Abstract
A subset of patients with IBS have visceral hypersensitivity and/or somatic hypersensitivity. Visceral hypersensitivity might have use as a clinical marker of IBS and could account for symptoms of urgency for bowel movements, bloating and abdominal pain. The mechanisms that lead to chronic visceral hypersensitivity in patients who have IBS are unclear. However, several working models may be considered, including: nociceptive input from the colon that leads to hypersensitivity; increased intestinal permeability that induces a visceral nociceptive drive; and alterations in the expression of microRNAs in gastrointestinal tissue that might be delivered via blood microvesicles to other target organs, such as the peripheral and/or central nervous system. As such, the chronic visceral hypersensitivity that is present in a subset of patients with IBS might be maintained by both peripheral and central phenomena. The theories underlying the development of chronic visceral hypersensitivity in patients with IBS are supported by findings from new animal models in which hypersensitivity follows transient inflammation of the colon. The presence of somatic hypersensitivity and an alteration in the neuroendocrine system in some patients who have IBS suggests that multisystemic factors are involved in the overall disorder. Thus, IBS is similar to other chronic pain disorders, such as fibromyalgia, chronic regional pain disorder and temporomandibular joint disorder, as chronic nociceptive mechanisms are activated in all of these disorders.
Introduction
IBS is a gastrointestinal disorder that affects up to 20% of the population of the USA and is associated with an alteration in bowel habits (such as diarrhea and constipation). Patients with IBS have a considerably decreased quality of life and utilize large amounts of health-care resources. A subset of patients who have IBS, varying from 30% to 40%, are reported to exhibit enhanced sensitivity to colonic distension, which is noticeable through their reduced threshold for pain, increased intensity of sensations and/or exaggerated viscerosomatic referral in response to colonic distension.1–4 Thus, visceral hypersensitivity is a clinical marker in a subset of patients who have IBS and could account for the symptoms of urgency for bowel movements, bloating and abdominal pain experienced by these patients. However, the correlation between hypersensitivity and clinical symptoms and outcomes is still not clearly defined.5 The findings of a study published in 2006 revealed that habituation of visceral hypersensitivity by repeated exposure to noxious stimuli occurred without any change in the severity of IBS symptoms.6
The cause of visceral hypersensitivity is unknown; however, a number of mechanisms have been postulated, such as inflammation or sensitization after an injury.1 Several studies have shown that some patients develop IBS symptoms following enteric infection of the gut.7–9 Although the exact mechanism or mechanisms underlying postinfectious IBS are not clearly understood, increased intestinal permeability has been reported in these patients.11
Interestingly, a study published in 2010 demonstrated that a subset of patients with IBS have increased intestinal permeability that is associated with decreased levels of glutamine synthetase in the gut.11 In this Review, we discuss the mechanisms associated with somatic and visceral hypersensitivity in patients who have IBS. We review the experimental evidence for both visceral and secondary somatic hypersensitivity in patients with IBS and then discuss several possible underlying mechanisms—impulse input from the colon in the induction and maintenance of hypersensitivity, increased intestinal permeability, and alterations in microRNA (miRNA) expression in gastrointestinal tissues that might be delivered via blood microvesicles to other target organs. These unifying mechanisms suggest that there is a synergistic interaction between peripheral and central nervous system mechanisms that may have an important role in the pathophysiology of the pain and hypersensitivity often seen in patients who have IBS.
Neurobiology of visceral afferents
The responses of visceral afferents are elicited by chemical stimuli, local luminal stimuli and by mechanical stimuli (such as gastrointestinal distension). Silent nociceptors also exist that are mechanically insensitive until tissue injury occurs, after which they develop spontaneous activity and mechanosensitivity.12 For example, acute instillation of bile salts into the colon considerably increases the activity of mechanosensitive colonic afferents in response to colonic distension.13,14 When silent nociceptors are activated, they could then contribute to chronic visceral hypersensitivity via both peripheral and central nervous system mechanisms. A superior understanding of the mechanisms that lead to chronically altered sensations from the viscera has come from an improved understanding of primary visceral afferent physiology. Disorders in which patients experience altered sensations include ureteric colic, functional bowel disorders (such as IBS) and interstitial cystitis.1,13
As mentioned above, although after acute injury visceral afferents can develop a state of acute mechano-sensitization, mounting evidence indicates that chronic hyperalgesia is a consequence of persistent tissue injury. Animal models of visceral hypersensitivity exist in which irritation of the colon causes chronic visceral hypersensitivity and central sensitization that is associated with allodynia and hyperalgesia.15–17 Similarly, IBS symptoms develop in ~20–25% of patients after Salmonella infection of the gut.18,19
Although many acute symptoms typically disappear within several weeks of the initiation of symptoms, bloating, diarrhea and abdominal symptoms do not. Transient inflammation of the colon might sensitize the gut, and the sensitization can persist long after resolution of the inflammation. This pattern is similar to that seen in the animal models of functional gastrointestinal disorders noted above.15–17 However, our understanding of colonic and/or peripheral afferents that become sensitized and contribute to chronic visceral hypersensitivity is still poor. Hyperalgesia involves elements from the peripheral and central nervous systems and might be entirely maintained by either central or peripheral mechanisms.
The neuroendocrine system in IBS
Some of the early investigations of somatic hypersensitivity in patients with IBS suggested that the hypersensitivity was limited to the gut.20–22 Sensitivity in other areas of the body was either reduced or unaffected in patients with IBS compared with healthy control participants matched for age and sex.20–22 The findings of these early studies might be explained by the stimuli that were used, as discomfort following electrical, cold pressor and/or mechanical stimulation might not have necessarily stimulated nociceptive receptors. In addition, the brief nociceptive stimuli used in these studies might not have activated C fibers for a sufficient time to assess neural mechanisms that involved N-methyl, D-aspartate (NMDA) receptors or other neuropeptides.23 Prolonged nociceptive stimuli, such as thermal pulses, activate cutaneous C fibers and NMDA receptor mechanisms. Similar to other painful conditions that probably depend on peripheral impulse input (for example, fibromyalgia, postherpetic neuralgia and complex regional pain syndrome), a proportion of patients who have IBS experience widespread hypersensitivity, which might involve chronic nociceptive input from the gastrointestinal tract.24
A study published in 2010 investigated the perception of somatic pain in patients who have IBS.4 In this study, several experimental procedures were used to induce somatic pain and look at different perceptual qualities and stimulus intensities across body sites that provided input to the spine at differing levels. Some patients with IBS had greater sensitivity to somatic (for example, thermal, cold pressor or ischemic) stimuli than control individuals did. After thermal and cold pressor stimuli this difference was noticeable for the foot, but not the hand, which suggests that thermal hypersensitivity is somatotopically organized in patients who have IBS, with dermatomes on the lower body showing greater hypersensitivity than dermatomes on the upper body. Thus, a subset of patients with IBS have greater sensitivity to somatic stimuli than control individuals. Interestingly, there were no differences in somatic sensitivity between patients who have IBS and control individuals when mechanical stimuli were used.
These results support the findings from a study that tested the pain perception of patients with IBS who either did or did not have fibromyalgia.25 During an ascending series of pressure (mechanical) stimuli, patients with IBS had similar pain thresholds to control individuals.25 A possible reason for this finding in patients who have IBS that differs from the reasons underlying the response to thermal, cold pressor and ischemic stimuli, might be related to the differences in stimulus modalities. Mechanical stimuli activate non-nociceptive mechano-receptors at higher levels in the cerebral cortex, which could be inhibited by chronic visceral nociceptive input in patients who have IBS.24 Thus, total nociceptive input with mechanical stimuli might be reduced in patients who have IBS compared with nociceptive input from thermal stimuli.
The cold pressor test has also been used to test somatic hypersensitivity in patients who have IBS.21,22 These patients have shown a notably higher perception of pain in response to the cold pressor applied to the foot compared with when it is applied to the hand.4 Interestingly, these findings are different from one study that showed hypersensitivity in patients who have IBS using cold pressor testing applied to the hand and to two studies that did not find any hypersensitivity in patients who have IBS.3,21,22 These differences might be explained by the fact that there is a subset of patients who have IBS whose pain threshold for cold water is similar to control individuals, and a small group of patients with IBS who have a much lower pain threshold for the cold pressor test than either control individuals or other patients with IBS. Further analysis of subsets of sensitive patients who have IBS might, therefore, prove interesting and could enable the identification of the frequency distribution of hypersensitivity in patients with IBS.
The modified submaximal effort tourniquet procedure (an ischemic test) has been used to evaluate somatic hypersensitivity in patients who have IBS.26 The ischemic test activates a large number of C fibers in the muscle and produces a diffuse, deep pain. This activation is different from other nociceptive stimuli that primarily involve Aδ fibers, as the cutaneous stimuli were of shorter duration than in the ischemic test.26,27 Compared with the thermal and cold pressor stimuli in a recent study,4 the ischemic test revealed a larger subset of patients who have IBS with hypersensitivity. Other studies have reported ischemic hypersensitivity using the modified submaximal effort tourniquet procedure in patients with temporomandibular disorders, fibromyalgia or inter stitial cystitis.27–29 All of these disorders have considerable comorbidity and overlap of symptoms with IBS.
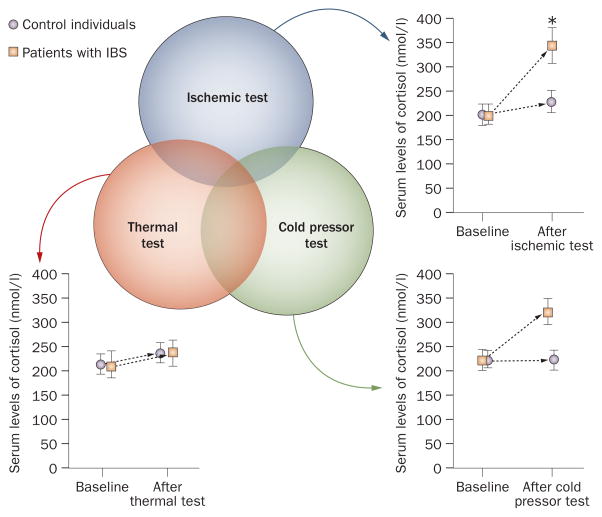
As there is a robust subset of patients who have IBS with hypersensitivity during the ischemic test, changes in the levels of cortisol and adrenocorticotropic hormone (ACTH) were measured in the study by Zhou et al.4 Following the ischemic test, patients with IBS, but not control individuals, had a considerable increase in levels of both cortisol and ACTH. The changes in the levels of cortisol and/or ACTH following the thermal or cold pressor stimuli were not statistically significant (Figure 1).4 The increase in the levels of cortisol and ACTH following an ischemic test suggests that patients who have IBS might experience this stimulus as more stressful than patients who do not have IBS.30 These results are similar to those of another study that demonstrated differences in the adrenocortical response to stress in patients with IBS compared with the response in control individuals.31
Figure 1.
Hypersenstivity in IBS. The three circles represent the distribution of overlap between subsets of patients who have IBS with somatic hypersensitivity in response to thermal, ischemic and cold pressor stimuli. Changes in serum levels of cortisol are also shown following the thermal, ischemic and cold pressor stimuli. The largest change in cortisol levels is seen following the ischemic test. Although there was a rise in serum cortisol levels following the cold pressor test, the changes were not statistically significant. *Significance level of <0.05. This figure has been reproduced with permission of the International Association for the Study of Pain® (IASP®) Zhou, Q. Pain 148, 454–461 (2011). The figure may not be reproduced for any other purpose without permission.
Overall, the findings of the above studies are consistent with the idea that a subset of patients who have IBS display somatic hypersensitivity.4 Given that the somatic hypersensitivity in patients with IBS is somatotopically organized, it is tempting to conclude that afferent nociceptive input from the gastrointestinal tract can sensitize spinal neurons that overlap somatotopically with the gastrointestinal tract. As subsets of patients who have IBS and hypersensitivity to cold pressor, ischemic and thermal stimuli exhibited only a small amount of overlap, several mechanisms could be involved. Overlap of cold pressor, ischemic and thermal hypersensitivity was only found in a small subset of patients who have IBS. In these patients, the largest overlap in somatic hypersensitivity was observed between the thermal and cold pressor tests (Figure 1). Differing modalities and mechanisms of hypersensitivity might, therefore, underlie this unique subset of patients who have IBS with somatic hypersensitivity.
Gastrointestinal afferent input
One plausible mechanism underlying secondary somatic hypersensitivity in patients who have IBS is a chronic afferent barrage from the colon to the spinal cord. This mechanism has been studied in both humans and in animal models of visceral hypersensitivity, as discussed above. Several animal models of neuropathic pain have been used to demonstrate that persistent afferent input to the spinal cord from peripheral sources sustains altered central processing and leads to spontaneous motor abnormalities, hyperalgesia, pain and allodynia.15–17 These models are based on the finding that blockade of nociceptive input from specific somatic foci by peripheral anesthetics abrogates pain and allodynia induced by cold or mechanic stimuli within widespread regions of the body in patients with complex regional pain syndrome.32 As widespread zones of hypersensitivity are present in patients with fibromyalgia, neuropathic pain and IBS, these regions of hypersensitivity could be maintained, at least in part, by tonic impulses from nociceptive colonic afferent neurons.
Two studies have evaluated the effects of lidocaine (a local anesthetic) on colonic distension. One study demonstrated that administration of rectal lidocaine did not alter sensation to rectal distension (either phasic or ramp distension) in patients with IBS.33 By contrast, another study showed that rectal lidocaine inhibited sensation to slow ramp but not rapid phasic distension in healthy individuals; patients with IBS were not tested in this study.34
The role of tonic impulse input from the gut of patients who have IBS has also been tested by administering lidocaine jelly to the colon in a double-blind crossover trial.35 Interestingly, intracolonic lidocaine diminished hypersensitivity to both nociceptive colonic distension and thermal stimuli applied to the foot. The same results have been demonstrated in an animal model of IBS in which intracolonic lidocaine normalized both colonic and thermal hypersensitivity in hypersensitive rats.36 In both of these studies, there were no detectable levels of lidocaine in the blood for up to 50 min following administration, which suggests that the findings were not caused by lidocaine being systemically absorbed. These results are in accordance with a role for peripheral impulse activity in conditions of neuropathic pain. Such conditions are present in situations in which tonic impulses from a peripheral source dynamically sustain the primary hypersensitivity caused by input from the gut as well as the secondary somatic hypersensitivity that is distant (for example, in an extremity) from the peripheral source of impulse input (in this case, the gut).32
Currently, there are few ways to test spinal cord mechanisms of hypersensitivity in patients who have IBS. One study used electromyographic recordings of somatic nociceptive flexion reflexes (R-III) after painful electric shocks in the foot area to evaluate the effect of colonic distension.37 Distension of the colon induced inhibition of this nociceptive reflex in 10 healthy volunteers; it accentuated this reflex in 14 patients who had IBS. These results strongly suggest that hyperexcitability of spinal nociceptive processing might occur in patients with IBS. Another study of patients who have IBS showed that a subset of these patients have marked temporal summation of second pain (also known as windup) in response to a series of noxious heat pulses with a low peak intensity (47 °C).38 The presence of windup in this subset of patients who have IBS is interesting and was blocked by the NMDA receptor antagonist, dextromethorphan. The presence of windup is important in the induction and/or maintenance of chronic disorders that involve allodynia and hyperalgesia.23,24 So, the somatic hypersensitivity that is present in a subset of patients with IBS could result from temporal summation via excessive activation of central NMDA receptors. Heightened central sensitization could explain the chronic pain present in the distinct subsets of patients who have IBS described above.4
The strongest evidence for spinal cord mechanisms of hypersensitivity in patients with IBS comes from several animal studies. One model shows that ~24% of rats treated with intracolonic trinitrobenzene sulfonic acid (TNBS) develop hypersensitivity to both nociceptive colonic distension and thermal stimuli following resolution of TNBS-induced colitis.15 Similar to findings in patients with IBS, the somatic hypersensitivity was greater in the lumbosacral dermatomes compared with the cervicothoracic dermatomes. Another study, which used an animal model that was IBS-like, showed that expression of the subunits of the NMDA NR1 protein in hypersensitive animals was greater in the L4–S1 region (laminae I and II) of the spinal cord than in the T10–L1 region of the spinal cord.23 In this animal model, the somatic hypersensitivity is organized somatotopically, with dermatomes in the lower body showing a greater hypersensitivity than those in the upper body. In another animal model, neonatal rats were injected with mustard oil, which induced delayed visceral and somatic hypersensitivity, similar to the effects of treating animals with TNBS.16 In the same study, dorsal horn neurons that received both colonic and somatic input had increased levels of evoked impulse activity from colonic distension and somatic stimuli as well as increased spontaneous activity. This finding provides further evidence that both visceral and somatic hypersensitivity in patients with IBS might be dynamically maintained by primary colonic afferent neurons and consequent sensitization of the dorsal horn neurons on which they synapse.
The role of the central nervous system in modulating visceral hypersensitivity has also gained increasing importance over the past decade. Activation of the central nervous system in animal models of visceral hypersensitivity has been shown in response to increased levels of c-Fos in the central nervous system.39 Levels of corticotropin-releasing factor, an important regulator of the stress response, are increased in several brain structures in rat models of functional bowel disorders.40,41 Thus, targeting central nervous system structures with cognitive behavioral therapy or other central therapies might lead to important treatment modalities for IBS, such as using amitriptyline.42
Increased intestinal permeability
The purposes of the gastrointestinal tract are to help nutrients to be absorbed and to act as a barrier to the absorption of toxic compounds, macromolecules and bacteria.43,44 Disruption of the gut barrier can cause local gastrointestinal dysfunction and systemic abnormalities (for example, bacterial translocation and sepsis). Alterations in the mechanical barrier or the immune barrier contribute to an increased uptake into the systemic circulation of pathogenic bacteria and inflammatory luminal macromolecules. Increased intestinal barrier permeability seems to correlate with several clinical conditions, including food allergies, IBD, rheumatoid arthritis, allergic disorders, celiac disease and a few chronic dermatological conditions.43 In the latest literature, there has been a focus on enhanced intestinal permeability as a possible causative factor in IBS.10
In patients with postinfectious IBS, it is hypothesized that ongoing subclinical inflammation with an increase in intestinal permeability, mast cells, proinflammatory cytokines, serotonin-containing enterochromatin cells and T lymphocytes could be involved.45,46 The intestinal mucosa of some patients with IBS contains an increased number of immunocytes (such as T lymphocytes and mast cells) and there is evidence of an increase in the release of cytokines, histamine, proteases and prostaglandins.45 These mediators are known to signal to epithelial, neuronal and muscle cells, which leads to intestinal dysfunction. The activation of these inflammatory factors might lead to increased intestinal permeability, especially in a subset of patients with diarrhea- predominant IBS.11 Hyperpermeability permits bacteria and antigens to translocate through the gut mucosa. Such translocation can activate symptoms seen in patients who have IBS, including abdominal pain, mucosal immune responses and the attendant chronic diarrhea. Interestingly, animal models have shown that increased intestinal permeability induces hypersensitivity. In one study, acute partial restraint stress considerably increased colonic permeability in addition to abdominal muscle electromyography in response to colonic distension.47
Increased intestinal permeability has been evaluated in a subset of patients with diarrhea-predominant IBS.48 Those who had intestinal hyperpermeability also had higher Functional Bowel Disorder Severity Index (FBDSI) scores than those without hyperpermeability. The increase in the FBDSI scores correlated positively with increases in visceral and thermal hypersensitivity. In the subset of patients who have IBS with diarrhea as the predominant symptom, intestinal hyper permeability could contribute to a chronic nociceptive drive from the gastrointestinal tract to the spinal cord, and lead to central sensitization. The study also showed a correlation between the severity of IBS symptoms (as monitored by the FBDSI scale) and the presence of both visceral and somatic hypersensitivity. In addition, patients who have IBS with somatic and visceral hypersensitivity exhibit increased intestinal permeability.48 The intestinal hyperpermeability could permit passage of inflammatory agents and bacteria through the gut wall, which would sensitize the myenteric plexus and overlapping common spinal segments. This spinal sensitization in patients with IBS could be created and/or maintained by tonic impulse input from the colon to the spinal cord. The FBDSI score in patients with IBS was positively correlated with both an increase in intestinal permeability and visceral and thermal hypersensitivity. Patients who have IBS, a high FBDSI score and increased intestinal permeability might, therefore, have central sensitization that would justify alternative treatment modalities aimed at the central nervous system.
The human gastrointestinal tract is the major site of glutamine utilization in the body. Glutamine is a major energy source for rapidly dividing intestinal mucosal cells of the digestive tract. Glutamine also helps to protect the lining of the gastrointestinal tract. Depletion of glutamine results in epithelial atrophy and a subsequent increase in intestinal permeability. Glutamine supplementation decreases bacterial translocation and intestinal permeability after intestinal injury.49 Glutamine supplementation also decreases intestinal permeability and improves gastrointestinal function in patients with Crohn’s disease and in patients with advanced esophageal cancer who are undergoing radiochemotherapy.50,51
Glutamine synthetase catalyzes the conversion of ammonia and glutamate to glutamine. This enzyme has a major role in cell signaling, ammonia detoxification, interorgan nitrogen flux and acid–base homeostasis. Congenital glutamine deficiencies have been reported in children with glutamine synthetase mutations.52 Because of the multiple functions and importance of glutamine synthetase in cellular metabolism, catalytic activities and synthesis are both highly regulated. Glutamine synthetase is also important for cell proliferation in the rat intestinal crypt cells.53 Mucosal epithelial cells that line the gastrointestinal tract have a small free glutamine pool and rely on glutamine synthetase to convert glutamate and ammonia to glutamine. Inhibition of glutamine synthetase decreased proliferation of cultured rat intestinal cells.53 Consequently, conditions in which decreased levels of intestinal glutamine synthetase are present might lead to low levels of available glutamine and increased intestinal permeability, as is seen in some patients with diarrhea-predominant IBS.11
Alterations in microRNA expression
miRNAs were identified in 1993 as small (~21–23 nucleotides long), endogenous, noncoding RNAs that have the capacity for gene regulation in larval development.54,55 Mature miRNAs are cleaved from 70–100-nucleotide hairpin pre-miRNA precursors. Single-stranded miRNAs bind through partial sequence homology to the 3′ untranslated region of target messenger (m)RNAs and cause a block of translation or, less frequently, mRNA degradation. Over the past decade, miRNAs have emerged as regulators involved in biological processes, such as cellular development, differentiation, proliferation, apoptosis and metabolism.56 Several studies have indicated that miRNAs can be used as a marker in patients with gastrointestinal diseases. In patients who have IBS, miRNA expression is altered in colonic tissue and these miRNAs regulate intestinal pathways, which results in epigenetic and genetic events. These changes could also lead to alterations in intestinal permeability, serotonin signaling, or visceral/somatic hypersensitivity.11,57,58 Alterations in gastrointestinal functional processes, such as intestinal permeability, can be altered by the expression of specific miRNAs, such as miR-29a.11 Interestingly, miR-29a has a complementary site in the 3′ untranslated region of the gene that encodes glutamine synthetase, as predicted by a well-established database that predicts miRNA target genes.59 Because of the complementary site in the GLUL gene, altered miR-29a expression leads to decreased glutamine synthetase levels. Decreased levels of glutamine synthetase might lead to decreased intestinal levels of glutamine, which results in an increased intestinal permeability.11 This finding supports a role for miR-29a in regulating intestinal permeability in patients with diarrhea-predominant IBS. This new discovery links the pathophysiology of IBS to a specific miRNA that modulates the integrity of the intestinal barrier.
Blood microvesicles are spherical membrane fragments that act as cell-to-cell and cell-to-organ communicators and are shed from the surface of most cells.60,61 Most cells release microvesicles from their plasma membrane (microparticles) or from multivesicular endocytic compartments (exosomes). Microvesicles directly target cells via receptor-mediated interactions or potentially by transferring various bioactive molecules, including membrane receptors, proteins, mRNAs, miRNAs and organelles. Shedding of membrane-derived microvesicles accompanies cell activation.60,61 Changes in the molecular signature of microvesicles could also be used to identify certain disease processes.
Unique blood microvesicles with increased expression of the miRNA miR-29a have been identified in patients with diarrhea-predominant IBS.11 Alterations in miRNA expression found in circulating blood micro-vesicles in patients who have IBS might be derived from colonic tissue. Alteration of miRNA expression probably occurs at the site of tissue injury (the colon) in patients who have IBS. These altered microvesicles might then travel to distant sites where they directly modulate downstream targets.
Conclusions
IBS is a common gastrointestinal disorder and a subset of patients with IBS have visceral and somatic hypersensitivity. The mechanisms underlying this hypersensitivity are unknown; however, there are several possible working models: impulse input from the colon in the induction and maintenance of hypersensitivity; increased intestinal permeability that induces and/or maintains a visceral nociceptive drive; and alterations in miRNA and microvesicles in target tissues. Other, as yet undiscovered, mechanisms may also be involved. How these potential mechanisms interact and produce a synergistic relationship between peripheral and central mechanisms in patients who have IBS requires further study. Thus, the mechanisms of hypersensitivity in IBS are still unclear but are probably induced by physiologic stimuli, such as transient inflammation of the gut or increased intestinal permeability. A subset of patients with IBS have persistent hypersensitivity in response to experimental pain stimuli. Future studies need to be done to determine the underlying neurobiology that is present in this subset of patients. These studies might then lead to the development of more effective and targeted therapies than those currently available for this group of hypersensitive patients.
Key points.
Visceral and somatic hypersensitivity are present in some patients with functional gastrointestinal disorders
Injury to visceral afferents is the most common underlying cause of visceral hypersensitivity that is maintained by either peripheral and/or central nervous system mechanisms
Animal models of hypersensitivity have been used to examine the neural mechanisms of hypersensitivity following inflammatory injury, such as alterations in the N-methyl, D-aspartate receptor, dorsal horn neurons or c-Fos
Increased intestinal permeability might lead to hypersensitivity and abdominal pain in patients with functional gastrointestinal disorders
Functional gastrointestinal disorders are similar to other chronic pain disorders in which persistent nociceptive mechanisms are activated
Review criteria.
PubMed was searched using the terms “visceral hypersensitivity” and “irritable bowel syndrome” for articles published over the past 25 years. Only English-language articles with an abstract were included.
Acknowledgments
The authors would like to acknowledge the support of NIH grants (NS053090, AT005291) and a VA Merit Review Award from the Medical Research Service of the Department of Veterans Affairs.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Both authors contributed equally to all aspects of this review.
References
- 1.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 2.Mayer EA, et al. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouin M, Meunier P, Riberdy-Poitras M, Poitras P. Pain hypersensitivity in patients with functional gastrointestinal disorders: a gastrointestinal-specific defect or a general systemic condition? Dig Dis Sci. 2001;46:2542–2548. doi: 10.1023/a:1012356827026. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q, Fillingim RB, Riley JL, Malarkey WB, Verne GN. Central and peripherial hypersensitivity in the irritable bowel syndrome. Pain. 2010;148:454–461. doi: 10.1016/j.pain.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piché M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010;148:49–58. doi: 10.1016/j.pain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Naliboff BD, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 8.Mearin F, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Dupont AW. Post-infectious irritable bowel syndrome. Curr Gastroenterol Rep. 2007;9:378–384. doi: 10.1007/s11894-007-0046-8. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545–552. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Souba WW, Croce C, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–784. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol. 1998;80:2632–2644. doi: 10.1152/jn.1998.80.5.2632. [DOI] [PubMed] [Google Scholar]
- 13.Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 14.Traub RJ, et al. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology. 2008;135:2075–2083. doi: 10.1053/j.gastro.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Caudle RM, Price DD, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-Induced colitis. Pain. 2008;134:9–15. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 17.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 18.Gwee KA, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 19.McKendrick MW, Read NW. Irritable bowel syndrome—post salmonella infection. J Infect. 1994;29:1–3. doi: 10.1016/s0163-4453(94)94871-2. [DOI] [PubMed] [Google Scholar]
- 20.Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108:636–643. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead WE, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 22.Zighelboim J, Talley NJ, Phillips SF, Harmsen WS, Zinsmeister AR. Visceral perception in irritable bowel syndrome. Rectal and gastric responses to distension and serotonin type 3 antagonism. Dig Dis Sci. 1995;40:819–827. doi: 10.1007/BF02064986. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Price DD, Caudle RM, Verne GN. Spinal NMDA NR1 subunit expression following transient TNBS colitis. Brain Res. 2009;1279:109–120. doi: 10.1016/j.brainres.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price DD, et al. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage. 2009;47:995–1001. doi: 10.1016/j.neuroimage.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84:297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 26.Moore PA, Duncan GH, Scott DS, Gregg JM, Ghia JN. The submaximal effort tourniquet test: its use in evaluating experimental and chronic pain. Pain. 1979;6:375–382. doi: 10.1016/0304-3959(79)90055-1. [DOI] [PubMed] [Google Scholar]
- 27.Carli G, Suman AL, Biasi G, Marcolongo R. Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. Pain. 2002;100:259–269. doi: 10.1016/S0304-3959(02)00297-X. [DOI] [PubMed] [Google Scholar]
- 28.Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris MB. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain. 1996;12:260–269. doi: 10.1097/00002508-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychological evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005;173:1983–1987. doi: 10.1097/01.ju.0000158452.15915.e2. [DOI] [PubMed] [Google Scholar]
- 30.Posserud I, et al. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang L, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterolol Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- 33.Lembo T, et al. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994;107:1686–1696. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 34.Sabate JM, Coffin B, Jian R, Le Bars D, Bouhassira D. Rectal sensitivity assessed by a reflexologic technique: further evidence for two types of mechanoreceptors. Am J Physiol Gastrointest Liver Physiol. 2000;279:G692–G699. doi: 10.1152/ajpgi.2000.279.4.G692. [DOI] [PubMed] [Google Scholar]
- 35.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain. 2008;139:218–224. doi: 10.1016/j.pain.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coffin B, Bouhassira D, Sabaté JM, Barbe L, Jian R. Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut. 2004;53:1465–1470. doi: 10.1136/gut.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q, Price DD, Callam CS, Woodruff MA, Verne GN. Effects of the N-methyl-D-aspartate receptor on temporal summation of second pain (wind-up) in irritable bowel syndrome. J Pain. 2011;12:297–303. doi: 10.1016/j.jpain.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R, et al. Elevated expression of c-fos in central nervous system correlates with visceral hypersensitivity in irritable bowel syndrome (IBS): a target for IBS treatment. Int J Colorectal Dis. doi: 10.1007/s00384-011-1153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers B, Greenwood-Van Meerveld B. Divergent effects of amygdala glucocorticoid and minealcorticoid receptors in the regulation of visceal and somatic pain. Am J Physiol Gastrointest Liver Physiol. 2010;298:G295–G303. doi: 10.1152/ajpgi.00298.2009. [DOI] [PubMed] [Google Scholar]
- 41.Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disease. Int J Neuropsycholopharmacol. 2010;22:1–18. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- 42.Thoua NM, et al. Amitriptyline modifies the visceral hypersensitivity repsonse to acute stress in the irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:552–560. doi: 10.1111/j.1365-2036.2008.03918.x. [DOI] [PubMed] [Google Scholar]
- 43.Bjarnason I, MacPherson A, Hollander D. Intestinal permeabiity: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 44.Macdonald TT, Montelenone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 45.Spiller RC, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunlop SP, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 47.Ait-Belgnaoui A, Bradesi S, Fioramonit J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113:141–147. doi: 10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–46. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souba WW, et al. The role of glutamine in maintaning a healthy gut and supporting the metabolic response to injury and infection. J Surg Res. 1990;48:383–391. doi: 10.1016/0022-4804(90)90080-l. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida S, et al. Effects of glutamine supplements and radiochemotherapy on systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann Surg. 1998;227:485–491. doi: 10.1097/00000658-199804000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sido B, Seel C, Hochlehnert A, Breitkreutz R, Dröge W. Low intestinal glutamine level and low glutaminase activity in Crohn’s disease: a rational for glutamine supplementation? Dig Dis Sci. 2006;51:2170–2179. doi: 10.1007/s10620-006-9473-x. [DOI] [PubMed] [Google Scholar]
- 52.Häberle J, et al. Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med. 2005;353:1926–1933. doi: 10.1056/NEJMoa050456. [DOI] [PubMed] [Google Scholar]
- 53.DeMarco V, Dyess K, Strauss D, West CM, Neu J. Inhibition of glutamine synthetase decreases proliferation of cultured rat intestinal epithelial cells. J Nutr. 1999;129:57–62. doi: 10.1093/jn/129.1.57. [DOI] [PubMed] [Google Scholar]
- 54.Kim J, et al. Identification of many microRNAs that copurify with polyribodomes in mammalian neurons. Proc Natl Acad Sci USA. 2004;101:360–365. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farh KK, et al. The widespread impact of mammalian microRNAs on mRNA represssion and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 56.Garson R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies, and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapeller J, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 58.Wu F, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2α. Gastroenterology. 2008;135:1624–1635. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 59.miRBase. The microRNA database. 2011 [online], http://microrna.sanger.ac.uk/sequences/
- 60.Valadi H, et al. Exosome-mediated transfer of mRNA and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 61.Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]