Abstract
Objective:
The objective of this study was to evaluate exposure to disease-modifying therapies (DMTs) during pregnancy in 335 pregnancies of multiple sclerosis (MS) patients and to further determine whether exclusive breastfeeding of MS mothers has any relevant influence on postpartum relapse rate.
Background:
Only limited data are available on whether DMT exposure during pregnancy affects relapse rate during pregnancy or after birth. Currently, findings on beneficial effect of exclusive breastfeeding on MS disease course are controversially discussed.
Methods:
We enrolled pregnant women with MS who contacted us directly or via their treating physicians to be included in our nationwide MS and pregnancy database.
Results:
We identified 78 pregnancies under interferon-beta (IFNβ) preparations, 41 under glatiramer acetate (GLAT), and 216 pregnancies without DMT exposure during pregnancy. As expected, annualized relapse rate (ARR) decreased continuously during pregnancy in nonexposed mothers (p < 0.001) to then increase after birth. In IFNβ- or GLAT-exposed women this typical pattern was not as obvious. Congenital anomalies were within normal ranges in exposed pregnancies. In total, 170 women were identified who exclusively breastfed (EBF). Significantly reduced postpartum relapse rate during the first 3 months after birth were registered in the EBF group as compared with nonexclusively breastfeeding (NEBF) or nonbreastfeeding women (NBF) women with MS (p < 0.0001). Relapse rate (RR) in the year before pregnancy had been similar throughout all groups. We did not observe any significant differences in RR of NEBF and NBF women.
Conclusion:
Exclusive breastfeeding showed some beneficial effects on postpartum relapse rate in our cohort. Our data support that IFNβ and GLAT do not seem to represent a major teratogenic risk in pregnancy.
Keywords: breastfeeding, disease-modifying therapy (DMT), relapse rate
Introduction
Multiple sclerosis (MS) is the most common disabling neurological disease in young women of childbearing age with an increasing incidence in females [Orton et al. 2006]. Data available so far shows no negative long-term impact of pregnancy on MS progression [Confavreux et al. 1998; Koch et al. 2009; Ramagopalan et al. 2012; Vukusic et al. 2004]. However, typically a continuous short-term reduction of relapse rate (RR) in the course of pregnancy occurs, followed by increased RR after delivery. Known risk factors for enhanced disease activity after birth are relapses in the year before and during pregnancy [Vukusic et al. 2004]. Nevertheless, about 25% of the women suffer relapses during 40 weeks of pregnancy, whereas nearly 30% of all patients suffer from relapses during the initial 3 months after birth [Hellwig et al. 2008; Vukusic et al. 2004]. These data were mainly driven from studies with untreated women during and after pregnancy. Little is known whether treatment of MS with disease-modifying therapies (DMTs) has an impact on RR during and after pregnancy. More recently published studies assessed mainly the safety of DMT- pregnancy exposures [Amato et al. 2010; Boskovic et al. 2005; Weber-Schoendorfer and Schaefer, 2009], or followed only small numbers of DMT-exposed pregnancies while describing epidemiological characteristics of pregnant women with MS [De Las Heras et al. 2007; Fernandez Liguori et al. 2009]. Therefore, the objective of our present study was to evaluate the effects of DMT exposition during pregnancy on RR and to simultaneously assess safety data on pregnancy outcomes.
Another important, currently controversially discussed issue is the possible benefit of exclusive breastfeeding on the postpartum RR [Airas et al. 2010; Hellwig et al. 2009b; Langer-Gould et al. 2009; Portaccio et al. 2011]. We therefore elucidated also the role of exclusive, nonexclusive and nonbreastfeeding on postpartum RR.
Patients and methods
This study was approved by the ethical committee of the Ruhr-University Bochum, Germany (314108). Participants gave written or oral witnessed consent.
Data from our nationwide MS pregnancy database from female relapsing–remitting MS (RRMS) patients with pregnancy or child delivery during the last 10 years were analysed. Previous studies of our group confirmed the reliability of the data in the sense of reproducing the typical course of relapses during and after pregnancy of non-DMT exposed pregnancies [Hellwig et al. 2008, 2009a]. This independent database has achieved wide acceptance in view of the obvious legal constraints to perform systematic studies. Similar to teratologic information services (TIS), we are contacted by neurologists and MS patients who seek advice for any reproductive question concerning MS and especially MS therapies. In a prospective follow up, we accompany MS pregnancies systematically by either telephone interviews every pregnancy trimester until 3 months after delivery or by visits in our university outpatient clinic. All information is obtained via standardized and structured interviews with standardized topics including obstetrical/breastfeeding and neurologic (MS) history and characteristics of the newborns by a MS-specialized consultant neurologist. For this study we differentiated between women who became pregnant under any interferon-beta (IFNβ) preparation (IFN mothers), under glatiramer acetate (GLAT mothers) and women with MS who became pregnant without DMT (non-DMT mothers).
To assess exclusivity of breastfeeding we additionally asked when the first regular formula food was introduced and differentiated between: (a) women breastfeeding exclusively for a minimum of 4 months (EBF mothers), (b) women breastfeeding nonexclusively, defined if at least one breastfeeding meal was replaced by formula meal (NEBF mothers), and (c) women who did not breastfeed at all (NBF mothers). Aside from patients’ information, we obtained additional information of MS disease course and RR from treating neurologists. In cases of retrospective follow up in patients who had been pregnant during the last 10 years, we invited these individuals to contact us for personal interviews with above-mentioned items by announcements on websites of the national German MS Society (www.dmsg.de) and other MS organizations including advertisements in journals for MS patients. We defined a pregnancy as DMT exposed if the last injection was administered after the last menstruation period (LMP).
Statistical analysis
For the annualized relapse rate (ARR) during three trimesters of pregnancy and the first 3 months after birth, RR was subdivided into three monthly intervals; a nonparametric Mann–Whitney U-test was performed for statistical analyses as presented data sets did not pass normality testings (GraphPad Prism, La Jolla, CA, USA).
Results
We followed n = 335 MS pregnancies in total, with n = 109 of them in a prospective fashion. A total of 78 pregnancies were exposed to IFNβ (n = 15 IFNβ-1b, n = 63 IFNβ-1a), with a mean exposure of IFNβ to gestational week (gw) of 8.8 ± 0.5 weeks. A total of n = 38 of these patients were followed prospectively. Of n = 41 GLAT-exposed pregnancies (mean exposure to gw 6.5 ± 6.7 weeks), n = 13 were followed prospectively.
Five patients continued treatment throughout pregnancy (n = 4 IFNβ, n = 1 GLAT) due to the advice of the treating neurologist. One patient accidently injected IFNβ (22 μg Rebif®) until the second pregnancy trimester. All other DMT-exposed women stopped DMT during the first pregnancy trimester.
As a control group, n = 216 non-DMT exposed pregnancies were included, n = 58 of which were followed prospectively. A total of n = 88 (40.7%) women in the control group were treated with DMT prior to pregnancy, but had stopped treatment in advance to pregnancy.
There were no significant differences in age between the groups; MS disease duration was significantly longer for GLAT-exposed women (Table 1).
Table 1.
Characteristics of cohorts.
| Non-DMT-exposed (n = 216) | IFNβ (n = 78) | GA exposed (n = 41) | p-value | |
|---|---|---|---|---|
| Age, mean (SD) in years | 31.01 (± 4.57) | 31.03 (± 4.05) | 31.29 (± 3.42) | |
| Disease duration, median (range) in years | 4.95 (± 3.74) | 5.38 (± 3.87) | 6.58 (± 3.93) | |
| RR prior 1 year, mean (SD) | 0.9 (± 1.8) | 1.04 (± 0.75) | 0.68 (± 4.7) | |
| RR during pregnancy mean (SD), p-values (as compared with prepregnancy RR) | ||||
| 1. Trimester | 0.37 (± 1.2) | 0.38 (± 1.2) | 0.2 (± 0.8) | |
| p < 0.0001 | p < 0.0001 | p = 0.002 | ||
| 2. Trimester | 0.26 (± 1) | 0.1 (± 0.7) | 0.3 (± 1.1) | |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | ||
| 3. Trimester | 0.15 (± 8.8) | 0.38 (± 1.2) | 0.1 (± 0.6) | |
| p < 0.0001 | p < 0.0001 | p = 0.01 | ||
| RR postpartum mean (SD), p-values (as compared with last trimester pregnancy RR, respectively) | 1.3 (± 1.9) | 0.8 (± 1.6) | 0.6 (± 1.4) | |
| p < 0.0001 | p < 0.0001 | p < 0.02 | ||
| EBF n (%) | 113 (53) | 37 (49) | 20 (51) | ns/ns |
| MS treatment | 20 (9.3) | 30 (38.5) | 16 (39) | p < 0.001 |
| IVIG n (%) postpartum | p < 0.001 | |||
| DMT (IFNβ, GA) Within 1 month postpartum | 41 (19) | 25 (32.05) | 6 (10) | p < 0.001 |
| ns |
DMT, disease-modifying therapy; IVIG, intravenous immunoglobulin; MS, multiple sclerosis; IFN, interferon; GA, glatiramer acetate; RR, relapse rate; EBF, exclusively breastfed.
Data are given as mean ± SD, respectively as total number.
p < 0.001 of the analysis of variance (ANOVA) between the three cohorts; age, duration and total time is given in years.
Last column: first p-value: non-DMT exposed compared with IFN; second p-value: non-DMT-exposed compared with GA.
Relapses
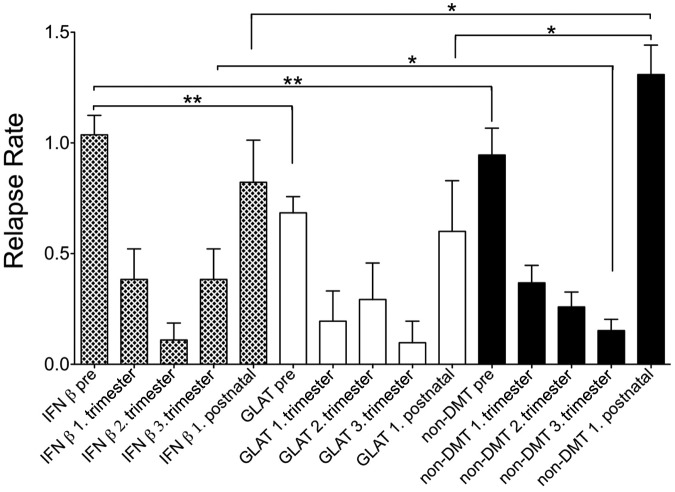
As described in more detail in Table 1, RR decreased significantly during pregnancy (Figure 1; 1.–3.trimester) in all three groups compared with the RR in the year before pregnancy (Figure 1; pre).
Figure 1.
Annual relapse rate before and during pregnancy.
I, II, III = I, II, III Trimester, ARR = annual relapse rate. * = p < 0.05, ** = p < 0.01; *** = p < 0.001 of the comparison ARR before versus ARR I respectively, ARR II, ARR III; data are given as mean ± SD.
After birth, RR during the first trimester increased significantly in all three groups (IFNβ, GLAT and controls) compared with the last pregnancy trimester (Table 1). In the control group (non-DMT mothers) we observed the typical pattern with a continuous decrease in relapses during pregnancy that increased after birth (Figure 1). This typical pattern was not seen as clearly for IFNβ- and GLAT-exposed women (Figure 1). We did not observe any significant differences in RR during pregnancy between the three groups. Interestingly, we observed a significantly lower RR during first trimester after birth in the IFNβ- and GLAT-exposed pregnancies as compared with non-DMT exposed pregnancies (Table 1 and Figure 1). Although the number of women who breastfed exclusively between the three groups did not differ significantly, significantly more women in the IFNβ- and GLAT-exposed group were treated with intravenous immunoglobulin (IVIG) after birth, and significantly more women in the IFNβ-exposed group were re-treated with IFNβ during the first weeks after birth (Table 1).
Effects of exclusive, nonexclusive and nonbreastfeeding on disease activity
In total n = 170 women throughout all groups breastfed exclusively. EBF was associated with significantly reduced RR postpartum as compared NEBF (RR-EBF 0.68±1.5 as compared with RR-NEBF 1.5 ± 2.1, p < 0.001; Figure 2). This difference was also seen when comparing RR postpartum between EBF and NBF (RR-NBF 1.68± 2.1 p<0.001; Fig.2). RR between NEBF and NBF women did not differ significantly (Figure 2). RR before pregnancy between EBF (0.8 ± 0.7), NEBF (0.9 ± 1.2) and NBF (0.9 ± 0.7) did not reveal significant differences.
Figure 2.
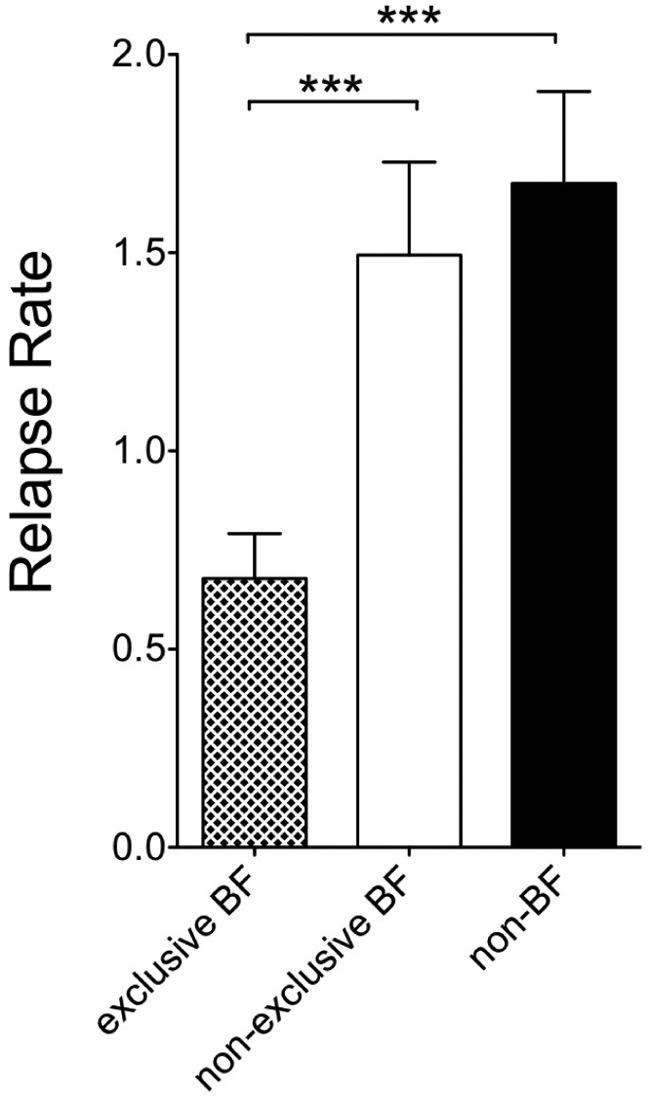
Annual relapse rate in the first trimester postpartum according to breastfeeding status.RR = relapse rate.
*** = p < 0.001 data are given as mean ± SD.
Comparing RR in IFNβ-, GLAT- and non-DMT-exposed mothers in the context of EBF, NEBF and NBF we observed differences for non-DMT-exposed mothers: during the third trimester after birth EBF led to significantly reduced RR (RR-EBF 0.1 ± 0.7) as compared with RR-NEBF (1.7 ± 2.3) and RR-NBF (2 ± 2.2) (p < 0.0001).
For IFNβ-exposed mothers only the comparison between RR-EBF and RR-NBF did reach statistical significance (p = 0.001), with a trend towards higher RR in NEBF women.
Pregnancy outcomes
Characteristics of neonates
No differences regarding birth weight (IFNβ-exposed mothers, 3260 ± 606 g; GLAT-exposed mothers, 3295 ± 688g; and non-DMT-exposed mothers, 3383 ± 544 g), body length of the newborn babies (IFNβ-exposed mothers, 51.0 ± 2.3 cm; GLAT-exposed mothers, 51.5 ± 2.7 cm; and non-DMT-exposed mothers 51.4 ± 2.6 cm) and gestational age (IFNβ-exposed mothers, 38.9 ± 2.4 gw; GLAT-exposed mothers, 39.2 ± 1.7 gw; and non-DMT-exposed mothers, 39.1 ± 2.3 gw) were observed between groups. Preterm birth was equally contributed in all groups.
Pregnancy, abnormalities and DMT exposure
Abnormalities of babies of MS mothers who were exposed to DMT during pregnancy occurred in n = 6 newborns. These included:
IFNβ (n = 3/78): ventricular septal defect (VSD), valvular stenosis of the pulmonary artery and hip dysplasia;
GLAT (n = 2/41): abnormality of urinary bladder valves and hip dysplasia;
Non-DMT (n = 7/216): VSD, medium chain acetyl CoA dehydrogenase deficiency, enzymatic deficiency of the glycogen metabolism, Down’s syndrome and VSD, large nevus cell nevi, periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) and a rare genetic Wolf Hirschhorn syndrome.
Discussion
To the best of the authors’ knowledge, this observational study is the largest reported study so far on MS patients evaluating the effects of basic immunomodulatory drugs on relapse rate and pregnancy outcome, taking into account the role of breastfeeding on disease activity.
Our data confirm the typical and known pattern of RR reduction during pregnancy, with an increase after birth, which held generally true for all groups indicating the reliability of the database [Confavreux et al. 1998; Nelson et al. 1988]. While non-DMT-exposed women showed a continuous decrease of relapses during pregnancy this pattern was not as evident for DMT-exposed mothers; the smaller sample size might account for this observation. Despite the official approval of IFNβ during pregnancy, most treating physicians, including those at our centre, advise their patients to stop treatment during pregnancy as the natural disease-reducing effect of pregnancy is well known [Nelson et al. 1988; Vukusic et al. 2004]. Since the vast majority of the women included in our study had stopped treatment during the first trimester we cannot draw any conclusions on DMT exposure beyond that time point.
Interestingly, RR during first postpartum trimester was significantly lower in both GLAT- and IFNβ-exposed mothers when compared with non-DMT-exposed mothers. Thus, we could not identify any factors accounting for this difference, e.g. disease activity before/during pregnancy or exclusive breastfeeding, other than early DMT use in IFNβ-exposed women and the use of IVIG postpartum in GLAT- and IFNβ-exposed mothers.
Even when considering a false-positive rate of 5%, these results provide new insights into interactions between pregnancy, MS and DMT exposure. Drug exposure is of particular interest as available data on MS course after pregnancy have been mainly obtained from unexposed women. A second important finding of our study is a further confirmation of data previously published by our group and others [Hellwig et al. 2009b; Langer-Gould et al. 2009], showing that women who breastfed exclusively had significantly lower postpartum disease activity during first postpartum trimester postpartum as compared with women who did not breastfed exclusively or not at all. Similar to the pilot study by Langer-Gould and colleagues [Langer-Gould et al. 2009], we did not observe a significant difference in those women who breastfed partially versus those who did not breastfeed at all. A possible explanation might be that only exclusive breastfeeding on demand suppresses ovarian function, with consecutive amenorrhea through high prolactin levels and reduced luteinizing hormone pulsatility [McNeilly, 2001]; in this context, high prolactin levels were shown to promote remyelination in experimental settings [Gregg et al. 2007].
So far, we recommend that mothers with MS who wish to breastfeed their child to do so exclusively during the first 4–6 months after birth. There is no strong evidence suggesting the optimal time point to ablactate and to reintroduce DMT. Breastfeeding mothers are recommended not to start DMT after birth, as there are no reliable data available on drug transfer into milk and their effects on newborns [Coyle et al. 2004]. If women choose not to breastfeed, we recommend to start DMT as soon as possible after birth as there is sufficient evidence for INFβ and GLAT have a delayed onset of efficacy [Comi et al. 2001; Li and Paty, 1999]. Prospective data evaluating the effect of very early DMT reintroduction are needed.
Concerning drug safety, our data further support the evidence that there is no obvious pattern of malformation attributable to DMT exposure and none of the first-line MS drugs (IFNβ and GLAT) was teratogenic in our cohort [Amato et al. 2010; Hellwig and Gold, 2011; Sandberg-Wollheim et al. 2011; Weber-Schoendorfer and Schaefer, 2009]. A study evaluating the effect of IFNβ in human and animal pregnancy showed higher risks for abortion under IFNβ treatment [Boskovic et al. 2005], which has not been confirmed by others [Amato et al. 2010; Lu et al. 2011; Sandberg-Wollheim et al. 2011; Weber-Schoendorfer and Schaefer, 2009]. Thus, the risk for abortions in women treated with IFNβ is not fully clarified. Larger pregnancy registries by respective pharmaceutical companies imply a low abortifacient risk for IFNβ [Sandberg-Wollheim et al. 2011]; however small the risk, female MS patients should be informed that the abortifacient potential of IFNβ is not entirely excluded.
We did not observe an increased risk for premature birth, birth weight reduction or abnormalities in neonates of DMT-exposed mothers, neither did we observe increase of foetal malformation in our study cohort. However, according to current guidelines, we clearly advise that DMT should be stopped during pregnancy. The number of the current study participants is not large enough to evaluate the risks related to DMT administration or to allow firm conclusions with regard to safety during pregnancy.
Its observational and partially retrospective character limits this study. For instance paraclinical disease parameters as MRI activity are not included in our database yet. Furthermore, selection bias and, particularly for duration of breastfeeding, a recall bias cannot be excluded. However, our results partially answer and raise some pressing questions concerning pregnancy in MS patients.
In conclusion, our results confirm reduced RR during pregnancy, followed by increased RR after birth in non-DMT-exposed women. The course of pregnancies developed under DMT needs further investigation. Whether IVIG has any additional beneficial effect together with EBF, or whether EBF alone exerts protective features will be investigated in ongoing prospective studies.
Acknowledgments
We thank the MS patients for their voluntary participation and the German MS society (DMSG) as well as diverse patients’ internet platforms and referring neurologists for their support. Aiden Haghikia completed the statistical analyses.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This study database was partly supported by Bayer-Schering Healthcare, Biogen-Idec Germany, Merck-Serono, Teva, Sanofi Aventis and Novartis Pharma.
Conflict of interest statement: This MS and pregnancy database was partly supported by Bayer-Schering Healthcare, Biogen-Idec Germany, Merck-Serono, Teva Pharma, Novartis Pharma and Sanofi Aventis. Dr Hellwig has received speakers honoraria and research support from Bayer-Schering Healthcare, Biogen-Idec Germany, Merck-Serono, Teva Pharma, Novartis Pharma and Sanofi Aventis. Dr Haghikia has received limited research support from Biogen Idec and speakers honoraria from Bayer Healthcare, Biogen Idec, Teva and Merck Serono. Milena Rockhoff has nothing to disclose. Professor Gold has received speakers honoraria and research support from Bayer-Schering Healthcare, Biogen-Idec Germany, Merck-Serono, Teva Pharma, Novartis Pharma and Sanofi Aventis.
Contributor Information
Kerstin Hellwig, Department of Neurology, St. Josef Hospital, Ruhr University Bochum, Gudrunstrasse 56, 44791 Bochum, Germany.
Aiden Haghikia, Department of Neurology, Ruhr University, Bochum, Germany.
Milena Rockhoff, Department of Neurology, Ruhr University, Bochum, Germany.
Ralf Gold, Department of Neurology, Ruhr University, Bochum, Germany.
References
- Airas L., Jalkanen A., Alanen A., Pirttila T., Marttila R. (2010) Breast-feeding, postpartum and prepregnancy disease activity in multiple sclerosis. Neurology 75: 474–476 [DOI] [PubMed] [Google Scholar]
- Amato M., Portaccio E., Ghezzi A., Hakiki B., Zipoli V., Martinelli V., et al. (2010) Pregnancy and fetal outcomes after interferon-beta exposure in multiple sclerosis. Neurology 75: 1794–1802 [DOI] [PubMed] [Google Scholar]
- Boskovic R., Wide R., Wolpin J., Bauer D., Koren G. (2005) The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology 65: 807–811 [DOI] [PubMed] [Google Scholar]
- Comi G., Filippi M., Wolinsky J. (2001) European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging-measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group; Ann Neurol 49: 290–297 [PubMed] [Google Scholar]
- Confavreux C., Hutchinson M., Hours M., Cortinovis-Tourniaire P., Moreau T. (1998) Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group; N Engl J Med 339: 285–291 [DOI] [PubMed] [Google Scholar]
- Coyle P., Christie S., Fodor P., Fuchs K., Giesser B., Gutierrez A., et al. (2004) Multiple sclerosis gender issues: clinical practices of women neurologists. Mult Scler 10: 582–588 [DOI] [PubMed] [Google Scholar]
- De Las Heras V., De Andres C., Tellez N., Tintore M. (2007) Pregnancy in multiple sclerosis patients treated with immunomodulators prior to or during part of the pregnancy: a descriptive study in the Spanish population. Mult Scler 13: 981–984 [DOI] [PubMed] [Google Scholar]
- Fernandez Liguori N., Klajn D., Acion L., Caceres F., Calle A., Carra A., et al. (2009) Epidemiological characteristics of pregnancy, delivery, and birth outcome in women with multiple sclerosis in Argentina (EMEMAR study). Mult Scler 15: 555–562 [DOI] [PubMed] [Google Scholar]
- Gregg C., Shikar V., Larsen P., Mak G., Chojnacki A., Yong V., et al. (2007) White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci 27: 1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig K., Beste C., Schimrigk S., Chan A. (2009a) Immunomodulation and postpartum relapses in patients with multiple sclerosis. Ther Adv Neurol Disord 2: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig K., Brune N., Haghikia A., Muller T., Schimrigk S., Schwodiauer V., et al. (2008) Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand 118: 24–28 [DOI] [PubMed] [Google Scholar]
- Hellwig K., Gold R. (2011) Glatiramer acetate and interferon-beta throughout gestation and postpartum in women with multiple sclerosis. J Neurol 258: 502–503 [DOI] [PubMed] [Google Scholar]
- Hellwig K., Haghikia A., Agne H., Beste C., Gold R. (2009b) Protective effect of breastfeeding in postpartum relapse rate of mothers with multiple sclerosis. Arch Neurol 66: 1580–1581; author reply 1581 [DOI] [PubMed] [Google Scholar]
- Koch M., Uyttenboogaart M., Heersema D., Steen C., De Keyser J. (2009) Parity and secondary progression in multiple sclerosis. J Neurol Neurosurg Psych 80: 676–678 [DOI] [PubMed] [Google Scholar]
- Langer-Gould A., Huang S., Gupta R., Leimpeter A., Greenwood E., Albers K., et al. (2009) Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol 66: 958–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Paty D. (1999) Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsing-remitting multiple sclerosis. Prevention of Relapses and Disability by Interferon-beta1a Subcutaneously in Multiple Sclerosis Ann Neurol 46: 197–206 [DOI] [PubMed] [Google Scholar]
- Lu E., Dahlgren L., Sadovnick A., Sayao A., Synnes A., Tremlett H. (2011) Perinatal outcomes in women with multiple sclerosis exposed to disease-modifying drugs. Mult Scler, in press [DOI] [PubMed] [Google Scholar]
- McNeilly A. (2001) Lactational control of reproduction. Reprod Fertil Develop 13: 583–590 [DOI] [PubMed] [Google Scholar]
- Nelson L., Franklin G., Jones M. (1988) Risk of multiple sclerosis exacerbation during pregnancy and breast-feeding. JAMA 259: 3441–3443 [PubMed] [Google Scholar]
- Orton S., Herrera B., Yee I., Valdar W., Ramagopalan S., Sadovnick A., et al. (2006) Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 5: 932–936 [DOI] [PubMed] [Google Scholar]
- Portaccio E., Ghezzi A., Hakiki B., Martinelli V., Moiola L., Patti F., et al. (2011) Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology, in press [DOI] [PubMed] [Google Scholar]
- Ramagopalan S., Yee I., Byrnes J., Guimond C., Ebers G., Sadovnick D. (2012) Term pregnancies and the clinical characteristics of multiple sclerosis: a population based study. J Neurol Neurosurg Psychiatry, in press [DOI] [PubMed] [Google Scholar]
- Sandberg-Wollheim M., Alteri E., Moraga M., Kornmann G. (2011) Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler 17: 423–430 [DOI] [PubMed] [Google Scholar]
- Vukusic S., Hutchinson M., Hours M., Moreau T., Cortinovis-Tourniaire P., Adeleine P., et al. (2004) Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain 127: 1353–1360 [DOI] [PubMed] [Google Scholar]
- Weber-Schoendorfer C., Schaefer C. (2009) Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult Scler 15: 1037–1042 [DOI] [PubMed] [Google Scholar]