Abstract
A large proportion of patients with multiple sclerosis (MS) have spasticity, which has a marked impact on their quality of life. Anecdotal evidence suggests a beneficial effect of cannabis on spasticity as well as pain. Recently, randomized, double-blind, placebo-controlled studies have confirmed the clinical efficacy of cannabinoids for the treatment of spasticity in patients with MS. Based on these data, nabiximols (Sativex), a 1:1 mix of Δ-9-tetrahydrocannabinol and cannabidiol extracted from cloned Cannabis sativa chemovars, received approval for treating MS-related spasticity in various countries around the globe. In this article we review the current understanding of cannabinoid biology and the value of cannabinoids as a symptomatic treatment option addressing spasticity in patients with MS.
Keywords: Δ-9-tetrahydrocannabinol, cannabidiol, multiple sclerosis, spasticity, nabiximols
Introduction
Based on individual case reports, the consumption of plant parts, specifically, the resin of the Cannabis sativa hemp plant, has, for years, been attributed to the capacity to reduce the symptoms of multiple sclerosis (MS), such as spasticity, neuropathic pain, tremor, and disturbed bladder function. As characterization of the endocannabinoid system and its role in the motor system and pain processing continue to advance, there is increasing evidence of a scientific basis for the postulated therapeutic effect of cannabis derivatives. The most important active components of C. sativa were identified as the cannabinoids Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), the effects of which are mediated through cannabinoid receptors of the endocannabinoid system. Along with synthetic cannabinoids and oral phytocannabinoids, the drug nabiximols (Sativex, Almirall, Barcelona, Spain), a plant extract from C. sativa, was developed as a pharmaceutical product to make medicinal use of the effects of cannabinoids (Table 1). Nabiximols contains THC and CBD in defined quantities and is administered as an oromucosal spray. Nabiximols received approval in many countries around the globe for treatment of spasticity in MS when conventional antispastic therapy is not adequately effective (Table 2).
Table 1.
Exogenous and endogenous cannabinoids.
| Exogenous cannabinoids |
|---|
| Phytocannabinoids |
| Δ-9-tetrahydrocannabinol (THC) |
| Cannabidiol |
| Synthetic cannabinoids |
| Synthetic THC dronabinol |
| Nabilone |
| Endogenous cannabinoids |
| Arachidonylethanolamide (anandamide) |
| 2-Arachidonoylglycerol |
Table 2.
Approval status for nabiximols around the globe.
| Country | Indication | Approval status* |
|
|---|---|---|---|
| Approved | Submitted | ||
| Canada | Cancer pain, spasticity | x | |
| Austria | spasticity | x | |
| Czech Republic | spasticity | x | |
| Denmark | spasticity | x | |
| Germany | spasticity | x | |
| Israel | spasticity | x | |
| Italy | spasticity | x | |
| Spain | spasticity | x | |
| Sweden | spasticity | x | |
| UK | spasticity | x | |
| New Zealand | spasticity | x | |
| Belgium | spasticity | x | |
| Finland | spasticity | x | |
| Iceland | spasticity | x | |
| Ireland | spasticity | x | |
| Luxembourg | spasticity | x | |
| Norway | spasticity | x | |
| Poland | spasticity | x | |
| Portugal | spasticity | x | |
| The Netherlands | spasticity | x | |
| Slovakia | spasticity | x | |
| Switzerland | spasticity | x | |
| Australia | spasticity | x | |
Approval status by June 2012.
Spasticity = multiple sclerosis-related spasticity, as add-on therapy.
Methods
We performed an extensive MEDLINE search covering publications on ‘cannabinoids’, ‘cannabidiol’, ‘Δ-9-tetrahydrocannabinol’, and ‘spasticity’ combined with ‘multiple sclerosis’ during the period from 1980 to April 2012. Randomized, controlled studies were considered to have the highest level of evidence, followed by one or more well documented clinical studies, such as case-controlled or cohort studies, and the lowest level of evidence was assigned to nonrandomized historical controls, case reports, or expert opinions. Levels of evidence were defined according to the standards of the European Federation of Neurology [Brainin et al. 2004].
The endocannabinoid system
The effect of exogenous cannabinoids is mediated primarily by cannabinoid receptors in the endocannabinoid system. The receptors in this system, which are characterized by seven transmembrane domains, belong to the family of G-protein-coupled receptors. Currently, the existence of two classes of cannabinoid receptors has been verified [Console-Bram et al. 2012]: the CB1 receptors, which are expressed primarily in the central nervous system; and the CB2 receptors that primarily occur in immune cells [Cabral et al. 2008]. CB1 receptors are primarily found in the frontal cortex, in the basal ganglia, the cerebellum, hypothalamus, and hippocampus as well as in the nucleus accumbens and the ventral tegmental area, that is, the regions of the brain that play a key role in the motor system, in the reward system, and in learning processes. In addition, CB1 receptors have been identified in peripheral and central pain pathways, specifically in the terminals of primary afferent nociceptive neurons and in the spinal cord [Fernandez-Ruiz et al. 2010].
CB1 receptors in the central nervous system, which are expressed primarily in the presynaptic membrane of the neurons, utilize a negative feedback mechanism to regulate transmitter release at GABAergic, glutamatergic, and dopaminergic synapses. Two arachidonic acid derivatives, arachidonylethanolamide (anandamide) and 2-arachidonylglycerol, have been identified as the most important endocannabinoids, that is, endogenous ligands at cannabinoid receptors. Cellular activity of the postsynaptic neuron promotes continuous synthesis and release of the endocannabinoid from phospholipids of the cell membrane, which then bind to the CB1 receptors in the presynaptic membrane. Stimulation of the CB1 receptors, which are coupled to inhibitory G proteins, blocks the activity of the presynaptic neurons through activation of A-type potassium channels and potassium inward rectifiers and through the inhibition of voltage-dependent calcium channels and adenylate cyclase. Reduced transmitter release in the presynaptic terminals and reduced cellular excitability are the outcomes [Wegener and Koch, 2009]. The endocannabinoid effect is regulated through the transport of endocannabinoids into the cell via specific transporters and subsequent degradation through the membrane-bound enzyme fatty acid hydrolase [Petrosino and Di Marzo, 2010].
Various cells of the immune system CB2 receptors are endowed with CB2 receptors. These predominantly are B lymphocytes, natural killer cells, and monocytes. However, CB2 receptors can also be detected in the central nervous system, where among others microglia are predominant cellular sources [Basu and Dittel, 2011]. Immunosuppressive mechanisms, such as the induction of apoptosis, the inhibition of cell proliferation and cytokine synthesis, are mediated and regulatory T lymphocytes are induced mainly through inhibition of adenylate cyclase and the consecutive reduction of intracellular cAMP concentrations [Rieder et al. 2010].
Cannabinoids for the treatment of spasticity associated with multiple sclerosis
Studies in animal models and in patients suggest that changes occur in the endocannabinoid system in MS. For instance, in experimental autoimmune encephalomyelitis (EAE), a lowered CB1 receptor density was noted in the basal ganglia and in the cerebellum, that is, regions of the brain that play a key role in the motor system. Interestingly, in the EAE model, an antispastic effect was observed following administration of nonselective CB1/CB2 receptor agonists in wild-type mice with EAE, whereas the effect was no longer detected in CB1 receptor-deficient mice. Therefore, it may be concluded that the antispastic effect of cannabinoids in the mouse model of multiple sclerosis is mediated primarily by CB1 receptors [Pryce and Baker, 2007]. The antispastic effect of CB2 receptor agonists described above was explained in this study by their lack of specificity with cross-linking activity for CB1 receptors.
Furthermore, various experimental models have shown that the activation of cannabinoid receptors in the inflammatory demyelinative process characterizing MS may cause a neuroprotective effect through a CB1 receptor-mediated inhibition of excitotoxicity and through a CB2 receptor-mediated inhibition of neuroinflammation [Sánchez and García-Merino, 2012; Kubajewska and Constantinescu, 2010; Gowran et al. 2010]. In a study of patients with MS by Jean-Gilles and colleagues, increased endocannabinoid levels were found in the plasma of patients that was attributed to a neuroprotective regulatory mechanism of the endocannabinoid system [Jean-Gilles et al. 2009].
Cannabinoids as pharmaceutical products
In the medical use of cannabinoids, smoking and oral administration of hemp plant parts appear to be unsuitable due to the nonstandardized composition of the plant product. Beyond this, smoking cannabis products is a form of drug administration that poses a potential health risk: cannabinoids, and especially smoked inhaled cannabis, are strongly implicated in oncogenesis by several molecular pathways. A population-based, case–control study provided evidence of a relationship between smoking cannabis and lung cancer in young adults. For each joint-year of cannabis smoking the risk of lung cancer was estimated to increase by 8% [Aldington et al. 2008].
A significant argument against therapeutic use is not least the fact that C. sativa is considered an illegal drug in most countries and the related potential lack of societal acceptance. The identification and isolated administration of therapeutically active components of C. sativa would, therefore, be desirable.
The C. sativa hemp plant contains more than 60 cannabinoids [Zajicek et al. 2003], which have various action profiles. In 1964, the cannabinoid THC was identified as the primary psychoactive substance of the hemp plant. In addition to producing psychotropic effects, THC is attributed to have analgesic, muscle relaxant, antiemetic, and appetite-stimulating effects [Novotna et al. 2011]. THC exerts its effects primarily by binding to CB1 receptors [Kubajewska and Constantinescu, 2010]. Only the (–)-trans isomer of THC occurs naturally in the hemp plant. Synthetically produced dronabinol, administered orally, is approved in the USA under the brand name Marinol (Solvay Pharmaceuticals, Brussels, Belgium) for treatment of anorexia among patients with acquired immune deficiency syndrome and as an antiemetic for therapy-resistant nausea induced by chemotherapy. Nabilone, a derivative of THC, is also approved as an orally administered antiemetic under the brand name Cesamet (Valeant Pharmaceuticals International, Toronto, Canada). Rimonabant was the first selective CB1 receptor blocker to be approved in Europe as an antiobesity drug for use in specific patient groups in conjunction with diet and exercise. Because of concerns over suicidality, depression, and other related side effects associated with use of the drug, rimonabant was suspended from the market.
There is also mounting evidence that the unwanted psychotropic effects of treatment with synthetic THC, such as intoxication, sedation, memory impairment, and dysphoria, are mitigated through the administration of plant extracts from C. sativa, which contain a number of other cannabinoids in addition to THC [Russo and Guy, 2006]. This modulation of the THC effect is attributed primarily to the phytocannabinoid CBD, which, on the one hand, inhibits the conversion of THC into its particularly psychoactive metabolites 11-hydroxy-THC, yet, on the other, works synergistically with THC (Figure 1). Experimental evidence suggests that THC as well as CBD exhibit anti-inflammatory and immunomodulatory properties, pointing to additive or potentiating effects of the combination of the two [Jamontt et al. 2010]. Also, it is suspected that CBD delays the absorption of THC, and consequently peak serum concentrations that are associated with the occurrence of unwanted side effects are avoided. CBD is presumed to have antipsychotic, anxiolytic, and anticonvulsant effects. Combination preparations of THC and CBD have been developed taking into consideration the synergistic actions and the reduction of possible side effects. The orally administered plant extract Cannador (Weleda, Arlesheim, Switzerland), which was not sufficiently standardized and contains THC and CBD in a ratio of 2:1, was in use only as a study drug and is not approved. With the advent of the nabiximols in an oromucosal spray formulation a highly standardized approved combination preparation of THC and CBD became available for the treatment of spasticity in MS (Table 3).
Figure 1.
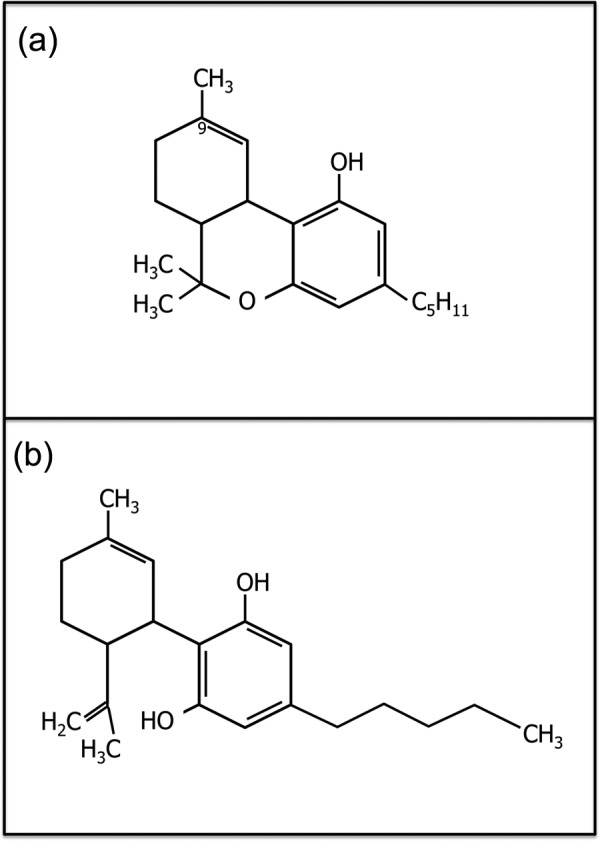
Chemical structure of Δ-9-tetrahydrocannabinol (a) and cannabidiol (b).
Table 3.
Pharmaceutical products with cannabinoid receptor-mediated effects.
| Product | Contents or active substance | Pharmaceutical company | Administration | Approval |
|---|---|---|---|---|
| Sativex | Plant extract, Δ-9-tetrahydrocannabinol (THC) and cannabidiol, 1:1 ratio | GW/Almirall (EU ex. UK)/Bayer (UK, Canada)/Otsuka (USA)/Novartis (other) | Oromucosal spray | Add-on therapy in the treatment of spasticity in multiple sclerosis |
| Cannador | Plant extract, Δ-9-tetrahydrocannabinol and cannabidiol, approx. 2:1 ratio | Society for Clinical Research, Germany | Oral | Study drug, not approved |
| Marinol | Synthetic THC dronabinol [(–)-trans isomer of Δ-9-tetrahydrocannabinol] | Solvay Pharmaceuticals, Belgium | Oral | For therapy of anorexia among patients with acquired immune deficiency syndrome and as antiemetic for chemotherapy-induced, therapy-resistant nausea in the USA |
| Cesamet | Nabilone (synthetic derivative of Δ-9-tetrahydrocannabinol) | Valeant Pharmaceuticals International | Oral | As antiemetic for chemotherapy-induced, therapy-resistant nausea |
| Acomplia | Rimonabant (CB1 receptor antagonist) | Sanofi-Aventis | Oral | As appetite suppressant for obesity and risk factors; removed from the market in 2008 |
Pharmacokinetics and use of nabiximols
Nabiximols is a plant extract that is obtained basically from the dried female flowers of natural C. sativa subspecies, which each produce a high content of THC or CBD. These cannabinoids are extracted from cloned plants, which contain a significantly more uniform cannabinoid profile as well as a higher cannabinoid yield, specifically of THC, compared with those grown from seed. The hydrophobic THC and CBD phytocannabinoids dissolved in ethanol constitute about 70% of the ingredients in nabiximols, but also contain small quantities of other components of the plant extract, such as other cannabinoids and terpenoids. Each dose of the oromucosal spray contains 2.7 mg THC, 2.5 mg CBD, and 0.04 g ethanol [Novotna et al. 2011].
Smoking cannabis products is associated with a very rapid onset, high peak, and short duration of plasma THC concentration, and thus favors the occurrence of side effects; however, absorption of cannabinoids through oral administration is subject to variability due to first pass effects. The oromucosal form of administration has the advantage that first pass effects are reduced and, compared with smoking, the maximum plasma concentration of cannabinoids is lower and increases more slowly, within 2–4 h [Karst et al. 2010]. Yet, the relatively rapid onset of the effect within approximately 15–40 min after administration facilitates treatment adjustment.
Currently the recommendation is to initiate therapy with a dose titration phase of approximately 2 weeks, during which the individually active and tolerable dose should be identified through gradual titration. Individual distribution of the dose over the course of a day is possible along with a dose adjustment in the course of the disease, depending on the severity of spasticity. One dose equals one spray, the maximum dose per day is limited to 12 sprays, and the time between sprays should not be shorter than 15 min. In the study by Novotna and colleagues, in which a maximum dose of 12 sprays a day of nabiximols was similarly recommended, the average daily dose was around 8 sprays [Novotna et al. 2011].
Efficacy of cannabinoids in the therapy of spasticity in multiple sclerosis
Methodological limitations
Assessment of the efficacy of an antispastic therapy is complicated by the fact that spasticity is a symptom that is difficult to quantify. The most commonly used measure of spasticity is the modified Ashworth Scale, in which the degree of spasticity is rated on a scale of 0–4 based on passive movements of the extremities. In most of the published studies so far, even commonly approved antispastic drugs exhibited no significant reduction in the score on the Ashworth Scale, which questions at least whether the Ashworth Scale is appropriate as a measure of spasticity. One of the few exceptions, for example, is the UK Tizanidine study, where a reduction in total Ashworth Score of 3.2 in addition to placebo was found [United Kingdom Tizanidine Trial Group, 1994]. Thus, few authors have now even rejected using this scale due to insufficient validity and reliability [Fleuren et al. 2010; Sunnerhagen 2010].
Evaluation procedures based on the severity of spasticity as subjectively perceived by patients are also available, such as a visual analogue scale. The advantage of subjective scales is that ‘severity’ of spasticity does not necessarily correlate with the functional requirements of the individual patient. When the degree of spasticity in a patient with simultaneously occurring motor weakness is reduced, ambulation may become impossible for a patient who was just able to walk with the help of the existing spasticity. This might result in a different perception in an objective rater and a subjective patients scoring system.
Assessment of the therapeutic effect may be confounded by psychotropic or analgesic effects of cannabinoid therapy, which limits the validity of such subjective scoring procedures.
Overview of relevant clinical studies
In the 1980s and 1990s only isolated studies were published on the efficacy of cannabinoids in treating spasticity in MS. Due to the low case numbers and the heterogeneity of the examined cannabinoids, these studies carried only little weight (for an overview of previously published studies, see the work of Karst and colleagues and Correia de Sa and colleagues [Karst et al. 2010; Correia de Sa et al. 2011]) (Table 4).
Table 4.
Larger studies on efficacy of cannabinoids in the therapy of spasticity in multiple sclerosis.
| Study/author | Product | Design | No. of patients | Primary endpoint | Secondary endpoint |
|---|---|---|---|---|---|
| Killestein et al. [2002] | THC versus Cannabis sativa plant extract | 20 weeks, randomized, double blind, placebo controlled, twofold crossover | 16 | No change in Ashworth Score or EDSS score. Worsening in MSFC. | |
| CAMS Zajicek et al. [2003] | Marinol, Cannador weight-adjusted | 15 weeks, randomized, placebo-controlled; 5 weeks, titration phase, plateau phase in weeks 6–13, downtitration in week 14, follow up in week 15 | 667 | Difference in average reduction of Ashworth Score (from baseline to end of week 13) between active and placebo groups: no significant therapeutic effect | Significant reduction in 10 m walking time in the Marinol group, improvement in spasticity and pain in self evaluations by the patients (p = 0.003) |
| Vaney et al. [2004] | THC plus CBD | Prospective, randomized, double blind, placebo controlled crossover | 57 | No statistically significant differences, trends in favor of active treatment for spasm frequency, mobility and getting to sleep | |
| CAMS-Extension Zajicek et al. [2005] | Marinol, Cannador weight adjusted | Up to 12 months, multicentre, double blind, placebo-controlled extension study of the CAMS study | 502 (80% of CAMS study patients) | Difference in average reduction of Ashworth Score (from baseline to the 12th month) between active and placebo groups: small treatment effect in the Marinol and Cannador groups (p = 0.01) | No significant treatment effects |
| Wade et al. [2006] | Sativex (up to max. 48 sprays per day) | Open label, on average 434 days (21–814) subsequent to a 6-week placebo-controlled study | 137 | Stable reduction of the VAS score (measure of spasticity). Other primary endpoints, including pain, tremor and bladder all negative | 58 patients (42.3%) withdrew due to lack of efficacy |
| Collin et al. [2007] | Sativex (up to max. 48 sprays per day) | 6 weeks, double blind, placebo controlled | 189 | Difference between active and placebo group in the reduction in spasticity with decrease in NRS score. Significant therapeutic effect (p = 0.048) | Among others, Ashworth Score without significant therapeutic effect |
| Novotna et al. [2011] | Sativex (up to max. 12 sprays per day) | 19 weeks, multicentre, phase III | 572 in phase A; 241 in phase B | Difference in NRS score in phase B, when the phase A Sativex initial responders were randomized into going on with Sativex or placebo: highly significant (p = 0.0002) | Among others, significant therapeutic effect in regard to frequency of spasms (spasm frequency sore, p = 0.005) and to sleep disturbances (sleep disturbance NRS, p < 0.0001) |
| Phase A*: 4 weeks, single blind | |||||
| Phase B: randomization of responders from phase A for 12-week, double-blind, placebo-controlled study with subsequent 2-week follow up |
In this study, ‘initial responders’ were defined as those who experience a reduction in spasticity by at least 20% in the NRS from screening until the end of phase A.
CAMS, Cannabinoids in Multiple Sclerosis; CBD, cannabidiol; EDSS Expanded Disability Status Scale; MSFC Multiple Sclerosis Functional Composite; NRS numerical rating scale; THC, Δ-9-tetrahydrocannabinol; VAS visual analogue scale.
In the multicentre, randomized, placebo-controlled study on cannabinoids in multiple sclerosis (CAMS) published in 2003, a large population sample with 630 patients was examined for the first time over the course of more than 15 weeks [Zajicek et al. 2003]. The primary endpoint of the study was the change in spasticity based on the Ashworth Scale under the influence of orally administered cannabis extract (Cannador), orally applied synthetic THC (Marinol), or a placebo following a 4-week dose titration phase. In comparison to the placebo group, no significant improvement in spasticity was detected in the two active groups based on the Ashworth Scale. For the secondary endpoint, however, a significant improvement in the 10 m walking time was observed in the Marinol group. In the self evaluations conducted, the patients in the active groups were significantly more likely to report an improvement in spasticity, quality of sleep, and pain in comparison to those in the placebo groups. As a significant unblinding of both the study patients and the study physicians was identified as a result of the side effects of the cannabinoid therapy, the significance of these study results was only limited. In an extension study over a period of 12 months, in which 80% of the participants from the initial CAMS study participated, a significant, albeit only moderate improvement in spasticity with a reduced Ashworth Score and a subjective improvement of symptoms was identified in the two treatment groups.
Wade and colleagues published another open-label extension study involving 137 patients diagnosed with MS, in which the efficacy and tolerability of an oromucosally administered THC-CBD 1:1 mixture (Sativex) was examined for utility in the treatment of spasticity and other symptoms over an average of 434 days (range 21–814 days) [Wade et al. 2006]. A visual analogue scale was used as a measure of spasticity. The effect of nabiximols to significantly reduce spasticity, confirmed in the initial 10-week, placebo-controlled study phase, proved to be stable even over a longer time period. Among the study participants who took the study drug for at least a year, and even after 74 weeks, a reduction of the scale value from 69.5 (baseline) to 31.6 on the 0–100 visual analogue scale was identified.
Building on the experience gained with the Ashworth Scale in the CAMS study, the randomized, placebo-controlled study published by Collin and colleagues on the efficacy of nabiximols in antispastic therapy in MS used, for the first time, the change in spasticity according to a numerical rating scale (NRS) of 0–10 as the primary endpoint, applied by the patients themselves [Collin et al. 2007]. Originally, the primary outcome measure was the Ashworth Scale but publication of the CAMS study provided confirmation of its lack of reliability and sensitivity to measure significant functional change in spasticity, in agreement with recent systematic reviews [Richards et al. 2002; Shakespeare et al. 2004]. During patient recruitment an application was made to the independent ethics committees to reorder the outcome data; NRS became the primary measure of efficacy. This amendment was finalized 2 months before the last patient was recruited for the study. Data lock and analysis occurred 4 months after implementation of the amendment with full ethical approval. In comparison to the placebo group, the active group showed a significant reduction in spasticity in the NRS score (decrease in NRS score by 1.18 points in the active group versus 0.63 points in the placebo group), while only a nonsignificant decrease in the active group was identified on the Ashworth Score. Approximately 40% of the study participants randomized to the active group were classified as responders experiencing at least a 30% reduction in the NRS score.
Novotna and colleagues devised a study design in which only the study participants who emerged as early therapy responders in a 4-week, single-blind treatment phase with nabiximols were randomized for the 12-week, placebo-controlled, double-blind study phase (Figure 2) [Novotna et al. 2011]. The participating patients with MS who showed an improvement in spasticity, with at least a 20% reduction in the NRS scores compared with the baseline value during screening under nabiximols at the end of the first 4-week treatment period, were defined as early responders. On the advice of the regulator an enriched design through the exclusion of nonresponders was implemented in order to demonstrate therapeutic efficacy of cannabinoid therapy. Of the 572 study participants, 272 proved to be early responders, of whom 241 were randomized for the second study phase. Even though the dose of nabiximols was limited to a maximum of 12 sprays per day in this study (in the study by Collin and colleagues the maximum dose was 48 sprays per day) a significant improvement in spasticity was seen on this drug with an average reduction of the NRS score from baseline by 3.01, from 6.91 to 3.9, in the early responders [Collin et al. 2007]. In the subsequent placebo-controlled study phase, therapeutic superiority of the active drug over placebo was identified. Also, the secondary endpoints, frequency of spasms and sleep disturbances, demonstrated superiority of nabiximols over placebo.
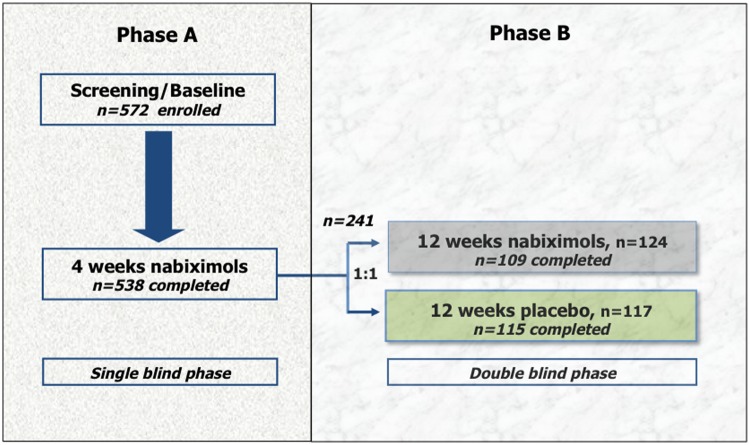
Figure 2.
Disposition of patients in the phase II study by Novotna et al. [2011].
A total of 660 patients with multiple sclerosis were screened and 572 enrolled into a single-blind phase during which all patients received nabiximols; 538 patients completed 4 weeks of treatment. All responders (n = 241) were randomised into a second double-blind phase, during which 124 patients received nabiximols and 117 placebo.
Assessment of the current studies
The potential role of cannabinoids in the treatment of spasticity in MS was highly controversial following publication of the first studies [Smith, 2007]. Their inconsistent results can be attributed to the heterogeneity of the study drugs used as well as to the various, sometimes unsuitable measurement parameters used to quantify the symptoms of spasticity. A meta-analysis of three studies on the therapeutic efficacy of nabiximols in the treatment of MS including a total of 666 participants found overall good efficacy of nabiximols as an antispastic therapeutic [Wade et al. 2010]. However, the phase III study published by Novotna and colleagues was the first to identify a clinically highly significant reduction in spasticity [Novotna et al. 2011]. The design applied in this study, with the exclusion of therapy nonresponders in the placebo-controlled study phase, facilitated demonstration of a therapeutic effect of nabiximols on spasticity. In view of the initial 4-week open-label phase with single-blind therapy of all study participants with nabiximols, a significant unmasking of the therapy responders randomized for the placebo-controlled phase cannot be ruled out as a result of side effects of nabiximols [Rog, 2010]. Reduction of spasticity perceived by the patients and reflected in the subjective analogue scales can likely be attributed, in part, to the known analgesic effect of nabiximols. Nevertheless, in a recently published 5-week placebo-controlled, parallel-group, randomized withdrawal study in patients with ongoing benefit from nabiximols, long-term symptomatic improvement of spasticity mediated by this drug could be confirmed [Notcutt et al. 2012].
Adverse effects and contraindications
General tolerability and profile of side effects
Overall, reports in the literature have shown a good tolerability of pharmaceutically used cannabinoids. In a systematic analysis of the previously published studies on the drug safety of oromucosally or orally administered THC or CBD, there were no indications of a significantly increased frequency of serious adverse events under therapy with cannabinoids in comparison to the control groups. However, there was an increased incidence of nonserious adverse events, which in most instances were related to side effects on the central nervous system. The most frequently reported nonserious adverse event was dizziness [Wang et al. 2008].
Similar results indicating good tolerability were obtained in safety studies of nabiximols. In a long-term study only 17 of the 137 patients discontinued the study due to adverse events under therapy with nabiximols, with a maximum 48 sprays per day for an average of 434 days [Wade et al. 2006]. In a 6-week, placebo-controlled study involving 189 randomized study participants and in which nabiximols was similarly administered at a maximum daily dose of 48 sprays, only 4.6% of the active group discontinued the study due to adverse events [Colin et al. 2007]. In this trial patients treated with nabiximols reported for the most part mild to moderate adverse events, primarily affecting the central nervous system. In an analysis of all placebo-controlled study data on the drug safety of nabiximols, an increased incidence of dizziness, drowsiness, disorientation, impaired concentration, and impaired balance was seen in comparison with the placebo group (Table 5). A specific profile of the side effects of nabiximols was identified and attributed to the oromucosal form of administration: increased occurrence of dry mouth, paraesthesiae in the region of the oral mucous membranes, impaired taste, and discoloration of teeth.
Table 5.
Adverse effects* of nabiximols.
| Gastrointestinal tract | Central nervous system | General symptoms | Cardiovascular system |
|---|---|---|---|
| Dryness of the mouth (6.1% versus 3.1%) | Dizziness (25% versus 8%) | Fatigue (12.5% versus 8.4%) | Tachycardia (1.0% versus 0.4%) |
| Drowsiness (8.2% versus 2.3%) | |||
| Ulcerations of oral mucosa (1.5% versus 0.8%) | Disorientation (4.1% versus 0.8%) | General physical weakness (asthenia, 5.6% versus 3.1%) | |
| Impaired concentration (3.9% versus 0.1%) | |||
| Nausea (9.6% versus 5.7%) | Impaired balance (2.9% versus 1.8%) | ||
| Diarrhea (5.5% versus 3.9%) | Blurry vision (1.9% versus 0.4%) | ||
| Increased appetite (1.4% versus 0.4%) | |||
| Euphoria (2.2% versus 0.9%) | |||
| Depression (2.9% versus 2.0%) | |||
| Psychosis (a total of 3 cases) | |||
| Hallucinations (a total of 11 cases) |
Data from the Public Assessment Report from a patient sample with multiple sclerosis.
Percentage values in brackets: percentage in the active group versus percentage in the placebo group.
Included among the commonly reported (in at least 5% of the patients diagnosed with MS) and usually mild to moderate adverse events experienced during treatment with nabiximols are feeling dazed, fatigue, nausea, urinary tract infections, drowsiness, dizziness, headache, and dryness of the mouth. In the long-term study by Wade and colleagues, with an observation period of up to 814 days, adverse events most frequently noted were pain in the region of the mouth (20.4%), dizziness (17%), diarrhea (17%), and nausea (15%) [Wade et al. 2006]. The incidence of serious adverse events was slightly increased during therapy with nabiximols (4.6% in the active group, 3.2% in the placebo group). Four incidents of severe psychiatric adverse events were observed, suicidal ideation in two cases and disorientation and paranoia in one case each. In this long-term study four epileptic seizures occurred during treatment with nabiximols, of which two were regarded as possibly related to therapy. In the CAMS extension study, there was one death in the Cannador group due to an epileptic seizure. With regard to cardiovascular side effects, isolated cases of syncope and cardiac arrhythmia were reported.
No meaningful data are available on the therapeutic range of nabiximols, dose dependency of adverse events, or the risk of overdose. A slow, individual dose titration and dose adjustment can prevent the occurrence of significant adverse events and dose reduction can resolve any side effects that occur.
Psychotropic side effects and possible contraindications
The psychotropic effects of cannabinoids known from recreational cannabis consumption, such as euphoria, tolerance development, withdrawal symptoms, induction of psychosis or depression, and impairments in cognitive function, are feared primarily with long-term therapy of spasticity using cannabinoids. These concerns are even more serious in patients with MS that are intrinsically vulnerable to the development of symptoms related to depression, cognitive impairment, and fatigue. Common cognitive deficits associated with MS are reduced information processing speed and impaired verbal memory. Among the cognitive disorders caused by cannabis consumption, in particular, is verbal learning impairment. In the context of the CAMS study, a substudy called CAMSPEC was conducted to specifically evaluate neuropsychological effects. This substudy found a significant increase in verbal learning impairment during cannabinoid therapy in comparison with placebo.
However, other studies failed to identify any influence of cannabinoid therapy on cognitive function (reviewed by Papathanasopoulos et al. [2008]). In a meta-analysis carried out by GW Pharmaceuticals of all scientific publications on nabiximols, the addictiveness and the risk of relevant psychoactive side effects of nabiximols were seen as minimal. For instance, the occurrence of euphoria and depression was observed in only 2.2% and 2.9% of the patients respectively [Robson, 2011]. No single case of defined cannabis withdrawal syndrome or tolerance development was verified. Yet, in an open-label study 46% of patients (11 of 25 patients) did report symptoms, including fatigue, emotional instability, and vivid dreams for an interval of 2 weeks following a scheduled, abrupt discontinuation of the study drug [Wade et al. 2006]. Overall, reports of psychoses (three cases) and hallucinations (11 cases) under therapy with nabiximols are relatively rare, and the symptoms remitted after discontinuation or dose reduction. Addiction and previous psychiatric illness are contraindications for treatment with nabiximols. The only exception is the presence of a depressive disorder associated with MS. The impact of nabiximols treatment on driving ability is unclear, and in case of doubt patients must be advised against driving a motor vehicle during therapy (Table 6).
Table 6.
Contraindications for treatment with nabiximols.*
| Contraindications |
|---|
| Allergies against cannabinoids |
| Pregnancy/breastfeeding |
| Psychiatric illnesses, in particular, schizophrenia, or other psychoses in the patient’s or family history, with the exception of depression associated with the underlying disease |
| Other possible contraindications/warnings |
| A past history of epileptic seizures |
| A past history of addiction |
| Serious cardiovascular diseases |
Current therapeutic options for spasticity
Treatment of this symptom is difficult in MS as use of the drugs is always associated with diminution of the existing muscle strength. For mild to moderate spasticity, oral antispastic drugs, such as the GABAB agonist baclofen and the α-2 agonist tizanidine, are usually the first choice; second-choice drugs are tetrazepam, tolperisone, memantine, and dantrolene [Samkoff and Goodman, 2011]. For severe spasticity, intrathecal baclofen therapy is an option, once the maximum doses of oral antispastic drugs have become ineffective. In addition, the intrathecal administration of triamcinolon acetonid is often used successfully in reducing spasticity. Focal spasticity may be treated successfully by local botulinum toxin injection (also in combination with oral/intrathecal baclofen therapy) [Snow et al. 1990].
Nabiximols is, therefore, a supplement to existing therapeutic options.
Conclusions
The oromucosal administration of THC and CBD in a 1:1 ratio has proven to be a well tolerated therapeutic option for treating spasticity in patients with MS who respond poorly to conventional antispastic drugs. Assessment of the efficacy is limited by the fact that spasticity as a symptom is very difficult to measure reliably, objectively, and validly. Current study data support the position that the beneficial effects of nabiximols on subjective and objective endpoints in a selected patient sample outweigh the adverse pharmaceutical effects. The effects of long-term nabiximols treatment on neuropsychological processes and the structure of the endocannabinoid system need to be further characterized.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Drs Hartung and Kieseier have received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer Health Care, Biogen Idec, Merck Serono, Novartis, Sanofi Aventis, and TEVA.
Contributor Information
Verena Isabell Leussink, Department of Neurology, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany.
Leila Husseini, Department of Neurology, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany.
Clemens Warnke, Department of Neurology, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany.
Erasmia Broussalis, Department of Neurology, Paracelsus Medical University, Salzburg, Austria.
Hans-Peter Hartung, Department of Neurology, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany.
Bernd C. Kieseier, Department of Neurology, Heinrich-Heine University, Moorenstrasse 5, 40225 Düsseldorf, Germany
References
- Aldington S., Harwood M., Cox B., Weatherall M., Beckert L., Hansell A., et al. Cannabis and Respiratory Disease Research Group (2008) Cannabis use and risk of lung cancer: a case–control study. Eur Respir J 31: 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Dittel B. (2011) Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res 51: 26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainin M., Barnes M., Baron J., Gilhus N., Hughes R., Selmaj K., et al. Guideline Standards Subcommittee of the EFNS Scientific Committee (2004) Guidance for the preparation of neurological management guidelines by EFNS scientific task forces – revised recommendations 2004. Eur J Neurol 11: 577–581 [DOI] [PubMed] [Google Scholar]
- Cabral G., Raborn E., Griffin L., Dennis J., Marciano-Cabral F. (2008) CB2 receptors in the brain: role in central immune function. Br J Pharmacol 153: 240–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C., Davies P., Mutiboko I., Ratcliffe S. (2007) Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 14: 290–296 [DOI] [PubMed] [Google Scholar]
- Console-Bram L., Marcu J., Abood M. (2012) Cannabinoid receptors: nomenclature and pharmacological principles. Prog Neuropsychopharmacol Biol Psychiatry 28 February (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia de Sa J., Airas L., Bartholome E., et al. (2011) Symptomatic therapy in multiple sclerosis – a review for a multimodal approach in clinical practice. Therap Adv Neurol Disord 4: 139–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J., Hernandez M., Ramos J. (2010) Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16: e72–e91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuren J., Voerman G., Erren-Wolters C., Snoek G., Rietman J., Hermens H., et al. (2010) Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 81: 46–52 [DOI] [PubMed] [Google Scholar]
- Gowran A., Noonan J., Campbell V. (2011) The multiplicity of action of cannabinoids: implications for treating neurodegeneration. CNS Neurosci Ther 17: 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamontt J., Molleman A., Pertwee R., Parsons M. (2010) The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol 160: 712–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Gilles L., Feng S., Tench, et al. (2009) Plasma endocannabinoid levels in multiple sclerosis. J Neurol Sci 287: 212–215 [DOI] [PubMed] [Google Scholar]
- Karst M., Wippermann S., Ahrens J. (2010) Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 70: 2409–2438 [DOI] [PubMed] [Google Scholar]
- Killestein J., Hoogervorst E., Reif M., Kalkers N., Van Loenen A., Staats P., et al. (2002) Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 58: 1404–1407 [DOI] [PubMed] [Google Scholar]
- Kubajewska I., Constantinescu C. (2010) Cannabinoids and experimental models of multiple sclerosis. Immunobiology 215: 647–657 [DOI] [PubMed] [Google Scholar]
- Notcutt W., Langford R., Davies P., Ratcliffe S., Potts R. (2012) A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Mult Scler 18: 219–228 [DOI] [PubMed] [Google Scholar]
- Novotna A., Mares J., Ratcliffe S., et al. (2011) A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex((R)) ), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 18: 1122–1131 [DOI] [PubMed] [Google Scholar]
- Papathanasopoulos P., Messinis L., Lyros E., Kastellakis A., Panagis G. (2008) Multiple sclerosis, cannabinoids, and cognition. J Neuropsychiat Clin Neurosci 20: 36–51 [DOI] [PubMed] [Google Scholar]
- Petrosino S., Di Marzo V. (2010) FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs 11: 51–62 [PubMed] [Google Scholar]
- Pryce G., Baker D. (2007) Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol 150: 519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder S., Chauhan A., Singh U., Nagarkatti M., Nagarkatti P. (2010) Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 215: 598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P. (2011) Abuse potential and psychoactive effects of delta-9-tetrahydrocannabinol and cannabidiol oromucosal spray (Sativex), a new cannabinoid medicine. Expert Opin Drug Saf 10: 675–685 [DOI] [PubMed] [Google Scholar]
- Rog D. (2010) Cannabis-based medicines in multiple sclerosis – a review of clinical studies. Immunobiology 215: 658–672 [DOI] [PubMed] [Google Scholar]
- Russo E., Guy G. (2006) A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 66: 234–246 [DOI] [PubMed] [Google Scholar]
- Samkoff L., Goodman A. (2011) Symptomatic management in multiple sclerosis. Neurol Clin 29: 449–463 [DOI] [PubMed] [Google Scholar]
- Sánchez A., García-Merino A. (2010) Neuroprotective agents: cannabinoids. Clin Immunol 142: 57–67 [DOI] [PubMed] [Google Scholar]
- Smith P. (2007) Symptomatic treatment of multiple sclerosis using cannabinoids: recent advances. Expert Rev Neurother 7: 1157–1163 [DOI] [PubMed] [Google Scholar]
- Snow B.J., Tsui J. K., Bhatt M.H., Varelas M., Hashimoto S.A., Calne D.B. (1990) Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol 28: 512–515 [DOI] [PubMed] [Google Scholar]
- Sunnerhagen K. (2010) Stop using the Ashworth scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 81: 2. [DOI] [PubMed] [Google Scholar]
- United Kingdom Tizanidine Trial Group (1994) A double-blind, placebo-controlled trial of tizanidine in the treatment of spasticity caused by multiple sclerosis. Neurology 44: S70–S78 [PubMed] [Google Scholar]
- Vaney C., Heinzel-Gutenbrunner M., Jobin P., Tschopp F., Gattlen B., Hagen U., et al. (2004) Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler 10: 417–424 [DOI] [PubMed] [Google Scholar]
- Wade D., Collin C., Stott C., Duncombe P. (2010) Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler 16: 707–714 [DOI] [PubMed] [Google Scholar]
- Wade D., Makela P., House H., Bateman C., Robson P. (2006) Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler 12: 639–645 [DOI] [PubMed] [Google Scholar]
- Wang T., Collet J., Shapiro S., Ware M. (2008) Adverse effects of medical cannabinoids: a systematic review. CMAJ 178: 1669–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener N., Koch M. (2009) Neurobiology and systems physiology of the endocannabinoid system. Pharmacopsychiatry 42(Suppl. 1): S79–S86 [DOI] [PubMed] [Google Scholar]
- Zajicek J., Fox P., Sanders H., Wright D., Vickery J., Nunn A., et al. ; UK MS Research Group (2003) Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet 362: 1517–1526 [DOI] [PubMed] [Google Scholar]
- Zajicek J.P., Sanders H.P., Wright D.E., Vickery P.J., Ingram W.M., Reilly S.M., et al. (2005) Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 76: 1664–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]