Abstract
Intratumoral injection of Semliki Forest virus encoding interleukin-12 (SFV-IL-12) combines acute expression of IL-12 and stressful apoptosis of infected malignant cells. Agonist antibodies directed to costimulatory receptor CD137 (4-1BB) strongly amplify pre-existing cellular immune responses toward weak tumor antigens. In this study, we provide evidence for powerful synergistic effects of a combined strategy consisting of intratumoral injection of SFV-IL-12 and systemic delivery of agonist anti-CD137 monoclonal antibodies (mAbs), which was substantiated against poorly immunogenic B16 melanomas (B16-OVA and B16.F10) and TC-1 lung carcinomas. Effector CD8β+ T cells were sufficient to mediate complete tumor eradications. Accordingly, there was an intensely synergistic in vivo enhancement of cytotoxic T lymphocytes (CTL)-mediated immunity against the tumor antigens OVA and tyrosine-related protein-2 (TRP-2). This train of phenomena led to long-lasting tumor-specific immunity against rechallenge, attained transient control of the progression of concomitant tumor lesions that were not directly treated with SFV-IL-12 and caused autoimmune vitiligo. Importantly, we found that SFV-IL-12 intratumoral injection induces bright expression of CD137 on most tumor-infiltrating CD8+ T lymphocytes, thereby providing more abundant targets for the action of the agonist antibody. This efficacious combinatorial immunotherapy strategy offers feasibility for clinical translation since anti-CD137 mAbs are already undergoing clinical trials and development of clinical-grade SFV-IL-12 vectors is in progress.
Introduction
Interleukin-12 (IL-12) is a potent immunotherapeutic cytokine usually expressed by activated macrophages and dendritic cells. IL-12 has shown strong antitumoral activity mediated by activation of cytotoxic T lymphocytes (CTL), T-helper cell type 1 responses, NK and NKT cells, as well as by inhibition of angiogenesis.1,2 Most of these effects are mediated by the induction of interferon γ (IFNγ).3 Several viral vectors, such as adenovirus, retrovirus, or alphavirus, have been used to deliver IL-12 to animal tumor models, resulting in localized expression of the cytokine and antitumor efficacy.4,5,6 However, in spite of successful preclinical studies, phase I clinical trials performed with adenovirus or canarypox vectors-expressing IL-12 only showed minor therapeutic effect.7,8 These results indicated a need for more potent vectors and for the development of combinatorial strategies.9 Alphavirus vectors based on Semliki Forest virus (SFV) have shown some advantages over adenoviral vectors in preclinical studies of cancer treatment, such as higher expression levels, broad tropism, and induction of immunogenic apoptosis in tumor cells.10,11 This last property can lead to the release of tumor antigens that can be uptaken by antigen-presenting cells, favoring an ensuing antitumor immune response.12 The SFV vector is based on a viral RNA genome in which the region coding for the structural proteins has been replaced by an heterologous gene.13 SFV vectors-expressing IL-12 have shown to be very efficient in inducing therapeutic antitumor responses in tumor models of colon adenocarcinoma, sarcoma, and glioma in mice,10,14,15 orthotopic hepatocellular carcinoma in rats,11 or spontaneous hepatocellular carcinoma in woodchucks.16
In vivo treatment with agonist agents acting on CD137 (4-1BB) expressed on primed T cells results in enhancement of tumor-eradicating cytotoxic T-cell responses.17 These therapeutic effects have been observed with conventional monoclonal antibodies (mAbs) and single chain Fv antibodies attached to tumor cells.18 Although originally described as an inducible molecule on activated T-cells,19 CD137 is not only expressed on antigen-activated T-cells but also on other cell types such as activated NK cells,20 dendritic cells,21 and endothelial cells in tumor vessels.22 Agonist mAbs given as monotherapy to tumor-bearing mice rely mainly on CTL antitumor responses, although an involvement for NK cells has been reported in a number of cases.23 Particularly, therapeutic CD137 stimulation greatly enhanced the NK-mediated ADCC activity of mAbs recognizing tumor-associated surface molecules.24 Moreover, anti-CD137 mAbs enhanced lymphocyte infiltration into tumors as a result of stimulating tumor endothelial cells to behave as those in inflamed tissue.22 Agonist mAbs directed to human CD137 (BMS663516 and PF-05082566) are undergoing clinical trials for cancer treatment, the results of which are eagerly awaited.25
Synergistic treatments that involved adenoviral gene transfer of IL-12 and agonist antibodies anti-CD137 or gene transfer of soluble forms of the natural CD137 ligand have been previously reported.26 The synergistic effects were dependent on T cells and NK cells.27,28 Our hypothesis was to exploit the powerful proimmunogenic effects of a suicidal but cytopathic RNA virus encoding IL-12 with systemic costimulation of CD8 T cells by agonist anti-CD137 mAb.
Results
Intratumoral SFV-IL-12 synergizes with systemic CD137 costimulation
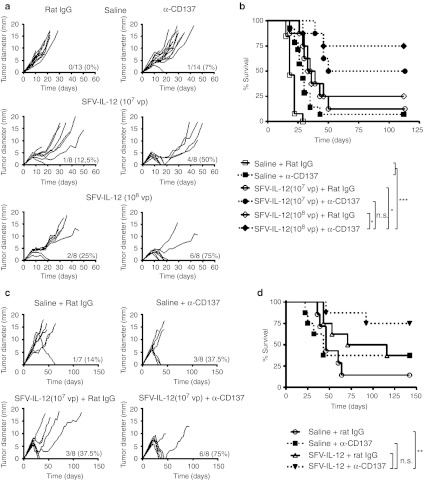
Although the SFV-IL-12 vector has shown a curative antitumoral efficacy in some murine models,10,14,15 its efficacy has been lower in other tumor models, such as B16 melanoma.29 We tested whether costimulation with an agonist mAb against CD137 could augment the antitumor potency of SFV-IL-12 vector in B16-OVA tumors. To study a potential synergy, we first determined the dose of SFV-IL-12 that was able to provide a suboptimal therapeutic effect in this melanoma model (Supplementary Figure S1). Since both 107 and 108 viral particles (vp) of SFV-IL-12 were able to provide a suboptimal antitumoral effect, mice bearing established B16-OVA tumors were treated intratumorally with each of the selected vector doses, or with saline, in combination with an agonist mAb against CD137 given intraperitoneally (Figure 1a). Control animals were treated with the same doses of SFV-IL-12 in combination with irrelevant rat immunoglobulin G (IgG). Mice treated with the CD137 agonist mAb as a single agent experienced tumor rejection in only 7% of the cases. As expected, SFV-IL-12 administration in combination with control IgG induced a modest response in treated animals, which rejected 12.5% and 25% of tumors intralesionally injected with 107 and 108 vp, respectively. In contrast, combined administration of both SFV-IL-12 and CD137 mAb greatly increased the therapeutic efficacy observed in single-agent treatments, inducing 50% and 75% of complete tumor remissions (Figure 1a,b) when the vector dose used was 107 or 108 vp, respectively. In order to determine the role of SFV cytopathic replication (Supplementary Figure S2a) in the antitumoral effect, we treated B16-OVA bearing mice with a single injection of 108 vp UV-inactivated SFV-LacZ + 15 ng of recombinant murine IL-12 in combination with systemic CD137 agonist mAb. As shown in Supplementary Figure S2b, SFV replication seemed to be necessary to achieve a good antitumoral synergy with CD137 mAb.
Figure 1.
Treatment efficacy of SFV-IL-12 + anti-CD137 combination. (a) C57BL/6 female mice were inoculated in the flank with 5 × 105 B16-OVA cells on day 0 and then received an intratumoral injection of saline (top panels), 107 viral particles (vp) (middle panels), or 108 vp of SFV-IL-12 (bottom panels) on day 7. On days 7, 10, and 14 mice received intraperitoneally 100 µg of rat immunoglobulin G (IgG) (left panels) or anti-CD137 mAb (right panels). (c) C57BL/6 female mice were inoculated in the flank with 5 × 105 TC-1 cells on day 0 and then received an intratumoral injection of saline (top panels) or 107 vp of SFV-IL-12 (bottom panels) on day 18. On days 18, 22, and 26 mice received intraperitoneally 100 µg of rat immunoglobulin G (IgG) (left panels) or anti-CD137 mAb (right panels). (a,c) Each curve represents the evolution of the mean tumor diameter for each individual mouse. The numbers in the right lower corner of each graph indicate the fraction of tumor-free mice on day 60 (for the B16-OVA model) or on day 121 (for the TC-1 model) relative to the total number of animals in each group, and the percentage of complete tumor regressions, respectively. (b,d) Kaplan–Meier plots of mouse survival. The SFV-IL-12 + anti-CD137-treated group (108 vp in b) was compared with the rest of the groups with the log-rank test. n.s., not significant; *P < 0.05; ** P < 0.01;*** P < 0.001. (a,b) The graphs correspond to pooled data of two independent experiments with similar results. α, anti-. SFV-IL-12, Semliki Forest virus encoding interleukin-12.
The combinatorial strategy was also tested in the murine lung TC-1 tumor model.30 This tumor model expresses human papilloma virus (HPV) E6 and E7 genes and has been frequently used as a surrogate for human tumors transformed by HPV-16. We first determined the dose of SFV-IL-12 that was able to provide a minimal therapeutic effect in this model and that would permit a demonstration of synergy on established TC-1 tumors of more than 5 mm of diameter grafted for 21 days31 (Supplementary Figure S3). Accordingly, we proceeded to treat TC-1 tumors by combining 107 vp of SFV-IL-12 intratumorally, or saline as control, with anti-CD137 mAb. Control mice were treated with the same dose of SFV-IL-12 in combination with a nonrelevant rat IgG. Animals receiving either SFV-IL-12 or anti-CD137 mAb treatment eradicated 37.5% of tumors. In contrast, combined treatment with SFV-IL-12 and anti-CD137 mAb was able to eradicate 75% of tumors (Figure 1c). All animals that eliminated tumors remained tumor-free until the end of the experiment 4 months later (Figure 1d).
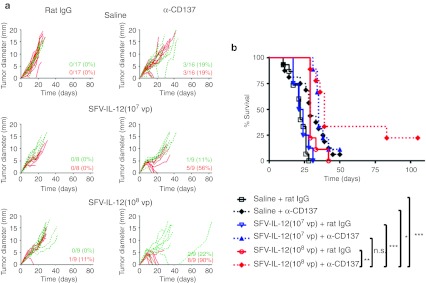
To determine whether SFV gene transfer of IL-12 was required on the same tumor nodule to display synergistic effects with anti-CD137 mAb, we conducted experiments in which two tumor nodules were generated by B16-OVA subcutaneous inoculation into opposite flanks of the same mouse. Once tumors reached 4–5 mm of diameter, one of the nodules in each mouse was inoculated with 107 or 108 vp of SFV-IL-12, or saline as control, leaving the other nodule untreated. Mice receiving SFV-IL-12 in combination with anti-CD137 mAb were again able to dramatically increase the number of complete regressions of SFV-treated nodules in comparison with animals treated with either agent by itself (Figure 2a, red lines). Although the effect of SFV-IL-12 + anti-CD137 on nontreated nodules was more modest, it was able to delay their growth in comparison to nontreated tumors from animals treated with SFV-IL-12 only (P = 0.0001 for 107 vp and P = 0.021 for 108 vp) (Figure 2a, green lines). This delay in tumor growth resulted in a longer survival of animals treated with the combined therapy (Figure 2b). To check whether this effect could be the result of SFV vector reaching nontreated nodules we inoculated mice bearing two tumors with 108 vp of SFV-Luc into one nodule. Luciferase expression was detected 24 hours later only in the injected nodules, suggesting that the antitumoral effect observed with SFV-IL-12+antiCD137 in nontreated tumors could be due to induction of a systemic immune response rather than dissemination of the viral vector to the contralateral tumor (Supplementary Figure S4). This was confirmed by the fact that a higher CD3+ infiltrate was observed in nontreated tumors in the SFV-IL-12+antiCD137 group as compared to nontreated tumors in the SFV-IL-12 or saline groups (Supplementary Figure S4b).
Figure 2.
Treatment efficacy of SFV-IL-12 + anti-CD137 combination on bilateral B16-OVA tumors. (a) C57BL/6 female mice were inoculated in contralateral flanks with 5 × 105 B16-OVA cells on day 0 and then received in the right tumor an injection of saline (top panels), 107 viral particles (vp) (middle panels), or 108 vp of SFV-IL-12 (bottom panels) on day 8. On days 8, 11, and 15 mice received intraperitoneally 100 µg of rat immunoglobulin G (IgG) (left panels) or anti-CD137 mAb (right panels). Red curves represent the evolution of SFV-IL-12-treated tumor diameter and green dotted curves represent the evolution of the nontreated tumor diameter for each individual mouse. Red numbers in the right lower corner of each graph indicate the number of completely rejected treated-tumors and green numbers indicate the number of completely rejected contralateral tumors on day 84 relative to the total number of animals in each group, and the percentage of complete tumor regressions, respectively. (b) Kaplan–Meier plot of mouse survival. The SFV-IL-12 (108 vp) + anti-CD137-treated group was compared with the rest of the groups with the log-rank test. n.s., not significant; *P < 0.05; **P < 0.01;***P < 0.001. The graphs correspond to pooled data from two independent experiments with similar results. α, anti-; SFV-IL-12, Semliki Forest virus encoding interleukin-12.
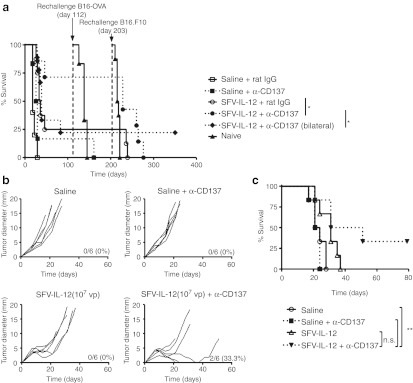
Cured animals from these experiments were rechallenged with B16-OVA and subsequently with B16.F10 cells, with an interval of 3 months between rechallenges. All mice that had been cured with SFV-IL-12, with or without anti-CD137, resisted the B16-OVA rechallenge, in contrast to the sole survivor treated with anti-CD137 monotherapy (Figure 3a). However, only mice that had been able to eliminate also the untreated concomitant tumors were resistant to the challenge with B16.F10, suggesting that the memory immune response generated in those animals was more potent.
Figure 3.
Evaluation of the generation of a protective immune memory response after SFV-IL-12 + anti-CD137 treatment and efficacy in the B16.F10 model. (a) Survivor mice from experiments shown in Figures 1a and 2 were rechallenged in a first step with 5 × 105 B16-OVA cells at day 112 and in a second step with 5 × 105 B16.F10 cells at day 203. When indicated, a group of naive animals were inoculated with the same tumor cells to control each rechallenge experiment. Mice survival was monitored and analyzed by sequential Kaplan–Meier plots. The different groups were compared with the log-rank test. (b) C57BL/6 female mice were inoculated in the flank with 5 × 105 B16.F10 cells on day 0 and then received an intratumoral injection of saline or 107 viral particles (vp) of SFV-IL-12 on day 7. On days 7, 10, and 14 mice received intraperitoneally 100 µg of anti-CD137 mAb as indicated in the figure. (c) Kaplan–Meier plot of mouse survival. The SFV-IL-12 + anti-CD137-treated group was compared with the rest of the groups with the log-rank test. n.s., not significant; *P < 0.05; **P < 0.01. α, anti-; SFV-IL-12, Semliki Forest virus encoding interleukin-12.
The expression of OVA in B16 tumor cells provides a xenoantigen which raises the antigenicity of the tumor. An experiment on established tumors derived from the B16.F10 cell line was carried out to disregard the need for xenoantigen. B16.F10 tumors were treated with each single agent (SFV-IL-12 or agonist CD137 mAb) or with the combination of both agents. As it can be seen in Figure 3b and c the combined treatment was able to eradicate 2 out of 6 tumors, while each single agent failed to cure any of the mice. Additionally, a noticeable retardation of tumor growth was observed in all mice treated with the combination therapy (Figure 3b).
CD8+ T cells are essential for the antitumor efficacy of combined treatment
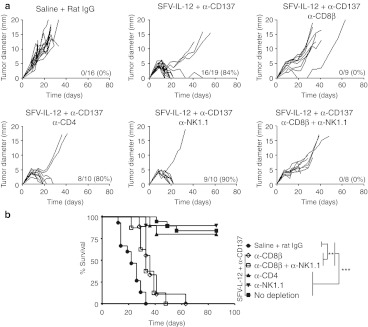
In order to determine which immune cell types were involved in the antitumoral effect mediated by SFV-IL-12 + anti-CD137 mAb therapy, we depleted CD4+, CD8+ T-cells, or NK cells in B16-OVA tumor-bearing animals before treatment. CD8+ T-cell depletion completely abrogated the antitumoral effect of the combined treatment, while CD4+ T-cell or NK depletion did not (Figure 4a). Although CD8+ T-cell depletion with anti-CD8β mAb prevented tumor eradications, a moderate delay in tumor growth was still observed in these mice, which led to a significantly longer survival in comparison to saline-treated animals (Figure 4b). We wondered whether the tumor growth delay observed in CD8+ T-cell-depleted mice could be due to NK cells. Double depletion of CD8+ T and NK cells resulted in a delay in tumor growth similar to that observed in CD8+ T-cell-depleted animals, indicating that NK cells were not involved in this effect, in contrast to previous observations with IL-12 gene therapy + CD137 agonist antibodies.27
Figure 4.
CD8+ T cell requirement for SFV-IL-12 + anti-CD137 treatment efficacy. (a) C57BL/6 female mice were inoculated in the flank with 5 × 105 B16-OVA cells on day 0 and then received intratumorally saline (first panel) or 108 viral particles (vp) of SFV-IL-12 (rest of the panels) on day 7. On days 7, 10, and 14 mice treated with saline received intraperitoneally 100 µg of rat immunoglobulin G (IgG) and mice treated with SFV-IL-12 received a course of anti-CD137 mAb as in Figure 1. Depletions of CD4+, CD8β+, and NK1.1+ cells were performed by intraperitoneal injection of 100 µg of specific antibodies administered at days 6, 10, 15, 21, and 28 for αCD4+ and αCD8+ and at days 6, 8, 10, 12, 15, 21, and 28 for αNK1.1+. Each curve represents the evolution of the mean tumor diameter for each individual mouse. The numbers in the right lower corner of each graph indicate the number of tumor-free mice on day 70 relative to the total number of animals in each group, and the percentage of sustained complete tumor regressions, respectively. (b) Kaplan–Meier plot of mouse survival. The different groups were compared with the log-rank test. **P < 0.01;***P < 0.001. The graphs correspond to pooled data from two independent experiments with similar results. α, anti-; SFV-IL-12, Semliki Forest virus encoding interleukin-12.
Combined therapy induces dramatic increases in CD8+ T-cell numbers and tumor-specific CTLs
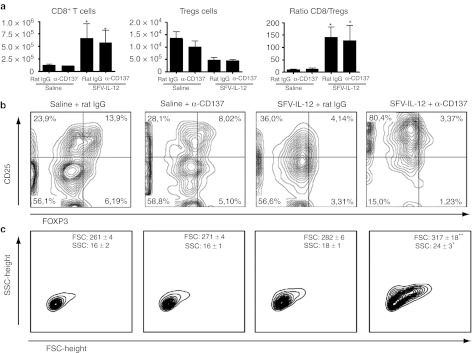
To further determine the immune cellular mechanisms that are taking place in the tumor environment, we characterized tumor infiltrating lymphocytes (TILs). It is broadly accepted that a positive ratio between effector CD8+ T and Treg cells within the tumor is a good sign of prognosis. Indeed, this was the case in all animals treated with SFV-IL-12, where a significant increase in the number of CD8+ T-cells was accompanied by a reduction in the number of Treg cells (Figure 5a,b) both in terms of percentage and absolute numbers. Interestingly, tumor infiltrating CD8+ T-cells from animals treated with the combined therapy exhibited a blast transformation phenotype, showing higher size and complexity upon fluorescence-activated cell sorting (FACS) examination (Figure 5c). The analysis of tumors also showed that mice receiving anti-CD137 mAb, with or without SFV-IL-12, had reduced numbers of tumor-infiltrating CD4+ T-cells (Supplementary Figure S5). NK and NKT cells were diminished in tumors of mice treated with SFV-IL-12, agonist anti-CD137 mAb, or both. Some changes were also observed in the myeloid-derived compartment of the tumor stroma, encompassing an elevation of Ly6G+ Ly6Cint or low cells in mice treated with the combination therapy (Supplementary Figure S5). However, in spite of these changes selective depletions of Ly6G+ and Ly6C+ cells did not alter the combined therapeutic efficacy (Supplementary Figure S6).
Figure 5.
SFV-IL-12 + anti-CD137 therapy augments the CD8/Treg ratio and the quality of CD8+ T cells. C57BL/6 female mice were flank-inoculated with 5 × 105 B16-OVA cells on day 0 and then received intratumorally saline or 108 viral particles (vp) of SFV-IL-12 on day 7. On days 7 and 10 mice received intraperitoneally 100 µg of rat immunoglobulin G (IgG) or anti-CD137 mAb. On day 11 mice were sacrificed and tumors were harvested, processed, and analyzed by flow cytometry. (a) Graphs show the number of CD8+ T cells (left), Tregs (middle), and the ratio between CD8+ T and Treg lymphocytes (right). The results are represented as the mean ± standard error of mean (SEM). (b) Representative dot plots of CD25 and FoxP3 expression on CD4+ T cells. Numbers indicate the percentages of cells in each quadrant analyzed by fluorescence-activated cell sorting (FACS). Tregs are identified in the upper-right quadrants as CD4+CD25+Foxp3+ cells. (c) Plots showing FACS-assessed size and complexity of intratumoral CD8+ T cells. The values on the right upper corner of each panel represent the mean ± SD for FSC or SSC. *P < 0.05; **P < 0.01; α, anti-; SFV-IL-12, Semliki Forest virus encoding interleukin-12.
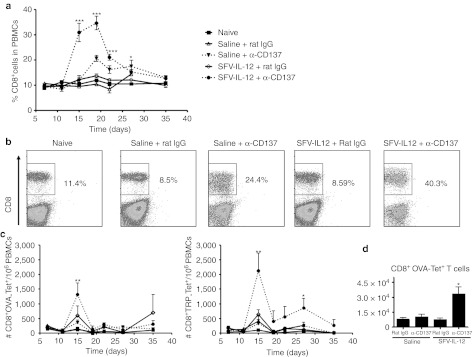
Mice treated with the combination of SFV-IL-12 and anti-CD137 mAb exhibited a huge increase in the percentage of circulating CD8+ lymphocytes which reached a maximum of 40% at 19 days after tumor treatment (Figure 6a,b). Agonist CD137 mAb by itself also boosted the percentage of circulating CD8+ T cells but in a much less pronounced manner. Importantly, CD8+ T-cells specific for OVA peptide SIINFEKL and for tyrosine-related protein-2 (TRP)-derived peptide presented by H2-Kb were significantly increased at the peak of the response in mice that had been treated with both agents in combination (Figure 6c). In good correlation with these results, a significant increment in OVA-specific CD8+ T cells was also observed in the spleen of mice treated with the combination (Figure 6d).
Figure 6.
Combined treatment increases CD8+ T cell and tumor-specific CD8+ T cell numbers. (a–c) C57BL/6 female mice were inoculated in the flank with 5 × 105 B16-OVA cells on day 0 and then received intratumorally saline or 108 viral particles (vp) of SFV-IL-12 on day 7. On days 7, 10, and 14 mice received intraperitoneally 100 µg of rat immunoglobulin G (IgG) or anti-CD137 mAb. Mice were bled at days 7, 11, 15, 19, 22, 27, and 35 and percentages of CD8+ T cells (a,b) or the number of CD8+ T cells per 106 peripheral blood mononuclear cells which were OVA- (c, left panel) or tyrosine-related protein-2 (TRP-2)-specific (c, right panel) were determined by flow cytometry. (d) C57BL/6 female mice were inoculated in the flank with 5 × 105 B16-OVA cells on day 0 and then received intratumorally saline or 108 vp of SFV-IL-12 on day 7. On days 7 and 10 mice received intraperitoneally 100 µg of rat IgG or anti-CD137 mAb. On day 11 mice were sacrificed and spleens were harvested, processed, and analysed by flow cytometry. The graph shows the number of OVA-specific CD8+ T cells in the spleens of treated mice. *P < 0.05; **P < 0.01; ***P < 0.001. α, anti-; SFV-IL-12, Semliki Forest virus encoding interleukin-12.
Combination therapy enhanced CD8+ T-cell effector responses
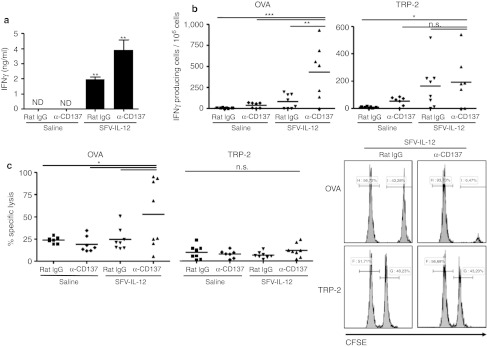
Since both IL-12 and CD137 stimulation can lead to T and NK cell activation, the combination of both agents could result in enhanced IFNγ expression. To check this point, mice were bled one day after treatment and serum IFNγ concentrations were determined by enzyme-linked immunosorbent assay. IFNγ was only detected in SFV-IL-12-treated mice, showing further enhanced concentrations in mice treated also with anti-CD137 mAb (Figure 7a). An enzyme-linked immunospot (ELISPOT) assay was also performed at day 7 after treatment by incubating splenocytes in the presence of major histocompatibility complex-I restricted peptides from OVA and TRP-2 proteins. For both peptides, mice treated with the combination therapy showed the highest number of IFNγ-producing spots, especially in the case of the OVA antigenic determinant (Figure 7b).
Figure 7.
Enhancement of immune responses with SFV-IL-12 + anti-CD137 combined therapy. C57BL/6 female mice were subcutaneously inoculated with 5 × 105 B16-OVA cells on day 0 and then received intratumorally saline or 108 viral particles (vp) of SFV-IL-12 on day 7. On days 7 and 10 mice received intraperitoneally 100 µg of rat immunoglobulin G (IgG) or anti-CD137. (a) At day 1 after treatment onset, mice were bled and sera samples were analyzed by enzyme-linked immunosorbent assay (ELISA) to determine interferon γ (IFNγ) concentrations. The results are represented as the mean ± SEM (n = 4 per group). ND, not detected. (b) On day 14, mice were sacrificed and spleens were processed to carry out an IFNγ enzyme-linked immunospot (ELISPOT) assay using OVA or tyrosine-related protein (TRP)-specific peptides for stimulation. The data are represented as number of IFNγ-producing spots per 106 splenocytes. (c) In-vivo killing assay. On day 13, tumor peptide-pulsed splenocytes from naive CD45.1 mice were injected to treated mice and 20 hours later mice were sacrificed and spleens collected. The percentage of specific cell lysis was quantified by flow cytometry. The set histograms in the right show representative cases of this experiment. n.s., not significant; *P < 0.05; **P < 0.01. α, anti-; SFV-IL-12, Semliki Forest virus encoding interleukin-12.
In-vivo cytotoxicity assays were performed at the same time-point revealing that mice receiving an intratumoral injection of SFV-IL-12 and anti-CD137 mAb intraperitoneally exhibited higher specific lysis when challenged with SIINFEKL-loaded splenocytes (Figure 7c, left graph). However, we did not observe an increase in specific killing when cells were loaded with TRP-2 peptide (Figure 7c, central and right graphs). The reasons for the discrepancy between in vivo killing and ELISPOT assays remain unknown, but lower TCR avidity in the CTLs recognizing an endogenous antigen such as TRP-2, as opposed to a foreign (chicken) antigen might be involved.
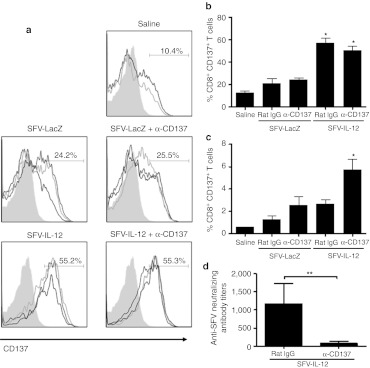
The enhanced therapeutic effect of SFV-IL-12 in combination with anti-CD137 mAb is mediated by upregulation of CD137 mAb
To understand the mechanisms underlying the synergistic effects exhibited by the combination of SFV-IL-12 and CD137 stimulation, we checked the expression levels of CD137 on CD8+ T cells in different experimental groups. An SFV vector encoding a LacZ irrelevant gene was also included as control to rule out the possible effects induced by SFV infection. Interestingly, SFV-IL-12 administration induced a significant upregulation of CD137 on the surface of tumor infiltrating CD8+ T cells 48 hours after intratumoral injection (Figure 8a,b). In contrast, CD137 was only significantly upregulated on CD8+ T-cells from tumor-draining lymph nodes when both SFV-IL-12 and anti-CD137 mAb were given simultaneously (Figure 8c). These results suggest that intratumoral IL-12 expression could increase the availability of target molecules for CD137-agonistic mAbs on CD8+ T cells both in tumor and draining lymph node environments. More abundant CD137 expression would be at least one of the cooperative mechanisms that explain why combined treatments attain synergy.
Figure 8.
SFV-IL-12 + anti-CD137 combined therapy up-regulates CD137 on CD8+ T cells and reduces anti-SFV humoral immune responses. (a–c) B16-OVA tumor-bearing mice were treated intratumorally with saline or 108 viral particles (vp) of SFV-LacZ or SFV-IL-12 on day 7. The same day mice received intraperitoneally 100 µg of rat immunoglobulin G (IgG) or anti-CD137 mAb. Two days later, mice were sacrificed and tumor nodules and draining lymph nodes (DLNs) were processed and analyzed by flow cytometry. (a) Plots showing CD137 expression level on CD8+ T cells from tumor nodules. (b,c) Graphs showing the percentage of CD8+ T cells that upregulate CD137 expression on their surface in tumor nodules or DLNs, respectively. (d) B16-OVA tumor-bearing mice treated with SFV-IL-12 + rat IgG, or anti-CD137 mAb, as described in Figure 1, were bled at day 21 and sera samples were analyzed to determine anti-SFV neutralizing antibody titers. The results are represented as mean ± SEM. *P < 0.05; **P < 0.01. α, anti-; SFV-IL-12, Semliki Forest virus encoding interleukin-12.
Combined therapy reduces humoral immune responses against the vector
The presence of SFV-neutralizing antibodies was analyzed at day 21 after treatment in mice that received SFV-IL-12 in combination with anti-CD137 mAb, or with a nonrelevant rat IgG as control. Interestingly, those animals treated with the combined therapy developed SFV neutralizing antibodies at titers that were >14-fold lower than those present in animals treated only with SFV-IL-12 (Figure 8d). This finding, which is in line with previous reports on inhibition of humoral immunity by CD137 mAb,32,33 represents an additional advantage of the combined therapy because lower SFV neutralizing antibody titers would allow higher infectivity if several doses of the vector are to be used repeatedly.
Discussion
Combinatorial treatments are perceived as a major pathway for progress in cancer immunotherapy.34 Synergistic effects, rather than additive effects are searched for, taking into account the complementarities of the elicited mechanisms. In this study, we have explored and documented the efficacy of combined intratumoral injections of a SFV-based vector encoding IL-12 and systemic treatment with an agonist anti-CD137 mAb. Such a strategy is certainly feasible because two human antibodies directed to CD137 are currently undergoing clinical trials, and the production of clinical-grade alphaviruses has been reported35 and is in progress in our institution.
Previous work had defined that IL-12-based gene transfer with adenoviral vectors and stimulation of the CD137 pathway can be combined in mouse models.27 In our studies we set out to exploit several of the unique features of SFV encoding IL-12 to turn a tumor lesion into an immunogenic vaccine. In this regard SFV-IL-12: (i) releases locally abundant IL-12, (ii) kills infected malignant cells in a stressful fashion,36 (iii) fills the cells with replicating viral RNA which turns on the innate immune response.4 Tumor antigens under these conditions should dramatically increase their immunogenicity. This probably explains why SFV-IL-12 achieves potent therapeutic results in immunotherapy of transplanted tumors and partial efficacy in spontaneous hepatocellular carcinomas in woodchucks.4,10,16
Immunostimulatory mAbs provide versatile tools to release the brakes or step on the gas pedals of immune system cells.25 There is panoply of examples in which these agents synergize with cancer vaccines.37 CD137 costimulation is one of the most potent examples in this regard.37 In this study, we changed this paradigm to locally transform at least part of the tumor lesion into a potent immunogen, whose effect would be multiplied by providing CD137 costimulation to primed T lymphocytes. We have previously tried to pursue a similar goal injecting tumor lesions with type I IFN.38 However, it is clear that intratumoral treatment with SFV-IL-12 is endowed with manifold more immunogenic characteristics than a local release of a single recombinant cytokine. Efficacy of CD137 agonists in monotherapy relies on dendritic cell-mediated tumor antigen crosspriming39 and thereby enhancing immunogenic crosspresentation of tumor antigens upon SFV infection should attain more efficacious therapeutic outcomes. It is likely that tumor cell death provides antigen material ready for crosspresentation by surrounding DCs. In this regard, we have observed that both treatment with antibody and the combined treatment enhanced the level of dendritic cell activation in the tumor tissue (data not shown).
In this study, efficacy was defined using established B16-OVA and B16.F10 melanomas as well as TC-1 lung carcinomas. In both cases, intrinsic immunogenicity is low and defined tumor antigens are available to monitor the immune response. Doses of SFV-IL-12 were titrated first to allow us to observe the synergy. Reducing vector doses offers also a safety advantage because of the toxicity profile of IL-12.9 It is of interest that the synergistic combination achieved efficacy in all tested models, including established B16.F10 melanomas devoid of any model antigen. Efficacy on this immunotherapy-refractory tumor is a promising feature for clinical translation.
The most prominent observation is that combined treatment with SFV-IL-12 and CD137 agonist mAb elicits a very robust CTL-mediated immune response able to completely eliminate most treated tumors and retard growth in concomitant malignant lesions. In contrast to studies from the group of Chen and colleagues with IL-12-encoding adenovirus, in our case NK cells were dispensable for antitumor activity.27 NK were not needed in previous reports of efficacious treatments with SFV-IL-12 in monotherapy,10 while the effects of CD137 agonist antibodies are NK-dependent in various models.20 Upon CD8β depletion some retardation of the tumors was still observed that could be related to the described antiangiogenic effects of IL-12 encoded by SFV vectors.29 Surprisingly, tumor infiltrates showed reductions in absolute numbers of NK/NKT cells, in spite of the well known IL-12 effects on these lymphocyte subsets. Although we do not have yet a mechanistic explanation it is of note that recent information suggests that NK cells can be harmful for the elicitation of systemic CD8 memory.40
In the case of B16-OVA, the CTL response against OVA is readily detected in terms of ELISPOT and in-vivo killing assays while the response to the melanosomal protein TRP-2 seems weaker. However, we have observed vitiligo surrounding the areas where the tumor had been grafted in about half of the mice cured from B16-OVA (Supplementary Figure S7).
The mechanisms of synergy between CD137 stimulation and SFV-IL-12 intratumoral injection can be multiple. The simplest synergistic mechanism would be that SFV-IL-12 injection would prime tumor-specific CTL to express CD137 on the surface. Once the target for the immunostimulatory mAb becomes available and is ligated by mAb, such CTL are protected from apoptosis and enhanced in their performance. Other proinflammatory mediators elicited by SFV at the tumor microenvironment beyond IL-12 are likely to be involved, including type I IFN and chemokines. Such factors acting in concert are known to enhance CTL responses while attracting effector lymphocytes toward infected tissue. Indeed other ongoing studies from our group show the absolute need of the type I IFN system for the antitumor effects of SFV-IL-12 (S. Hervas-Stubbs et al., manuscript in preparation). In agreement with these observations, we also showed that SFV replication in tumors was a requirement to achieve a good synergy between IL-12 and anti-CD137 antibodies (Supplementary Figure S2b).
Another effect of anti-CD137 agonist antibodies is the suppression of ongoing humoral responses acting on T helper cells32 and the network of follicular dendritic cells.33 We have documented that the response against SFV particles is markedly downsized by anti-CD137 mAb, reaching levels that would allow an efficient readministration of the SFV vector.41 This has implications for treatment repetitions that might extend the therapeutic effects.
Overall, we have defined a clinically feasible and potent therapeutic combination encompassing intratumoral treatments with SFV-IL-12 and systemic costimulation with anti-CD137 mAb. The mechanisms are viewed as the result of enhanced immunogenic antigen presentation that primes CTL, which upon becoming activated receive the stimuli of IL-12 and anti-CD137 mAb in a context that resembles many of the danger signals featuring viral infections that so potently elicit CTL responses.
Materials and Methods
Cell lines and animals. The BHK-21 cell line (ATCC CCL-10) was cultured as described previously.10 The murine melanoma cell line B16.F10 and the OVA-expressing variant B16-OVA (H-2b) and the TC-1 cell line, a lung epithelial cell line transformed by the HPV-16 E6 and E7 oncogenes,30 were tested to be mycoplasma-free and verified for identity by Johns Hopkins Genetic Resources Core Facility. Five-week-old female C57BL/6 mice were purchased from Harlan Laboratories (Barcelona, Spain). Animal studies were approved by the institutional ethical committee for animal experimentation under Spanish regulations.
Vector production. Plasmids pSFV-enhLacZ, pSFV-Luc, and pSFV-enhIL-12 have been described previously.10,42 RNA synthesis from SFV plasmids, transfection into BHK-21 cells by electroporation, and packaging of recombinant RNA into SFV vp were performed as described previously.36 Briefly, BHK-21 cells were co-electroporated with the recombinant RNA, together with two helper RNAs (i.e., SFV-helper-C-S219A and SFV-helper-S2 RNAs), which provided in trans the capsid and the spike proteins, respectively.43 SFV vp were harvested and purified by ultracentrifugation as described previously.44 Indirect immunofluorescence was applied to SFV-infected BHK-21 cells to determine the titer of SFV-Luc, SFV-enhLacZ, and SFV-enhIL-12 (designated in this manuscript as SFV-LacZ and SFV-IL-12, respectively) recombinant virus stocks as described previously.45 The cytopathic effect induced by SFV-IL-12 on tumor cell lines was determined by infecting cell monolayers of B16-OVA and TC-1 with a multiplicity of infection of 1,000 (Supplementary Figure S2a).
In-vivo tumor experiments. Mice were injected with B16-OVA, B16.F10, or TC-1 cells (5 × 105 cells per animal) subcutaneously in the right flank or in both flanks as detailed in each experiment. Treatments were given from 7 to 8 days (B16) and 18 to 21 days (TC-1) after tumor inoculation as detailed for each experiment, when tumors reached 4–5 mm of average diameter. SFV-IL-12 resuspended in 30 µl of phosphate-buffered saline was injected intratumorally with 28G needles. Anti-CD137 mAb was produced from the 2A hybridoma (kindly provided by Dr Lieping Chen, Yale University, New Haven, CT). The mAb was purified from culture supernatant, dialyzed and quality controlled including determinations of lipopolysaccharide concentration (Antibody BCN, Barcelona, Spain, as a contractor). Control IgG from rat serum was obtained from Sigma-Aldrich (Dorset, UK). Tumor growth was monitored every 3–4 days by measuring two perpendicular tumor diameters, and considering the average diameter as an indicator of tumor size.
Depletion of immune cells. Depletions of CD4+, CD8+, or NK1.1+ were performed by intraperitoneal injections of 100 µg of specific antibodies against CD4 (GK 1.5), CD8β (H3S-17-2), and NK1.1 (PK136). Anti-CD4 and anti-CD8β antibodies were injected at days 6, 10, 15, 21, and 28 after tumor inoculation. Anti-NK1.1 antibody was injected at days 6, 8, 10, 12, 15, 21, and 28 after tumor cell inoculation. Depletions were monitored by FACS analysis and only mice with a depletion efficacy higher than 99% for each specific cell type were used.
In-vivo killing assay. Splenocytes from naive C57BL/6 mice congeneic for the CD45.1 allele were divided into three different samples. Two of these samples were pulsed with 10 µg/ml of the OVA257–264 peptide (SIINFEKL; NeoMPS, Strasbourg, France) or the TRP-2180–188 peptide (SVYDFFVWL) for 30 minutes at 37 °C in 5% CO2 and washed extensively. The third part remained without any peptide as an additional control. OVA-pulsed, TRP-2-pulsed, and nonpulsed splenocytes were then labeled with 1 µmol/l (CFSEhi), 100 nmol/l (CFSEmed), or 10 nmol/l (CFSElow) of CFSE (Sigma-Aldrich), respectively. The three samples were mixed at the same ratio and injected intravenously (5 × 106 cells of each population) into B16-OVA-bearing or naive mice. Twenty hours later, spleens were harvested and transferred target cells were gated using an anti-CD45.1 PE-conjugated antibody. Specific cytotoxicity was analyzed by flow cytometry and calculated as follows: 100 − [100 × (% CFSEhi or CFSEmed tumor-bearing mice/% CFSElow tumor-bearing mice)/(%CFSEhi or CFSEmed naive mice/% CFSElow naive mice)].
Serum determination of IFNγ by enzyme-linked immunosorbent assay and IFNγ-ELISPOT assays. IFNγ concentrations were determined in sera using a mouse IFNγ-specific enzyme-linked immunosorbent assay kit (BD Bioscience, Franklin Lakes, NJ). ELISPOT assays for the detection of tumor-specific interferon-γ-producing spleen cells were performed as described.46 4 × 105 spleen mononuclear cells from the different groups of mice were incubated for 24 hours with or without the specific peptide (OVA257–264 or TRP-2180–188). Spots were quantified using the automated CTL-ImmunoSpot S5 Micro Analyzer (CTL-Europe, Bonn, Germany) and results were expressed as number of spots/106 spleen mononuclear cells.
Isolation of mononuclear cells from lymph node and tumors and flow cytometry. To obtain unicellular cell suspensions, tumor-draining lymph nodes from pooled inguinal, brachial, and axilar lymph nodes from tumor-bearing mice, subcutaneous tumor nodules, and spleens were surgically harvested. These organs were incubated in Collagenase-D and DNase-I (Roche, Basel, Switzerland) for 15 minutes at 37 °C. Dissociated cells were passed through a 70-µm nylon mesh filter (BD Falcon, BD Bioscience, San Jose, CA). Single-cell suspensions were pretreated with FcR-Block (anti-CD16/32 clone 2.4G2; BD Biosciences-Pharmigen, San Jose, CA). Afterwards, cells were stained with specific antibodies for CD8, CD4, CD3, CD137, NK1.1, CD11b (M1/70), Ly6G (1A8), Ly6C (AL21), CD25, and FoxP3, as well as using Mouse regulatory T cell staining kit purchased from e-bioscience (catalog number 88–8,111). To identify tumor-specific CD8 T lymphocytes, cells were stained with the iTAg major histocompatibility complex class I tetramer loaded with the SIINFEKL synthetic peptide and the TRP-2-specific pentamer conjugated with PE. For tetramer kinetics, blood samples were extracted at indicated time points by tail bleeding. Cells were analyzed with a FACSCalibur or FACSCanto (BD, San Agustin de Guadalix, Spain) and FlowJo software.
Evaluation of anti-SFV neutralizing antibodies in SFV-treated mice. The presence of SFV neutralizing antibodies in serum of SFV-treated animals was analyzed by performing an in vitro infection assay of BHK cells with SFV-Luc in the presence of different amounts of sera from the SFV-treated animals.10
Statistical analyses. All error terms are expressed as the SEM. Monolix software (http://www.monolix.org/) was used for analysis of tumor growth by non-linear mixed effect models in bilateral experiments. Prism software (GraphPad Software, San Diego, CA) was employed for the rest of statistical analysis. Survival of tumor bearing animals was represented by Kaplan–Meier plots and analyzed by log-rank tests. Data were analyzed first by the Kolmogorov–Smirnov Normality test. Mann–Whitney U-test was used to compare two experimental groups. One-way ANOVA or Kruskal–Wallis tests followed by Bonferroni or Dunn's Multiple comparison tests were used to compare four experimental group experiments, depending on whether the data were or were not from a normally distributed sample, respectively. P values <0.05 were considered to be statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Evaluation of antitumor efficacy of SFV-IL-12 in B16-OVA tumors. Figure S2. Analysis of cytopathic effect of SFV-IL-12 in tumor cells and role of SFV replication on antitumoral efficacy in combined treatment. Figure S3. Evaluation of antitumor efficacy of SFV-IL-12 in TC-1 tumors. Figure S4. Treatment efficacy of SFV-IL-12 + anti-CD137 combination on bilateral tumors is not mediated by dissemination of the viral vector but immune system mediated. Figure S5. Tumor infiltrating leukocytes. Figure S6. Depletion of myeloid cells does not affect antitumor efficacy of SFV-IL-12 + anti-CD137 mAb treatment. Figure S7. Vitiligo occurrence in cured mice.
Acknowledgments
We acknowledge Puri Fortes, Ester Larrea, Pedro Berraondo, and Jesús Prieto for scientific discussions and long-term support and reagents. The excellent technical assistance of Arantza Azpilikueta, Erkuden Casales, Elixabet Bolaños, Eneko Elizalde, and Laura Guembe is also acknowledged. The financial support was provided by MICINN (SAF2011-22831), the Spanish Health Ministry (FIS-PI081660 and PI11/02190), Departamento de Educación del Gobierno de Navarra (grants Ortiz de Landázuri and GNE-VECTORES ALFAVIRUS), RETIC (RD06/0020/0065), and CIMA-UTE Project. J.I.Q. was the recipient of a Torres Quevedo contract, M.F.-S. is a recipient of a Rio Ortega contract from ISCIII and S.H.-S. receives a Ramon y Cajal contract from MICINN.
Supplementary Material
Evaluation of antitumor efficacy of SFV-IL-12 in B16-OVA tumors.
Analysis of cytopathic effect of SFV-IL-12 in tumor cells and role of SFV replication on antitumoral efficacy in combined treatment.
Evaluation of antitumor efficacy of SFV-IL-12 in TC-1 tumors.
Treatment efficacy of SFV-IL-12 + anti-CD137 combination on bilateral tumors is not mediated by dissemination of the viral vector but immune system mediated.
Tumor infiltrating leukocytes.
Depletion of myeloid cells does not affect antitumor efficacy of SFV-IL-12 + anti-CD137 mAb treatment.
Vitiligo occurrence in cured mice.
REFERENCES
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ., and, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- Mazzolini G, Narvaiza I, Martinez-Cruz LA, Arina A, Barajas M, Galofré JC.et al. (2003Pancreatic cancer escape variants that evade immunogene therapy through loss of sensitivity to IFNgamma-induced apoptosis Gene Ther 101067–1078. [DOI] [PubMed] [Google Scholar]
- Quetglas JI, Ruiz-Guillen M, Aranda A, Casales E, Bezunartea J., and, Smerdou C. Alphavirus vectors for cancer therapy. Virus Res. 2010;153:179–196. doi: 10.1016/j.virusres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Barajas M, Mazzolini G, Genové G, Bilbao R, Narvaiza I, Schmitz V.et al. (2001Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12 Hepatology 3352–61. [DOI] [PubMed] [Google Scholar]
- Tahara H, Zitvogel L, Storkus WJ, Zeh HJ 3rd, McKinney TG, Schreiber RD.et al. (1995Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector J Immunol 1546466–6474. [PubMed] [Google Scholar]
- Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I.et al. (2004Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors J Clin Oncol 221389–1397. [DOI] [PubMed] [Google Scholar]
- Triozzi PL, Strong TV, Bucy RP, Allen KO, Carlisle RR, Moore SE.et al. (2005Intratumoral administration of a recombinant canarypox virus expressing interleukin 12 in patients with metastatic melanoma Hum Gene Ther 1691–100. [DOI] [PubMed] [Google Scholar]
- Melero I, Mazzolini G, Narvaiza I, Qian C, Chen L., and, Prieto J. IL-12 gene therapy for cancer: in synergy with other immunotherapies. Trends Immunol. 2001;22:113–115. doi: 10.1016/s1471-4906(00)01824-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Madoz JR, Prieto J., and, Smerdou C. Semliki forest virus vectors engineered to express higher IL-12 levels induce efficient elimination of murine colon adenocarcinomas. Mol Ther. 2005;12:153–163. doi: 10.1016/j.ymthe.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Guan M, Rodriguez-Madoz JR, Alzuguren P, Gomar C, Kramer MG, Kochanek S.et al. (2006Increased efficacy and safety in the treatment of experimental liver cancer with a novel adenovirus-alphavirus hybrid vector Cancer Res 661620–1629. [DOI] [PubMed] [Google Scholar]
- Ying H, Zaks TZ, Wang RF, Irvine KR, Kammula US, Marincola FM.et al. (1999Cancer therapy using a self-replicating RNA vaccine Nat Med 5823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P., and, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (NY) 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Chikkanna-Gowda CP, Sheahan BJ, Fleeton MN., and, Atkins GJ. Regression of mouse tumours and inhibition of metastases following administration of a Semliki Forest virus vector with enhanced expression of IL-12. Gene Ther. 2005;12:1253–1263. doi: 10.1038/sj.gt.3302561. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Zullo SA, Tanaka R, Ramsey J, Blaese M., and, Xanthopoulos KG. Induction of a therapeutic antitumor immunological response by intratumoral injection of genetically engineered Semliki Forest virus to produce interleukin-12. Neurosurg Focus. 2000;9:e7. doi: 10.3171/foc.2000.9.6.8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Madoz JR, Liu KH, Quetglas JI, Ruiz-Guillen M, Otano I, Crettaz J.et al. (2009Semliki forest virus expressing interleukin-12 induces antiviral and antitumoral responses in woodchucks with chronic viral hepatitis and hepatocellular carcinoma J Virol 8312266–12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE.et al. (1997Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors Nat Med 3682–685. [DOI] [PubMed] [Google Scholar]
- Ye Z, Hellström I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA., and, Hellström KE. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT.et al. (1993Inducible T cell antigen 4-1BB. Analysis of expression and function J Immunol 150771–781. [PubMed] [Google Scholar]
- Melero I, Johnston JV, Shufford WW, Mittler RS., and, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- Choi BK, Kim YH, Kwon PM, Lee SC, Kang SW, Kim MS.et al. (20094-1BB functions as a survival factor in dendritic cells J Immunol 1824107–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazón A, Teijeira A, Martínez-Forero I, Hervás-Stubbs S, Roncal C, Peñuelas I.et al. (2011Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes Cancer Res 71801–811. [DOI] [PubMed] [Google Scholar]
- Murillo O, Arina A, Hervas-Stubbs S, Gupta A, McCluskey B, Dubrot J.et al. (2008Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma Clin Cancer Res 146895–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J.et al. (2011CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies Blood 1172423–2432. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ascierto PA, Simeone E, Sznol M, Fu YX., and, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Chen SH, Pham-Nguyen KB, Martinet O, Huang Y, Yang W, Thung SN.et al. (2000Rejection of disseminated metastases of colon carcinoma by synergism of IL-12 gene therapy and 4-1BB costimulation Mol Ther 239–46. [DOI] [PubMed] [Google Scholar]
- Xu D, Gu P, Pan PY, Li Q, Sato AI., and, Chen SH. NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer. 2004;109:499–506. doi: 10.1002/ijc.11696. [DOI] [PubMed] [Google Scholar]
- Martinet O, Ermekova V, Qiao JQ, Sauter B, Mandeli J, Chen L.et al. (2000Immunomodulatory gene therapy with interleukin 12 and 4-1BB ligand: long-term remission of liver metastases in a mouse model J Natl Cancer Inst 92931–936. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C, Lassau N, Guinebretière JM, Zhang J, Gay F, Bex F.et al. (1999Transfer of the murine interleukin-12 gene in vivo by a Semliki Forest virus vector induces B16 tumor regression through inhibition of tumor blood vessel formation monitored by Doppler ultrasonography Gene Ther 6606–615. [DOI] [PubMed] [Google Scholar]
- Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM.et al. (1996Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen Cancer Res 5621–26. [PubMed] [Google Scholar]
- Berraondo P, Nouzé C, Préville X, Ladant D., and, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res. 2007;67:8847–8855. doi: 10.1158/0008-5472.CAN-07-0321. [DOI] [PubMed] [Google Scholar]
- Mittler RS, Bailey TS, Klussman K, Trailsmith MD., and, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med. 1999;190:1535–1540. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Blink SE, Chen JH., and, Fu YX. Regulation of follicular dendritic cell networks by activated T cells: the role of CD137 signaling. J Immunol. 2005;175:884–890. doi: 10.4049/jimmunol.175.2.884. [DOI] [PubMed] [Google Scholar]
- Takeda K, Okumura K., and, Smyth MJ. Combination antibody-based cancer immunotherapy. Cancer Sci. 2007;98:1297–1302. doi: 10.1111/j.1349-7006.2007.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DI, Reap EA, Katen K, Watson A, Smith K, Norberg P.et al. (2009Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers Vaccine 28484–493. [DOI] [PubMed] [Google Scholar]
- Liljeström P., and, Garoff H.Expression of proteins using Semliki Forest virus vectors. Current Protocols in Molecular Biology F. Ausubel et al. eds.)16.20.11–16.20.16.Greene Publishing Associates and Wiley Interscience: New York, NY [Google Scholar]
- Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM., and, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- Dubrot J, Milheiro F, Alfaro C, Palazón A, Martinez-Forero I, Perez-Gracia JL.et al. (2010Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ Cancer Immunol Immunother 591223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo O, Dubrot J, Palazón A, Arina A, Azpilikueta A, Alfaro C.et al. (2009In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb Eur J Immunol 392424–2436. [DOI] [PubMed] [Google Scholar]
- Soderquest K, Walzer T, Zafirova B, Klavinskis LS, Polic B, Vivier E.et al. (2011Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses J Immunol 1863304–3308. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Madoz JR, Prieto J., and, Smerdou C. Biodistribution and tumor infectivity of semliki forest virus vectors in mice: effects of re-administration. Mol Ther. 2007;15:2164–2171. doi: 10.1038/sj.mt.6300274. [DOI] [PubMed] [Google Scholar]
- Quetglas JI, Fioravanti J, Ardaiz N, Medina-Echeverz J, Baraibar I, Prieto J.et al. (2012A Semliki Forest virus vector engineered to express IFNa induces efficient elimination of established tumors Gene Ther 19271–278. [DOI] [PubMed] [Google Scholar]
- Smerdou C., and, Liljeström P. Two-helper RNA system for production of recombinant Semliki forest virus particles. J Virol. 1999;73:1092–1098. doi: 10.1128/jvi.73.2.1092-1098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleeton MN, Sheahan BJ, Gould EA, Atkins GJ., and, Liljeström P. Recombinant Semliki Forest virus particles encoding the prME or NS1 proteins of louping ill virus protect mice from lethal challenge. J Gen Virol. 1999;80 (Pt 5):1189–1198. doi: 10.1099/0022-1317-80-5-1189. [DOI] [PubMed] [Google Scholar]
- Salminen A, Wahlberg JM, Lobigs M, Liljeström P., and, Garoff H. Membrane fusion process of Semliki Forest virus. II: Cleavage-dependent reorganization of the spike protein complex controls virus entry. J Cell Biol. 1992;116:349–357. doi: 10.1083/jcb.116.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón MR, Berraondo P, Crettaz J, Ochoa L, Vera M, Lasarte JJ.et al. (2005Induction of gp120-specific protective immune responses by genetic vaccination with linear polyethylenimine-plasmid complex Vaccine 231384–1392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of antitumor efficacy of SFV-IL-12 in B16-OVA tumors.
Analysis of cytopathic effect of SFV-IL-12 in tumor cells and role of SFV replication on antitumoral efficacy in combined treatment.
Evaluation of antitumor efficacy of SFV-IL-12 in TC-1 tumors.
Treatment efficacy of SFV-IL-12 + anti-CD137 combination on bilateral tumors is not mediated by dissemination of the viral vector but immune system mediated.
Tumor infiltrating leukocytes.
Depletion of myeloid cells does not affect antitumor efficacy of SFV-IL-12 + anti-CD137 mAb treatment.
Vitiligo occurrence in cured mice.