Abstract
Objective
To examine a) ethnic differences in blood alcohol level (BAL) and pre-injury chronic alcohol use (PI-ETOH) within a severe closed head injury (CHI) sample, and b) the main and interaction effects of BAL, PI-ETOH, and ethnicity on functional outcome following severe CHI.
Participants
434 Hispanic, Anglo-Caucasian, and African American individuals with severe CHI.
Design
Retrospective cohort study.
Setting
Consecutive admissions to a level one trauma center.
Main measures
BAL upon admission to the trauma center was collected for each patient. Additional information regarding PI-ETOH was collected in a subset of patients (N=116). Functional outcome was measured using the Disability Rating Scale (DRS) at 6 months post-injury.
Results
A one-way ANOVA revealed ethnic differences in mean BAL. Hierarchical multiple regression indicated that BAL did not predict DRS outcomes after controlling for pertinent covariates. An interaction effect between PI-ETOH and ethnicity was observed, such that presence of chronic alcohol use predicted worse functional outcome for Anglo-Caucasians and African Americans, but more favorable outcome for Hispanics.
Conclusions
Ethnic differences in BALs within our severe TBI sample mirrored ethnic drinking patterns observed in the general population, with Hispanics having the highest BALs. A paradoxical relationship between PI-ETOH and functional outcome was observed for Hispanics.
Keywords: traumatic brain injury, blood alcohol level, pre-injury alcohol use, racial/ethnic diversity
It is widely known and accepted that alcohol use is associated with traumatic brain injury (TBI). One-third to one-half of patients with TBI arrive at the hospital with a positive blood alcohol level (BAL), and approximately one-fourth of these individuals have a BAL higher than the legally recognized limit (.08 to .10 mg/dL).1-4 Alcohol use shortly prior to injury is a commonly cited predisposing factor of head trauma due to its direct and indirect involvement in motor vehicle accidents, falls, and assaults, which are among the most common mechanisms of head injury.5 The incidence of pre-injury chronic alcohol use (PI-ETOH) is also higher among patients with TBI than the general population, with as many as one half to two thirds of patients with TBI having a history of heavy drinking.2
Less is known regarding the effect of alcohol use, both chronically and acutely, on subsequent recovery from TBI. Intoxication at the time of injury has been found to increase complications during the acute medical management of TBI.6-12 This has led some researchers to investigate the effect that alcohol-related variables may have on early outcome indicators. Results from these studies have been inconsistent. Some studies have shown that acute alcohol intoxication is related to increased length of hospitalization,3,5,13 longer coma duration,13 and lower global cognitive and functional outcome,5,14,15 while other investigations have not shown these effects.5-7,15,16 Likewise, studies investigating the relationship between PI-ETOH and early outcome have found that PI-ETOH was associated with lower initial Glasgow Coma Scale (GCS) scores,15 increased length of hospitalization,17 longer post traumatic amnesia (PTA),17 and poorer acute global outcome,6 although there is again conflicting evidence.15,16,18
While there is some evidence of adverse effects of intoxication on acute functioning following TBI, these effects may be ameliorated over time as recovery progresses.1,3 Investigators have been unable to detect an association between BAL and global outcome, as measured by the Glasgow Outcome Scale (GOS), at 3, 6, and 12 months post-injury.6,17,19-21 Moreover, one study reported the paradoxical finding that acute alcohol intoxication was related to better global outcome, as measured by the Functional Independence Measure (FIM).22 A notable exception to this body of literature consisting of largely null and paradoxical results, is a recent study by Vickery and colleagues in which increased BAL was associated with higher Disability Rating Scale (DRS) scores (i.e. more disability), but not FIM scores, at the time of admission to acute rehabilitation. 15 Thus, it appears that with the use of a measure with a wider range of values (i.e., DRS), differences in global functional outcome may be detected. However, in this study, a higher BAL was associated with only a .85-unit increase in DRS scores after controlling for other factors, a small effect that may have gone undetected had their sample size not been so large (N = 1748).
Even fewer studies have examined the potential effects of PI-ETOH on global outcome following the acute stages of recovery. While there is some evidence that a history of PI-ETOH is related to poorer global outcomes,6,23 others report no relationship.17 Furthermore, Vickery et al. reported the paradoxical finding that pre-injury “binge drinking” was actually associated with better global outcome on the DRS.15 The authors explained the latter finding by positing that individuals who are more productive pre-injury might have fewer occasions to drink but tend to consume in higher quantities when they do drink. As a result, the relationship found between binge drinking and better global outcome may be an artifact of the relationship between pre-injury productivity and outcome.
Further complication arises when one considers the substantial overlap between BAL and history of PI-ETOH. Those TBI patients with a positive BAL are more likely to have a history of PI-ETOH than those who are not intoxicated at the time of injury.2,6 This presents a challenge in disentangling the unique and combined effects of these two variables on outcome. Vickery and colleagues emphasize the importance of studying these two alcohol variables both in isolation and in conjunction in order to assess for relative contributions and possible interaction effects.15
Like alcohol use, ethnicity is also related to the incidence of TBI, with minorities having a higher risk of acquiring a TBI compared to Anglo-Caucasians.24,25 Additionally, there is a substantial and growing body of evidence suggesting that ethnicity may have a significant and unique effect on TBI outcome, such that minorities appear vulnerable to poorer outcomes.26-31 Moreover, drinking patterns vary between the three major ethnic groups in the US, with African Americans tending to drink less often and in lower quantities compared to Anglo-Caucasians and Hispanics.32,33 Patterns in the frequency and quantity of alcohol consumption between Anglo-Caucasians and Hispanics are less distinguishable, though there is some evidence suggesting that Hispanics may drink in higher quantities, though less frequently, relative to Anglo-Caucasians.32-35Studies that explore the possible relationships between ethnicity, alcohol use, and TBI outcomes are needed. There is a general lack of ethnic diversity in samples of published TBI studies in which alcohol variables are examined. The ethnicity of the samples in the vast majority of the studies we reviewed was either predominantly Anglo-Caucasian or not reported, with no study having large enough samples of more than one ethnic group to consider ethnicity as a variable in their analyses. Moreover, we could not find any published studies from nations outside of North America that have examined the relationship between alcohol-related variables and global outcome from TBI, though a handful studies have examined the affects of alcohol consumption on other dimensions of outcome following head injury. A study out of Glasgow, Scotland, by Brooks and colleagues18, examined the effect of alcohol-related variables on early functional status (i.e. length of PTA) and specific cognitive functions (e.g., memory) following severe head injury. An additional Finnish cohort study investigated the relationship between intoxication at the time of a first TBI and subsequent reoccurring TBI36. Finally, a Taiwanese study examined the role of alcohol intoxication in morbidity following motor vehicle accident (not specific to those resulting in head injury)37. In each of these three studies, the ethnic make-up of the patient populations was not given, and so it is likely that these study samples, like those originating from the US and Canada, reflected the majority culture from their respective countries. A more substantial body of international research would make comparison across studies a viable option for studying potential cultural differences in the relationship between alcohol use and TBI outcomes, but this has not been possible as of yet. Thus, due to the scarcity of diverse TBI samples and cross-cultural/international research in alcohol-related TBI research, potential ethnic/cultural differences in the relationships between alcohol-related variables and outcome from TBI are largely unexplored at this point.
The present study is unique in that we have collected data on BAL, PI-ETOH, and global outcome from individuals with severe TBI in a diverse, metropolitan city where there is no majority culture. A recent survey conducted by the U.S. Census Bureau reported this metropolitan population to be made up of 33.3% Hispanics, 43% non-Hispanic Whites, 16.6% non-Hispanic Blacks and 5.7% Asians.38 Because of the similarly low number of Asians and others in our TBI study population, we have only included Hispanics, Anglo-Caucasians, and African-Americans in this study, with their percentages being relatively representative of the general population of the city. This provides an opportunity to examine the relationships between ethnicity, alcohol use, and TBI outcome within a single sample. The main objectives of this study are to examine a) ethnic differences in BAL and PI-ETOH among individuals with severe TBI, and b) the main and interaction effects of BAL, PI-ETOH, and ethnicity on global outcome following severe TBI.
Our specific hypotheses were that, in our overall sample: (1) Anglo-Caucasians would have the highest rate of positive BALs (i.e., BAL > 0.00 mg/dL), followed by Hispanics, with African Americans having the lowest, and (2) Hispanics would have the highest mean BAL, followed by Anglo-Caucasians, with African Americans having the lowest. Additionally, in a subset of our sample, we hypothesized that: (3) Both Anglo-Caucasians and Hispanics would have higher rates of PI-ETOH relative to African Americans; (4) Hispanic and African American ethnicity, a history of PI-ETOH, and increasing BAL would be associated with poorer global outcome, after accounting for other pertinent characteristics; and (5) interactions between BAL, PI-ETOH, and ethnicity would exist, such that these factors in combination would have an additive connection to global outcome.
Methods
Participants
Participants were 434 patients with severe closed head injury (CHI), admitted to the Neurosurgery Intensive Care Unit (NICU) of a Level 1 Trauma Center in a diverse, metropolitan city. The study sample was obtained retrospectively from a database of 640 patients with severe TBI that were consecutively admitted to the NICU and enrolled in National Institute of Health-National Institute of Neurological Disorders and Stroke (NIH-NINDS) funded grants studying aspects of cerebrovascular functioning after TBI during the years 1987-2009. Severe TBI was defined as having a GCS motor score of 5 or less upon admission to the NICU. Patients were excluded from enrolling in grant studies if they had a history of a previous TBI or a major psychiatric disorder (e.g., Schizophrenia or Bipolar Disorder). The data for this research were collected in compliance with regulations of the affiliated academic institutions and their respective Committees for the Protection of Human Subjects and Institutional Review Boards.
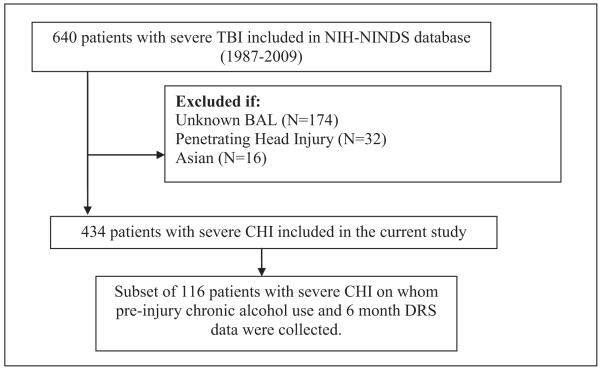
Inclusion criteria for the current study required (1) diagnosis of a CHI; (2) BAL testing upon admission to the EC; and (3) African American, Anglo-Caucasian, or Hispanic ethnic/race identification. A subset of these patients (N=116) had data on both PI-ETOH and functional outcome at 6 months post-injury. Figure 1 provides a flow chart of the sample and subsample selection process. Table 1 provides demographic and injury-related characteristics of the study sample (N=434) and subsample (N=116).
Figure 1.
Flow chart depicting the process by which the current study sample was derived.
Table 1.
Characteristics of study sample and subsample.
| Characteristic | Study sample (N = 434) |
Subset (N = 116) |
|---|---|---|
| Gender | ||
| Male, n (%) | 368 (84.8) | 98 (84.5) |
| Female, n (%) | 66 (15.2) | 18 (15.5) |
| Age | ||
| Mean years, (SD) | 34.38 (14.1)a | 31.28 (11.9)a |
| Range | 14 - 85 | 15 – 73 |
| Ethnicityb | ||
| Hispanic, n (%) | 206 (47.5)c | 43 (37.1)c |
| Anglo-Caucasian, n (%) | 126 (29.0)c | 44 (37.9)c |
| African American, n (%) | 102 (23.5) | 29 (25.0) |
| Education | ||
| 1-8 years, n (%) | 38 (8.8) | 9 (8.8) |
| Some high school, n (%) | 67 (15.4)d | 31 (30.4)d |
| High school graduate, n (%) | 56 (12.9)d | 20 (19.6)d |
| Some college, n (%) | 29 (6.7)d | 14 (13.7)d |
| College graduate, n (%) | 10 (2.3) | 4 (3.9) |
| Post graduate, n (%) | 2 (.5) | 0 (0.00) |
| Vocational, n (%) | 10 (2.3) | 2 (2.0) |
| Unknown/missing, n (%) | 222 (51.2) | 36 (31.0) |
| Injury mechanism | ||
| Automobile, n (%) | 217 (50.0) | 54 (46.6) |
| Assault, n (%) | 71 (16.4) | 22 (19.0) |
| Fall/jump, n (%) | 61 (14.1) | 18 (15.5) |
| Motorcycle, n (%) | 29 (6.7) | 10 (8.6) |
| Other/unknown, n (%) | 56 (12.9) | 12 (10.3) |
| EC Admission GCS | ||
| Mean (SD) | 6.54 (3.1) | 6.69 (3.1) |
| Range | 3 - 15 | 3 – 15 |
| Best Day 1 GCS | ||
| Mean (SD) | 7.35 (2.5) | 7.70 (2.4) |
| Range | 3 - 15 | 3 – 15 |
| BAL | ||
| 0.0 mg/dL, n (%) | 119 (27.4) | 32 (44.8) |
| Between 0.0 and .08 mg/dL | 138 (31.8) | 32 (27.6) |
| Greater than .08 mg/dL | 177 (40.8) | 52 (44.8) |
| Mean (SD)f | .135 (.12) | .138 (.12) |
| Range | .000 - .474 | .000 -.465 |
| Pre-injury ETOHg | ||
| Clear evidence of pre-injury drinking problem, n (%) |
-- | 26 (22.4) |
| Frequent of heavy use greater than occasional use, n (%) |
-- | 31 (26.7) |
| No to mild use that is clearly non-problematic, n (%) |
-- | 59 (50.9) |
| 6 Month DRS, | ||
| Missing, n (%) | 107 (24.7) | 0 (0.0) |
| Mean (SD) | 13.57 (12.1)h | 7.65 (8.2)h |
| Range | 0 - 30 | 0 – 30 |
Individuals in the subsample were significantly younger than those in the study sample.
Asians were selected out of the study sample.
There were significantly less Hispanics and significantly more Anglo-Caucasians in the subsample relative to the study sample.
Individuals in the subsample were significantly more educated relative to the study sample.
Individuals were only included in the study sample and subsample if they were tested for BAL upon admission.
Mean BAL was calculated only including those BALs that were greater than 0.00 mg/dL.
The subsample was selected for study because data were collected on pre-injury alcohol use.
Individuals in the subsample had significantly lower DRS (better) scores than those in the study sample.
Procedure
Demographic information was determined from available medical records and by interviewing patients and their family members. Several measures related to initial injury severity were included in this study: post-resuscitation emergency center (EC) and best day 1 GCS scores (i.e., the highest GSC score in the first 24 hours after admission to the NICU), corresponding pupillary response scores (i.e., number of reactive pupils), as well as EC admission and worst Marshall CT classification scores.
BALs were obtained upon admission to the EC. Information about patients’ history of PI-ETOH was obtained during a clinical interview using the Pre-Injury Head Injury Family Interview (HI-FI).39 The interviewer asked the patient/family member/significant other about the frequency, amount, and duration of the patient’s alcohol use prior to injury, and then rated the patient on a 3-point scale: “1” indicating clear evidence that the individual had a pre-injury drinking problem; “2” indicating frequent or heavy use of alcohol that appears to be greater than occasional use, but is less than a clear cut addiction; or “3” indicating no to mild use of alcohol that was clearly not problematic.
Functional outcome at approximately 6 months post-injury was measured by the Disability Rating Scale (DRS).40 The DRS is widely used with individuals with severe TBI because it is able to capture increments of functional improvement throughout the span of the recovery process (i.e., “from coma to community”). The individual is assessed and rated on 8 items: best eye opening, communication ability, and motor response; cognitive ability for feeding; cognitive ability for toileting; cognitive ability for grooming; level of functioning; and employability at that time. These 8 ratings are then summed to give a total score that ranges from 0 to 30, with 0 representing no disability and 30 representing death; thus, lower scores on the DRS are associated with better outcome. Information necessary to rate each individual on this outcome measure at 6 months post-injury was obtained from the patient, family member, significant other, and/or caretaker in person or during a telephone phone interview by individuals trained to reliably rate the DRS.
All data were quality controlled in regular meetings with the clinical neuropsychologist [HJH] and the staff members in charge of data collection.
Statistical Data Analysis
The relationship between BAL and ethnicity was examined in 434 patients with severe CHI. Chi-square analyses were used to test for ethnic differences in frequency of positive BAL (> 0.00 mg/dL) and frequency of BAL at or over the legal limit for intoxication (≥ 0.08 mg/dL). Cramer’s V was used as a measure of effect size for these chi-square analyses, with values of .1, .3, and .5 representing small, medium, and large effects, respectively41. A one-way Welch’s ANOVA with ethnicity as the independent factor (three levels: Hispanic, Anglo-Caucasian, and African American) and BAL as the continuous dependent variable was used to test the omnibus null hypothesis that the population mean BALs of Hispanics, Anglo-Caucasians, and African Americans individuals with severe CHI were equal to one another. Only individuals with positive BALs (i.e., BAL > 0.00 mg/dL) were included in this analysis (N = 315). Fisher’s LSD procedure was utilized to test post hoc pairwise comparisons; thus, p values of .05 or less were considered statistically significant. Cohen’s f2 was used as a measure of effect size for the overall ANOVA; by convention, f2 values of .02, .15., and .35 are termed small, medium, and large effects, respectively42. Pearson’s r was used as a measure of effect size for post hoc pairwise comparisons, with values of .1, .3, and .5 representing small, medium, and large effects, respectively42.
The relationship between PI-ETOH and ethnicity was examined in a subsample of patients (N =116) on whom data were available regarding PI-ETOH and 6 month DRS outcomes. Demographic and injury characteristics of the subsample were compared statistically to the characteristics of those not included in the subsample using chi-square tests for categorical variables and t-tests for continuous variables. Chi-square analyses were used to test for ethnic differences in PI-ETOH ratings. Again, Cramer’s V was used as a measure of effect size for this analysis.
To evaluate the degree of association between our 3 independent variables (ethnicity, BAL, and PI-ETOH) and DRS scores at 6 months post injury, a hierarchical linear multiple regression analysis was performed on a subsample of patients who possessed data for each of these variables and had survived to 6 months post-injury (N=111). Change in R2 was examined to determine if predictors in each block significantly contributed to the model above and beyond those variables entered in previous blocks. As with the ANOVA, Cohen’s f2 was used as a measure of effect size for each block of predictors in the hierarchical multiple regression. Variance inflation factors (VIF) and tolerance values were examined to assess for potential issues with multicollinearity using the cut-off guidelines set forth by Myers43 (VIF < 10) and Menard44 (tolerance > .2), respectively.
The interaction effects between BAL, PI-ETOH, and ethnicity were explored. Six month DRS scores were regressed on the two variables and their interaction term(s). The interaction term was examined for significance (p ≤ .05) and interpretability.
Results
BAL and ethnicity
The frequency with which Hispanics, Anglo-Caucasians, and African Americans presented to the EC with a BAL greater than 0.00 mg/dL did not differ significantly (χ22 = .436, = .804, V = .032). Likewise, there were no significant ethnic differences in the relative frequencies of those who presented to the EC with a BAL at or above the legal limit for intoxication (i.e., 0.08 mg/dL; χ22 = .850, p = .654, V = .044).
Mean BALs, however, differed significantly across the three ethnic groups (F2, 183.751 = 4.406, P = .014, f2 = .03), with Hispanics having the highest mean BAL (mean = .155 mg/dL, SD = .128), followed by Anglo-Caucasians (mean = .120 mg/dL, SD = .113), and African Americans having the lowest (mean = .112 mg/dL, SD = .106). Post-hoc comparisons revealed that the mean BAL of Hispanics was significantly higher than that of Anglo-Caucasians (t203.549 = 2.223, P = .027, r = .154) and African Americans (t178.680 = 2.740, P = .007, r = .201), who did not differ significantly from one another (t161.664 = .498, P = .619, r = .039).
Pre-injury alcohol use and ethnicity
The relationship between PI-ETOH and ethnicity was examined in the subsample of individuals on whom PI-ETOH data was available (N=116). Individuals with a positive BAL were more likely to have a history of PI-ETOH (χ21 = 5.658, P = .017, V = .22). Individuals in the PI-ETOH subsample were, on average, younger, more educated, and less disabled at 6 months post-injury compared to those who were not included in the subsample. In addition, there were significantly more Anglo-Caucasians and less Hispanics in the PI-ETOH subsample relative to the group of individuals not included in the subsample. No significant ethnic differences were found in PI-ETOH ratings (χ24 = 3.902, P = .419, V = .13).
Predicting DRS outcomes at 6 months
Injury severity variables (best day 1 GCS, best day 1 pupillary response, and worst Marshall CT classification score) were entered in step 1 of the hierarchical liner regression and found to account for 11.2% of the variance in 6 month DRS scores (adj. R2 = .112, F5, 98 = 3.591, P = .005, f2 = .18). Age was entered in the second step and failed to significantly account for additional variance in DRS scores above and beyond that accounted for in step 1 (ΔR2 = .021, ΔF1, 97 = 2.447, P =.121, f2 = .02). Ethnicity was entered into the model in the third step and found to significantly increase the variance accounted for by the model from 12.5% to 19.5% (ΔR2 = .082, ΔF2, 95 = 5.260, p=.007, f2 = .09). In the fourth step, pre-injury ETOH was entered into the model and did not significantly improve the model (ΔR2 = .000, ΔF1, 94 = .038, p =.846, f2 = .00). In the fifth step, BAL was entered into the model as a continuous predictor and did not yield any additional improvement in the model (ΔR2 = .006, ΔF1, 93 = .783, p =.379, f2 = .01). To ensure that multicollinearity was not biasing the regression model, VIF and tolerance values were examined and found to be within the appropriate limits.
Exploratory interaction analyses
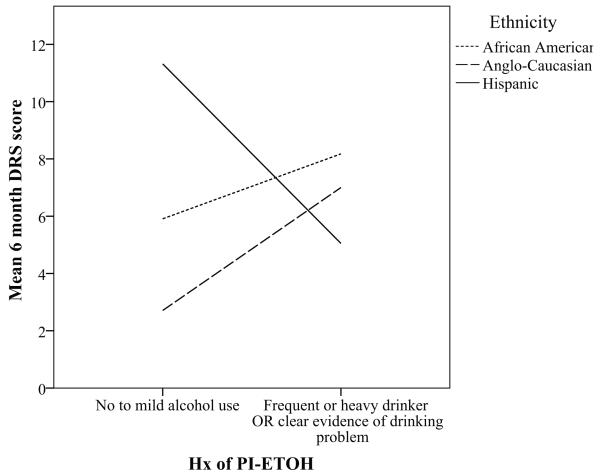
Only the interaction between ethnicity and PI-ETOH was found to be significant (F5,105 = 4.851, P < .001) and interpretable. Further examination of the nature of interaction between PI-ETOH and ethnicity revealed that Anglo-Caucasians and African Americans show the predicted pattern in which PI-ETOH was associated with higher DRS scores (i.e., worse outcome), while Hispanics showed a counterintuitive pattern in which PI-ETOH was associated with more favorable outcome (Fig 2).
Figure 2.
Interaction between PI-ETOH and ethnicity in predicting 6 month DRS scores.
When the PI-ETOH by ethnicity interaction terms were added in the final step of the hierarchical regression, the model was significantly improved (ΔR2 = .064, ΔF2,91 = 4.325, P = .016, f2 = .07), producing the final model that predicted 24.0% of the variance in 6 month DRS scores (adj. R2 = .240, F12, 91 = 3.704, P < .001).
Discussion
Hispanics, Anglo-Caucasians, and African Americans in our sample of patients with severe CHI did not differ in frequency of positive BAL or legal intoxication (BAL ≥ .08 mg/dL) at the time of admission to the EC. However, ethnic groups differed in the quantity of alcohol found in the blood serum. As hypothesized, Hispanics in our sample had the highest BALs, followed by Anglo-Caucasians, with African Americans having the lowest. The mean BALs of individuals in our CHI sample therefore, mirrored the patterns of heavy alcohol consumption and/or binge drinking that have been reported to exist within the general population.32-35 With regard to PI-ETOH, we did not find evidence to support the hypothesis that African Americans would have lower rates of PI-ETOH compared to Anglo-Caucasians and Hispanics, as no one ethnic group was more or less likely to endorse a history of PI-ETOH. The reduced sample size for testing this hypothesis could explain the lack of significance reported (p=.419) despite the presence of a small effect size (V=.13). Indeed, a post hoc achieved power calculation revealed that the analysis was under-powered for detecting an effect of this size (power = .33). Worth noting, the pattern of frequencies observed was not consistent with our original hypothesis and we really cannot infer what the relationship between PI-ETOH and ethnicity might have been with a larger sample.
In considering reasons why ethnic patterns of positive BAL frequency and PI-ETOH in our sample did not mirror those seen in the general public, one should note the inherent differences between our severe TBI sample and the general population. Individuals in our sample were involved in motor vehicle accidents, assaults, falls, and other events that led to severe injury, alcohol intoxication being a risk factor for the occurrence of such events. Additionally, we know that individuals who abuse alcohol are over-represented in TBI populations relative to the general population. Therefore, alcohol trends seen in the general public may not apply to this biased subsample of the population. Finally, in our sample, just as in previous research, those who had alcohol present in the blood serum upon admission to the EC were more likely to be same individuals who had a history of chronic alcohol use, and so it follows that patterns of positive BAL frequency and PI-ETOH within TBI samples would be consistent with one another.
Our hypotheses regarding utility of ethnicity, PI-ETOH, and BAL in predicting global DRS outcome scores at 6 months post-injury were only partially supported. Hispanics who had survived to 6 months had DRS scores that were approximately four points higher (i.e., indicating more disability) relative to Anglo-Caucasians who survived. Additionally, African Americans had 6 month DRS scores that were approximately two points higher than Anglo-Caucasians, though this difference did not reach statistical significance. This finding is in agreement with previous research reporting ethnic discrepancies in outcome following TBI. 26-31
There was no main effect of PI-ETOH on DRS scores at 6 months post-injury after controlling for injury severity and demographics variables. These results are contrary to what have been reported in some previous investigations,6,31 but similar to the modest paradoxical relationship reported by Vickery et al.15 and lack of relationship reported by De Guise et al.17 However, through exploratory analysis of potential interaction between our three main independent variables of interest, it was found that ethnicity and PI-ETOH interacted in predicting 6 month post-injury DRS scores. More specifically, Anglo-Caucasians and African Americans showed the predicted pattern in which PI-ETOH was associated with higher DRS scores (i.e., worse outcome). Hispanics, on the other hand, showed a counterintuitive pattern in which PI-ETOH was associated with more favorable outcome. Given evidence that Hispanics tend to drink in higher quantities than Anglo-Caucasians and African Americans,32-35 it would be interesting to know if a higher percentage of our Hispanic individuals fell into the category of “binge drinkers” and were exhibiting the paradoxical relationship between binge drinking and functional outcome reported by Vickery et al.15 In light of this interaction, the relationship between PI-ETOH and DRS outcomes may be moderated by ethnicity. It also remains possible that PI-ETOH is associated with more subtle neuropsychological deficits that were not captured by the global measure of outcome that we used.
BAL was not related to outcome at 6 months post-injury measured by the DRS after controlling for injury severity variables, demographics variables, and PI-ETOH use. This finding is consistent with the results of many past studies,6,17,19-22 though not with other studies that reported paradoxical (“protective”) findings17 or that BAL was predictive of poorer outcome.15 We did not replicate the finding of Vickery et al.15 despite our similar use of the DRS in measuring outcome; however, they had an extremely large sample size and detected only a small difference in DRS scores of less than one point. We might also point out that Vickery and colleagues measured DRS outcomes at time to admission to rehabilitation, which varied and on the average was much sooner after injury than our stable time point of 6 months post-injury for every patient. As the DRS was developed to include items that capture increments of change “from coma to community,” it is possible that the mechanism through which BAL was related to DRS scores in Vickery’s study is no longer operating during the later stages of recovery. More thorough investigation into the potentially changing relationship between alcohol-related variables and DRS outcomes over time is an interesting topic for future research.
There were several limitations to this study. The smaller subset was comprised of individuals with relatively less severe injuries and higher education compared to a “typical” severe TBI population, which may limit the generalizability of results from analyses done on this sample. Another limitation of this retrospective study is that the alcohol variables were originally collected with the intention of being included as covariates, rather than main variables of interest; therefore, we did not have the opportunity to ask more specific questions about PI-ETOH that might have been more informative. Also, this study did not consider the potential effects of co-occurring drug intoxication and history of drug abuse, variables which might be considered in future research. Finally, our study considered only one global outcome measure. It is entirely possible that BAL, PI-ETOH, and/or ethnicity are related to other constructs of recovery that were not captured by our measure.
It is important to emphasize that this study of ethnic differences in alcohol use and TBI outcomes took place within the context of a metropolitan US city, in which there are number of complex social and economic factors present that are related to ethnicity and unique to this society. Results may not generalize to multicultural populations outside of the US or to individuals living in their native country (e.g., Mexicans living in Mexico).
Conclusion
Ethnic differences in BAL in severe CHI patients partially mirrored ethnic drinking patterns observed in the general US population, with Hispanics having higher BALs than Anglo-Caucasians and African Americans. BAL was not related to 6 month post-injury DRS scores. An interesting interaction between ethnicity and PI-ETOH use was observed such that Hispanics with a history of PI-ETOH showed the counterintuitive pattern of more favorable outcome.
Acknowledgments
This work was supported by grants from the National Institutes of Health - National Institute of Neurological Disorders and Stroke (grant no. P01-NS38660). Special thanks to the patients and staff of Harris County Hospital District’s Ben Taub General Hospital.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Keira M. O’Dell, Department of Psychology, University of Houston
H. Julia Hannay, Department of Psychology, University of Houston Department of Neurosurgery, Baylor College of Medicine
Fedora O. Biney, Department of Psychology, University of Houston
Claudia S. Robertson, Department of Neurosurgery, Baylor College of Medicine
T. Siva Tian, Department of Psychology, University of Houston.
References
- 1.Bombardier CH, Thurber CA. Blood alcohol level and early cognitive status after traumatic brain injury. Brain Inj. 1998;12:725–734. doi: 10.1080/026990598122124. [DOI] [PubMed] [Google Scholar]
- 2.Dikmen SS, Machamer JE, Donovan DM, Winn HR, Temkin NR. Alcohol use before and after traumatic head injury. Ann Emerg Med. 1995;26:167–176. doi: 10.1016/s0196-0644(95)70147-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan CP, Corrigan JD. Effect of blood alcohol level on recovery from severe closed head injury. Brain Inj. 1992;6:337–349. doi: 10.3109/02699059209034948. [DOI] [PubMed] [Google Scholar]
- 4.Tate PS, Freed DM, Bombardier CH, Harter SL, Brinkman S. Traumatic brain injury: influence of blood alcohol level on post-acute cognitive function. Brain Inj. 1999;13:767–784. doi: 10.1080/026990599121160. [DOI] [PubMed] [Google Scholar]
- 5.Sparadeo FR, Gill D. Effects of prior alcohol use on head injury recovery. J Head Trauma Rehabil. 1989;4:75–82. [Google Scholar]
- 6.Ruff RM, Marshall LF, Klauber MR, et al. Alcohol abuse and neurological outcome of the severely head injured. J Head Trauma Rehabil. 1990;5:21–31. [Google Scholar]
- 7.Gurney JG, Rivara FP, Mueller BA, Newell DA, Copass MK, Jurkovich GJ. The effects of alcohol intoxication on the initial treatment and hospital course of patients with acute brain injury. J Trauma. 1992;33:709–713. doi: 10.1097/00005373-199211000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Jurkovich GJ, Rivara FP, Gurney JG, Seguin D, Fligner CL, Copass M. Effects of alcohol intoxication on the initial assessment of trauma patients. Ann EmergMed. 1992;21:704–708. doi: 10.1016/s0196-0644(05)82783-0. [DOI] [PubMed] [Google Scholar]
- 9.Steinbok P, Thompson G. Metabolic disturbances after head injury: abnormalities of sodium and water balance with special reference to the effects of alcohol intoxication. Neurosurgery. 1978;3:9–15. doi: 10.1227/00006123-197807000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Leonard BE. The involvement of neuromediation in alcohol abuse and alcoholism. Neurochem Int. 1995;26:343–346. doi: 10.1016/0197-0186(94)00141-g. [DOI] [PubMed] [Google Scholar]
- 11.Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int. 1995;26:305–306. doi: 10.1016/0197-0186(94)00139-l. [DOI] [PubMed] [Google Scholar]
- 12.Markwalder T. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637–645. doi: 10.3171/jns.1981.54.5.0637. [DOI] [PubMed] [Google Scholar]
- 13.Kraus JF, Morgenstern H, Fife D, Conroy C, Nourjah P. Blood alcohol tests, prevalence of involvement, and outcomes following brain injury. Am J Public Health. 1989;7:294–299. doi: 10.2105/ajph.79.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drubach DA, Kelly MP, Winslow MM, Flynn JP. Substance abuse as a factor in the causality, severity, and recurrence rate of traumatic brain injury. Md Med J. 1993;4:989–993. [PubMed] [Google Scholar]
- 15.Vickery CD, Sherer M, Nick TG, et al. Relationships among premorbid alcohol use, acute intoxication, and early functional status after traumatic brain injury. Arch Phys Med Rehabil. 2008;89:48–55. doi: 10.1016/j.apmr.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 16.Christensen MA, Janson S, Seago JA. Alcohol, head injury, and pulmonary complications. J Neurosci Nurs. 2001;33:184–189. doi: 10.1097/01376517-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 17.De Guise E, LeBlanc J, Dagher J, et al. Early outcome in patients with traumatic brain injury, pre-injury alcohol abuse and intoxication at time of injury. Brain Inj. 2009;23:853–865. doi: 10.1080/02699050903283221. [DOI] [PubMed] [Google Scholar]
- 18.Brooks N, Symington C, Beattie A, Campsie L, Bryden J, McKinlay W. Alcohol and other predictors of cognitive recovery after severe head injury. Brain Inj. 1989;3:235–246. doi: 10.3109/02699058909029638. [DOI] [PubMed] [Google Scholar]
- 19.Alexander S, Kerr ME, Yonas H, Marion DW. The effects of admission alcohol level on cerebral blood flow and outcomes after severe traumatic brain injury. J Neurotraum. 2004;2:575–583. doi: 10.1089/089771504774129900. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri A, Servadei F, Marchesini G, Stein SC, Vandelli A. Early predictors of unfavorable outcome in subjects with moderate head injury in the emergency department. J Neurol Neurosur Ps. 2007;7:567–573. doi: 10.1136/jnnp.2007.120162. [DOI] [PubMed] [Google Scholar]
- 21.Nath FP, Beastal G, Teasdale GM. Alcohol and traumatic brain damage. Injury. 1986;17:150–153. doi: 10.1016/0020-1383(86)90320-7. [DOI] [PubMed] [Google Scholar]
- 22.Labi MLC, Brentjens M, Coad ML, Flynn WJ, Zielezny M. Development of a longitudinal study of complications and functional outcomes after traumatic brain injury. Brain Inj. 2003;17:265–278. doi: 10.1080/0269905021000038410. [DOI] [PubMed] [Google Scholar]
- 23.Corrigan JD. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil. 1995;76:302–309. doi: 10.1016/s0003-9993(95)80654-7. [DOI] [PubMed] [Google Scholar]
- 24.Cooper KD, Tabaddor K, Hauser WA, Shulman K, Feiner C, Factor PR. The epidemiology of head injury in the Bronx. Neuroepidemiology. 1983;2:70–88. [Google Scholar]
- 25.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Center for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta: 2006. [Google Scholar]
- 26.Arango-Lasprilla JC, Rosenthal M, Deluca J, Cifu DX, Hanks R, Komaroff E. Functional outcomes from inpatient rehabilitation after traumatic brain injury: how do Hispanics fare? Arch Phys Med Rehabil. 2007;8:11–18. doi: 10.1016/j.apmr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Arango-Lasprilla JC, Rosenthal M, Deluca J, Komaroff E, Sherer M, Cifu DX, Hanks R. Traumatic brain injury and functional outcomes: does minority status matter? Brain Inj. 2007;21:701–708. doi: 10.1080/02699050701481597. [DOI] [PubMed] [Google Scholar]
- 28.Hanks RA, Wood DL, Millis S, et al. Violent traumatic brain injury: occurrence, patient characteristics, and risk factors from the Traumatic Brain Injury Model Systems Project. Arch Phys Med Rehabil. 2003;84:249–254. doi: 10.1053/apmr.2003.50096. [DOI] [PubMed] [Google Scholar]
- 29.Hart T, O’Neil-Pirozzi TM, Williams KD, Rapport LJ, Hammond F, Kreutzer J. Racial difference in caregiving patterns, caregiver emotional function, and source of emotional support following traumatic brain injury. J Head Trauma Rehabil. 2007;22:122–131. doi: 10.1097/01.HTR.0000265100.37059.44. [DOI] [PubMed] [Google Scholar]
- 30.Staudenmayer KL, Diaz-Arrastia R, de Oliveira A, Gentilello LM, Shafi S. Ethnic disparities in long-term functional outcome after traumatic brain injury. J Trauma. 2007;63:1364–1369. doi: 10.1097/TA.0b013e31815b897b. [DOI] [PubMed] [Google Scholar]
- 31.Wagner AK, Hammond FM, Sasser HC, Wiercisiewski D, Norton HJ. Use of injury severity variables in determining disability and community integration after traumatic brain injury. J Trauma. 2000;49:411–419. doi: 10.1097/00005373-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Neff J. Alcohol consumption and psychological distress among Anglos, Hispanics, and blacks. Alcohol Alcoholism. 1986;2:337–351. [PubMed] [Google Scholar]
- 33.Substance Abuse and Mental Health Services Administration [Accessed November 21st, 2010];Results from the 2002 national survey on drug use and health: detailed tables. http://www.oas.samhsa.gov/nhsda/2k2nsduh/Sect2peTabs55to69.pdf. Published 2005.
- 34.Neff J, Hoppe SK. Acculturation and drinking patterns among U.S. Anglos, Blacks, and Mexican Americans. Alcohol Alcoholism. 1992;27:293–308. [PubMed] [Google Scholar]
- 35.Neff J, Hoppe SK, Perea P. Acculturation and alcohol use: drinking patterns and problems among Anglo and Mexican American male drinkers. Hispanic J Behav Sci. 1987;9:151–181. [Google Scholar]
- 36.Winqvist S, Luukinen H, Jokelainen J, Lehtilahti M, Näyhä S, Hillborn M. Recurrent traumatic brain injury is predicted by the index injury occurring under the influence of alcohol. Brain Inj. 2008;22:780–785. doi: 10.1080/02699050802339397. [DOI] [PubMed] [Google Scholar]
- 37.Shih HC, Hu SC, Yang CC, Ko TJ, Wu JK, Lee CH. Alcohol intoxication increases morbidity in drivers involved in motor vehicle accidents. Am J Emerg Med. 2003;21:91–94. doi: 10.1053/ajem.2003.50025. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Census Bureau [Retrieved August 25th, 2010];2006-2008 American Community Survey 3-year estimates. http://factfinder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenu Id=&_lang=en&_ds_name=ACS_2008_3YR_G00_&ts=. Published 2008.
- 39.Kay T, Cavallo MM, Ezrachi O, Vavagiakis P. The Head Injury Family Interview: a clinical and research tool. J Head Trauma Rehabil. 1995;10:12–31. [Google Scholar]
- 40.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118–123. [PubMed] [Google Scholar]
- 41.Field A. Discovering Statistics using SPSS. 3rd ed Sage; London: 2009. [Google Scholar]
- 42.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Academic Press; New York: 1988. [Google Scholar]
- 43.Myers R. Classical and Modern Regression with Applications. 2nd ed Duxbury; Boston, MA: 1990. [Google Scholar]
- 44.Menard S. Applied Logistic Regression Analysis. Sage; Thousand Oaks, CA: 1995. Sage University Paper Series on Quantitative Applications in the Social Sciences, 07-106. [Google Scholar]