Summary
Although the contribution of bone marrow-derived cells to regenerating skeletal muscle has been repeatedly documented, there remains considerable debate as to whether this incorporation is exclusively a result of inflammatory cell fusion to regenerating myofibers or whether certain populations of bone marrow-derived cells have the capacity to differentiate into muscle. The present study uses a dual-marker approach in which GFP+ cells were intravenously transplanted into lethally irradiated β-galactosidase+ recipients to allow for simple determination of donor and host contribution to the muscle. FACS analysis of cardiotoxin-damaged muscle revealed that CD45+ bone-marrow side-population (SP) cells, a group enriched in hematopoietic stem cells, can give rise to CD45−/Sca-1+/desmin+ cells capable of myogenic differentiation. Moreover, after immunohistochemical examination of the muscles of both SP- and whole bone marrow-transplanted animals, we noted the presence of myofibers composed only of bone marrow-derived cells. Our findings suggest that a subpopulation of bone marrow SP cells contains precursor cells whose progeny have the potential to differentiate towards a muscle lineage and are capable of de novo myogenesis following transplantation and initiation of muscle repair via chemical damage.
Keywords: Skeletal Muscle, Bone Marrow, Side Population, Progenitor
Introduction
Duchenne muscular dystrophy (DMD), which is caused by mutations in the dystrophin gene, is a progressive muscle-wasting disorder characterized by continuous degeneration of muscle fibers and an eventual loss of muscle function owing to the infiltration of connective tissue (Koenig et al., 1987). Among other therapeutic approaches, over the past two decades there has been considerable experimentation with cell-based therapy as a means to treat DMD (Law et al., 1988; Karpati et al., 1989; Partridge et al., 1989). This strategy involves transplanting cells that produce a functional copy of dystrophin into an affected individual so that the donor cells can provide gene products normally absent or non-functional in the recipient. Although myoblasts or muscle precursor cells (Smythe et al., 2001; Partridge, 2002; Price et al., 2007) were used as donor cells in initial studies, bone marrow-derived cells (BMDCs) emerged as an attractive alternative for multiple reasons. The contribution of bone marrow cells to regenerating muscle was initially documented by Ferrari et al. (Ferrari et al., 1998) and, shortly thereafter, corroborated by a number of studies (Gussoni et al., 1999; Fukada et al., 2002; LaBarge and Blau, 2002). Additionally, bone marrow transplantation (BMT) may bypass some of the potential pitfalls of myoblast transplantation through convenient systemic delivery and avoidance of immune rejection (Partridge, 2002). As BMT is currently approved to treat a number of human diseases, including Fanconi anemia (Kohli-Kumar et al., 1994), immunological deficiencies (Gatti et al., 1968), metabolic diseases (Parkman et al., 1995) and osteogenesis imperfecta (Horwitz et al., 2001), investigations into the ability of BMDCs to contribute to regenerating muscle fibers have intensified.
Hematopoietic stem cells (HSCs) are the prototypical adult stem cell in that they are capable of multipotent differentiation into all blood and immune cell lineages, including T cells, B cells and myelomonocytes (Spangrude et al., 1988). HSCs can be efficiently isolated based on a combination of phenotypic markers yielding a Thy1.1loLin−Sca-1+ fraction of pure multilineage stem cells (Spangrude et al., 1988) that, even as single cells, can radioprotect a lethally irradiated mouse (Krause et al., 2001). Analogous cells have been identified in humans, though some of the specific markers involved differ; for example, HSCs from mice are CD34−, whereas those from humans are CD34+ (Baum et al., 1992). An alternative purification method involving the vital DNA-binding dye Hoechst 33342 yields bone-marrow side-population (SP) cells that, isolated on the basis of their ability to efflux the dye, are enriched for hematopoietic stem cells (HSCs) that are capable of radioprotection and hematopoietic lineage reconstitution even at low cell number (Goodell et al., 1996).
Though experiments involving either the intramuscular or intravenous delivery of BMDCs, including whole bone marrow (WBM) and bone marrow SP cells, have not resulted in therapeutically significant levels of engraftment, they have demonstrated that these cells home to muscle in addition to the bone marrow compartment (Ferrari et al., 1998; Gussoni et al., 1999; LaBarge and Blau, 2002; Wagers et al., 2002; Brazelton et al., 2003; Camargo et al., 2003; Corbel et al., 2003; Dreyfus et al., 2004; Rivier et al., 2004; Sherwood et al., 2004). Additionally, donor cells can be found incorporated into regenerating myofibers following injury to the muscle (Ferrari et al., 1998; Fukada et al., 2002; LaBarge and Blau, 2002; Brazelton et al., 2003). The connection between muscle repair and local proliferation of cells with limited myogenic potential has been strengthened by reports of increases in both bone marrow SP (Musarò et al., 2004) and muscle resident Sca-1+/CD45+ (Polesskaya et al., 2003) populations in response to chemical damage. In addition, transplantations of single HSCs yield progeny that contribute to blood and muscle (Corbel et al., 2003). However, the mechanism behind the incorporation of BMDCs into regenerating muscle remains unclear; there is considerable debate as to whether the engraftment of these cells is exclusively due to random, stochastic fusion of immune cells into regenerating fibers (Camargo et al., 2004) or, as initial studies suggested, whether certain populations of BMDC have the capacity to activate myogenic transcription programs and differentiate into muscle (Ferrari et al., 1998; LaBarge and Blau, 2002; Dreyfus et al., 2004).
To dissect the biological steps behind the incorporation of BMDCs into regenerating skeletal muscle, we lethally irradiated ROSA26 mice and intravenously transplanted either WBM or bone marrow SP cells isolated from GFP+ donor mice. Seven weeks after BMT, mice were injected intramuscularly with cardiotoxin (CTX). Five or 14 days after CTX damage, muscles were analyzed by fluorescence-activated cell sorting (FACS), immunocytochemistry and immunohistochemistry for donor cell presence and contribution to myofibers. The transplantation of GFP-labeled cells into mice with a marker for host tissue, β-galactosidase (β-gal), proved to be a simple yet effective way for distinguishing between donor and recipient contribution for individual myofibers. Analysis of muscles from mice transplanted with bone marrow SP cells revealed a population of donor-derived cells that had lost the hematopoietic marker CD45 but expressed the myogenic marker desmin. In addition, immunohistochemistry of both WBM and SP transplants showed newly formed myofibers composed only of donor-derived cells, as well as instances in which donor cells had fused into regenerating host fibers. Together, these results suggest that a subpopulation of adult bone marrow-derived stem cells have the capacity to undergo myogenic differentiation under regenerative conditions. These cells are enriched within the bone marrow SP fraction and are not myeloid precursors, as they are capable of undertaking a myogenic fate as mononuclear cells.
Results
Lethally irradiated female ROSA26 mice were transplanted with GFP+ WBM or GFP+ bone marrow SP cells and subjected to CTX damage 7 weeks later. Five and 14 days after CTX-induced muscle damage, recipient bone marrow and muscles were analyzed as schematically outlined in Fig. 1. Seven rounds of transplantations were performed with at least three mice per group.
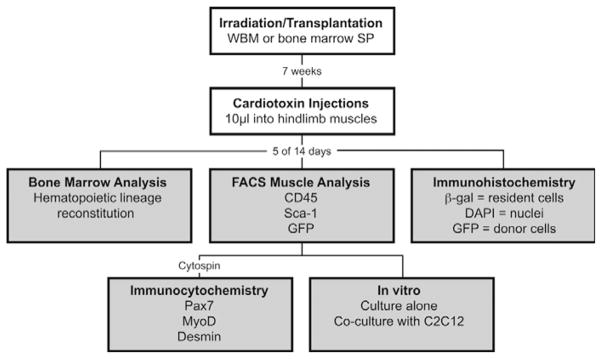
Fig. 1.
Experimental set-up and methods of analysis. ROSA26 mice were lethally irradiated and transplanted with either GFP+ WBM or bone marrow SP cells. Seven weeks after transplantation, recipient muscles were damaged with intramuscular cardiotoxin injections. Five and 14 days later, bone marrow and cardiotoxin-damaged muscles were harvested for various analyses (shaded boxes). Recipient bone marrow was analyzed for lineage reconstitution by transplanted cells via FACS. FACS was also used to analyze mononuclear muscle cells for donor cell contribution and for the markers CD45 and Sca-1. Cells from this muscle analysis were sorted based on GFP and CD45 expression, and transferred to slides via cytospin where they were stained for myogenic markers Desmin, MyoD and Pax7. Whole TA muscles were also frozen, sectioned for immunohistochemistry, stained for β-galactosidase and assayed for engraftment of donor-derived cells. Overall, at least seven rounds of irradiation/transplantation were carried out with at least three mice.
Lineage reconstitution of transplanted mice
It is known that radioprotection of lethally irradiated mice can be achieved through transplantations of WBM and bone marrow SP cells (Goodell et al., 1996), which are purified by virtue of their ability to exclude the vital dye Hoechst 33342 and comprise only a small fraction (0.01%–0.06%) of bone marrow (data not shown) (Goodell et al., 1996). To ensure that transplantation of WBM or bone marrow SP cells not only rescued the mice from irradiation but also resulted in equivalent reconstitution of the hematopoietic lineages, the bone marrow of transplanted mice was analyzed for hematopoietic lineage composition 7 weeks after lethal irradiation and transplantation. The percentage of lineage-positive cells was determined using FACS analysis with antibodies for B220 (B cells), CD3 (T lymphocytes), Gr-1 (mature granulocytes), Mac-1 (monocytes/macrophages) and Ter119 (erythrocytes) (Fig. 2). For each marker, the percent reconstitution in WBM-transplanted mice was normalized to 1, and the relative corresponding percentages for SP-transplanted mice were calculated (Fig. 2). Statistical analysis (t-test) of three independent experiments revealed that there were no significant differences in the percent reconstitution of any hematopoietic lineage in mice transplanted with SP cells compared to those that received a WBM transplant (Fig. 2). These results suggest that transplantation with either WBM or bone marrow SP cells yields equal reconstitution of the lineages examined.
Fig. 2.
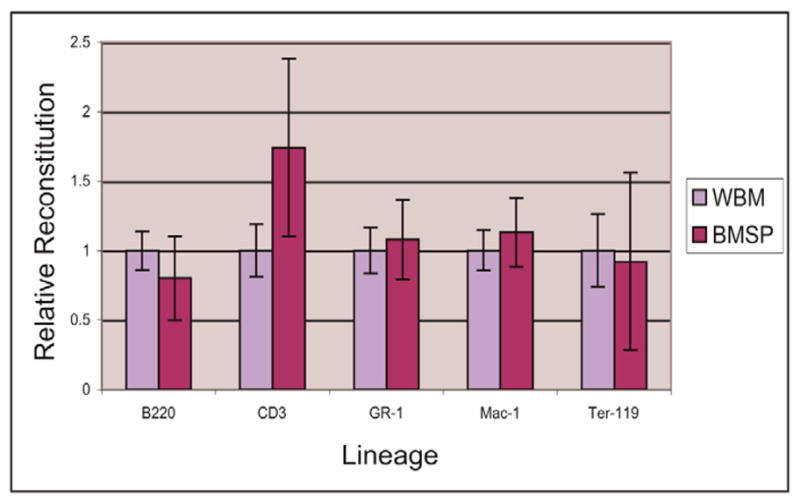
WBM and SP transplantations result in equivalent hematopoietic lineage reconstitution. Markers for B cell (B220), T lymphocyte (CD3), mature granulocyte (Gr-1), monocyte/macrophage (Mac-1) and erythroid (Ter-119) lineages were tested using a mouse lineage panel. For each marker, the percentage of lineage-positive cells as determined by FACS in WBM-transplanted mice was normalized to 1, and the corresponding percentage in bone marrow SP transplanted mice was adjusted accordingly. Transplantation of WBM and SP cells resulted in equivalent reconstitution of these lineages as no significant differences in the percent of lineage-positive cells for a given marker were seen between the two transplant types. A t-test was used for statistical analysis and error bars represent the s.d. from three independent experiments.
Muscles of SP-transplanted mice contain GFP+/CD45−/Sca+ cells
To determine whether BMDCs were capable of exhibiting myogenic transcription programs, GFP+ donor cells were examined for the pan-hematopoietic marker CD45 and stem cell antigen 1 (Sca-1), a hematopoietic stem cell marker (Spangrude et al., 1988), before transplantation. Antibody staining and FACS analysis of freshly isolated WBM revealed that 83.5% of cells were positive for CD45, whereas only 5.4% were Sca-1+ (data not shown). In agreement with previous studies (Goodell et al., 1996; Montanaro et al., 2004), isolated bone marrow SP cells were enriched for CD45 and Sca-1, as ~98% were positive for CD45 and 89.5–100% were positive for Sca-1 (data not shown).
Seven weeks after bone marrow transplantation, muscle regeneration was initiated through intramuscular injections of 10 μM CTX. Mononuclear muscle cells of recipient mice were analyzed by FACS for GFP and CD45 expression 5 (Fig. 3A) and 14 days (Fig. 3B) after CTX damage. At both time points, the muscles of mice transplanted with WBM and SP cells contained populations of GFP−/CD45+ and GFP+/CD45+ cells (Fig. 3A,B). GFP−/CD45+ cells are host non-myogenic, muscle-resident cells that proliferate in response to an acute injury such as CTX (Polesskaya et al., 2003), while the large populations of GFP+/CD45+ cells are due to the influx of donor-derived immune/inflammatory cells that migrate to the muscle after chemical damage. As expected, these populations decreased substantially over time from 5 to 14 days after CTX injection in both transplant types (Fig. 3A,B) as myofibers completed regeneration and the immune response waned.
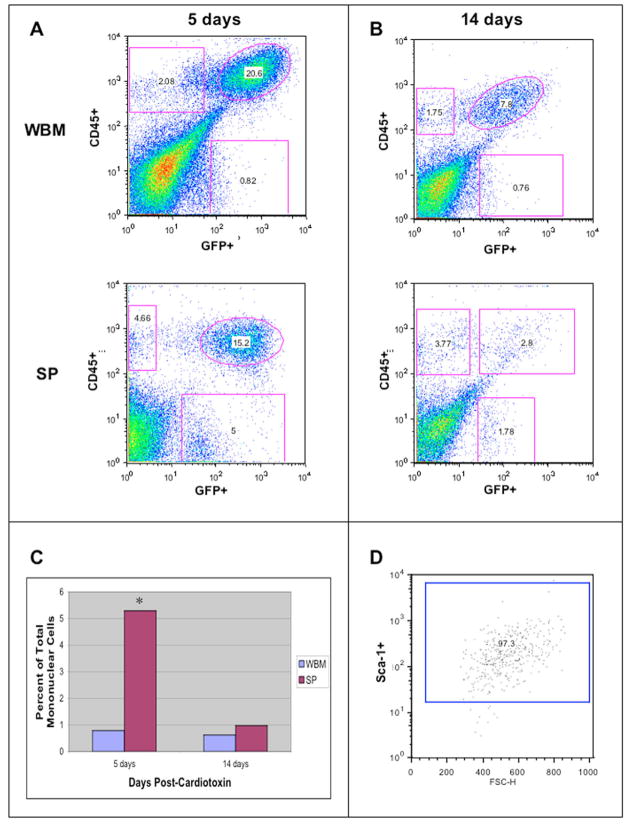
Fig. 3.
Regenerating muscles of SP-transplanted mice contain a population of GFP+/CD45−/Sca-1+ cells. (A) Analysis of muscle mononuclear cells 5 days post-CTX of both transplant types revealed a large population of infiltrating immune cells (upper right quadrant) as well as a smaller population of muscle-resident CD45+ cells (upper left quadrant). SP-transplanted mice also contained a population of donor-derived cells that have lost CD45 (lower right quadrant). (B) 14 days after CTX damage, the percentages of GFP+/CD45− and GFP−/CD45+ populations (in both transplant types), as well as GFP+CD45− cells from the SP-transplanted mice, were reduced. In WBM transplants, the percentage of GFP+/CD45− cells was stable. (C) Comparing the abundance of this population of donor-derived CD45− cells across the two transplant types, SP-transplanted mice contained significantly more GFP+/CD45− cells than did their WBM-transplanted counterparts 5 days after damage (P=0.1, Wilcoxon ranksum test, n=4). We believe that these donor-derived cells are not an inflammatory population because the cells do not express the hematopoietic marker CD45. Analysis carried out 14 days post-CTX did not show any significant difference between the two transplant types. (D) GFP+/CD45− cells isolated from SP-transplanted mice sacrificed 5 days post-CTX were analyzed for Sca-1 expression and were 97.3% positive for this marker.
FACS analysis also revealed a distinct population of GFP+/CD45− cells found in significantly higher percentages in the muscle of mice transplanted with bone marrow SP cells compared with those transplanted with WBM. Analysis of four independent experiments 5 days post-CTX revealed that this population comprises 0.78±0.48% of mononuclear cells in the muscles of WBM-transplanted mice versus 5.29±3.46% in SP-transplanted mice (P=0.1, Wilcoxon Rank Sum Test, Fig. 3C). Again, this population was reduced two weeks after damage when compared with 5 days after damage; percentages dropped to 0.62±0.28% for WBM-transplants and 0.97±1.1% for SP-transplants. These GFP+ progeny of donor bone marrow SP cells no longer express the hematopoietic marker CD45 and, as FACS revealed, are 97% positive for Sca-1 (Fig. 3D).
GFP+/CD45−/Sca-1+ cells from SP-transplanted mice express muscle markers
The presence of bone marrow-derived GFP+/CD45− cells in the regenerating muscle of mice transplanted with bone marrow SP cells prompted us to investigate whether progeny of donor cells expressed myogenic lineage markers. FACS sorted cells were transferred to slides via cytospin and stained for the muscle-specific marker desmin, early muscle marker MyoD and satellite cell marker Pax7 (Table 1, average of three independent experiments). No freshly isolated cells from the GFP+/CD45+ or GFP+/CD45− populations of WBM and bone marrow SP transplants stained positive for Pax7 (Table 1), and all of these four populations were less than 4% positive for MyoD (Table 1). In WBM-transplanted mice, the vast majority of donor-derived cells did not express desmin: 2.5% of GFP+/CD45− and 6.4% GFP+/CD45+ were desmin-positive (Table 1). Whereas only a small population (5.1%) of GFP+/CD45+ isolated from the SP-transplanted mice were desmin positive, 42.5% of the GFP+/CD45− cells isolated from the muscles of the same animals expressed desmin (Table 1).
Table 1.
Muscle marker expression of sorted mononuclear muscle cells isolated 5 days post-CTX from WBM- and SP-transplanted mice
| Sorted cell type | Marker | WBM-transplanted | SP-transplanted |
|---|---|---|---|
| GFP+/CD45+ | Pax7 | 0.0% | 0.0% |
| MyoD | 3.7% | 2.2% | |
| Desmin | 6.4% | 5.1% | |
| GFP+/CD45− | Pax7 | 0.0% | 0.0% |
| MyoD | 3.9% | 3.7% | |
| Desmin | 2.5% | 42.5%* |
Percentages represent the average of three independent experiments.
P<0.01 relative to the other transplant model and sorted cell type.
Isolated GFP+/CD45− cells were cultured alone in proliferation medium to assess gross myogenic morphology. Analysis using phase-contrast (Fig. 4A) and fluorescent (Fig. 4B) microscopy demonstrated that donor-derived cells adhered to culture dishes and elongated in a manner characteristic of myoblasts. In addition, owing to a low number of cells obtained, a co-culture scenario was used to better estimate myogenic marker expression; sorted GFP+/CD45− cells were cultured in a 1:1 ratio with C2C12 myoblasts. Staining with MyoD (Fig. 4C) and desmin (Fig. 4D) confirmed that BMDCs that have lost CD45 can express muscle markers as mononuclear cells when in a proliferative myogenic environment. Importantly, GFP+/CD45−/Sca-1+ bone marrow cells from non-transplanted mice did not express Pax7, MyoD or desmin when stained either directly after isolation, or after 7 days under myogenic proliferation or differentiation conditions (data not shown). Along with the FACS data presented above, these findings indicate that the progeny of bone marrow SP cells home to muscle not only as inflammatory cells, but also as cells capable of adopting myogenic markers.
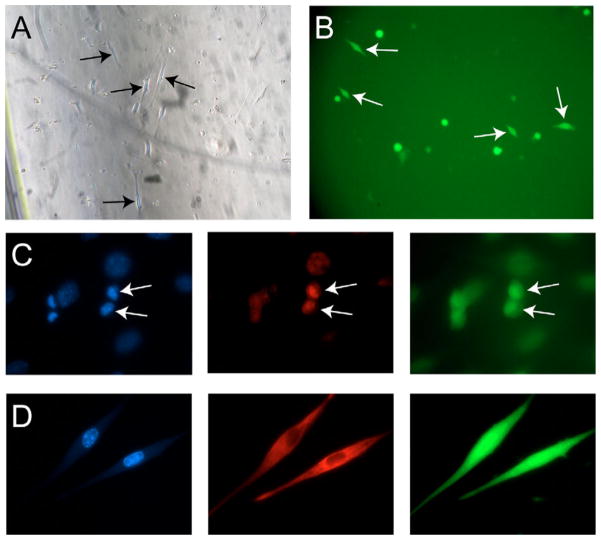
Fig. 4.
GFP+/CD45− cells from regenerating muscle appear myogenic in vitro. (A,B) Following FACS sorting, GFP+/CD45− cells from SP-transplanted mice analyzed 5 days after CTX damage were cultured alone in proliferation medium. (A) Observation by phase contrast microscopy showed that these cells displayed a myogenic morphology (arrows), and (B) visualization by fluorescence microscopy revealed that cells with this morphology were, indeed, donor-derived, GFP+ (arrows). (C,D) Expression of myogenic markers by isolated GFP+/CD45− cells from multiple time points co-cultured in 1:1 ratio with C2C12 myoblasts. (C) MyoD staining (red) performed on GFP+/CD45− cells from a WBM-transplanted mouse 5 days post-CTX injury revealed that these cells can express this muscle marker when cultured in a myogenic environment (arrows). (D) Desmin staining (red) showed that even 7 weeks after CTX injection, the regenerated muscle still contains mononuclear cells capable of adopting myogenic markers. DAPI stained in blue.
GFP+/β-gal− fibers suggest de novo myogenesis from bone marrow cells
Recent reports claim that transplanted BMDCs incorporate into regenerating muscle tissue only by fusing with existing myofibers, not through de novo myogenesis (Wagers and Conboy, 2005). However, as our experimental design differed from previous studies, we decided to address this issue further. Our model, which used both a donor tracking marker and host marker, allowed for easy differentiation between donor-derived and recipient cells when assaying for engraftment. Muscles of ROSA26 mice transplanted with GFP+ WBM and bone marrow SP cells were analyzed via immunohistochemistry; 10 μm histological sections of CTX-damaged (as well as uninjured, contralateral) TA muscles were taken from recipients sacrificed 5 and 14 days after CTX injection. At both time points, undamaged TAs contained no examples of GFP+ muscle fibers, though rare GFP+ mononuclear cells were observed (data not shown).
At 5 days post-CTX, immunohistochemistry showed considerable inflammatory infiltration and substantial necrosis in the injured muscles of WBM- and SP-transplanted mice. In both cases, the vast majority of donor contribution was in the form of interstitial mononuclear GFP+ cells, similar to those found in uninjured muscles although at much higher levels. As expected, these donor-derived cells were found in a greater density close to the site of CTX injection. Laminin staining revealed that these cells lay outside the basal lamina (not shown) and the fact that donor-derived mononuclear cells were Pax7− (Table 1), confirmed that they were not quiescent satellite cells (Asakura et al., 2002). Laminin and Pax7 staining did reveal endogenous GFP− satellite cells (supplementary material, Fig. S1). However, rare instances of GFP+ muscle fibers were also seen at this time point: a mean of 2.67 GFP+ fibers per WBM-transplanted mouse and 6.67 GFP+ fibers per SP-transplanted mouse (Table 2). GFP+ fibers fell into one of two categories: ‘yellow’ fibers that co-expressed β-gal (GFP+/β-gal+), indicating donor cell fusion into existing or newly formed myofibers (Fig. 5A, arrowhead); and ‘green’ fibers that expressed only GFP (GFP+/β-gal−), indicating the fusion of donor cells with each other in the same manner as resident myoblasts (Fig. 5A, arrow). Five days after CTX, approximately half of the observed GFP+ myofibers were positive solely for GFP and not for β-gal (Table 2).
Table 2.
Average number of GFP+ and β-gal− muscle fibers per damaged mouse TA
| Mice | Fiber type | Mean number of fibers (range) |
|---|---|---|
| WBM 5 d | Total GFP+ | 2 (0–5) |
| GFP+/β-gal+ | 1 | |
| GFP+/β-gal− | 1 | |
| GFP−/β-gal− | 1.67 (0–4) | |
| SP 5 d | Total GFP+ | 6.67 (2–15) |
| GFP+/β-gal+ | 4 | |
| GFP+/β-gal− | 2.67 | |
| GFP−/β-gal− | 1.33 (0–3) | |
| WBM 14 d | Total GFP+ | 4 (3–5) |
| GFP+/β-gal+ | 2.5 | |
| GFP+/β-gal− | 1.5 | |
| GFP−/β-gal− | 2.5 (1–4) | |
| SP 14 d | Total GFP+ | 9.5 (5–14) |
| GFP+/β-gal+ | 8 | |
| GFP+/β-gal− | 1.5 | |
| GFP−/β-gal− | 1.5 (0–3) |
GFP+/β-gal+ fibers co-expressed GFP and β-gal. GFP+/β-gal− fibers expressed only GFP and showed no sign of host-cell contribution. GFP+ fibers were followed from section to section for as long as the same fiber could be definitively identified. If no β-gal co-expression was observed on any section, fibers were scored as ‘GFP+/β-gal−’. Otherwise, fibers were deemed ‘GFP+/β-gal+’. n≥2. These results were not significant.
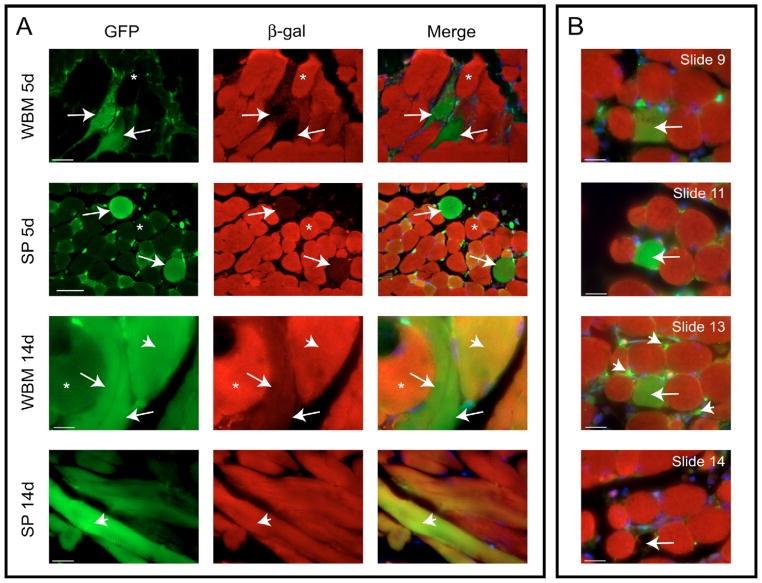
Fig. 5.
Bone marrow-derived cells can form de novo myofibers. (A) Examples of GFP+ myofibers in WBM- and SP-transplanted mice at 5 and 14 days following CTX are shown. Three different myofiber types were visible in each condition: ‘red’ GFP−/β-Gal+ (asterisk), ‘yellow’ GFP+/β-Gal+ (arrowhead) and ‘green’ GFP+/β-Gal− (arrow). (B) Serial sectioning was used to track certain ‘green’ fibers throughout the length of the muscle. This particular fiber (found in an SP-transplanted mouse 5 days post-CTX) stayed ‘green’ for its entire length (>290 μm) and contained at least four distinct nuclei. As the bottom panel illustrates, in the following section (slide 14), the surrounding ‘red’ fibers were observed, but the ‘green’ fiber in question was gone (arrow). The last section in which this ‘green’ fiber was visible (slide 13) showed many GFP+ mononuclear cells in the immediate vicinity of the ‘green’ fiber (arrowhead). (A) Scale bars: 65 μm in the first and second rows, 20 μm in the third row and 31 μm in the fourth row. (B) Scale bars, 20 μm.
Two weeks post-CTX, when there was reduced necrosis and greater total numbers of regenerated myofibers, an increase in GFP+ fibers in both transplant conditions was observed: four fibers per WBM-transplanted mouse, 9.5 fibers per SP transplanted mouse (Table 2). Between 5 and 14 days, the frequency of ‘green’ fibers increased in WBM-transplanted mice and decreased in SP-transplanted mice (Table 2).
While the overall percentage of GFP+ fibers in the transplanted mice was quite low, the presence of entirely ‘green’ fibers implied that progeny of donor bone marrow cells fused with each other to form new myofibers. Serial sectioning was performed to determine the length of myofibers composed only of donor cells and to establish whether endogenous myogenic cells contributed at all to the formation of these GFP+ fibers. An example of the same GFP+/β-gal− fiber traced along its entire length is illustrated in Fig. 5B. Additionally, the following section, slide 14, showed the surrounding GFP−/β-gal+ myofibers, and the area where the GFP+ fiber had been is marked with an arrow (Fig. 5B). GFP+ mononuclear cells were visible in the immediate vicinity of this fiber in the last section in which it was identified (Fig. 5B, arrowheads). It is possible that these cells are of the same type that fused with each other to form this fiber and, had the muscle been analyzed at a later date, may have further contributed to the growth of this ‘green’ fiber. Moreover, intermediate sections revealed no co-expression of β-gal with GFP for this particular fiber (Fig. 5B), implying that not only are BMDCs capable of fusing with each other in a manner similar to myoblasts, but that their fusion can result in a syncytial muscle fiber independent of host cell contribution.
It is necessary to note that following careful examination of histological sections used for quantitation of GFP+ fibers, isolated examples of GFP− myofibers that did not stain positive for the host marker β-gal were also observed in some of the injured muscles. The appearance of these fibers, which were observed at an overall lower frequency than GFP+ fibers (Table 2), is probably due to silencing of transcription from the lacZ gene in individual host myofibers, resulting in a lack of β-gal expression. In conclusion, these results suggest that, after injury in an environment conducive to myofiber repair and formation, a small percentage of transplanted BMDCs migrate to muscle and aid in myofiber regeneration, either by dividing and fusing into regenerating host fibers or, as the presence of myofibers composed only of donor-derived cells intimates, through de novo myogenesis.
Discussion
This study used a comparative approach involving both bone marrow SP cells and WBM, along with a dual-marker strategy for distinguishing between donor and recipient cells that has not yet been tested in conjunction with muscle damage. Our results suggest that, following CTX damage, WBM- and bone marrow SP-derived cells yield progeny capable of engraftment into regenerating muscle via myogenic differentiation. Past studies attributed engraftment of BMDCs into regenerating skeletal muscle to random stochastic fusion of infiltrating immune cells (Wagers and Conboy, 2005). Although we do not dismiss the idea of cell fusion in this context, our data strongly support the hypothesis that there is indeed a biological mechanism behind the incorporation of BMDCs into regenerating muscle (LaBarge and Blau, 2002; Brazelton et al., 2003) and that the cells responsible are enriched within a subpopulation of HSCs (Doyonnas et al., 2004): the SP fraction. Specifically, our findings that donor-derived cells (1) showed a loss of CD45, (2) stained positive for a muscle-specific marker and (3) displayed a morphology suggesting non-hematopoietic differentiation, support the notion that transplanted BMDCs have the potential to differentiate into myogenic cells in a regenerative environment.
It has been reported that CD45− donor-derived cells are found in muscle after BMT (Issarachai et al., 2002). The presence of muscle markers on these cells was not explored, though the fact that they were not capable of radioprotection supports our claim that they are myogenic (Issarachai et al., 2002). Adoption of myogenic markers in a non-myogenic population that had lost CD45 has also been documented (Polesskaya et al., 2003); in times of stress, regenerative signals in the muscle recruit resident CD45+/Sca-1+ muscle cells to progress down a myogenic lineage through Wnt signaling and subsequent Pax7 expression (Polesskaya et al., 2003; Seale et al., 2004). Furthermore, 2 days after CTX injury in another study, Pax7+ cells were observed in the muscle of mice transplanted with bone marrow SP cells (Musarò et al., 2004). In these injury models, myogenic activation was reportedly restricted to Sca-1+ cells (CD45+/Sca-1+ or CD45−/Sca-1+) (Polesskaya et al., 2003; Musarò et al., 2004). Similarly, in the present study, transplantations of cells enriched in Sca-1 (SP cells) yielded a significantly greater population of donor-derived cells that no longer expressed CD45 and seemed to have activated a myogenic transcription program (Fig. 3, Table 1). This is unsurprising considering that, unlike lineage-committed main population cells, SP cells are enriched in progenitors. However, immunostaining of GFP+/CD45− cells isolated from damaged muscle did not reveal any cells that express Pax7 in our study. Therefore a different Pax7-independent mechanism might be involved in the myogenic differentiation of these BMDCs in response to injury. Indeed, although Pax7 is necessary for myogenic commitment of satellite cell progeny, it is not required for prenatal muscle formation (Seale et al., 2000).
Regardless, the progeny of donor GFP+ cells no longer express CD45 and are positive for Sca-1, clearly distinguishing them from the differentiated myeloid cells purported in multiple studies to be the sole hematopoietic contributors to regenerating muscle (Camargo et al., 2003; Sherwood et al., 2004). A potential explanation for the discrepancy between the results of those studies and ours lays in the fact that bone marrow SP cells are a heterogeneous population, even when further isolated by Sca-1 and CD45 expression. These cells seem to contain progenitors for both blood and muscle lineages (Corbel et al., 2003), although it appears that marrow precursor cells far outnumber those for muscle, as single HSC transplants yield only blood cells in regenerating muscle (Camargo et al., 2003), and a greater percentage of GFP+/CD45+ compared with GFP+/CD45− cells were identified in our recipients (Fig. 3). Although it is usually enough to radioprotect and reconstitute all blood lineages in mice, one SP cell may not reflect the full potential of the entire side population. In contrast to the present study in which 300–500 SP cells were transplanted, isolation of individual SP cells (Camargo et al., 2003), or single c-kit+Thy1.1loLin−Sca-1+ HSCs (Sherwood et al., 2004) most probably overlooks (either through sheer probability or the particular markers used) bone marrow cells that are able to differentiate into muscle.
Musarò et al. put forward a model describing a step-wise process in which the migration of undifferentiated bone marrow subpopulations to damaged muscle and their myogenic differentiation are accelerated by a local insulin-like growth factor (mIGF-1) (Musarò et al., 2004). Although no mIGF-1 was used in this study, our results corroborate the hypothesized multi-step model. We noted the loss of CD45 in BMDCs that had migrated to muscle; this step is described as a ‘muscle “stem” cell’ (presumably originating in the bone marrow) becoming an ‘early myogenic progenitor’ (Musarò et al., 2004). Another of our observations, the adoption of myogenic markers in bone marrow-derived CD45−/Sca-1+ cells, is hypothesized to be the formation of a ‘committed myogenic progenitor’ which eventually fully differentiates to become a myoblast (Musarò et al., 2004).
At the time of analysis, 5 days post-CTX, donor-derived CD45− cells in our study appeared to be committed to the muscle lineage. This population was found at significantly lower percentages in the muscles of bone marrow SP-transplanted mice 14 days compared with 5 days post-CTX. As the proliferation of myogenic cells in response to CTX peaks between 3 and 5 days after injury (Polesskaya et al., 2003; Yan et al., 2003), it is reasonable to theorize that donor mononuclear cells present 5 days post-CTX ceased proliferating and, instead, increasingly fused into regenerating fibers, thereby reducing their relative abundance when viewed by FACS 2 weeks after injury. This idea is supported by our immunohistochemical data, which illustrated an increase in donor cell fusion into existing myofibers and decrease in GFP+ mononuclear cells 14 days (compared with 5 days) after damage (Table 2, data not shown), and our immunocytochemical results, which indicated that these were later stage muscle cells [Pax7−/MyoD−/Desmin+ (Table 1)]. Overall, short-term analysis (5 days) revealed more myogenic mononuclear cells in the muscles of SP-transplanted mice compared with their WBM-transplanted counterparts. However, this did not directly translate into a higher rate of donor-derived myofibers in these animals – as demonstrated by analysis after 14 days. The roughly equivalent number of GFP+/β-gal− (de novo) myofibers found in whole bone marrow-transplanted and bone marrow SP-transplanted mice is largely a testament to the rarity of these fibers – although the fact that they are found at the same frequency in SP-transplanted mice suggests that the cells responsible for these occurrences are found within the SP fraction. Additionally, recipient mice were not dystrophic; their endogenous regenerative capability was uncompromised, and host mononuclear muscle cells could readily fuse into the de novo fibers creating GFP+/β-gal+ fibers.
The presence of GFP−/β-gal− myofibers in regenerating ROSA26 muscle precludes us from stating definitively that the observed GFP+/β-gal− fibers were formed from donor cells alone. It is possible that GFP+/β-gal− fibers arise from the fusion of donor cells into myofibers that have been damaged and also happen to have silenced β-gal expression. However, several pieces of evidence have led us to believe that some, if not all, of the GFP+/β-gal− fibers we saw were legitimate de novo myofibers of donor origin. Not only do GFP+ fibers outnumber GFP−/β-gal− fibers in WBM- and bone marrow SP-transplanted mice both 5 and 14 days after CTX treatment, but the average number of GFP−/β-gal− fibers remains relatively constant across all transplant types and time points, while the frequency of GFP+ fibers varies over these parameters (Table 2). Most importantly, the muscles of some mice contained GFP+/β-gal− fibers but no examples of GFP−/β-gal− myofibers, suggesting that the ‘green’ fibers observed in these mice are the result of de novo myogenesis – although these fibers may contain very rare host nuclei resulting in the production of β-gal at levels that are undetectable when diluted by the cytoplasm of the myofiber. The myogenic potential of donor-derived cells was further supported by the results obtained through in vitro experiments. When co-cultured with myoblasts, GFP+/CD45− cells isolated from muscles of recipient mice displayed a myogenic morphology and expressed muscle specific markers MyoD and desmin (Fig. 4).
Although it does not appear that BMDCs play a significant role in muscle repair under non-transplant conditions in injured or dystrophic mice (Wakeford et al., 1991; Quinlan et al., 1995; Wagers and Conboy, 2005), our data suggest that the bone marrow side population is enriched in progenitor cells capable of differentiating down a myogenic lineage and contributing to muscle regeneration through a mechanism other than inflammatory cell fusion. If this subpopulation can be isolated and expanded, transplantation of these BMDCs may be optimized for future cell-based therapy experiments.
Materials and Methods
Animals
C57BL/6-Tg(ACTB-EGFP)1Osb/J (GFP+) (Okabe et al., 1997) and B6.129S7-Gt(ROSA)26Sor/J (ROSA26) (Friedrich and Soriano, 1991) mice were used. GFP+ heterozygotes, which express eGFP under the control of a ubiquitous β-actin promoter, provided donor cells. ROSA26 female mice, which ubiquitously express β-galactosidase, were used as recipients. Animals were housed in Animal Resources Children’s Hospital (ARCH) and all procedures were approved by ARCH protocols.
Bone marrow cell isolation
GFP+ mice between 6 and 8 weeks were euthanized and whole bone marrow (WBM) was harvested. Briefly, WBM was flushed from tibias and femurs using a 25G needle with 10 ml of bone marrow medium [Dulbecco’s modified Eagle’s Medium (DMEM), 2% fetal bovine serum (FBS, Hyclone), 2% penicillin-streptomycin (Sigma), 1 mM HEPES buffer, filtered]. Red blood cells (RBCs) were removed using RBC lysis solution (Gentra Systems) and a single-cell suspension was achieved. Bone marrow SP cells were prepared as previously described (Montanaro et al., 2004). Briefly, 106 cells/ml suspension of WBM was incubated for 90 minutes with Hoechst 33342 (Sigma) at a concentration of 5 μg/ml. An aliquot was incubated with 50 μM verapamil (Sigma) as a negative control to set the SP gate prior to sorting. Propidium iodide was added to samples at a concentration of 2 μg/ml to exclude dead cells. SP cells were detected in a FACSVantage flow cytometer (Becton Dickinson) via excitation with a dual laser and collected in phosphate-buffered saline (PBS). Flow cytometry data was analyzed using Flowjo software version 6.3.3 (Treestar).
Bone marrow transplantation and muscle damage
Recipient female ROSA26 mice (5–7 weeks) were irradiated with two doses of 549 rads, enough for a lethal dose (Frasca et al., 2000), given ~3 hours apart (Uchida et al., 1994) using a Gammacel irradiator (Atomic Energy of Canada Ltd) prior to transplantation with either WBM or SP cells. 10×106 WBM cells or 300–500 SP cells were isolated from GFP+ mice, resuspended in 150 μl of sterile PBS and injected into the tail vein of restrained mice using an insulin syringe. This relatively small number of SP cells was shown previously to be sufficient for reconstitution of the bone marrow (Goodell et al., 1996). Transplanted mice were housed individually with autoclaved food and water bottles supplemented with antibiotics (Sulfatrim/Bactrim). Seven weeks after BMT, hind limb muscles [tibialis anterior (TA), quadriceps, gastrocnemius] were injected with 12–20 μl of 10 μM CTX, Najanigricollis (Calbiochem Cat#217504), while mice were anesthetized via isoflurane inhalation. The contralateral TA was left uninjured as a control.
Muscle FACS analysis and sorting
Hind limb muscles were isolated from transplanted ROSA26 mice 5 and 14 days post-CTX damage. Muscles were cleaned and minced under a tissue culture hood, and digested with 3.5 ml/g of tissue of both 2.4 U/ml neutral protease/dispase and 10 mg/ml collagenase IV (Worthington Biochemical) for 45 minutes at 37°C. Digests were triturated with a 5 ml disposable pipette every 15 minutes. Following digestion, cells were passed through 70 μm and 40 μm filters, resuspended in muscle medium [F-10, 20% FBS (Hyclone), 1% penicillin-streptomycin (Sigma)], and RBCs were removed with RBC lysis solution. Cells were resuspended in PBS 0.5% bovine serum albumin [PBS/BSA; PBS 2.5g BSA (Sigma), filtered] at a final concentration of 106 cells/ml for antibody staining (20 minutes on ice) with the following BD PharMingen antibodies: PE anti-mouse CD45 (2.5 μl/million), PE rat IgG2aκ (5 μl/million), Biotin anti-mouse Ly-6A/E (Sca-1) (1 μl/million), Streptavidin-APC (2.5 μl/million) and Biotinylated rat IgG2aκ (2 μl/million). IgG isotypes were used as negative controls.
Bone marrow lineage analysis
Bone marrow of recipient mice was examined for hematopoietic lineage reconstitution following transplantation and CTX damage. A single cell suspension of bone marrow was prepared as described above. Prior to FACS analysis, cells were incubated with either Biotinylated-CD3, -Mac-1, -B220, -Gr-1 or -Ter-119 (2 μl/million, mouse lineage panel) (BD PharMingen Cat#559971) for 20 minutes on ice. Cells were washed with ice-cold PBS/BSA and incubated for an additional 20 minutes on ice with Streptavidin PE (5 μl/million) (BD PharMingen).
Immunohistochemistry
CTX damaged and uninjured TA muscles were harvested from ROSA26 recipients and fixed overnight in 4% paraformaldehyde (PFA) followed by successive overnight soakings in 5% and 20% sucrose in PBS – all at 4°C on a rocking platform. Tissues were then frozen in liquid nitrogen-cooled isopentane. 10 μm sections were cut using a cryostat (Microm HM505E) and placed on Superfrost Plus slides (VWR). Sections were fixed at room temperature in 4% PFA in water for 5 minutes then transferred to ice cold PBS for 20 minutes (or overnight in PBS at 4°C) before blocking in PBS 10% FBS + 0.1% Triton X-100 for 1 hour at room temperature. Sections were stained overnight at 4°C with one of the following primary antibodies diluted in blocking buffer: rabbit anti-mouse β-galactosidase 616 1:1000 (AbCam Cat#ab616-1) and rabbit anti-laminin 1:2000 (Sigma Cat#L9393). After three 10-minute room temperature washes in PBS + 0.1% Triton X-100, sections were stained with goat anti-rabbit Rhodamine (TRITC) 1:50 (Jackson ImmunoResearch Cat#111-025-003) diluted in blocking buffer. Slides were washed three more times as described above and mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear counterstain.
Immunostaining
Freshly sorted GFP+/CD45+ and GFP+/CD45− cells were transferred onto Colorfrost slides (VWR) using a cytospin centrifugation (Thermo Electron) for 3 minutes and fixed in 4% PFA at 4°C for 20 minutes, after which slides were kept in PBS at 4°C until cells were stained with muscle markers. Just prior to staining, cells were permeabilized in PBS + 0.5% Triton X-100 for 3 minutes at room temperature and blocked in PBS 10% horse serum (Hyclone) + 0.5% Triton X-100 on a rocker for 1 hour at room temperature. Slides were then incubated overnight at 4°C with the following antibodies: monoclonal mouse anti-human desmin clone D33 (1:100) (DakoCytomation Cat#M0760), mouse anti-chicken Pax7 (1:100, Developmental Studies Hybridoma Bank) or purified mouse monoclonal MyoD (1:20) (BD PharMingen Cat#554130). Slides were washed with PBS + 0.1% Triton X-100 three times for 10 minutes at room temperature and incubated for 1 hour at room temperature with AlexaFluor 405 goat anti-mouse (Invitrogen Cat# A31553) secondary antibody diluted 1:200 in blocking medium. Slides were washed three more times as described above and mounted in Vectashield.
Culture of muscle cells isolated from transplanted mice
Sorted GFP+/CD45− cells were counted and resuspended in proliferation medium [DMEM, 20% FBS (Atlanta Biological), 1% L-glutamine (Sigma), 1% penicillin-streptomycin (Sigma)]. Cells were grown in a 96-well, human fibronectin-coated plate (Becton Dickenson) supplemented with 10ng/μl basic fibroblast growth factor (bFGF) or plated in plastic chamber slides (without bFGF) with C2C12 myoblasts in a 1:1 ratio. Proliferation medium was changed every other day after a period of 6 days during which cells had attached to the plate surface. Pictures of cells cultured alone were obtained after 7 days in culture. After 9 days, co-cultured cells were fixed in 4% PFA for 20 minutes at room temperature and premeabilized by washing with PBS + 0.5% Triton X-100 for 5 minutes at room temperature. Slides were blocked in PBS 10% horse serum (Hyclone) + 0.5% Triton X-100 on a rocker for 1 hour at room temperature. Primary antibody staining with either mouse anti-human desmin clone D33 (1:100) (DakoCytomation Cat#M0760) or rabbit polyclonal anti-MyoD (1:50) (Santa Cruz Cat#sc-304) was carried out overnight at 4°C. The following day, slides were washed with PBS + 0.1% Triton X-100 three times for 10 minutes at room temperature and incubated for 90 minutes at room temperature with either donkey anti-mouse Texas Red (Jackson ImmunoResearch Cat#715-075-150) or donkey anti-rabbit Texas Red (Jackson ImmunoResearch Cat#711-075-152) secondary antibody diluted 1:100 in blocking medium. Slides were washed three more times as described above and mounted in Vectashield with DAPI for nuclear counterstain.
Microscopy
Immunofluorescence and immunocytochemistry images were acquired on a Nikon Eclipse E1000 fluorescent microscope using an Orca camera (Hamamatsu) with Openlab software (ImproVision). Contrast adjustment was performed using Adobe Photoshop (Adobe Systems).
Supplementary Material
Acknowledgments
The authors thank Alan Flint for technical assistance with flow cytometry in a core facility supported by a DDRC, NIH grant HD018655 and Dr Juan Carlos Casar for helpful suggestions during the preparation of the manuscript. The Pax7 antibody (developed by Atsushi Kawakami) was obtained from the Development Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development, and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. The authors have no conflicting financial interests. This work was supported by a grant from the Muscular Dystrophy Association and the generous contribution of the Bernard F. and Alva B. Gimbel Foundation. E.G. is supported by NIH 5R01NS047727 and L.M.K. is an investigator with the Howard Hughes Medical Institute.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/121/9/1426/DC1
References
- Asakura A, Seale S, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TR, Nystrom M, Blau HM. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev Biol. 2003;262:64–74. doi: 10.1016/s0012-1606(03)00357-9. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9:1520–1527. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Chambers SM, Goodell MA. Stem cell plasticity: from transdifferentiation to macrophage fusion. Cell. 2004;37:55–65. doi: 10.1111/j.1365-2184.2004.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel SY, Lee A, Yi L, Duenas J, Brazelton TR, Blau HM, Rossi FM. Contribution of hematopoietic stem cells to skeletal muscle. Nat Med. 2003;9:1528–1532. doi: 10.1038/nm959. [DOI] [PubMed] [Google Scholar]
- Doyonnas R, LaBarge MA, Sacco A, Charlton C, Blau HM. Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors. Proc Natl Acad Sci USA. 2004;101:13507–13512. doi: 10.1073/pnas.0405361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus PA, Chretien F, Chazaud B, Kirova Y, Caramelle P, Garcia L, Butler-Browne G, Gherardi RK. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am J Pathol. 2004;164:773–779. doi: 10.1016/S0002-9440(10)63165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Cussella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Frasca D, Guidi F, Arbitrio M, Pioli C, Poccia F, Cicconi R, Doria G. Hematopoietic reconstitution after lethal irradiation and bone marrow transplantation: effects of different hematopoietic cytokines on the recovery of thymus, spleen and blood cells. Bone Marrow Transplant. 2000;25:427–433. doi: 10.1038/sj.bmt.1702169. [DOI] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Fukada S, Miyagoe-Suzuki Y, Tsukihara H, Yuasa K, Higuchi S, Ono S, Tsujikawa K, Takeda S, Yamamoto H. Muscle regeneration by reconstitution with bone marrow or fetal liver cells from green fluorescent protein-gene transgenic mice. J Cell Sci. 2002;115:1285–1293. doi: 10.1242/jcs.115.6.1285. [DOI] [PubMed] [Google Scholar]
- Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Soneonka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- Issarachai S, Priestley GV, Nakamoto B, Papayannopoulou T. Bone marrow-derived CD45+ and CD45− cells reside in skeletal muscle. Blood Cells Mol Dis. 2002;29:69–72. doi: 10.1006/bcmd.2002.0541. [DOI] [PubMed] [Google Scholar]
- Karpati G, Pouliot Y, Zubrzycka-Gaarn E, Carpenter S, Ray PN, Worton RG, Holland P. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989;135:27–32. [PMC free article] [PubMed] [Google Scholar]
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Kohli-Kumar M, Morris C, DeLaat C, Sambrano J, Masterson M, Mueller R, Shahidi NT, Yanik G, Desantes K, Friedman DJ, et al. Bone marrow transplantation in Fanconi anemia using matched sibling donors. Blood. 1994;84:2050–2054. [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- Law PK, Goodwin TG, Wang MG. Normal myoblast injections provide genetic treatment for murine dystrophy. Muscle Nerve. 1988;12:525–533. doi: 10.1002/mus.880110602. [DOI] [PubMed] [Google Scholar]
- Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res. 2004;298:144–154. doi: 10.1016/j.yexcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Musarò A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, Ottolenghi S, Cossu G, Bernardi G, Battistini L, et al. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci USA. 2004;101:1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Parkman R, Crooks G, Kohn DB, Lenarsky C, Weinberg K. Bone marrow transplantation for metabolic diseases. Cancer Treat Res. 1995;76:87–96. doi: 10.1007/978-1-4615-2013-9_4. [DOI] [PubMed] [Google Scholar]
- Partridge TA. Myoblast transplantation. Neuromuscul Disord. 2002;12(Suppl 1):S3–S6. doi: 10.1016/s0960-8966(02)00076-7. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Price FD, Kuroda K, Rudnicki MA. Stem cell based therapies to treat muscular dystrophy. Biochim Biophys Acta. 2007;1772:272–283. doi: 10.1016/j.bbadis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Quinlan JG, Lyden SP, Cambier DM, Johnson SR, Michaels SE, Denman DL. Radiation inhibition of mdx mouse muscle regeneration: dose and age factors. Muscle Nerve. 1995;18:201–206. doi: 10.1002/mus.880180209. [DOI] [PubMed] [Google Scholar]
- Rivier F, Alkan O, Flint AF, Muskiewicz K, Allen PD, Leboulch P, Gussoni E. Role of bone marrow cell trafficking in replenishing skeletal muscle SP and MP cell populations. J Cell Sci. 2004;117:1979–1988. doi: 10.1242/jcs.01051. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Seale P, Ishibashi J, Scimè A, Rudnicki MA. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2004;2:664–672. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Weissman IL, Wagers AJ. Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells. Stem Cells. 2004;22:1292–1304. doi: 10.1634/stemcells.2004-0090. [DOI] [PubMed] [Google Scholar]
- Smythe GM, Hodgetts SI, Grounds MD. Problems and solutions in myoblast transfer therapy. J Cell Mol Med. 2001;5:33–47. doi: 10.1111/j.1582-4934.2001.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Uchida N, Aguila HL, Fleming WH, Jerabek L, Weissman IL. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1loLin−Sca-1+ hematopoietic stem cells. Blood. 1994;83:3758–3779. [PubMed] [Google Scholar]
- Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wakeford S, Watt DJ, Partridge TA. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991;14:42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- Yan Z, Choi S, Liu X, Zhang M, Schagemann JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem. 2003;278:8826–8836. doi: 10.1074/jbc.M209879200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.