Abstract
Atrial fibrillation (AF) has been considered as a growing epidemiological problem in the world, with a substantial impact on morbidity and mortality. Ambulatory electrocardiography (e.g., Holter) monitoring is commonly used for AF diagnosis and therapy and the automated detection of AF is of great significance due to the vast amount of information provided. This study presents a combined method to achieve high accuracy in AF detection. Firstly, we detected the suspected transitions between AF and sinus rhythm using the delta RR interval distribution difference curve, which were then classified by a combination analysis of P wave and RR interval. The MIT-BIH AF database was used for algorithm validation and a high sensitivity and a high specificity (98.2% and 97.5%, respectively) were achieved. Further, we developed a dataset of 24-h paroxysmal AF Holter recordings (n=45) to evaluate the performance in clinical practice, which yielded satisfactory accuracy (sensitivity=96.3%, specificity=96.8%).
Keywords: Atrial fibrillation, Delta RR interval distribution difference curve, Holter monitoring
1. Introduction
Atrial fibrillation (AF) is a growing epidemiological problem in the world, with a substantial impact on morbidity and mortality (Wolf et al., 1978; Chugh et al., 2001). However, a significant portion of patients with AF has no obvious clinical symptoms, which makes them at risk of other diseases (e.g., cryptogenic stroke) (Humphries et al., 2001; Page et al., 2003; Kaufman and Waldo, 2004). Thus, AF diagnosis, especially asymptomatic AF detection, is necessary in clinical practice.
Ambulatory electrocardiography (ECG) (e.g., Holter) monitoring is usually used to diagnose AF or evaluate AF therapy (Roche et al., 2002; Schaer et al., 2004). Since this technology provides a vast amount of valuable information, automated detection of AF is of great significance. Several algorithms have been developed to detect AF based on two ECG traces: (1) absence of P waves, and/or (2) irregularly irregular RR intervals. The absence of P waves is the most significant marker of AF; however, since locating the P wave fiducial point is often difficult when extensive noise is present, the results relied on this hallmark are moderate to high (73.0% to 91.0%, 71.0% to 80.0%, respectively) (Fukunami et al., 1991; Opolski et al., 1997; Budeus et al., 2003). The methods based on RR intervals are, therefore, preferable, with a higher sensitivity and specificity compared to the former method (93.7% vs. 80.7%, 93.0% vs. 75.7% on average, respectively) (Moody and Mark, 1983; Tateno and Glass, 2001; Duverney et al., 2002; Dash et al., 2009). However, it is important to note that regular RR intervals occur in the presence of an atrioventricular (AV) block or ventricular or AV junctional tachycardia when AF is present (Levy et al., 1998). In addition, irregular RR intervals also can be seen in the other arrhythmias (e.g., atrial impure flutter, multifocal atrial tachycardia, sinus arrhythmia), and therefore this method cannot completely distinguish AF from other arrhythmias. A combined approach of these two ECG characteristics, therefore, may be the most optimal approach to appropriate AF diagnosis (Couceiro et al., 2008; Babaeizadeh et al., 2009). Previous studies have shown that the analysis of P waves leads to improved performance (Babaeizadeh et al., 2009), though the improvement is limited. This study presents our findings on achieving high accuracy for AF detection. The proposed method is based on the combination analysis of P wave and RR interval according to our previously published methods (Huang et al., 2011).
2. Materials and methods
2.1. Databases
2.1.1. Dataset of 24-h paroxysmal AF Holter recordings
For algorithm development, we gathered 45 24-h Holter recordings with paroxysmal AF from the ECG Department of the Sir Run Run Shaw Hospital and the First Affiliated Hospital, School of Medicine, Zhejiang University, China. All the recordings, recorded using V1, V5 and aVF leads and sampled at 200 Hz, were first analyzed by the Holter system and AF episodes were independently annotated by two cardiologists. All the atrial flutter (AFL) episodes were treated as non-AF. Thus, a list of beat annotations and their labels (AF or non-AF) was obtained for further processing. Of the total 45 recordings, most were in association with other arrhythmias, e.g., AFL, AV block, and atrial tachycardia. The detailed statistics are shown in Table 1.
Table 1.
Statistics of the used databases
| Database name | Record | AF |
AF (>30 s)*
|
Non-AF |
||
| Beat | Episode | Beat | Episode | Beat | ||
| 24-h paroxysmal AF Holter recordings | 45 | 1 322 402 | 893 | 1 310 598 | 541 | 3 380 906 |
| MIT-BIH AF database | 23 | 508 419 | 291 | 506 872 | 226 | 620 123 |
The 24-h paroxysmal AF Holter recordings are used for algorithm development. All records have paroxysmal AF and some of them contain more than one other type of arrhythmia. The MIT-BIH AF database is used for algorithm validation. Of the total 23 records, 21 have paroxysmal AF and the other 2 have persistent AF. All the atrial flutter episodes were treated as non-AF
AF (>30 s) indicates AF episodes larger than 30 s
2.1.2. MIT-BIH AF database (Goldberger et al., 2000)
This database is commonly used for validation of AF detection algorithms. It includes 23 10-h multi-lead ECG recordings with AF (21 paroxysmal and 2 persistent) and each record is digitized at a sample rate of 250 Hz. The summary of AF episodes and beats is also listed in Table 1.
2.2. Methods
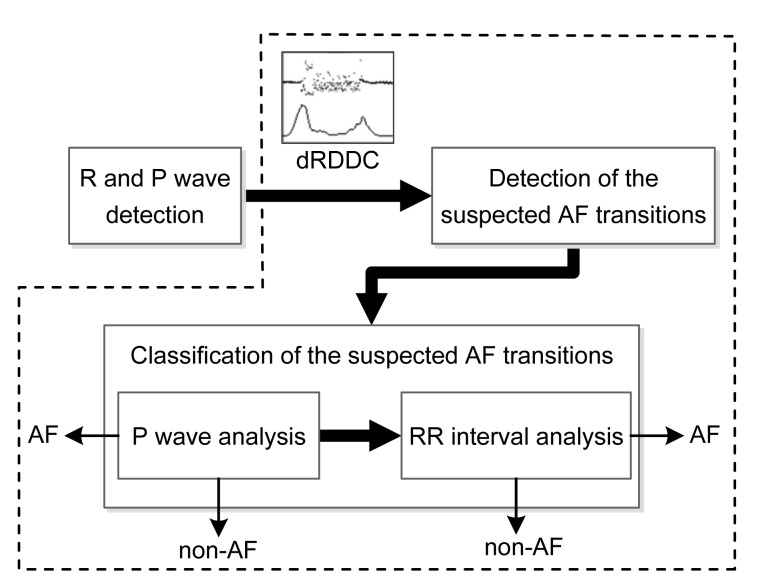
The proposed method starts with the detection of R and P waves (Fig. 1), and then goes on to detect AF in two steps: (1) detection of the suspected AF transitions using the delta RR interval distribution difference curve (dRDDC); (2) classification of the suspected AF transitions by a combination analysis of P wave and RR interval. Here, the AF transition represents the transition between AF and sinus rhythm, the delta RR interval is defined as the difference between two successive RR intervals and the dRDDC is defined as the difference between the distribution of the delta beRRs and delta afRRs (beRRs: RR intervals before the current RR interval; afRRs: RR intervals after the current RR interval). In this study, since the annotations of the R wave have already been prepared according to the databases, we solely focused on the detection of P waves and AF.
Fig. 1.
Flowchart of the proposed method
The steps surrounded by dotted line box are the procedure for AF detection. The dRDDC is the abbreviation of the delta RR interval distribution difference curve
2.2.1. P wave detection
The morphological transform method as proposed by Sun et al. (2005) was used to detect P waves. The procedures were performed as: (1) signal preprocessing, (2) multi-scale morphological derivative transform, and (3) detection of P waves of each beat in morphological derivative transformed signals according to the annotations of the R wave. Since P waves are absent in atrial arrhythmia, especially in AF, the P waves would not always be detected. These undetected P waves were marked as invalid for further processing.
2.2.2. AF detection
We first detected the suspected AF transitions using the dRDDC. The dRDDC reflects the variability of RR interval and its peaks (local maximum points) represent the major changes including the transitions between two rhythms, especially between AF and sinus rhythm. Thus, the suspected AF transitions could be located by detecting the peaks in the dRDDC. In this study, we used the density histogram of the 50 delta beRRs and the 50 delta afRRs to calculate the dRDDC by the following equation (Huang et al., 2011):
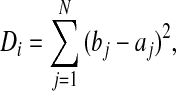 |
where Di is the value of dRDDC of each beat; j is the bin index of histogram and N is the bin number of histogram; bj and aj are the bin counts in the density histogram of the 50 delta beRRs and the 50 delta afRRs, respectively. All the delta RR intervals were limited to the range of −1 200 to 1 200 ms and the histogram parameters were fixed as: range=−1 200 to 1 200 ms, bin number=21, bin width=114.3 ms.
When a suspected AF transition was detected, a classifier was used to determine if it was the transition between AF and sinus rhythm or not. In this study, the classifier was based on a combination analysis of P wave and RR interval surrounding the 50 afRRs.
In the P wave analysis, we used the P-R intervals to distinguish AF and non-AF rhythms. Here, the P-R interval is defined as the time between the peak of the P wave and the peak of the R wave. Since some of the peaks would be invalid due to the absence of P waves during AF, the P-R intervals included two types: valid (>0) and invalid (=0). Of the valid P-R intervals, we calculated the minimum standard deviation (MinSD) of 16 consecutive P-R intervals to quantify the variation of the P-R interval. In sinus rhythm, the variation is relatively small but during AF or strong noise affected signals, it is very large due to the easy detection of pseudo-P waves (P-wave-like artifact). Therefore, we used the MinSD smaller than a fixed threshold (SDThresh=10) to determine non-AF rhythms. Of the invalid P-R intervals, we counted their numbers to quantify the absence of P waves and if the number was sufficiently larger than a fixed threshold (PRThresh=24), it was considered to be an AF rhythm. These two strategies ensure that some of the apparent AF and non-AF rhythms be detected, with the remaining to be classified by RR interval analysis.
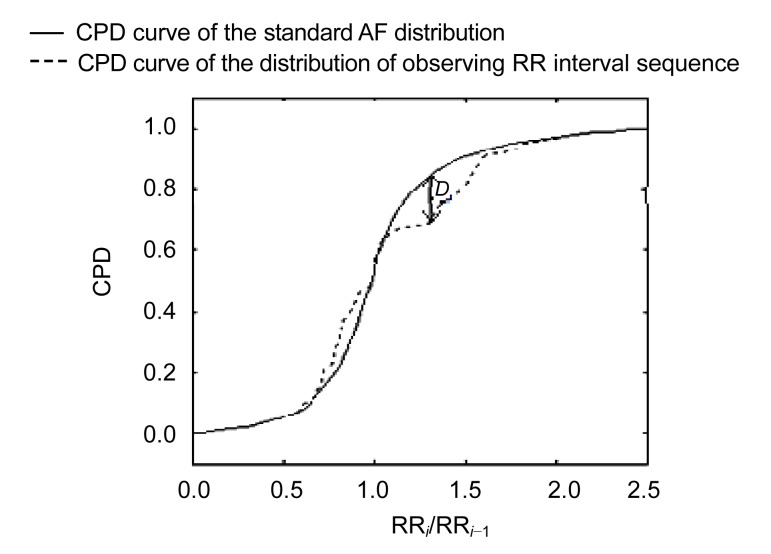
The RR interval analysis involved four steps as proposed in the previously published algorithm (Huang et al., 2011): (1) histogram analysis, (2) standard deviation analysis, (3) numbering aberrant rhythms recognition, and (4) Kolmogorov-Smirnov (K-S) test. The first three steps were used to filter some apparent non-AF rhythms (especially the numbering aberrant rhythms) and the final step is the core of classification. The assessment of the K-S test reflects the difference between two distributions. Thus, we developed a standard AF distribution to identify AF rhythm and evaluated the difference between the distribution of observed RR interval sequence and the standard AF distribution by the K-S test (Fig. 2).
Fig. 2.
Example of evaluation of difference between the distribution of observing RR interval sequence and the standard AF distribution using the K-S test
D: the greatest distance between the two curves. The cumulative probability distribution (CPD) is derived from ratio of two successive RR intervals (RRi/RRi −1)
The significance level (prob) is given as follows (Press et al., 1992):
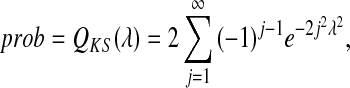 |
where,
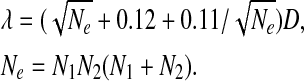 |
D is the greatest distance between two distributions; N e is the effective number of data points; N 1 and N 2 are the numbers of data points of two distributions, respectively.
A small prob signifies the significant difference between two distributions. Therefore, we used a fixed threshold (ProbThresh=0.1) to distinguish the AF and non-AF rhythms according to the previous study.
3. Results
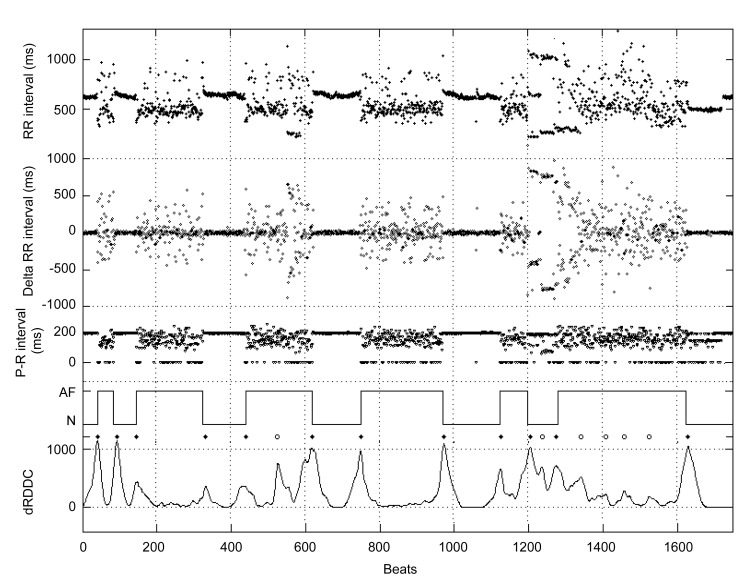
We used the single-lead annotations of R and P waves to detect AF. Fig. 3 shows an example of the intermediate results of the proposed method. The delta RR interval filters out the baseline drift of RR interval, which makes the histogram of delta RR interval vary symmetrically and thus the calculated dRDDC performs more stable. Also, since the variation of the delta RR interval is larger than that of the RR interval, the transitions between two rhythms can be shown more prominently in the dRDDC. From the dRDDC, we detected several peaks as shown in Fig. 3 and many of the peaks were true AF transitions. Note that the P-R intervals are always invalid (=0) during AF, while they only vary slightly during the sinus rhythm. We could classify some of the transitions by analyzing the P waves, with the remaining transitions to be confirmed using RR interval analysis procedures.
Fig. 3.
Intermediate results of the proposed method
The RR/delta RR intervals were calculated from subject 04043 of the MIT-BIH AF database. The P-R intervals were derived from the proposed P wave detection method and the value of zero indicates the invalidation of P-R interval. The solid line in the fourth row shows the assessment of AF as published in the MIT-BIH AF database. ♦: the detected peaks represented the AF transitions in the dRDDC; ○: the detected peaks represented the false AF transitions in the dRDDC. The bottom line shows the dRDDC calculated by the density histograms of the 50 delta beRRs and the 50 delta afRRs
The performance results are listed in Table 2. We applied the algorithm to all single-lead annotations of the MIT-BIH AF database to evaluate the performance and the results are reported in two parts: AF and AF (>30 s). The AF part reports the statistics of all AF episodes and the AF (>30 s) part ignores episodes shorter than 30 s. Looking at the results of the MIT-BIH AF database, we achieved a high sensitivity and specificity in all leads (98.2% and 97.5% on average in AF part, respectively). Note that in the part of AF (>30 s), the sensitivity is larger than that of AF. Moreover, of the total 291 AF episodes, 260 were correctly detected in the AF part, and of the total 226 episodes in the AF (>30 s) part, the number of correctly detected episodes was 224. This shows that accuracy increases with the duration of AF episodes.
Table 2.
Performance results of the proposed method
| Database name | AF |
AF (>30 s) |
|||||
| Beat |
Episode |
Beat |
Episode |
||||
| SE (%) | SP (%) | TP | SE (%) | SP (%) | TP | ||
| MIT-BIH AF database | Lead 1 | 98.0 | 97.6 | 260 | 98.1 | 97.4 | 224 |
| Lead 2 | 98.3 | 97.4 | 260 | 98.4 | 97.2 | 224 | |
| Average | 98.2 | 97.5 | 260 | 98.3 | 97.3 | 224 | |
| 24-h paroxysmal AF Holter recordings | Lead 1 | 96.2 | 96.8 | 757 | 96.4 | 96.4 | 499 |
| Lead 2 | 96.3 | 96.9 | 732 | 96.6 | 96.5 | 487 | |
| Lead 3 | 96.5 | 96.8 | 738 | 96.7 | 96.5 | 494 | |
| Average | 96.3 | 96.8 | 742 | 96.6 | 96.5 | 493 | |
SE: sensitivity; SP: specificity; TP: true positive
Furthermore, we tested the proposed method on the dataset of 24-h paroxysmal AF Holter recordings (Table 2). The average sensitivity and specificity were 96.3% and 96.8%, respectively. Since the single-lead P wave annotation of the same record is not the same, the accuracy slightly varies with different leads.
4. Discussion
This study presents a combined method to AF detection. The method first detects the suspected AF transitions and then classifies these transitions using a combination analysis of P wave and RR interval. A comparison with previously published algorithms on the MIT-BIH AF database is listed in Table 3. The proposed method achieved the highest performance, with 98.2% sensitivity and 97.5% specificity. In comparison with our previously published results (Huang et al., 2011), the sensitivity significantly increased (2.1%), while the specificity has a moderate decrease of −0.6%.
Table 3.
Comparison of algorithm performance on the MIT-BIH AF database
| Methods | SE (%) | SP (%) |
| The proposed method | 98.2 | 97.5 |
| Huang et al., 2011 * | 96.1 | 98.1 |
| Tateno and Glass, 2001 * | 94.4 | 97.2 |
| Dash et al., 2009 * | 94.4 | 95.1 |
| Couceiro et al., 2008 | 93.8 | 96.1 |
| Babaeizadeh et al., 2009 | 92.0 | 98.0 |
SE: sensitivity; SP: specificity
These methods are based on RR intervals and the remains are based on analysis of P wave and RR interval
The irregularity of RR intervals is the most accessible ECG characteristic for AF detection (Babaeizadeh et al., 2009). However, since irregular RR intervals can be seen in other arrhythmias and regular RR intervals are possible in AF, some of the AF and non-AF rhythms may be misclassified if only analyzing the RR intervals. Analyzing the P wave location and/or morphology can improve the performance of algorithm. However, since the detection of P wave is easily affected by noise, the P wave analysis would be unreliable under certain circumstances. In this study, we proposed a simple and conservative P wave strategy to classify AF and non-AF rhythms. The strategy is based on two P-R interval characteristics: (1) small variation in sinus rhythm, and (2) large number of invalid P-R intervals during AF. These characteristics could distinguish some of the apparent AF and non-AF rhythms in good signal conditions but would fail in the presence of noise. Thus, the P wave strategy ensures the accuracy even in the strong noise situations. The purpose of P wave analysis is to filter some apparent AF and non-AF rhythms; therefore, the thresholds should be applied strictly and kept constant in order to prevent misclassifying the AF and non-AF rhythms.
5. Conclusions
In this study, we proposed an automatic AF detection method based on the combination analysis of P wave and RR interval and achieved high accuracy on both validation and development databases. The proposed algorithm has been applied for Holter monitoring in clinical practice.
References
- 1.Babaeizadeh S, Gregg RE, Helfenbein ED, Lindauer JM, Zhou SH. Improvements in atrial fibrillation detection for real-time monitoring. J Electrocardiol. 2009;42(6):522–526. doi: 10.1016/j.jelectrocard.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Budeus M, Hennersdorf M, Perings C, Strauer BE. Detection of atrial late potentials with P wave signal averaged electrocardiogram among patients with paroxysmal atrial fibrillation. Z Kardiol. 2003;92(5):362–369. doi: 10.1007/s00392-003-0921-8. (in German) [DOI] [PubMed] [Google Scholar]
- 3.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37(2):371–378. doi: 10.1016/S0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 4.Couceiro R, Carvalho P, Henriques J, et al. Detection of Atrial Fibrillation Using Model-Based ECG Analysis. 19th International Conference on Pattern Recognition; 2008. pp. 2225–2229.pp. 4005 [DOI] [Google Scholar]
- 5.Dash S, Chon KH, Lu S, Raeder EA. Automatic real time detection of atrial fibrillation. Ann Biomed Eng. 2009;37(9):1701–1709. doi: 10.1007/s10439-009-9740-z. [DOI] [PubMed] [Google Scholar]
- 6.Duverney D, Gaspoz JM, Pichot V, Roche F, Brion R, Antoniadis A, Barthelemy JC. High accuracy of automatic detection of atrial fibrillation using wavelet transform of heart rate intervals. Pace-Pacing Clin Electrophysiol. 2002;25(4):457–462. doi: 10.1046/j.1460-9592.2002.00457.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukunami M, Yamada T, Ohmori M, Kumagai K, Umemoto K, Sakai A, Kondoh N, Minamino T, Hoki N. Detection of patients at risk for paroxysmal atrial-fibrillation during sinus rhythm by P-wave triggered signal-averaged electrocardiogram. Circulation. 1991;83(1):162–169. doi: 10.1161/01.CIR.83.1.162. [DOI] [PubMed] [Google Scholar]
- 8.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–E220. doi: 10.1161/01.CIR.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 9.Huang C, Ye SM, Chen H, Li DL, He FT, Tu YW. A novel method for detection of the transition between atrial fibrillation and sinus rhythm. IEEE Trans Biomed Eng. 2011;58(4):1113–1119. doi: 10.1109/TBME.2010.2096506. [DOI] [PubMed] [Google Scholar]
- 10.Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New-onset atrial fibrillation—sex differences in presentation, treatment, and outcome. Circulation. 2001;103(19):2365–2370. doi: 10.1161/01.CIR.103.19.2365. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman ES, Waldo AL. The impact of asymptomatic atrial fibrillation. J Am Coll Cardiol. 2004;43(1):53–54. doi: 10.1016/j.jacc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Levy S, Breithardt G, Campbell RW, Camm AJ, Daubert JC, Allessie M, Aliot E, Capucci A, Cosio F, Crijns H, et al. Atrial fibrillation: current knowledge and recommendations for management. Working Group on Arrhythmias of the European Society of Cardiology. Eur Heart J. 1998;19(9):1294–1320. doi: 10.1053/euhj.1998.1050. [DOI] [PubMed] [Google Scholar]
- 13.Moody GB, Mark RG. A new method for detecting atrial fibrillation using R-R intervals. Comput Cardiol. 1983;10:227–230. [Google Scholar]
- 14.Opolski G, Scislo P, Stanislawska J, Gorecki A, Steckiewicz R, Torbicki A. Detection of patients at risk for recurrence of atrial fibrillation after successful electrical cardioversion by signal-averaged P-wave ECG. Int J Cardiol. 1997;60(2):181–185. doi: 10.1016/S0167-5273(97)02982-3. [DOI] [PubMed] [Google Scholar]
- 15.Page RL, Tilsch TW, Connolly SJ, Schnell DJ, Marcello SR, Wilkinson WE, Pritchett ELC, Arrhyth AS. Asymptomatic or “silent” atrial fibrillation. Frequency in untreated patients and patients receiving azimilide. Circulation. 2003;107(8):1141–1145. doi: 10.1161/01.CIR.0000051455.44919.73. [DOI] [PubMed] [Google Scholar]
- 16.Press WH, Flannery BP, Teukolsky SA, et al. 2nd Ed. Cambridge University Press; 1992. Numerical Recipes in C: The Art of Scientific Computing; pp. 623–628. [Google Scholar]
- 17.Roche FDR, Gaspoz JM, da Costa A, Isaaz K, Duverney D, Pichot V, Costes F, Lacour JR, Barthelemy JC. Frequent and prolonged asymptomatic episodes of paroxysmal atrial fibrillation revealed by automatic long-term event recorders in patients with a negative 24-hour Holter. Pace-Pacing Clin Electrophysiol. 2002;25(11):1587–1593. doi: 10.1046/j.1460-9592.2002.01587.x. [DOI] [PubMed] [Google Scholar]
- 18.Schaer BA, Zellweger MJ, Cron TA, Kaiser CA, Osswald S. Value of routine Holter monitoring for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemic events. Stroke. 2004;35(3):68–70. doi: 10.1161/01.STR.0000117568.07678.4B. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Chan KL, Krishnan SM. Characteristic wave detection in ECG signal using morphological transform. BMC Cardiovasc Disord. 2005;5(1):28. doi: 10.1186/1471-2261-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tateno K, Glass L. Automatic detection of atrial fibrillation using the coefficient of variation and density histograms of RR and delta RR intervals. Med Biol Eng Comput. 2001;39(6):664–671. doi: 10.1007/BF02345439. [DOI] [PubMed] [Google Scholar]
- 21.Wolf PA, Dawber TR, Thomas HE, Kannel WB. Epidemiologic assessment of chronic atrial-fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28(10):973–977. doi: 10.1212/WNL.28.10.973. [DOI] [PubMed] [Google Scholar]