Abstract
Hypoxia-inducible factors (HIFs) mediate adaptive physiological responses to hypoxia. In human cancers that are accessible for O2 electrode measurements, intratumoral hypoxia is common and is associated with increased risk of mortality. HIF activity in regions of intratumoral hypoxia mediates angiogenesis, epithelial-mesenchymal transition, stem cell maintenance, invasion, metastasis, and resistance to radiation therapy and chemotherapy. A growing number of drugs have been identified that inhibit HIF activity by a variety of molecular mechanisms. Because many of these drugs are already FDA-approved for other indications, clinical trials can (and should) be initiated to test the hypothesis that incorporation of HIF inhibitors into current standard-of-care therapy will increase the survival of cancer patients.
Hypoxia and cancer
Human cells require adequate supplies of O2 on a continuous basis for use as the terminal electron acceptor in the process of mitochondrial respiration that generates ATP, which is used to power most biochemical reactions. Both the delivery and consumption of O2 are precisely regulated through the activity of hypoxia-inducible factors (HIFs) [1]. As cells proliferate, increased O2 consumption results in hypoxia (reduced O2 levels), which activates HIFs, leading to transcription of the VEGF gene, which encodes vascular endothelial growth factor, a secreted protein that stimulates angiogenesis and thereby increases O2 delivery. Cancer cells are characterized by dysregulated cell proliferation, and the blood vessels that form within solid tumors are often structurally and functionally abnormal, resulting in severe hypoxia. To adapt to the hypoxic microenvironment, cancer cells co-opt physiological responses to hypoxia that are mediated by HIFs. In the process of doing so, hypoxic cancer cells acquire invasive and metastatic properties as well as resistance to chemotherapy and radiation therapy, which together constitute the lethal cancer phenotype. Despite ample data to support this model, there are few drugs in the cancer armamentarium that target hypoxic cancer cells. Not coincidentally, the options for treatment of advanced metastatic disease (and their efficacy) are extremely limited, and this year over 570,000 Americans will die of cancer [2]. Given the magnitude of this unmet clinical need, novel therapeutic strategies that are not limited to those few approaches employed by the pharmaceutical industry must be considered. This review will summarize the molecular mechanisms by which HIF activity is regulated in an O2-dependent manner, the roles of HIFs in cancer progression, the chemical compounds that have been shown to inhibit HIF activity, and their potential use as anti-cancer agents.
Molecular biology of HIFs
The nucleated cells of all metazoan species analyzed to date express HIF-1, which is a heterodimer that is composed of HIF-1α and HIF-1β subunits [1]. Certain cell types of vertebrate organisms also express HIF-2, which is composed of HIF-2α and HIF-1β subunits. A principal mechanism by which O2 regulates HIF activity is through proline and asparagine hydroxylation [3, 4]. The hydroxylation of two proline residues in HIF-1α and HIF-2α (Pro402 and Pro564 in human HIF-1α) by prolyl hydroxylase domain protein 2 (PHD2) is required for the binding of the von Hippel-Lindau protein (VHL), which leads to HIF-α ubiquitination and proteasomal degradation. Hydroxylation of an asparagine residue (Asn803 in human HIF-1α) by factor inhibiting HIF-1 (FIH-1) blocks the recruitment of the coactivator p300. These hydroxylation reactions use O2 and α-ketoglutarate as substrates and enzyme activity is inhibited under hypoxic conditions, leading to increased HIF-α stability and transcriptional activity. HIFs bind to hypoxia response elements that contain the consensus sequence 5'-RCGTG-3' [5]. Based on genome-wide chromatin immunoprecipitation combined with DNA sequencing or mRNA microarrays (ChIP-seq and ChIP-chip, respectively), the number of direct HIF target genes is currently greater than 800, (i.e. at least 1 out of every 30 human genes)[6, 7]. HIFs also indirectly regulate gene expression by transactivating genes encoding microRNAs [8] and chromatin modifying enzymes [6, 9].
HIFs in cancer progression
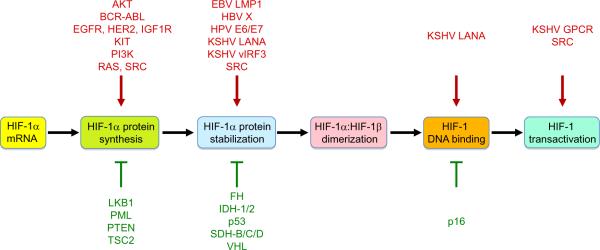
HIFs play key roles in many critical aspects of cancer biology including angiogenesis [10–12], stem cell maintenance [13–15], metabolic reprogramming [16, 17]; autocrine growth factor signaling [18, 19]; epithelial-mesenchymal transition [9, 20–22], invasion [23, 24], metastasis [25–27], and resistance to radiation therapy [28] and chemotherapy [29]. An extensive body of experimental and clinical data has validated HIFs as targets for cancer therapy: first, in addition to intratumoral hypoxia, loss-of-function for tumor suppressor genes (most notably, VHL) and gain-of-function for oncogenes and viral transforming genes increase HIF activity (Figure 1); and second, levels of HIF-1α or HIF-2α are correlated with tumor growth, vascularization, and metastasis both in animal models and in clinical studies [30]. In different cancers, the HIF-dependent expression of these genes may be increased either by genetic alterations or by intratumoral hypoxia. Several specific HIF-regulated genes that play key roles in critical aspects of cancer biology are discussed in greater detail below.
Figure 1.
Regulation of HIF-1 activity by oncoproteins and tumor suppressors. HIF-1 activity is stimulated by oncoprotein (red) gain-of-function. HIF-1 activity is inhibited by tumor suppressors (green) and their loss-of-function therefore stimulates HIF-1 activity.
Increased cell proliferation and survival
Among the first changes that distinguish neoplastic from normal cells are an increased rate of cell proliferation and a decreased rate of cell death, due to increased expression of secreted growth/survival factors. Often, the same cells express the cognate membrane receptors for these factors, resulting in autocrine signaling. Among the growth/survival factors that are encoded by HIF-regulated genes (italicized and in parentheses below and in Figure 2A) and participate in autocrine signaling are: transforming growth factor-α (TGFA) in clear-cell renal carcinoma [31]; insulin-like growth factor-2 (IGF2) in colorectal carcinoma [32, 33]; vascular endothelial growth factor (VEGF) in colorectal, gastric, and pancreatic cancer [34, 35]; endothelin 1 (EDN1) in breast, prostate, and ovarian cancer [36, 37]; adrenomedullin in pancreatic and prostate cancer [38, 39], and erythropoietin (EPO) in breast, prostate, and renal cancer and melanoma [40]. Immortalization of cancer stem cells also requires the expression of telomerase (TERT), pluripotency factors such as NANOG and OCT4, and factors that block cellular senescence such as the glycolytic enzymes glucosephosphate isomerase (GPI) and phosphoglycerate mutase (PGM) [41–43].
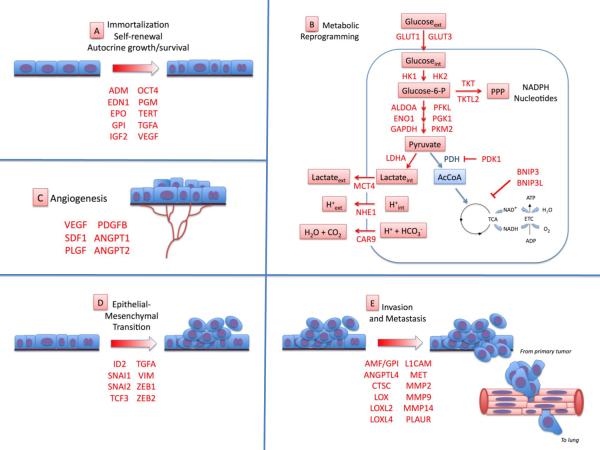
Figure 2.
HIF target genes encode proteins involved in critical aspects of cancer progression. The list of HIF-regulated genes (shown in red) is intended to be illustrative rather than comprehensive. (A) HIF target genes (locus abbreviations in parentheses) that promote cell immortalization, stem cell self-renewal, and autocrine growth and survival include those encoding adrenomedullin (ADM), endothelin 1 (EDN1), erythropoietin (EPO), glucose-6-phosphate isomerase (GPI), insulin-like growth factor 2 (IGF2), octamer binding protein 4 (OCT4), phosphoglycerate mutase (PGM), telomerase (TERT), transforming growth factor α (TGFA), and vascular endothelial growth factor (VEGF). (B) HIF-1 target genes involved in metabolic reprogramming include glucose transporter 1 and 3 (GLUT1, GLUT3), hexokinase 1 and 2 (HK1, HK2), glycolytic enzymes aldolase A (ALDOA), enolase 1 (ENO1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphofructokinase L (PFKL), phosphoglycerate kinase 1 (PGK1), pyruvate kinase M2 (PKM2), and lactate dehydrogenase A (LDHA). The enzymatic activity of pyruvate dehydrogenase (PDH) is inhibited by PDH kinase 1 (PDK1), thereby blocking the conversion of pyruvate to acetyl coenzyme A (AcCoA) for entry into the tricarboxylic acid (TCA) cycle. Two members of the BCL2 family of mitochondrial proteins (BNIP3, BNIP3L) trigger mitochondrial selective autophagy. Lactate and hydrogen ion (H+) generated by glycolysis are effluxed from the cell through the activity of the monocarboxylate transporter 4 (MCT4), sodium-hydrogen exchanger 1 (NHE1), and carbonic anhydrase 9 (CAR9). HIF-1 may also regulate the expression of the transketolase (TKT) and TKT-like 2 (TKTL2) enzymes of the non oxidative arm of the pentose phosphate pathway (PPP). (C) HIFs stimulate tumor vascularization by activating transcription of the genes encoding VEGF, stromal-derived factor 1 (SDF1), placental growth factor (PGF), platelet-derived growth factor B (PDGFB), and angiopoietin 1 and 2 (ANGPT1, ANGPT2). (D) HIF target genes that promote epithelial mesenchymal transition include inhibitor of differentiation 2 (ID2), snail 1 and 2 (SNAI1, SNAI2), transcription factor 3 (TCF3), TGFA, vimentin (VIM), and zinc finger E-box-binding homeobox 1 and 2 (ZEB1, ZEB2). (E) HIF target genes promoting invasion and metastasis include those encoding autocrine motility factor (AMF; also known as GPI), angiopoietin- like 4 (ANGPTL4), cathepsin C (CTSC), lysyl oxidase (LOX), LOX-like 2 and 4 (LOXL2, LOXL4), L1 cell adhesion molecule (L1CAM), Met proto-oncogene/hepatocyte growth factor receptor (MET), matrix metalloproteinase 2, 9, and 14 (MMP2, MMP9, MMP14), and the urokinase plasminogen activator receptor (PLAUR).
Metabolic reprogramming
The uptake of glucose by metastatic cancer cells is so reliably and markedly increased relative to normal cells that it serves as the basis for the clinical test that is used to screen cancer patients for occult metastases, in which 18F-fluorodeoxyglucose is imaged by positron emission tomography (FDG-PET). HIF-1 mediates expression of genes encoding glucose transporters (GLUT1, GLUT3) and glycolytic enzymes (ALDOA, ENO1, GAPDH, HK1, HK2, PFKL, PGK1, PKM2, LDHA) that convert glucose to lactate (Figure 2B). HIF-1 also actively suppresses mitochondrial oxidative metabolism by increasing the expression of pyruvate dehydrogenase kinase 1 (PDK1), which phosphorylates and inactivates pyruvate dehydrogenase (PDH), the enzyme that converts pyruvate to acetyl coA for entry into the TCA cycle [44]. HIF-1 also downregulates oxidative metabolism by activating the expression of BNIP3 and BNIP3L, which mediate mitochondrial-selective autophagy [45, 46]. Recent data suggest that in some cell types HIF-1 may also mediate expression of transketolase enzymes (TKT, TKTL2) of the pentose phosphate pathway (PPP), which non-oxidatively catalyze the production of NADPH and nucleotides that are required for lipid and nucleic acid synthesis [47]. Angiogenesis. HIF-1 controls the expression of multiple genes encoding angiogenic growth factors, including vascular endothelial growth factor (VEGF), stromal-derived factor 1 (SDF1), placental growth factor (PGF), platelet-derived growth factor B (PDGFB), and angiopoietin (ANGPT) 1 and 2 [48] (Figure 2C). In mouse models, inhibition of HIF-1 activity dramatically inhibits tumor vascularization [11, 12].
Epithelial-mesenchymal transition
HIF-1 activates the transcription of genes encoding repressors (ID2, SNAI1, SNAI2, TCF3, ZEB1, ZEB2) that block the expression of E-cadherin and other proteins that contribute to the rigid cytoskeleton, cell-cell adhesion, and other differentiated characteristics of epithelial cells [9, 20, 21]. HIF-1 also mediates expression of genes (TGFA, VIM) that promote the flexible cytoskeleton and other characteristics of the mesenchymal phenotype [31] (Figure 2D).
Invasion and metastasis
HIF-1 activates transcription of genes encoding: proteases that degrade (CTSC, MMP2, MMP9, MMP14, PLAUR) or remodel (LOX, LOXL2, LOXL4) the extracellular matrix within the primary tissue and at distant sites of metastasis [25, 26]; motility factors (AMF, MET); permeability factors (VEGF, ANGPT2) that promote the intravasation of cancer cells into blood vessels; and cell surface (L1CAM) and secreted (ANGPTL4) proteins that promote extravasation of cancer cells into the parenchyma at metastatic sites such as the lung [27] (Figure 2E).
HIF-1 inhibitors for cancer therapy
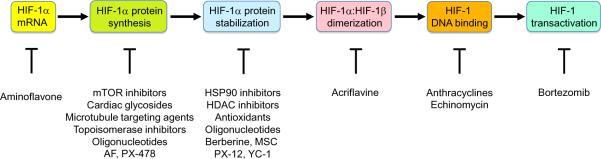
A growing number of chemical compounds have been shown to block tumor xenograft growth and inhibit HIF activity through a wide variety of molecular mechanisms, including decreased HIF-1α mRNA levels, decreased HIF-1α protein synthesis, increased HIF-1α degradation, decreased HIF subunit heterodimerization, decreased HIF binding to DNA, and decreased HIF transcriptional activity (Figure 3), as described below. Many of these are drugs that are in clinical cancer trials or are already approved for the treatment of cancer or other diseases. The list of drugs discussed below is meant to be illustrative rather than comprehensive.
Figure 3.
Molecular mechanism of action of drugs that inhibit HIF-1. The steps required for the transactivation of target genes by HIF-1 are shown in the colored ovals and the drugs that inhibit each step are shown below. Abbreviations: AF, aminoflavone, MSC, Se-methylselenocysteine.
HIF-1α mRNA expression
Aminoflavone, the active component of the prodrug AFP-464, which is currently in phase I cancer trials, partially inhibits HIF-1α mRNA expression but almost completely blocks HIF-1α protein expression [49], suggesting that it decreases both the stability and translation of HIF-1α mRNA.
HIF-1α synthesis
Drugs that inhibit the translation of HIF-1α mRNA into protein include: (i) mTOR inhibitors, such as rapamycin, temsirolimus (CCI-779), and everolimus (RAD-001) [50]; (ii) cardiac glycosides, such as digoxin [51], which have been used for decades to treat heart disease; (iii) microtubule targeting agents, such as 2-methoxyestradiol and taxotere [52]; (iv) topoisomerase I and II inhibitors, such as topotecan [53] and NSC-644221 [54], respectively; and (v) synthetic oligonucleotides, such as EZN-2968, a locked nucleic acid oligonucleotide that binds to HIF-1α mRNA and blocks its translation, which is currently in phase I clinical trials [55]. This category also includes anti-cancer drugs that inhibit receptor tyrosine kinases, such as BCR/ABL, EGFR, and HER2, and thereby indirectly inhibit mTOR activity. YC-1 [3-(5'-hydroxy methyl-2'-furyl)-1-benzylindazole] may also block HIF-1α protein expression by inhibiting mTOR [56]. PX-478 (S-2-amino-3-[4′-N,N,-bis(2-chloroethyl)amino]phenyl propionic acid N-oxide dihydrochloride) inhibits HIF-1α translation by an undetermined mechanism and sensitizes tumor xenografts to radiation therapy [57]. Many of the drugs in this category show preferential activity against HIF-1α, with less or no effect on HIF-2α.
HIF-1α stability
Drugs that induce the degradation of HIF-1α protein include: (i) HSP90 inhibitors, such as 17-allylamino-17-demethoxygeldanamycin, which cause VHL-independent, RACK1-dependent ubiquitination and proteasomal degradation of HIF-1α [58]; (ii) antioxidants, such as ascorbate (vitamin C) and N-acetyl cysteine, which block tumor xenograft growth by promoting HIF-1α degradation through the PHD2-VHL-proteasome pathway [59]; (iii) the thioredoxin inhibitor PX-12, which induces HIF-1α degradation that may be due in part to increased expression of SSAT1, a protein that binds to HIF-1α and promotes RACK1-dependent ubiquitination and proteasomal degradation [60, 61]; (iv) Class II histone deacetylase (HDAC) inhibitors, such as LAQ824, which stimulate ubiquitination of HIF-1α by an undetermined mechanism [62]; (v) G-rich oligonucleotides, which represent another nucleic acid-based strategy; but in this case, the target is HIF-1α protein rather than mRNA [63]; (vi) berberine, a natural product that induces HIF-1α degradation, has anti-angiogenic effects both in cancer cells and in endothelial cells [64]; (vii) Se-methylselenocysteine, which induces HIF-1α degradation and sensitizes hypoxic cancer cells to the effects of the chemotherapy drug irinotecan [65]; and (viii) YC-1, a guanylate cyclase activator that induces HIF-1α degradation by an unknown mechanism [66]. YC-1 and deguelin, an HSP90 inhibitor, sensitize tumor xenografts to radiation therapy [66, 67]. Most of the drugs in this category show similar activity against HIF-1α and HIF-2α.
HIF heterodimerization
Acriflavine, a drug that was used clinically as an antibacterial agent prior to the discovery of penicillin, binds directly to the PAS-B subdomain of HIF-1α and HIF-2α and blocks their interaction with HIF-1β, thereby blocking HIF-dependent gene transcription, leading to impaired tumor growth and vascularization [12].
HIF DNA-binding activity
Anthracyclines, such as doxorubicin (Adriamycin) and daunorubicin, bind to DNA and block the binding of HIF-1 and HIF-2 in cultured cells and block HIF-1-dependent expression of angiogenic growth factors leading to impaired tumor growth and vascularization [11]. Echinomycin, another DNA intercalating agent that inhibits HIF-1 activity, has been shown to block lymphoma and acute myelogenous leukemia growth by eradicating cancer stem cells [15].
HIF-1α-dependent transactivation
The proteasome inhibitor bortezomib, which is approved by the FDA for treatment of mantle cell lymphoma and multiple myeloma [68], inhibits HIF-1 transcriptional activity by targeting the carboxyl-terminal transactivation domain of HIF-1α that interacts with the coactivator p300, although drug treatment does not disrupt the interaction [69]. In prostate cancer cells, bortezomib blocks HIF-1α protein expression by inhibiting phosphatidylinositol-3-kinase/AKT/mTOR and ERK signaling [70].
Pursuing “failed” drugs?
Several known chemotherapeutic agents, such as topotecan, have been shown to inhibit HIF-1. One of the criticisms of proposed trials involving compounds such as topotecan is that these are drugs that have already failed to show significant anti-cancer effects in clinical trials. However, the key distinction that must be made between prior and current trials is that these drugs have previously been administered episodically at maximum tolerated dose as cytotoxic agents, whereas their current utilization as HIF inhibitors involves frequent administration at lower doses in an effort to maintain continuous inhibition of HIF activity. In a recently completed pilot pharmacodynamic study involving 16 patients with advanced cancer and biopsy-proven HIF-1α overexpression, the administration of topotecan orally at 1.6 mg/m2/day × 5 days/week × 2 weeks/28-day cycle resulted in decreased tumor blood flow by DCE-MRI in 7 of 10 patients and loss of HIF-1α expression on repeat biopsy in 4 of 7 patients, suggesting that the drug hit the target in vivo [53]. During the trial, the topotecan dose was reduced to 1.2 mg/m2/day due to myelosuppression, although it is not known whether this side effect reflected cytotoxicity due to DNA damage or was a direct consequence of HIF inhibition, independent of DNA damage. In either case, it remains to be determined (i) whether 2-week-on/2-week-off inhibition of HIF will be an effective anti-cancer strategy; and (ii) whether there is a therapeutic window that will allow chronic use of topotecan as a HIF inhibitor. Digoxin is an appealing candidate in this regard because it has been used for decades in the treatment of heart disease and blood levels of the drug that are safe and therapeutic (in the context of heart disease) are well established.
No drug has a single effect
Another means of disparaging the use of existing (and off-patent) drugs is the claim that novel anti-cancer agents are “specific” whereas repurposed drugs are “non-specific”. It does not require much understanding of pharmacology to appreciate that this claim represents a triumph of hope over data and marketing over science; i.e. the older the drug, the more likely it is that additional targets have been identified, which is a reflection of time, not inherent drug specificity. In the case of digoxin, it is not clear whether inhibition of HIF-1α synthesis is dependent on its known activity as a Na+/K+ ATPase inhibitor. However, mouse xenograft studies have demonstrated that the arrest of tumor growth that occurred when mice bearing prostate cancer xenografts were treated with digoxin was lost when the prostate cancer cells were engineered to express HIF-1α from a vector that was not inhibited by digoxin, demonstrating that the ability of digoxin to block tumor growth was dependent upon its ability to inhibit HIF-1α expression [51]. Hence, the activity of digoxin as an anti-cancer agent can be directly attributed to its activity as a HIF inhibitor. Combining several HIF inhibitors may allow lower doses of each individual drug, thereby reducing the likelihood of off-target effects. Combination therapy may also reduce the probability of selecting for drug resistant cancer cells.
Can HIF inhibitors improve current therapies?
In addition to extensive data indicating that HIFs mediate resistance to radiation therapy [28] and chemotherapy [29], there is mounting evidence that HIF-1 activity may contribute to the development of resistance to novel targeted therapies, such as imatinib treatment of chronic myeloid leukemia [47]. HIF-1 appears to mediate resistance to imatinib through metabolic reprogramming, by activating expression of transketolase and thereby increasing glucose flux through the non-oxidative arm of the pentose phosphate pathway [47]. The switch from oxidative to reductive metabolism that is mediated by HIF-1 [44] has the effect of reducing cellular ROS levels [16, 44], which may increase resistance to cytotoxic chemotherapy [71].
In the case of VEGF receptor inhibitors, data from several mouse models indicate that treatment, either with the anti-VEGFR2 antibody DC-101 or the small molecule tyrosine kinase inhibitor sunitinib, reduced primary tumor growth and vascularization but increased metastasis, probably because impaired angiogenesis led to increased intratumoral hypoxia and increased HIF activity [72–74]. The failure of the anti-VEGF antibody bevacizumab to affect breast cancer progression, which led to revocation of approval by the FDA [75], may involve HIF-1-dependent expression of other angiogenic growth factors. In contrast, HIF inhibitors dramatically decreased the spontaneous metastasis of human breast cancer cells to the lungs in mouse orthotopic transplantation models by affecting multiple steps in the metastatic process [26, 27]. Taken together, these results suggest that combination treatment with HIF inhibitors may improve the efficacy of anti-angiogenic agents, a conclusion that is supported by data from mouse models [76].
Traditional chemotherapy may also be more effective when administered with a HIF inhibitor and many different molecular mechanisms underlie this effect in a cell-type and chemotherapy-specific manner. First, HIFs have been shown to regulate the expression of genes encoding ATP-binding cassette multidrug transporters, including MDR1 (ABCB1) and BCRP (ABCG2), which efflux chemotherapy drugs from cancer cells [77, 78]. Second, HIF-1 inhibits chemotherapy-induced cancer cell senescence [79]. Third, HIF-1 inhibits expression of pro-apoptotic mitochondrial proteins (BAX, BID) and caspases (CASP3, CASP8, CASP10) and induces expression of anti-apoptotic proteins (BCL2, BIRC5) [80–84]. Fourth, HIF-1 prevents chemotherapy-induced DNA damage by inhibiting the expression of topoisomerase IIα protein [85] or the DNA-dependent protein kinase complex [86]. Fifth, HIF-1-dependent metabolic reprogramming [16, 17, 30, 44, 45, 87, 88] may decrease ROS levels and thereby inhibit chemotherapy-induced cell death [89].
Caveats
There are important caveats regarding the safety and efficacy of HIF inhibitors as anti-cancer agents. A major safety issue is that patients with severe ischemic cardiovascular disease may experience exacerbation of their condition, and HIF inhibitors are contraindicated in such cases. With respect to efficacy, many of the inhibitors described have been found to inhibit HIF activity in some but not all cancer cell lines tested. This may be due to several potential mechanisms. First, drug levels in resistant cell lines may not be high enough to inhibit HIF activity. Second, the targeted pathway may not be contributing to HIF activity in the resistant cell line. Third, an alternative pathway may be activated in response to treatment. Strategies for monitoring HIF activity in vivo to test for drug response early in the treatment course would be valuable as a means of demonstrating that the drug is hitting its target. Although indirect and highly expensive, imaging techniques that monitor tumor perfusion (e.g. DCE MRI) or glucose uptake (FDG-PET) may be useful for this purpose.
Concluding remarks
Preclinical data provide compelling evidence that HIFs play important roles in many critical aspects of cancer biology and that inhibition of HIFs, both in cancer and stromal cells, inhibits tumor growth, vascularization, metabolic reprogramming, invasion, metastasis, and resistance to radiation therapy and chemotherapy. Clinical data indicate that HIF-1α overexpression is associated with increased risk of patient mortality in many cancers. The addition of HIF inhibitors to existing therapeutic regimens is likely to improve their efficacy, particularly in cancers in which HIF-1α overexpression is documented in the diagnostic tumor biopsy and in those cases in which the existing therapy induces HIF activity, as in the case of anti-angiogenic and vascular targeting agents. Preclinical data from relevant mouse models is particularly important in providing scientific justification to support clinical trials. Finally, the HIF inhibitor acriflavine, which potently inhibits prostate cancer xenograft growth [12] and breast cancer metastasis to the lungs [90] in mouse models, was used as an antimicrobial agent in the 1930s [91] but is not currently available in a pharmaceutical preparation suitable for administration to patients. Since pharmaceutical companies are unlikely to manufacture such off-patent drugs, the NCI has an obligation to the oncology community to perform this function.
Acknowledgments
Work in the author's laboratory is supported by grants from the American Cancer Society, Johns Hopkins Institute for Cell Engineering, National Cancer Institute, and Susan G. Komen Foundation. G.L.S. is the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine and an American Cancer Society Research Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Semenza GL. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer Facts & Figures 2011. American Cancer Society; Atlanta: 2011. http://www.cancer.org. [Google Scholar]
- 3.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Majmundar AJ, et al. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 6.Xia X, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc. Natl. Acad. Sci. USA. 2009;106:4260–4265. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SchÖdel J, et al. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosby ME, et al. Emerging roles of microRNAs in the molecular responses to hypoxia. Curr. Pharm. Des. 2009;15:3861–3866. doi: 10.2174/138161209789649367. [DOI] [PubMed] [Google Scholar]
- 9.Wu MZ, et al. Interplay between HDAC3 and WDR5 is essential for hypoxia-induced epithelial-mesenchymal transition. Mol. Cell. 2011;43:811–822. doi: 10.1016/j.molcel.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, et al. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc. Natl. Acad. Sci. USA. 2009;106:2353–2358. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Lee K, et al. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl. Acad. Sci. USA. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Barnhart BC, Simon MC. Metastasis and stem cell pathways. Cancer Metastasis Rev. 2007;26:261–271. doi: 10.1007/s10555-007-9053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suda T, et al. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Luo W, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franovic A, et al. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc. Natl. Acad. Sci. USA. 2007;104:13092–13097. doi: 10.1073/pnas.0702387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau CK, et al. An Akt/hypoxia-inducible factor 1α/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance inliver cancer cells and tumorigenic hepatic progenitor cells. Clin. Cancer Res. 2009;15:3462–3471. doi: 10.1158/1078-0432.CCR-08-2127. [DOI] [PubMed] [Google Scholar]
- 20.Esteban MA, et al. Regulation of E-cadherin expression by VHL and hypoxiainducible factor. Cancer Res. 2006;66:3567–3575. doi: 10.1158/0008-5472.CAN-05-2670. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamachary B, et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 22.Mak P, et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26:319–331. doi: 10.1007/s10555-007-9062-2. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamachary B, Semenza GL. Analysis of hypoxia-inducible factor 1α expression and its effects on invasion and metastasis. Methods Enzymol. 2007;435:347–354. doi: 10.1016/S0076-6879(07)35017-9. [DOI] [PubMed] [Google Scholar]
- 25.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 26.Wong CC, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc. Natl. Acad. Sci. USA. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2011 doi: 10.1038/onc.2011.365. 2011 Aug 22. doi: 10.1038/onc.2011.365. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Moeller BJ, et al. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007;26:241–248. doi: 10.1007/s10555-007-9056-0. [DOI] [PubMed] [Google Scholar]
- 29.Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011;14:191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunaratnam L, et al. Hypoxia inducible factor activates the transforming growth factor-α/epidermal growth factor receptor growth stimulatory pathway in VHL–/– renal cell carcinoma cells. J. Biol. Chem. 2003;278:44966–44974. doi: 10.1074/jbc.M305502200. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 33.Feldser D, et al. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- 34.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dallas NA, et al. Functional significance of vascular endothelial growth factor receptors on gastrointestinal cancer cells. Cancer Metastasis Rev. 2007;26:433–441. doi: 10.1007/s10555-007-9070-2. [DOI] [PubMed] [Google Scholar]
- 36.Wülfing P, et al. Expression of endothelin-1, endothelin-A, and endothelin-B receptor in human breast cancer and correlation with long-term follow-up. Clin. Cancer Res. 2003;9:4125–4131. [PubMed] [Google Scholar]
- 37.Grimshaw MJ. Endothelins and hypoxia-inducible factor in cancer. Endocr. Relat. Cancer. 2007;14:233–244. doi: 10.1677/ERC-07-0057. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran V, et al. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67:2666–2675. doi: 10.1158/0008-5472.CAN-06-3362. [DOI] [PubMed] [Google Scholar]
- 39.Berenguer C, et al. Adrenomedullin, an autocrine/paracrine factor induced by androgen withdrawal, stimulates `neuroendocrine phenotype' in LNCaP prostate tumor cells. Oncogene. 2008;27:506–518. doi: 10.1038/sj.onc.1210656. [DOI] [PubMed] [Google Scholar]
- 40.Szenajch J, et al. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells From clinic to bench - a critical review. Biochim. Biophys. Acta. 2010;1806:82–95. doi: 10.1016/j.bbcan.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Nishi H, et al. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT) Mol. Cell. Biol. 2004;24:6076–6083. doi: 10.1128/MCB.24.13.6076-6083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathieu J, et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondoh H, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 44.Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb. Symp. Quant. Biol. 2011 doi: 10.1101/sqb.2011.76.010678. 2011 Jul 22. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Zhang H. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Bellot G, et al. Hypoxia-induced autophagy is mediated through hypoxiainducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao F. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1α-induced metabolic reprogramming. Oncogene. 2010;29:2962–2972. doi: 10.1038/onc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terzuoli E, et al. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1α expression in an AhR-independent fashion. Cancer Res. 2010;70:6837–6848. doi: 10.1158/0008-5472.CAN-10-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shackelford DB, et al. mTOR and HIF-1α-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc. Natl. Acad. Sci. USA. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carbonaro M, et al. Microtubule disruption targets HIF-1α mRNA to cytoplasmic P-bodies for translational repression. J. Cell Biol. 2011;192:83–99. doi: 10.1083/jcb.201004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kummar S, et al. Multihistology, target-driven pilot trial of oral topotecan as an inhibitor of hypoxia-inducible factor-1α in advanced solid tumors. Clin. Cancer Res. 2011;17:5123–5131. doi: 10.1158/1078-0432.CCR-11-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Creighton-Gutteridge M, et al. Cell type-specific, topoisomerase II-dependent inhibition of hypoxia-inducible factor-1α protein accumulation by NSC 644221. Clin. Cancer Res. 2007;13:1010–1018. doi: 10.1158/1078-0432.CCR-06-2301. [DOI] [PubMed] [Google Scholar]
- 55.Greenberger LM, et al. A RNA antagonist of hypoxia-inducible factor 1α, EZN-2968, inhibits tumor cell growth. Mol. Cancer Ther. 2008;7:3598–3608. doi: 10.1158/1535-7163.MCT-08-0510. [DOI] [PubMed] [Google Scholar]
- 56.Sun HL, et al. YC-1 inhibits HIF-1 expression in prostate cancer cells: contribution of Akt/NF-κB signaling to HIF-1α accumulation during hypoxia. Oncogene. 2007;26:3941–3951. doi: 10.1038/sj.onc.1210169. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz DL, et al. Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer. Mol. Cancer Ther. 2010;9:2057–2067. doi: 10.1158/1535-7163.MCT-09-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu YV, et al. RACK1 competes with HSP90 for binding to HIF-1α and is required for O2-independent and HSP90 inhibitor-induced degradation of HIF-1α. Mol. Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao P, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baek JH, et al. Spermidine/spermine N1-acetyltransferase-1 binds to hypoxia-inducible factor-1α (HIF-1α) and RACK1 and promotes ubiquitination and degradation of HIF-1α. J. Biol. Chem. 2007;282:33358–33366. doi: 10.1074/jbc.M705627200. [DOI] [PubMed] [Google Scholar]
- 61.Kim YH, et al. Antitumor agent PX-12 inhibits HIF-1α protein levels through an Nrf2/PMF-1-mediated increase in spermidine/spermine acetyl transferase. Cancer Chemother. Pharmacol. 2011;68:405–413. doi: 10.1007/s00280-010-1500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian DZ, et al. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1α. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 63.Guan Y, et al. G-rich oligonucleotides inhibit HIF-1α and HIF-2α and block tumor growth. Mol. Ther. 2010;18:188–197. doi: 10.1038/mt.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamsa TP, Kuttan G. Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor 1, VEGF, and proinflammatory mediators. Drug Chem. Toxicol. 2012;35:57–70. doi: 10.3109/01480545.2011.589437. [DOI] [PubMed] [Google Scholar]
- 65.Chintala S, et al. Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor 1α. Cancer Chemother. Pharmacol. 2010;66:899–911. doi: 10.1007/s00280-009-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harada H, et al. Treatment regimen determines whether an HIF-1 inhibitor enhances or inhibits the effect of radiation therapy. Br. J. Cancer. 2009;100:747–757. doi: 10.1038/sj.bjc.6604939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim WY, et al. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor 1α. Cancer Res. 2009;69:1624–1632. doi: 10.1158/0008-5472.CAN-08-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molineaux SM. Molecular pathways: targeting proteasomal protein degradation in cancer. Clin. Cancer Res. 2012;18:15–20. doi: 10.1158/1078-0432.CCR-11-0853. [DOI] [PubMed] [Google Scholar]
- 69.Kaluz S, et al. Proteasomal inhibition attenuates transcriptional activity of hypoxia-inducible factor 1 (HIF-1) via specific effect on the HIF-1α C-terminal activation domain. Mol. Cell. Biol. 2006;26:5895–5907. doi: 10.1128/MCB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Befani CD, et al. Bortezomib represses HIF-1α protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J. Mol. Med. 2012;90:45–54. doi: 10.1007/s00109-011-0805-8. [DOI] [PubMed] [Google Scholar]
- 71.Santamaría G, et al. Efficient execution of cell death in non-glycolytic cells requires the generation of ROS controlled by the activity of mitochondrial H+-ATP synthase. Carcinogenesis. 2006;27:925–935. doi: 10.1093/carcin/bgi315. [DOI] [PubMed] [Google Scholar]
- 72.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loges S, et al. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Pàez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanne JH. FDA cancels approval for bevacizumab in advanced breast cancer. BMJ. 2011;343:d7684. doi: 10.1136/bmj.d7684. doi: 10.1136/bmj.d7684. [DOI] [PubMed] [Google Scholar]
- 76.Rapisarda A, et al. Antiangiogenic agents and HIF-1 inhibitors meet at the crossroads. Cell Cycle. 2009;15:4040–4043. doi: 10.4161/cc.8.24.10145. [DOI] [PubMed] [Google Scholar]
- 77.Comerford KM, et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 78.Krishnamurthy P, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 79.Sullivan R, et al. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor 1 activity. Mol. Cancer Ther. 2008;7:1961–1973. doi: 10.1158/1535-7163.MCT-08-0198. [DOI] [PubMed] [Google Scholar]
- 80.Erler JT, et al. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol. Cell. Biol. 2004;24:2875–2889. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng XH, et al. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1α signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J. Biol. Chem. 2006;281:25903–25914. doi: 10.1074/jbc.M603414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown LM, et al. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia-inducible factor 1. Mol. Pharmacol. 2006;69:411–418. doi: 10.1124/mol.105.015743. [DOI] [PubMed] [Google Scholar]
- 83.Liu L, et al. Hypoxia-inducible factor-1α contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008;99:121–128. doi: 10.1111/j.1349-7006.2007.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flamant L, et al. Anti-apoptotic role of HIF-1 and AP-1 in paclitaxel exposed breast cancer cells under hypoxia. Mol. Cancer. 2010;9:191. doi: 10.1186/1476-4598-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sullivan R, Graham CH. Hypoxia prevents etoposide-induced DNA damage in cancer cells through a mechanism involving hypoxia-inducible factor 1. Mol. Cancer Ther. 2009;8:1702–1713. doi: 10.1158/1535-7163.MCT-08-1090. [DOI] [PubMed] [Google Scholar]
- 86.Wirthner R, et al. Impaired DNA double-strand break repair contributes to chemoresistance in HIF-1α-deficient mouse embryonic fibroblasts. Carcinogenesis. 2008;29:2306–2316. doi: 10.1093/carcin/bgn231. [DOI] [PubMed] [Google Scholar]
- 87.Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 88.Rey S, et al. Metabolic reprogramming by HIF-1 promotes the survival of bone marrow-derived angiogenic cells in ischemic tissue. Blood. 2010;117:4988–4998. doi: 10.1182/blood-2010-11-321190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rohwer N, et al. Hypoxia-inducible factor 1α determines gastric cancer chemosensitivity via modulation of p53 and NF-κB. PLoS One. 2010;5:e12038. doi: 10.1371/journal.pone.0012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong CC, et al. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J. Mol. Med. 2012 doi: 10.1007/s00109-011-0855-y. 2012 Jan 10 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Assinder EU, Birm NM. Acriflavine as urinary antiseptic. Lancet. 1936;i:304–306. [Google Scholar]