Abstract
The human epidermal receptor-2 (HER-2) is amplified in up to 25% of patients with gastroesophageal adenocarcinomas. Although the presence of this amplification does not appear to confer a poor prognosis, it provides a valuable novel therapeutic target for this group of patients. Trastuzumab is a fully humanized monoclonal antibody directed at HER-2 which binds the external domain of the receptor and exerts its action via a combination of antibody-dependent cytotoxicity, reduced shedding of the extracellular domain, inhibition of dimerization and possibly receptor downregulation. The ToGA trial was an international multicentre randomized phase III study which evaluated the addition of trastuzumab to a cisplatin plus fluoropyrimidine chemotherapy doublet in 594 patients with HER-2-positive advanced gastric or oesophagogastric junction adenocarcinoma. The combination of the antibody with chemotherapy significantly improved response rate, median progression-free survival and median overall survival without additional toxicity or adversely affecting quality of life. Accordingly, trastuzumab plus chemotherapy is now a standard first-line treatment option for patients with advanced HER-2-positive gastroesophageal cancer. Unfortunately, many patients with HER-2-positive cancer exhibit primary resistance to trastuzumab and the remainder will acquire resistance to the antibody; therefore, urgent investigation into novel agents which may circumvent resistance mechanisms is warranted. Small molecule inhibitors of HER-2, which commonly also target other members of the HER family of receptors, such as EGFR and HER-3, are currently undergoing evaluation in gastroesophageal cancer as first-line alternatives to trastuzumab and second-line salvage treatments for trastuzumab-resistant disease. Extrapolating the successful use of trastuzumab in the advanced disease setting, clinical trials are underway to assess the role of this antibody in the perioperative and adjuvant settings, where it is hoped that it will have a meaningful impact upon the currently poor survival rates.
Keywords: adenocarcinoma, gastric cancer, HER-2, monoclonal antibody, oesophagogastric junction (OGJ), trastuzumab, tyrosine kinase inhibitor
Background
Worldwide, gastric cancer is the fourth most commonly diagnosed cancer and the second most common cause of cancer death; accounting for almost 10% of all cancer deaths in 2008 [Ferlay et al. 2010]. The majority of patients present with advanced, inoperable disease, where treatment is palliative and the median survival is just 3 months with supportive care alone [Murad et al. 1993; Pyrhonen et al. 1995]. Chemotherapy improves survival in patients with advanced gastric cancer and combination regimens are superior in this respect to monotherapy [Wagner et al. 2010]. Whilst there is no globally accepted standard first-line regimen, a platinum-fluoropyrimidine doublet [Kang et al. 2009], with or without epirubicin [Cunningham et al. 2008] or docetaxel [Tebbutt et al. 2010; Van Cutsem et al. 2006], is most commonly used. However, even with optimal combination chemotherapy, the median survival in Western studies remains less than 1 year [Cunningham et al. 2010; Van Cutsem et al. 2006]. A longer median survival has been reported in a Japanese phase III study comparing fluoropyrimidine monotherapy, with the oral agent, S-1, with a S-1/cisplatin doublet. However, this is likely to reflect the use of second- and third-line chemotherapy, evidenced by the relatively short median progression-free survival (PFS), and possibly more patients with a lower burden of disease at study entry, rather than the efficacy of the S-1+/- cisplatin regimen itself [Koizumi et al. 2008]. Until recently, there were no supporting phase III trial data for second-line chemotherapy for patients with advanced gastric cancer, however, a survival benefit for irinotecan or docetaxel monotherapy has now been reported in patients previously treated with a platinum and fluoropyrimidine [Kang et al. 2012], confirming the efficacy of second-line irinotecan that had been reported in a small German study, which had closed prior to completing planned accrual [Thuss-Patience et al. 2011]. Although modest increments in survival may be possible from further refinements of first- and second-line chemotherapy regimens, attention has turned to potential molecular targets in oesophagogastric cancer. Like advanced breast cancers, 15–25% of advanced gastroesophageal cancers overexpress the human epidermal receptor 2 (HER-2). Trastuzumab, a monoclonal antibody targeting HER-2, improves survival in HER-2-positive advanced [Slamon et al. 2001] and early breast cancer [Slamon et al. 2011; Smith et al. 2007] and has been a key component of breast cancer treatment of for over a decade. Following the successful phase III evaluation of the antibody in HER-2-positive advanced gastric and oesophagogastric junction (OGJ) cancers, demonstrating improved radiological response rate, PFS and overall survival with the addition of trastuzumab to a cisplatin/fluoropyrimidine doublet [Bang et al. 2010], trastuzumab has become a standard treatment option for patients with this disease also. Whether a similar benefit will be attained from HER-2 inhibition with trastuzumab in patients with operable gastroesophageal cancers is currently unknown.
This review will discuss the current and future roles of trastuzumab in the treatment of gastroesophageal cancer, resistance mechanisms to the antibody, strategies to overcome trastuzumab resistance and novel anti-HER-2 targeted agents.
The HER-2 receptor
HER-2 (also known as erbB-2) is one of a family of four identified human epidermal receptors, which also includes the epidermal growth factor receptor (EGFR, HER-1), the HER-3 and HER-4 receptors. The four receptors and their respective ligands form a complex, key signalling network for cell proliferation and survival [Yarden, 2001]. The oncogene encoding the HER-2 receptor (HER-2/neu or erbB2) was first described in 1984 [Schechter et al. 1984] and mapped to q21 of chromosome 17 [Coussens et al. 1985; Schechter et al. 1985]. The gene product is a 185 kDa tyrosine kinase receptor [Akiyama et al. 1986; Toyoshima et al. 1986]. The first description of HER-2 amplification in gastric cancer was in the MKN-7 gastric cancer cell line [Fukushige et al. 1986], with a subsequent report in two of nine gastric cancer resections [Yokota et al. 1986], with amplification most commonly detected in tubular (intestinal) cancers [Yokota et al. 1988].
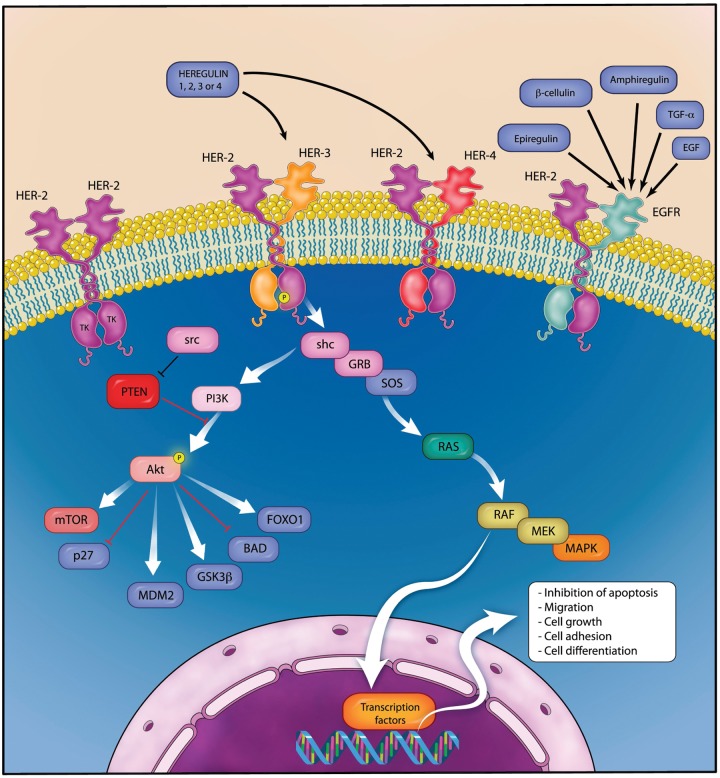
The HER-2 receptor protein consists of four extracellular domains, one transmembrane domain and a cytoplasmic tyrosine kinase domain [Cho et al. 2003]. HER-2 differs from the other three HER family members in that no ligand has been identified. Activation of the receptor can be either ligand-independent with the formation of HER-2 homodimers in the presence of tumour HER-2 overexpression [Worthylake et al. 1999] or ligand-dependent heterodimerization with EGFR, HER-3 or HER-4 following receptor-specific ligand binding. Heregulin is a key ligand for the HER-3 receptor [Carraway et al. 1994] and co-expression of HER-2 and HER-3 leads to high-affinity heregulin binding and tyrosine phosphorylation. Despite the HER-3 receptor possessing no functional tyrosine kinase activity itself [Guy et al. 1994], the heterodimer comprising HER-2/HER-3 is thought to produce the most potent mitogenic signal [Pinkas-Kramarski et al. 1996], stimulating cell proliferation and inhibiting apoptosis via the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K) pathways [Daly et al. 1999]. A simplified version of HER-2 dimers and the intracellular signalling pathway is depicted in Figure 1.
Figure 1.
Simplified diagram of the HER family signalling network. Ligand binding to HER-3 stimulates heterodimerization with HER-2 and transphosphorylation of HER-3, initiating the intracellular signalling cascade, resulting in cell growth and survival. (Illustration courtesy of Alessandro Baliani, Copyright © 2012.)
Prognostic effect of HER-2 overexpression and amplification
The presence of HER-2 overexpression or HER-2/neu amplification is associated with an aggressive phenotype in breast cancer, conferring a poor prognosis [Slamon et al. 1987, 1989]. Although initial studies in gastric cancer suggested a similarly negative impact on prognosis [Yonemura et al. 1991], more recent studies have reported no significant prognostic effect [Shah et al. 2011; Terashima et al. 2011; Yoon et al. 2011].
These studies are summarized in Table 1. The reason for these conflicting data is most likely the previous lack of standardized testing and scoring of HER-2 in gastric cancer, leading to marked heterogeneity in the definition of HER-2-positive cases. Overall, it appears likely that in contrast to breast cancer, HER-2-positive gastric cancer is not associated with an adverse prognosis. This suggests that HER-2 amplification exerts distinct biological effects according to the primary tumour site, which at present are not fully understood. However, as inhibition of HER-2 signalling is beneficial in patients with HER-2 amplified gastric and OGJ cancers, at least partial dependence upon this oncogenic signalling through HER-2 can be reasonably assumed. High levels of concordance between primary tumours and paired metastatic sites suggest that HER-2 amplification is an earlier event and not simply acquired by cells with metastatic potential [Bozzetti et al. 2011]. Disconcordance, where observed, is largely due to primary tumour heterogeneity rather than a true difference in HER-2 gene copy number between the primary and metastatic site [Kim et al. 2011].
Table 1.
Recent large studies examining HER-2 as a prognostic marker in gastric cancer.
| Reference | Population (n) | Gastric cancer scoring used? | Method of HER-2 testing and definition of HER-2 positive | Percentage HER-2 positive | Effect of HER-2 on prognosis |
|---|---|---|---|---|---|
| Hsu et al. [2011] | Resected gastric cancer (1036) | Yes | IHC, FISH in 2+ cases (IHC 3+ or IHC2+ and FISH HER-2:CEP17 ratio >2) | 6.1% | No significant effect on prognosis |
| Begnami et al. [2011] | Resected gastric cancers (221) | Yes | IHC (2+ or 3+) FISH (HER-2 copy number >10 or ratio ≥2) |
12% 8% |
Significantly reduced overall survival in FISH + compared with patients with nonamplified tumours (p = 0.023) |
| Shah et al. [2011] | Advanced gastric cancer (338) | Yes | IHC and FISH or dual (D)ISH (IHC3+ or ISH+ =positive) |
20.6% | Correlates with improved survival on univariate analysis (p = 0.05) but not on multivariate analysis (p = 0.243) |
| Terashima et al. [2011] | Stage II/III resected gastric cancer (829) | Yes | IHC and DISH and mRNA expression IHC 3+ or IHC 2+ and DISH+ |
13.6% | No correlation with overall survival (p = 0.406) |
| Yoon et al. [2011] | Resected oesophageal cancer (713) | Yes | IHC and FISH (IHC 3+ or IHC2+ and FISH+) | 17% | Improved survival in HER-2 positive patients (p = 0.24), but not significant when adjusted for T, N and grade (p = 0.17) |
| Grabsch et al. [2010] | Resected gastric cancers (924) | No | IHC (2+ or 3+) | 4% | No correlation with survival (p = 0.903) |
| Barros-Silva et al. [2009] | Resected gastric cancers (463) | No | IHC (2+ or 3+) FISH ratio ≥2 or signal cluster) |
9.3% 8.2% |
Nonsignificant trend towards worse survival (p = 0.222) |
| Kim et al. [2007] | Resected gastric cancer (248) | No | IHC (2+ or 3+) FISH (ratio ≥2) quantitative PCR |
22.6% 7.7% |
Nonsignificant trend towards inferior survival in subgroup of intestinal cancers (p = 0.152) |
| Tanner et al. [2005] | Resected gastric cancers (131) OGJ tumours stage I–IV (100) |
No | CISH (CISH cut-off not stated) | 12.2% 24.0% |
Reduced cancer-specific survival (p = 0.0089). |
| Pinto-de-Sousa et al. [2002] | Resected gastric cancers (157) | No | IHC (any expression of Her-2 at the membrane) | 15.3% | Significant reduction in 5-year survival (p = 0.004) |
IHC, immunohistochemistry; FISH, fluorescent in situ hybridization; CISH, chromogenic in situ hybridization; DISH, DNA in situ hybridization; OGJ, oesophagogastric junction; PCR, polymerase chain reaction.
HER-2 testing in gastric cancer
A separate gastric cancer HER-2 immunohistochemistry (IHC) scoring system has been developed due to key differences in HER-2 staining patterns in gastric compared with breast cancer, noted in a study designed to validate HER-2 testing for the phase III trial of trastuzumab in gastric cancer. In particular, an increased frequency of tumour heterogeneity and incomplete basolateral reactivity led the authors to recommend that incomplete strong staining could be IHC 3+ and for biopsy samples, the 10% required cut-off used in breast cancer should be replaced by a minimum of 5 cohesive cells staining with 3+ intensity [Hofmann et al. 2008]. These guidelines were further validated in a larger study [Ruschoff et al. 2010] and have been accepted into routine clinical practice. The gastric cancer HER-2 scoring is summarized in Table 2.
Table 2.
Recommended scoring for HER-2 in gastric cancer.
| Characteristic | IHC score | ISH | |
|---|---|---|---|
| Resections | No reactivity or faint reactivity in <10% of cells | 0 (negative) | 20 cohesive cells showing highest gene count. Positive =HER-2:CEP17 ratio ≥2.0 Count 40 cells if ratio on 20 cells 1.8–2.2 |
| Faint reactivity in >10% of cells (membranous staining only confirmed at ×40 magnification. | 1+ (negative) | ||
| Weak–moderate complete or basolateral reactivity in >10% of cells. Unequivocal membranous staining only clear at medium magnification (×10) | 2+ (equivocal, requires confirmation by ISH) | ||
| Moderate–strong complete or basolateral reactivity in >10% of cells. Visible to the naked eye, unequivocal membranous staining at low magnification (×2.5 or ×5) | 3+ (positive) | ||
| Biopsies | ≥5 cells showing strong reactivity | 3+ (positive) | ISH+ clones considered positive irrespective of size. |
IHC, immunohistochemistry; ISH, in situ hybridization.
Tumours of the OGJ (15–33%) [Bang et al. 2009; Boers et al. 2011; Terashima et al. 2011; Yoon et al. 2011] and oesophagus (16–21%) [Thompson et al. 2010; Yoon et al. 2011] are most frequently HER-2 positive, with the lowest rates reported in distal gastric cancers (7%) [Boers et al. 2011]. This may in part be due to the association with intestinal histology, where HER-2-positive diffuse cancers are uncommon [Bang et al. 2009].
Development of trastuzumab
Following the development of specific anti-HER-2 monoclonal antibodies [Fendly et al. 1990], antiproliferative effects in both HER-2-positive breast and gastric cancer cell lines and xenografts were reported, with synergy with chemotherapy agents including cisplatin noted [Hancock et al. 1991; Kasprzyk et al. 1992]. A humanized antibody directed at the extracellular domain of HER-2 was developed, with increased affinity to the HER-2 receptor, improved antibody-dependent cytotoxicity (ADCC) and equal antiproliferative potency in HER-2-positive breast cancer cell lines to the original murine antibody [Carter et al. 1992]. Similar efficacy of trastuzumab has since been reported in HER-2-positive gastric cancer cells lines and xenografts [Matsui et al. 2005; Tanner et al. 2005]. Trastuzumab binds the HER-2 receptor on the C-terminal of domain IV, the juxtamembrane region, facilitating endocytosis of the receptor-antibody complex and blocking constituent activation of HER-2, which can occur via proteolysis of the extracellular domain, leaving a truncated HER-2 receptor known as p95 HER-2 [Cho et al. 2003]. Trastuzumab is also believed to exert its anticancer effects by direct blockade of dimerization, thereby preventing tyrosine kinase activation, ADCC via recruitment of host myeloid cells [Clynes et al. 2000] and possibly via anti-angiogenic effects [Petit et al. 1997]. Figure 2 is a diagrammatic representation of the HER-2 receptor.
Figure 2.
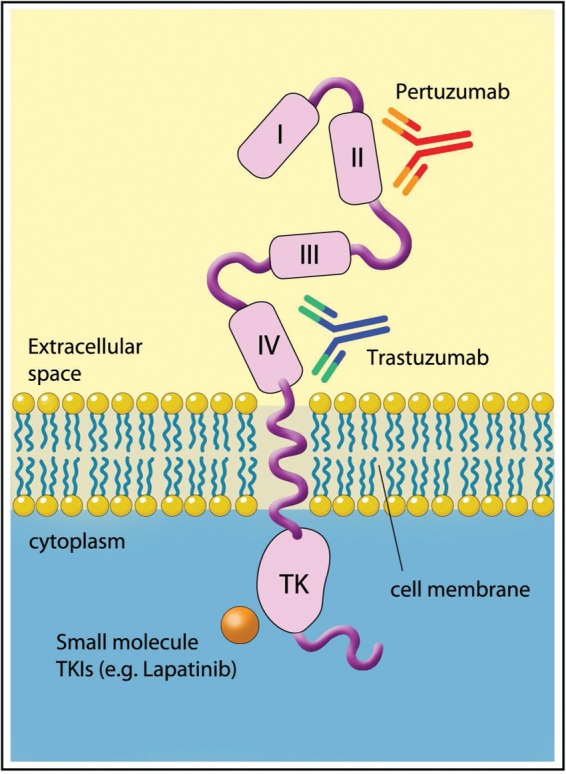
The HER-2 receptor and binding sites for trastuzumab, pertuzumab and small molecule inhibitors. (Illustration courtesy of Alessandro Baliani, Copyright © 2012.)
Further preclinical studies of trastuzumab provided the rationale for the clinical evaluation of trastuzumab in combination with chemotherapy for HER-2-positive gastric cancer; with enhanced antitumour effect observed with capecitabine, cisplatin, paclitaxel, docetaxel and irinotecan [Fujimoto-Ouchi et al. 2007], and a synergistic effect reported with cisplatin in HER-2 positive gastric cancer cell lines [Kim et al. 2008b].
Clinical evaluation of trastuzumab in gastroesophageal cancer
A single phase II study evaluating trastuzumab in advanced gastric cancer has been recently published, 4 years after the results were initially presented. A total of 228 patients with locally advanced unresectable or metastatic adenocarcinoma of the stomach or OGJ were screened to select 22 patients with HER-2-positive cancers for treatment with trastuzumab (8 mg/kg loading dose then 6 mg/kg q 21 days) plus cisplatin monotherapy (75 mg/m2 q 21 days). The response rate (32%) and median PFS (5.1 months) [Gravalos et al. 2011] do not compare favourably with those reported with combination chemotherapy, probably reflecting the suboptimal chemotherapy backbone selected. For the phase III ToGA trial, a chemotherapy doublet comprising cisplatin (80 mg/m2 day 1) with either infused 5-fluororacil (5-FU; 800 mg/m2 days 1–5) or capecitabine (2000 mg/m2/day days 1–14) was chosen for combination with trastuzumab (8 mg/kg loading dose then 6 mg/kg) every 21 days. A total of 3665 patients were centrally screened for HER-2 overexpression by IHC or amplification by fluorescent in situ hybridization (FISH; HER2:CEP17 ratio ≥2), of whom 810 were HER-2 positive, defined as IHC3+, or FISH+ with any IHC result. Of those, 584 patients were randomized to the chemotherapy doublet, with or without trastuzumab in this open-label phase III study designed to detect an increase in overall survival from 10 to 13 months with trastuzumab (hazard ratio [HR] 0.77) with 80% power and a two-sided log rank test α = 0.05. The statistical calculations had been amended due to a lower than expected event rate during the study; the anticipated poor prognosis of HER-2-positive patients and projected median survival of just 7 months with chemotherapy alone (and expected increase to 10 months with the addition of trastuzumab, HR 0.70) was incorrect. In the intent-to-treat population, at the final analysis, median survival was 11.1 months with the chemotherapy doublet, significantly increased to 13.8 months with the addition of trastuzumab (HR 0.74, 95% confidence interval [CI] 0.60–0.91, p = 0.0046). Median PFS (6.7 compared with 5.5 months, HR 0.71, 95% CI 0.59–0.85, p = 0.0002) and radiological response rate (47% compared with 35%, odds ratio 1.70, 95% CI 1.22–2.38, p = 0.0017) were similarly improved with the antibody. Patients with the strongest expression of HER-2 gained the greatest benefit in a preplanned subgroup analysis, with an unprecedented median survival of 17.9 months in patients with IHC3+ and FISH-positive tumours who received trastuzumab, compared with 12.3 months with chemotherapy. Furthermore, in a post hoc exploratory analysis, the subgroup of 446 patients with IHC2+/FISH+ or IHC3+ cancers had a 4.2 month prolongation of median overall survival with the addition of trastuzumab to chemotherapy (16.0 versus 11.8 months), which has formed the basis of the EMEA approval of trastuzumab in gastric cancer. Efficacy outcomes for the ITT population and these molecular defined subgroups who benefitted most are recorded in Table 3.
Table 3.
Efficacy outcomes in the ToGA trial.
| Population (n) | Response rate (%) |
Median PFS/months |
Median OS/months |
|||
|---|---|---|---|---|---|---|
| FP | FP+T | FP | FP+T | FP | FP+T | |
| All patients | ||||||
| IHC3+ or FISH+ (584) | 35% | 47% OR 1.70, 95% CI1.22–2.38, p = 0.0017 |
5.5 | 6.7 HR 0.71, 95% CI 0.59–0.85, p = 0.0002 |
11.1 | 13.8 HR 0.74, 95% CI 0.60–0.91, p = 0.0046 |
| Predefined subgroup | ||||||
| IHC3+ and FISH positive (256) | NR | NR | NR | NR | 12.3 | 17.9 HR 0.58, 95% CI 0.41–0.81 |
| Exploratory subgroups | ||||||
| IHC 0–1+/ FISH+ (131) | NR | NR | NR | NR | 8.7 | 10.0 |
| IHC 2+/FISH+ or IHC 3+ (446) | NR | NR | NR | NR | 11.8 | 16.0 HR 0.65, 95% CI 0.51–0.83 |
IHC, immunohistochemistry; FISH, fluorescent in situ hybridization; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; OR, odds ratio; CI, confidence interval; NR, not reported.
Whilst some clinicians would not consider a platinum–fluoropyrimidine doublet the optimal chemotherapy regimen, it is well tolerated and remains an internationally accepted first-line treatment option. Furthermore, additive cardiac toxicity might have been expected had the investigators selected an epirubicin-based triplet based upon previous studies of trastuzumab with doxorubicin in breast cancer [Seidman et al. 2002], although more recent studies have safely combined trastuzumab with moderate cumulative doses (360 mg/m2) of the less cardiotoxic epirubicin [Untch et al. 2010]. With the doublet regimen selected for the ToGA trial, trastuzumab added little toxicity, with no increase in chemotherapy related grade 3–4 toxicities (68% both arms) or cardiac events (6% both arms), although a numerical increase in treatment related deaths was observed (3% compared with 1%). The only increase in cardiotoxicity reported with trastuzumab was a modest increase in asymptomatic left ventricular dysfunction defined as a drop of left ventricular ejection fraction (LVEF) of ≥10% to below 50% (5% compared with 1% with chemotherapy alone) detected on 12-weekly cardiac assessments. Whilst this is of little relevance to patients with advanced disease whose median survival is so limited, it may be more important in the curative-intent setting where, as we have seen in studies of early breast cancer [Slamon et al. 2011], cardiac failure could occur in a small proportion of long-term survivors. Current studies of trastuzumab in gastroesophageal cancer are summarized in Table 4. Notably at present, none evaluate trastuzumab with an anthracycline-based triplet, although two phase II studies are combining the antibody with a novel triplet chemotherapy backbone comprising docetaxel, oxaliplatin plus a fluoropyrimidine, one with the addition of the anti-VEGF-A monoclonal antibody, bevacizumab.
Table 4.
Selected current phase II and III studies evaluating trastuzumab in gastroesophageal cancer.
| Study, Sponsor, (ClinicalTrials. gov ID) | Phase | n | Population | Treatment | Primary endpoint |
|---|---|---|---|---|---|
| Operable disease | |||||
| RTOG-1010 NCI/RTOG, (NCT01196390) |
III | 480 | Operable adenocarcinoma of the middle or lower oesophagus or OGJ | Weekly carboplatin, paclitaxel ± trastuzumab + radical radiotherapy. Adjuvant trastuzumab × 39 weeks (all patients). | DFS |
| AIO-STO-0310 AIO-Studien-gGmbH (NCT01472029) |
II | 53 | Locally advanced resectable adenocarcinoma of the OGJ or stomach | Perioperative 5-FU, leucovorin, docetaxel, and oxaliplatin + trastuzumab | pCR rate |
| ML25189 Hoffmann-La Roche, (NCT01130337) |
II | 45 | Resectable gastric adenocarcinoma | 3 × CAPOX + trastuzumab before and after surgery, 12 months adjuvant trastuzumab | DFS |
| Advanced disease: first line | |||||
| Hoffman-La Roche (NCT01450696) | III | 400 | HER-2-positive metastatic adenocarcinoma of OGJ and stomach | Cisplatin + capecitabine + trastuzumab 8mg loading dose then 6 mg versus 10 mg/kg q 21 days | OS |
| AMC-ONCGI-1101 Asan Medical Centre (NCT01396707) |
II | 56 | HER-2-positive locally advanced or metastatic adenocarcinoma of OGJ and stomach | CAPOX + trastuzumab | ORR |
| M10B-DOCT The Netherlands Cancer Institute (NCT01359397) |
II | Not stated | HER-2-positive locally advanced or metastatic adenocarcinoma of OGJ and stomach | Docetaxel, oxaliplatin, capecitabine + bevacizumab ± trastuzumab | PFS |
| 09-457 Dana-Farber Cancer Institute (NCT01191697) |
II | 36 | HER-2-positive locally advanced or metastatic adenocarcinoma of OGJ and stomach | CAPOX + bevacizumab + trastuzumab | ORR |
| BP27836 Hoffmann-La Roche (NCT01461057) |
II | 30 | HER-2-positive locally advanced or metastatic adenocarcinoma of OGJ and stomach | Cisplatin + capecitabine + trastuzumab + pertuzumab | Safety |
| National University Hospital, Singapore (NCT01228045) | II | 30 | Advanced HER-2 positive gastric cancer | S-1 + cisplatin + trastuzumab | Not stated |
| Advanced disease: second line | |||||
| CAUY922A2205 Novartis (NCT01402401) |
II | 48 | HER-2-positive advanced gastric or OGJ cancer, previously treated with chemotherapy + trastuzumab | Trastuzumab + AUY922 (HSP90 inhibitor) | ORR |
DFS, disease-free survival; PFS, progression-free survival; pCR, pathological complete remission; OS, overall survival; HR, hazard ratio; OR, odds ratio; CI, confidence interval; OGJ, oesophagogastric junction; ORR, overall response rate; CAPOX, capecitabine + oxaliplatin; ECF, epirubicin, cisplatin + infused 5-fluorouracil; ECX, epirubicin, cisplatin + capecitabine.
Other agents targeting HER-2
Trastuzumab emtansine (T-DM1)
Trastuzumab emtansine (T-DM1) is a novel antibody–drug conjugate linking trastuzumab to the cytotoxic agent, DM1, a derivative of maytansine, a potent microtubule inhibitor developed in the early 1970s [Kupchan et al. 1972]. As a systemic therapy, severe, mainly gastrointestinal toxicity was dose-limiting and efficacy was minimal at tolerable doses [Issell and Crooke, 1978]. Therefore, attention turned to strategies to deliver the cytotoxicity directly into tumour cells, via conjugation with antibodies. Initial conjugates utilizing disulphide bonds, which were anticipated to undergo endosomal reduction thereby releasing the active drug within the target tumour cell [Chari et al. 1992], were limited by insufficient cleavage of the disulphide linker with consequent poor drug delivery [Austin et al. 2005]. These have been superseded by a thioether linkage, which despite being nonreducible, show improved efficacy and reduced toxicity [Lewis Phillips et al. 2008]. T-DM1 is well tolerated and has encouraging monotherapy activity in patients with advanced breast cancer, who are resistant to trastuzumab. The most common toxicities are hypokalaemia, thrombocytopenia, transaminitis and fatigue [Burris et al. 2011; Krop et al. 2010a, 2010b]. Two phase III studies are currently underway in the first- [ClinicalTrials.gov identifier: NCT01120184] and second-line treatment [ClinicalTrials.gov identifier: NCT00829166] of advanced breast cancer. With promising preclinical activity reported in HER-2-positive gastric cancer cell lines [Barok et al. 2011], we are hopeful that evaluation in patients HER-2-positive advanced gastroesophageal cancer will soon follow.
Pertuzumab
Pertuzumab is a monoclonal antibody targeting HER-2 in domain II, the extracellular subdomain required to dimerize with other HER family receptors, preventing formation of the highly mitogenic HER-2/HER-3 dimer. Like trastuzumab, the antibody is not effective in patients without HER-2 amplification [Gianni et al. 2012]. However, in HER-2 positive disease, promising results from a phase II study combining pertuzumab with trastuzumab in patients with previously trastuzumab-treated advanced breast cancer [Baselga et al. 2010] have translated into a 6-month improvement in PFS from the addition of pertuzumab to first-line docetaxel and trastuzumab in the recent phase III CLEOPATRA study in advanced breast cancer [Baselga et al. 2012]. Computer models and molecular dynamic simulations undertaken to investigate the apparent synergy with trastuzumab have reported that the HER-2 receptor is altered by trastuzumab binding, increasing the plasticity of the receptor and enhancing association with pertuzumab. Similarly, pertuzumab may enhance trastuzumab binding [Fuentes et al. 2011]. The landmark CLEOPATRA study could be considered as significant an advance as the initial phase III study of trastuzumab in advanced breast cancer, with a comparable magnitude of benefit reported with each additional antibody over standard therapy: the HR for the addition of trastuzumab to chemotherapy for time to disease progression was 0.51 (95% CI 0.41–0.63) [Slamon et al. 2001]. Similarly, the HR for the addition of pertuzumab to docetaxel plus trastuzumab for PFS was 0.62 (95% CI 0.51–0.75) [Baselga et al. 2012]. A trend towards improved survival was noted at this preplanned interim analysis 0.64 (95% CI 0.47–0.88; p = 0.005) which did not meet the study’s early stopping rule, allowing completion of planned accrual. The second antibody adds moderate toxicity, with higher rates of diarrhoea, rash and febrile neutropenia with the triplet regimen, but no increase in left ventricular dysfunction was evident [Baselga et al. 2012]. As a result of these data, an adjuvant study adding pertuzumab to trastuzumab has opened to accrual in early breast cancer (APHINITY). Evaluation in advanced gastric cancer is also planned, with a phase II study of cisplatin, capecitabine and trastuzumab, randomizing patients to two different pertuzumab dose schedules, expected to open in 2012 [ClinicalTrials.gov identifier: NCT01461057].
Lapatinib
Lapatinib is an orally active small molecule inhibitor of HER-2 and EGFR, which is licensed for the treatment of HER-2-positive advanced breast cancer. Preclinical efficacy in HER-2 amplified gastric cancer cell lines has been reported, with synergy observed with trastuzumab [Wainberg et al. 2010], oxaliplatin [Kim et al. 2008a] and the oral fluoropyrimidine S-1 [Tanizaki et al. 2010]. Of interest, paclitaxel and cisplatin demonstrated a sequence-dependent synergistic effect, where the cytotoxic agent was optimally delivered before lapatinib [Kim et al. 2008a]. Lapatinib also appears to reverse irinotecan resistance in vitro [Yashiro et al. 2011], providing rationale for its use in the second- or third-line treatment of advanced gastroesophageal cancer.
As monotherapy, lapatinib has been evaluated in a phase II study of previously untreated patients with advanced gastric cancer. Perhaps unsurprisingly, the drug had limited efficacy in the unselected cohort (confirmed partial responses reported in 4 of 47 patients, median survival 4.8 months), and a significantly longer median survival was noted in patients with HER-2 gene expression above the median value (6.8 compared with 3.0 months in patients with expression less than median, p = 0.0031) [Iqbal et al. 2011]. However, even a median survival of 6.8 months in the high HER-2 expressers compares poorly to first-line studies of combination chemotherapy [Cunningham et al. 2008]. A small phase II study of lapatinib monotherapy in 25 patients with previously treated EGFR or HER-2-positive advanced gastric cancer (measured by FISH or IHC) reported no objective responses, and remains unpublished [Hecht et al. 2008]. Owing to the limited efficacy as monotherapy, combination of lapatinib with chemotherapy is now being investigated: a placebo-controlled randomised phase III study (LOGiC) will determine whether the addition of lapatinib to capecitabine and oxaliplatin improves survival in previously untreated patients with HER-2-positive advanced gastric or OGJ cancer. Early blinded safety results suggested no unmanageable toxicity, with grade 3 diarrhoea in 2 of 21 evaluable patients (9.5%) [Hecht et al. 2009], despite potential concerns regarding overlapping gastrointestinal toxicities of the three agents. A phase III study in the second-line setting (TYTAN) has also reported blinded safety data for the combination of paclitaxel with or without lapatinib, with grade 3 diarrhoea similarly reported in 10% of patients.[Satoh T, 2010] The clinical applicability of the TYTAN study results will largely depend upon the efficacy in the subgroup of patients who received first-line trastuzumab, which became standard therapy during the study accrual. An EORTC randomized phase II study is evaluating the combination of lapatinib with a combination of epirubicin, cisplatin and either 5-FU or capecitabine (ECF or ECX) in patients with HER-2 positive advanced gastric cancer, which will also be investigated in the operable disease setting in a planned amendment to the phase III MRC ST03 trial. These studies in progress are summarized in Table 5.
Table 5.
Current phase II and III studies evaluating other HER-2 targeted agents in gastroesophageal cancer.
| Study, Sponsor, (Clinical trials.gov ID) | Phase | n | Population | Treatment | Primary endpoint |
|---|---|---|---|---|---|
| Operable disease | |||||
| ST03 MRC (NCT00450203)* |
III | 370 | HER-2 positive adenocarcinoma of oesophagus, OGJ, stomach | ECX ± lapatinib | OS |
| Advanced disease: first line | |||||
| LOGiC GSK (NCT00680901) | III | 535 | HER-2 positive adenocarcinoma of oesophagus, OGJ, stomach | CAPOX + lapatinib or placebo | OS |
| EORTC -40071, EORTC (NCT01123473) | II | 192 | HER-2 or EGFR positive adenocarcinoma of OGJ or stomach, | ECF/X ± lapatinib | PFS |
| GSK, (NCT00526669) | II | 64 | Adenocarcinoma of OGJ and stomach, not selected by HER-2 status | Lapatinib + capecitabine | Biomarkers + ORR |
| CASE2310 Case Comprehensive Cancer Centre (NCT01395537) |
I/II | 43 | Adenocarcinoma of the oesophagus or OGJ, not selected by HER-2 status | Carboplatin, paclitaxel + lapatinib | ORR |
| Advanced disease: second line | |||||
| TYTAN GSK (NCT00486954) |
III | 314 | HER-2 positive adenocarcinoma of stomach | Paclitaxel ± lapatinib | OS |
| GastroLap National Center for Tumor Diseases, Heidelberg (NCT01145404) |
II | 76 | HER-2 positive adenocarcinoma of oesophagus, OGJ, stomach | Lapatinib ± capecitabine | ORR |
| H-1004-031-315 Seoul National University (NCT01152853) |
II | 28 | HER-2 positive adenocarcinoma of OGJ or stomach, | PF-00299804 monotherapy | PFS |
| Memorial Sloan-Kettering Cancer Center (NCT01522768) | II | 27 | HER-2 positive metastatic oesophagogastric cancer, refractory to trastuzumab. | Afatinib monotherapy | ORR |
Planned amendment to the current study of ECX ± bevacizumab, subject to completion of pilot safety study.
DFS, disease-free survival; PFS, progression-free survival; pCR, pathological complete remission; OS, overall survival; HR, hazard ratio; OR, odds ratio; CI, confidence interval; OGJ, oesophagogastric junction; ORR, overall response rate; 5-FU, 5-fluorouracil; CAPOX, capecitabine + oxaliplatin; ECF, epirubicin, cisplatin + infused 5-fluorouracil; ECX, epirubicin, cisplatin + capecitabine.
Other small molecule inhibitors of HER-2
Dacomitinib (PF00299804) is a ‘pan-HER’ small molecule inhibitor, with antitumour activity reported in HER-2-positive gastric cancer cell lines and xenografts, and synergy observed with several commonly used cytotoxics (5-FU, cisplatin, docetaxel and paclitaxel), targeted agents (trastuzumab and investigational inhibitors of IGF1R, ERK1/2 and PI3K/mTOR) [Nam et al. 2011a]. A phase II study will determine its monotherapy activity in patients with previously treated HER-2-positive advanced gastric or OGJ cancers.
A phase II study of Afatinib (BIBW2992), an irreversible inhibitor of EGFR and HER-2 has recently been initiated in trastuzumab-refractory advanced oesophagogastric cancer [ClinicalTrials.gov identifier: NCT01522768]. HM781-36B is an irreversible pan-HER inhibitor, which also demonstrates potent preclinical activity in HER-2-positive gastric cancer cell lines and xenografts. Of interest, although the targeted agent was inactive as monotherapy in HER-2-negative cell lines, synergy with chemotherapy was reported in both HER-2 amplified and nonamplified models [Nam et al. 2011b]. The drug is currently undergoing phase I evaluation in solid tumours [ClinicalTrials.gov identifiers: NCT01455584 and NCT01455571].
Other pan-HER inhibitors, including AZD8931 and BMS-599626, are undergoing early phase clinical testing, but currently lack published data in gastroesophageal cancers, with no trials in this disease registered as yet.
Resistance to trastuzumab
Whilst some patients with HER-2-positive advanced gastric cancer exhibit primary resistance to trastuzumab, all acquire resistance after a relatively short treatment duration (median PFS 6.7 months) [Bang et al. 2010]. At present, we are not aware of any published studies which have evaluated mechanisms of trastuzumab resistance in gastroesophageal cancers.
In the decade since the integration of trastuzumab into the routine management of breast cancer, much research has focused on the mechanisms of primary and acquired resistance, the latter of which develops in the majority of patients. Whilst resistance remains poorly elucidated on an individual patient basis, important mechanisms in breast cancer have been established. These can be broadly divided into aberrations in the downstream signalling PI3K/AKT/mTOR pathway, shedding of the extracellular domain of the receptor with formation of the constitutively activated p95 HER-2, and redirection of signalling via other growth factor receptors.
PTEN (phosphatase and tensin homologue protein), is the protein product of the PTEN tumour suppressor gene which antagonizes the function of PI3K, thereby inhibiting signal transduction through the PI3K/AKT pathway. Loss of expression occurs in approximately one third of breast cancers [Perez-Tenorio et al. 2007] and renders the tumour at least partially resistant to trastuzumab [Nagata et al. 2004]. This resistance can potentially be overcome by PI3K inhibitors, which abrogate the constitutive signalling through this pathway in preclinical studies [Nagata et al. 2004]. Similarly, combination of trastuzumab with AKT or mTOR inhibitors in preclinical studies reverses PTEN loss-induced trastuzumab resistance [Lu et al. 2007]. PTEN loss has also been reported to predict benefit from lapatinib [Dave et al. 2011].
Activating mutations in the gene encoding the p110 catalytic subunit α of PI3K (PIK3CA) also switch on signalling through this important pathway, negating the upstream inhibitory effect of trastuzumab [Berns et al. 2007] and additionally conferring resistance to lapatinib [Wang et al. 2011]. Such mutations are relatively common in breast cancer, reported in approximately 25% of patients and associated with HER-2 overexpression [Saal et al. 2005]. Again, PI3K, AKT and mTOR inhibitors are under evaluation in advanced breast cancer in combination with trastuzumab to circumvent this resistance mechanism. A phase I study in patients with HER-2-positive advanced breast cancer previously treated with trastuzumab reported a 44% response rate to the mTOR inhibitor, everolimus (RAD001) combined with paclitaxel and trastuzumab [Andre et al. 2010], despite the majority of patients having progressed on or within 3 months of receiving the antibody for advanced disease or within 12 months of adjuvant trastuzumab. A 15% response rate was reported in a phase II study of the chemotherapy-free combination of trastuzumab and everolimus in patients with a more stringent definition of trastuzumab resistance, where all patients had progressed on trastuzumab-based therapy [Morrow et al. 2011]. Of interest, neither loss of PTEN nor PIK3CA mutations was predictive for benefit from everolimus.
P95 HER-2, a truncated form of the receptor formed by cleavage of the extracellular domain, lacks a binding site for trastuzumab, bestowing resistance to the antibody. Preclinical studies have confirmed that breast cancer cell lines expressing p95 HER-2 are resistant to trastuzumab, but remain sensitive to the small molecule inhibitor, lapatinib. Correlation with archival tumour from 47 patients with advanced breast cancer treated with trastuzumab, with or without chemotherapy, revealed expression of p95 in 9 out of 46 patients, 8 of whom had not responded to trastuzumab [Scaltriti et al. 2007]. The combination of trastuzumab and lapatinib has been evaluated in a phase III study of 296 patients with trastuzumab-resistant advanced breast cancer, in whom the combination was superior to lapatinib alone (HR for progression 0.73, 95% CI 0.57–0.93, p = 0.008). Unfortunately, correlation with p95 expression was not performed [Blackwell et al. 2010]. This combination is particularly attractive for patients with trastuzumab-resistant gastric cancer, potentially providing a chemotherapy-free treatment option after first-line chemotherapy plus trastuzumab. Inhibitors of the chaperone protein, HSP90, have also been reported to abrogate signalling through p95 HER-2 in vivo [Chandarlapaty et al. 2010]. A study evaluating an HSP90 inhibitor in combination with trastuzumab in the second-line treatment of HER-2-positive advanced gastric cancer [ClinicalTrials.gov identifier: NCT01402401] is underway.
Increased signalling through other receptor tyrosine kinases including EGFR [Diermeier et al. 2005], MET [Shattuck et al. 2008] and the insulin-like growth factor 1 receptor (IGF1R) [Lu et al. 2001] has been described in HER-2-resistant cells. Inhibition of IGF1R improves trastuzumab sensitivity in preclinical studies [Browne et al. 2011], providing rationale for a dual HER-2/IGF1R targeted strategy to prevent or at least delay acquired resistance via this mechanism.
Cyclin E overexpression has also been recently correlated with reduced trastuzumab sensitivity in HER-2-positive cell lines [Scaltriti et al. 2011], but we are not aware of any clinical confirmation of this putative mechanism.
A recently described further mechanism of resistance, is hyperactivation of the nonreceptor tyrosine kinase, SRC, which may modulate trastuzumab resistance induced by the other mechanisms described above, potentially providing a more broadly applicable therapeutic target. The investigators demonstrated that inhibition of SRC by the small molecule saracatinib in combination with trastuzumab, inhibited AKT activation, a surrogate marker of HER-2 signalling, in both PTEN-deficient and EGFR-overexpressing HER-2-positive cells [Zhang et al. 2011].
Correlative translational studies are vital to identify whether these, or other, novel mechanisms of trastuzumab resistance are also relevant to gastric and OGJ cancers, to allow rationally designed clinical trials for patients with primary or acquired trastuzumab-resistant disease.
Future directions
Two phase II and one phase III study of trastuzumab have already been initiated in early oesophagogastric cancer, to determine whether the benefit observed in the advanced disease setting will translate into a meaningful increase in survival in patients with operable disease. At present, possibly due to concerns over cardiotoxicity, none will evaluate the antibody with epirubicin-based chemotherapy in advanced or early disease. Similarly, no study of trastuzumab beyond first disease progression has yet been initiated to the best of the authors’ knowledge. This common practice in advanced breast cancer is supported by retrospective studies and small prospective studies [von Minckwitz et al. 2009], but should now be robustly tested in advanced oesophagogastric cancer, evaluating continuation of trastuzumab with second-line irinotecan or docetaxel chemotherapy. The patent life of intravenous trastuzumab will inevitably limit investment in large studies in gastroesophageal cancer, and whether the new subcutaneous formulation of the drug can be substituted currently remains unproven.
Attention has naturally turned to the trastuzumab–pertuzumab dual antibody blockade, which will soon be tested in a phase II study in advanced gastric and OGJ cancers, which it is hoped will lead to phase III evaluation. However, the cost–efficacy analysis of this combination will be critical to determine its applicability and will inevitably limit its utility in countries where health resources are finite. The combination of T-DM1 and pertuzumab may be even more effective and potentially provide a systemic chemotherapy-free strategy, which would be popular with both patients and physicians if efficacious. Certainly, accrual to two phase III studies evaluating this combination in advanced breast cancer has been extremely rapid due to widespread expectation of improved outcomes. It is our hope that the evaluation of T-DM1 in advanced gastroesophageal cancer will follow swiftly, avoiding the almost decade-long delay experienced with trastuzumab.
Correlative translational studies are now integral to prospective clinical trials of targeted agents, therefore the identification of mechanisms of resistance to HER-2-targeted agents should be expedient, and novel agents to circumvent these mechanisms swiftly tested in molecularly selected patients.
Whilst only 15–25% of patients with gastroesophageal cancer are HER-2 positive, the integration of trastuzumab into the routine treatment of this molecularly selected group represents an important advance towards personalized medicine for these patients, which can perhaps be replicated once further subgroups of patients with druggable molecular aberrations can be identified.
Acknowledgments
The authors acknowledge NHS funding to the NIHR Biomedical Research Centre.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors for this work.
Conflict of interest statement: Dr Okines previously received an honorarium from Roche and travel support to attend international scientific meetings from Roche, Bayer and Amgen. Professor Cunningham has received research funding from Roche, Amgen and Merck and have attended advisory boards for Roche and Amgen and acted as an expert witness for Amgen (all uncompensated).
Contributor Information
Alicia F.C. Okines, Department of Medicine, Royal Marsden Hospital, Fulham Road, London SW3 6JJ, UK
David Cunningham, Department of Medicine, Royal Marsden Hospital, London, UK.
References
- Akiyama T., Sudo C., Ogawara H., Toyoshima K., Yamamoto T. (1986) The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 232: 1644-1646 [DOI] [PubMed] [Google Scholar]
- Andre F., Campone M., O’Regan R., Manlius C., Massacesi C., Sahmoud T., et al. (2010) Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol 28: 5110–5115 [DOI] [PubMed] [Google Scholar]
- Austin C.D., Wen X., Gazzard L., Nelson C., Scheller R.H., Scales S.J. (2005) Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc Natl Acad Sci U S A 102: 17987–17992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang Y., Chung H., Xu J., Lordick F., Sawaki A., Lipatov O., et al. for the Seoul National University Hospital, Seoul, Republic of Korea; Yonsei University College of Medicine, Seoul, Republic of Korea; Affiliated Hospital (307 Hospital) Cancer Centre, Beijing, China; National Centre for Tumour Diseases, Heidelberg, Germany; Aichi Cancer Center, Nagoya, Japan; Bashkirian Republican Clinical Oncology Dispensary, Ufa, Russia; F. Hoffmann-La Roche, Basel, Russia; Roche Products Ltd, Welwyn Garden City, United Kingdom; TARGOS Molecular Pathology GmbH, Kassel, Germany; and University Hospital Gasthuisberg, Leuven, Belgium (2009) Pathological features of advanced gastric cancer (GC): Relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial. J Clin Oncol 27(15 Suppl.): abstract 4556 [Google Scholar]
- Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697 [DOI] [PubMed] [Google Scholar]
- Barok M., Tanner M., Koninki K., Isola J. (2011) Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett 306: 171–179 [DOI] [PubMed] [Google Scholar]
- Barros-Silva J.D., Leitao D., Afonso L., Vieira J., Dinis-Ribeiro M., Fragoso M., et al. (2009) Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer 100: 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J., Cortes J., Kim S.B., Im S.A., Hegg R., Im Y.H., et al. (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366: 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J., Gelmon K.A., Verma S., Wardley A., Conte P., Miles D., et al. (2010) Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 28: 1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begnami M.D., Fukuda E., Fregnani J.H.T.G., Nonogaki S., Montagnini A.L., da Costa W.L., et al. (2011) Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol, in press [DOI] [PubMed] [Google Scholar]
- Berns K., Horlings H.M., Hennessy B.T., Madiredjo M., Hijmans E.M., Beelen K., et al. (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12: 395–402 [DOI] [PubMed] [Google Scholar]
- Blackwell K.L., Burstein H.J., Storniolo A.M., Rugo H., Sledge G., Koehler M., et al. (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28: 1124–1130 [DOI] [PubMed] [Google Scholar]
- Boers J.E., Meeuwissen H., Methorst N. (2011) HER2 status in gastro-oesophageal adenocarcinomas assessed by two rabbit monoclonal antibodies (SP3 and 4B5) and two in situ hybridization methods (FISH and SISH). Histopathology 58: 383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzetti C., Negri F.V., Lagrasta C.A., Crafa P., Bassano C., Tamagnini I., et al. (2011) Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer 104: 1372–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne B.C., Crown J., Venkatesan N., Duffy M.J., Clynes M., Slamon D., et al. (2011) Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Ann Oncol 22: 68–73 [DOI] [PubMed] [Google Scholar]
- Burris H.A., 3rd, Rugo H.S., Vukelja S.J., Vogel C.L., Borson R.A., Limentani S., et al. (2011) Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 29: 398–405 [DOI] [PubMed] [Google Scholar]
- Carraway K.L., III, Sliwkowski M.X., Akita R., Platko J.V., Guy P.M., Nuijens A., et al. (1994) The erbB3 gene product is a receptor for heregulin. J Biol Chem 269: 14303–14306 [PubMed] [Google Scholar]
- Carter P., Presta L., Gorman C.M., Ridgway J.B., Henner D., Wong W.L., et al. (1992) Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 89: 4285–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S., Scaltriti M., Angelini P., Ye Q., Guzman M., Hudis C.A., et al. (2010) Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene 29: 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari R.V., Martell B.A., Gross J.L., Cook S.B., Shah S.A., Blattler W.A., et al. (1992) Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res 52: 127–131 [PubMed] [Google Scholar]
- Cho H.S., Mason K., Ramyar K.X., Stanley A.M., Gabelli S.B., Denney D.W., Jr., et al. (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421: 756–760 [DOI] [PubMed] [Google Scholar]
- Clynes R.A., Towers T.L., Presta L.G., Ravetch J.V. (2000) Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 6: 443–446 [DOI] [PubMed] [Google Scholar]
- Coussens L., Yang-Feng T.L., Liao Y.C., Chen E., Gray A., McGrath J., et al. (1985) Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230: 1132–1139 [DOI] [PubMed] [Google Scholar]
- Cunningham D., Okines A.F., Ashley S. (2010) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 362: 858–859 [DOI] [PubMed] [Google Scholar]
- Cunningham D., Starling N., Rao S., Iveson T., Nicolson M., Coxon F., et al. (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358: 36–46 [DOI] [PubMed] [Google Scholar]
- Daly J.M., Olayioye M.A., Wong A.M., Neve R., Lane H.A., Maurer F.G., et al. (1999) NDF/heregulin-induced cell cycle changes and apoptosis in breast tumour cells: role of PI3 kinase and p38 MAP kinase pathways. Oncogene 18: 3440–3451 [DOI] [PubMed] [Google Scholar]
- Dave B., Migliaccio I., Gutierrez M.C., Wu M.F., Chamness G.C., Wong H., et al. (2011) Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol 29: 166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diermeier S., Horvath G., Knuechel-Clarke R., Hofstaedter F., Szollosi J., Brockhoff G. (2005) Epidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activation. Exp Cell Res 304: 604–619 [DOI] [PubMed] [Google Scholar]
- Fendly B.M., Winget M., Hudziak R.M., Lipari M.T., Napier M.A., Ullrich A. (1990) Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res 50: 1550–1558 [PubMed] [Google Scholar]
- Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893–2917 [DOI] [PubMed] [Google Scholar]
- Fuentes G., Scaltriti M., Baselga J., Verma C.S. (2011) Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res 13(3): R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto-Ouchi K., Sekiguchi F., Yasuno H., Moriya Y., Mori K., Tanaka Y. (2007) Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol 59: 795–805 [DOI] [PubMed] [Google Scholar]
- Fukushige S., Matsubara K., Yoshida M., Sasaki M., Suzuki T., Semba K., et al. (1986) Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol 6: 955–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni L., Llado A., Bianchi G., Cortes J., Kellokumpu-Lehtinen P.L., Cameron D.A., et al. (2012) Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28: 1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabsch H., Sivakumar S., Gray S., Gabbert H.E., Muller W. (2010) HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol 32: 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravalos C., Gomez-Martin C., Rivera F., Ales I., Queralt B., Marquez A., et al. (2011) Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol 13: 179–184 [DOI] [PubMed] [Google Scholar]
- Guy P.M., Platko J.V., Cantley L.C., Cerione R.A., Carraway K.L., III (1994) Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A 91: 8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock M.C., Langton B.C., Chan T., Toy P., Monahan J.J., Mischak R.P., et al. (1991) A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res 51: 4575–4580 [PubMed] [Google Scholar]
- Hecht J., Bang Y., Sobrero A., Elme A., Patel G., Park J., et al. (2009) A phase III study of CapeOx +/- lapatinib in HER2 positive locally-advanced/metastatic upper gastrointestinal adenocarcinoma: interim safety results. In: Proceedings of the Joint ECCO 15 - 34TH ESMO Multidisciplinary Congress, Berlin, September 2009 (Eur J Cancer Suppl 7(2): 385). [Google Scholar]
- Hecht J.R., Urba S.G., Koehler M., Ellis C., Gagnon R., Kemner A., et al. (2008) Lapatinib monotherapy in recurrent upper gastrointestinal malignancy: Phase II efficacy and biomarker analyses. In: Proceedings of the Gastrointestinal Cancers Symposium [Google Scholar]
- Hofmann M., Stoss O., Shi D., Buttner R., van de, Vijver M., Kim W., et al. (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52: 797–805 [DOI] [PubMed] [Google Scholar]
- Hsu J.T., Chen T.C., Tseng J.H., Chiu C.T., Liu K.H., Yeh C.N., et al. (2011) Impact of HER-2 overexpression/amplification on the prognosis of gastric cancer patients undergoing resection: a single-center study of 1,036 patients. Oncologist 16: 1706–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S., Goldman B., Fenoglio-Preiser C.M., Lenz H.J., Zhang W., Danenberg K.D., et al. (2011) Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol 22: 2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issell B.F., Crooke S.T. (1978) Maytansine. Cancer Treat Rev 5: 199–207 [DOI] [PubMed] [Google Scholar]
- Kang J.H., Lee S.I., Lim D.H., Park K.W., Oh S.Y., Kwon H.C., et al. (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol, in press [DOI] [PubMed] [Google Scholar]
- Kang Y.K., Kang W.K., Shin D.B., Chen J., Xiong J., Wang J., et al. (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20: 666–673 [DOI] [PubMed] [Google Scholar]
- Kasprzyk P.G., Song S.U., Di Fiore P.P., King C.R. (1992) Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res 52: 2771–2776 [PubMed] [Google Scholar]
- Kim J.W., Kim H.P., Im S.A., Kang S., Hur H.S., Yoon Y.K., et al. (2008a) The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett 272: 296–306 [DOI] [PubMed] [Google Scholar]
- Kim M.A., Jung E.J., Lee H.S., Lee H.E., Jeon Y.K., Yang H.K., et al. (2007) Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum Pathol 38: 1386–1393 [DOI] [PubMed] [Google Scholar]
- Kim M.A., Lee H.J., Yang H.K., Bang Y.J., Kim W.H. (2011) Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology 59: 822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Kim H.P., Kim Y.J., Oh do Y., Im S.A., Lee D., et al. (2008b) Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol 32: 89–95 [PubMed] [Google Scholar]
- Koizumi W., Narahara H., Hara T., Takagane A., Akiya T., Takagi M., et al. (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9: 215–221 [DOI] [PubMed] [Google Scholar]
- Krop I., Lorusso P., Miller K.D., Modi S., Yardley D., Rodriguez G., et al. (2010a) A phase 2 study of the HER2 antibody-drug conjugate trastuzumab-DM1 (T-DM1) in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab, lapatinib, and chemotherapy. In: Proceedings of the ESMO Congress, Milan (Ann Oncol 21(Suppl. 8)). [Google Scholar]
- Krop I.E., Beeram M., Modi S., Jones S.F., Holden S.N., Yu W., et al. (2010b) Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 28: 2698–2704 [DOI] [PubMed] [Google Scholar]
- Kupchan S.M., Komoda Y., Court W.A., Thomas G.J., Smith R.M., Karim A., et al. (1972) Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc 94: 1354–1356 [DOI] [PubMed] [Google Scholar]
- Lewis Phillips G.D., Li G., Dugger D.L., Crocker L.M., Parsons K.L., Mai E., et al. (2008) Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 68: 9280–9290 [DOI] [PubMed] [Google Scholar]
- Lu C.H., Wyszomierski S.L., Tseng L.M., Sun M.H., Lan K.H., Neal C.L., et al. (2007) Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res 13: 5883–5888 [DOI] [PubMed] [Google Scholar]
- Lu Y., Zi X., Zhao Y., Mascarenhas D., Pollak M. (2001) Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 93: 1852–1857 [DOI] [PubMed] [Google Scholar]
- Matsui Y., Inomata M., Tojigamori M., Sonoda K., Shiraishi N., Kitano S. (2005) Suppression of tumor growth in human gastric cancer with HER2 overexpression by an anti-HER2 antibody in a murine model. Int J Oncol 27: 681–685 [PubMed] [Google Scholar]
- Morrow P.K., Wulf G.M., Ensor J., Booser D.J., Moore J.A., Flores P.R., et al. (2011) Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol 29: 3126–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad A.M., Santiago F.F., Petroianu A., Rocha P.R., Rodrigues M.A., Rausch M. (1993) Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72: 37–41 [DOI] [PubMed] [Google Scholar]
- Nagata Y., Lan K.H., Zhou X., Tan M., Esteva F.J., Sahin A.A., et al. (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6: 117–127 [DOI] [PubMed] [Google Scholar]
- Nam H.J., Ching K.A., Kan J.L., Kim H.P., Han S.W., Im S.A., et al. (2011a) Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther, in press [DOI] [PubMed] [Google Scholar]
- Nam H.J., Kim H.P., Yoon Y.K., Hur H.S., Song S.H., Kim M.S., et al. (2011b) Antitumor activity of HM781-36B, an irreversible Pan-HER inhibitor, alone or in combination with cytotoxic chemotherapeutic agents in gastric cancer. Cancer Lett 302: 155–165 [DOI] [PubMed] [Google Scholar]
- Perez-Tenorio G., Alkhori L., Olsson B., Waltersson M.A., Nordenskjold B., Rutqvist L.E., et al. (2007) PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res 13: 3577–3584 [DOI] [PubMed] [Google Scholar]
- Petit A.M., Rak J., Hung M.C., Rockwell P., Goldstein N., Fendly B., et al. (1997) Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 151: 1523–1530 [PMC free article] [PubMed] [Google Scholar]
- Pinkas-Kramarski R., Soussan L., Waterman H., Levkowitz G., Alroy I., Klapper L., et al. (1996) Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. Embo J 15: 2452–2467 [PMC free article] [PubMed] [Google Scholar]
- Pinto-de-Sousa J., David L., Almeida R., Leitao D., Preto J.R., Seixas M., et al. (2002) c-erb B-2 expression is associated with tumor location and venous invasion and influences survival of patients with gastric carcinoma. Int J Surg Pathol 10: 247–256 [DOI] [PubMed] [Google Scholar]
- Pyrhonen S., Kuitunen T., Nyandoto P., Kouri M. (1995) Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 71: 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschoff J., Dietel M., Baretton G., Arbogast S., Walch A., Monges G., et al. (2010) HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 457: 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal L.H., Holm K., Maurer M., Memeo L., Su T., Wang X., et al. (2005) PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 65: 2554–2559 [DOI] [PubMed] [Google Scholar]
- Satoh T., Bang Y., Wang J., Xu J., Chung H.C., Yeh K., et al. (2010) Interim safety analysis from TYTAN: A phase III Asian study of lapatinib in combination with paclitaxel as second-line therapy in gastric cancer. In: Proceedings of the ASCO Annual Meeting, Chicago, IL (J Clin Oncol 28(15 Suppl.): abstract 4057). [Google Scholar]
- Scaltriti M., Eichhorn P.J., Cortes J., Prudkin L., Aura C., Jimenez J., et al. (2011) Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A 108: 3761–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaltriti M., Rojo F., Ocana A., Anido J., Guzman M., Cortes J., et al. (2007) Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99: 628–638 [DOI] [PubMed] [Google Scholar]
- Schechter A.L., Hung M.C., Vaidyanathan L., Weinberg R.A., Yang-Feng T.L., Francke U., et al. (1985) The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science 229: 976–978 [DOI] [PubMed] [Google Scholar]
- Schechter A.L., Stern D.F., Vaidyanathan L., Decker S.J., Drebin J.A., Greene M.I., et al. (1984) The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 312: 513–516 [DOI] [PubMed] [Google Scholar]
- Seidman A., Hudis C., Pierri M.K., Shak S., Paton V., Ashby M., et al. (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20: 1215–1221 [DOI] [PubMed] [Google Scholar]
- Shah M.A., Janjigian Y.Y., Pauligk C., Werner D., Kelsen D.P., Jaeger E., et al. (2011) Prognostic significance of human epidermal growth factor-2 (HER2) in advanced gastric cancer: A U.S. and European international collaborative analysis. In: Proceedings of the ASCO Annual Meeting, Chicago, IL (J Clin Oncol 29(Suppl.): abstract 4014). [Google Scholar]
- Shattuck D.L., Miller J.K., Carraway K.L., III, Sweeney C. (2008) Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res 68: 1471–1477 [DOI] [PubMed] [Google Scholar]
- Slamon D., Eiermann W., Robert N., Pienkowski T., Martin M., Press M., et al. (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365: 1273–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182 [DOI] [PubMed] [Google Scholar]
- Slamon D.J., Godolphin W., Jones L.A., Holt J.A., Wong S.G., Keith D.E., et al. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712 [DOI] [PubMed] [Google Scholar]
- Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792 [DOI] [PubMed] [Google Scholar]
- Smith I., Procter M., Gelber R.D., Guillaume S., Feyereislova A., Dowsett M., et al. (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369: 29–36 [DOI] [PubMed] [Google Scholar]
- Tanizaki J., Okamoto I., Takezawa K., Tsukioka S., Uchida J., Kiniwa M., et al. (2010) Synergistic antitumor effect of S-1 and HER2-targeting agents in gastric cancer with HER2 amplification. Mol Cancer Ther 9: 1198–1207 [DOI] [PubMed] [Google Scholar]
- Tanner M., Hollmen M., Junttila T.T., Kapanen A.I., Tommola S., Soini Y., et al. (2005) Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 16: 273–278 [DOI] [PubMed] [Google Scholar]
- Tebbutt N.C., Cummins M.M., Sourjina T., Strickland A., Van Hazel G., Ganju V., et al. (2010) Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: the AGITG ATTAX trial. Br J Cancer, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima M., Ochiai A., Kitada K., Ichikawa W., Kurahashi I., Sakuramoto S., et al. (2011) Impact of human epidermal growth factor receptor (EGFR) and ERBB2 (HER2) expressions on survival in patients with stage II/III gastric cancer, enrolled in the ACTS-GC study. In: Proceedings of the ASCO Annual Meeting, Chicago, IL (J Clin Oncol 29(Suppl.): abstract 4013). [Google Scholar]
- Thompson S.K., Sullivan T.R., Davies R., Ruszkiewicz A.R. (2010) Her-2/neu gene amplification in esophageal adenocarcinoma and its influence on survival. Ann Surg Oncol 18: 2010–2017 [DOI] [PubMed] [Google Scholar]
- Thuss-Patience P.C., Kretzschmar A., Bichev D., Deist T., Hinke A., Breithaupt K., et al. (2011) Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer - a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 47: 2306–2314 [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Semba K., Akiyama T., Ikawa S., Yamamoto T. (1986) The c-erbB-2 gene encodes a receptor-like protein with tyrosine kinase activity. Cold Spring Harb Symp Quant Biol 51: 977–982 [DOI] [PubMed] [Google Scholar]
- Untch M., Muscholl M., Tjulandin S., Jonat W., Meerpohl H.G., Lichinitser M., et al. (2010) First-line trastuzumab plus epirubicin and cyclophosphamide therapy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: cardiac safety and efficacy data from the Herceptin, Cyclophosphamide, and Epirubicin (HERCULES) trial. J Clin Oncol 28: 1473–1480 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E., Moiseyenko V.M., Tjulandin S., Majlis A., Constenla M., Boni C., et al. (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24: 4991–4997 [DOI] [PubMed] [Google Scholar]
- von Minckwitz G., du Bois A., Schmidt M., Maass N., Cufer T., de Jongh F.E., et al. (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol 27: 1999–2006 [DOI] [PubMed] [Google Scholar]
- Wagner A.D., Unverzagt S., Grothe W., Kleber G., Grothey A., Haerting J., et al. (2010) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 3: CD004064. [DOI] [PubMed] [Google Scholar]
- Wainberg Z.A., Anghel A., Desai A.J., Ayala R., Luo T., Safran B., et al. (2010) Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res 16: 1509–1519 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang Q., Zhang J., Sun S., Guo H., Jia Z., et al. (2011) PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer 11: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthylake R., Opresko L.K., Wiley H.S. (1999) ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem 274: 8865–8874 [DOI] [PubMed] [Google Scholar]
- Yarden Y. (2001) The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 37(Suppl. 4): S3-S8 [DOI] [PubMed] [Google Scholar]
- Yashiro M., Qiu H., Hasegawa T., Zhang X., Matsuzaki T., Hirakawa K. (2011) An EGFR inhibitor enhances the efficacy of SN38, an active metabolite of irinotecan, in SN38-refractory gastric carcinoma cells. Br J Cancer 105: 1522–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Yamamoto T., Miyajima N., Toyoshima K., Nomura N., Sakamoto H., et al. (1988) Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene 2: 283–287 [PubMed] [Google Scholar]
- Yokota J., Yamamoto T., Toyoshima K., Terada M., Sugimura T., Battifora H., et al. (1986) Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet 1: 765–767 [DOI] [PubMed] [Google Scholar]
- Yonemura Y., Ninomiya I., Yamaguchi A., Fushida S., Kimura H., Ohoyama S., et al. (1991) Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res 51: 1034–1038 [PubMed] [Google Scholar]
- Yoon H.H., Shi Q., Sukov W.R., Wiktor A.E., Khan M., Sattler C.A., et al. (2011) HER2 expression/amplification: Frequency, clinicopathologic features, and prognosis in 713 patients with esophageal adenocarcinoma (EAC). In: Proceedings of the ASCO Annual Meeting, Chicago, IL (J Clin Oncol 29(Suppl.): abstract 4012). [Google Scholar]
- Zhang S., Huang W.C., Li P., Guo H., Poh S.B., Brady S.W., et al. (2011) Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med 17: 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]