Abstract
Colorectal cancer remains one of the most common types of cancer and leading causes of cancer death worldwide. Although we have made steady progress in chemotherapy and targeted therapy, evidence suggests that the majority of patients undergoing drug therapy experience severe, debilitating, and even lethal adverse drug events which considerably outweigh the benefits. The identification of suitable biomarkers will allow clinicians to deliver the most appropriate drugs to specific patients and spare them ineffective and expensive treatments. Prognostic and predictive biomarkers have been the subjects of many published papers, but few have been widely incorporated into clinical practice. Here, we want to review recent biomarker data related to colorectal cancer, which may have been ready for clinical use.
Keywords: Colorectal cancer, Biomarker, Predictive value, Prognostic value, Chemotherapy, Targeted therapy
1. Introduction
Colorectal cancer (CRC) remains one of the most common types of cancer and leading causes of cancer death worldwide. The American Cancer Society estimates that 1 596 670 new cases of colon cancer (822 300 in men and 774 370 in women) were diagnosed in 2011 (Siegel et al., 2011). Approximately 20% of CRC patients have already present with metastasis disease at the time of their diagnosis, and surgery cannot always extirpate the recurrence of advanced CRC. This has prompted researchers to establish active drug treatment strategies (Weitz et al., 2005). The recent introduction of cytotoxic drugs such as oxaliplatin or irinotecan in addition to fluorouracil and the development of targeted agents have caused significant progress in the treatment of advanced CRC. Standard treatment has evolved from 5-fluorouracil (5-FU), with a median overall survival (OS) of 10–12 months and an overall response rate of 10%, to combinations of oxaliplatin and irinotecan that have dramatically improved survival to 14–16 months (Best et al., 2000; Colucci et al., 2005). With the introduction of targeted therapy, the OS of metastasized CRC (mCRC) patients has been further prolonged (Köhne and Lenz, 2009). No benefit of targeted therapy has been observed in adjuvant therapy with advanced CRC (Chibaudel et al., 2010). Although we have made steady progress in chemotherapy and CRC-targeted therapy, there is sufficient evidence to suggest that the majority of patients undergoing drug therapy will not benefit but will rather experience severe, debilitating, even lethal adverse drug events (Gill et al., 2004). Recent studies have suggested an association between genetic variants and efficacy or adverse events of drug therapy (Watson and McLeod, 2011). The identification of suitable biomarkers would allow clinicians to deliver the most appropriate drugs to targeted patients and spare them ineffective and expensive treatment.
Biomarkers may have prognostic value (that is, the ability to estimate a patient’s outcome regardless of treatment) or predictive value (that is, the ability to estimate the efficacy and toxicity of a given treatment). Despite a very large number of publications dealing with prognostic and predictive biomarkers in CRC, only a few molecular biomarkers have been implemented in clinical practice (Table 1). Here we want to review recent biomarker data related to CRC, which may have been ready for clinical use.
Table 1.
Summary of potential molecular biomarkers for colorectal cancer
| Biomarker | Predictive and prognostic values |
| Biomarkers now incorporated into clinical practice | Predictive value*: KRAS mutation; UGT1A1*28 polymorphism |
| Biomarkers very likely for clinical practice | Prognostic value*: MSI (dMMR); prognostic recurrence score |
| Predictive value#: mutation of BRAF and PIK3CA; mutation or lack of expression of PTEN | |
| Biomarkers that may have clinical use | Prognostic value#: BRAF mutation |
| Predictive value#: polymorphism of TS, TP, DPD, MTHFR, UGT1A1*6, GSTP1, ERCC1, ERCC2 and XRCC1; EGFR copy number; VGPs |
Based on data from studies that prospectively defined biomarker analysis and included a large number of patients
Based on data from retrospective studies needed to be validated in large patient datasets with prospective study design
2. Prognostic value
Chemotherapy is now the standard treatment for post-surgical patients with stage III colon cancer. However, there is an ongoing controversy as to whether adjuvant chemotherapy should be advised for patients with stage II colon cancer. The quick and simple and reliable (QUASAR) study showed that adjuvant chemotherapy with FU plus leucovorin (LV) produces a small (approximately 3.6%) survival benefit in stage II colon cancer, which must be balanced against its toxicity (Gray et al., 2007). Many attempts have been made to identify the subset of patients at higher risk of relapse in stage II CRC, which would facilitate better selection of high-risk patients and patients who would benefit the most from adjuvant therapy. Currently, anatomical and pathologic staging, such as pathologic stage T4, the presence of lymphatic or vascular invasion, and grade are still the most accurate predictors of patient outcome. The problem of this approach is that the studies linking these variables to outcomes are retrospective and sometimes conflicting. They do not adequately assess the risk of recurrence in individual patients. We believe that recent biomarker data shifts the paradigm for management of stage II colon cancer and should have an influence on clinical decision-making.
2.1. Molecular markers
Most early studies focused on single molecular markers using hypothesis-driven research with limited success in terms of prognostic information. For example, TP53 mutations are found in up to 70% of sporadic CRCs. In these cases, inactivating mutations (29% of all CRCs) are correlated with advanced stage and vascular and lymphatic involvement. Diep et al. (2003) showed that TP53 mutations affecting the L3 zinc-binding domain and lower survival rate in the subclassification of Dukes’ B and C patients and may have an impact on the ideal treatment strategy. However, the prognostic role of TP53-inactivating mutations is still in question (Iacopetta et al., 2006). Similarly, the impacts of epidermal growth factor receptor (EGFR) over-expression, loss of heterozygosity in chromosome 18q, somatic adenomatous polyposis coli (APC), and KRAS mutations on survival remain unclear (Diep et al., 2003; Spano et al., 2005; Walther et al., 2009).
The presence of defective DNA mismatch repair (dMMR, that is, loss of expression of hMSH2, hMLH1, hPMS1, hPMS2, hMSH6, or hMLH3 gene), as assessed by the presence of tumor microsatellite instability (MSI), continues to be one of the most promising molecular markers of colon cancer. Three distinct MSI phenotypes have been described: MSS (none of the examined loci demonstrate instability), MSI-L (MSI at <30% of loci examined), and MSI-H (MSI at ≥30% of loci examined). Within sporadic CRC, the majority of MSI-H cases are due to inactivation of hMLH1 (~95%), with hMSH2 and hMSH6 accounting for a smaller percentage, ~5% and <1%, respectively (Boland et al., 1998). An association between MSI-H and favorable prognosis has been detected in several randomized clinical trials, and confirmed in a meta-analysis comprising 7 642 patients, 1 277 of whom had MSI-H tumors (Popat et al., 2005). Furthermore, MSI status is also a predictor for 5-FU-based adjuvant chemotherapy. Ribic et al. (2003) suggested that only patients with MSS or MSI-L could derive a benefit from 5-FU-based adjuvant chemotherapy. Sinicrope et al. (2011) suggested that MMR deficiency may identify a small percentage (approximately 15%) of patients with stage II disease who receive little benefit from FU/LV. Thus, histopathologically stage II patients with T3 disease and no signs of metastatic disease should be considered for dMMR testing in order to select patients who should receive 5-FU-based adjuvant chemotherapy and exclude those who should not.
BRAF mutations in CRC have been reported to occur more frequently in cases characterized by the presence of dMMR and to confer a poor prognosis (Rajagopalan et al., 2002; Roth et al., 2010). French et al. (2008) examined the prognostic significance of MMR deficiency and the presence of a specific mutation in BRAF (V600E) in a group of patients (n=533), who were then grouped in four categories for further analysis: dMMR/BRAF (−), dMMR/BRAF (+), pMMR/BRAF (−), and pMMR/BRAF (+). The dMMR/BRAF (−) group had a significantly improved OS rate compared to all others (5-year OS of 100% vs. 73%; P=0.002).
2.2. Gene expression signature
The development of high-throughput technologies such as microarrays and next-generation sequencing has facilitated systematic study of biomarkers through unbiased interrogation of the whole genome, transcriptome, and proteome. This allows the development of multigene algorithms for estimating risk of recurrence. Gene expression signatures (mammaPrint® and Oncotype Dx®) in breast cancer are the typical models. Oncotype DX® is a 21-gene commercialized signature that predicts prognosis (such as OS and relapse-free survival) in women with node-negative, estrogen receptor (ER)-positive tumors who have been treated with tamoxifen. Several molecular signatures have been developed to identify patients that could be spared from adjuvant chemotherapy in stage II CRC. On the basis of gene-expression profiling, Wang et al. (2004) generated a 23-gene signature that identified patients with Dukes’ B type tumors likely to develop recurrent disease. When this signature was validated on an independent data set, however, the accuracy was only 67%. Several other signatures have been proposed, including a 43-gene signature and a 50-gene signature, but their applicability to clinical decision-making is limited owing to a lack of large-scale validation (Eschrich et al., 2005; Garman et al., 2008; Jorissen et al., 2009).
O′Connell et al. (2010) studied the relationship between quantitative tumor gene expression and risk of cancer recurrence in patients with stage II or III colon cancer treated with surgery alone or surgery plus FU and LV. They aimed to develop multigene algorithms to quantify the risk of recurrence as well as the likelihood of differential treatment benefit of FU/LV adjuvant chemotherapy for individual patients. During initial development, the assay analyzed 761 candidate genes in the context of stage, grade, nodes examined, and MSI status, and yielded an 18-gene panel that included seven genes related to relapse-free survival in colon cancer, yielding a prognostic recurrence score (RS), six genes related to response to 5-FU/LV chemotherapy, yielding a predictive treatment score (TS), and five reference genes. The clinical utility of these algorithms has been evaluated in the QUASAR study (Gray et al., 2011). It has showed that RS, T stage, and mismatch repair (MMR) deficiency are key independent predictors of recurrence in stage II colon cancer. It was believed that the RS would have the greatest clinical utility when used in conjunction with T stage and MMR status. However, to date there has not been enough evidence for a full evaluation of this assay. Although Genomic Health launched the worldwide Oncotype Dx® colon cancer assay in January 2010, which includes seven genes related to relapse-free survival and five reference genes to calculate the prognostic RS, further validation in adequately powered prospective trials is necessary before the value of this assay for clinical practice can be determined.
3. Predictive value
Variability in drug response and toxicity is one of the largest challenges for chemotherapy and targeted therapy for CRC. Genetics is believed to account for between 20% and 95% of the variability in drug disposition and effects (Kalow et al., 1998). With the development of pharmacogenomics, inter-individual differences in drug response have been linked to variations in gene sequence and differences in expression of drug-metabolizing enzymes, drug transporters, and drug targets.
3.1. Biomarkers for chemotherapy
3.1.1. Molecular markers of fluoropyrimidine
Fluoropyrimidine therapy is the foundation of most first-line CRC treatments. Doctors and patients should choose between intravenous fluoropyrimidine 5-FU and the orally available prodrug, capecitabine. The molecular targets and drug-metabolizing enzymes of fluoropyrimidine can be used as biomarkers for drug response.
Thymidylate synthase (ThS) is a critical enzyme for DNA synthesis, so it is an important molecular target for many chemotherapy agents including 5-FU which mediates its cytotoxicity through inhibition of ThS. Numerous studies have shown that over-expression of ThS is linked to resistance to 5-FU. The causes of high ThS levels vary, including copy number and the number of the so-called thymidylate synthase promoter enhancer region (TSER), a tandem repeat polymorphism present in the 5′-promoter enhancer region. Kawakami et al. (1999) showed that three copies of the tandem repeat (TSER*3) in the promoter region of the ThS enhancer region give greater ThS expression than two copies (TSER*2). CRC patients who have TSER*2/TSER*2 or TSER*2/TSER*3 genotypes have improved response to 5-FU and survival over those TSER*3-homozygous patients (Marsh and McLeod, 2001; Villafranca et al., 2001). In addition, many copies of the ThS gene result in over-expression of ThS, which has been implicated in poor 5-FU response and survival in mCRC patients (Wang et al., 2004).
Dihydropyrimidine dehydrogenase (DPD) mediates the initial and rate-limiting steps of 5-FU catabolism. More than 80% of 5-FU is catabolized by DPD. Studies have indicated that patients with DPD deficiency often experience severe 5-FU toxicity, including death in some cases (Milano et al., 1999; Coursier et al., 2010). Studies on the mechanistic link between DPD deficiency and 5-FU toxicity have identified more than 30 polymorphisms in DPYD, the gene encoding DPD (Raida et al., 2001; Mattison et al., 2002; Seck et al., 2005). Most of these polymorphisms have no functional effect on DPD activity except for DPYD*2A allele, a G>A splice site transition that results in the skipping of exon 14. Wei et al. (1996) found that patients heterozygous or homozygous for this polymorphism have low DPD activity and increased toxicity to 5-FU. van Kuilenburg et al. (2002) found that 40% to 50% of patients with low DPD activity are heterozygous or homozygous for the DPYD*2A variant compared with only 4% of patients with normal DPD activity. Thus, the DPYD*2A variant seems to be predictive for DPD deficiency and increased risk of 5-FU toxicity.
Thymidine phosphorylase (TP) encodes a protein that catalyzes the reversible phosphorolysis of thymidine, deoxyuridine, and their analogs to their respective bases and 2-deoxyribose-1-phosphate, which is then dephosphorylated to 2-deoxy-D-ribose. TP expression was found to be elevated from 10- to 260-fold in nearly all biopsies examined from carcinomas relative to non-neoplastic regions (Hotta et al., 2004). Prior studies of the effects of TP expression on FU response have not been able to conclusively determine whether TP expression is a predictive factor for FU response, but recent studies have shown that TP expression is associated with response to capecitabine in many cancers (Andreetta et al., 2009; Petrioli et al., 2010; Gao et al., 2011). Capecitabine is an orally-administered chemotherapeutic agent designed to generate 5-FU preferentially in tumors, with similar response, progression-free survival (PFS) and OS rates to 5-FU in CRC patients (Cassidy et al., 2004). It is a prodrug that is converted to 5-FU in the tumors through a pathway with three enzymatic steps and two intermediary metabolites. At the last enzymatic step, the metabolite 5′-deoxy-5-fluorouridine (5′-DFUR) is converted to 5-FU by TP. The over-expression of TP in tumor tissues can increase the concentration of 5-FU, so patients with high levels of intratumoral TP expression are the ideal candidates for capecitabine-based chemotherapy.
Methylenetetrahydrofolatereductase (MTHFR) is a key regulatory enzyme in the metabolism of folate and 5-FU cytotoxicity. Two common single nucleotide polymorphisms (SNPs) cause reduced enzyme activity in homozygous individuals: MTHFR 677C>T polymorphism induces an Ala-to-Val substitution in the catalytic domain (70% reduction in activity), whereas the MTHFR 1 298A>C polymorphism induces a Glu-to-Ala substitution in a regulatory domain (30%–40% reduction). Compound heterozygous individuals have a 40%–50% reduction in enzyme activity (Weisberg et al., 1998). Various studies have been carried out to test the association between MTHFR polymorphisms and 5-FU treatment effect (Cohen et al., 2003; Jakobsen et al., 2005; Zhang et al., 2007; Afzal et al., 2009). The results of these studies are not completely consistent. Afzal et al. (2009) showed that MTHFR polymorphisms likely do not predict efficacy of adjuvant 5-FU treatment in CRC after complete resection. However, 677C>T polymorphism may be associated with lower toxicity. All these results indicate that MTHFR polymorphisms are inadequate in predicting outcome in adjuvant 5-FU treatment.
3.1.2. Molecular markers of irinotecan
Irinotecan has been recognized as one of the pivotal agents of randomized trials against CRC. Increases in median survival time by irinotecan in combination with folicacid and FU (FOLFIRI regimen) have led to its approval for use as first-line treatments for metastatic CRC (Fuchs et al., 2007). However, principal-dose limiting toxicities of irinotecan including severe delayed diarrhea and leukoneutropenia often preclude continued treatment.
Similar to the way that genetic variation in 5-FU catabolism has been linked to 5-FU toxicity, strong genetic variations in irinotecan’s metabolism may play a role in irinotecan’s toxicity. Irinotecan is a prodrug and is hydrolyzed into SN-38, which is further metabolized by uridine diphosphate glucuronosyl transferases (UGTs) into an inactive product, SN-38G. In the UGT superfamily, UGT1A1 is thought to be the most predominant catalyst. More than 50 genetic variations of UGT1A1 have been identified, among which UGT1A1*28, associated with a significant increase risk of neutropenia, now have been recommended to be used to guide treatment decisions (Hoskins et al., 2007; Palomaki et al., 2009). This genotype is caused by variations in the “TATAA” element of the 5′-promoter region. Wild-type UGT1A1 contains six TA repeats (A(TA)6TAA), whereas UGT1A1*28 genes contain seven TA repeats. Innocenti et al. (2004) found that 50% of patients who had the TA indel 7/7 genotype had grade four neutropenia, compared with 12.5% of patients with the TA 6/7 genotype and 0% of patients homozygous for the TA 6/6 genotype (P<0.001). Although the link between UGT1A1*28 and diarrhea seems less evident, the United States Food and Drug Administration (USFDA) recommends that UGT1A1*28-homozygous patients receive a lower starting dose of irinotecan because they are more likely to experience neutropenia than patients who have one or two copies of the wild-type allele. The allele frequency of UGT1A1*28 varies across individuals from various geographic areas. It is highest among African Americans (0.38–0.45), followed by Caucasians (0.29–0.39) and Asians (0.02–0.14) (Beutler et al., 1998; Sugatani et al., 2002; Kaniwa et al., 2005). Even among Asians, the occurrence of the UGT1A1*28 homozygote varies across the continent at frequencies ranging from <5% in the Southeast Asia to around 20% in the Indian subcontinent (Kaniwa et al., 2005).
UGT1A1*6 is a single-nucleotide substitution of G by A at base position 211, resulting in the replacement of glycine with arginine (211G>A (G71R)), which causes decreased glucuronidation. It occurs most frequently in Asians, especially in the Chinese and Japanese populations, but is absent or very rare in Caucasians and African Americans. As such, it has been suggested necessary to analyze UGT1A1*6 in addition to UGT1A1*28 to predict irinotecan-related toxicity in Asian patients (Jada et al., 2007). A Japanese prospective study examined the distributions of UGT1A1*28 and UGT1A1*6 (n=300) and showed that the allele (UGT1A1*28 and/or UGT1A1*6), which exists in 10% of Japanese patients, might lead to an increased risk of irinotecan-related neutropenia (Akiyama et al., 2008).
Many other enzymes and transporters are also involved in irinotecan metabolism. These include the organic anion transporting polypeptide C (OATP1B1), the cytochrome P450 3A system, and the adenosine-triphosphate-binding cassette transporter system (Innocenti et al., 2009). Continued efforts toward discovering irinotecan metabolism genotype sets and haplotypes must be made before personalized dosing of irinotecan becomes common practice.
3.1.3. Molecular markers of oxaliplatin
Oxaliplatin is one of the most widely used agents in adjuvant and palliative chemotherapy in patients with CRC. However, oxaliplatin-induced chronic neuropathy and intrinsic and acquired resistance continue to be problematic. SNPs involved in drug metabolism and DNA repair systems, including nucleotide excision repair (NER) and base excision repair (BER), have been investigated for associations with clinical outcomes and oxaliplatin toxicity (Moreno et al., 2006; Yin et al., 2011).
Glutathione S-transferase P1 (GSTP1) is an important host-defense molecule against a range of toxins including oxaliplatin, which participates directly in the detoxification of platinum compounds. An A-to-G polymorphism at codon 105 of the GSTP1 gene alters the specificity and activity of the GSTP1 enzyme by changing isoleucine (Ile) to valine (Val). This influences the geometry of the hydrophobic binding site of the enzyme. The relationship between GSTP1 Ile105Val polymorphisms and survival or therapeutic response in CRC patients remains controversial. However, most studies have shown consistent results, indicating that GSTP1 Ile105Val polymorphism is associated with the risk of oxaliplatin-induced neuropathy, which is cumulative and dose-limiting (Lecomte et al., 2006; Mcleod et al., 2010). Inada et al. (2010) first analyzed the association of the excision repair cross-complementing group 1 (ERCC1) and GSTP1 polymorphisms against the time elapsed before onset of neuropathy and found that GSTP1 Ile105Val and ERCC1 C118T polymorphisms were more strongly related to the time until onset of neuropathy than to the grade of toxicity in CRC patients who received oxaliplatin-based chemotherapy.
NER pathways, which include ERCC1 and ERCC2 or XPD, play a decisive role in platinum-based chemotherapeutic efficacy by repairing drug-produced DNA damage (Weaver et al., 2005). Because previous published reports of an association between NER SNPs and clinical outcomes of platinum-based chemotherapy from individual studies were not consistent, Yin et al. (2011) carried out a meta-analysis of gastric cancer and CRC for the commonly studied polymorphisms ERCC1 C118T and ERCC2 T751G. They found consistent and clinically substantial risks to be associated with TR, PFS, and OS in oxaliplatin-treated gastric cancer and CRC patients, though exact levels of risk varied along ethnic lines. For ERCC1 C118T, the T allele was found to be associated with reduced response and poor PFS and OS in Asians. For ERCC2 T751G, the G allele was associated with reduced response and poor PFS and OS in Caucasians. Results showed that ERCC1 C118T and ERCC2 T751G polymorphisms are useful predictive factors in the oxaliplatin-based treatment of CRC.
Another noteworthy pathway involves the X-ray repair cross-complementing group 1 (XRCC1), which is a rate-limiting member of the BER family. It interacts with other proteins involved in several stages of BER pathways, including repairing specific base damage caused by oxaliplatin (Moreno et al., 2006). Suh et al. (2006) showed that XRCC1 Arg399Gln was a significant predictive factor for the response to FOLFOX chemotherapy and short-term survival in Asian mCRC patients.
However, this predictive value of XRCC1 Arg399Gln (1 196G>A) was inconsistent with the results from a multiple genetic polymorphism study of biomarkers to predict response to a fluorouracil-oxaliplatin regimen in patients with metastatic CRC (Lamas et al., 2011). It is a frequent phenomenon in biomarker research. For example, the use of a comprehensive panel of biomarkers to predict response to the combination chemotherapy regimens, the biomarkers with potential predictive values for 5-FU, such as ThS, DPD, and MTHFR have also failed to show clinical association with OS and/or PFS in CRC patients treated with first-line FOLFOX-4 regimens (Huang et al., 2011). This research showed that genetic polymorphisms of ERCC1 and XRCC1 might be useful in predicting clinical outcomes in Taiwanese mCRC patients treated with FOLFOX-4.
3.2. Biomarkers for targeted therapy
3.2.1. Molecular markers of EGFR inhibitors
The EGFR is a tyrosine kinase receptor from the ErbB family. It is abnormally activated in epithelial tumors, including 25%–80% of CRCs (Mayer et al., 1993). Monoclonal antibodies specifically inhibiting EGFR, such as cetuximab and panitumumab, are now an integral part of CRC therapy. Clinical evidence has shown a correlation between the severity of rash, a common side effect of these agents, and response to the therapy (Segaert et al., 2009). This information, however, cannot help clinicians optimize their selection of patients. The EGFR-I’s small responsive patient population and high costs have pushed clinicians to investigate other predictive biomarkers.
It is now clear that tumor growth can be driven by constitutive activation of signaling pathways downstream of the EGFR, such as the RAS-MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Scaltriti and Baselga, 2006). Oncogenic activation of components in these pathways can bypass the EGFR-driven signaling cascade and impair the clinical efficacy of anti-EGFR monoclonal antibodies. Such activation can occur via mutations in oncogenes such as KRAS and BRAF on one side of the EGFR-mediated pathway and by PIK3CA mutations and loss of tumor suppressor genes, such as PTEN on the opposite side of the cascade. Lièvre et al. (2006) first reported a link between KRAS mutations and a lack of response to EGFR-targeted monoclonal antibodies. Data from a number of large randomized phase II and III trials have provided convincing evidence that activated mutations of KRAS, which account for 35%–55% of sporadic CRC, predict lack of response to anti-EGFR monoclonal antibody treatment, regardless of first-line FOLFOX (folinic acid, fluorouracil, and oxaliplatin), FOLFIRI (folinic acid, fluorouracil, and irinotecan), or EGFR-I monotherapy (Karapetis et al., 2008; Bokemeyer et al., 2009). The osteoporosis and ultrasound study (OPUS) indicated that the addition of EGFR-targeted treatment to chemotherapy might even be detrimental to patients with KRAS mutations (Bokemeyer et al., 2009). More recently, it has been suggested that not all KRAS mutations have equal effects on EGFR therapy. Patients with a G13D mutation showed superior survival over patients with other KRAS mutations (de Roock et al., 2010a). Despite this predictive impact, KRAS mutations do not appear to confer any prognostic value in CRC patients (Walther et al., 2009). KRAS testing is now being integrated into clinical practice. The identification of wild-type KRAS as a selection biomarker for panitumumab and cetuximab therapy represents an important step toward fulfilling the promise of individualized treatment for metastatic CRC.
More than 50% of patients bearing wild-type (WT) KRAS expressed resistance to panitumumab and cetuximab (Khambata-Ford et al., 2007). Because of the complexity of the EGFR signaling system, no one biomarker can be identified to reveal which patients will benefit from anti-EGFR therapy in practice. It is likely that predictive algorithms that incorporate several molecular biomarkers will be developed for metastatic CRC. For instance, combining analysis of KRAS status with BRAF and PIK3CA status and PTEN expression may identify about 70% of CRC patients who are unlikely to respond to EGFR-I (Siena et al., 2009). de Roock et al. (2010b) showed that response rates improve from 36.3% in KRAS-WT to 41.2% in the KRAS/BRAF/NRAS/PIK3CA exon20 WT subpopulation. However, these additional biomarkers require further validation before incorporation into clinical practice.
The initial assumption that EGFR over-expression, as assessed by immunohistochemistry, would predict response to anti-EGFR therapies has not been supported by studies (Walther et al., 2009). There is evidence that tumors with an increased EGFR gene copies, as assessed by fluorescence in situ hybridization (FISH) or chromogenic in situ hybridization (CISH), may predict increased likelihood of response (Sartore-Bianchi et al., 2007). These data suggest that patients with fewer than three EGFR gene copies per nucleus are less likely to respond to EGFR-I. Further research on larger groups of patients is required to validate this biomarker (Moroni et al., 2008).
3.2.2. Molecular markers of VEGF inhibitor
Vascular endothelial growth factor (VEGF) plays a pivotal role in cancer neoangiogenesis and its role in cancer including CRC growth and development has been extensively documented (Stockmann et al., 2008). Several anti-VEGF therapies, such as bevacizumab, sunitinib, and sorafenib have been developed. MoAb bevacizumab is currently the only antiangiogenic agent licensed by the USFDA for use in mCRC. Hurwitz et al. (2004) demonstrated that bevacizumab significantly improved OS in mCRC patients, with median OS (mOS) of 20.3 months in patients receiving irinotecan, bolus fluorouracil, and leucovorin (IFL) plus bevacizumab, relative to 15.6 months in patients who received IFL alone. They also retrospectively analyzed plasma and tumor VEGF levels and KRAS mutational status but found no association between these biomakers and survival benefits from bevacizumab (Hurwitz et al., 2009).
Scartozzi et al. (2009) suggested a possible correlation between bevacizumab-induced hypertension and clinical outcomes in CRC patients treated with first-line bevacizumab. Unlike other targeted therapies, however, no molecular biomarkers clearly predict bevacizumab efficacy. Recent studies have investigated the influence of VEGF gene polymorphisms (VGPs) on the response to bevacizumab in breast and ovarian cancer (Schneider et al., 2008; Schultheis et al., 2008). Formica et al. (2011) first reported a predictive value for PFS of VGPs located in the promoter/5′ UTR in bevacizumab-treated mCRC patients. They investigated eight VGPs showing the highest polymorphic rates and found a statistically significant association between germline −2 578, −1 512, −1 451, −1 411, −460, −152, and −1 154 VGPs and PFS. They also found a significant association between germline VGP −634 and objective response rate (ORR), but not between VGP −634 and PFS. The study was, however, retrospective with a small sample size (n=40). Prospective trials with larger sample sizes assessing chemotherapy+bevacizumab versus chemotherapy alone are required to definitively determine how predictive VGPs are in mCRC.
4. Future perspectives
The identification of biomarkers that would help clinicians practice personalized medicine in CRC is a more complex process than was previously believed. Biomarkers have been the subject of many published papers, but other than KRAS mutation and UGT1A1*28 polymorphism, no widely accepted molecular markers of prognosis and prediction have found their way into clinical decision-making (Table 1).
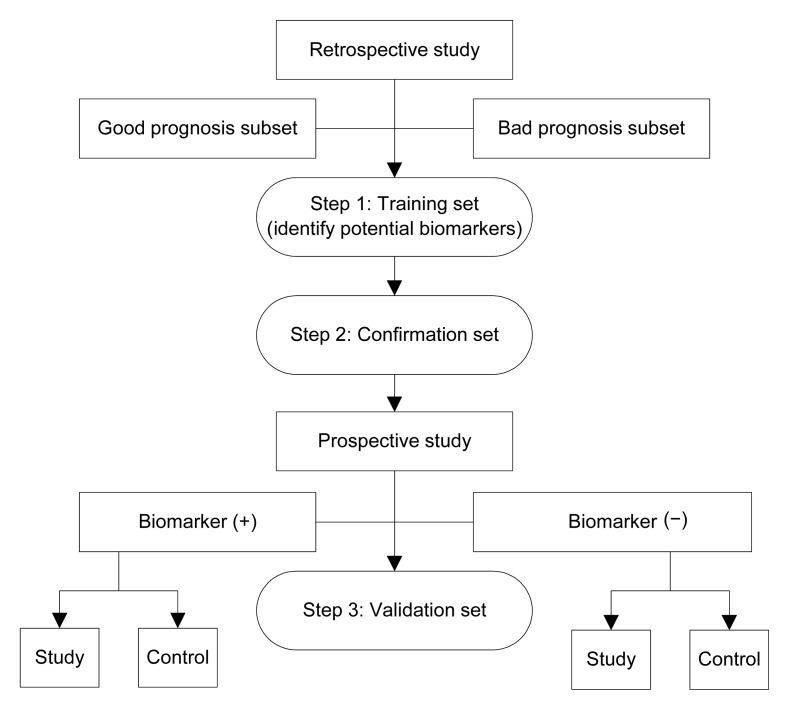
One reason may be due to the complex process of biomarker research, which often involves three steps (Fig. 1). First, the potential biomarkers must be identified through a retrospective study (the “training set”). Subsequently, the potential biomarkers must be confirmed in another population (the “confirmation set”). Finally, the biomarkers must be validated through prospective, randomized controlled trials (the “validation set”). The majority of translational studies of biomarkers are small, retrospective studies with training and confirmation sets, with poorly defined protocols for sample collection and insufficient statistical power. These studies have a high risk of bias, resulting in unreliable conclusions. Few biomarker studies have gone through validation sets. Randomized controlled trials are needed, in which specimens are collected, processed, and archived using generic standard operating procedures (SOPs).
Fig. 1.
Process of biomarker research
With the advent of combination regimens, the process of elucidating net outcomes of various biomarker polymorphisms that alter the efficacy and toxicity of combination regimens has become more complex. Studies aimed at examining the feasibility of multigene predictors of drug response combination regimens such as FOLFOX, FOLFIRI are still rare.
There is no standard method of evaluating corresponding biomarkers. For example, studies have shown that tumors with increased EGFR gene copies, as assessed by FISH or CISH, may predict increased response to EGFR-I in mCRC patients. However, when EGFR gene copies were evaluated by polymerase chain reaction, no association was found between this parameter and the clinical outcomes of panitumumab- or cetuximab-based treatment (Moroni et al., 2005; de Roock et al., 2008). This may be because of tumor DNA dilution by DNA from normal cells during DNA extraction. These results limit the predictive value in the number of EGFR gene copies.
In conclusion, the quest for prognostic and predictive biomarkers has allowed more rational use of medication, which should provide benefits to patients and health-care providers alike by sparing patients unnecessary treatment and allowing better use of health care resources. Prospective biomarker-driven studies are now under way, moving beyond single-gene polymorphisms to a more comprehensive evaluation of pathways to identify genomic variants and patterns via high-throughput molecular technology. The community, however, is still a long way from true personalized medicine.
References
- 1.Afzal S, Jensen SA, Vainer B, Vogel U, Matsen JP, Sørensen JB, Andersen PK, Poulsen HE. MTHFR polymorphisms and 5-FU-based adjuvant chemotherapy in colorectal cancer. Ann Oncol. 2009;20(10):1660–1666. doi: 10.1093/annonc/mdp046. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama Y, Fujita K, Nagashima F, Yamamoto W, Endo H, Sunakawa Y, Yamashita K, Ishida H, Mizuno K, Araki K, et al. Genetic testing for UGT1A1*28 and *6 in Japanese patients who receive irinotecan chemotherapy. Ann Oncol. 2008;19(12):2089–2090. doi: 10.1093/annonc/mdn645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreetta C, Puppin C, Minisini A, Valent F, Pegolo E, Damante G, di Loreto C, Pizzolitto S, Pandolfi M, Fasola G, et al. Thymidine phosphorylase expression and benefit from capecitabine in patients with advanced breast cancer. Ann Oncol. 2009;20(2):265–271. doi: 10.1093/annonc/mdn592. [DOI] [PubMed] [Google Scholar]
- 4.Best L, Simmonds P, Baughan C, Buchanan R, Davis C, Fentiman I, George S, Gosney M, Northover J, Williams C. Palliative chemotherapy for advanced or metastatic colorectal cancer. Cochrane Database Syst Rev. 2000;(2):CD001545. doi: 10.1002/14651858.CD001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? PNAS. 1998;95(14):8170–8174. doi: 10.1073/pnas.95.14.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 7.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, et al. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. [PubMed] [Google Scholar]
- 8.Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22(11):2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 9.Chibaudel B, Tournigand C, André T, Larsen AK, de Gramont A. Targeted therapies as adjuvant treatment for early-stage colorectal cancer: first impressions and clinical questions. Clin Colorectal Cancer. 2010;9(5):269–273. doi: 10.3816/CCC.2010.n.039. [DOI] [PubMed] [Google Scholar]
- 10.Cohen V, Panet-Raymond V, Sabbaghian N, Morin I, Batist G, Rozen R. Methylenetetrahydrofolate reductase polymorphism in advanced colorectal cancer: a novel genomic predictor of clinical response to fluoropyrimidine-based chemotherapy. Clin Cancer Res. 2003;9(5):1611–1615. [PubMed] [Google Scholar]
- 11.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell′Italia Meridionale. J Clin Oncol. 2005;23(22):4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 12.Coursier S, Martelet S, Guillermet A, Emptoz J, Villier C, Bontemps H. Severe toxicity following capecitabine administration because of dihydropyrimidine deshydrogenase (DPD) deficiency. Gastroenterol Clin Biol. 2010;34(3):218–223. doi: 10.1016/j.gcb.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 13.de Roock W, Piessevaux H, de Schutter J, Janssens M, de Hertogh G, Personeni N, Biesmans B, van Laethem JL, Peeters M, Humblet Y, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19(3):508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 14.de Roock W, Jonker DJ, di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304(16):1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 15.de Roock W, Claes B, Bernasconi D, de Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, et al. Effects of KRAS, BRAF, NRAS, and PIK3A, mutations on the efficacy of cetuximab plus chemotherapy in chemotehrapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 16.Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO, Lothe RA. Genetic tumor markers with prognostic impact in Dukes’ stages B and C colorectal cancer patients. J Clin Oncol. 2003;21(5):820–829. doi: 10.1200/JCO.2003.05.190. [DOI] [PubMed] [Google Scholar]
- 17.Eschrich S, Yang I, Bloom G, Kwong KY, Boulware D, Cantor A, Coppola D, Kruhøffer M, Aaltonen L, Orntoft TF, et al. Molecular staging for survival prediction of colorectal cancer patients. J Clin Oncol. 2005;23(15):3526–3535. doi: 10.1200/JCO.2005.00.695. [DOI] [PubMed] [Google Scholar]
- 18.Formica V, Palmirotta R, del Monte G, Savonarola A, Ludovici G, de Marchis ML, Grenga I, Schirru M, Guadagni F, Roselli M. Predictive value of VEGF gene polymorphisms for metastatic colorectal cancer patients receiving first-line treatment including fluorouracil, irinotecan, and bevacizumab. Int J Colorectal Dis. 2011;26(2):143–151. doi: 10.1007/s00384-010-1108-1. [DOI] [PubMed] [Google Scholar]
- 19.French AJ, Sargent DJ, Burgart LJ, Foster NR, Kabat BF, Goldberg R, Shepherd L, Windschitl HE, Thibodeau SN. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res. 2008;14(11):3408–3415. doi: 10.1158/1078-0432.CCR-07-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol. 2007;25(30):4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Lu M, Yu JW, Li YY, Shen L. Thymidine Phosphorylase/β-tubulin III expressions predict the response in Chinese advanced gastric cancer patients receiving first-line capecitabine plus paclitaxel. BMC Cancer. 2011;11:177. doi: 10.1186/1471-2407-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garman KS, Acharya CR, Edelman E, Grade M, Gaedcke J, Sud S, Barry W, Diehl AM, Provenzale D, Ginsburg GS, et al. A genomic approach to colon cancer risk stratification yields biologic insights into therapeutic opportunities. PNAS. 2008;105(49):19432–19437. doi: 10.1073/pnas.0806674105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois SJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22(10):1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 24.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 25.Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, Beaumont C, Clark-Langone KM, Yoshizawa CN, Lee M, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol. 2011;29(35):4611–4619. doi: 10.1200/JCO.2010.32.8732. [DOI] [PubMed] [Google Scholar]
- 26.Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst. 2007;99(17):1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 27.Hotta T, Taniguchi K, Kobayashi Y, Johata K, Sahara M, Naka T, Watanabe T, Ochiai M, Tanimura H, Tsubota YT. Increased expression of thymidine phosphorylase in tumor tissue in proportion to TP-expression in primary normal tissue. Oncol Rep. 2004;12(3):539–541. [PubMed] [Google Scholar]
- 28.Huang MY, Huang ML, Chen MJ, Lu CY, Chen CF, Tsai PC, Chuang SC, Hou MF, Lin SR, Wang JY. Multiple genetic polymorphisms in the prediction of clinical outcome of metastatic colorectal cancer patients treated with first-line FOLFOX-4 chemotherapy. Pharmacogenet Genomics. 2011;21(1):18–25. doi: 10.1097/FPC.0b013e3283415124. [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of KRAS mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14(1):22–28. doi: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]
- 31.Iacopetta B, Russo A, Bazan V, Dardanoni G, Gebbia N, Soussi T, Kerr D, Elsaleh H, Soong R, Kandioler D, et al. Functional categories of TP53 mutation in colorectal cancer: results of an International Collaborative Study. Ann Oncol. 2006;17(5):842–847. doi: 10.1093/annonc/mdl035. [DOI] [PubMed] [Google Scholar]
- 32.Inada M, Sato M, Morita S, Kitagawa K, Kawada K, Mitsuma A, Sawaki M, Fujita K, Ando Y. Associations between oxaliplatin-induced peripheral neuropathy and polymorphisms of the ERCC1 and GSTP1 genes. Int J Clin Pharmacol Ther. 2010;48(11):729–734. doi: 10.5414/CPP48729. [DOI] [PubMed] [Google Scholar]
- 33.Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramírez J, Rudin CM, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22(8):1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 34.Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramírez J, Relling M, Chen P, Das S, Rosner GL, Ratain MJ. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol. 2009;27(16):2604–2614. doi: 10.1200/JCO.2008.20.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jada SR, Lim R, Wong CI, Shu X, Lee SC, Zhou Q, Goh BC, Chowbay B. Role of UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan induced neutropenia in Asian cancer patients. Cancer Sci. 2007;98(9):1461–1467. doi: 10.1111/j.1349-7006.2007.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakobsen A, Nielsen JN, Gyldenkerne N, Lindeberg J. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphism in normal tissue as predictors of fluorouracil sensitivity. J Clin Oncol. 2005;23(7):1365–1369. doi: 10.1200/JCO.2005.06.219. [DOI] [PubMed] [Google Scholar]
- 37.Jorissen RN, Gibbs P, Christie M, Prakash S, Lipton L, Desai J, Kerr D, Aaltonen LA, Arango D, Kruhøffer M, et al. Metastasis-associated gene expression changes predict poor outcomes in patients with Dukes stage B and C colorectal cancer. Clin Cancer Res. 2009;15(24):7642–7651. doi: 10.1158/1078-0432.CCR-09-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalow W, Tang B, Endrenyi L. Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8(4):283–289. doi: 10.1097/00008571-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Kaniwa N, Kurose K, Jinno H, Tanaka-Kagawa T, Saito Y, Saeki M, Sawada J, Tohkin M, Hasegawa R. Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C>T (P229L) found in an African-American. Drug Metab Dispos. 2005;33(3):458–465. doi: 10.1124/dmd.104.001800. [DOI] [PubMed] [Google Scholar]
- 40.Karapetis CS, Khambata-Ford S, Jonker DJ, O′Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, et al. KRAS mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 41.Kawakami K, Omura K, Kanehira E, Watanabe Y. Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer Res. 1999;19(4B):3249–3252. [PubMed] [Google Scholar]
- 42.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, et al. Expression of epiregulin and amphiregulin and KRAS mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25(22):3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 43.Köhne CH, Lenz HJ. Chemotherapy with targeted agents for the treatment of metastatic colorectal cancer. Oncologist. 2009;14(5):478–488. doi: 10.1634/theoncologist.2008-0202. [DOI] [PubMed] [Google Scholar]
- 44.Lamas MJ, Duran G, Balboa E, Bernardez B, Touris M, Vidal Y, Gallardo E, Lopez R, Carracedo A, Barros F. Use of a comprehensive panel of biomarkers to predict response to a fluorouracil-oxaliplatin regimen in patients with metastatic colorectal cancer. Pharmacogenomics. 2011;12(3):433–442. doi: 10.2217/pgs.10.196. [DOI] [PubMed] [Google Scholar]
- 45.Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006;12(10):3050–3056. doi: 10.1158/1078-0432.CCR-05-2076. [DOI] [PubMed] [Google Scholar]
- 46.Lièvre A, Bachet JB, le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 47.Marsh S, McLeod H. Thymidylate synthase pharmacogenetics in colorectal cancer. Clin Colorectal Cancer. 2001;1(3):175–178. doi: 10.3816/CCC.2001.n.018. [DOI] [PubMed] [Google Scholar]
- 48.Mattison LK, Soong R, Diasio RB. Implications of dihydropyrimidine dehydrogenase on 5-fluorouracil pharmacogenetics and pharmacogenomics. Pharmacogenomics. 2002;3(4):485–492. doi: 10.1517/14622416.3.4.485. [DOI] [PubMed] [Google Scholar]
- 49.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71(8):2454–2460. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 50.Mcleod HL, Sargent DJ, Marsh S, Green EM, King CR, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Thibodeau SN, et al. Pharmacogenetic predictors of adverse events and response to chemotherapy in metastatic colorectal cancer: results from North American Gastrointestinal Intergroup Trial N9741. J Clin Oncol. 2010;28(20):3227–3233. doi: 10.1200/JCO.2009.21.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milano G, Etienne MC, Pierrefite V, Barberi-Heyob M, Deporte-Fety R, Renée N. Dihydropyrimidine dehydrogenase deficiency and fluorouracil-related toxicity. Br J Cancer. 1999;79:627–630. doi: 10.1038/sj.bjc.6690098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno V, Gemignani F, Landi S, Gioia-Patricola L, Chabrier A, Blanco I, González S, Guino E, Capellà G, Canzian F. Polymorphisms in genes of nucleotide and base excision repair: risk and prognosis of colorectal cancer. Clin Cancer Res. 2006;12(7Pt1):2101–2108. doi: 10.1158/1078-0432.CCR-05-1363. [DOI] [PubMed] [Google Scholar]
- 53.Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, di Nicolantonio F, Gambacorta M, Siena S, Bardelli A. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6(5):279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 54.Moroni M, Sartore-Bianchi A, Veronese S, Siena S. EGFR FISH in colorectal cancer: what is the current reality? Lancet Oncol. 2008;9(5):402–403. doi: 10.1016/S1470-2045(08)70109-8. [DOI] [PubMed] [Google Scholar]
- 55.O′Connell MJ, Lavery I, Yothers G, Paik S, Clark-Langone KM, Lopatin M, Watson D, Baehner FL, Shak S, Baker J, et al. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol. 2010;28:3937–3944. doi: 10.1200/JCO.2010.28.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palomaki GE, Bradley LA, Douglas MP, Kolor K, Dotson WD. Can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? An evidence-based review. Genet Med. 2009;11(1):21–34. doi: 10.1097/GIM.0b013e31818efd77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrioli R, Bargagli G, Lazzi S, Pascucci A, Francini E, Bellan C, Conca R, Martellucci I, Fiaschi AI, Lorenzi B, et al. Thymidine phosphorylase expression in metastatic sites is predictive for response in patients with colorectal cancer treated with continuous oral capecitabine and biweekly oxaliplatin. Anticancer Drugs. 2010;21(3):313–319. doi: 10.1097/CAD.0b013e328334d88a. [DOI] [PubMed] [Google Scholar]
- 58.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 59.Raida M, Schwabe W, Hausler P, van Kuilenburg AB, van Gennip AH, Behnke D, Höffken K. Prevalence of a common point mutation in the dihydropyrimidine dehydrogenase (DPD) gene within the 5′-splice donor site of intron 14 in patients with severe 5-fluorouracil (5-FU)-related toxicity compared with controls. Clin Cancer Res. 2001;7(9):2832–2839. [PubMed] [Google Scholar]
- 60.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418(6901):934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 61.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28(3):466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 63.Sartore-Bianchi A, Moroni M, Veronese S, Carnaghi C, Bajetta E, Luppi G, Sobrero A, Barone C, Cascinu S, Colucci G, et al. Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol. 2007;25(22):3238–3245. doi: 10.1200/JCO.2007.11.5956. [DOI] [PubMed] [Google Scholar]
- 64.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12(18):5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 65.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20(2):227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 66.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26(28):4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schultheis AM, Lurje G, Rhodes KE, Zhang W, Yang D, Garcia AA, Morgan R, Gandara D, Scudder S, Oza A, et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin Cancer Res. 2008;14(22):7554–7563. doi: 10.1158/1078-0432.CCR-08-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seck K, Riemer S, Kates R, Ullrich T, Lutz V, Harbeck N, Schmitt M, Kiechle M, Diasio R, Gross E. Analysis of the DPYD gene implicated in 5-fluorouracil catabolism in a cohort of Caucasian individuals. Clin Cancer Res. 2005;11(16):5886–5892. doi: 10.1158/1078-0432.CCR-04-1784. [DOI] [PubMed] [Google Scholar]
- 69.Segaert S, Chiritescu G, Lemmens L, Dumon K, van Cutsem E, Tejpar S. Skin toxicities of targeted therapies. Eur J Cancer. 2009;45(Suppl.1):295–308. doi: 10.1016/S0959-8049(09)70044-9. [DOI] [PubMed] [Google Scholar]
- 70.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 71.Siena S, Sartore-Bianchi A, di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101(19):1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, Yothers G, Allegra C, Moore MJ, Gallinger S, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103(11):863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spano JP, Fagard R, Soria JC, Rixe O, Khayat D, Milano G. Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol. 2005;16(2):189–194. doi: 10.1093/annonc/mdi057. [DOI] [PubMed] [Google Scholar]
- 74.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugatani J, Yamakawa K, Yoshinari K, Machida T, Takagi H, Mori M, Kakizaki S, Sueyoshi T, Negishi M, Miwa M. Identification of a defect in the UGT1A1 gene promoter and its association with hyperbilirubinemia. Biochem Biophys Res Commun. 2002;292(2):492–497. doi: 10.1006/bbrc.2002.6683. [DOI] [PubMed] [Google Scholar]
- 76.Suh KW, Kim JH, Kim DY, Kim YB, Lee C, Choi S. Which gene is a dominant predictor of response during FOLFOX chemotherapy for the treatment of metastatic colorectal cancer, the MTHFR or XRCC1 gene? Ann Surg Oncol. 2006;13(11):1379–1385. doi: 10.1245/s10434-006-9112-y. [DOI] [PubMed] [Google Scholar]
- 77.van Kuilenburg AB, Meinsma R, Zoetekouw L, van Gennip AH. Increased risk of grade IV neutropenia after administration of 5-fluorouracil due to a dihydropyrimidine dehydrogenase deficiency: high prevalence of the IVS14+1G>A mutation. Int J Cancer. 2002;101(3):253–258. doi: 10.1002/ijc.10599. [DOI] [PubMed] [Google Scholar]
- 78.Villafranca E, Okruzhnov Y, Dominguez MA, García-Foncillas J, Azinovic I, Martínez E, Illarramendi JJ, Arias F, Martínez Monge R, Salgado E, et al. Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J Clin Oncol. 2001;19(6):1779–1786. doi: 10.1200/JCO.2001.19.6.1779. [DOI] [PubMed] [Google Scholar]
- 79.Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9(7):489–499. doi: 10.1038/nrc2645. [DOI] [PubMed] [Google Scholar]
- 80.Wang TL, Diaz LA, Jr, Romans K, Bardelli A, Saha S, Galizia G, Choti M, Donehower R, Parmigiani G, Shih IM, et al. Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients. PNAS. 2004;101(9):3089–3094. doi: 10.1073/pnas.0308716101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y, Jatkoe T, Zhang Y, Mutch MG, Talantov D, Jiang J, McLeod HL, Atkins D. Gene expression profiles and molecular markers to predict recurrence of Dukes’ B colon cancer. J Clin Oncol. 2004;22(9):1564–1571. doi: 10.1200/JCO.2004.08.186. [DOI] [PubMed] [Google Scholar]
- 82.Watson RG, McLeod HL. Pharmacogenomic contribution to drug response. Cancer J. 2011;17(2):80–88. doi: 10.1097/PPO.0b013e3182147432. [DOI] [PubMed] [Google Scholar]
- 83.Weaver DA, Crawford EL, Warner KA, Elkhairi F, Khuder SA, Willey JC. ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer. 2005;4(1):18. doi: 10.1186/1476-4598-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei X, McLeod HL, McMurrough J, Gonzalez FJ, Fernandez-Salguero P. Molecular basis of the human dihydropyrimidine dehydrogenase deficiency and 5-fluorouracil toxicity. J Clin Invest. 1996;98(3):610–615. doi: 10.1172/JCI118830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64(3):169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 86.Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365(9454):153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 87.Yin M, Yan J, Martinez-Balibrea E, Graziano F, Lenz HJ, Kim HJ, Robert J, Im SA, Wang WS, Etienne-Grimaldi MC, et al. ERCC1 and ERCC2 polymorphisms predict clinical outcomes of oxaliplatin-based chemotherapies in gastric and colorectal cancer: a systemic review and meta-analysis. Clin Cancer Res. 2011;17(6):1632–1640. doi: 10.1158/1078-0432.CCR-10-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang W, Press OA, Haiman CA, Yang DY, Gordon MA, Fazzone W, El-Khoueiry A, Iqbal S, Sherrod AE, Lurje G, et al. Association of methylenetetrahydrofolate reductase gene polymorphisms and sex-specific survival in patients with metastatic colon cancer. J Clin Oncol. 2007;25(24):3726–3731. doi: 10.1200/JCO.2007.11.4710. [DOI] [PubMed] [Google Scholar]