Abstract
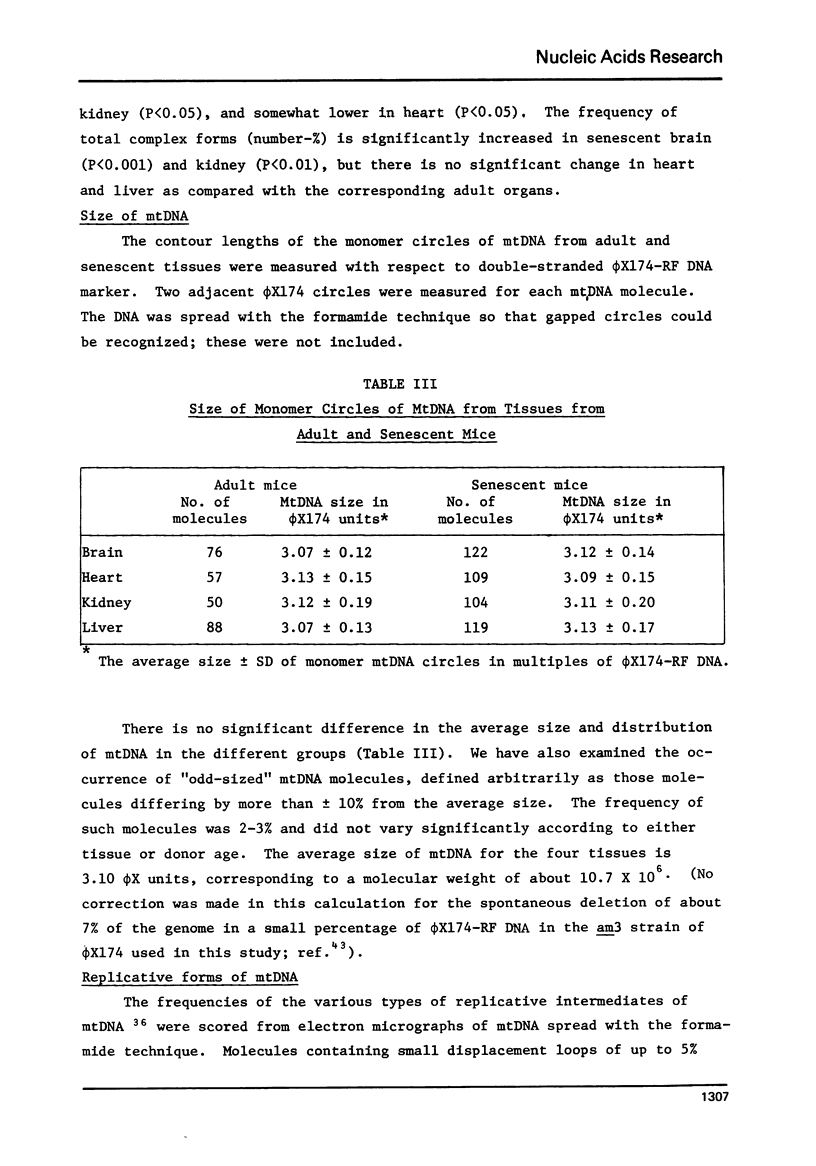
The occurrence and types of complex forms and replicative intermediates of mitochondrial DNA (mtDNA) were investigated in tissues from C57BL/6J mice aged 10-11 months or 29-30 months. Total mtDNA from brain, heart, kidney and liver was isolated in ethidium bromide-CsCl gradients and examined by electron microscopy after aqueous or formamide spreading. Contour length measurements indicated no difference in the monomer size of mtDNA according to either tissue or donor age. The frequencies of catenated mtDNA, ranging from 4 to 8%, varied significantly according to tissue but changed relatively little as a result of donor age. The main age-related effect observed in this study was a significant increase in the frequency of circular dimers, from about 0.05% in adult tissues to 0.3% in kidney, 0.5% in liver, 0.6% in heart and 1.9% in brain of senescent mice. The frequency of D-loop DNA varied from 30 to 60% and that of larger replicative intermediates from 1 to 10%, suggesting differences in the rate of mtDNA replication according to tissue. The frequencies and types of the various replicative intermediates were unaffected by donor age.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M., Eisenberg M., Sinsheimer R. L. Multiple length DNA molecules of bacteriophage phi-X174. Nat New Biol. 1972 May 31;237(74):141–144. doi: 10.1038/newbio237141a0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: asynchronous replication of strands, segregation of circular daughter molecules, aspects of topology and turnover of an initiation sequence. J Mol Biol. 1974 Jul 15;86(4):801–824. doi: 10.1016/0022-2836(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: topology of circular daughter molecules and dynamics of catenated oligomer formation. J Mol Biol. 1976 Jan 5;100(1):85–92. doi: 10.1016/s0022-2836(76)80036-8. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Chen J. C., Warshaw J. B., Sanadi D. R. Regulation of mitochondrial respiration in senescence. J Cell Physiol. 1972 Aug;80(1):141–148. doi: 10.1002/jcp.1040800115. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Smith C. A. Complex mitochondrial DNA. Int Rev Exp Pathol. 1975;14:1–67. [PubMed] [Google Scholar]
- Clayton D. A., Smith C. A., Jordan J. M., Teplitz M., Vinograd J. Occurrence of complex mitochondrial DNA in normal tissues. Nature. 1968 Dec 7;220(5171):976–979. doi: 10.1038/220976a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Vinograd J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature. 1967 Nov 18;216(5116):652–657. doi: 10.1038/216652a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Vinograd J. Complex mitochondrial DNA in leukemic and normal human myeloid cells. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1077–1084. doi: 10.1073/pnas.62.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch C. E. Catecholamine metabolism in the brains of ageing male mice. Brain Res. 1973 Mar 30;52:261–276. doi: 10.1016/0006-8993(73)90663-x. [DOI] [PubMed] [Google Scholar]
- Finch C. E., Foster J. R. Hematologic and serum electrolyte values of the C57BL-6J male mouse in maturity and senescence. Lab Anim Sci. 1973 Jun;23(3):339–349. [PubMed] [Google Scholar]
- Finch C. E., Foster J. R., Mirsky A. E. Ageing and the regulation of cell activities during exposure to cold. J Gen Physiol. 1969 Dec;54(6):690–712. doi: 10.1085/jgp.54.6.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch C. E., Girgis F. G. Enlarged seminal vesicles of senescent C57BL-6J mice. J Gerontol. 1974 Mar;29(2):134–138. doi: 10.1093/geronj/29.2.134. [DOI] [PubMed] [Google Scholar]
- Finch C. E. The regulation of physiological changes during mammalian aging. Q Rev Biol. 1976 Mar;51(1):49–83. doi: 10.1086/409053. [DOI] [PubMed] [Google Scholar]
- Flory P. J., Jr, Vinograd J. 5-bromodeoxyuridine labeling of monomeric and catenated circular mitochondrial DNA in HeLa cells. J Mol Biol. 1973 Feb 25;74(2):81–94. doi: 10.1016/0022-2836(73)90100-9. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Getz G. S., Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem. 1969 Mar 25;244(6):1552–1562. [PubMed] [Google Scholar]
- Hobom G., Hogness D. S. The role of recombination in the formation of circular oligomers of the lambda plasmid. J Mol Biol. 1974 Sep 5;88(1):65–87. doi: 10.1016/0022-2836(74)90295-2. [DOI] [PubMed] [Google Scholar]
- Hudson B., Vinograd J. Catenated circular DNA molecules in HeLa cell mitochondria. Nature. 1967 Nov 18;216(5116):647–652. doi: 10.1038/216647a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Mehler W. R., Miquel J. A fine structural study of degenerative changes in the dorsal column nuclei of aging mice. Lack of protection by vitamin E. J Gerontol. 1975 Jul;30(4):395–411. doi: 10.1093/geronj/30.4.395. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Replication of circular DNA in eukaryotic cells. Annu Rev Biochem. 1974;43(0):695–719. doi: 10.1146/annurev.bi.43.070174.003403. [DOI] [PubMed] [Google Scholar]
- Kim J., Sharp P. A., Davidson N. Electron microscope studies of heteroduplex DNA from a deletion mutant of bacteriophage phiX-174. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1948–1952. doi: 10.1073/pnas.69.7.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto L., Kasamatsu H., Pikó L., Vinograd J. Mitochondrial DNA replication in sea urchin oocytes. J Cell Biol. 1974 Oct;63(1):146–159. doi: 10.1083/jcb.63.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto L., Pikó L., Vinograd J. Complex mitochondrial DNA in animal thyroids. A comparative study. Biochim Biophys Acta. 1976 May 19;432(3):257–266. doi: 10.1016/0005-2787(76)90134-9. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Abnormal DNA patterns in animal mitochondria: ethidium bromide-induced breakdown of closed circular DNA and conditions leading to oligomer accumulation. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1926–1933. doi: 10.1073/pnas.67.4.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Reversible generation of circular dimer and higher multiple forms of mitochondrial DNA. Nature. 1969 Sep 13;223(5211):1124–1129. doi: 10.1038/2231124a0. [DOI] [PubMed] [Google Scholar]
- Paoletti C., Riou G., Pairault J. Circular oligomers in mitochondrial DNA of human and beef nonmalignant thyroid glands. Proc Natl Acad Sci U S A. 1972 Apr;69(4):847–850. doi: 10.1073/pnas.69.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Blair D. G., Tyler A., Vinograd J. Cytoplasmic DNA in the unfertilized sea urchin egg: physical properties of circular mitochondrial DNA and the occurrence of catenated forms. Proc Natl Acad Sci U S A. 1968 Mar;59(3):838–845. doi: 10.1073/pnas.59.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol. 1976 Mar;49(1):1–10. doi: 10.1016/0012-1606(76)90253-0. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E., Hutchison C. A., 3rd, Edgell M. H. Specific cleavage analysis of mammalian mitochondrial DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4496–4500. doi: 10.1073/pnas.72.11.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A., Morrow J. F. Cleavage of replicating forms of mitochondrial DNA by EcoRI endonuclease. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4447–4451. doi: 10.1073/pnas.71.11.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor B., Shimada Y. Degenerative changes in the mitochondria of flight muscle from aging blowflies. J Cell Biol. 1972 Feb;52(2):465–477. doi: 10.1083/jcb.52.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Vinograd J. Complex mitochondrial DNA in human tumors. Cancer Res. 1973 May;33(5):1065–1070. [PubMed] [Google Scholar]
- Wilson P. D., Franks L. M. The effect of age on mitochondrial ultrastructure and enzyme cytochemistry. Biochem Soc Trans. 1975;3(1):126–128. doi: 10.1042/bst0030126. [DOI] [PubMed] [Google Scholar]
- Wohlrab H. Age-related changes in the flight muscle mitochondria from the blowfly Sarcophaga bullata. J Gerontol. 1976 May;31(3):257–263. doi: 10.1093/geronj/31.3.257. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Koike K., Cochran-Fouts P. Replication of mitochondrial DNA: replicative forms of molecules from rat tissues and evidence for discontinuous replication. Cold Spring Harb Symp Quant Biol. 1974;38:267–280. doi: 10.1101/sqb.1974.038.01.030. [DOI] [PubMed] [Google Scholar]
- Zuccarelli A. J., Benbow R. M., Sinsheimer R. L. Deletion mutants of bacteriophage phiX174. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1905–1910. doi: 10.1073/pnas.69.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]




