Abstract
The mechanism by which Suppressor of Cytokine Signaling-3 (SOCS3) negatively regulates cytokine signaling has been widely investigated using over-expression studies in cell lines and is thought to involve interactions with both the gp130 receptor and JAK1. Here, we compare the endogenous JAK/STAT signaling pathway downstream of Leukemia Inhibitory Factor (LIF) signaling in wild type (WT) Embryonic Stem (ES) cells and in ES cells lacking either the entire Socs3 gene or bearing a truncated form of SOCS3 (SOCS3DSB) lacking the C-terminal SOCS box motif (SOCS3DSB/DSB). In SOCS3DSB/DSB cells phosphorylated JAK1 accumulated at much higher levels than in WT cells or even cells lacking SOCS3 (SOCS3-/-). In contrast enhanced activation of STAT3 and SHP2 was seen in SOCS3-/- cells. Size exclusion chromatography of cell extracts showed that in unstimulated cells, JAK1 was exclusively associated with receptors but following cytokine stimulation hyperphosphorylated JAK1 (pJAK1) appeared to dissociate from the receptor complex in a manner independent of SOCS3. In WT and SOCS3DSB/DSB cells SOCS3 was associated with pJAK1. The data suggest that dissociation of activated JAK1 from the receptor results in separate targeting of JAK1 for proteasomal degradation through a mechanism dependent on the SOCS3 SOCS box thus preventing further activation of STAT3.
Keywords: SOCS3, JAK/STAT, LIF, SOCS box, embryonic stem cells
Introduction
Over expression of the Suppressor of Cytokine Signaling 3 (SOCS3) protein has been shown to negatively regulate signaling of many cytokines by attenuating the JAK/STAT signaling pathway in various experimental systems [1]. However, gene targeting experiments have revealed greater specificity with SOCS3 regulating physiological levels of G-CSF and gp130-dependent cytokines. Mice lacking the Socs3 gene die at approximately embryonic day (E) 12.5 due to a placental defect resulting from dysregulated Leukemia Inhibitory Factor (LIF) signalling [2-4]. Conditional Socs3 deletion has demonstrated important functions for SOCS3 in the hematopoietic and immunological systems, osteoclasts, T cell function, brain, spinal cord, mammary gland, retina, intestinal epithelium, and liver [5-21].
SOCS3, like SOCS1, has an N terminal region, which contains a putative Kinase Inhibitory Region (KIR), a central Src homology 2 (SH2) domain, and a highly conserved C-terminal region termed the SOCS box. The SH2 domain of SOCS3 is thought to determine target protein-binding specificity and binds with highest affinity to tyrosine phosphorylated sequences in the cytokine receptors [22]. How this results in signal attenuation is presently unclear although mechanisms involving KIR mediated inhibition of JAK activity and proteasome mediated degradation of receptor complexes have been proposed [23, 24]. The SOCS box of SOCS3 is thought to participate in the formation of an E3 ubiquitin ligase complex that is assumed to degrade the activated signaling complex [1]. The SOCS box is a C-terminal sequence of approximately 40 amino acids with two conserved regions termed the BC box and the Cul5 box [25]. Binding studies have shown that the conserved BC box forms a platform for binding the Elongin B/C complex, while the Cul5 box serves to bind the Cullin5:Rbx2 complex [25, 26]. Together the SOCS:Elongin B/C:Cullin:Rbx2 complex forms an E3 ubiquitin ligase, which acts in concert with an E1 ubiquitin activating enzyme and an E2 ubiquitin conjugating enzyme to ubiquitinate proteins, targeting them for degradation by the proteasome.
The SOCS box also appears to play a role in the regulation of SOCS protein stability. Kamura et al (1998) demonstrated that disrupting the SOCS box/Elongin B/C interaction decreased the half-life of the SOCS1 protein, and others have shown that phosphorylation of Y204 and Y221 within the SOCS3 SOCS box disrupts stabilising SOCS3:Elongin B/C interactions, resulting in a reduction in SOCS3 half-life [27]. More recently over-expression analyses have demonstrated that SOCS3, when hyper phosphorylated by the JAK2 V617F mutant, found in patients with myeloproliferative disorders, does not undergo degradation indicating that in some instances phosphorylation may be insufficient to promote protein destabilisation [23]. In over-expression studies both the SOCS box and PEST sequences of SOCS3 contributed to SOCS3 degradation [28] .
Early studies using protein over-expression systems suggested that the SOCS box was not essential for the inhibition of cytokine signaling by SOCS1 and SOCS3 [29-31]. More recently, in vitro studies demonstrated that the SOCS3 SOCS box is required for complete negative regulation of STAT3 and STAT5 activation downstream of G-CSF signaling [32].
To date, two studies have demonstrated a role for the SOCS box in vivo [33, 34]. Mice lacking full-length SOCS1 succumbed to an inflammatory disease at around three weeks of age resulting in perinatal lethality [35]. In mice expressing a truncated form of SOCS1, lacking the SOCS box, this phenotype was somewhat ameliorated but the mice still displayed significant inflammatory disease [34]. Subsequently we demonstrated that in contrast to SOCS3-/- mice, mice expressing a truncated version of SOCS3 lacking the SOCS box (SOCS3DSB/DSB) survived the perinatal period [3, 33], but showed altered responsiveness to cytokine signaling in vivo and in vitro [33].
SOCS3 is expressed in ES cells grown in standard culture with LIF and is upregulated following LIF stimulation [8, 36, 37]. To further study the consequences of SOCS box deletion we derived murine Embryonic Stem (ES) cells expressing only a truncated form of SOCS3 lacking the C-terminal SOCS box (SOCS3DSB/DSB). This provided us with a tool to test the role of the SOCS box in SOCS3 protein stability and in regulating cytokine signaling in a physiological system.
Materials and Methods
Generation of SOCS3DSB/DSB ES cells
SOCS3-/- ES cells have been previously described [37]. ES cell lines lacking the SOCS3 SOCS box were generated from blastocysts derived from SOCS3DSB/DSB mice as described [33, 38]. After thawing, all lines were maintained in ES cell medium (Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10−4 M β-mercapto ethanol, 1 mM non essential amino acids, 1 mM L glutamine, 1 mM Sodium Pyruvate) supplemented with 15% Fetal Calf Serum (FCS) and 100 ng/mL LIF (Chemicon International) on gelatinised tissue culture plates without a feeder layer.
Antibodies
The following antibodies were used: Rabbit anti SOCS3 (Immunobiological Laboratories), Mouse anti SOCS3 (Jian Guo Zhang, Walter and Eliza Hall Institute, Victoria, Australia), Rabbit anti pY1022/pY1023 JAK1 (Invitrogen Life Technologies), Rabbit anti JAK1, Rabbit anti gp130, Rabbit anti SHPTP2, Rabbit anti His (Santa Cruz Biotechnology), Rabbit anti pERK (Thr202/Tyr204), Rabbit anti ERK, Rabbit anti SHP2, Rabbit anti pSHP2 (Tyr542), Rabbit anti pSTAT3 (Tyr705), and Rabbit anti SHP2 (Cell Signaling Technology), and Mouse-α-βCatenin (BD Sciences Pharmingen). Primary antibodies for western immunoblotting were used at 1:1000 in 5% Skim milk with the exception of anti pJAK1 and anti pERK1/2 antibodies, which were used at 1:500. Horseradish Peroxidase (HRP) conjugated sheep anti rabbit immunoglobulin (Chemicon International) secondary antibody was used at 1:5000 in 5% Skim milk powder/Mouse Tonicity Phosphate Buffered Saline (MTPBS). HRP conjugated sheep anti mouse immunoglobulin (GE Health sciences) secondary antibody was used at 1:10000 in 5% Skim milk powder/MTPBS.
Immunoprecipitation and Western Immunoblotting
Cells were lysed in RIPA buffer (1% Triton X-100, 0.1% SDS, 1% Sodium deoxycholate, 150 mM NaCl, 10 mM Tris HCl, pH7.5, 0.01% (w/v) sodium azide) supplemented with 2 mM sodium vanadate, 10 mM sodium fluoride, 1 mM PMSF and complete protease inhibitor (Roche) for direct protein detection, or KALB lysis buffer (1% (v/v) Triton X-100, 50 mM Tris HCl pH7.4, 150 mM NaCl, 1 mM EDTA) supplemented with 1 mM Na3VO4, 10 mM NaF and complete protease inhibitor (Roche) for immunoprecipitation. Protein quantitation was determined by Bicinchoninic Acid (BCA) assay (Pierce). Lysates were incubated with 5 Kg of primary antibody and immunoprecipitated with 50% Protein A Sepharose (PAS) slurry. Proteins were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS PAGE) under reducing conditions and electrophorectically transferred onto PVDF membranes. Membranes were blocked for 1 h in 10% w/v skim milk and incubated with primary antibody for 2 h. Antibody binding was visualized with either peroxidase conjugated sheep anti rabbit immunoglobulin (Chemicon) or peroxidase conjugated goat anti mouse immunoglobulin (GE Health Sciences), and the enhanced chemiluminescence (ECL) system (Millipore). For re probing, membranes were stripped of antibodies with either 0.25 M Tris, 1.92 M Glycine, 0.035 M SDS supplemented with 100 mM β-mercapto ethanol, or 0.1 M glycine, pH 2.9. For some experiments, membranes were analysed by Odyssey Infrared imaging system (LI COR Biosciences). Samples were separated by SDS PAGE as above and electrophorectically transferred to low fluorescence PVDF membrane (Millipore). Membranes were blocked in Odyssey reagent (1:1 MTPBS; LI COR Biosciences) for 1 h and incubated with primary antibody for 2 h. Antibody binding was visualised with either Alexa Fluor 680-allophycocyanin conjugated goat anti mouse immunoglobulin (Molecular Probes) or IRDye 800-conjugated affinity purified anti rabbit immunoglobulin. Antibodies for Odyssey scanning were diluted in Odyssey reagent (1:1 MTPBS) supplemented with 0.1% Tween20. Membranes were rinsed repeatedly with MTPBS/0.1% Tween20, rinsed in MTPBS, and scanned on the Odyssey Infrared imaging system (LI COR Biosciences).
Metabolic labelling
For analysis of SOCS3 half-life, confluent cells were washed with MTPBS and incubated in cysteine/methionine deficient ES cell media supplemented with 0.5% FCS for 6 h. Cells were pulsed with ES cell media supplemented with 0.138 mCi/mL [35S]-Cysteine/Methionine (Perkin Elmer), 0.5% FCS (Sigma Aldrich), and 100 ng/mL LIF (Chemicon) for 1 h at 37°C. For analysis of phosphorylated JAK1 half-life WT and SOCS3DSB/DSB ES cells were washed with MTPBS and incubated in cysteine/methionine deficient ES cell media (Invitrogen Life Technologies) supplemented with 0.5% FCS for 6 h. Cells were pulsed with 0.138 mCi/mL [35S]-Cysteine/Methionine for 1 h and 100 ng/mL LIF (Chemicon) was added for the final 15 min to induce JAK1 phosphorylation. Cells were then incubated in ES cell media + 0.5% FCS + 2 mM cysteine + 2 mM methionine for various times upon which cells were lysed in KALB and the amount of [35S]-labelled protein was determined by immunoprecipitation for phosphorylated JAK1 or SOCS3. Detection and quantification were carried out using a PhosphoImager (Fujifilm FLA-3000) and associated software. Band intensity was expressed as a percentage of the amount of protein present at the conclusion of pulse (0′). Background radioactivity was subtracted from each band to control for differences in immunoprecipitation.
Analysis of protein production
ES cells were washed with MTPBS and incubated in cysteine/methionine deficient ES cell media with 0.5% FCS for 6 h at 37°C. Cells were pulsed with 0.138 mCi/mL [35S]-cysteine/methionine and 100 ng/mL LIF for various times upon which cells were lysed in KALB and immunoprecipitated for SOCS3. Proteins were separated by 15% SDS PAGE and electrophorectically transferred to PVDF membrane. Incorporation of [35S]-cysteine/methionine was analysed using a PhosphoImager. Membranes were then analysed by Western Immunoblot for SOCS3 protein. Band intensity of the resulting scans was determined with ImageGauge software and background radioactivity was subtracted from each band to control for differences in immunoprecipitation.
Size Exclusion Chromatography
Confluent ES cells were either starved of LIF and FCS for 6 h, or starved of LIF and FCS for 6 h then re stimulated with 100 ng/mL LIF for 15 min or 1 hr. After treatment, cells were lysed in KALB and protein concentration was determined by the BCA assay. Equal quantities of lysates were made up to 500 KL with KALB and chromatographed using a Waters HPLC system on a pre packed Superose 6 column (300 mm x 10 mm internal diameter, GE Healthcare) operated at 0.4 mL/min in a running buffer containing 20 mM HEPES, pH 7.2, 200 mM NaCl, 0.5% Triton X-100 (v/v), 1 mM PMSF, 0.1 mM Na3VO4, and complete protease inhibitor mixture. Protein standards including thyroglobulin, MW 670,000; bovine γ-globulin, MW 158,000; chicken ovalbumin, MW 44,000; and equine myoglobin, MW 17,000 (BioRad) were used to generate the calibration curve. Additional standards used to calibrate the column included a rabbit-α-mIL-6 polyclonal antibody, MW ~158,000; anti SOCS1 monoclonal antibody, MW ~158,000; bovine serum albumin (BSA), MW 66,000; and BSA dimer, MW 132,000. Column fractions (0.4 mL) were collected, mixed with 4 × reducing SDS sample buffer (250 mM Tris HCl, pH 6.8, 40% (v/v) glycerol, 8% SDS, 0.01% bromophenol blue, 0.03% DTT) and snap frozen in dry ice. Samples were separated by SDS PAGE, transferred to PVDF membranes, and immunoblotted with antibodies against phosphorylated JAK1, phosphorylated STAT3, JAK1, gp130, SOCS3, His, and LIFR. Full-length His-tagged SOCS3 protein was purified as described previously [22] and reconstituted at [1 Kg/KL] in H2O. D1-21 SOCS3DSB was cloned as described previously [39] 200 KL of SOCS3 protein was mixed with 400 KL KALB and centrifuged at 100 000 x g for 1 h at 4°C to remove precipitate formed upon mixing with KALB. Supernatant was recovered and chromatographed as outline above.
In vitro kinase assay
WT, SOCS3DSB/DSB, and SOCS3-/- ES cells were starved of FCS and LIF for 6 h at 37°C and re stimulated with 100 ng/mL LIF and 10 KM MG132 for 1 hr at 37°C. Cells were lysed and protein quantitated using a BCA assay. Lysates were pre cleared with PAS for 45 min at 4°C and incubated with an antibody to JAK1. Antibody:protein complexes were immunoprecipitated by incubation with PAS. JAK1 in vitro kinase assays were performed essentially as described [40] with the inclusion of 1 mM Dithiothreitol in the kinase reaction. Proteins were separated by 8% SDS PAGE and electrophorectically transferred to PVDF membrane. Incorporation of [γ-32P]-ATP was analysed using a PhosphoImager. Membranes were then analysed by Western Immunoblot for phosphorylated and total JAK1 protein.
Statistical analysis
Data was analysed with the GraphPad Prism software. Statistical significance was determined using either a two tailed Student’s t-test. Values are presented as the mean ± the standard deviation.
Results
Effect of deletion of the SOCS3 SOCS box on SOCS3 protein levels
Studies with over-expressed protein have implicated the SOCS box motif in regulation of SOCS protein stability [26, 27, 41-44]. We observed increased levels of SOCS3DSB protein compared to full-length SOCS3 in G-CSF treated bone marrow cells [33]. Full-length SOCS3 and SOCS3DSB proteins were detectable in ES cells when analysed during routine culture (NT), with greater amounts of SOCS3 DSB protein detected than full-length SOCS3 protein (Wild type; WT). Re stimulation of starved WT and SOCS3DSB/DSB ES cell lines with LIF induced SOCS3 protein in both lines, and the level of SOCS3DSB protein present in routine culture and stimulated cells was increased compared to full-length SOCS3 protein (Fig. 1A). SOCS3DSB protein was present at 3 to 4 h after stimulation, compared to full-length SOCS3 protein, which was not detectable after 2 h. Therefore, deletion of the SOCS box affected both the quantity and duration of SOCS3 protein expression in response to LIF stimulation.
Figure 1. Effect of deletion of the SOCS box on SOCS3 protein half-life and SOCS3 protein production.
(A) WT and SOCS3ΔSB/ΔSBES cells were left untreated (NT), starved for 6 hours (SO), or stimulated with LIF for 1 h (0′), then washed and cultured in starvation media for 15 min to 4 h. Cells were lysed at the indicated time points, immunoprecipitated with anti SOCS3 antibody and subjected to SDS PAGE. Membranes were immunoblotted with anti SOCS3 antibody to detect full-length SOCS3 (SOCS3) and SOCS box deleted SOCS3 (SOCS3DSB) proteins. (B) WT and SOCS3ΔSB/ΔSBES cells were starved of LIF, FCS, cysteine and methionine for 6 h, then stimulated with LIF in the presence of [35S]-cysteine/methionine for 1 h. Cells were either lysed at the conclusion of stimulation (0′) or washed and chased for 30 120 min in ES cell media supplemented with unlabelled cysteine/methionine. Whole cell lysates were immunoprecipitated with an anti SOCS3 antibody and subjected to SDS PAGE on a polyacrylamide gel. Incorporation of [35S]-was measured using a PhosphoImager. Band intensity was quantified from four separate experiments and graphed as a percentage of the starting amount of protein at the conclusion of stimulation (0′). (C) WT and SOCS3ΔSB/ΔSB ES cells were starved of LIF, FCS, cysteine, and methionine for 6 h and then stimulated with LIF in the presence of [35S]-cysteine/methionine for 15 90 min. Cells were processed as in B and the results of three separate experiments graphed as a percentage of the total amount of protein at the 90 min stimulation (90′). (NT ES cells cultured in ES cell medium with LIF (100ng/ml), SO ES cells starved for 6 h) * indicates p < 0.05.
The effect of SOCS box deletion on SOCS3 protein stability was further assessed by pulse chase analysis (Fig. 1B). To examine the rate of degradation of newly synthesised proteins, WT and SOCS3DSB/DSB ES cells were stimulated with LIF to induce expression of SOCS3 protein in the presence of [35S]-cysteine/methionine and then chased for 30 min to 2 h in unlabelled ES cell culture medium. At 30 mins after stimulation, the percent of SOCS3 protein remaining was greater in WT ES cells compared to SOCS3DSB/DSB ES cells, however, overall there was little difference in the half life of the SOCS3 and SOCS3 DSB protein (Fig. 1B).
Pulse chase experiments did not provide an explanation for the observation that, in Western blot analyses, SOCS3 DSB protein was clearly present at greater levels than full-length SOCS3 protein. To investigate protein production, WT and SOCS3DSB/DSB ES cells were stimulated with LIF and incorporation of [35S]-cysteine/methionine monitored for the indicated time period (Fig. 1C). The level of [35S]-labelled SOCS3DSB protein was significantly greater than the level of [35S]-labelled SOCS3 protein at 30 min after stimulation (Fig. 1C), indicating that SOCS box deletion causes a slight increase in the rate of SOCS3DSB protein synthesis.
It is hypothesised that SOCS proteins are degraded via the proteasome after SOCS box induced poly ubiquitination. Addition of proteasomal inhibitors has previously been demonstrated to prolong the half-life of the SOCS3 protein [26]. To assess this in an endogenous system, the half-life of the SOCS3 protein in WT and SOCS3DSB/DSB ES cell lines was analysed in the presence of the proteasomal inhibitor MG132. Addition of MG132 to WT ES cells prolonged the half-life of full-length SOCS3 protein from 45 min to approximately 1 h (Fig. 2A). Addition of MG132 to SOCS3DSB/DSB ES cells also prolonged the half-life of SOCS3 DSB protein from 30 min to approximately 1 h, indicating that in the absence of the SOCS box, the SOCS3 protein is still degraded by the proteasome (Fig. 2A). Proteasome inhibition was confirmed by Western blot analysis for β-Catenin, which accumulated in the presence of MG132 (Fig. 2B). The specificity of this protein accumulation was confirmed by Western blot for ERK1/2 proteins, which are not proteasomally degraded (Fig. 2B).
Figure 2. SOCS3 protein is degraded by the proteasome.
(A) WT and SOCS3ΔSB/ΔSB ES cells were incubated in ES cell media without cysteine, methionine or LIF for 6 h, after which SOCS3 protein was no longer detectable. Cultures were then stimulated with LIF in the presence of [35S]-cysteine/methionine for 1 h. Cells were lysed after stimulation (0′) or washed and chased for 30 to 120 min after stimulation in the absence (control) or presence of MG132. Whole cell lysates were immunoprecipitated with anti SOCS3 antibody and subjected to SDS PAGE. Labeled full-length SOCS3 and SOCS3DSB proteins were detected by PhosphoImager. Bands were quantitated and are expressed as a percentage of the starting amount of protein at the conclusion of stimulation (0′). The mean ± SD of three experiments with full-length SOCS3 protein (WT) and with MG132 treated SOCS3 (WT + MG132) is shown on one graph and of three SOCS3DSB and MG132 treated SOCS3DSB (SOCS3DSB + MG132) experiments on another. (B) Remaining lysates from (A) were subject to SDS PAGE and immunoblotted with antibodies against β-Catenin, and ERK1/2 to confirm proteasomal inhibition by MG132 and equal protein loading. * indicates p < 0.05, ** indicates p < 0.01
JAK1 phosphorylation is prolonged in ES cells lacking the SOCS3 SOCS box
Analysis of JAK/STAT signaling downstream of LIF stimulation in WT, SOCS3DSB/DSB, and SOCS3-/- ES cells demonstrated enhanced phosphorylation of JAK1 (pJAK1) in SOCS3DSB/DSB ES cells cultured in normal conditions (NT) (Fig. 3). In WT ES cells, pJAK1 protein was observed at 5 and 15 min following stimulation with LIF, whereas pJAK1 protein was present to at least 240 min following LIF stimulation in SOCS3DSB/DSB ES cells (Fig. 3). The duration of JAK1 phosphorylation was also prolonged in SOCS3-/- ES cells compared to WT, although not to the extent observed in SOCS3DSB/DSB ES cells. Phosphorylation of STAT3 (pSTAT3) was not markedly increased in SOCS3DSB/DSB ES cells compared to WT, despite the dramatic change in JAK1 phosphorylation (Fig. 3). Phosphorylation of JAK2 was not altered in either SOCS3DSB/DSB or SOCS3-/- ES cells compared to WT and JAK3 and TYK2 phosphorylation was not detectable in any of the three genotypes (data not shown). Analysis of the Ras/MAPK signaling pathway downstream of LIF stimulation revealed no increase and possibly a decrease in phosphorylation of SHP2 (pSHP2) in SOCS3DSB/DSB ES cells compared to WT (Fig. 3). These observations were confirmed in three independently derived SOCS3DSB/DSB ES cell lines. As previously reported, both STAT3 and SHP2 phosphorylation were dramatically increased in SOCS3-/- ES cells [37].
Figure 3. Enhanced JAK1 phosphorylation in SOCS3DSB/DSB ES cells in response to LIF.
WT, SOCS3DSB/DSB, and SOCS3-/- ES cells were washed, starved of LIF and FCS for 6 h and restimulated with LIF for 5 to 120 min. Lysates were separated by SDS PAGE and probed with antibodies to phosphorylated JAK1 (pJAK1), phosphorylated STAT3 (pSTAT3), and phosphorylated SHP2 (pSHP2) protein. Membranes were stripped and re probed with antibodies to total JAK1, STAT3, and SHP2 protein.
Phosphorylated JAK1 remains kinase active in ES cells lacking the SOCS3 SOCS box
Interestingly, the increased phosphorylation of JAK1 in SOCS3DSB/DSB ES cells did not lead to increased STAT3 activation. A possible explanation for this is that, whilst pJAK1 accumulates in the absence of the SOCS3 SOCS box, the KIR is still able to inhibit kinase activity thereby preventing further STAT3 phosphorylation. To determine whether the increased JAK1 phosphorylation correlated with increased kinase activity, WT, SOCS3DSB/DSB, and SOCS3-/- ES cells were stimulated with LIF in the presence of a proteasomal inhibitor for 1 hour, lysed and immunoprecipitates subjected to an in vitro kinase assay. SOCS3DSB/DSB ES cells contained more kinase active JAK1 than both WT and SOCS3-/- ES cells as measured by 32P incorporation into JAK1 (Fig. 4A). Immunoblotting confirmed the presence of increased levels of both phosphorylated and total JAK1 in SOCS3DSB/DSB ES cells, with the least amount detected in WT ES cells (Fig. 4A). This result confirms that the phosphorylated JAK1 in SOCS3DSB/DSB ES cells remains kinase active and indicates that the intact regulation of STAT3 activation is probably not a result of SOCS3 KIR mediated inhibition of JAK1.
Figure 4. The half-life of phosphorylated JAK1 is prolonged in the absence of the SOCS box.
(A) WT, SOCS3DSB/DSB and SOCS3-/- ES cells were starved of LIF and FCS for 6 h then restimulated with LIF in the presence of 10 KM MG132 for 1 h. Whole cell lysates were prepared, quantitated and immunoprecipitated with an antibody to JAK1. Immunoprecipitates were subject to an in vitro kinase assay, radiolabeled proteins were then separated by SDS PAGE, transferred to PVDF membranes and incorporation of [γ32P]-ATP analysed using a PhosphoImager. Membranes were subsequently immunoblotted with antibodies to phosphorylated and total JAK1. Result shown is representative of three separate experiments. (B) WT and SOCS3DSB/DSB ES cells were starved of LIF, FCS, cysteine and methionine for 6 h, incubated with [35S]-cysteine/methionine for 45 min, then stimulated with LIF in the presence of [35S]-cysteine/methionine for 15 min. Cells were either lysed at the conclusion of stimulation (0′) or washed and chased for 5 120 min. Whole cell lysates were immunoprecipitated with an antibody to pJAK1, subjected to SDS PAGE, and incorporation of [35S] was analysed on a PhosphoImager. Bands were quantitated from three separate experiments and graphed as a percentage of the starting amount of protein at the conclusion of stimulation (0′). (C) WT and SOCS3DSB/DSB ES cells were either left untreated (NT), starved for 6 h of LIF and FCS (SO), or re stimulated with LIF for 5 120 min in the presence or absence of MG132. Cells were lysed and separated by SDS PAGE, then probed with an antibody to pJAK1. Membranes were re blotted with an antibody to total JAK1. D WT and SOCS3DSB/DSB ES cells were treated and analysed as in (B) with the exception that the chase period was performed in the presence or absence of MG132. * indicates p<0.05, ** indicates p<0.01
The apparent half-life of phosphorylated JAK1 is prolonged in SOCS3DSB/DSB ES cells
To determine whether the increased levels of phosphorylated JAK1 observed in SOCS3DSB/DSB ES cells resulted from the inability of the SOCS3 DSB protein to effectively target phosphorylated JAK1 protein for proteasomal degradation, the apparent half-life of the phosphorylated JAK1 protein was examined. The half-life of phosphorylated JAK1 protein in WT ES cells was approximately 30 min, however this was extended to greater than 2 h in SOCS3DSB/DSB ES cells (Fig. 4B). This indicates that the phosphorylated JAK1 protein is degraded by SOCS3 in a SOCS box dependent manner and is likely to be degraded by the proteasome. To confirm that phosphorylated JAK1 was indeed being degraded by the proteasome the experiment was repeated in the presence and absence of the proteasomal inhibitor, MG132. In WT ES cells, following LIF stimulation, the phosphorylation of JAK1 was prolonged to at least 120 min upon addition of MG132 (Fig. 4C). This correlated with an increased apparent half-life of the phosphorylated JAK1 protein in WT ES cells from 30 min to approximately 2 h in the presence of MG132 (Fig. 4D). Addition of MG132 had no significant effect on the half-life or expression of pJAK1 in SOCS3DSB/DSB ES cells (Fig. 4C & D).
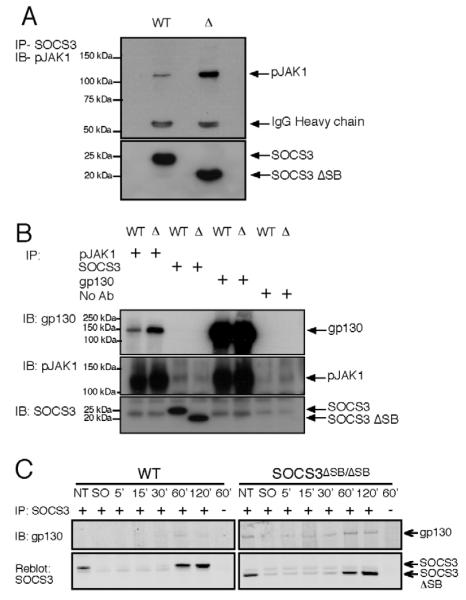
SOCS3 association with gp130 and phosphorylated JAK1 in ES cells is independent of the SOCS box
In 293T cells, over-expressed SOCS3 is thought to bind the phosphorylated gp130 receptor via its SH2 domain prior to inhibiting JAK activity through its KIR [22]. Co-immunoprecipitation studies were used to determine whether endogenous SOCS3, phosphorylated JAK1, and gp130 interact in ES cells. Both full-length SOCS3 and SOCS3DSB proteins co-immunoprecipitated with phosphorylated JAK1 protein. An increased amount of phosphorylated JAK1 co-immunoprecipitated with the SOCS3 DSB protein compared to the full-length protein (Fig. 5A). Phosphorylated JAK1 co-immunoprecipitated with gp130 in both WT and SOCS3DSB/DSB ES cells, although initially co-immunoprecipitation of gp130 was not observed with either full-length SOCS3 or SOCS3DSB protein (Fig. 5B). To increase sensitivity the gp130:SOCS3 interaction was examined using the Odyssey scanner. Full-length SOCS3 and SOCS3DSB protein were immunoprecipitated from WT and SOCS3DSB/DSB ES cells grown in normal media (NT) and following 60 and 120 min of LIF stimulation in the presence of MG132 and immunoblotted for the presence of gp130. Weak but reproducible co-immunoprecipitation of gp130 with SOCS3 was observed following 30, 60 and 120 min of LIF stimulation (Fig. 5C). The SOCS3 DSB protein co-immunoprecipitated with gp130 during routine culture (NT) and following 5 120 min of LIF stimulation, with maximal co-immunoprecipitation occurring at 60 and 120 min (Fig. 5C). Together this demonstrates that a portion of endogenous SOCS3 is complexed with gp130 after stimulation and that complex formation is enhanced in the absence of the SOCS box.
Figure 5. SOCS3, gp130 and phosphorylated JAK1 associate downstream of LIF.
(A) WT and SOCS3DSB/DSB ES cells were starved of LIF and FCS for 6 h and subsequently stimulated with LIF for 1 h and lysed. Lysates were immunoprecipitated with an antibody to SOCS3, separated by SDS PAGE and probed with an antibody to pJAK1. The membrane was stripped and re probed with an antibody to SOCS3. (B) WT and SOCS3DSB/DSB ES cells were starved of LIF and FCS for 6 h and re stimulated with LIF for 1 h and lysed. Lysates were immunoprecipitated with antibodies to phosphorylated JAK1, SOCS3, or gp130, separated by SDS PAGE and immunoblotted with an antibody to gp130. The membrane was stripped and re probed with an antibody to pJAK1 and subsequently with an antibody to SOCS3. Control lysates received no antibody for immunoprecipitation (No Ab). (C) WT and SOCS3DSB/DSB ES cells were starved of LIF and FCS (SO) for 6 h then re stimulated with 100 ng/mL LIF and 10 KM MG132 for 5 120 min, lysed, and immunoprecipitated with an antibody to SOCS3. Lysates were separated by SDS PAGE and probed with an antibody to gp130 then scanned on the LiCor scanner. Membranes were stripped and re probed with antibodies to phosphorylated JAK1 and subsequently SOCS3. ( indicates lysates received no IP antibody, IP immunoprecipitated, IB immunoblotted, NT ES cells in routine culture, SO starved ES cells)
Formation of SOCS3, phosphorylated JAK1, and gp130 complexes
Co immunoprecipitation studies demonstrated an association between SOCS3, gp130, and phosphorylated JAK1 protein (Fig. 5A C). To further investigate how SOCS3, gp130 and phosphorylated JAK1 associate, size exclusion chromatography (SEC) was used to examine the complexes formed in unstimulated (Fig. 6A) and ES cells stimulated with LIF for 1 hr (Fig. 6B). In the absence of signaling, gp130 co-localised with LIFR and JAK1 in SEC fractions #30-32 from WT, SOCS3DSB/DSB, and SOCS3-/- ES cells (Fig. 6A). In particular there was no evidence of receptor free JAK1 in fractions #36-40. LIFR and gp130 were also present in smaller complexes in fraction #34 in the apparent absence of JAK1 in all three genotypes. In SOCS3DSB/DSB ES cells the pattern was similar.
Figure 6. During the attenuation of JAK/STAT signaling, SOCS3, gp130 and phosphorylated JAK1 serially form complexes.
WT, SOCS3DSB/DSB, and SOCS3-/- ES cells were either (A) starved of LIF and FCS for 6 h or (B) starved for 6 h then restimulated with LIF for 1 h. Cells were lysed and whole cell lysates fractionated by size exclusion chromatography. The column was calibrated with BioRad gel filtration standard containing equine myoglobin (17 kDa), chicken ovalbumin (44 kDa), bovine γ-globulin (158 kDa), and thyroglobulin dimer (670 kDa). Retention time of standards was graphed against molecular weight to generate a standard curve in order to determine MW of fractions as shown. Every second fraction from #20-50 was separated by SDS PAGE and membranes were immunoblotted with antibodies to gp130, LIFR, pJAK1, pSTAT3, JAK1 and SOCS3. The boxes indicate the formation of a SOCS3:pJAK1 complex upon LIF stimulation which is absent in starved cells. (C) Recombinant SOCS3 protein was fractionated by size exclusion chromatography and immunoblotted with an antibody to SOCS3. (D) A recombinant His-tagged SOCS3 protein lacking the first 21 amino acids and the SOCS box was chromatographed and immunoblotted with an antibody to the His-tag.
Following stimulation with LIF for 1 h, four discernible complexes were observed in WT and SOCS3DSB/DSB ES cells (Fig. 6B). LIFR and gp130 formed high molecular weight complexes greater than approximately 700 kDa. LIFR and gp130 were also observed in complex with phosphorylated JAK1 and SOCS3 in fractions #32-34. In fractions #36-40, pJAK1 comigrated with SOCS3 and SOCS3 DSB proteins in the absence of the receptor, suggesting that SOCS3 forms a SOCS box-independent complex with pJAK1, which is independent of receptor association. As highlighted in Fig. 6, this complex was completely absent in the absence of stimulation. Interestingly the apparent molecular weight of pJAK1 appeared slightly different in the receptor associated versus non associated forms suggesting that additional post-translational modifications (perhaps phosphorylation at additional sites) are associated with the release of JAK1 from the receptor. To confirm that SOCS3 was present in these complexes and was not simply co-migrating as an aggregated form, purified full-length SOCS3 protein was chromatographed under identical conditions (Fig. 6C). In the absence of other proteins full-length His-tagged recombinant SOCS3 eluted only in fractions #42-50, indicating that the complexes formed with SOCS3 and SOCS3 DSB protein are true complexes and not a result of SOCS3 aggregation or micelle formation. This was not affected by SOCS box deletion as a recombinant SOCS3 protein lacking the N terminal 21 amino acids and the SOCS box eluted in fractions #46-50.
In SOCS3-/- ES cells, LIFR:gp130 and LIFR:gp130:pJAK1 complexes were observed as in WT and SOCS3DSB/DSB ES cells (Fig. 6B). Interestingly, in SOCS3-/- ES cells, pJAK1 eluted in the same fractions as observed in SOCS3DSB/DSB ES cells, indicating that hyper phosphorylation and release of pJAK1 from the receptor complex can occur in the absence of SOCS3. Furthermore, pSTAT3 was observed to elute in fractions corresponding to a molecular weight of approximately 160 kDa, which supports the hypothesis that pSTAT3 dissociates from the receptor and dimerises following LIF stimulation.
The above data suggest that pJAK1 dissociates from the receptor complex in a SOCS3 independent manner. Therefore we repeated the above experiments after only 15 min LIF stimulation a time point at which detectable SOCS3 has not been induced, (Fig. 3). As shown in Fig. 7, pJAK1 release from the receptor was clearly evident at this time point for all three genotypes of ES cells and the higher apparent molecular weight of pJAK1 released from the receptor (fractions #36-40) compared to that still associated with the receptor (fractions #32-34) was clearly evident. Since this higher apparent molecular weight form of pJAK1 was only seen in Bis/Tris electrophoresis gels and not Tris glycine gels, it is likely that it represents a hyperphosphorylated form of pJAK1.
Figure 7. pJAK1 dissociation occurs independently of SOCS3.
This experiment was performed exactly as for Fig. 6 except that the LIF stimulation time was 15 min, a time at which SOCS3 is not induced.
Discussion
Whilst several studies have examined the molecular detail of SOCS protein regulation of cytokine signaling, most have relied on over-expression systems in cell lines with all the attendant caveats associated with such studies. In an attempt to separate the roles of the SH2/KIR domains and the SOCS box we have studied protein levels and interactions of endogenous proteins in primary ES cells with full-length Socs3, SOCS box-deleted Socs3, or entire Socs3-deleted genes. Our results show that phosphorylated JAK1 is targeted for proteasomal degradation via the SOCS3 SOCS box and this regulation occurs independently of STAT3 regulation. Further, our results suggest that SOCS3 and pJAK1 form an independent complex post LIF-stimulation.
Western blot analyses revealed that the SOCS3DSB protein was present at increased levels compared to full-length SOCS3 protein. The elevated levels of SOCS3DSB protein did not appear to be a result of decreased clearance as might have been expected by the loss of the SOCS box – if anything clearance rates were slightly increased compared to full-length SOCS3 and were dependent on the proteasome. This suggests that SOCS box-independent mechanisms are primarily responsible for SOCS3 clearance and may involve the previously described PEST domain sequence in the SH2 domain [28]. The increased levels of SOCS3DSB protein instead appeared to result from an increased synthesis rate that could in turn result from increased STAT3 signaling.
Dysregulation of the Ras/MAPK signaling pathway, as a consequence of Socs3 deletion in murine ES cells, causes spontaneous differentiation of ES cells under conditions that normally support ES cell self renewal and it is likely that the increased levels of activated SHP2 (a known stimulator of this pathway) seen in SOCS3-/- cells are responsible for the increased activation of the Ras/MAPK pathway [37]. Deletion of the SOCS3 SOCS box did not affect ES cell morphology during routine culture (data not shown) and Western blot analyses revealed that Ras/MAPK signaling was not enhanced in SOCS3DSB/DSB ES cells, therefore the SOCS3 SOCS box is dispensable for Ras/MAPK regulation downstream of gp130. SOCS3 and SHP2 bind the same phosphorylated tyrosine on gp130 [22, 45, 46]. Therefore the increase in activated SHP2 seen in the absence of SOCS3 is likely the result of increased recruitment of SHP2 to the receptor in the absence of the competitive binding inhibitor, SOCS3. Since the SOCS3DSB protein retains the ability to bind to this site it is not surprising that activated SHP2 is not elevated in SOCS3DSB/DSB ES cells and its reduction in these cells despite elevated levels of activated JAK1 may well be a result of the elevated levels of SOCS3DSB protein compared to full-length SOCS3 in wild type cells.
Contrary to the current paradigm, which states that SOCS3 binds to phosphorylated gp130 and inhibits JAK1 kinase activity, thereby inhibiting further STAT3 phosphorylation, Western blot analyses indicated that activated JAK1 and STAT3 levels may be negatively regulated by different mechanisms. In the absence of SOCS3, JAK1 activation was mildly elevated while STAT3 and SHP2 activation were highly elevated. In contrast, when only the SOCS box of SOCS3 is deleted, JAK1 activation was highly elevated, STAT3 activation was mildly elevated and SHP2 activation was decreased relative to wild type cells. These results are not consistent with a model in which SOCS3 simply binds to the activated receptor and targets the entire signaling complex for proteasomal degradation, but instead, suggest specific targeting of JAK1.
The elevated levels of pJAK1 in SOCS3DSB/DSB ES cells correlated with reduced clearance of pJAK1, which was proteasome dependent in wild type cells, suggesting that SOCS3 normally targets pJAK1 for degradation through its SOCS box. As pSTAT3 was not similarly elevated in these cells, the pathways for degradation of activated STAT3 and JAK1 appear to be separable and not simply the result of degradation of the entire signaling complex. In support of this, activated JAK1 co-immunoprecipitated with both gp130 and SOCS3 but it appeared that there was a significant fraction of gp130 that was not associated with SOCS3 or pJAK1. SOCS3, like SOCS1, contains a KIR, which has been reported to be essential for the inhibition of JAK kinase activity and therefore necessary for the negative regulation of JAK/STAT signalling [24, 31]. As the SOCS3DSB protein retains both the SH2 and KIR domains of SOCS3, it should be able to bind to activated gp130 and/or LIFR with similar affinity to full-length SOCS3 and inhibit JAK1 kinase activity. Kinase assays revealed that the increase in JAK1 phosphorylation in SOCS3DSB/DSB ES cells correlated with increased kinase activity, suggesting that it could still be able to phosphorylate a substrate, yet this did not translate to increased STAT3 phosphorylation downstream. Therefore, we propose that SOCS3 may negatively regulate STAT3 activation by complexing with receptor dissociated pJAK1 and targeting it for proteasomal degradation, thus ensuring kinase active JAK1 is separated from its STAT3 substrate.
The present studies suggest that a hyperphosphorylated form of JAK1 is released from the receptor complex independently of SOCS3. This is consistent with recent data from Funakoshi Tago et al showing that the autophosphorylation status of a single tyrosine in the FERM domain of JAK2 (Tyr119) dictates whether JAK2 remains bound to the erythropoietin receptor or is released to be degraded by (then) unknown mechanisms [48]. Moreover, the same authors showed that Tyr119 phosphorylation was dependent on prior activation of JAK1 through phosphorylation of the activation loop Tyr1007 and this two step hyper phosphorylation of JAK1 required for its release from the receptor is consistent with the studies described here [47, 48]. In unstimulated cells it appears that all cytokine receptors have JAKs bound to them and all JAKs are bound to receptor [49] so the release appears to be specific to the signaling response.
It is unclear why pJAK persisted longer in SOCS3DSB/DSB cells than in cells lacking SOCS3 altogether. However, it is possible that alternate mechanisms also exist to degrade or dephosphorylate pJAK1. For example, in SOCS3-/- cells pJAK1 Tyr1007 would not be bound by the SOCS3 SH2 domain and would therefore be vulnerable to dephosphorylation whereas in SOCS3DSB/DSB cells the pJAK1 is not degraded (due to absence of the SOCS box) nor is it vulnerable to phosphatase activity.
Studies utilising mice with a mutated Tyr757 on gp130 support the hypothesis that SOCS3 must first bind to gp130 in order to inhibit JAK1 kinase activity [50]. Macromolecular complex analysis using size exclusion chromatography suggested that SOCS3 and SOCS3DSB proteins were each associated with several different complexes, some containing gp130:LIFR:pJAK1 (Fraction #32) and some containing only phosphorylated JAK1 (Fraction #40). Furthermore, LIF stimulation led to higher order complexes containing LIFR and gp130 (Fraction # 28) that did not appear to contain pJAK1 or SOCS3 and these higher order complexes were enhanced in ES cells containing the SOCS3DSB protein.
These observations are consistent with a model in which SOCS3 binds transiently to phosphorylated gp130 and/or LIFR through its SH2 domain (Fig. 8A), then binds to phosphorylated JAK1 (which has already dissociated from the receptor; Fig. 8B) and is released as a SOCS3:pJAK1 complex that is targeted for proteasomal degradation (Fig. 8C). A higher order gp130:LIFR complex, which was observed by SEC following LIF stimulation, may then be separately targeted for degradation or may be routed through the lysosomal pathway as has previously been demonstrated for the G-CSFR [23]. Together these observations form the first endogenous study to demonstrate that the SOCS3 SOCS box targets phosphorylated JAK1 for proteasomal degradation and that SOCS3 negatively regulates JAK1/STAT3 signaling by sequestering phosphorylated JAK1 from the receptor as a means of terminating STAT3 activation.
Figure 8. The mechanism of phosphorylated JAK1 degradation by SOCS3 and the SOCS box.
(A) LIF binding to the gp130:LIFR heterodimer induces auto phosphorylation of associated JAK1, which subsequently phosphorylates receptor tyrosines. STAT3 binds to phosphorylated tyrosines on gp130, becomes phosphorylated, dimerises and translocates to the nucleus where it induces transcription of SOCS3. SOCS3 binds to phosphorylated tyrosine 757 on gp130 and may undergo a conformational change. (B) SOCS3 then binds to pJAK1, which is destined for release from the receptor. (C) SOCS3:pJAK1 complex is released from the receptor complex, thereby inhibiting further STAT3 activation as tyrosines within the intracellular domain are no longer being phosphorylated by JAK1. SOCS3 then promotes the proteasomal degradation of pJAK1 most likely through interactions with Elongin B/C and Cullin5/Rbx2 to ubiquitinate pJAK1 and target it for degradation thus preventing re binding to the receptor.
Acknowledgements
This work was supported by grants from the National Institute of Health (CA 22556) and the National Health and Medical Research Council of Australia (Program Grant 461219).
The abbreviations used are
- ES
embryonic stem
- LIF
leukemia inhibitory factor
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alexander WS. Nature Immunology Reviews. 2002;2:1–7. [Google Scholar]
- [2].Robb L, Boyle K, Rakar S, Hartley L, Lochland J, Roberts AW, Alexander WS, Metcalf D. Proc Natl Acad Sci U S A. 2005;102(45):16333–16338. doi: 10.1073/pnas.0508023102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA, Metcalf D, Hilton DJ, Alexander WS. PNAS. 2001;98(16):9324–9329. doi: 10.1073/pnas.161271798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN. The EMBO Journal. 2003;22(3):372–384. doi: 10.1093/emboj/cdg057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cappellen D, Nguyen NH Luong, Bongiovanni S, Grenet O, Wanke C, Susa M. J Biol Chem. 2002;277(24):21971–21982. doi: 10.1074/jbc.M200434200. [DOI] [PubMed] [Google Scholar]
- [6].Chen Z, Laurence A, Kanno Y, Zavisin M Pacher, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Proc Natl Acad Sci U S A. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. Nature Immunology. 2003;4(6):540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- [8].Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, Zhang JG, Hilton DJ, Nicola NA, Alexander WS, Roberts AW. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- [9].Emery B, Cate HS, Marriott M, Merson T, Binder MD, Snell C, Soo PY, Murray S, Croker B, Zhang JG, Alexander WS, Cooper H, Butzkueven H, Kilpatrick TJ. Proc Natl Acad Sci U S A. 2006;103(20):7859–7864. doi: 10.1073/pnas.0602574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Cell Metab. 2006;4(2):123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [11].Kimura A, Kinjyo I, Matsumura Y, Mori H, Mashima R, Harada M, Chien KR, Yasukawa H, Yoshimura A. The Journal of Biological Chemistry. 2004;279(8):6905–6910. doi: 10.1074/jbc.C300496200. [DOI] [PubMed] [Google Scholar]
- [12].Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T, Yoshimura A. J Exp Med. 2006;203(4):1021–1031. doi: 10.1084/jem.20052333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Nat Med. 2004;10(7):739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- [14].Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A. Oncogene. 2006;25(17):2520–2530. doi: 10.1038/sj.onc.1209281. [DOI] [PubMed] [Google Scholar]
- [15].Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Nat Med. 2006;12(7):829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- [16].Ozawa Y, Nakao K, Shimazaki T, Shimmura S, Kurihara T, Ishida S, Yoshimura A, Tsubota K, Okano H. Dev Biol. 2007;303(2):591–600. doi: 10.1016/j.ydbio.2006.11.032. [DOI] [PubMed] [Google Scholar]
- [17].Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Oncogene. 2007;26(33):4833–4841. doi: 10.1038/sj.onc.1210286. [DOI] [PubMed] [Google Scholar]
- [18].Robinson GW, Zavisin M Pacher, Zhu BM, Yoshimura A, Hennighausen L. Dev Dyn. 2007;236(3):654–661. doi: 10.1002/dvdy.21058. [DOI] [PubMed] [Google Scholar]
- [19].Seki YI, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K, Ohara K Inagaki, Cacalano N, O’Garra A, Oshida T, Saito J, Johnston JA, Yoshimura A, Kubo M. Nature Medicine. 2003;9(8):1047–1054. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- [20].Sutherland KD, Vaillant F, Alexander WS, Wintermantel TM, Forrest NC, Holroyd SL, McManus EJ, Schutz G, Watson CJ, Chodosh LA, Lindeman GJ, Visvader JE. Embo J. 2006;25(24):5805–5815. doi: 10.1038/sj.emboj.7601455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Torisu T, Sato N, Yoshiga D, Kobayashi T, Yoshioka T, Mori H, Iida M, Yoshimura A. Genes Cells. 2007;12(2):143–154. doi: 10.1111/j.1365-2443.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- [22].Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, Silva A, Asimakis M, Farley A, Nash AD, Metcalf D, Hilton DJ, Nicola NA, Baca M. Proc Natl Acad Sci U S A. 2000;97(12):6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Irandoust MI, Aarts LH, Roovers O, Gits J, Erkeland SJ, Touw IP. Embo J. 2007;26(7):1782–1793. doi: 10.1038/sj.emboj.7601640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, Sasaki M, Johnston JA, Yoshimura A. Genes Cells. 1999;4(6):339–351. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- [25].Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC, Conaway JW, Nakayama KI. Genes Dev. 2004;18(24):3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G, Kile BJ, Kent SB, Alexander WS, Metcalf D, Hilton DJ, Nicola NA, Baca M. Proc Natl Acad Sci U S A. 1999;96(5):2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Haan S, Ferguson P, Sommer U, Hiremath M, McVicar DW, Heinrich PC, Johnston JA, Cacalano NA. J Biol Chem. 2003;278(34):31972–31979. doi: 10.1074/jbc.M303170200. Epub 32003 Jun 31973. [DOI] [PubMed] [Google Scholar]
- [28].Babon JJ, McManus EJ, Yao S, DeSouza DP, Mielke LA, Sprigg NS, Willson TA, Hilton DJ, Nicola NA, Baca M, Nicholson SE, Norton RS. Mol Cell. 2006;22(2):205–216. doi: 10.1016/j.molcel.2006.03.024. [DOI] [PubMed] [Google Scholar]
- [29].Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T, Yoshizaki K, Naka T, Kishimoto T. Proc Natl Acad Sci U S A. 1998;95(22):13130–13134. doi: 10.1073/pnas.95.22.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, Alexander WS, Metcalf D, Hilton DJ, Nicola NA. The EMBO Journal. 1999;18(2):375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. Embo J. 1999;18(5):1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].van de Geijn GJ, Gits J, Aarts LHJ, Antonissen C Heijmans, Touw IP. Blood. 2004;104(3):667–674. doi: 10.1182/blood-2003-08-2913. [DOI] [PubMed] [Google Scholar]
- [33].Boyle K, Egan P, Rakar S, Willson TA, Wicks IP, Metcalf D, Hilton DJ, Nicola NA, Alexander WS, Roberts AW, Robb L. Blood. 2007;110(5):1466–1474. doi: 10.1182/blood-2007-03-079178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang JG, Metcalf D, Rakar S, Asimakis M, Greenhalgh CJ, Willson TA, Starr R, Nicholson SE, Carter W, Alexander WS, Hilton DJ, Nicola NA. PNAS. 2001;98(23):13261–13265. doi: 10.1073/pnas.231486498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TWH, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- [36].Duval D, Reinhardt B, Kedinger C, Boeuf H. Faseb J. 2000;14(11):1577–1584. doi: 10.1096/fj.14.11.1577. [DOI] [PubMed] [Google Scholar]
- [37].Forrai A, Boyle K, Hart AH, Hartley L, Rakar S, Willson TA, Simpson KM, Roberts AW, Alexander WS, Voss AK, Robb L. Stem Cells. 2006;24(3):604–614. doi: 10.1634/stemcells.2005-0323. [DOI] [PubMed] [Google Scholar]
- [38].Voss AK, Thomas T, Gruss P. Exp Cell Res. 1997;230(1):45–49. doi: 10.1006/excr.1996.3418. [DOI] [PubMed] [Google Scholar]
- [39].Babon JJ, Yao S, DeSouza DP, Harrison CF, Fabri LJ, Liepinsh E, Scrofani SD, Baca M, Norton RS. Febs J. 2005;272(23):6120–6130. doi: 10.1111/j.1742-4658.2005.05010.x. [DOI] [PubMed] [Google Scholar]
- [40].Nicholson SE, Oates AC, Harpur AG, Ziemiecki A, Wilks AF, Layton JE. Proc Natl Acad Sci U S A. 1994;91(8):2985–2988. doi: 10.1073/pnas.91.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hanada T, Yoshida T, Kinjyo I, Minoguchi S, Yasukawa H, Kato S, Mimata H, Nomura Y, Seki Y, Kubo M, Yoshimura A. J Biol Chem. 2001;276(44):40746–40754. doi: 10.1074/jbc.M106139200. [DOI] [PubMed] [Google Scholar]
- [42].Kamizono S, Hanada T, Yasukawa H, Minoguchi S, Kato R, Minoguchi M, Hattori K, Hatakeyama S, Yada M, Morita S, Kitamura T, Kato H, Nakayama K, Yoshimura A. The Journal of Biological Chemistry. 2001;276(16):12530–12538. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- [43].Kamura T, Sato S, Haque D, Liu L, Kaelin WG, Jr., Conaway RC, Conaway JW. Genes Dev. 1998;12(24):3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ungureanu D, Saharinen P, Junttila I, Hilton DJ, Silvennoinen O. Mol Cell Biol. 2002;22(10):3316–3326. doi: 10.1128/MCB.22.10.3316-3326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lehmann U, Schmitz J, Weissenbach M, Sobota RM, Hortner M, Friederichs K, Behrmann I, Tsiaris W, Sasaki A, Mergener J Schneider, Yoshimura A, Neel BG, Heinrich PC, Schaper F. J Biol Chem. 2003;278(1):661–671. doi: 10.1074/jbc.M210552200. [DOI] [PubMed] [Google Scholar]
- [46].Schmitz J, Weissenbach M, Haan S, Heinrich PC, Schaper F. The Journal of Biological Chemistry. 2000;275(17):12848–12856. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- [47].Tago M Funakoshi, Pelletier S, Moritake H, Parganas E, Ihle JN. Mol Cell Biol. 2008;28(5):1792–1801. doi: 10.1128/MCB.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tago M Funakoshi, Tago K, Kasahara T, Parganas E, Ihle JN. Cell Signal. 2008 doi: 10.1016/j.cellsig.2008.07.008. [DOI] [PubMed] [Google Scholar]
- [49].Giese B, Au Yeung CK, Herrmann A, Diefenbach S, Haan C, Kuster A, Wortmann SB, Roderburg C, Heinrich PC, Behrmann I, Newen G Muller. J Biol Chem. 2003;278(40):39205–39213. doi: 10.1074/jbc.M303347200. [DOI] [PubMed] [Google Scholar]
- [50].Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, Baumann H, Judd LM, Giraud AS, Boussioutas A, Zhu HJ, Ernst M. Nat Med. 2005;11(8):845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]