rowan, 1995a, 1995b) and that pattern structural complexity predicts pattern learning difficulty (Fountain & Rowan, 1995a; Fountain & Benson, 2006). Researchers of these studies interpret these results to mean that behavior is governed by an internal abstract representation or a pattern structure.
Our data suggest that responding to within-chunk elements was controlled by some internal representation. The evidence for this is that none of the transfer conditions changed responding on within-chunk elements. If within-chunk element responding was controlled by discriminative cues such as intra-maze cues, transferring rats to a new box should have increased errors on within-chunk elements, as well as on the violation element.
The rule learning hypothesis alone (Restle & Brown,1970a, 1970b; Fountain & Rowan, 1995a, 1995b; Fountain et al., 2010) cannot account for our chunk-boundary data as it assumes that what is learned is a representation of the symbolic relationships between stimuli, regardless of temporal cues. Stempowski et al. (1999) presented evidence that contradicted the idea that serial pattern learning in this paradigm depends on rule learning alone. She showed that performance on chunk-boundary elements was impaired when phrasing cues were removed. This showed that when phrasing cues were available rats used them to guide performance on those elements, with little evidence that rats used the structure of the pattern to guide responding at chunk boundaries. According to rule learning theory, phrasing cues should simply facilitate detecting and encoding structure, but clearly our results in this and other studies indicate that discrimination learning for phrasing cues and sensitivity to serial position cues, implicating some sort of timing or counting mechanism, also play important roles in chunk-boundary learning and performance.
Violation element responding was not mediated by systems of rules in Experiment 1. When the rats were transferred to a new box, violation errors increased. According to rule-learning theory, rats should have coded violations in relation to patterns structure. If they had done so, they should have been able to generalize what they learned in their training box to another, highly similar box or situation. It should not have mattered which box the rat was in because pattern structure would be the same for any box. Experiment 1 provides stronger evidence for discriminative cues controlling responding at the violation element since only animals that had training phase location cues available to them were able to learn where to make the violation response. These results suggest that despite the fact that there is ample evidence for rule learning in this paradigm, rats did not use a rule learning strategy exclusively for encoding and performing responses at chunk boundaries and the violation element.
5.2 Discrimination and Generalization in Serial Pattern Learning
In Experiment 1, responding to the violation element was shown to be governed by item memories (e.g., Capaldi & Molina,1979) that consisted of locations in or around the chamber. In this case, something about the chamber (e.g., levers, wires, grid flooring, etc.) served as a cue for when to make the correct response on the violation element. When location cues were removed in Experiment 1, by transferring rats to a different box for testing, anticipation of the violation element was lost. Similarly, earlier research showed that responding to chunk boundaries is mediated by phrasing cues when they are available (Stempowski et al., 1999). In that study, when phrasing cues were removed, performance on chunk boundaries was impaired. In other words, removing the between chunk phrasing cue resulted in errors on chunk-boundary elements. Although discrimination does a nice job of accounting for the foregoing data, they have more difficulty explaining responding on the within-chunk elements. If within-chunk element responding was controlled by discriminative cues such as inter-maze cues, transferring rats to a new box should have increased errors on within-chunk elements, as well as on the violation element. Rotating the box should also have had a detrimental effect on within-chunk element performance if rats were using discriminative cues as predictors. An alternative hypothesis could be that rats used motor cues as item cues to guide behavior on within-chunk elements. For example, the items could have been left and right turns, so that a left turn predicts a right turn, and a right turn predicts another right turn. This hypothesis suffers from other problems, particularly the “branching” where a single cue predicts different responses on multiple different occasions, which we have discussed in detail before (e.g., Fountain & Rowan, 1995a).
5.3 Sensitivity to Serial Position in Serial Pattern Learning
The results of Experiment 1 suggest that rats do not use serial position even when it is the only valid cue available. However, other research shows that animals including rats (Burns et al., 1999;2000) and monkeys (Chen et al., 1997) are capable of using serial position as a cue. It may be the case that in Experiment 1 the serial position cue for the violation element occurred too far into the sequence for animals to track. Experiment 2 was conducted to see if there was a condition under which rats could use serial position to track the violation element in a pattern. We found that rats could use serial position as a cue when the violation element occurred at SP6, but not when it occurred at SP12.
The mechanism underlying sensitivity to serial position is still unknown. One hypothesis is that animals use a timing mechanism to track important events. Research has shown that rats are very good at timing intervals in different tasks (Meck & Church, 1983). Rats might reasonably be expected to adopt a timing strategy when faced with a serial position problem. In a 24-item sequence, SP6 is near the beginning of the pattern, whereas SP12 is further along. Weber’s law states that the further up the stimulus dimension a stimulus is, the harder it is to discriminate from stimuli in that same region. The notion that SP12 may be too far into the sequence for rats to track using a timing mechanism is consistent with Weber’s law and might serve to explain the results. Another consideration regarding the nature of our task was that other serial learning research using animals employed much smaller arrays for the animals to track (Burns et al., 1999; Burns et al., 2000; Capaldi et al., 1979, 1984). The overall length of our pattern (24 items) may have had an effect on violation element performance. If the length of the pattern were only 12 items long results may have been very different. Another explanation for how animals use serial position as a cue is that they use some sort of counting mechanism (Capaldi & Miller, 1988). Our data would suggest that animals are unable to count up to12, though our understanding of rats’ timing and counting capabilities in this situation is limited. A future study investigating the possibility that rats use a counting strategy in this task is needed. However, the fact that rats in SP6 were able to predict the violation element and rats in SP12 were not poses problems for timing or counting accounts as general explanatiosn for serial pattern learning in this paradigm.
Rats demonstrated sensitivity to serial position in tracking a violation in SP6 of their pattern in Experiment 2 and in responding to phrasing cues in the probe patterns of Experiment 3, yet they were apparently unable to learn the serial position of violation elements in SP24 and SP12 in Experiments 1 and 2, respectively. Clearly rats were employing cognitive processes that allowed them to use serial position information in learning their patterns, but this explanation is not sufficient to account for all aspects of learning and performance in this task.
5.4 Concurrent Cognitive Processes in Serial Pattern Learning
The present study demonstrated which stimuli control responding for the different elements of our pattern, namely within-chunk elements, chunk-boundary elements, and the violation element. External cues appeared to be controlling responding at the violation element of the sequence when they were available. When such cues were not present, rats seemed to be capable of anticipating the violation element using serial position as a cue. This ability was limited, in that rats anticipated the violation element when it was in SP6, but not when it was in SP12. How rats used SP as a cue is still unknown, but some hypotheses can be offered. For example rats could have used a timing or a counting mechanism to anticipate the violation element in Experiment 2. Either one of these mechanisms presumably requires the animal to use some sort of cognitive timing or counting ability. Although our study did not directly examine how chunk-boundary elements were anticipated, earlier work in our lab has established that performance on chunk-boundary elements comes under the control of phrasing cues acting as discriminative cues when phrasing cues are available (Fountain et al., 2000a; Stempowski et al., 1999), though phrasing cues may also affect encoding of the pattern by biasing rats’ perception of pattern structure (Fountain, Rowan, & Carman, 2007). Performance on within-chunk elements has not been disrupted by any cue manipulation we have attempted and is resistant to drug challenges that impair chunk-boundary and violation element learning and performance (Fountain et al., 2000b).
Psychobiological evidence has also been consistent with the view that learning about structured elements involves processes dissociable from those mediating learning about violation elements. Consistent with this view is research from behavioral neuroscience and related fields providing neurobehavioral evidence for more than one process and more than one brain area mediating sequential learning. Nissen and Bullemer's (1987) paper on sequence learning has been particularly influential. Nissen's work on brain correlates of her human serial reaction time task (Knopman & Nissen, 1987; Nissen, Knopman, & Schacter, 1987) has supported the idea that serial learning is subserved by both declarative and nondeclarative memory systems. Nissen and co-workers showed that human Alzheimer's patients and scopolamine-treated experimental participants could improve their reaction times for a repeating 10-element pseudo-random response sequence (Knopman et al., 1987; Nissen et al., 1987; Nissen et al., 1987). Both groups showed no recognition that they were learning a repeating sequence, thus suggesting that learning could occur implicitly (or "procedurally"). Huntington's disease patients, however, exhibited no improvement; that is, they showed a deficit in serial learning described as a procedural memory deficit (Knopman & Nissen, 1991). Given that basal ganglia are severely affected by Huntington's disease, the results contribute to the growing evidence implicating the basal ganglia in sequence learning. For example, Huntington's disease and Parkinson's disease patients who suffer basal ganglia dysfunction have motor learning deficits that are characterized as deficits in sequencing and sequence learning independent of general motor performance deficits (Heindel, Butters, & Salmon, 1988; Willingham, 1998).
Fountain and Rowan (Fountain et al., 2000b) examined the effects of the drug, MK-801, on serial learning. MK-801 is an NMDA receptor antagonist that blocks long-term potentiation in the hippocampus and blocks NMDA-mediated plasticity in the hippocampus and other brain areas. Rats learned either a structurally “perfect” pattern, or a pattern that contained a violation at the end. Rats in one set of groups learned the Perfect pattern 123-234-345-456-567-678-781-812, while rats in another set of groups learned the Violation pattern 123-234-345-456-567-678-781-818. Rats from one group for each pattern were administered MK-801 each day before training began. Acquisition of chunk-boundary, within-chunk and violation elements was measured to see if MK-801 would have an effect on learning. The results indicated that MK-801 had no effect on within-chunk elements, but greatly impaired performance on chunk boundaries and the violation element. These findings indicate that the process or processes that control responding to rule-based elements are located in a different area of the brain than the processes that control responding to associative-based elements.
The general goal of the foregoing studies was to identify which behavioral processes contribute to control of rats' responses in serial patterns of behavior on an element-by-element basis in order to develop a more complete picture of how sequential behavior is acquired, represented, and produced. The approach was to determine the extent to which rats' sequential behavior is controlled by extra-sequence stimuli through associative processes, such as temporal or spatial cues acting as discriminative cues, by some other mechanisms, such as internal motor patterns or cognitive structures, or by multiple processes acting concurrently. The behavioral evidence that we have accumulated strongly supports the view that rats concurrently track multiple interoceptive, exteroceptive, and cognitive sources of information to organize their behavior through time.
Acknowledgements
Preparation of this article was supported in part by National Institute on Drug Abuse Grant DA023349 to Stephen B. Fountain. We thank Jennifer Knick and John Flavelle for assistance in collecting data and Denise P. A. Smith for assistance in conducting surgery. Correspondence concerning this article should be addressed to Melissa D. Muller, Department of Psychology, University of Mount Union, Alliance, OH 44601-3993 (e-mail: mullermd@mountunion.edu), or Stephen B. Fountain, Department of Psychology, Kent State University, Kent, Ohio 44242-0001 (e-mail: sfountai@kent.edu, FAX: +1 330-672-3786).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melissa D. Muller, Department of Psychology, University of Mount Union, Alliance, OH 44601-3993, USA
Stephen B. Fountain, Department of Psychology, Kent State University, Kent, OH 44242-0001, USA
References
- Bruner JS. Going beyond the information given. In: Bruner JS, Brunswik E, Festinger L, Heider F, Muenzinger KF, Osgood CE, Rapaport D, editors. Contemporary approaches to cognition. Cambridge, MA: Harvard University Press; 1957. pp. 41–69. [Google Scholar]
- Burns RA, Dunkman JA, Jr, Detloff SL. Ordinal position in the serial learning of rats. Animal Learning & Behavior. 1999;27:272–279. [Google Scholar]
- Burns RA, Gordon WU. Some further observations on serial enumeration and categorical flexibility. Animal Learning & Behavior. 1988;16:425–428. [Google Scholar]
- Burns RA, Hulbert LG, Cribb D. A test for order relevance in a three-element serial learning task. Journal of General Psychology. 1990;117:91–98. doi: 10.1080/00221309.1990.9917776. [DOI] [PubMed] [Google Scholar]
- Burns RA, Kinney BA, Criddle CR. Position cues and reward memories as compatible components of serial learning. Learning and Motivation. 2000;31:236–250. [Google Scholar]
- Burns RA, Johnson KS, Harris BA, Kinney BA, Wright SE. Functional cues for position learning effects in animals. Psychological Record. 2004;54:233–254. [Google Scholar]
- Capaldi EJ. Serial learning and trimethyltin: An unfortunate case of ad hoc conclusions. Physiological Psychology. 1986;14:71–72. [Google Scholar]
- Capaldi EJ. Animal number abilities: Implications for a hierarchical approach to instrumental learning. In: Boysen ST, Capaldi EJ, editors. The development of numerical competence: Animal and human models. Hillsdale, NJ: Erlbaum; 1993. pp. 191–209. [Google Scholar]
- Capaldi EJ, Miller DJ. Counting in rats: Its functional significance and the independent cognitive processes that constitute it. Journal of Experimental Psychology: Animal Behavior Processes. 1988a;14:3–17. [Google Scholar]
- Capaldi EJ, Miller DJ. Number tags applied by rats to reinforcers are general and exert powerful control over responding. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 1988b;40:279–297. [Google Scholar]
- Capaldi EJ, Miller DJ. Rats classify qualitatively different reinforcers as either similar or different by enumerating them. Bulletin of the Psychonomic Society. 1988c;26:149–151. [Google Scholar]
- Capaldi EJ, Miller DJ, Alptekin S. Numerical aspects of nonreinforcement: The same-phase nonreinforcement procedure. Animal Learning & Behavior. 1988;16:411–416. [Google Scholar]
- Capaldi EJ, Miller DJ. Number tags applied by rats to reinforcers are general and exert powerful control over responding. Quarterly Journal of Experimental Psychology: Comparative & Physiological Psychology. 1988d;40:279–297. [Google Scholar]
- Capaldi EJ, Molina P. Element discriminability as a determinant of serial-pattern learning. Animal Learning & Behavior. 1979;7:318–322. [Google Scholar]
- Capaldi EJ, Nawrocki TM, Miller DJ, Verry DR. An examination into some variables said to affect serial learning. Animal Learning & Behavior. 1985;13:129–136. [Google Scholar]
- Capaldi EJ, Nawrocki TM, Verry DR. Difficult serial anticipation learning in rats: Rule-encoding vs. memory. Animal Learning & Behavior. 1982;10:167–170. [Google Scholar]
- Capaldi EJ, Verry DR, Davidson T. Memory, serial anticipation pattern learning, and transfer in rats. Animal Learning & Behavior. 1980a;8:575–585. [Google Scholar]
- Capaldi EJ, Verry DR, Davidson T. Why rule encoding by animals in serial learning remains to be established. Animal Learning & Behavior. 1980b;8:691–692. [Google Scholar]
- Chen S, Swartz KB, Terrace HS. Knowledge of the ordinal position of list items in rhesus monkeys. Psychological Science. 1997;8:80–86. [Google Scholar]
- Fountain SB. The structure of sequential behavior. In: Wasserman EA, Zentall TR, editors. Comparative cognition: Experimental explorations of animal intelligence. Oxford: Oxford University Press; 2006. pp. 439–458. [Google Scholar]
- Fountain SB, Benson AM, Wallace DG. Number, but not rhythmicity, of temporal cues determines phrasing effects in rat serial-pattern learning. Learning and Motivation. 2000a;31:301–322. [Google Scholar]
- Fountain SB, Benson DM., Jr Chunking, rule learning, and multiple item memory in rat interleaved serial pattern learning. Learning and Motivation. 2006;37:95–112. [Google Scholar]
- Fountain SB, Krauchunas SM, Rowan JD. Serial-pattern learning in mice: Pattern structure and phrasing. Psychological Record. 1999;49:173–192. [Google Scholar]
- Fountain SB, Rowan JD. bSensitivity to violations of "run" and "trill" structures in rat serial-pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 1995a;21:78–81. [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Coding of hierarchical versus linear pattern structure in rats and humans. Journal of Experimental Psychology: Animal Behavior Processes. 1995b;21:187–202. doi: 10.1037//0097-7403.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Sensitivity to violations of “run” and “trill” structures in rat serial-pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 1995c;21:78–81. [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Differential impairments of rat serial-pattern learning and retention induced by MK-801, an NMDA receptor antagonist. Psychobiology. 2000b;28:32–44. [Google Scholar]
- Fountain SB, Rowan JD, Carman HM. Encoding structural ambiguity in rat serial pattern learning: The role of phrasing. International Journal of Comparative Psychology. 2007;20:25–34. [Google Scholar]
- Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley EP. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Experimental Brain Research. 2008;187:651–656. doi: 10.1007/s00221-008-1346-4. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Muller MD, Kundey SMA, Pickens LRG, Doyle KE. The organization of sequential behavior: Conditioning, memory, and abstraction. In: Wasserman EA, Zentall TR, editors. Handbook of Comparative Cognition. Oxford: Oxford University Press; 2010. p. xxx. [Google Scholar]
- Fountain SB, Wallace DG, Rowan JD. The organization of sequential behavior. In: Fountain SB, Bunsey M, Danks JH, McBeath MK, editors. Animal cognition and sequential behavior: Behavioral, biological, and computational perspectives. Boston, MA: Kluwer Academic; 2002. pp. 115–150. [Google Scholar]
- Heindel WC, Butters N, Salmon DP. Impaired learning of a motor skill in patients with Huntington's disease. Behavioral Neuroscience. 1988;102:141–147. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- Hull CL. Goal attraction and directing ideas conceived as habit phenomena. Psychological Review. 1931;38:487–506. [Google Scholar]
- Hulse SH. Patterned reinforcement. In: Bower G, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 7. New York: Academic Press; 1973. pp. 313–362. [Google Scholar]
- Hulse SH. Cognitive structure and serial pattern learning by animals. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. 1 ed. Hillsdale, NJ: Erlbaum; 1978. pp. 311–340. [Google Scholar]
- Hulse SH. The case of the missing rule: Memory for reward vs. formal structure in serial-pattern learning by rats. Animal Learning & Behavior. 1980;8:689–690. [Google Scholar]
- Hulse SH, Campbell CE. "Thinking ahead" in rat discrimination learning. Animal Learning & Behavior. 1975;3:305–311. [Google Scholar]
- Hulse SH, Dorsky NP. Structural complexity as a determinant of serial pattern learning. Learning and Motivation. 1977;8:488–506. [Google Scholar]
- Hulse SH, Dorsky NP. Serial pattern learning by rats: Transfer of a formally defined stimulus relationship and the significance of nonreinforcement. Animal Learning & Behavior. 1979;7:211–220. [Google Scholar]
- Knopman D, Nissen MJ. Procedural learning is impaired in Huntington's disease: Evidence from the serial reaction time task. Neuropsychologia. 1991;29:245–254. doi: 10.1016/0028-3932(91)90085-m. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Nissen MJ. Implicit learning in patients with probable Alzheimer's disease. Neurology. 1987;37:784–788. doi: 10.1212/wnl.37.5.784. [DOI] [PubMed] [Google Scholar]
- Kundey SMA, Fountain SB. Blocking in Rat Serial Pattern Learning. Journal of Experimental Psychology: Animal Behavior Processes. 2010 doi: 10.1037/a0016523. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Nissen MJ, Knopman DS, Schacter DL. Neurochemical dissociation of memory systems. Neurology. 1987;37:789–794. doi: 10.1212/wnl.37.5.789. [DOI] [PubMed] [Google Scholar]
- Restle F, Burnside BL. Tracking of serial patterns. Journal of Experimental Psychology. 1972;95:299–307. doi: 10.1037/h0033619. [DOI] [PubMed] [Google Scholar]
- Roitblat HL, Pologe B, Scopatz RA. The representation of items in serial position. Animal Learning & Behavior. 1983;11:489–498. [Google Scholar]
- Skinner BF. The extinction of chained reflexes. Proceedings of the National Academy of Sciences. 1934;20:234–237. doi: 10.1073/pnas.20.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempowski NK, Carman HM, Fountain SB. Temporal phrasing and overshadowing in rat serial-pattern learning. Learning and Motivation. 1999;30:74–100. [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychological Review. 1998;105:558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]