1,2-Dihydro-1,2-azaborine 1 is a benzene isostere in which a CC unit of benzene is replaced with an isoelectronic BN unit.[1,2] As part of our program to develop the basic science and applications of 1,2-azaborine heterocycles,[3–6] we have focused on expanding the scope of synthetically accessible 1,2-azaborines and investigating the aromatic character of this family of heterocycles.
Among the four commonly investigated criteria of aromaticity (structure, magnetism, energy, and reactivity), we have determined that 1,2-azaborines exhibit delocalized bonding,[7] have appropriate predicted NICS values,[8] and an experimentally determined resonance stabilization energy of 16.6 kcal/mol,[9] consistent with significant aromatic character. With regard to the reactivity criterion, Ashe has demonstrated that substituted 1,2-azaborines undergo electrophilic aromatic substitution reactions.[10] The parent 1,2-dihydro-1,2-azaborine 1 has recently been isolated.[8] However, there have been no reactivity studies performed on 1 to date. We are particularly interested in investigating the reactivity of parent 1 for two reasons: 1) to explore the fundamental reactivity differences between 1,2-dihydro-1,2-azaborine 1 and benzene, and 2) to develop new synthetic methods to access novel 1,2-azaborine derivatives. In this communication, we report that 1 readily undergoes nucleophilic aromatic substitution (SNAr) reactions to furnish new 1,2-azaborine compounds. We also present evidence that the substitution involves an addition-elimination mechanism consistent with SNAr.
In our initial investigation, we discovered that when 1 was treated with 1.0 equiv. n-BuLi in Et2O followed by 4.0 equiv. of trimethylsilyl chloride, substituted heterocycle 2 was formed in 17% yield (Table 1, entry 1). A dramatic increase in product yield was observed when 2.0 equivalents of the nucleophile were used (entry 2). However, a further increase in the amount of nucleophile used led to a substantial decrease in product yield (entry 3). A survey of solvents revealed that Et2O is the solvent of choice among etheral and hydrocarbon solvents (entries 4–7 vs. entry 2). We also determined that the optimal temperature for performing this substitution reaction is −30 °C; increasing or lowering the reaction temperature resulted in diminished yield of 2 (entries 8–9 vs. entry 2).
Table 1.
Optimization Survey of SNAr Reaction
 | ||||
|---|---|---|---|---|
| entry | solvent | equiv. of n-BuLi | temp. (°C) | product (%)a |
| 1 | Et2O | 1 | Š30 | 17 |
| 2 | Et2O | 2 | Š30 | 94 |
| 3 | Et2O | 3 | Š30 | 71 |
| 4 | THF | 2 | Š30 | 67 |
| 5 | Pentane | 2 | Š30 | 11 |
| 6 | Toluene | 2 | Š30 | 53 |
| 7 | DME | 2 | Š30 | 86 |
| 8 | Et2O | 2 | 25 | 46 |
| 9 | Et2O | 2 | Š78 | 77 |
Yields determined by GC analysis of the reaction mixture versus pentadecane as a calibrated internal standard. Yields are average of two runs.
The formation of compound 2 is consistent with a nucleophilic aromatic substitution in which the hydride on boron is serving as a leaving group. The ease with which this substitution occurs (i.e., at −30 °C) is distinct from the reactivity of benzene. The corresponding substitution reaction with benzene typically requires a stronger nucleophile (e.g., t-BuLi) and much harsher conditions (e.g., reflux in decalin at 165 °C for 20 hours).[11,12]
Having established the optimal reaction condition for this new substitution reaction, we then sought to expand its substrate scope. As can be seen from Table 2, oxygen-based nucleophiles are suitable for this reaction, including sodium tert-butyloxide (entry 1) and potassium allyloxide (entry 2). Carbon nucleophiles are very effective reaction partners. Hindered branched (entry 3), less hindered linear (entry 4) sp3-hybridized organolithium reagents as well as sp2-hybridized phenyllithium (entry 5) furnish the desired substituted products in high yield. Grignard reagents also give the corresponding products in moderate to good yield (entries 6 and 7). Noteworthy is the synthesis of BN styrene[13] (entry 6) and a novel BN tolan derivative (entry 7). The scope with respect to the electrophile at the nitrogen position includes H, TMS, Me, and Bn (entries 4, 8, 9, and 10).
Table 2.
Substrate Scope of SNAr Reaction
 | |||
|---|---|---|---|
| entry | M-Nu | E-X | yield (%)a |
| 1 | Na-OtBu | H-Cl | 63 |
| 2 | K-Oallyl | H-Cl | 79 |
| 3 | Li-tBu | H-Cl | 81 |
| 4 | Li-nBu | H-Cl | 80 |
| 5 | Li-Ph | H-Cl | 98 |
| 6 | BrMg-vinyl | H-Cl | 59 |
| 7 | H-Cl | 71 | |
| 8 | Li-nBu | TMS-Cl | 89 |
| 9 | Li-nBu | Me-I | 67 |
| 10 | Li-nBu | Bn-Br | 60 |
Isolated yield. Average of two runs.
Scheme 1 illustrates four possible mechanistic scenarios for the observed substitution reaction. Mechanism 1 involves a simple displacement of the B–H bond with the nucleophile (via intermediate A) followed by intermolecular deprotonation by the released metal hydride and quenching with the electrophile. In Mechanism 2, intermediate A releases H2 in an intramolecular fashion to generate intermediate B, which is then quenched with the electrophile. In Mechanisms 3 and 4, one equivalent of the nucleophile serves first as a base to remove the N–H proton to produce C. Subsequently in pathway 3, the second equivalent of nucleophile displaces the B–H bond (via a “di-anion”) to produce intermediate B. Alternatively, intermediate C can eliminate a hydride to yield a “benzyne”-type 1,2-azaborine[14] which then reacts with the nucleophile to produce B (Mechanism 4).
Scheme 1.
Possible reaction pathways of the substitution reaction.
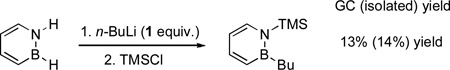
In our mechanistic studies we initially focused on the reaction of 1 with n-BuLi and TMSCl. In order to test the role of the NH group, we synthesized the N-benzyl protected 1,2-azaborine 3 (eq 1), which was then subjected to the SNAr reaction conditions (1 and 2 equivalents of n-BuLi followed by quenching with TMSCl). Interestingly, substituted product 4 was not formed (eq 1).[15] This experimental observation is inconsistent with Mechanism 1, which should be largely independent of the nature of the N-substituent. We determined that 2 equivalents of nucleophile are necessary to achieve high yield of 2 (eq. 2 vs. 3). This is incompatible with Mechanism 2, which requires only 1 equiv. of the nucleophile. Furthermore, when 1 was treated with 2 equiv. of n-BuLi, a fine white powder precipitated out of solution (eq 4). IR analysis of this powder indicates formation of LiH.[16,17] The observation of LiH is again inconsistent with Mechanism 2.
 |
(1) |
 |
(2) |
 |
(3) |
 |
(4) |
Whereas the experiments illustrated in eqs. 1–4 are inconsistent with the proposed Mechanisms 1 and 2, they are in agreement with Mechanisms 3 and 4. We were not successful in trapping the “benzyne”-type 1,2-azaborine intermediate using a number of trapping agents.[18] Therefore, we used calculations to help determine the most likely mechanism for the SNAr reaction. The computationally determined energy diagram (Figure 1) at the G3MP2[19] level indicates that the formation of the “benzyne”-type 1,2-azaborine is a high-energy process. On the other hand, the formation of the “di-anion” intermediate is energetically very favorable. Based on all of the available data, we believe that Mechanism 3 is the most likely mechanism for the conversion of 1 to 2.
Figure 1.
Calculated free energies in Et2O (G3MP2 + COSMO solvation model at the B3LYP-DZVP2 level) of the proposed intermediates in the SNAr reaction at 298 K.
The pKa of the N–H proton in 1,2-azaborines has been determined to be ~26.[20] Alkoxide nucleophiles are not basic enough to deprotonate the N–H of 1. Consequently, Mechanisms 3 and 4 cannot be used to explain the SNAr reactivity with alkoxide nucleophiles (Table 2, entries 1–2). To investigate the mechanism for oxygen-based nucleophiles, we focused on the reaction of 1 with Na-OtBu and TMSCl. In this case, we determined that one equivalent of nucleophile is sufficient to furnish the substituted product 5 in comparable yield as when two equivalents of nucleophile were used (eq 5 vs. Table 2, entry 1). Furthermore, addition of Na-OtBu to 1 results in release of significant amount of gas consistent with H2 formation. Mechanisms 1 and 2 are both consistent with these observations, the difference being whether H2 is released in an intramolecular fashion (Mechanism 2) or intermolecularly through the formation of NaH (Mechanism 1). To address this, we added NaH to compound 6 followed by addition of TMSCl (eq 6); starting material 6 was the only observed species of this reaction by NMR. If Mechanism 1 were operating, we would expect formation of 5. Based on these observations, we conclude that substitution reactions of 1 with alkoxide nucleophiles are most consistent with Mechanism 2.
 |
(5) |
 |
(6) |
In summary, we have presented the first reactivity study of 1,2-dihydro-1,2-azaborine 1. We demonstrated that 1 can readily undergo nucleophilic aromatic substitution reactions under mild conditions, a reactivity pattern that is distinct from its isostere benzene. This new reactivity allows access to novel 1,2-azaborine structures, including a BN tolan derivative. Using a combined experimental and computational approach, we determined the most likely substitution mechanisms of 1 with both carbon- and oxygen-based nucleophiles. Current efforts are directed at utilizing this reactivity for incorporating 1,2-azaborines into biologically relevant and materials related molecules.
Experimental Section
Compound 2. In a glove box, a 4 mL vial was charged with a solution of 1 (0.020, 0.26 mmol), and ether (1.0 mL). n-BuLi (1.6 M in Et2O, 0.320 mL, 0.510 mmol) was added to the solution at −30 °C, and the mixture was allowed to stand at −30 °C for 3 hours. Subsequently, a cold solution of trimethylsilyl chloride (0.111 g, 1.02 mmol in 0.5 mL Et2O) was slowly added to the reaction mixture. The resulting mixture was allowed to stand for 1 hour at −30 °C, then it was allowed to warm to room temperature and stir for an additional hour. At the conclusion of the reaction, the mixture was concentrated under reduced pressure, and the crude material was subjected to silica gel chromatography using pentane as eluent, yielding 2 (0.047 g, 89%) as a clear colorless oil.
1H NMR (500 MHz, C6D6): δ 7̃59 (dd, 3JHH = 6.3, 4.8 Hz, 1H), 7.14 (d, 3JHH = 6.5 Hz, 1H), 7.03 (d, 3JHH = 11.1 Hz, 1H), 6.22 (d t, 3JHH =1.18, 5.23 Hz, 1H), 1.71(m, 2H), 1.48 (m, 2H), 1.38 (t, 3JHH = 8.3 Hz, 2H), 0.99 (t, 3JHH = 7.4 Hz, 3H), 0.17 (s, 9H). 13C NMR (125 MHz, C6D6): δ 143.2, 136.8, 130 (br), 111.3, 30.1, 26.4, 21 (br), 14.4, 1.5. 11B NMR (96.3 MHz, C6D6): δ 4̃14. FTIR (thin film) 2958, 2872, 1608, 1508, 1448, 1401, 1286, 1253, 1216, 1149, 1105, 1007, 991, 845, 765, 736, 685 cm–1. HRMS (EI) calcd for C11H22BNSi (M+) 207.16146, found 207.16073.
Footnotes
Support for this research has been provided by the National Science Foundation (Grant DGE-0742540; A.N.L), the National Institutes of Health (Grant R01-GM094541), the U.S. Department of Energy, and the Robert Ramsay Foundation of The University of Alabama. We thank Dr. A. J. V. Marwitz for helpful discussions.
Supporting information for this article is available on the WWW under http://www.angewandte.org
Contributor Information
Ashley N. Lamm, Department of Chemistry, University of Oregon, Eugene, OR 97403-1253 (USA)
Edward B. Garner, III, Department of Chemistry, Shelby Hall, University of Alabama, Tuscaloosa, AL 35487-0336.
David A. Dixon, Email: dadixon@bama.ua.edu, Department of Chemistry, Shelby Hall, University of Alabama, Tuscaloosa, AL 35487-0336.
Shih-Yuan Liu, Email: lsy@uoregon.edu, Department of Chemistry, University of Oregon, Eugene, OR 97403-1253 (USA).
References
- 1.Bosdet MJD, Piers WE. Can. J. Chem. 2009;87:8–29. [Google Scholar]
- 2.Liu Z, Marder TB. Angew. Chem. Int. Ed. 2008;47:242–244. doi: 10.1002/anie.200703535. [DOI] [PubMed] [Google Scholar]
- 3.For fundamental and synthetic contributions, see: Tanjaroon C, Daly A, Marwitz AJV, Liu S-Y, Kukolich S. J. Chem. Phys. 2009;131 doi: 10.1063/1.3270157. 224312. Daly AM, Tanjaroon C, Marwitz AJV, Liu S-Y, Kukolich SG. J. Am. Chem. Soc. 2010;132:5501–5506. doi: 10.1021/ja1005338. Marwitz AJV, Abbey ER, Jenkins JT, Zakharov LN, Liu SY. Org. Lett. 2007;9:4905–4908. doi: 10.1021/ol702383u. Marwitz AJV, McClintock SP, Zakharov LN, Liu SY. Chem. Commun. 2010;46:779–781. doi: 10.1039/b919632c. Lamm AN, Liu SY. Mol. BioSyst. 2009;5:1303–1305. doi: 10.1039/b904120f.
- 4.For work related to materials science, see: Marwitz AJV, Jenkins JT, Zakharov LN, Liu S-Y. Angew. Chem. Int. Ed. 2010;49:7444–7447. doi: 10.1002/anie.201004084.
- 5.For potential biomedical applications, see: Abbey ER, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2010;132:16340–16342. doi: 10.1021/ja107312u. Liu L, Marwitz AJV, Matthews BW, Liu S-Y. Angew. Chem. Int. Ed. 2009;48:6817–6819. doi: 10.1002/anie.200903390.
- 6.For the use of BN heterocycles as potential hydrogen storage materials, see: Campbell PG, Zakharov LN, Grant DJ, Dixon DA, Liu S-Y. J. Am. Chem. Soc. 2010;132:3289–3291. doi: 10.1021/ja9106622.
- 7.Abbey ER, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2008;130:7250–7252. doi: 10.1021/ja8024966. [DOI] [PubMed] [Google Scholar]
- 8.Marwitz AJV, Matus MH, Zakharov LN, Dixon DA, Liu S-Y. Angew. Chem. Int. Ed. 2009;48:973–977. doi: 10.1002/anie.200805554. [DOI] [PubMed] [Google Scholar]
- 9.Campbell PG, Abbey ER, Neiner D, Grant DJ, Dixon DA, Liu S-Y. J. Am. Chem. Soc. 2010;132:18048–18050. doi: 10.1021/ja109596m. [DOI] [PubMed] [Google Scholar]
- 10.Pan J, Kampf JW, Ashe AJ., III Org. Lett. 2007;9:679–681. doi: 10.1021/ol062968r. [DOI] [PubMed] [Google Scholar]
- 11.For nucleophilic alkylation reactions of benzene and other aromatic hydrocarbons, see: Dixon JA, Fishman DH. J. Am. Chem. Soc. 1963;85:1356–1357.
- 12.For reactions of B- and N-substituted borazines with nucleophiles, see: Smalley JH, Stafiej SF. J. Am. Chem. Soc. 1959;81:582–586. Nöth H, Rojas-Lima S, Troll A. Eur. J. Inorg. Chem. 2005:1895–1906. Nöth H, Troll A. Eur. J. Inorg. Chem. 2005:3524–3535. For reactions of borazine with organomagnesium and organolithium compounds, see: Moews PC, Jr, Laubengayer AW. Inorg. Chem. 1963;2:1072–1073. Powell P, Semlyen JA, Blofeld RE, Phillips CSG. J. Chem. Soc. 1964:280–283.
- 13.Pan J, Kampf JW, Ashe AJ., III J. Organomet. Chem. 2009;694:1036–1040. [Google Scholar]
- 14.For a computational study on “benzyne”-type 1,2-azaborine, see: Fazen PJ, Burke LA. Inorg. Chem. 2006;45:2494–2500. doi: 10.1021/ic051649d.
- 15.Similarly, no reaction occurred when 3 was reacted with Na-OtBu as the nucleophile. For experimental details, see Supporting Information.
- 16.The IR stretching frequency of LiH has been determined to be ~1400 cm−1, see: Dixon DA, Gole JL, Komornicki A. J. Phys. Chem. 1988;92:2134–2136. Wang X, Andrews L. J. Phys. Chem. A. 2007;111:6008–6019. doi: 10.1021/jp071251y.
- 17.Nucleophilic substitution reactions involving aromatic hydrocarbon and pyridine derivatives have been shown to produce LiH, see: Eppley RL, Dixon JA. J. Am. Chem. Soc. 1968;90:1606–1612. Abramovitch RA, Giam C-S. Can. J. Chem. 1963;41:3127–3131.
- 18.Benzyl azide, anthracene, furan, and cyclopentadiene were used as trapping agents. See Supporting Information for experimental details.
- 19.Curtiss LA, Redfern PC, Raghavachari K, Rassolov V, Pople JA. J. Chem. Phys. 1999;110:4703–4709. [Google Scholar]
- 20.Pan J, Kampf JW, Ashe AJ., III Organometallics. 2004;23:5626–5629. [Google Scholar]





