Abstract
Hepatitis B virus (HBV) reactivation is well documented in previously resolved or inactive HBV carriers who receive cancer chemotherapy. The consequences of HBV reactivation range from self-limited conditions to fulminant hepatic failure and death. HBV reactivation also leads to premature termination of chemotherapy or delay in treatment schedules. This review summarizes current knowledge of management of HBV reactivation in patients receiving cancer chemotherapy. HBV surface antigen (HBsAg) testing should be performed in patients who require cancer chemotherapy. Four meta-analyses support lamivudine prophylaxis for HBV reactivation during chemotherapy in HBsAg-positive patients. Randomized controlled trials to compare different HBV antiviral agents are needed to define optimal regimens for the prevention and treatment of HBV reactivation in patients receiving cancer chemotherapy.
Keywords: cancer, chemotherapy, HBV reactivation, hepatitis B virus, lamivudine, prophylaxis
Introduction
There are 400 million chronic carriers of hepatitis B virus (HBV) worldwide and 800,000 to 1.4 million in the US [Sorrell et al. 2009; Weinbaum et al. 2009]. Approximately half to two thirds of HBV carriers in US were born in other countries [Weinbaum et al. 2009]. HBV reactivation has been well documented in previously resolved or inactive HBV carriers who receive cancer chemotherapy. The consequences of HBV reactivation range from a self-limited hepatitis to fulminant hepatic failure and death [Liang et al. 1990; Galbraith et al. 1975; Pinto et al. 1990; Yeo et al. 2009]. HBV reactivation also necessitates premature termination of chemotherapy or delay in treatment schedules [Yeo et al. 2003, 2005]. Several factors have served to further accentuate the clinical importance of HBV reactivation: (1) advances in potent chemotherapy for cancer; (2) the increasing utilization of immunosuppressive agents in transplantation or autoimmune diseases; (3) the increasing influx of immigrants from HBV high endemic to low endemic areas; and (4) a potentially fatal outcome that is readily preventable with prophylactic antiviral treatment. This work reviews current concepts regarding management of HBV reactivation in patients receiving cancer chemotherapy. A PubMed search with the keywords ‘hepatitis B’ AND ‘chemotherapy’ was conducted to retrieve published articles focused on natural history, pathogenesis, prevention and treatment of HBV reactivation.
Natural history of HBV reactivation
Definition of HBV reactivation
HBV reactivation is defined as an abrupt increase in serum HBV DNA and alanine transaminase (ALT) levels in a patient with resolved or inactive HBV infection [Hoofnagle, 2009; Lok and McMahon, 2009]. In patients with negative HBV surface antigen (HBsAg) and positive antibody to HBV core antigen (anti-HBc), HBV reactivation, termed de novo hepatitis, is defined as HBsAg reappearance with threefold elevated ALT on two consecutive tests 5 days apart and accompanied by an increase in HBV DNA to more than 105 copies/ml [Hui et al. 2006].
Epidemiology
The incidence of HBV reactivation in HBsAg positive patients receiving cancer chemotherapy has been reported to range from 20% to 57% [Kim et al. 2007; Kumagai et al. 1997; Yeo et al. 2000a, 2000b, 2003]. HBV reactivation was observed in 50% of HBsAg-positive patients receiving rituximab-based therapy [Mendez-Navarro et al. 2011]. Reactivation does not necessarily require receipt of systemic chemotherapy. For instance, the reactivation rate in those receiving transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) has been reported to be as high as 34% [Jang et al. 2004].
Reactivation can be seen in persons with past exposure who do not harbor active HBV. The HBV reactivation rate in subjects with negative HBsAg and positive anti-HBc was 3% [Hui et al. 2006], and ranges from 2% to 25% in those receiving rituximab-based therapy [Ji et al. 2010; Koo et al. 2011; Matsue et al. 2010; Yeo et al. 2009].
Clinical features of HBV reactivation
In hematologic malignancies, early reports with serial follow up of HBV serologic markers observed reduction of antibody titers to HBV with subsequent reappearance of HBV antigen in 29% of patients under cancer chemotherapy. In addition, HBsAg was persistent in 40% of them and an increase in antigen titer was associated with elevated ALT [Wands et al. 1975]. Elevation in HBsAg titer was noted in 83% of patients with hematologic malignancies who received glucocorticoids [Ohtsu et al. 1991]. During follow up for up to 1 year after cancer chemotherapy, loss of antibody to HBV surface antigen (anti-HBs) was observed in 1% [Alexopoulos et al. 1999].
In breast cancer patients receiving doxorubicin and cyclophosphamide who were premedicated with dexamethasone, serum HBV DNA peaked prior to ALT by nearly 2 weeks [Yeo et al. 2001]. In contrast, serum HBV DNA rose 5–8 weeks prior to the ALT in chronic HBV patients with spontaneous reactivation [Maruyama et al. 1993] and in those treated with corticosteroid alone, 4 weeks in advance of peak ALT [Nair et al. 1986].
In de novo HBV-related hepatitis, a hundredfold rise in serum HBV DNA preceded threefold elevated ALT by 12 to 28 weeks [Hui et al. 2006]. HBsAg seroreversion developed after rise in serum HBV DNA and before ALT elevation [Hui et al. 2006]. Rituximab plus steroid is an independent factor associated with de novo HBV-related hepatitis [Hui et al. 2006]. Thus, patients with negative HBsAg and positive anti-HBc need a much longer interval to develop clinical hepatitis as compared with those with positive HBsAg. This late onset of clinical hepatitis suggests a delayed immune recovery because of the prolonged suppressive effects of rituximab [Dai et al. 2004a]. Rituximab-induced deficiency in antigen-presenting cells which led the HBV to escape the control of cytotoxic T-lymphocyte [Hui et al. 2006].
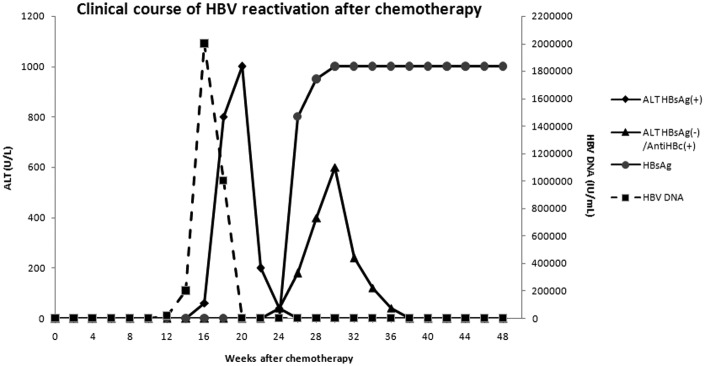
Serial serum HBV DNA and ALT monitoring before and during chemotherapy is important for a timely diagnosis of HBV reactivation. Since HBV DNA elevation precedes ALT elevation in both HBsAg-positive and HBsAg-negative/anti-HBc-positive patients (Figure 1), vigilance for HBV DNA during chemotherapy is essential so that prompt preemptive antiviral therapy may be instituted.
Figure 1.
Typical clinical course of HBV reactivation in HBsAg-positive and HBsAg-negative/anti-HBc-positive individuals receiving chemotherapy. In HBsAg-positive patients, serum HBV DNA was undetectable at the time of peak ALT, instead, it peaked prior to ALT by around 2 weeks [Yeo et al. 2001]. In HBsAg-negative/anti-HBc-positive subjects, serum HBV DNA preceded elevated ALT by 12–28 weeks [Hui et al. 2006]. HBsAg seroreversion developed after rise in serum HBV DNA and before ALT elevation [Hui et al. 2006].
HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; ALT, alanine transaminase.
Risk factors for HBV reactivation
HBV reactivation has been observed in patients receiving chemotherapy for hematologic malignancy [Galbraith et al. 1975; Yeo et al. 2009; Hwang et al. 2011], breast cancer [Kim et al. 2007; Yeo et al. 2003], hepatocellular carcinoma (HCC) [Nagamatsu et al. 2003; Yeo et al. 2004c], nasopharyngeal carcinoma [Yeo et al. 2005], and chemoradiation in postgastrectomy for gastric/esophageal adenocarcinoma [Cheng et al. 2004].
The risks of HBV reactivation in patients with positive HBsAg and negative HBsAg/positive anti-HBc are shown in Table 1. The cutoff level of serum HBV DNA between HBsAg-positive patients with versus without reactivation was 3 × 105 copies/ml [Zhong et al. 2004]. One of the modifiable risks among the risk factors for HBsAg-positive patients may be glucocorticoid use. This may be due to steroid increased HBV replication through glucocorticoid responsive element in HBV genome which enhances HBV replication greatly. In this regard, steroid-free regimens in HBsAg-positive patients with lymphoma were associated with reduced incidence and severity of HBV reactivation [Cheng et al. 2003].
Table 1.
Risks for HBV reactivation in patients with positive HBsAg and negative HBsAg/positive anti-HBc.
| Risks for HBV reactivation in patients with positive HBsAg | [References] | |
| Patient specific | ||
| Male gender | [Yeo et al. 2000a] | |
| Younger age | [Yeo et al. 2000a] | |
| Elevated baseline ALT | [Yeo et al. 2004c] | |
| Viral related | ||
| Positive HBeAg | [Yeo et al. 2000a] | |
| Precore mutation | [Yeo et al. 2000b] | |
| Prechemotherapy HBV DNA | [Yeo et al. 2004d; | |
| Zhong et al. 2004] | ||
| Treatment related | ||
| Glucocorticoid use | [Cheng et al. 2003; | |
| Nakamura et al. 1996; | ||
| Ohtsu et al. 1991; | ||
| Yeo et al. 2004d] | ||
| Anthracyclines use | [Yeo et al. 2004d] | |
| TACE treatment for HCC | [Jang et al. 2004] | |
| Cancer type | ||
| Lymphoma | [Yeo et al. 2000a; | |
| Yeo et al. 2004d] | ||
| Breast cancer | [Yeo et al. 2004d] | |
| Risks for HBV reactivation in patients with negative HBsAg / positive anti-HBc | ||
| Male gender | [Yeo et al. 2009] | |
| Rituximab use and negative anti-HBs | [Matsue et al. 2010; | |
| Yeo et al. 2009] | ||
| Multivariate analysis of risks for HBV reactivation in patients with positive HBsAg | ||
| [Yeo et al. 2004d] | ||
| Variable | OR (95% CI) | |
| Prechemotherapy detectable HBV DNA | 8.4 (2.6–27.2) | |
| Lymphoma | 5.0 (1.1–23.5) | |
| Breast cancer | 4.2 (1.6–11.0) | |
| Glucocorticoid use | 2.7 (1.0–7.2) | |
| Multivariate analysis of risks for HBV reactivation in patients with negative HBsAg | ||
| [Hui et al. 2006] | ||
| Variable | ARR (95% CI) | p-value |
| Rituximab plus steroid-containing regimen: | 0.001 | |
| Yes | 13.8 (2.8–68.3) | |
| No | 1 | |
| Steroid-containing regimen: | 0.105 | |
| Yes | 5.0 (0.6–40.9) | |
| No | 1 | |
| Rituximab-containing regimen: | 0.263 | |
| Yes | 1.3 (0.1–20.4) | |
| No | 1 | |
| Anti-HBc status prechemotherapy: | 0.190 | |
| Positive | 3.7 (0.5–30.2) | |
| Negative | 1 | |
HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B extracellular antigen; ALT, alanine transaminase; TACE, transarterial chemoembolization; HCC, hepatocellular carcinoma; OR, odds ratio; ARR, adjusted relative risk; CI, confidence interval.
Consequences of HBV reactivation
HBV reactivation has been associated with jaundice in 10% of patients [Liang et al. 1990], fulminant hepatic failure in 6% [Liang et al. 1990] and death [Galbraith et al. 1975; Pinto et al. 1990; Yeo et al. 2009]. In fulminant hepatic failure or severe hepatitis, patients harboring precore mutations had increased risk compared with those without these mutants [Dai et al. 2001; Steinberg et al. 2000]. The overall liver related mortality associated with reactivation has been estimated to be 5% [Liang et al. 1990], with high mortality rates (30%) in patients with HCC [Yeo et al. 2004c]. Around 11% of HCC patients receiving TACE died of hepatic decompensation due to HBV reactivation [Jang et al. 2004]. In patients with de novo HBV-related hepatitis, fulminant hepatic failure developed in 38% [Hui et al. 2006]. In addition, patients with HBV reactivation had delays or interruption of chemotherapy schedule or premature termination in 68–71% versus 19–33% of those without [Yeo et al. 2003, 2005], which may itself be associated with decreased cancer survival.
Pathogenesis of HBV reactivation
The underlying mechanism of HBV reactivation during cancer chemotherapy remains unclear. The reactivation is consistent with replication induced from latent forms of HBV even after serologic evidence of viral clearance [Hoofnagle, 2009]. It has been generally hypothesized that cancer chemotherapy leads to widespread HBV infection in hepatocytes by enhancing replication and suppressing the cellular immune response to the virus. Subsequent resurgence of immune function following chemotherapy discontinuation then results in rapid destruction of all infected hepatocytes [Galbraith et al. 1975; Xunrong et al. 2001].
In support of this premise are data demonstrating that direct exposure of HBV-expressing human hepatoma 1.3ES2 cells to doxorubicin or etoposide increased HBV DNA replication and HBV pregenomic transcription [Chung and Tsai, 2009]. Furthermore, HBV replication during chemotherapy is associated with activation of DNA repair pathways. Activation of chemotherapy-induced DNA repair signaling upregulated promyelocytic leukemia protein in its nuclear body (PML-NB) and the upregulated PML-NB initiated HBV replication [Chung and Tsai, 2009].
Prevention and treatment
Screening
The National Institutes of Health Consensus Development Conference Statement recommends routine screening for hepatitis B of newly arrived immigrants to the US from countries where the HBV prevalence rate is intermediate or high (i.e. greater than 2%) [Sorrell et al. 2009]. The US Centers for Disease Control and Prevention (CDC) also recommend testing of persons born in geographic regions with HBsAg prevalence of greater than 2% [Weinbaum et al. 2009]. The CDC recommends testing for HBsAg, anti-HBc and anti-HBs for all patients before the receipt of chemotherapy [Weinbaum et al. 2009]. The American Society of Clinical Oncology provisional clinical opinion suggested testing for HBsAg on high risk of previous HBV exposure or reactivation and testing for anti-HBc in some but not all patients [Artz et al. 2010]. However, testing for anti-HBs was not supported by evidence [Artz et al. 2010]. In patients with solid tumors who require chemotherapy, testing for HBsAg alone was the most economical strategy [Day et al. 2011]. For patients with reported HBV reactivation rates of more than or equal to 41% as in HBsAg-positive patients receiving rituximab-based therapy and in populations with expected HBsAg prevalence of 2.7%, testing for HBsAg and anti-HBc maximized cost-effectiveness [Day et al. 2011].
In clinical practice, a retrospective study in US showed that only 17% of cancer patients were screened for HBsAg and anti-HBc prior to chemotherapy [Hwang et al. 2011]. Testing for HBV has not been adequately performed in patients at risk for HBV in US.
Prevention and treatment
Lamivudine is a nucleoside analogue that effectively suppresses HBV replication. Four meta-analyses have supported lamivudine prophylaxis for HBV reactivation during chemotherapy in HBsAg-positive patients (Table 2). All studies included in these four meta-analyses showed a lower rate of HBV reactivation in patients receiving lamivudine prophylaxis (Table 2). The earliest analysis revealed prophylactic lamivudine decreased HBV reactivation by fourfold to sevenfold [Kohrt et al. 2006]. Two other meta-analyses, with much overlap in the studies included in both analyses, showed that lamivudine reduced the risk for HBV reactivation by 80–87% and reactivation-related mortality by 70% over no or deferred lamivudine use [Loomba et al. 2008; Martyak et al. 2008]. Preventive lamivudine also reduced treatment delays and premature termination of chemotherapy by 59% [Martyak et al. 2008]. A recent meta-analysis focused on lymphoma confirmed the efficacy of prophylactic lamivudine in reducing HBV reactivation [Ziakas et al. 2009]. Prophylactic lamivudine also reduced the severity of chemotherapy-related HBV reactivation in patients with solid tumors as well as lymphoma in a randomized trial and a retrospective study [Hsu et al. 2008; Yun et al. 2011]. A more recent retrospective study did not support the use of prophylactic lamivudine in HBsAg-negative anti-HBc-positive individuals receiving chemotherapy or rituximab [Koo et al. 2010] given the low frequency of this event [Hui et al. 2006].
Table 2.
Meta-analyses on lamivudine prophylaxis for HBV reactivation during chemotherapy in HBsAg-positive patients.
| Meta-analyses |
Studies included |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | RCT | Lmv E/N | No E/N | RR [95%CI] | Prospective | Lmv E/N | No E/N | RR [95%CI] | Retrospective | Lmv E/N | No E/N | RR [95%CI] |
| Kohrt et al. [2006] | Lau et al. [2003] | 0/15 | 8/15 | Idilman et al. [2004] | 0/8 | 5/10 | ||||||
| Dai et al. [2004b] | 1/6 | 0 | ||||||||||
| Dai et al. [2004c] | 0/11 | 5/9 | ||||||||||
| Yeo et al. [2004b] | 2/31 | 19/61 | ||||||||||
| Yeo et al. [2005] | 0/16 | 6/21 | ||||||||||
| el-Sayed et al. [2004] | 0/10 | 0 | ||||||||||
| Hui et al. [2005] | 11/46 | 0 | ||||||||||
| Rossi et al. [2001] | 1/20 | 0 | ||||||||||
| Vassiliadis et al. [2005] | 0/10 | 0 | ||||||||||
| Loomba et al. [2008] | Lau et al. [2003] | 0/15 | 8/15 | 0.00 [0.00–0.39] | Jia and Lin [2004] | 1/8 | 7/8 | 0.14 [0.01–0.67] | Lim et al. [2002] | 0/16 | 7/19 | 0.00 [0.00–0.56] |
| Jang et al. [2006] | 1/36 | 15/37 | 0.07 [0.01–0.35] | Idilman et al. [2004] | 0/8 | 5/10 | 0.00 [0.00–0.79] | Leaw et al. [2004] | 0/11 | 17/53 | 0.00 [0.00–0.86] | |
| Hsu et al. [2006] | 3/26 | 14/25 | 0.21 [0.04–0.59] | Shibolet et al. [2002] | 0/9 | 2/5 | 0.00 [0.00–1.31] | Nagamatsu et al. [2004] | 0/8 | 6/9 | 0.00 [0.00–0.66] | |
| Dai et al. [2004c] | 0/11 | 5/9 | 0.00 [0.00–0.61] | Lee et al. [2003] | 1/11 | 17/20 | 0.11 [0.01–0.46] | |||||
| Yeo et al. [2004a] | 3/65 | 47/193 | 0.19 [0.04–0.52] | |||||||||
| Yeo et al. [2005] | 0/16 | 6/21 | 0.00 [0.00–0.75] | |||||||||
| Martyak et al. [2008] | Lau et al. [2003] | 0/15 | 8/15 | Idilman et al. [2004] | 0/8 | 5/10 | Lim et al. [2002] | 0/16 | 7/19 | |||
| Jang et al. [2006] | 1/36 | 15/37 | Dai et al. [2004c] | 0/11 | 5/9 | Lee et al. [2003] | 1/11 | 17/20 | ||||
| Yeo et al. [2004b] | 2/31 | 19/61 | Sugimoto et al. [2004] | 0/5 | 6/6 | |||||||
| Yeo et al. [2004a] | 3/65 | 47/193 | ||||||||||
| Yeo et al. [2005] | 0/16 | 6/21 | ||||||||||
| Ziakas et al. [2009]* | Lau et al. [2003] | 0/15 | 8/15 | 0.06 [0.00–0.94] | Idilman et al. [2004] | 0/4 | 2/3 | 0.16 [0.01–2.47] | Leaw et al. [2004] | 0/11 | 17/53 | 0.13 [0.01–1.99] |
| Hsu et al. [2008] | 3/26 | 14/25 | 0.21 [0.07–0.63] | Shibolet et al. [2002] | 0/7 | 2/4 | 0.13 [0.01–2.10] | Lee et al. [2003] | 1/11 | 17/20 | 0.11 [0.02–0.70] | |
| Li et al. [2006] | 7/40 | 60/116 | 0.34 [0.17–0.68] | |||||||||
| Persico et al. [2002] | 0/3 | 12/18 | 0.19 [0.01–2.59] | |||||||||
| Tsutsumi et al. [2009] | 0/10 | 4/15 | 0.16 [0.01–2.71] | |||||||||
RCT, randomized controlled trial; Lmv, lamivudine prophylaxis; No, no prophylaxis; E/N, events/total number; RR, relative risk; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen.
Lymphoma patients.
Timing and duration of therapy
The optimal duration of preventive lamivudine remains unclear. Discontinuation of preventive lamivudine at 3 months after completion of chemotherapy resulted in a 24% rate of HBV reactivation [Hui et al. 2005]. Prechemotherapy serum HBV DNA of ≥104 copies/ml predicted reactivation after discontinuation [Hui et al. 2005]. In a decision analysis, extended lamivudine prophylaxis for 2 years throughout rituximab maintenance therapy improved 3-year overall survival rates by 2.4% in HBsAg-positive patients [Ziakas et al. 2009]. A cost-effectiveness study supported prophylactic lamivudine in HBsAg-positive patients with lymphoma 1 week before chemotherapy and for 6 months after the discontinuation of chemotherapy [Saab et al. 2007].
The use of lamivudine is limited by the development of resistance mutations that may contribute to hepatic decompensation and liver failure. The frequency of lamivudine resistance ranges from 3% to 8% in HBsAg-positive patients with hematologic malignancies under prophylactic use [Hsu et al. 2008; Pelizzari et al. 2004]. Adefovir has lower primary resistance rates and has been shown to be effective against lamivudine-resistant HBV strains. Two case reports of successful adefovir use either alone or in combination with lamivudine in patients with HBV reactivation under cancer chemotherapy have been reported [Cortelezzi et al. 2006; Perez-Roldan et al. 2005]. Entecavir is a more potent inhibitor of HBV replication with a very low long-term rate of primary resistance [Chang et al. 2006]. A retrospective multicenter study indicated that entecavir was superior to lamivudine in preventing HBV reactivation in lymphoma patients [Li et al. 2011]. Based on the anticipated duration of treatment, AASLD Practice Guidelines currently recommend lamivudine or telbivudine for a planned duration of ≤12 months, while tenofovir or entecavir are recommended for longer planned duration [Lok and McMahon, 2009]. Randomized controlled trials to compare different HBV antiviral agents are needed to establish optimal drug regimens for prevention and treatment of HBV reactivation in patients receiving cancer chemotherapy. Case reports described liver transplantation as a life-saving option for patients with liver failure due to HBV reactivation when the prognosis of the coexistent malignancy is good [Kim et al. 2010; King and Chung, 2010; Noterdaeme et al. 2011].
Discussion
The time to discontinue prophylactic lamivudine has been suggested as 6 months after completion of chemotherapy in a cost-effectiveness study [Saab et al. 2007]. A shorter duration of 3 months resulted in high HBV reactivation rate in a longitudinal follow-up study [Hui et al. 2005], whereas an extended duration for 2 years improved 3-year overall survival rates in a decision analysis by only 2.4% [Ziakas et al. 2009]. Therefore, prophylactic lamivudine should be continued for 6 months after completion of chemotherapy (Table 3), however, in patients with prechemotherapy serum HBV DNA of ≥2000 IU/ml, extended lamivudine use is recommended (Table 3). Further study is needed to determine the timing of treatment cessation in: (1) prophylactic use of antiviral agents with higher potency; and (2) HBsAg-negative patients receiving antiviral agents for serum HBV DNA elevation during chemotherapy. Data on monitoring following treatment discontinuation as well as management of reactivation in hepatitis B and delta co-infection have also been limited.
Table 3.
Recommendations for management of HBV reactivation in patients receiving cancer chemotherapy.
| 1. HBsAg testing should be performed in patients who require cancer chemotherapy or a finite course of immunosuppressive agents. |
| 2. Patients who require rituximab therapy or transplantation should be tested for HBsAg and anti-HBc. |
| 3. Serum HBV DNA and ALT should be monitored every 1–3 months during and at least 6 months after completion of chemotherapy or immunosuppressive agents. Since HBV DNA precedes ALT, vigilance for HBV DNA is essential. |
| 4. Prophylactic antiviral medication should be given to HBsAg positive patients before cancer chemotherapy or immunosuppressive agents. |
| 5. Antiviral medication should be continued for 6 months after completion of cancer chemotherapy or immunosuppressive agents. |
| 6. Chronic HBV patients with prechemotherapy serum HBV DNA of ≥2000 IU/ml should continue antiviral medication until: |
| a. HBeAg-positive patients: 6 months after HBeAg loss and anti-HBe detection. |
| b. HBeAg-negative patients: HBsAg clearance. |
| 7. Antiviral medication should be given to HBsAg negative patients as soon as abrupt serum HBV DNA elevation is detected during cancer chemotherapy or immunosuppressive agents. Antiviral medication may be given to HBsAg-negative, anti-HBc-positive, anti-HBs-negative patients who receive rituximab. |
| 8. The choice of antiviral medication can be based on the anticipated duration of treatment: lamivudine or telbivudine for duration of ≤12 months with undetectable serum HBV DNA and entecavir or tenofovir for longer duration or for patients with detectable HBV DNA prior to chemotherapy. |
HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B extracellular antigen; ALT, alanine transaminase.
Footnotes
Funding: Yi-Wen Huang is a research fellow at Massachusetts General Hospital and Harvard Medical School sponsored by National Science Council of Taiwan, Republic of China and Cathay General Hospital Medical Center, Taipei, Taiwan. Dr. Chung was supported by NIH DK078772 and DK082919.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Yi-Wen Huang, Gastrointestinal Unit, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Liver Center, Cathay General Hospital Medical Center, Taipei, Taiwan; School of Medicine, Taipei Medical University, Taipei, Taiwan; Division of Gastroenterology, Department of Internal Medicine, National Taiwan University College of Medicine and Hospital, Taipei, Taiwan.
Raymond T. Chung, Gastrointestinal Unit, Department of Medicine, Massachusetts General Hospital, Warren 1007, 55 Fruit Street, Boston, MA 02114, USA
References
- Alexopoulos C.G., Vaslamatzis M., Hatzidimitriou G. (1999) Prevalence of hepatitis B virus marker positivity and evolution of hepatitis B virus profile, during chemotherapy, in patients with solid tumours. Br J Cancer 81: 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz A.S., Somerfield M.R., Feld J.J., Giusti A.F., Kramer B.S., Sabichi A.L., et al. (2010) American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol 28: 3199–3202 [DOI] [PubMed] [Google Scholar]
- Chang T.T., Gish R.G., de Man R., Gadano A., Sollano J., Chao Y.C., et al. (2006) A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 354: 1001–1010 [DOI] [PubMed] [Google Scholar]
- Cheng J.C., Liu M.C., Tsai S.Y., Fang W.T., Jer-Min Jian J., Sung J.L. (2004) Unexpectedly frequent hepatitis B reactivation by chemoradiation in postgastrectomy patients. Cancer 101: 2126–2133 [DOI] [PubMed] [Google Scholar]
- Cheng A.L., Hsiung C.A., Su I.J., Chen P.J., Chang M.C., Tsao C.J., et al. (2003) Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology 37: 1320–1328 [DOI] [PubMed] [Google Scholar]
- Chung Y.L., Tsai T.Y. (2009) Promyelocytic leukemia nuclear bodies link the DNA damage repair pathway with hepatitis B virus replication: implications for hepatitis B virus exacerbation during chemotherapy and radiotherapy. Mol Cancer Res 7: 1672–1685 [DOI] [PubMed] [Google Scholar]
- Cortelezzi A., Vigano M., Zilioli V.R., Fantini N.N., Pasquini M.C., Deliliers G.L., et al. (2006) Adefovir added to lamivudine for hepatitis B recurrent infection in refractory B-cell chronic lymphocytic leukemia on prolonged therapy with Campath-1H. J Clin Virol 35: 467–469 [DOI] [PubMed] [Google Scholar]
- Dai M.S., Lu J.J., Chen Y.C., Perng C.L., Chao T.Y. (2001) Reactivation of precore mutant hepatitis B virus in chemotherapy-treated patients. Cancer 92: 2927–2932 [DOI] [PubMed] [Google Scholar]
- Dai M.S., Chao T.Y., Kao W.Y., Shyu R.Y., Liu T.M. (2004a) Delayed hepatitis B virus reactivation after cessation of preemptive lamivudine in lymphoma patients treated with rituximab plus CHOP. Ann Hematol 83:769–774 [DOI] [PubMed] [Google Scholar]
- Dai M.S., Wu P.F., Lu J.J., Shyu R.Y., Chao T.Y. (2004b) Preemptive use of lamivudine in breast cancer patients carrying hepatitis B virus undergoing cytotoxic chemotherapy: a longitudinal study. Support Care Cancer 12: 191–196 [DOI] [PubMed] [Google Scholar]
- Dai M.S., Wu P.F., Shyu R.Y., Lu J.J., Chao T.Y. (2004c) Hepatitis B virus reactivation in breast cancer patients undergoing cytotoxic chemotherapy and the role of preemptive lamivudine administration. Liver Int 24: 540–546 [DOI] [PubMed] [Google Scholar]
- Day F.L., Karnon J., Rischin D. (2011) Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. J Clin Oncol 29: 3270–3277 [DOI] [PubMed] [Google Scholar]
- el-Sayed M.H., Shanab G., Karim A.M., el-Tawil A., Black A., Dixon J.S. (2004) Lamivudine facilitates optimal chemotherapy in hepatitis B virus-infected children with hematological malignancies: a preliminary report. Pediatr Hematol Oncol 21: 145–156 [DOI] [PubMed] [Google Scholar]
- Galbraith R.M., Eddleston A.L., Williams R., Zuckerman A.J. (1975) Fulminant hepatic failure in leukemia and choriocarcinoma related to withdrawal of cytotoxic drug therapy. Lancet 2: 528–530 [DOI] [PubMed] [Google Scholar]
- Hoofnagle J.H. (2009) Reactivation of hepatitis B. Hepatology 49: S156-S165 [DOI] [PubMed] [Google Scholar]
- Hsu C., Hsiung C.A., Su I.J., Hwang W.S., Wang M.C., Lin S.F., et al. (2008) A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology 47: 844–853 [DOI] [PubMed] [Google Scholar]
- Hsu C., Sur I.J., Hwang W.S., Wang M.C., Lin S.F., Lin T.H., et al. (2006) A prospective comparative study of prophylactic or therapeutic use of lamivudine for chemotherapy-associated hepatitis B (HBV) reactivation in non-Hodgkin’s lymphoma patients [Abstract]. Gastroenterology 131: S297 [Google Scholar]
- Hui C.K., Cheung W.W., Au W.Y., Lie A.K., Zhang H.Y., Yueng Y.H., et al. (2005) Hepatitis B reactivation after withdrawal of pre-emptive lamivudine in patients with haematological malignancy on completion of cytotoxic chemotherapy. Gut 54: 1597–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C.K., Cheung W.W., Zhang H.Y., Au W.Y., Yueng Y.H., Leung A.Y., et al. (2006) Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 131: 59–68 [DOI] [PubMed] [Google Scholar]
- Hwang J.P., Fisch M.J., Zhang H., Kallen M.A., Routbort M., Lal L.S., et al. (2011) Reactivation of hepatitis B infection among patients with cancer [Abstract]. Hepatology 54: 445A [Google Scholar]
- Idilman R., Arat M., Soydan E., Toruner M., Soykan I., Akbulut H., et al. (2004) Lamivudine prophylaxis for prevention of chemotherapy-induced hepatitis B virus reactivation in hepatitis B virus carriers with malignancies. J Viral Hepat 11: 141–147 [DOI] [PubMed] [Google Scholar]
- Jang J.W., Choi J.Y., Bae S.H., Kim C.W., Yoon S.K., Cho S.H., et al. (2004) Transarterial chemo-lipiodolization can reactivate hepatitis B virus replication in patients with hepatocellular carcinoma. J Hepatol 41: 427–435 [DOI] [PubMed] [Google Scholar]
- Jang J.W., Choi J.Y., Bae S.H., Yoon S.K., Chang U.I., Kim C.W., et al. (2006) A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology 43: 233–240 [DOI] [PubMed] [Google Scholar]
- Ji D., Cao J., Hong X., Li J., Wang J., Chen F., et al. (2010) Low incidence of hepatitis B virus reactivation during chemotherapy among diffuse large B-cell lymphoma patients who are HBsAg-negative / HBcAb-positive: a multicenter retrospective study. Eur J Haematol 85: 243–250 [DOI] [PubMed] [Google Scholar]
- Jia J., Lin F. (2004) Lamivudine therapy for prevention of chemotherapy-induced reactivation of hepatitis B virus. Zhonghua Gan Zang Bing Za Zhi 12: 628–629 [PubMed] [Google Scholar]
- Kim M.K., Ahn J.H., Kim S.B., Im Y.S., Lee S.I., Ahn S.H., et al. (2007) Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution’s experience. Korean J Intern Med 22: 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.G., Chun J.M., Jin R., Kim J.Y., Won D.I., Hwang Y.J. (2010) Living donor liver transplantation for acute hepatic failure caused by reactivation of hepatitis B virus infection after chemotherapy for hematologic malignancy: case reports. Transplant Proc 42: 843–845 [DOI] [PubMed] [Google Scholar]
- King L.Y., Chung R.T. (2010) When treating cancer, please don’t forget hepatitis B. Oncologist 15: 826–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt H.E., Ouyang D.L., Keeffe E.B. (2006) Systematic review: lamivudine prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Aliment Pharmacol Ther 24: 1003–1016 [DOI] [PubMed] [Google Scholar]
- Koo Y.X., Tan D.S., Tan I.B., Tao M., Chow W.C., Lim S.T. (2010) Hepatitis B virus reactivation and role of antiviral prophylaxis in lymphoma patients with past hepatitis B virus infection who are receiving chemoimmunotherapy. Cancer 116: 115–121 [DOI] [PubMed] [Google Scholar]
- Koo Y.X., Tay M., The Y.E., Teng D., Tan D.S., Tan I.B., et al. (2011) Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis B core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann Hematol 90: 1219–1223 [DOI] [PubMed] [Google Scholar]
- Kumagai K., Takagi T., Nakamura S., Sawada U., Kura U., Kodama F., et al. (1997) Hepatitis B virus carriers in the treatment of malignant lymphoma: an epidemiological study in Japan. Ann Oncol 8(Suppl. 1): 107–109 [PubMed] [Google Scholar]
- Lau G.K., Yiu H.H., Fong D.Y., Cheng H.C., Au W.Y., Lai L.S., et al. (2003) Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 125: 1742–1749 [DOI] [PubMed] [Google Scholar]
- Leaw S.J., Yen C.J., Huang W.T., Chen T.Y., Su W.C., Tsao C.J. (2004) Preemptive use of interferon or lamivudine for hepatitis B reactivation in patients with aggressive lymphoma receiving chemotherapy. Ann Hematol 83: 270–275 [DOI] [PubMed] [Google Scholar]
- Lee G.W., Ryu M.H., Lee J.L., Oh S., Kim E., Lee J.H., et al. (2003) The prophylactic use of lamivudine can maintain dose-intensity of adriamycin in hepatitis-B surface antigen (HBsAg)-positive patients with non-Hodgkin’s lymphoma who receive cytotoxic chemotherapy. J Korean Med Sci 18: 849–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.R., Huang J.J., Guo H.Q., Zhang X., Xie Y., Zhu H.L., et al. (2011) Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat 18: 877–883 [DOI] [PubMed] [Google Scholar]
- Li Y.H., He Y.F., Jiang W.Q., Wang F.H., Lin X.B., Zhang L., et al. (2006) Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer 106: 1320–1325 [DOI] [PubMed] [Google Scholar]
- Liang R.H., Lok A.S., Lai C.L., Chan T.K., Todd D., Chiu E.K. (1990) Hepatitis B infection in patients with lymphomas. Hematol Oncol 8: 261–270 [DOI] [PubMed] [Google Scholar]
- Lim L.L., Wai C.T., Lee Y.M., Kong H.L., Lim R., Koay E., et al. (2002) Prophylactic lamivudine prevents hepatitis B reactivation in chemotherapy patients. Aliment Pharmacol Ther 16: 1939–1944 [DOI] [PubMed] [Google Scholar]
- Lok A.S., McMahon B.J. (2009) Chronic hepatitis B: update 2009. Hepatology 50: 661-662 (http://www.aasld.org). [DOI] [PubMed] [Google Scholar]
- Loomba R., Rowley A., Wesley R., Liang T.J., Hoofnagle J.H., Pucino F., et al. (2008) Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 148: 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyak L.A., Taqavi E., Saab S. (2008) Lamivudine prophylaxis is effective in reducing hepatitis B reactivation and reactivation-related mortality in chemotherapy patients: a meta-analysis. Liver Int 28: 28–38 [DOI] [PubMed] [Google Scholar]
- Maruyama T., Iino S., Koike K., Yasuda K., Milich D.R. (1993) Serology of acute exacerbation in chronic hepatitis B virus infection. Gastroenterology 105: 1141–1151 [DOI] [PubMed] [Google Scholar]
- Matsue K., Kimura S., Takanashi Y., Iwama K., Fujiwara H., Yamakura M., et al. (2010) Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer 116: 4769–4776 [DOI] [PubMed] [Google Scholar]
- Mendez-Navarro J., Corey K.E., Zheng H., Barlow L.L., Jang J.Y., Lin W., et al. (2011) Hepatitis B screening, prophylaxis and re-activation in the era of rituximab-based chemotherapy. Liver Int 31: 330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu H., Itano S., Nagaoka S., Akiyoshi J., Matsugaki S., Kurogi J., et al. (2004) Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol 99: 2369–2375 [DOI] [PubMed] [Google Scholar]
- Nagamatsu H., Kumashiro R., Itano S., Matsugaki S., Sata M. (2003) Investigation of associating factors in exacerbation of liver damage after chemotherapy in patients with HBV-related HCC. Hepatol Res 26: 293–301 [DOI] [PubMed] [Google Scholar]
- Nair P.V., Tong M.J., Stevenson D., Roskamp D., Boone C. (1986) A pilot study on the effects of prednisolone withdrawal on serum hepatitis B virus DNA and HBeAg in chronic active hepatitis B. Hepatology 6: 1319–1324 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Motokura T., Fujita A., Yamashita T., Ogata E. (1996) Severe hepatitis related to chemotherapy in hepatitis B virus carriers with hematologic malignancies. Survey in Japan, 1987–1991. Cancer 78: 2210–2215 [DOI] [PubMed] [Google Scholar]
- Noterdaeme T., Longree L., Bataille C., Deroover A., Lamprove A., Delwaide J., et al. (2011) Liver transplantation for acute hepatic failure due to chemotherapy-induced HBV reactivation in lymphoma patients. World J Gastroenterol 17: 3069–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsu T., Sai T., Oka M., Shgai Y., Tobinai K. (1991) Activation of hepatitis B virus infection by chemotherapy containing glucocorticoid in hepatitis B virus carriers with hematologic malignancies. Japan J Clin Oncol 21: 360–365 [PubMed] [Google Scholar]
- Pelizzari A.M., Motta M., Cariani E., Turconi P., Borlenghi E., Rossi G. (2004) Frequency of hepatitis B virus mutant in asymptomatic hepatitis B virus carriers receiving prophylactic lamivudine during chemotherapy for hematologic malignancies. Hematol J 5: 325–328 [DOI] [PubMed] [Google Scholar]
- Perez-Roldan F., Gonzalez-Carro P., Villafanez-Garcia M.C. (2005) Adefovir dipivoxil for chemotherapy-induced activation of hepatitis B virus infection. N Engl J Med 352: 310–311 [DOI] [PubMed] [Google Scholar]
- Persico M., De Marino F., Russo G.D., Morante A., Rotoli B., Torella R., et al. (2002) Efficacy of lamivudine to prevent hepatitis reactivation in hepatitis B virus-infected patients treated for non-Hodgkin lymphoma. Blood 99: 724–725 [DOI] [PubMed] [Google Scholar]
- Pinto P.C., Hu E., Bernstein-Singer M., Pinter-Brown L., Govindarajan S. (1990) Acute hepatic injury after the withdrawal of immunosuppressive chemotherapy in patients with hepatitis B. Cancer 65: 878–884 [DOI] [PubMed] [Google Scholar]
- Rossi G., Pelizzari A., Motta M., Puoti M. (2001) Primary prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HbsAg carriers with lymphoid malignancies treated with chemotherapy. Br J Haematol 115: 58–62 [DOI] [PubMed] [Google Scholar]
- Saab S., Dong M.H., Joseph T.A., Tong M.J. (2007) Hepatitis B prophylaxis in patients undergoing chemotherapy for lymphoma: a decision analysis model. Hepatology 46: 1049–1056 [DOI] [PubMed] [Google Scholar]
- Shibolet O., Ilan Y., Gillis S., Hubert A., Shouval D., Safadi R. (2002) Lamivudine therapy for prevention of immunosuppressive-induced hepatitis B virus reactivation in hepatitis B surface antigen carriers. Blood 100: 391–396 [DOI] [PubMed] [Google Scholar]
- Sorrell M.F., Belongia E.A., Costa J., Gareen I.F., Grem J.L., Inadomi J.M., et al. (2009) National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology 49: S4-S12 [DOI] [PubMed] [Google Scholar]
- Steinberg J.L., Yeo W., Zhong S., Chan J.Y., Tam J.S., Chan P.K., et al. (2000) Hepatitis B virus reactivation in patients undergoing cytotoxic chemotherapy for solid tumours: precore/core mutations may play an important role. J Med Virol 60: 249–255 [PubMed] [Google Scholar]
- Sugimoto R., Enjoji M., Kotoh K., Noguchi K., Tsuruta S., Nakamuta M., et al. (2004) Effect of lamivudine for hepatitis B virus reactivation in blood cancer patients undergoing immunosuppressive chemotherapy. Fukuoka Igaku Zasshi 95: 274–279 [PubMed] [Google Scholar]
- Tsutsumi Y., Shigematsu A., Hashino S., Tanaka J., Chiba K., Masauzi N., et al. (2009) Analysis of reactivation of hepatitis B virus in the treatment of B cell non-Hodgkin’s lymphoma in Hokkaido. Ann Hematol 88: 375–377 [DOI] [PubMed] [Google Scholar]
- Vassiliadis T., Garipidou V., Tziomalos K., Perifanis V., Giouleme O., Vakalopoulou S. (2005) Prevention of hepatitis B reactivation with lamivudine in hepatitis B virus carriers with hematologic malignancies treated with chemotherapy – a prospective case series. Am J Hematol 80: 197–203 [DOI] [PubMed] [Google Scholar]
- Wands J.R., Chura C.M., Roll F.J., Maddrey W.C. (1975) Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology 68: 105–112 [PubMed] [Google Scholar]
- Weinbaum C.M., Mast E.E., Ward J.W. (2009) Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology 49: S35-S44 [DOI] [PubMed] [Google Scholar]
- Xunrong L., Yan A.W., Liang R., Lau G.K. (2001) Hepatitis B virus (HBV) reactivation after cytotoxic or immunosuppressive therapy – pathogenesis and management. Rev Med Virol 11: 287–299 [DOI] [PubMed] [Google Scholar]
- Yeo W., Chan P.K.S., Chan H.L.Y., Mo F.K.F., Johnson P.J. (2001) Hepatitis B virus reactivation during cytotoxic chemotherapy-enhanced viral replication precedes overt hepatitis. J Med Virol 65: 473–477 [PubMed] [Google Scholar]
- Yeo W., Chan P.K.S., Ho W.M., Zee B., Lam K.C., Lei K.I., et al. (2004a) Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol 22: 927–934 [DOI] [PubMed] [Google Scholar]
- Yeo W., Chan P.K.S., Hui P., Ho W.M., Lam K.C., Kwan W.H., et al. (2003) Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol 70: 553–561 [DOI] [PubMed] [Google Scholar]
- Yeo W., Chan T.C., Leung N.W.Y., Lam W.Y., Mo F.K.F., Chu M.T., et al. (2009) Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 27: 605–611 [DOI] [PubMed] [Google Scholar]
- Yeo W., Chan P.K.S., Zhong S., Ho W.M., Steinberg J.L., Tam J.S., et al. (2000a) Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 62: 299–307 [DOI] [PubMed] [Google Scholar]
- Yeo W., Ho W.M., Hui P., Chan P.K., Lam K.C., Lee J.J., et al. (2004b) Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat 88: 209–215 [DOI] [PubMed] [Google Scholar]
- Yeo W., Hui E.P., Chan A.T.C., Ho W.M., Lam K.C., Chan P.K.S., et al. (2005) Prevention of hepatitis B virus reactivation in patients with nasopharyngeal carcinoma with lamivudine. Am J Clin Oncol 28: 379–384 [DOI] [PubMed] [Google Scholar]
- Yeo W., Lam K.C., Zee B., Chan P.S.K., Mo F.K.F., Ho W.M., et al. (2004c) Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol 15: 1661–1666 [DOI] [PubMed] [Google Scholar]
- Yeo W., Zee B., Zhong S., Chan P.K.S., Wong W.L., Ho W.M., et al. (2004d) Comprehensive analysis of risk factors associating with hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer 90: 1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo W., Zhong S., Chan P.K., Ho W.M., Wong H.T., Chan A.S., et al. (2000b) Sequence variations of precore/core and precore promoter regions of hepatitis B virus in patients with or without viral reactivation during cytotoxic chemotherapy. J Viral Hepat 7: 448–458 [DOI] [PubMed] [Google Scholar]
- Yun J., Kim K.H., Kang E.S., Gwak G.Y., Choi M.S., Lee J.E., et al. (2011) Prophylactic use of lamivudine for hepatitis B exacerbation in post-operative breast cancer patients receiving anthracycline-based adjuvant chemotherapy. Br J Cancer 104: 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Yeo W., Schroder C., Chan P.K., Wong W.L., Ho W.M., et al. (2004) High hepatitis B virus (HBV) DNA viral load is an important risk factor for HBV reactivation in breast cancer patients undergoing cytotoxic chemotherapy. J Viral Hepat 11: 55–59 [DOI] [PubMed] [Google Scholar]
- Ziakas P.D., Karsaliakos P., Mylonakis E. (2009) Effect of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in lymphoma: a meta-analysis of published clinical trials and a decision tree addressing prolonged prophylaxis and maintenance. Haematologica 94: 998–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]