Abstract
Objective
To determine predictors of fistula repair outcomes 3 months postsurgery.
Methods
We conducted a multicountry prospective cohort study between 2007 and 2010. Outcomes, measured 3 months postsurgery, included fistula closure, and residual incontinence in women with a closed fistula. Potential predictors included patient and fistula characteristics, and context of repair. Multivariable generalized estimating equation models were used to generate adjusted risk ratios (ARR) and 95% confidence intervals (CI).
Results
Women who returned for follow-up 3 month postsurgery were included in predictors of closure analyses (n=1,274). Small bladder size (ARR 1.57; 95% CI 1.39–1.79), prior repair (ARR 1.40; 95% CI 1.11–1.76), severe scarring (ARR 1.56; 95% CI 1.20–2.04), partial urethral involvement (ARR 1.36; 95% CI 1.11–1.66), and complete urethral destruction/circumferential defect (ARR 1.72; 95% CI 1.33–2.23) predicted failed fistula closure. Women with a closed fistula at 3 month follow-up were included in predictors of residual incontinence analyses (n=1041). Prior repair (ARR 1.37; 95% CI 1.13–1.65), severe scarring (ARR 1.35; 95% CI 1.10–1.67), partial urethral involvement (ARR 1.78; 95% CI 1.27–2.48), and complete urethral destruction or circumferential defect (ARR 2.06; 95% CI 1.51–2.81) were significantly associated with residual incontinence.
Conclusions
The prognosis for genital fistula closure is related to preoperative bladder size, previous repair, vaginal scarring, and urethral involvement.
It is estimated that up to 2 million women in resource poor countries are living with a urinary or recto-vaginal fistula (1,2), primarily as a result of prolonged obstructed labor.(3)
Despite the growing number of studies examining factors influencing fistula repair outcomes (4–7), there remains limited evidence on which to base clinical practice. More empirical evidence on independent predictors of repair outcomes would serve several purposes. Additional evidence of fistula and patient characteristics independently associated with repair outcomes would strengthen the movement towards developing a standardized fistula classification system by contributing evidence with regard to which characteristics should be included in such a system. Similarly, such information could guide discussions with fistula patients regarding possible outcomes of their repair. Finally, additional evidence regarding predictors of repair outcome would provide useful information to guide clinical practice, including decisions about how and by whom (i.e. the level of skill needed by the surgeon) a given fistula should be repaired.
Published evidence to-date fails to demonstrate an independent role of any patient characteristic in predicting repair outcomes(8,9,14,16,20,27). On the other hand, several fistula characteristics, most notably vaginal scarring and urethral involvement, have been found to predict poor prognosis of repair surgery (5,8,9,11,16,17). No published studies have examined the effect of contextual factors on repair outcomes, factors such as surgeon experience or whether the repair was conducted as part of outreach services or in the context of training. Most studies have been conducted at one center in a single country, often by a single surgeon, thereby limiting the ability of these studies to generalize beyond a very specific patient population and setting. Many of the studies had small sample sizes and were of varied quality, and few conducted analyses necessary to assess independent effects of predictors of repair outcomes.(4)
Against this background, we conducted a large, multi-country prospective cohort study to evaluate which patient-level (patient and fistula characteristics) and contextual factors predicted fistula repair surgery outcomes. We evaluated the influence of these factors on two outcomes at 3 months after fistula repair surgery: fistula closure and residual incontinence in women after successful fistula closure.
MATERIALS AND METHODS
Women seeking fistula repair services between September 2007 and September 2010 were recruited for this prospective cohort study at 11 sites in five countries; Bangladesh (three sites), Guinea (one site), Niger (two sites), Nigeria (three sites) and Uganda (two sites). Study sites included a mix of public, private, and faith-based institutions located in both urban and rural settings. All were receiving support to conduct fistula repairs from EngenderHealth’s Fistula Care project. The study protocol was reviewed and approved as required by institutional and government guidelines. Approvals were obtained from the Comite National D’Ethique pour la Recherché en Santé in Guinea; the Ministere de la Sante Publique in Niger; the National Health Research Ethics Committee, the Kebbi State Ministry of Health, the Sokoto State Ministry for Women Affairs, and the Zamfara State Hospital Services Management Board in Nigeria; and the National Council for Science and Technology in Uganda. The Bangladesh Medical Research Council declined to review the study due to its observational nature.
Women were eligible for the study if they freely consented to participate and signed an informed consent form, had a urinary fistula or rectovaginal fistula (RVF) (obstetric or traumatic in origin) and agreed to attend one follow-up visit three months following their fistula repair surgery. Women were excluded if they did not consent to participate, had incontinence unrelated to a fistula, or had a condition, which in the opinion of the site investigator prevented her from undergoing fistula repair surgery or contraindicated her participation in the study. Urinary fistula was broadly defined as an abnormal connection/communication between the genital and urinary tracts, including vesicovaginal (VVF), vesicouterine, urethrovaginal, and ureterovaginal fistula. Given that genital fistula does occur in young women/girls we did not have a lower age limit for study participation. Status as a “minor” was determined in accordance with legal age of adulthood in each country. Proxy consent was based on each country’s legislation and, where appropriate, was obtained from a relevant legal authority (i.e. parent or guardian). Proxy consent did not substitute for the minor’s consent, but rather supplemented it.
Given the observational nature of the study, no new interventions were introduced and clinical procedures were not standardized within or across sites for study purposes. Care of patients before, during and after their fistula repair surgery was at the discretion of the surgeon and other site staff. Repair surgeries were conducted either by trained fistula surgeons or by surgeons being trained to repair fistulas under the guidance of a fistula surgery trainer. The surgeons included some who were based at the study sites and others who were surgeons visiting the sites during focused outreach efforts.
Data were collected on standardized forms during participant’s hospital stay and at the 3 month follow-up exam. Interviews were carried out at admission, discharge and follow-up in English, French or local languages. Participants were given funds to cover their return transport for the follow-up visit, and at most sites a small gift to express our appreciation that they had returned for follow-up (e.g. a blanket, fabric for making a dress or a carrying basket). As is the standard practice at all study sites, repair services and follow-up care were provided at no cost.
The primary outcome was fistula closure 3 month postsurgery, determined by pelvic examination, with a dye test in women who had urine leakage. If no pelvic examination or dye test was conducted (186/1274, 14.6%) fistula closure was determined by providers’ response to the question “Does the client have continuous and uncontrolled leakage of urine.” The secondary outcome was residual incontinence among women with a closed fistula 3 month post-surgery. Residual incontinence, including overflow, stress and urge incontinence, was determined by the surgeons based on the participants history and clinical examination findings; additional specialized diagnostics were not done in most cases.
The potential predictors of fistula closure and residual incontinence included patient characteristics, fistula characteristics, and context of repair. Patient characteristics included age and years living with the fistula (both continuous variables), rural residence, education (dichotomized at primary education or higher), parity (dichotomized at greater than three), whether the patient had delivered via c-section, whether or not the patient had previously undergone surgery for the fistula, and whether the patient had female genital cutting (FGC). FCG status was assessed during a clinical exam; data for the other variables were derived from the participant interviews. Comorbidities assessed included malnutrition, determined through skin-fold measurement, body mass index or visual assessment; anemia, determined through hemoglobin level, hematocrit or visual assessment; and other conditions as reported by clinicians.
Fistula characteristics assessed included fistula location, bladder size (dichotomized as small versus normal or distended), fistula size, degree of scarring (none/mild, moderate or severe), number of fistulas (dichotomized at greater than one), ureter involvement (yes or no), and degree of urethral involvement. Urethral involvement was categorized as “partial” (urethra involved but not completely destroyed or separated), and “complete destruction or circumferential,” where circumferential referred to complete separation of the urethra from the bladder. Because there are no agreed upon standard definitions or objective measure for most of the fistula characteristics assessed, the operating surgeon made subjective assessments based on their clinical judgment and experience.
Variables related to context of repair included whether the repair was conducted during routine services or an outreach activity and whether it was conducted during a training session, as well as surgeon experience. Surgeon experience was measured by the number of complex repairs the surgeon reported ever conducting; complex was defined subjectively by each surgeon, and the variable was dichotomized at greater than 200 complex repairs.
For bivariable analyses, patient, fistula and contextual correlates of surgery outcome were compared using risk ratios (RRs) and corresponding 95% confidence intervals (CIs); these were derived using generalized estimating equations (GEE), accounting for clustering of patient outcomes within facilities. RRs and corresponding 95% CIs were generated using the logarithm link function and binomial distribution specification in SAS PROC GENMOD.(28)
Variables eligible for inclusion in multivariable models were conceptually associated with repair outcomes and statistically associated (p-value <0.20) with repair outcomes in bivariable analysis. In the event that variables were too highly correlated (r >0.4) only one was included. For example, prior repair was included instead of fistula duration. Multivariable GEE models using the log-binomial specification in GENMOD were used to generate adjusted RRs (ARR) and corresponding 95% CIs; where the log-binomial model failed to converge, SAS PROC GENMOD’s Poisson regression capability with a log link function and robust variance was used.(29) All data were analyzed using SAS version 9.2.
RESULTS
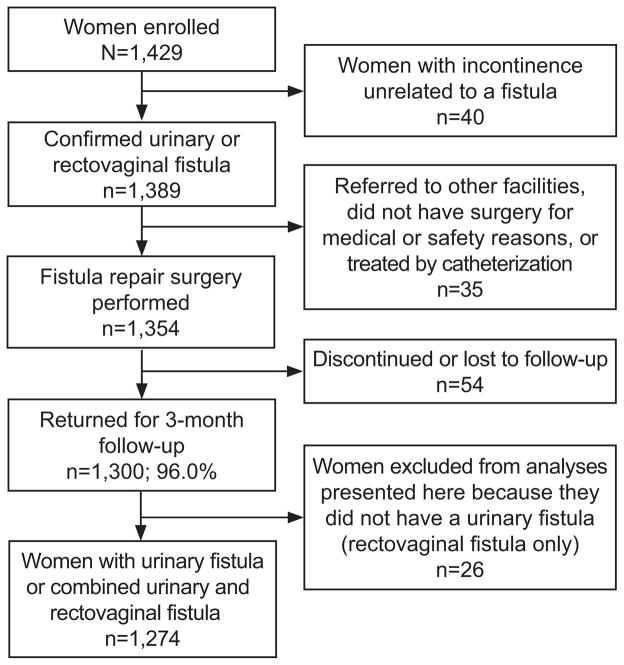
Flow of study participants is shown in Figure 1. A total of 1,429 women were enrolled, ranging from 51 to 261 women per site at ten sites. Variability was based primarily on the site’s caseload. At the eleventh site (one in Bangladesh), only five women were enrolled before fistula repair services were no longer offered due to staffing changes (data from these women were included in our analyses). Overall, 1300 (96.0%) women returned for the 3 month follow-up visit, with follow-up varying among sites, ranging from 74.2 – 100%. Median duration of follow-up was 88.0 days (interquartile range (IQR) 84.0–99.0; range 22.0–553.0). Data presented here are for the 1274 women who had a urinary fistula (n=1229) or a combined urinary fistula/RVF (n=37) and who returned for 3 month follow-up. This sample size enabled us to detect with over 95% power an absolute difference in failure of 7% between exposed and unexposed, assuming 8% failure among those unexposed to the characteristic in question, a 95% confidence level, and a 1:1 ratio of unexposed to exposed. Analyses examining predictors of residual incontinence include the 1041 women whose fistula was closed at the 3 month visit.
Figure 1.
Flow of study participants.
Repairs were conducted by 67 surgeons (resident, visiting and trainee); 10 reported conducting more than 200 complex repairs during their career. The latter were identified as the primary surgeon for nearly one-third (411/1274, 32.3%) of repairs. Just over half (721/1274, 56.7%) of all repairs were in the context of routine services, while more than one-third (477/1274, 37.5%) occurred during a training.
Table 1 shows selected baseline characteristics of study participants. Median age at repair was 25.0 (IQR 20.0–35.0; range 14.0–83.0). Most women were in their teens to mid-thirties, and were married or divorced/separated rural residents with limited education, who relied on their husband or other relatives for financial support. Nearly all fistulas (1241/1272, 97.6%) were obstetric in origin. Participants were healthy, apart from their fistula, with the majority of women (901/1274, 70.7%) exhibiting no other apparent medical conditions at admission to the study. FGC was present in 20.4% of the women (259/1274); the majority of these in Guinea.
Table 1.
Selected Patient and Fistula Characteristics of Women Undergoing Urinary Fistula Repair Surgery
| Patient Characteristic at Enrollment | n (%) | |
|---|---|---|
| Age (n=1267) | 14–19 | 289 (22.8) |
| 20–24 | 282 (22.3) | |
| 25–34 | 363 (28.7) | |
| 35 and older | 333 (26.3) | |
|
| ||
| Median age at first marriage (IQR) (n=1172) | 15.0 (14.0– 18.0) | |
|
| ||
| Marital status (n=1256) | Single | 23 ( 1.8) |
| Married/living as if married | 830 (66.1) | |
| Divorced/separated | 341 (27.1) | |
| Widowed | 62 ( 4.9) | |
|
| ||
| Education (n=1271) | Less than primary | 720 (56.6) |
| Primary or higher | 265 (20.8) | |
| Religious (eg Koranic studies) | 286 (22.5) | |
|
| ||
| Residence (n=1264) | Rural | 1088 (86.1) |
| Semiurban (town) | 109 ( 8.6) | |
| Urban (city) | 67 (5.3) | |
|
| ||
| Primary source of income (n=1268) | Husband | 657 (51.6) |
| Relatives | 429 (33.7) | |
| Self | 164 (12.9) | |
| Other | 23 ( 1.8) | |
|
| ||
| Median Parity (IQR) (n=1231) | 2.0 (1.0–5.0) | |
|
| ||
| Median age at fistula occurrence (IQR) (n=1273) | 20.3 (17.3– 26.8) | |
|
| ||
| Event after which fistula occurred (n=1272) | Delivery | 1241 (97.6) |
| Medical procedure | 20 ( 1.6) | |
| Genital cutting | 7 ( 0.6) | |
| Sexual violence | 0 ( 0) | |
| Other | 4 ( 0.3) | |
|
| ||
| Status of baby from causative delivery (n=1243) | Baby born dead | 1088 |
| Baby born alive | (84.4) | |
| Baby died shortly after birth | 155 | |
| (12.0) | ||
| 46 (3.6) | ||
|
| ||
| Years with fistula (median, IQR) (n=961) | 1.0 (0.3–3.0) | |
|
| ||
| Had prior repair surgery (n=1271) | 294 (23.1) | |
|
| ||
| Median previous repair attempts (IQR) (n=288) | 1 (1–2) | |
|
| ||
| Fistula characteristics at surgery | ||
|
| ||
| Fistula location and type (n=1274) | Midvaginal | 369 (29.1) |
| Juxtaurethral | 276 (21.8) | |
| (multiple responses possible) | Juxtacervical | 225 (17.8) |
| Circumferential | 214 (16.8) | |
| Intracervical | 81 ( 6.4) | |
| Trigonal | 66 ( 5.2) | |
| Vault | 35 ( 2.8) | |
| Supratrigonal | 32 ( 2.5) | |
| Vesicouterine | 21 ( 1.7) | |
| Ureteric | 20 ( 1.6) | |
| Ureterovaginal | 15 ( 1.2) | |
|
| ||
| Fistula size (cm) (n=1274) | Small (smaller than 2) | 348 (28.6) |
| Medium (2–3) | 612 (50.3) | |
| Large (4–5) | 198 (16.3) | |
| Extensive (6 or longer) | 58 ( 4.8) | |
|
| ||
| Urethral involvement (n=1274) | None | 759 (59.8) |
| Partial | 281 (22.1) | |
| Complete destruction or circumferential | 226 (17.9) | |
|
| ||
| Small bladder (n=1274) | 338 (28.6) | |
|
| ||
| Scarring (n=1274) | No or mild scarring | 806 (63.4) |
| Moderate scarring | 371 (29.2) | |
| Severe scarring | 95 ( 7.5) | |
|
| ||
| More than one urinary fistula (n=1274) | 75 ( 5.9) | |
IQR, interquartile range.
Table 1 also shows clinical characteristics of the fistulas seen in study participants. While few women had severe vaginal scarring, more than one quarter had a small bladder and approximately one-third had some involvement of the urethra. According to the surgeon’s subjective assessment at the time of surgery, similar numbers of fistulas were categorized as simple (363/1266, 28.7%) or complex (356/1266, 28.1%). The remaining 43.2% (547/1266) were classified as intermediate.
Overall fistula closure at 3 months was 81.7% (1041/1274; 95%CI: 79.5%–83.7%), varying among sites from 59.7–97.5%. Most of those women (823/1041, 79.1%; 95%CI: 76.5%–81.4%) were closed and dry (i.e. had no residual incontinence), however nearly 20% of the women with closed fistula had residual incontinence (193/1041, 18.5%; 95%CI: 16.3%–21.0%). Occurrence of residual incontinence varied among sites from 9.9–47.1%).
In unadjusted analyses, a number of patient characteristics, fistula characteristics and context of repair variables were significantly (p<0.05) associated with fistula closure at the 3 month follow-up visit (Table 2). However, after adjusting for other patient, fistula and contextual factors, only small bladder size, prior repair, severe vaginal scarring, and urethral involvement were found to predict failure of fistula closure at 3 months post-repair (Table 3). Women with small bladder size, prior repair or severe vaginal scarring had approximately a 1.5 times greater risk of failed fistula closure. Additionally, as degree of urethral involvement increased, so did risk of failed fistula closure.
Table 2.
Patient and Fistula Characteristics by Fistula Closure and Residual Incontinence at 3 Months Postrepair
| Fistula Closure (n=1274) | Residual Incontinence (n=1016) | |||
|---|---|---|---|---|
| Characteristics | Closed n (%) | Not closed n (%) | No residual incontinence n (%) | Residual incontinence n (%) |
| Total | 1039 | 235 | 823 | 193 |
| Patient characteristics | ||||
| Age (mean, SD) | 27.8 (11.0) | 29.8 (11.2) | 27.4 (10.8) | 29.0 (11.7) |
| Completed primary school or higher | 237 (22.8) | 28 (12.0)* | 183 (22.3) | 47 (24.4) |
| Rural residence | 887 (86.0) | 201 (86.3) | 705 (86.5) | 160 (82.9)† |
| Parity greater than 3 | 362 (35.9) | 83 (37.1) | 293 (36.9) | 59 (31.4) † |
| Delivered via c-section | 389 (38.5) | 92 (40.5) | 311 (38.9) | 70 (37.2)* |
| Prior repair | 210 (20.3) | 85 (36.3)† | 156 (19.0) | 48 (24.9)† |
| Genital cutting | 194 (18.7) | 65 (27.7) | 168 (20.4) | 25 (13.1)* |
| Patient comorbidities | ||||
| Anemia | 72 ( 6.9) | 19 ( 8.1) | 52 ( 6.3) | 16 ( 8.3) |
| Malnutrition | 58 ( 5.6) | 18 ( 7.7) | 42 ( 5.1) | 12 ( 6.2) |
| Malaria | 4 ( 0.4) | 2 ( 0.9) | 3 ( 0.4) | 1 ( 0.5) |
| HIV | 4 ( 0.4) | 0 ( 0.0) | 4 ( 0.5) | 0 ( 0.0) |
| UTI | 2 ( 0.2) | 0 ( 0.0) | 2 ( 0.2) | 0 ( 0.0) |
| Fistula characteristics | ||||
| Location and type | ||||
| Midvaginal | 318 (30.8) | 51 (21.9)* | 272 (33.2) | 43 (22.4)† |
| Juxtaurethral | 230 (22.3) | 46 (19.7) | 163 (20.0) | 61 (31.8)† |
| Juxtacervical | 187 (18.2) | 38 (16.3) | 149 (18.2) | 35 (18.2) |
| Circumferential | 143 (13.8) | 71 (30.2)† | 87 (10.6) | 50 (25.9)† |
| Intracervical | 71 ( 6.9) | 10 ( 4.3)† | 67 ( 8.2) | 4 ( 2.1)† |
| Trigonal | 55 ( 5.3) | 11 ( 4.7) | 46 ( 5.6) | 8 ( 4.2) |
| Vault | 29 ( 2.8) | 6 ( 2.6) | 22 ( 2.7) | 6 ( 3.1) |
| Supratrigonal | 27 ( 2.6) | 5 ( 2.1)* | 25 ( 3.1) | 1 ( 0.5)† |
| Vesicouterine | 19 ( 1.8) | 2 ( 0.9) | 17 ( 2.1) | 2 ( 1.0) |
| Ureteric | 18 ( 1.7) | 2 ( 0.9) | 12 ( 1.5) | 5 ( 2.6) |
| Ureterovaginal | 11 ( 1.1) | 4 ( 1.7)† | 9 ( 1.1) | 1 ( 0.5) |
| Fistula size (cm) | ||||
| Small (smaller than 2) | 295 (29.9) | 53 (23.1) | 246 (31.3) | 47 (26.1) |
| Medium (2–3) | 508 (51.5) | 104 (45.4) | 421 (53.5) | 81 (45.0) |
| Large (4–5) | 144 (14.6) | 60 (26.2)† | 93 (11.8) | 45 (25.0)† |
| Extensive (longer than 6) | 45 ( 4.6) | 13 ( 5.7) | 28 ( 3.6) | 10 ( 5.6)* |
| Urethral involvement | ||||
| None (reference category) | 664 (64.0) | 95 (40.8) | 566 (69.0) | 83 (43.5) |
| Partial | 220 (21.2) | 61 (26.2)† | 159 (19.4) | 56 (29.3)† |
| Complete destruction or circumferential | 150 (14.6) | 76 (32.9)† | 92 (11.3) | 52 (27.2)† |
| Small bladder | 241 (24.9) | 97 (45.1)† | 179 (23.2) | 57 (31.8)† |
| Scarring | ||||
| No or mild scarring (reference category) | 694 (66.9) | 112 (47.7) | 569 (69.3) | 115 (59.6) |
| Moderate scarring | 288 (27.8) | 83 (35.3)† | 221 (26.9) | 57 (29.5)* |
| Severe scarring | 55 ( 5.3) | 40 (17.0)† | 31 ( 3.8) | 21 (10.9)† |
| More than one urinary fistula | 50 ( 4.8) | 25 (10.6)† | 29 ( 3.5) | 16 ( 8.3)† |
| Context of repair | ||||
| Routine services | 120 (51.7) | 601 (57.8) | 482 (58.6) | 103 (53.6)* |
| Training | 89 (38.2) | 388 (37.4) | 302 (36.8) | 77 (40.1) † |
| Surgeon conducted more than 200 complex repairs | 61 (27.2) | 350 (35.5)* | 263 (33.6) | 73 (40.1)* |
SD, standard deviation; HIV, human immunodeficiency virus; UTI, urinary tract infection.
p<0.2.
p<0.05.
Table 3.
Bivariable and Multivariable Predictors of Failure of Fistula Closure and Residual Incontinence at 3 Months Postrepair
| Relative Risk (95%CI) | Adjusted Risk Ratio*(95%CI) | |
|---|---|---|
|
| ||
| Predictors of fistula closure | ||
|
| ||
| Midvaginal | 0.74 (0.56–0.99) | 0.93 (0.72–1.21) |
|
| ||
| Intracervical fistula | 0.69 (0.49–0.98) | 1.03 (0.73–1.45) |
|
| ||
| Supratrigonal | 0.69 (0.44–1.06) | 0.97 (0.68–1.39) |
|
| ||
| Small bladder | 2.08 (1.52–2.83) | 1.57 (1.39–1.79) |
|
| ||
| Prior repair | 1.53 (1.23–1.90) | 1.40 (1.11–1.76) |
|
| ||
| Scarring | ||
| None or mild | Ref | Ref |
| Moderate | 1.53 (1.02–2.28) | 1.15 (0.89–1.49) |
| Severe | 2.79 (1.81–4.28) | 1.56 (1.20–2.04) |
|
| ||
| Urethral involvement | ||
| None | Ref | Ref |
| Partial | 1.55 (1.28–1.88) | 1.36 (1.11–1.66) |
| Complete destruction or circumferential | 2.34 (1.72–3.16) | 1.72 (1.33–2.23) |
|
| ||
| More than one urinary fistula | 1.68 (1.11–2.53) | 1.39 (0.95–2.03) |
|
| ||
| Fistula size (diameter greater than 4 cm) | 1.59 (1.07–2.35) | 0.81 (0.61–1.07) |
|
| ||
| Ureter involvement | 1.52 (1.06–2.18) | 1.01 (0.83–1.25) |
|
| ||
| Surgeon conductedmore than 200 complex repairs | 1.27 (1.02–1.58) | 0.97 (0.72–1.30) |
|
| ||
| Predictors of residual incontinence | ||
|
| ||
| Prior repair | 1.27 (1.03–1.56) | 1.37 (1.13–1.65) |
|
| ||
| Scarring | ||
| None or mild | Ref | Ref |
| Moderate | 1.36 (1.03–1.80) | 1.09 (0.84–1.42) |
| Severe | 2.18 (1.81–2.62) | 1.35 (1.10–1.67) |
|
| ||
| Urethral involvement | ||
| None | Ref | Ref |
| Partial | 1.82 (1.41–2.34) | 1.78 (1.27–2.48) |
| Complete destruction or circumferential | 2.33 (1.72–3.16) | 2.06 (1.51–2.81) |
|
| ||
| Small bladder | 1.65 (1.27– 2.14) | 1.22 (1.00–1.49) |
|
| ||
| Surgeon conducted more than 200 complex repairs | 1.31 (0.93–1.85) | 1.11 (0.77–1.59) |
CI, confidence interval.
Each variable was adjusted for all other variables listed in the table
Similarly, a wide variety of patient characteristics, fistula characteristics and context of repair variables were significantly (p<0.5) associated with residual incontinence in women with closed fistula in unadjusted analyses (Table 2). Following adjustment for other patient, fistula and contextual factors, only prior repair, severe vaginal scarring and urethral involvement remained significant predictors of residual incontinence in women with closed fistula at 3 months post-repair (Table 3). Women with prior repair or severe vaginal scarring had a 1.3 times greater risk of residual incontinence, while women with urethral involvement (partial or complete involvement, or circumferential fistulas) had approximately a 2 times greater risk of residual incontinence.
None of the contextual factors were significantly associated with fistula closure at 3 months. Although women whose surgery was conducted during a training were significantly (p<0.05) more likely to have residual incontinence at follow-up, the association did not remain in multivariable analysis.
DISCUSSION
Our results provide further evidence supporting the role of vaginal scarring and urethral involvement in predicting failure to close the fistula and residual incontinence following fistula closure. (5,7,8,11,16,17) They also support the smaller number of studies suggesting a negative prognostic role of bladder size and prior repair. (6,7,11,16)
We found, as others have,(7,17,20) no relationship between fistula size and successfully closure after repair surgery. It is plausible that fistula size, independent of other factors, is not a predictor of repair outcome because even large defects can be surgically closed, whereas other factors such as prior repair or severe scarring (that may reduce the amount of viable tissue), or urethral involvement (that may affect sphincter mechanisms), cannot be easily addressed surgically. Our results also contribute to the growing body of evidence showing that patient characteristics and co-morbidities do not independently predict fistula closure or residual incontinence after successful closure.(7–9,16,20)
A systematic review of the MEDLINE database from 1970 to 2010, using the following topic headings: “obstetric fistula,” “vaginal fistula,” “urinary bladder fistula,” “vesicovaginal fistula” and “fistula” identified no published studies that reported the effect of contextual factors on repair outcomes; most studies have been conducted at a single site, which has not allowed for comparisons of different contexts. It is reassuring to note from our data that outreach models of service delivery, where relatively large numbers of women are repaired over a short period of time, appear to have no negative impact on repair outcome; neither do training sessions, presumably because of the availability of surgeons with advanced skills in both circumstances. Quality of services most likely also plays a role, although we did not assess that.
Although we found that the number of complex repairs previously performed by surgeons was not a significant predictor of repair outcomes, clearly, specialized skills are required for fistula surgery. All surgeries were conducted by trained fistula surgeons or by a trainee under the direct guidance of an experienced fistula surgeon. Surgeons who reported having conducted more than 200 complex repairs were repairing fistulas with a worse prognosis. While the analyses controlled for a variety of fistula characteristics we cannot exclude the possibility of residual confounding by prognosis.
Some limitations should be kept in mind. Although we accounted for a wide variety of patient and fistula characteristics and several contextual factors, the study was observational and the results may be subject to confounding by factors that were not measured or controlled for. Socio-demographic, obstetric history and causative delivery data were subject to the inaccuracies and reporting biases inherent in self-reported data. Laboratory measurements for some comorbidities and standard definitions for some fistula characteristics were not available, which may have led to underreporting or bias in our results. We believe that errors in exposure measurement were non-differential, and that any bias would be toward the null.
For some variables examined in the bivariable and multivariable analyses, we were missing data from some women. Provider experience was the only variable where we determined missing data could have been associated with outcome. Values were missing for 64 women repaired by nine surgeons. To assess potential bias this might have introduced, we conducted a sensitivity analysis, imputing different values for this variable for the nine surgeons. Our results remained unchanged, increasing our confidence that no bias was introduced by excluding these cases.
Loss to follow-up varied across sites and our results may have been biased if women who completed the study differed from those who were lost; while we cannot rule out this possibility, loss to follow-up was remarkably low. Given the exploratory nature of the study, we may not have had sufficient power to detect small significant differences in some analyses.
This study is one of the largest collections of data assessing fistula repair outcomes across multiple sites and countries, and one of few studies that followed women after discharge to determine predictors of longer-term repair outcomes. We hypothesize a number of reasons to explain the unusually high follow-up, primarily related to the unique nature of fistula patients and services. Fistula patients often develop a relationship with the staff. This is, for many, the first time since their fistula occurred that someone has listened to their concerns, addressed their condition and treated them with dignity and respect. Additionally, women often reside at the facility for extended periods of time before, during and after their repair. We asked staff to repeatedly stress the importance of returning for the follow-up visit. Women may also have wanted to take advantage of the opportunity for a check-up or, in the case of women with continued incontinence, to seek further care. It is possible that reimbursing transport costs and/or giving women a small gift at follow-up may have encouraged women to return. The high follow-up rate suggests that it might be possible to assess even longer-term outcomes in future studies with fistula clients.
Our results may help surgeons to make decisions about the skill level needed to repair individual patients as well as to communicate adequately to fistula patients about the possibility of a failed repair or remaining residual incontinence given the characteristic of their fistula. They also provide further evidence to support inclusion of certain fistula characteristics, particularly vaginal scarring and urethral involvement, in prognostic classification systems.
Acknowledgments
Funded by the U.S. Agency for International Development (USAID), under associate cooperative agreement GHS-A-00-07-00021-00. The second author’s doctoral research, which contributed analyses to this manuscript, was supported by a Ruth L. Kirschstein National Research Service Award. Views expressed here do not necessarily reflect those of USAID or the U.S. National Institutes of Health.
The authors thank the study coordinators, physicians, nurses, and social workers from the study sites for assistance with patient care and data collection: Mary Ellen Stanton, Erin Mielke, Patricia MacDonald, Neal Brandes, John Yeh (currently at Harvard Medical School) and Chelsea Smart from USAID for collaborating on and funding of the study; Drs. Florina Serbanescu and Paul Stupp from the U.S. Centers for Disease Control and Prevention for assistance with the sample size calculations; Karen Beattie and Evelyn Landry from Fistula Care for project management, contributions to interpretation of the analysis, and review of the manuscript; EngenderHealth staff in Bangladesh, Guinea, Nigeria, Uganda, and New York, and Fistula Care’s partner REF in Niger, for their assistance in supporting study implementation; and Altine Diop, Julianne Deitch and Sarah Burgess for assisting in data entry and cleaning. The individuals noted above provided their assistance as part of their routine work duties, with support from the institution that employs then or with funds from the study.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented in part at the International Society of Fistula Surgeons conference in Dakar Senegal, 7-9 December 2010.
References
- 1.AbouZahr C. Prolonged and obstructed labor. In: Murray CLA, editor. The health dimensions of sex and reproduction: the global burden of sexually transmitted diseases and HIV, maternal conditions, perinatal disorders and congenital anomalies. Boston, MA: Harvard University Press; 1998. pp. 242–66. [Google Scholar]
- 2.Waaldijk K, Armiya’u YD. The obstetric fistula: A major public health problem still unsolved. International Urogynecology Journal. 1993;4:126–8. [Google Scholar]
- 3.Wall LL. Obstetric vesicovaginal fistula as an international public-health problem. Lancet. 2006;368:1201–9. doi: 10.1016/S0140-6736(06)69476-2. [DOI] [PubMed] [Google Scholar]
- 4.Frajzyngier V, Ruminjo J, Barone MA. Factors influencing urinary fistula repair outcomes in developing countries: a systematic review. Am J Obstet Gynecol. 2012 doi: 10.1016/j.ajog.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayondo M, Wasswa S, Kabakyenga J, et al. Predictors and outcome of surgical repair of obstetric fistula at a regional referral hospital, Mbarara, western Uganda. BMC Urol. 2011;11:23. doi: 10.1186/1471-2490-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz O, Bowling CB, Gerten KA, et al. Factors influencing post-operative short-term outcomes of vesicovaginal fistula repairs in a community hospital in Liberia. 2011;4:259–65. doi: 10.1016/j.bjmsu.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjoveian S, Vangen S, Mukwege D, Onsrud M. Surgical outcome of obstetric fistula: a retrospective analysis of 595 patients. Acta Obstet Gynecol Scand. 2011;90:753–60. doi: 10.1111/j.1600-0412.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirschner C, Yost K, Du H, Karshima J, Arrowsmith S, Wall L. Obstetric fistula: the ECWA Evangel VVF Center surgical experience from Jos, Nigeria. International Urogynecology Journal. 2010;21:1525–33. doi: 10.1007/s00192-010-1231-0. [DOI] [PubMed] [Google Scholar]
- 9.Lewis A, Kaufman MR, Wolter CE, et al. Genitourinary fistula experience in Sierra Leone: review of 505 cases. J Urol. 2009;181:1725–31. doi: 10.1016/j.juro.2008.11.106. [DOI] [PubMed] [Google Scholar]
- 10.Muleta M, Tafesse B, Aytenfisu HG. Antibiotic use in obstetric fistula repair: single blinded randomized clinical trial. Ethiop Med J. 2010;48:211–7. [PubMed] [Google Scholar]
- 11.Nardos R, Browning A, Chen CC. Risk factors that predict failure after vaginal repair of obstetric vesicovaginal fistulae. Am J Obstet Gynecol. 2009:200–578. e1–4. doi: 10.1016/j.ajog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Nardos R, Browning A, Member B. Duration of bladder catheterization after surgery for obstetric fistula. Int J Gynaecol Obstet. 2008;103:30–2. doi: 10.1016/j.ijgo.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Olusegun AK, Akinfolarin AC, Olabisi LM. A review of clinical pattern and outcome of vesicovaginal fistula. J Natl Med Assoc. 2009;101:593–5. doi: 10.1016/s0027-9684(15)30946-9. [DOI] [PubMed] [Google Scholar]
- 14.Safan A, Shaker H, Abdelaal A, Mourad MS, Albaz M. Fibrin glue versus martius flap interpositioning in the repair of complicated obstetric vesicovaginal fistula. A prospective multi-institution randomized trial. Neurourol Urodyn. 2009;28:438–41. doi: 10.1002/nau.20754. [DOI] [PubMed] [Google Scholar]
- 15.Browning A. Lack of value of the Martius fibrofatty graft in obstetric fistula repair. Int J Gynaecol Obstet. 2006;93:33–7. doi: 10.1016/j.ijgo.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Browning A. Risk factors for developing residual urinary incontinence after obstetric fistula repair. BJOG. 2006;113:482–5. doi: 10.1111/j.1471-0528.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 17.Goh JT, Browning A, Berhan B, Chang A. Predicting the risk of failure of closure of obstetric fistula and residual urinary incontinence using a classification system. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1659–62. doi: 10.1007/s00192-008-0693-9. [DOI] [PubMed] [Google Scholar]
- 18.Holme A, Breen M, MacArthur C. Obstetric fistulae: a study of women managed at the Monze Mission Hospital, Zambia. BJOG. 2007;114:1010–7. doi: 10.1111/j.1471-0528.2007.01353.x. [DOI] [PubMed] [Google Scholar]
- 19.Morhason-Bello IO, Ojengbede OA, Adedokun BO, Okunlola MA, Oladokun A. Uncomplicated midvaginal vesico-vaginal fistula repair in Ibadan: A comparison of the abdominal and vaginal routes. Annals of Ibadan Postgraduate Medicine. 2008;6:39–43. doi: 10.4314/aipm.v6i2.64051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raassen TJ, Verdaasdonk EG, Vierhout ME. Prospective results after first-time surgery for obstetric fistulas in East African women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:73–9. doi: 10.1007/s00192-007-0389-6. [DOI] [PubMed] [Google Scholar]
- 21.Chigbu CO, Nwogu-Ikojo EE, Onah HE, Iloabachie GC. Juxtacervical vesicovaginal fistulae: outcome by route of repair. J Obstet Gynaecol. 2006;26:795–7. doi: 10.1080/01443610600984651. [DOI] [PubMed] [Google Scholar]
- 22.Melah GS, Massa AA, Yahaya UR, Bukar M, Kizaya DD, El-Nafaty AU. Risk factors for obstetric fistulae in north-eastern Nigeria. J Obstet Gynaecol. 2007;27:819–23. doi: 10.1080/01443610701709825. [DOI] [PubMed] [Google Scholar]
- 23.Murray C, Goh JT, Fynes M, Carey MP. Urinary and faecal incontinence following delayed primary repair of obstetric genital fistula. BJOG. 2002;109:828–32. doi: 10.1111/j.1471-0528.2002.00124.x. [DOI] [PubMed] [Google Scholar]
- 24.Rangnekar NP, Imdad Ali N, Kaul SA, Pathak HR. Role of the martius procedure in the management of urinary-vaginal fistulas. J Am Coll Surg. 2000;191:259–63. doi: 10.1016/s1072-7515(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 25.Tomlinson AJ, Thornton JG. A randomised controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 1998;105:397–9. doi: 10.1111/j.1471-0528.1998.tb10122.x. [DOI] [PubMed] [Google Scholar]
- 26.Bland KG, Gelfand M. The influence of urinary bilharziasis on vesico-vaginal fistula in relation to causation and healing. Trans R Soc Trop Med Hyg. 1970;64:588–92. doi: 10.1016/0035-9203(70)90081-7. [DOI] [PubMed] [Google Scholar]
- 27.Kriplani A, Agarwal N, Parul, Gupta A, Bhatla N. Observations on aetiology and management of genital fistulas. Arch Gynecol Obstet. 2005;271:14–8. doi: 10.1007/s00404-004-0680-4. [DOI] [PubMed] [Google Scholar]
- 28.Carter RE, Zhang X, Woolson RF, CCA Statistical analysis of correlated relative risks. Journal of Data Science. 2009;7:397–407. [Google Scholar]
- 29.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]