Abstract
We present here a description of early development in the onychophoran Euperipatoides kanangrensis with emphasis on processes that are ambiguously described in older literature. Special focus has been on the pattern of early cleavage, blastoderm and germinal disc development and gastrulation. The formation of the blastopore, stomodeum and proctodeum is described from sectioned material using light and transmission electron microscopy as well as whole-mount material stained for nuclei and gene expression. The early cleavages were found to be superficial, contrary to earlier descriptions of cleavage in yolky, ovoviviparous onychophorans. Also, contrary to earlier descriptions, the embryonic anterior-posterior axis is not predetermined in the egg. Our data support the view of a blastopore that becomes elongated and slit-like, resembling some of the earliest descriptions. From gene expression data, we concluded that the position of the proctodeum is the most posterior pit in the developing embryo. This description of early development adds to our knowledge of the staging of embryonic development in onychophorans necessary for studies on the role of developmental changes in evolution.
Keywords: Onychophora, Embryonic development, Cleavage, Blastopore, Gastrulation, Proctodeum, Wingless expression
Highlights
► Cleavages are superficial and appear identical to that described in the South-African yolk-less ovoviviparous species. ► The initial positioning of the anterior to posterior axis is not fixed in the egg. ► We propose the terms anterior and posterior part of the blastopore to replace older terms. ► The expression of the gene wingless shows the position of the proctodeum. ► This study has clarified the processes that occur in stage 1 of embryonic development in onychophorans.
1. Introduction
1.1. Phylogenetic position
Recent molecular and morphological studies propose a radical reorganisation of phyla within the Protostomia (Aguinaldo et al., 1997; Dunn et al., 2008; Wheeler et al., 1993). Despite these changes, most analyses confirm the status of the Onychophora as a sister group to the Arthropoda (see Campbell et al., 2011). Because of their phylogenetic position, onychophorans provide vital information about the evolution of arthropods. To facilitate such an investigation, it is essential to have a detailed description of their embryonic development. This will form the basis for comparative analyses of development between onychophorans and arthropods, studies that fit well with the resurgence of our appreciation of the role that developmental change plays in evolutionary innovation.
1.2. Reproductive strategies
Present day onychophorans are well known for the diversity of female reproductive strategies they display. As a consequence of their terrestrial lifestyle, these strategies provide adaptations for the protection and nourishment of their developing embryos. These range from oviparity with large (approximately 2 mm), yolky eggs surrounded by a thick shell (Dendy, 1902; Norman and Tait, 2008); ovoviviparity with large (approximately 1.5 mm), yolky eggs (Reid, 1996; Ruhberg, 1985) and smaller (approximately 0.5 mm), relatively yolk-free eggs surrounded by thin egg membranes (Manton, 1938, 1949; Ruhberg, 1985); to viviparity with minute (approximately 0.03 mm), non-yolky eggs that lack surrounding membranes (Anderson and Manton, 1972; Campiglia and Walker, 1995). This designation of reproductive strategies within the Onychophora is in accordance with the definition of viviparity, which includes the absence of egg membranes during embryonic development (Campiglia and Walker, 1995; Reid, 1996). Contrary to this, some authors have designated species of onychophorans with membrane-bound, non-yolky eggs as non-placental viviparous (Anderson, 1973; Ruhberg, 1985).
In oviparous species, some embryonic development occurs before egg deposition (Brockmann et al., 1997; Norman and Tait, 2008), but the vast majority of development takes place outside the body of the female within the protection of the shell (Brockmann et al., 1997; Dendy, 1893). On the other hand, ovoviviparous species complete their development within the uteri of the female and hatching and birth are simultaneous. While nourishment for the developing embryo in ovoviviparous species is primarily derived from yolk, even in very yolky eggs some nutrient is obtained from other source/s, perhaps from uterine secretions (Sunnucks et al., 2000). On the other hand, ovoviviparous species with relatively yolk-free eggs have eggs that contain a clear liquid. However, the origin of this fluid, its composition and its uptake and role in providing nutrient to the developing embryo requires investigation (Anderson, 1973; Manton, 1949). In true viviparity, the developing embryos receive their nutrient via a placental attachment to the uterine wall (Anderson and Manton, 1972; Walker and Campiglia, 1988).
Onychophoran embryology has been most extensively studied in the relatively yolk-free ovoviviparous Peripatopsis sp. and Opisthopatus roseus from South Africa (Manton, 1949; Mayer et al., 2005; Sedgwick, 1885, 1886, 1887, 1888) and Paraperipatus amboinensis from Indonesia (Pflugfelder, 1948), and the Neotropical viviparous Epiperipatus trinidadensis, Macroperipatus torquatus, Peripatus acacioi and Epiperipatus biolleyi (Anderson and Manton, 1972). Embryological studies on the early development of yolky, ovoviviparous species are limited (Anderson, 1966; Sheldon, 1887, 1888), while later development and general reproductive biology is more thoroughly documented (Anderson, 1966; Curach and Sunnucks, 1999; Eriksson et al., 2003, 2005, 2009, 2010; Evans, 1901; Janssen et al., 2010; Mayer et al., 2010; Sunnucks et al., 2000; Walker and Tait, 2004). The embryology of oviparous species is practically unknown (Brockmann et al., 1997; Dendy, 1902).
1.3. Cleavage
In viviparous species, cleavage is total and equal and, as cell division proceeds, a hollow morula with a single layer of cells is formed. This becomes attached to the uterine wall by specialised cells of the morula that form a hollow stalk that forms the placenta. These stalk cells are derived from dorsal extra-embryonic ectoderm. The remaining cells of the morula, at the free end of the placental stalk, continue to increase in number to form the blastoderm of the developing embryo (see Fig. 44 in Anderson, 1973; Kennel, 1885; Sclater, 1888). The few descriptions of early development in yolky, ovoviviparous onychophorans (Anderson, 1966; Evans, 1901; Sheldon, 1887, 1888; and summarized in Anderson, 1973) describe early cleavage as intralecithal with resulting nuclei migrating to the egg surface to form a small group of blastomeres partly covering the yolk. The blastomeres divide and spread to eventually cover the surface of the egg, forming a blastoderm. The eggs of yolk-free ovoviviparous species have a somewhat different pattern of cleavage, since the zygote nucleus is not centrally located but lies to one side of the egg. Consequently, it becomes membrane bound to form a blastomere without prior migration to the surface. Cleavage divisions follow to produce a saddle-shaped patch of cells. Subsequent events are variable with some species (P. moseleyi and P. sedgwicki) expanding the patch to form a blastoderm similar to that in yolky, ovoviviparous species. Other species (P. balfouri and P. capensis) are more modified in that there is ingression of cells at the perimeter of the patch prior to blastoderm formation. These cells are transient in P. balfouri but persist in P. capensis to add an inner lining of cells to the superficial blastoderm (see S. Fig. 6). It has been suggested that this precocious migration of cells in yolk-free, ovoviviparous species is confined to the presumptive anterior midgut region of the blastoderm and as such may be involved in the uptake of nutrients secreted into the uterus (Anderson, 1973; Manton, 1949). Apart from early migration of anterior midgut, yolk-free species also show a relative increase in the area of extra-embryonic ectoderm as compared with embryonic ectoderm again possibly a reflection of its role in absorption of nutrients.
1.4. Gastrulation
Gastrulation starts after the formation of a germinal disc somewhere on the surface of the egg (Fig. 1) (Anderson, 1973; Mayer et al., 2010; Manton, 1949). Cells start to aggregate in this germinal disc and a cone-shaped pit leading into the interior of the germinal disc is formed. A groove then extends from this pit, which is variously referred to as a groove, furrow or mouth-anus furrow. There are diverging views on the nature of the blastopore, stomodeum and proctodeum and their contribution to the gastrointestinal system between earlier authors (Balfour, 1883; Kennel, 1888; Sedgwick, 1887; Sheldon, 1887, 1888) and that of Manton (1949). The earlier authors except Kennel regarded the mouth-anus furrow as the blastopore, whereas Manton and Kennel favoured a different view. While this may very well be due to interspecific differences, it is possible that some differences are also due to errors in observation and evaluation of the details of embryonic development. In her work on yolk-free South African species, Manton (1949) found no evidence for the mouth-anus furrow (blastopore of earlier authors) producing endodermal cells and contributing to the anterior midgut and hence, she did not correlate this groove with the blastopore. She claimed that the blastopore, as the centre of origin of the endoderm and mesoderm as well as the ectoderm, is confined to the pit area posterior to the mouth-anus groove. The situation is, however, slightly different in yolky ovoviviparous species, where Anderson (1966, 1973) and Evans (1901) claimed that invagination of cells in the mouth-anus groove contributes to the endoderm of the anterior midgut, while the pit, the blastopore as defined by Manton, later takes over endoderm production as the gut extends (Fig. 1, S. figs. 6–7). Manton (1949) did not find that the blastopore was ever continuous with the mouth-anus groove, and hence, regarded the mouth-anus groove as a new opening in close proximity to, or at the site of the blastopore, depending on species. There is also a question of how the early proctodeum opening is moved to its adult position at the posterior of the body (Sedgwick, 1888), where the blastopore (Manton, 1949) or primitive streak (Sedgwick, 1888) is situated during gastrulation.
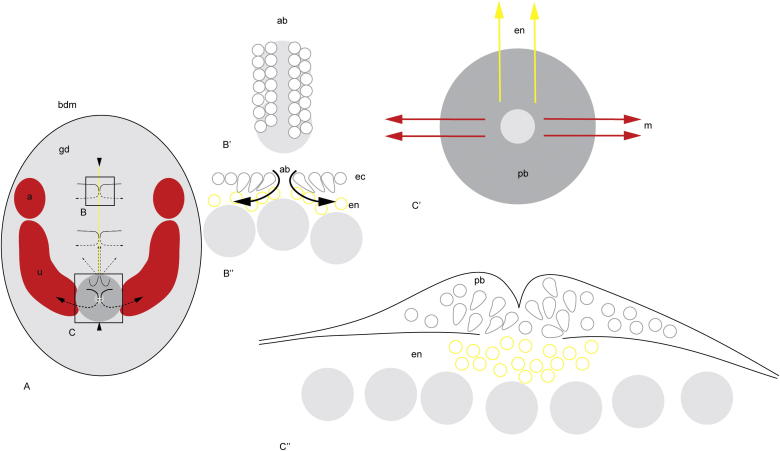
Fig. 1.
Euperipatoides kanangrensis. Diagrammatic representation of gastrulatory movements. (A) Ventral view of embryo showing germinal disc (gd) as encircled gray area within the surrounding blastoderm (bdm). Upper arrowhead indicates position of anterior end of the embryo. A mid-line groove (marked in yellow) is referred to as the blastopore by Sedgwick (1887) or the mouth-anus by Manton (1949) but here is called the anterior part of the blastopore. Upper two pairs of small, solid arrows show direction of migration of germinal disc cells as they move towards the midline then, as dashed arrows, as they descend into the groove and spread laterally taking on the function of vitellophages before becoming part of the anterior midgut. The posterior pair of small, solid arrows shows cells from the raised pit (dark grey circle), called here the posterior part of the blastopore, but referred to as the primitive streak by Sedgwick (1887) or blastopore area by Manton (1949). They become internalized and migrate anteriorly and laterally (dashed arrows), before ending up as part of the posterior midgut. Boxed area B is enlarged in ventral view B′ and in transverse section in B″. Lower arrowhead indicates position of the posterior end of the embryo. The large arrows on each side of the pit indicate the migration of the mesoderm as the germ bands on each side descend into the pit (dashed arrows) and migrate, at first laterally, and then anteriorly and parallel to the groove. Segmentation is first seen as the separation of the first somite, the antennal segment (a), from the unsegmented mesoderm (u). Boxed area C is enlarged in ventral view in C′ and transverse section in C″. (B′) Ventral view of the anterior part of the blastopore (ab), box B in A. The small, grey open circles represent germinal disc cells, on each side of the groove, before they become internalized and the grey area represents underlying yolk. (B″) Transverse section of the anterior part of the blastopore (ab), box B in A. Small, grey open circles indicate germinal disc cells at the surface and grey, open tear-like shapes indicate cells being internalized. Small, yellow open circles indicate internalized endodermal cells (en) migrating laterally. Large, grey-filled circles indicate yolk spheres. (C′) Ventral view of the posterior part of the blastopore (pb), box C in A. Grey circle indicates the posterior blastopore area (pb) and the lighter grey circle the central pit. The yellow arrows show the migration path of endoderm cells (en), while the red arrows show the migration of the mesoderm (m). (C″) Transverse section of the posterior part of the blastopore, box C in A. Open, grey circles indicate cells in the blastopore area (pb) and grey, open tear-like shapes indicate cells being internalized. Yellow, small open circles indicate internalized endodermal cells (en) migrating anteriorly, corresponding to yellow arrows in C′ and lower, small dashed arrows in A. Large, grey-filled circles indicate yolk spheres. Anterior is to the top in A, B′ and C′. Ventral is to the top in B″ and C″. This study recommends that the groove be henceforth referred to as the anterior part of the blastopore and the pit the posterior part of the blastopore, which removes the inappropriate use of vertebrate terminology.
As there is scarce data on the early development of the yolky eggs of ovoviviparous onychophorans, this study investigates cleavage and gastrulatory events in ovoviviparous Euperipatoides kanangrensis by morphology as well as gene expression. The wingless gene is expressed in the future proctodeum in representatives of several animal groups. These include Arthropoda–Diplopoda Glomeris marginata (Janssen et al., 2004), Coleoptera Tribolium castaneum (Nagy and Carroll, 1994), Diptera Drosophila melanogaster (Hoch and Pankratz, 1996), Crustacea Triops longicaudatus (Nulsen and Nagy, 1999); Annelida–Polychaeta Capitella sp. (Seaver and Kaneshige, 2006) and Platynereis dumerelii (Prud’homme et al., 2003); Chordata-Cephalochordata Branchiostoma floridae (Holland et al., 2000). Hence the control of wingless expression during early gastrulation is used here to define the location of the proctodeum.
2. Methods
2.1. Collection of specimens
Gravid female specimens of Euperipatoides kanangrensis, Reid (1996) selected for their large size, were collected in Kanangra Boyd National Park, NSW, Australia (33°59′S, 150°08′E) and maintained in the laboratory at ∼13 °C in moist sphagnum moss until processed.
2.2. Dissection of embryos
Specimens were relaxed and killed by exposure to ethyl acetate fumes in a Petri dish lined with moistened filter paper. After a mid-dorsal incision, the embryos were removed in sequence from each uterus into PBS with 0.1% Tween-20. Using fine watchmaker’s forceps, the two embryonic membranes, outer chorion and inner vitelline membrane, were removed. After the formation of the blastoderm, a slight space (the perivitelline space) is formed between the surface of the blastoderm and the vitelline membrane. Before this perivitelline space is formed, removal of the vitelline membrane without damaging the embryo is impossible. Consequently, embryos at this stage of development have to be photographed without staining.
2.3. Transmission electron microscopy (TEM) and light microscopy (LM)
Embryos were fixed in a mixture of 1.5% paraformaldehyde and 1.5% glutaraldehyde in 0.1 M phosphate buffer (19 mM NaH2PO4·H2O and 81 mM Na2HPO4·7H2O, pH 7.4) and post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h at 4 °C, then rinsed and dehydrated. Embryos for TEM and LM were put into acetone and then embedded in epoxy resin (TAAB 812). Sections for TEM were cut at ∼50 nm, stained for 30 min with uranyl acetate and then 10 min with lead citrate. Sections for LM were cut at 2 μm and stained with methylene blue.
2.4. Preparation of embryos for in situ hybridization and light microscopy
Embryos were fixed in 4% formaldehyde in phosphate buffered saline (PBS) (35.7 mM NaCl, 7.7 mM KCl, 16 mM NaH2PO4·2H2O and 3.5 mM KH2PO4) overnight at 4 °C. They were dehydrated in a graded series of methanol (25, 50 and 75% in PBS with 0.1% Tween-20 for 10 min each) and stored in 100% methanol at −20 °C. Some embryos were processed for paraffin embedding. Sections were cut at 8 μm. The sections were then stained with eosin/haematoxylin, mounted and photographed. For in situ hybridization, a previous cloned fragment of wingless was used as a template for probe preparation and subsequent in situ hybridization according to Eriksson et al. (2009).
2.5. Nuclear staining
Whole embryos, dissected germinal discs and germ bands were stained with SYBR-Green (Invitrogen) at a concentration of 1:10,000, cleared in 70% glycerol in PBS and photographed in a fluorescence microscope.
2.6. Live embryo culture and photography
Early cleavage stages were incubated in PBS at 13 °C and removed for photography twice a day. No staining was done but the light source was adjusted until sufficient contrast for the detection of nuclei was achieved. Embryos were subjected to shrinkage and hence, different salinities were included in the incubation media as well as a number of different media. This did not, however, have any noticeable effect on shrinkage. We propose that the embryos were subjected to a loss of mechanical support when they were removed from the uterus and therefore start to shrink a few minutes after removal. Cleavage continues for a few days in vitro before it stops. We do not know how much effect this shrinkage has on early development during cleavages and we must, therefore, stress that the time between cleavages reported here should be taken with caution.
3. Results
3.1. Cleavage
Large female E. kanangrensis may contain as many as 150 embryos in their paired uteri. These embryos are in series with embryos of later developmental stages towards the distal end (S. Fig. 1). This difference in developmental timing is relative, so that all embryos may be of early cleavage stages but differ in the number of nuclei on the surface. In another female, there may be embryos that are in the process of forming segments, so that the proximal embryos have just formed the first segment while the distal embryos have formed all segments. Sometimes each uterus contains two batches of embryos, one in late stages of embryogenesis and one with much earlier stages. The difference in developmental timing between these two batches may be several months.
Secondary oocytes are attached by a stalk to the haemocoelic surface of the fused ovary. The side of the oocyte that is attached to the stalk is somewhat flattened (Fig. 2A). This slightly bean-shaped form remains for some time while the oocytes pass through the lumen of the ovary and into the paired oviducts each of which bears a spermatheca. The eggs then pass into the uteri where the rest of their embryonic development occurs. Cleavage nuclei first appear on the flattened side of the egg (Fig. 2B). The dimensions of the eggs in the uteri are approximately 1.5 by 1 mm.
Fig. 2.
Euperipatoides kanangrensis. (A) An egg (e) situated in the ovary (o) and connected to its flat side with a stalk (arrow). (B) An embryo with approximately 60 nuclei, the nuclei rise to the flattened surface of the egg (arrow). Scale bars: A = 200 μm; B = 300.
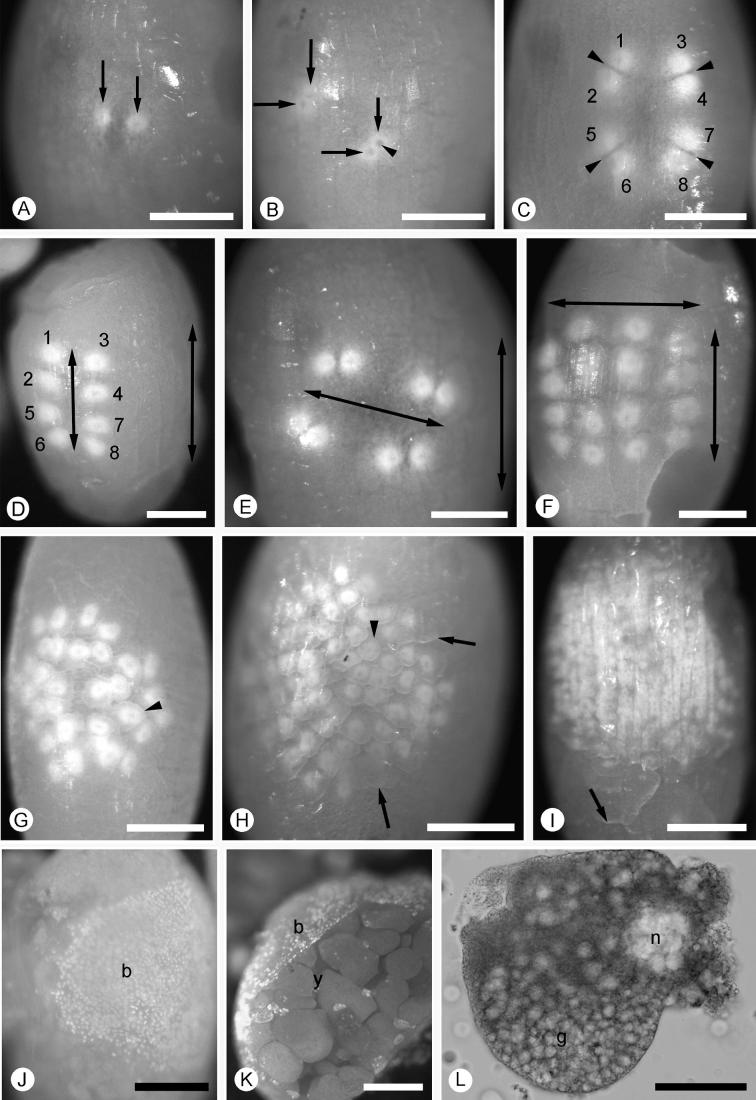
Eggs in the immediate proximal end of the uterus do not show any superficial signs of cleavage. The beginning of cleavage is indicated by the appearance of eggs with one or two nuclei visible at the surface (Fig. 3A). Each nucleus appears as a clear area within a larger zone of white cytoplasm (Fig. 3B). In dissected blastomeres, from a 32 cell embryo, this white zone appears granular (Fig. 3L). We never found consecutive cleavages in the developmental series of embryos in a single uterus, but rather, it would appear that two or three cleavages separate the eggs lying adjacent to each other. During culture of the eggs in vitro, the time between the earliest cleavages was roughly 24 h at 13 °C. This suggests that a new egg is fertilized and passed down to each uterus every two to three days. However, after the one nucleus stage, eggs with 2, 4, 8, 16 and 32 nuclei at the surface were found in the uteri of different females (Fig. 3A–G). We did not find any nuclei within the yolk mass of dissected early cleavage or sectioned blastoderm stage embryos. Cleavages up to 32 nuclei are synchronous but, at later stages, this synchronicity is abandoned and the nuclei loose their regular arrangement (Fig. 3G–I), such that eggs with exactly 64 nuclei could not be found. However, the island of nuclei on the yolk surface continues to grow (Fig. 3J) and eventually covers the egg as the blastoderm (Fig. 3K). The blastomeres become membrane enclosed, as can be seen in a dissected blastomere from a 32 cell embryo (Fig. 3L). The presence of a plasma membrane is also indicated by a sharp border to the cytoplasm containing a cleavage nucleus (Fig. 3H). We could not determine, however, if there is still a cytoplasmic connection towards the interior of the egg at this stage.
Fig. 3.
Euperipatoides kanangrensis. (A–I) Cleavage stages of unstained live material. (J–K) specimens fixed and then stained with DAPI. (A) Two nuclei at the surface (arrows). (B) Four nuclei stage, each nucleus is composed of a clear, circular area (arrowhead) surrounded by a white zone (arrows). (C) Eight nuclei (1–8) stage with cleavage planes indicated by arrowheads. (D) Same embryo as in C a few hours later when the newly divided nuclei have been rearranged into two rows; each numbered nucleus corresponds to the nucleus with the same number in C. The left arrow indicates the pattern of nuclei and the right arrow indicates the long axis of the egg. (E) Another eight nuclei embryo with the rows of nuclei arranged in a different orientation (left arrow) compared with long axis of the egg (right arrow). (F) 16 nuclei stage with the nuclei organized in an alignment (arrows). (G) 32 nuclei stage, the nuclei are starting to loose their organization and synchronization. The boundaries around the nuclei are starting to be sharp (arrowhead), indicating forming cell membranes (see also Fig. 3L). (H) An embryo with approximately fifty cells and the yolk starting to segment (arrows) forming yolk granules without nuclei. The cells containing a nucleus appear white (arrowhead). (I) Embryo with approximately 150 cells and yolk granules formed (arrow). (J) An embryo with the number of cells estimated to be around 1000 forming a saddle of blastoderm cells on one side of the egg (b). (K) An embryo with a blastoderm (b) covering the yolk granules (y). (L) A dissected cell from a 32 cell stage embryo stained with DAPI showing the nucleus (n) and granules (g). Scale bars: A–K 300 μm, L 50 μm.
As the number of blastomeres on the surface of the egg increases to approximately the 64-cell stage and onwards, aggregations of yolk granules into rounded clusters become apparent (Fig. 3K). Some of these yolk clusters contain a nucleus (Supp. Fig. 2).
3.2. Formation of germinal disc and beginnings of gastrulation
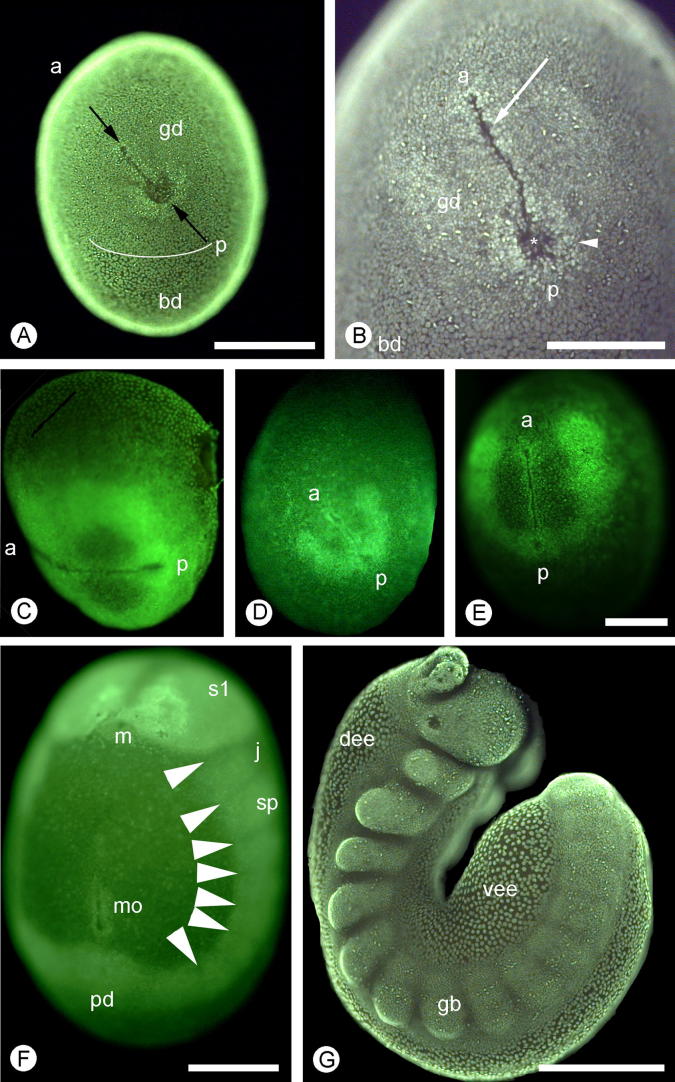
The germinal disc appears within the blastoderm as an area of more densely packed cells that contain smaller nuclei (Fig. 4A–B). The cells aggregate in the centre of the germinal disc to form a cluster that becomes elongated and bilaterally symmetrical (Fig. 4A–E). This cluster of cells is a few cells thick (Fig. 5A). One end of the blastoderm forms a circular mass of cells surrounding a pit. A groove, lined by aggregations of cells, extends from the pit in the germinal disc. This groove and pit form the axis of the embryo with the groove at the anterior and the pit at the posterior end. At this stage, the embryonic axis is not fixed in relation to the axis of the egg but varies in its position (Fig. 4C–E).
Fig. 4.
Euperipatoides kanangrensis. A and C–G embryos stained with SYBR-green, (B) embryo stained with DAPI. (A) An embryo with the blastopore (between arrows) formed in the germinal disc (gd). The border between the germinal disc and the rest of the blastoderm (bd) is not sharp but nuclei start to become smaller and more densely packed within the germinal disc, compare both sides of white line. (B) The blastopore has elongated and the posterior (p) end can be distinguished from the anterior (a) by the former having a circular aggregate of cells (arrowhead) surrounding the central pit (asterisk) and the latter is an elongated concentration of cells on both sides of a cell-free furrow (arrow). (C–E) The anterior–posterior axis is, at the early germ band stages, not fixed in relation to the long axis of the egg. (F) An embryo with eight somites formed as indicated by the segmental grooves (arrowheads). The blastopore has been split up into three parts, the mouth (m), a middle opening (mo), and the proctodeum (pd). The first somite, the antennal segment, (s1) has already started to grow considerably larger than the following jaw (J) and slime papilla (sp) somites. (G) An embryo with approximately 15 segments out of a total of 18 formed. The brain lobes have formed as a result of extensive cell proliferation of the neuroectoderm that covers most of the segment. The split germ band is separated by dorsal and ventral extra-embryonic ectoderm (dee and vee). Scale bars = 250 μm. Anterior is to the top in panels F–G otherwise as indicated in the images.
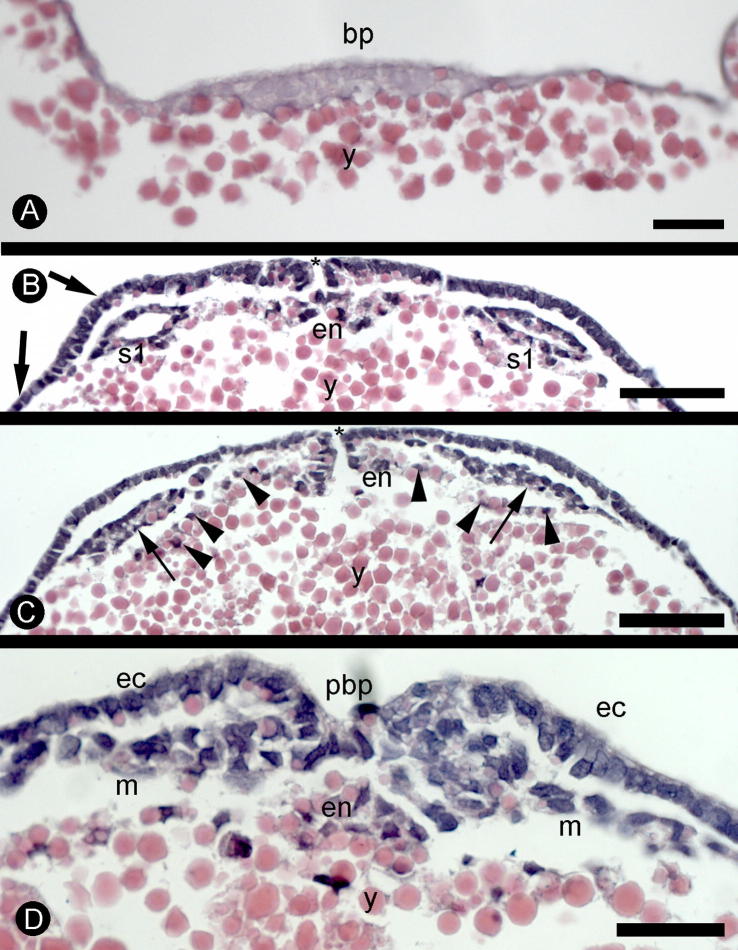
Fig. 5.
Euperipatoides kanangrensis. (A) Cross section of an embryo at the same stage as Fig. 4, A–B. The section is cut level with the blastopore (bp) just posterior to the central pit. (y = yolk). (B) Cross section at the level of the first somite (s1). The ectoderm over the somite is approximately two cell layers deep (upper arrow) as compared to the ectoderm on the flanking sides that is one cell layer thick (lower arrow). The anterior part of the blastopore (asterisk) appears to produce cells that migrate inwards (en) and then laterally beneath the ectoderm. (y = yolk) (C) Cross section at the level of the presomitic mesoderm that has not yet formed a somitic cavity (arrows). The anterior part of the blastopore (asterisk) provides free passage between the outside and the yolk. The continuous sheet of cells (en) stretching from the outside to the inside splits up into scattered cells (arrowheads) located below the mesoderm. (y = yolk). (D) Cross section at the level of the posterior part of the blastopore (pbp). There appear to be three cell groups originating from this area, the ectoderm (ec), the mesoderm (m) and the endoderm (en). Scale bars: A, D = 50 μm; B–C = 100 μm. Dorsal is to the top in all panels.
Sections taken from the anterior part of the groove indicate that cells become internalized and spread as a sheet on each side of the groove and, as they tuck back, they detach and migrate dorsally in the peripheral part of the yolk (Fig. 5B–D and see also Fig. 13 in Eriksson et al., 2003 and Fig. 21 plate 16 in Sheldon, 1888). It is possible that some internalisation by invagination occurs since bottle-shaped cells can be seen in some sections (Supp. Fig. 4) The internalisation of midgut cells into the groove and then up the sides of the embryo leaves the yolk exposed along the floor of the groove (Fig. 5B–C). This is in contrast to the posterior part of the blastopore, in the base of the pit (Fig. 5D, Supp. Fig. 5). The posterior part of the blastopore forms a multi-layered elevation with a central pit. It is not clear how cells get internalised in this area but mesoderm and endoderm appear to be spreading from this area (Supp. Fig. 5). During the early phases of development the groove merges imperceptibly into the pit (Fig. 4B). This connection is soon severed and, in later development, the groove itself splits into two (see below).
3.3. Germ band formation and segmentation
Cells that accumulate at the pit and pass inwards are mesodermal and eventually give rise to the split germ band on either side of the developing embryo. The split germ band extends from each side of the pit (Fig. 4C–E). It first moves away from the pit perpendicular to the midline of the embryo (Fig. 4C–E). The germ band bends anteriorly and thereafter become parallel to the embryonic axis (Fig. 4F, see also Fig. 1 for an explanation). The two halves of the germ band are never in contact along the midline of the embryo, only the most anterior somites that will form the brain segment will be joined anteriorly at the stomodeum. The germ band is at first a homogenous sheet of cells that in the LM cannot be separated into ectoderm and mesoderm (Fig. 5A). However, in the TEM it is possible to appreciate that the mesoderm is epithelium-like, and is surrounded by a basal lamina towards both the ectoderm and the endoderm (Fig. 6A–B). The one to two cell layered mesodermal strands underlying the similarly layered ectoderm elongates beneath the ectoderm. The first somites on each side form by the budding of the anterior-most mesodermal strands into a single-layered vesicle (Fig. 5B and Supp. Fig. 3).
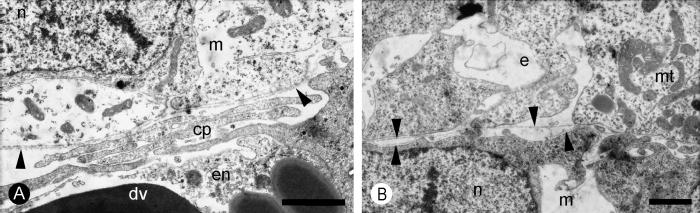
Fig. 6.
Euperipatoides kanangrensis. (A) TEM micrograph of the zone between the endoderm (en) and mesoderm (m). The endoderm contains electron dense vesicles (dv) presumed to be yolk inclusions and cellular processes (cp) that might facilitate transfer of nutrients from the yolk. The mesoderm is epithelial-like with a surrounding basal lamina (arrowheads). (B) TEM micrograph of the zone between the mesoderm (m) and ectoderm (e) showing the basal lamina around the mesoderm (lower arrowheads) and ectoderm (upper arrowheads). mt = mitochondrion; n = nucleus. Scale bars = 1 μm.
Earlier authors claim that at the same time as the split germ band extends from the lateral sides of the pit, endoderm forms from the anterior part of the pit. Consequently, three sheets of cells extend from the pit, two mesodermal sheets laterally and the endoderm medially and anteriorly (Anderson, 1973) (Fig. 1 and Supp. Fig. 5). Our investigation does not contradict this statement and newly formed endoderm at the pit can be identified by means of its position on top of the yolk, its possession of cellular protrusions and yolk inclusions (Fig. 6A and Supp. Fig. 5).
Somite formation continues in an anterior/posterior sequence with mesodermal vesicles being pinched off from the presomitic mesoderm. Somite proliferation in the split germ band of an embryo are synchronised although some degree of asymmetry was observed in the early stages of segmentation (Supp. Fig. 3). The yolk becomes extended as the trunk elongates, stretching from the stomodeum to the proctodeum (Fig. 4G). With further elongation of the germ band the groove splits up into three parts, an anterior opening that is the stomodeum, a posterior opening that this investigation claims to be the proctodeum based on the expression pattern of a wingless homologue (Fig. 7) and an opening (mo) in between with an unknown destiny (Fig. 4F). The split germ band is separated ventrally and dorsally by extra-embryonic ectoderm (Fig. 4G), we cannot conclude if this area is really extra-embryonic, i.e. does it deteriorate or does it become incorporated in the embryo. The term ‘extra-embryonic’, however, does occur in the old literature as summarised in Anderson (1973).
Fig. 7.
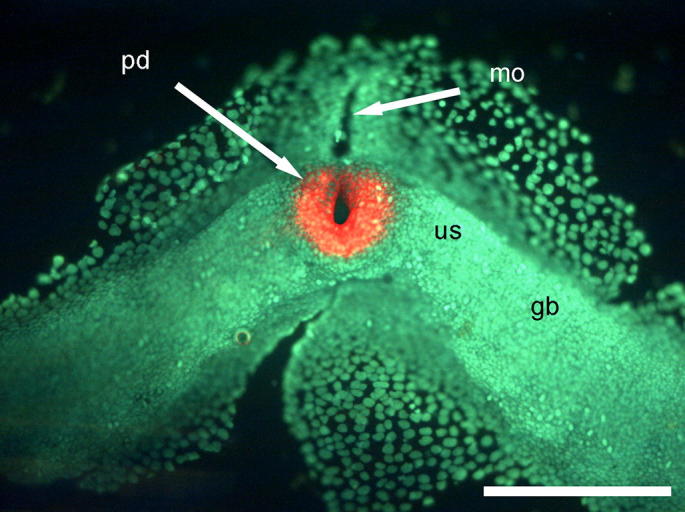
Euperipatoides kanangrensis. The posterior part of an embryo hybridized with a probe directed against wingless. Wingless expression can be seen around the presumed proctodeum (pd). Note that there is no staining around the opening (mo) previously believed to form the proctodeum. us = presomitic mesoderm; gb = germ band. Scale bar = 200 μm.
4. Discussion
4.1. Stages of early development
This description of early development now adds to previous studies on embryological development in onychophorans with yolky eggs (Norman and Tait, 2008; Walker and Tait, 2004). Stage 1 (Walker and Tait, 2004) includes embryos from cleavage through to gastrulation and the beginnings of segmentation. Because of the significance of the Onychophora in evolutionary studies, detailed knowledge of their embryological development is essential in determining the evolutionary origins of the arthropod body plan.
4.2. Cleavage
The fact that all nuclei are positioned close together, surrounded by a large amount of yolk and the lack of separated blastomeres suggest that cleavage is of the superficial type. This is not as has been reported in other species of yolky ovoviviparous onychophorans were cleavage is described as intralecithal (Evans, 1901; Sheldon, 1887, 1888) with cleavage occurring in the centre of the egg and with subsequent rising of the cleavage nuclei up to scattered positions at the surface. However, it is more or less identical to that described for some South-African Peripatopsis sp. Manton (1949). The cells are arranged in two columns at the eight nuclei stage and the long axis of these columns varies in relation to the long axis of the egg (Fig. 3C–E), which is also in agreement with Manton (1949). The pattern of formation of these nuclei was termed a saddle of blastomeres by Manton (1949). We cannot, of course, completely rule out the possibility that cleavages have occurred before the first nuclei appear at the surface and so giving rise to a population of nuclei within the yolk. At a later stage during blastoderm formation, yolk clusters containing nuclei were detected. We do not know how these nuclei enter the interior of the egg, or if they have remained in the interior as a population of nuclei descending from a very early cleavage stage (see discussion below).
4.3. Formation of germinal disc and blastopore
The accumulation of a number of densely packed cells within the blastoderm indicates the first appearance of the germinal disc in which the future embryo will develop. The inward movement of cells can be seen as a cone-shaped pit leading into the interior of the germinal disc. A groove then extends in an anterior direction from the pit (see Supp. Fig. 7 for an explanatory illustration). The axis of the germinal disc with its pit and groove varies in its orientation relative to the long axis of the egg. Intriguingly, the orientation of the pattern of early cleavage nuclei shows a similar variation. Whether or not these two observations are related cannot be determined because it was not possible to follow individual embryos in culture for sufficient time for them to pass from early cleavage to germinal disc formation. However, by the time 4–5 segments appear and regardless of the orientation of the early embryo, the AP axis of the embryo has become aligned with the long axis of the egg. These observations were also made in South African species (Manton, 1949). It is, therefore, unlikely that there is a fate map during the blastoderm stage and, therefore, also unlikely that segmental patterning genes would be detected, and indeed, the first sign of engrailed and wingless expression occur when the germ band has formed (Eriksson et al., 2009).
There is some degree of controversy about the role that the pit and groove play in early development and consequently there are a variety of terms applied to each of these structures depending on the author’s view of their significance. Sedgwick (1885) referred to the groove as the blastopore and to the pit with the rather vertebracentric term ‘primitive streak’ while Manton (1949) concluded that the groove played no part in the invagination of cells and consequently referred to the pit as the blastopore area. We propose that the entire structure should be termed the blastopore, since it is clearly formed as one unit, the groove could be identified as the anterior part of the blastopore and the pit as the posterior part of the blastopore.
4.4. Gastrulation and segmentation
From sections of the anterior part of the blastopore it would appear that cells invaginate, detach and then migrate dorsally in the peripheral part of the yolk (Fig. 5B–D and see also Fig. 13 in Eriksson et al., 2003 and Fig. 21 plate 16 in Sheldon, 1888). Labelling experiments of embryos in culture are required to confirm this movement of cells and to establish that they contribute to the anterior midgut. Earlier workers have concluded that these cells give rise to the anterior midgut by encircling the yolk (Anderson, 1966; Evans, 1901). However, further away from the furrow these cells seem to be too sparse and scattered to be able to form a sheet around the yolk and, at least in the anterior part of the embryo, the gut penetrates into the yolk rather than surrounding it (see Fig. 44 in Eriksson et al., 2003). It is, however, possible that these yolk cells or vitellophages will contribute in some way to the formation of the anterior midgut. The formation of the germ bands and their extension and segmentation by somite formation seems to concur with earlier investigations on the viviparous and ovoviviparous species (Anderson, 1973). However, one difference that can be identified in comparison with Peripatopsis sp. from South Africa is the asymmetrical formation of the early somites observed by Manton (1949). This difference in the number of somites formed on each of the side of the embryo is corrected during later development. In E. kanangrensis embryos, somites developing from the two sides of the germ band seem to be much more synchronised (Fig. 4E–G), with asynchronous somite formation only observed during the very first stages of somite formation (Supp. Fig. 3). Recently Mayer et al. (2010) showed that a growth zone around the posterior part of the blastopore with proliferating cells is lacking in two species of onychophorans, the ovoviviparous Euperipatoides rowelli and the viviparous Epiperipatus isthmicola. Instead, it appears as if the germ band elongates by intercalated cell divisions. Our own observations on E. kanangrensis embryos stained with nuclear dyes and antibodies against alpha-phospho histone 3 (a mitotic marker) corroborates the findings of Mayer et al. (2010) (data not shown).
4.5. Wingless expression and the position of proctodeum
Earlier authors have identified the mouth-anus furrow as the blastopore (Balfour, 1883; Sedgwick, 1885; Sheldon, 1888) and the more posterior thickening, the posterior part of the blastopore in this work that gives rise to the mesoderm as the primitive streak. However, Manton (1949) concluded that the mouth-anus furrow did not give rise to any endoderm by invagination and therefore should not be labelled as blastopore. She restricted the term blastopore for the posterior thickening we term the posterior part of the blastopore. In the present investigation, we show that the blastopore and mouth-anus furrow is actually a continuous depression in the germinal disc and stays that way during a large part of its elongation, only relatively late does it divide to form three openings. The most anterior opening is believed to be the stomodeum for reasons of its position. The second opening from the anterior is more problematic because of the present investigation. It was labelled the anus by earlier authors (Balfour, 1883; Sedgwick, 1885; Sheldon, 1888) even though it is situated well in front of the final position of the anus at the extreme posterior end. This problem was recognised by (Balfour, 1883) but he provided no explanation for the apparent difference in position of the embryonic and adult anus. According to our results, wingless is expressed around the posterior terminus in what we call the posterior part of the blastopore, but not around the opening traditionally referred to as the embryonic anus. Given the expression of wingless around the proctodeum in many animals (Janssen et al., 2004; Nagy and Carroll, 1994; Seaver and Kaneshige, 2006), we suggest that this is also the case in the E. kanangrensis embryo. It is still not known what happens to the opening referred to as the embryonic anus by the earlier authors. During development, it appears to move posteriorly and take up a position very close to the proctodeum before it disappears. It is possible that it actually merges with the proctodeum, or closes. It should be noted that the central pit of the posterior part of the blastopore is much less developed or even absent in the South African species (see figs. 54, 59 and 112 in Manton, 1949) and this may explain why the earlier authors never regarded this area as the proctodeal rudiment.
It is not unlikely that that the edges of the mouth-anus furrow give rise to endoderm by invagination as suggested by earlier workers (Balfour, 1883; Sedgwick, 1885; Sheldon, 1888). Manton (1949) claimed that the furrow did not contribute cells to the endoderm in the South African species she investigated. The endoderm had already formed and taken its position before the mouth-anus furrow opened. In E. kanangrensis, we see a continuous line of cells from the edges leading in to the periphery of the yolk. The final destination of these cells is unknown but it seems reasonable, on grounds of their location within the yolk, to assume that they at least temporarily have a function as vitellophages.
There is a discrepancy between the descriptions of early gastrulation in onychophorans, possibly due, at least in part, to species differences (Balfour, 1883; Kennel, 1885; Manton, 1949; Sedgwick, 1885; Sheldon, 1888). It appears that early phases of gastrulation are subject to variability due to a shift in the timing of the separation of the anterior part of the blastopore (mouth-anus) and, hence, also the possible contribution of endoderm from that structure.
4.6. Conclusion
-
•
Cleavages are superficial and appear identical to that described in the South-African yolk-less ovoviviparous species (earlier viviparous without placenta, see introduction) but not to other ovoviviparous species.
-
•
The initial positioning of the anterior to posterior axis is not fixed but the axis becomes aligned with the long axis of the egg during segmentation.
-
•
We propose that the term ‘blastopore’ should replace ‘mouth-anus groove’ and ‘primitive streak’. The anterior part of the blastopore corresponds with the mouth-anus groove and the posterior part of the blastopore corresponds to primitive streak. The reason for this proposal is that they are formed initially as one unit and they both indicate sites of ingression of cells during gastrulation.
-
•
The expression of the gene wingless suggests that the primitive streak/blastopore of earlier authors, the posterior part of the blastopore in the present investigation, gives rise to the future anus.
-
•
This study has clarified the processes that occur in stage 1 (stage table of Walker and Tait, 2004) of embryonic development in onychophorans.
Acknowledgment
This work was funded by the Marie Curie Training Network ZOONET (Cambridge) and the Austrian Science Fund (FWF): M1296-B17 to BJE under the Lise Meitner Programme. We are grateful to D.T. Anderson and two anonymous reviewers for constructive comments on the manuscript.
Footnotes
Supplementary data related to this article can be found online at doi:10.1016/j.asd.2012.02.009.
Appendix A. Supplementary material
The following are the Supplementary data related to this article:
S. Fig. 1.
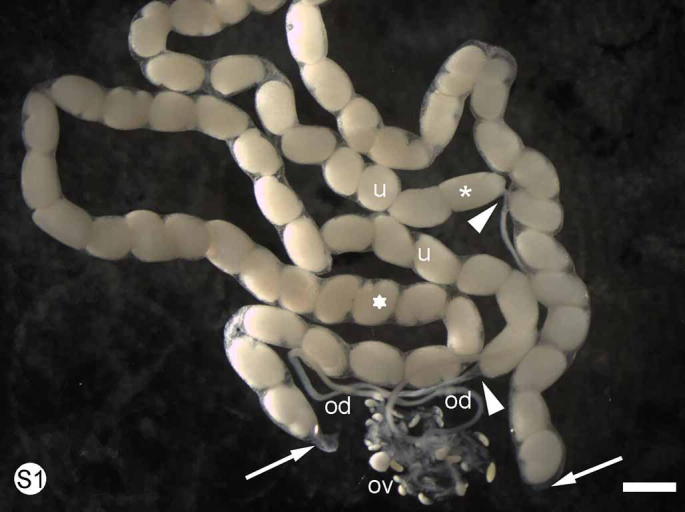
Euperipatoides kanangrensis. The fused ovaries (ov) lead to paired oviducts (od) each broadening into a hairpin-bent uterus (u) containing numerous embryos. This female had one batch of segmenting embryos in each uterus. The proximal end (white arrow heads) of each uterus contains relatively undeveloped embryos (asterisk) while the distal end (white arrows) contains embryos at a slightly later stage of development (star). The distal end of each uterus has been cut at the point where they fuse to form the unpaired vagina leading to the gonopore between the last pair of legs. Scale bar = 1 mm.
S. Fig. 2.
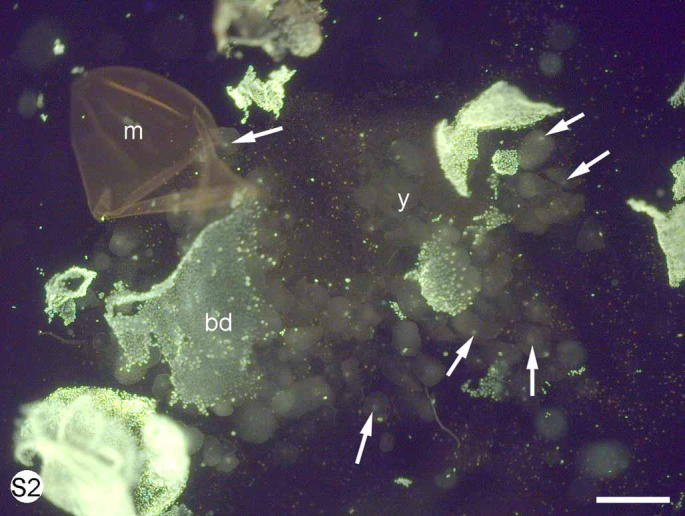
Euperipatoides kanangrensis. Stained with SYBR green. A dissected blastoderm stage embryo showing blastoderm (bd), egg membrane (m) and nuclei (arrows) within yolk clusters (y). Scale bar = 100 μm.
S. Fig. 3.
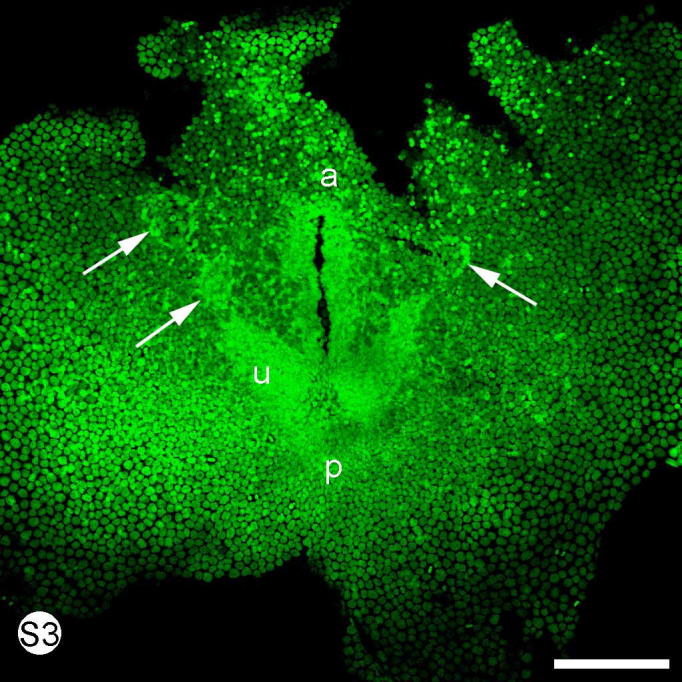
Euperipatoides kanangrensis. Stained with SYBR green. A surface view of a dissected early stage of gastrulation showing the paired germ band and blastopore. The initial phase of somite formation is often asynchronous with the left side slightly ahead with two somites formed (left arrows) while the right side only has one (right arrow). a = anterior part of blastopore, p = posterior part of blastopore, u = unsegmented germ band. Scale bar = 50 μm.
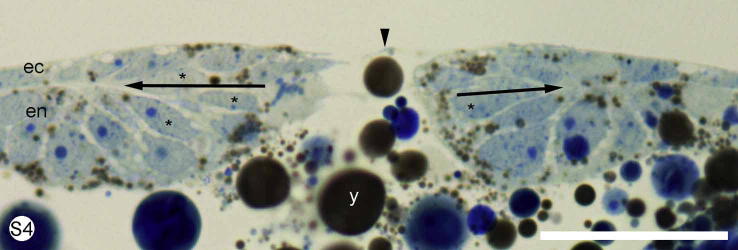
S. Fig. 4.
Euperipatoides kanangrensis. Stained with methylene blue. Cross section through the mid-part of the blastopore of an early segmenting embryo showing flask-shaped cells (stars), the surface ectoderm (ec) and the endoderm (en). Arrows mark the border between endoderm and ectoderm. y = yolk. Scale bar = 40 μm.
S. Fig. 5.
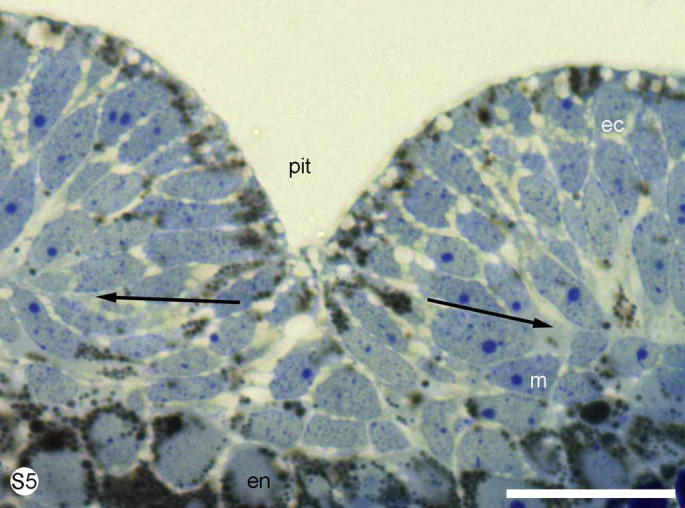
Euperipatoides kanangrensis. Stained with methylene blue. Cross section of the same embryo as above at the level of the pit of the posterior part of the blastopore to show the multi-layered appearance of the area. Arrows mark the border between the ectoderm (ec) and mesoderm (m). en = endoderm, y = yolk. Scale bar = 30 μm.
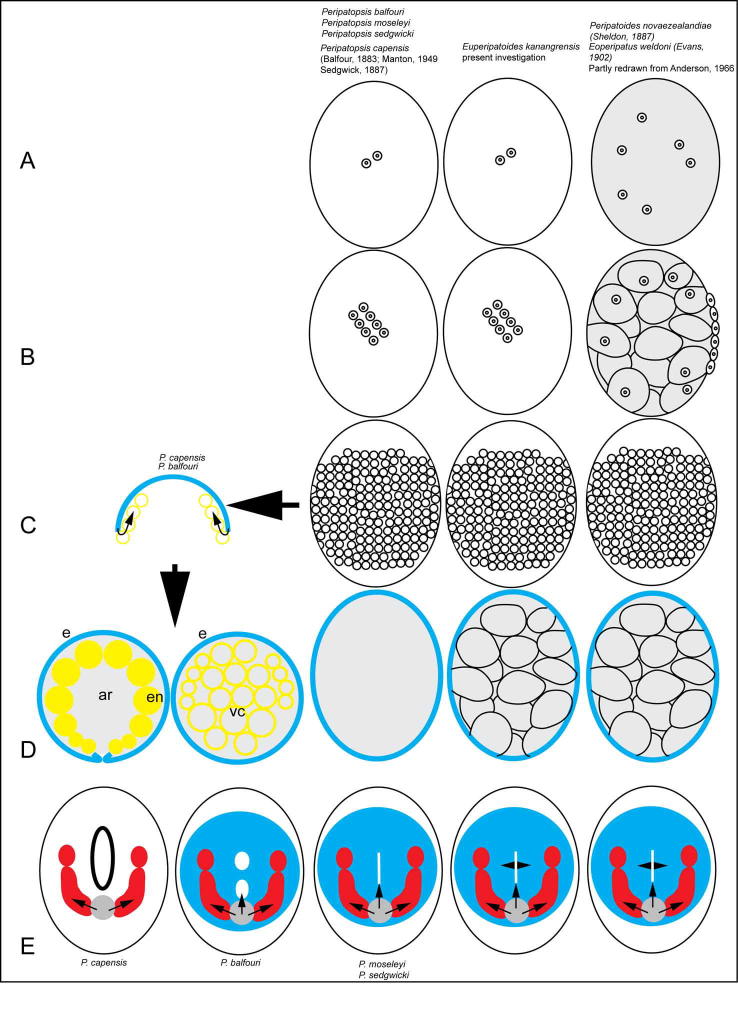
S. Fig. 6.
Diagrammatic representation of cleavage and gastrulatory movements in onychophoran embryos of the yolk-free South African species Peripatopsis balfouri, P. moseleyi, P. sedgwicki and P. capensis as well as the yolk-rich Australasian species Peripatoides novaezealandiae, Eoperipatus weldoni and Euperipatoides kanangrensis. (A) Early cleavage is similar in the South African species and E. kanangrensis with cleavage nuclei or blastomeres being generated at the periphery of the egg while cleavage nuclei are initially generated within the yolk mass in P. novaezealandiae and E. weldoni. (B) The first divisions result in a pattern of nuclei at the surface of the egg with a variable orientation with regard to the long axis of the egg in the South African species and E. kanangrensis, there is no report of this phenomenon in P. novaezealandiae and E. weldoni but nuclei gather at one location at the yolk surface. (C) The dividing blastomeres initially cover part (a saddle) of the egg surface in Australasian and South African species. Cells at the peripheral part of the expanding cell mass (blue) are internalized in P. capensis and P. balfouri (yellow circles) and this has been described as precocious gastrulation. (D) The resulting blastoderm (blue) covers yolk granules in the Australasian species and fluid in the South African species P. sedgwicki and P. moseleyi, while a gastrula has formed in P. capensis and P. balfouri. In P. capensis, this gastrula is open to the outside by a blastopore and a cavity, the archaenteron (ar) lined by endoderm (en), is present inside. In P. balfouri, the internalised cells are vacuolated (vc) and later disappear leaving a blastula-like embryo. (E) Gastrulation begins in all species with the formation of a blastopore in the germinal disc (blue). In P. capensis and P. balfouri, a secondary blastopore is opened, from where mesoderm and endoderm (P. balfouri) or only mesoderm (P. capensis) are formed (arrows). In P. capensis, there is no formation of a germinal disc and all endoderm derives from the previously formed endoderm. In the Australasian species, endoderm is formed from the anterior part of the blastopore (arrow heads). The shape and timing of the separation of the anterior part of the blastopore varies among species. In P. capensis, the anterior part of the blastopore is relatively large and has protruding edges (black, thick-lined oval). The anterior part of the blastopore in P. balfouri is separated very early into the stomodeum and the middle part of the blastopore (white ovals). In P. moseley, P. sedgwicki, E. kanangrensis, P. novaezealandiae and E. weldoni, the anterior blastopore remains slit-like (white, vertical bar) for some time after the first phases of segmentation. Panel A–C & E represent surface views except for grey-shaded embryos that show sagittal sections. Panel D shows sagittal (ovals) and cross sections (circles). The blue lining in panel D represent the cellular surface layer, ectoderm or blastoderm. The present investigation concerns E. kanangrensis only while details of early development in the other species have been taken from the literature as cited.
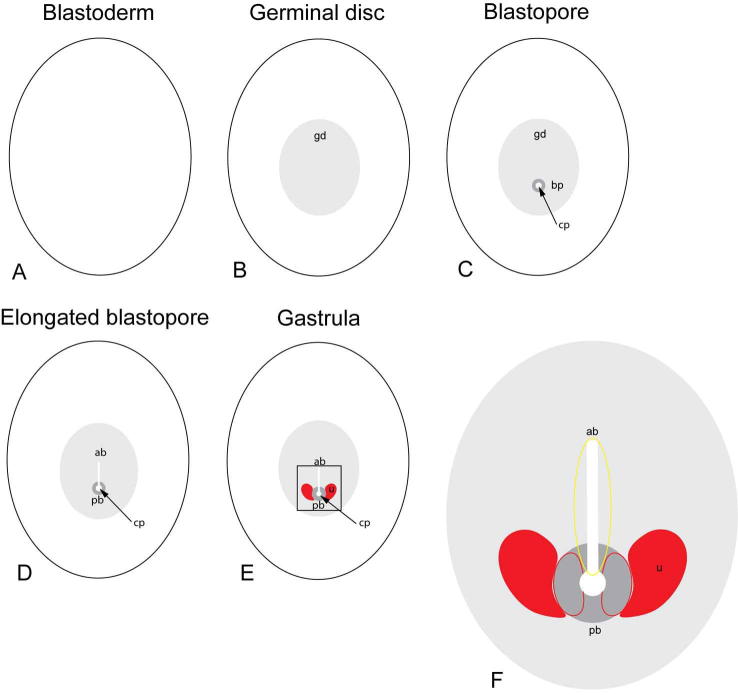
S. Fig. 7.
Euperipatoides kanangrensis. Schematic illustrations of early developmental stages. (A) A single layer of cells, the blastoderm, covers the yolk. (B) The germinal disc (gd) has formed as an area with more densely packed cells. (C) The blastopore (bp) forms as an elevation of cells with a central pit (cp) within the germinal disc. (D) The elongation of the blastopore pit forms a furrow in the anterior part of the blastopore (ab) that is continuous with the posterior part of the blastopore (bp). (E) The early gastrula has started to form unsegmented mesoderm (u) lateral to the central pit. (F) A magnification of the box in (E) showing the areas of mesoderm formation (red line) and the area of endoderm formation (yellow line).
References
- Aguinaldo A.M.A., Turbeville J.M., Linford L.S., Rivera M.C., Garey J.R., Raff R.A., Lake J.A. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature. 1997;387:489–493. doi: 10.1038/387489a0. [DOI] [PubMed] [Google Scholar]
- Anderson D.T. The comparative early embryology of the Oligochaeta, Hirudinae and Onychophora. Proceedings of the Linnean Society of New South Wales. 1966;91:10–43. [Google Scholar]
- Anderson D.T. Pergamon press; Oxford: 1973. Embryology and Phylogeny in Annelids and Arthropods. [Google Scholar]
- Anderson D.T., Manton S.M. Studies on the Onychophora. VIII. The relationship between the embryos and the oviduct in the viviparous placental onychophorans Eoperipatus trinidadensis Bouvier and Macroperipatus torquatus Kennel from Trinidad. Philosophical Transactions of the Royal Society of London B. 1972;264:161–189. [Google Scholar]
- Balfour F.M. The anatomy and development of Peripatus capensis. Quarterly Journal of Microscopical Sciences. 1883;23:213–259. [Google Scholar]
- Brockmann C., Mesibov R., Ruhberg H. Observations on Ooperipatellus decoratus, an oviparous onychophoran from Tasmania (Onychophora: Peripatopsidae) Entomologica Scandinavia Supplement. 1997;51:319–329. [Google Scholar]
- Campbell L.I., Rota-Stabelli O., Edgecombe G.D., Marchioro T., Longhorn S.J., Telford M.J., Philippe H., Rebecchi L., Peterson K.J., Pisani D. MicroRNAs and phylogenomics resolve the relationships of Tardigrada and suggest that velvet worms are the sister group of Arthropoda. Proceedings of the National Academy of Sciences United States of America. 2011;108:15920–15924. doi: 10.1073/pnas.1105499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campiglia S.S., Walker M.H. Developing embryo and cyclic changes in the uterus of Peripatus (Macroperipatus) acacioi (Onychophora, Peripatidae) Journal of Morphology. 1995;224:179–198. doi: 10.1002/jmor.1052240207. [DOI] [PubMed] [Google Scholar]
- Curach N., Sunnucks P. Molecular anatomy of an onychophoran: compartmentalized sperm storage and heterogeneous paternity. Molecular Ecology. 1999;8:1375–1385. doi: 10.1046/j.1365-294x.1999.00698.x. [DOI] [PubMed] [Google Scholar]
- Dendy A. The hatching of a peripatus egg. Nature. 1893;47:508–509. [Google Scholar]
- Dendy A. On the oviparous species of Onychophora. Quarterly Journal of Microscopical Sciences. 1902;45:363–416. [Google Scholar]
- Dunn C.W., Hejnol A., Matus D.Q., Pang K., Browne W.E., Smith S.A., Seaver E., Rouse G.W., Obst M., Edgecombe G.D., Sorensen M.V., Haddock S.H.D., Schmidt-Rhaesa A., Okusu A., Kristensen R.M., Wheeler W.C., Martindale M.Q., Giribet G. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–749. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- Eriksson B.J., Tait N.N., Budd G.E. Head development in the onychophoran Euperipatoides kanangrensis with particular reference to the central nervous system. Journal of Morphology. 2003;255:1–23. doi: 10.1002/jmor.10034. [DOI] [PubMed] [Google Scholar]
- Eriksson B.J., Larson E.T., Thornqvist P.O., Tait N.N., Budd G.E. Expression of engrailed in the developing brain and appendages of the onychophoran Euperipatoides kanangrensis (Reid) Journal of Experimental Zoology Part B-Molecular and Developmental Evolution. 2005;304B:220–228. doi: 10.1002/jez.b.21043. [DOI] [PubMed] [Google Scholar]
- Eriksson B.J., Tait N., Budd G., Akam M. The involvement of engrailed and wingless during segmentation in the onychophoran Euperipatoides kanangrensis (Peripatopsidae: Onychophora) (Reid 1996) Development Genes and Evolution. 2009;219:249–264. doi: 10.1007/s00427-009-0287-7. [DOI] [PubMed] [Google Scholar]
- Eriksson B.J., Tait N., Budd G., Janssen R., Akam M. Head patterning and Hox gene expression in an onychophoran and its implications for the arthropod head problem. Development Genes and Evolution. 2010;220:117–122. doi: 10.1007/s00427-010-0329-1. [DOI] [PubMed] [Google Scholar]
- Evans R. On the Malayan species of Onychophora. Part II. The development of Eoperipatus weldoni. Quarterly Journal of Microscopical Sciences. 1901;45:41–86. [Google Scholar]
- Hoch M., Pankratz M.J. Control of gut development by fork head and cell signaling molecules in Drosophila. Mechanisms of Development. 1996;58:3–14. doi: 10.1016/s0925-4773(96)00541-2. [DOI] [PubMed] [Google Scholar]
- Holland L.Z., Holland N.D., Schubert M. Developmental expression of AmphiWnt1, an amphioxus gene in the Wnt1/wingless subfamily. Development Genes and Evolution. 2000;210:522–524. doi: 10.1007/s004270000089. [DOI] [PubMed] [Google Scholar]
- Janssen R., Prpic N.-M., Damen W.G.M. Gene expression suggests decoupled dorsal and ventral segmentation in the millipede Glomeris marginata (Myriapoda: Diplopoda) Developmental Biology. 2004;268:89–104. doi: 10.1016/j.ydbio.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Janssen R., Eriksson B.J., Budd G.E., Akam M., Prpic N.-M. Gene expression patterns in an onychophoran reveal that regionalization predates limb segmentation in pan-arthropods. Evolution & Development. 2010;12:363–372. doi: 10.1111/j.1525-142X.2010.00423.x. [DOI] [PubMed] [Google Scholar]
- Kennel J. Entwicklungsgeschichte von Peripatus edwardsii Blanch. und Peripatus torquatus n.sp. I. Theil. Arbeiten aus dem Zoologisch-Zootomischen Institut in Würzburg. 1885;7:95–229. [Google Scholar]
- Kennel J. Entwicklungsgeschichte von Peripatus edwardsii Blanch. und Peripatus torquatus n.sp. II. Theil. Arbeiten aus dem Zoologisch-Zootomischen Institut in Würzburg. 1888;8:1–93. [Google Scholar]
- Manton S.M. Studies on the Onychophora. IV. The passage of spermatozoa into the ovary in Peripatopsis and the Early development of the ova. Philosophical Transactions of the Royal Society of London Series B. 1938;228:421–441. [Google Scholar]
- Manton S.M. Studies on the Onychophora VII. The early embryonic stages of Peripatopsis, and some general considerations concerning the morphology and phylogeny of the Arthropoda. Philosophical Transactions of the Royal Society of London. 1949;233:483–580. [Google Scholar]
- Mayer G., Bartolomaeus T., Ruhberg H. Ultrastructure of mesoderm in embryos of Opisthopatus roseus (Onychophora, Peripatopsidae): revision of the “long germ band” hypothesis for Opisthopatus. Journal of Morphology. 2005;263:60–70. doi: 10.1002/jmor.10289. [DOI] [PubMed] [Google Scholar]
- Mayer G., Kato C., Quast B., Chisholm R., Landman K., Quinn L. Growth patterns in Onychophora (velvet worms): lack of a localised posterior proliferation zone. BMC Evolutionary Biology. 2010;10:339. doi: 10.1186/1471-2148-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L.M., Carroll S. Conservation of wingless patterning functions in the short-germ embryos of Tribolium castaneum. Nature. 1994;367:460–463. doi: 10.1038/367460a0. [DOI] [PubMed] [Google Scholar]
- Norman J.M., Tait N.N. Ultrastructure of the eggshell and its formation in Planipapillus mundus (Onychophora: Peripatopsidae) Journal of Morphology. 2008;269:1263–1275. doi: 10.1002/jmor.10658. [DOI] [PubMed] [Google Scholar]
- Nulsen C., Nagy L.M. The role of wingless in the development of multibranched crustacean limbs. Development Genes and Evolution. 1999;209:340–348. doi: 10.1007/s004270050262. [DOI] [PubMed] [Google Scholar]
- Pflugfelder O. Entwicklung von Paraperipatus amboinensis n. sp. Zoologische Jahrbücher Abt 2 Anatomie und Ontogenie der Tiere. 1948;69:443–492. [Google Scholar]
- Prud’homme B., de Rosa R., Arendt D., Julien J.-F., Pajaziti R., Dorresteijn A.W.C., Adoutte A., Wittbrodt J., Balavoine G. Arthropod-like expression patterns of engrailed and wingless in the annelid Platynereis dumerilii suggest a role in segment formation. Current Biology. 2003;13:1876–1881. doi: 10.1016/j.cub.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Reid A. A review of the Peripatopsidae (Onychophora) in Australia, with comments on peripatopsid relationships. Invertebrate Taxononomy. 1996;10:663–936. [Google Scholar]
- Ruhberg H. Die Peripatopsidae (Onychophora). Systematik, Ökologie, Chorologie und phylogenetische Aspekte. Zoologica. 1985;137:1–183. [Google Scholar]
- Sclater W.L. On the early stages of the development of a South American species of Peripatus. Quarterly Journal of Microscopical Sciences. 1888;24:43–82. [Google Scholar]
- Seaver E.C., Kaneshige L.M. Expression of ‘segmentation’ genes during larval and juvenile development in the polychaetes Capitella sp. I and H. elegans. Developmental Biology. 2006;289:179–194. doi: 10.1016/j.ydbio.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. The development of Peripatus capensis. Part I. Quarterly Journal of Microscopical Sciences. 1885;25:449–466. [Google Scholar]
- Sedgwick A. The development of the Cape species of Peripatus. Part II. Quarterly Journal of Microscopical Sciences. 1886;26:175–212. [Google Scholar]
- Sedgwick A. The development of the Cape species of Peripatus. Part III. On the changes from stage A to stage F. Quarterly Journal of Microscopical Sciences. 1887;27:467–550. [Google Scholar]
- Sedgwick A. The Development of the Cape Species of Peripatus. Part IV. The changes from stage g to birth. Quarterly Journal of Microscopical Sciences. 1888;28:373–396. [Google Scholar]
- Sheldon L. On the development of Peripatus novae-zealandiae. Quarterly Journal of Microscopical Sciences. 1887;28:205–237. [Google Scholar]
- Sheldon L. On the development of Peripatus novae-zealandiae. Quarterly Journal of Microscopical Sciences. 1888;29:283–294. [Google Scholar]
- Sunnucks P., Curach N.C., Young A., French J., Cameron R., Briscoe D.A., Tait N.N. Reproductive biology of the onychophoran Euperipatoides rowelli. Journal of Zoology. 2000;250:447–460. [Google Scholar]
- Walker M., Campiglia S. Some aspects of segment formation and post-placental development in Peripatus acacioi Marcus and Marcus (Onycophora) Journal of Morphology. 1988;195:123–140. doi: 10.1002/jmor.1051950202. [DOI] [PubMed] [Google Scholar]
- Walker M.H., Tait N.N. Studies of embryonic development and the reproductive cycle in ovoviviparous Australian Onychophora (Peripatopsidae) Journal of Zoology. 2004;264:333–354. [Google Scholar]
- Wheeler W.C., Cartwright P., Hayashi C.Y. Arthropod phylogeny: a combined approach. Cladistics. 1993;9:1–39. doi: 10.1111/j.1096-0031.1993.tb00207.x. [DOI] [PubMed] [Google Scholar]