Glioblastoma (grade IV astrocytoma) is the most common primary adult brain tumor. Although these tumors rarely metastasize, they almost always recur locally. In spite of intensive treatment regimens consisting of surgery, radiotherapy and temozolomide chemotherapy, patients with these tumors have poor prognoses with a median survival of under one year 1,2. A number of factors may contribute to the resistance of these tumors to therapy. Conventional chemotherapy is limited by a relatively low drug penetration into the tumor interstitium, due to the need to cross the blood-brain and blood-tumor barriers 3. In addition, glioblastomas have regions of hypoxia 4,5 that can lead to resistance to both chemo- and radiotherapy.
Despite the presence of hypoxic regions, one well-recognized hallmark of glioblastomas is endothelial cell proliferation and robust neovascularization (Figure 1). This is likely due to the expression of a variety of angiogenic growth factors such as vascular endothelial growth factor (VEGF). Recent evidence suggests that stem cell-like glioblasts may be a crucial source of key angiogenic factors and that targeting proangiogenic factors from these populations may be a viable therapeutic option 6.
Figure 1.
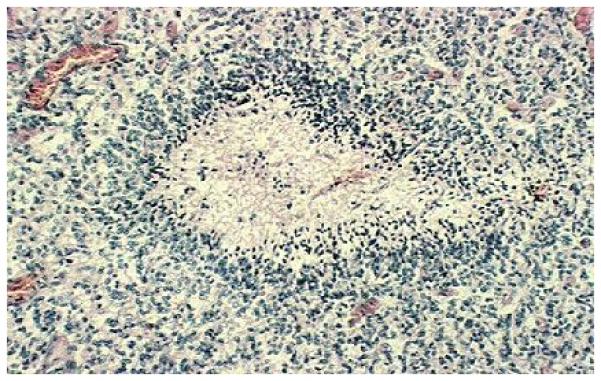
Hematoxyllin/eosin staining of human glioblastoma multiforme. Neoplastic cells stain blue and surround the area of central necrosis. Numerous microvessels are richly perfused with red blood cells.
Is the reliance of glioblastoma on angiogenesis the Achilles’ heel of this deadly neoplasm? Recently, this hypothesis has been put to rigorous tests in both pre-clinical and clinical settings. Xenograft models, where human glioma cells are implanted either ectopically (subcutaneously) or orthotopically (intracerebrally) into immunocompromised mice or rats, are particularly suited to assess the effects of anti-angiogenic molecules on tumor vessel density, overall growth, and survival 7. Numerous studies in animal models have shown that inhibiting VEGF function using neutralizing antibodies 8, dominant-negative VEGF receptor mutants 9 and antisense constructs 10 causes overt regression of blood vessels and thus precludes growth of glioma cells in vivo 11.
Subsequently, a few promising studies have been performed in human patients. For example, AZD2171, an oral tyrosine kinase inhibitor of the VEGF receptor, has afforded significant clinical benefits in alleviating edema and normalizing the tumor vasculature 12. However, in a recent Phase II clinical trial of bevacizumab (anti-VEGF antibody) plus irinotecan, six-month progression-free survival probability was only 38% 13. Thus, it appears that advanced glioblastomas do not rely exclusively on VEGF, and that other pathways involved in angiogenesis must be targeted in parallel.
One such approach would be the use of natural inhibitors of angiogenesis, whose action does not depend on neutralization of VEGF. One prominent member of this diverse family is thrombospondin-1 (Tsp1). This matricellular protein was the first naturally occurring angiogenic inhibitor discovered 14, and early on its anti-angiogenic effects were shown to limit tumor growth and metastasis 15. Many oncogenes, such as c-Myc, down-regulate Tsp1 to promote neovascularization 16,17. Anti-angiogenic activity of Tsp1 is mediated primarily through its interaction with the scavenger receptor CD36 18. This leads to the inhibition of endothelial cell migration 19 and the induction of p38 MAPK-dependent apoptosis 20. At the same time, treatment with thrombospondin-1 results in increased expression of Fas ligand on the surface of endothelial cells, which makes them vulnerable to Fas-mediated apoptosis 21. Thrombospondin-1 may be a particularly useful therapeutic agent for glioblastoma because its expression is often absent in these tumors, due to frequent loss of chromosome 10 on which the THBS1 gene resides. Importantly, when chromosome 10 is re-introduced, human glioblastoma cell lines switch to non-angiogenic phenotype and thus lose their ability to form tumors in athymic mice 22.
The use of thrombospondin-1 as a cancer therapeutic has been limited by its very large size (>450 kDa as a trimer) and the presence of multiple functional domains 23.
However, its anti-angiogenic potential is attributed mostly to the so-called type 1 repeats, or TSR 24. In early pre-clinical studies, a recombinant protein encompassing all three TSRs was found to inhibit the growth of experimental B16F10 melanomas, Lewis lung carcinomas 25 and human pancreatic cancer cells in an orthotopic mouse model 26. Within TSRs, the anti-angiogenic activity has been mapped mainly to the DGGWSHWSPWSSC and GVITRIR amino acid sequences 27, allowing the therapeutic use of even shorter peptides. The two modified peptides from the TSR region strongly suppressed tumor growth when administered intravenously to mice bearing MDA-MB435 breast carcinomas 28.
In the last few years, Abbott Laboratories has developed and championed the use of another TSR-based therapeutic peptide, ABT-510, which is derived from the GVITRIR sequence 29. Treatment with ABT-510 inhibits the growth of murine melanoma metastases in syngeneic animals and blocks the progression of orthotopic human bladder cell tumors 30. Its potency is markedly improved when CD36 is upregulated using ligands of peroxisome proliferator-activated receptor gamma 31 and when combined with metronomic low-dose chemotherapy 32 or the histone deacetylase inhibitor valproic acid 33. Last but not least, ABT-510 has proven safe in canine 34 as well as human cancer patients 35. Does it have the potential to become a drug of choice for glioblastoma?
In this issue of CB&T, Anderson et al describe the effects of ABT-510 on angiogenesis and tumor growth in mouse models of glioblastoma 36. The authors observe that ABT-510 induces apoptosis of human brain microvascular endothelial cells (MvEc) by a CD36-dependent, caspase-8 mediated mechanism. It also inhibits tubulogenesis by MvEc propagated in 3D-collagen gels. More importantly, ABT-150 inhibits growth of human and murine glioblastoma grafts in orthotopic locations while decreasing microvascular densities. This work echoes an earlier study where a WSHWSPW-containing peptide was shown to significantly slow the growth of rat C6 glioma and 9L gliosarcomas 37. While robust performance in mouse models certainly does not guarantee success in clinical settings, the authors’ data argue quite compellingly that ABT-510 should be evaluated in glioblastoma patients.
References
- 1.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de TN, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, Van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Tuettenberg J, Friedel C, Vajkoczy P. Angiogenesis in malignant glioma--a target for antitumor therapy? Crit Rev Oncol Hematol. 2006;59:181–193. doi: 10.1016/j.critrevonc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Evans SM, Judy KD, Dunphy I, Jenkins WT, Hwang WT, Nelson PT, Lustig RA, Jenkins K, Magarelli DP, Hahn SM, Collins RA, Grady MS, Koch CJ. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10:8177–8184. doi: 10.1158/1078-0432.CCR-04-1081. [DOI] [PubMed] [Google Scholar]
- 5.Lally BE, Rockwell S, Fischer DB, Collingridge DR, Piepmeier JM, Knisely JP. The interactions of polarographic measurements of oxygen tension and histological grade in human glioma. Cancer J. 2006;12:461–466. doi: 10.1097/00130404-200611000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 7.Goldbrunner RH, Bendszus M, Tonn JC. Models for angiogenesis in gliomas. Cancer Treat Res. 2004;117:115–135. doi: 10.1007/978-1-4419-8871-3_6. [DOI] [PubMed] [Google Scholar]
- 8.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 9.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SY, Huang HJ, Nagane M, Ji XD, Wang D, Shih CC, Arap W, Huang CM, Cavenee WK. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci USA. 1996;93:8502–8507. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batchelor TT, Sorensen AG, di TE, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 14.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994;54:6504–6511. [PubMed] [Google Scholar]
- 16.Watnick RS, Cheng YN, Rangarajan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell. 2003;3:219–231. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 17.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates αvβ3 function through integrin-associated protein. J Cell Biol. 1996;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 21.Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 22.Hsu SC, Volpert OV, Steck PA, Mikkelsen T, Polverini PJ, Rao S, Chou P, Bouck NP. Inhibition of angiogenesis in human glioblastomas by chromosome 10 induction of thrombospondin-1. Cancer Res. 1996;56:5684–5691. [PubMed] [Google Scholar]
- 23.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12:634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 24.Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn. 2000;218:280–299. doi: 10.1002/(SICI)1097-0177(200006)218:2<280::AID-DVDY4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Miao WM, Seng WL, Duquette M, Lawler P, Laus C, Lawler J. Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor-beta-dependent and - independent mechanisms. Cancer Res. 2001;61:7830–7839. [PubMed] [Google Scholar]
- 26.Zhang X, Galardi E, Duquette M, Lawler J, Parangi S. Antiangiogenic treatment with three thrombospondin-1 type 1 repeats versus gemcitabine in an orthotopic human pancreatic cancer model. Clin Cancer Res. 2005;11:5622–5630. doi: 10.1158/1078-0432.CCR-05-0459. [DOI] [PubMed] [Google Scholar]
- 27.Lawler J, Detmar M. Tumor progression: the effects of thrombospondin-1 and-2. Int J Biochem Cell Biol. 2004;36:1038–1045. doi: 10.1016/j.biocel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- 29.Haviv F, Bradley MF, Kalvin DM, Schneider AJ, Davidson DJ, Majest SM, McKay LM, Haskell CJ, Bell RL, Nguyen B, Marsh KC, Surber BW, Uchic JT, Ferrero J, Wang YC, Leal J, Record RD, Hodde J, Badylak SF, Lesniewski RR, Henkin J. Thrombospondin-1 mimetic peptide inhibitors of angiogenesis and tumor growth: design, synthesis, and optimization of pharmacokinetics and biological activities. J Med Chem. 2005;48:2838–2846. doi: 10.1021/jm0401560. [DOI] [PubMed] [Google Scholar]
- 30.Reiher FK, Volpert OV, Jimenez B, Crawford SE, Dinney CP, Henkin J, Haviv F, Bouck NP, Campbell SC. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int J Cancer. 2002;98:682–689. doi: 10.1002/ijc.10247. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Campbell SC, Bedford DF, Nelius T, Veliceasa D, Shroff EH, Henkin J, Schneider A, Bouck N, Volpert OV. Peroxisome proliferator-activated receptor gamma ligands improve the antitumor efficacy of thrombospondin peptide ABT510. Mol Cancer Res. 2004;2:541–550. [PubMed] [Google Scholar]
- 32.Yap R, Veliceasa D, Emmenegger U, Kerbel RS, McKay LM, Henkin J, Volpert OV. Metronomic low-dose chemotherapy boosts CD95-dependent antiangiogenic effect of the thrombospondin peptide ABT-510: a complementation antiangiogenic strategy. Clin Cancer Res. 2005;11:6678–6685. doi: 10.1158/1078-0432.CCR-05-0621. [DOI] [PubMed] [Google Scholar]
- 33.Yang Q, Tian Y, Liu S, Zeine R, Chlenski A, Salwen HR, Henkin J, Cohn SL. Thrombospondin-1 peptide ABT-510 combined with valproic acid is an effective antiangiogenesis strategy in neuroblastoma. Cancer Res. 2007;67:1716–1724. doi: 10.1158/0008-5472.CAN-06-2595. [DOI] [PubMed] [Google Scholar]
- 34.Rusk A, McKeegan E, Haviv F, Majest S, Henkin J, Khanna C. Preclinical evaluation of antiangiogenic thrombospondin-1 peptide mimetics, ABT-526 and ABT-510, in companion dogs with naturally occurring cancers. Clin Cancer Res. 2006;12:7444–7455. doi: 10.1158/1078-0432.CCR-06-0109. [DOI] [PubMed] [Google Scholar]
- 35.Hoekstra R, de Vos FY, Eskens FA, de Vries EG, Uges DR, Knight R, Carr RA, Humerickhouse R, Verweij J, Gietema JA. Phase I study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with 5-fluorouracil and leucovorin: a safe combination. Eur.J Cancer. 2006;42:467–472. doi: 10.1016/j.ejca.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JC, Grammer JR, Wang W, Nabors LB, Henkin J, Stewart JE, Jr., Gladson CL. ABT-510, a Modified Type 1 Repeat Peptide of Thrombospondin, Inhibits Malignant Glioma Growth In Vivo by Inhibiting Angiogenesis. Cancer Biol Ther. 2007;6 doi: 10.4161/cbt.6.3.3630. [DOI] [PubMed] [Google Scholar]
- 37.Bogdanov AJ, Marecos E, Cheng HC, Chandrasekaran L, Krutzsch HC, Roberts DD, Weissleder R. Treatment of experimental brain tumors with trombospondin-1 derived peptides: an in vivo imaging study. Neoplasia. 1999;1:438–445. doi: 10.1038/sj.neo.7900044. [DOI] [PMC free article] [PubMed] [Google Scholar]


