Abstract
Treatment of permissive tumors with the oncolytic virus (OV) VSV-Δ51 leads to a robust antitumor T-cell response, which contributes to efficacy; however, many tumors are not permissive to in vivo treatment with VSV-Δ51. In an attempt to channel the immune stimulatory properties of VSV-Δ51 and broaden the scope of tumors that can be treated by an OV, we have developed a potent oncolytic vaccine platform, consisting of tumor cells infected with VSV-Δ51. We demonstrate that prophylactic immunization with this infected cell vaccine (ICV) protected mice from subsequent tumor challenge, and expression of granulocyte–monocyte colony stimulating factor (GM-CSF) by the virus (VSVgm-ICV) increased efficacy. Immunization with VSVgm-ICV in the VSV-resistant B16-F10 model induced maturation of dendritic and natural killer (NK) cell populations. The challenge tumor is rapidly infiltrated by a large number of interferon γ (IFNγ)-producing T and NK cells. Finally, we demonstrate that this approach is robust enough to control the growth of established tumors. This strategy is broadly applicable because of VSV's extremely broad tropism, allowing nearly all cell types to be infected at high multiplicities of infection in vitro, where the virus replication kinetics outpace the cellular IFN response. It is also personalized to the unique tumor antigen(s) displayed by the cancer cell.
Introduction
The current standard of care for cancer treatment is associated with severe off-target effects due to poor selectivity of the agent for cancer cells. New targeted therapeutics often target only one gene or pathway in a cell, allowing for resistance to easily evolve.1 Likewise, cancer immunotherapies, though making great strides in recent years, are still focused on identifying one or very few tumor-associated antigens that can be targeted. However, tumors can rapidly evolve immune evasion and immune suppression mechanisms countering these therapies, leading to treatment failure.2,3 As well, tumors are antigenically heterogeneous4,5 as a result of high genetic instability.6 In theory, a vaccine presenting the spectrum of tumor antigens could allow for the in vivo selection of the optimum epitope(s) to target.
Oncolytic viruses (OVs) have emerged as a promising anticancer treatment platform, able to specifically replicate in and kill cancer cells while leaving normal cells unharmed. Though engineered for tumor-specific lysis, the multimodal nature of this platform is currently being revealed. Many of these viruses can be delivered systemically to reach distant tumor beds,7 be targeted to tumor vasculature to induce tumor vascular shutdown,8,9 and be engineered to carry genetic payloads. Importantly, preclinical and clinical evidence for OV-mediated antitumor immunity is emerging.10 Recent results from a phase II clinical trial with OncoVexGM-CSF, an oncolytic HSV expressing granulocyte–monocyte colony stimulating factor (GM-CSF), have demonstrated that patients treated with this platform have a very different tumor immune landscape. These tumors had significantly lower regulatory T cells and higher CD8+ effector T cells in the tumor.11
Previous research by our lab has demonstrated that a vesicular stomatitis virus (VSV) harboring a deletion in the M protein at position 51 (VSV-Δ51) is very sensitive to interferon (IFN)12 and neutralizing antibody,13 which act to clear virus from the host. Antitumor immune stimulation may be important for the ongoing tumor destruction once the virus is cleared, and offers the potential to restore immune surveillance mechanisms that can lead to complete responses and prevent recurrence. Wild-type VSV has been observed to induce antitumor immune responses in models expressing exogenous antigens14 and has now been demonstrated to be a potent boost in an elegant prime/boost oncolytic vaccination model.15,16 Strategies that allow us to exploit the antitumor immunity induced through virus replication and lysis will be vital to using the full potential of these viruses. Herein we describe an infected cell vaccine (ICV) platform that presents a multitude of tumor antigens in the context of a robust OV infection. We demonstrate that this leads to potent immune stimulation and ultimately activates both natural killer (NK) cells and T cells for tumor debulking and long-term cancer surveillance. In addition, no prior knowledge on the tumor antigens is required to make this vaccine.
Results
T cells are required for VSV-mediated long-term tumor regression
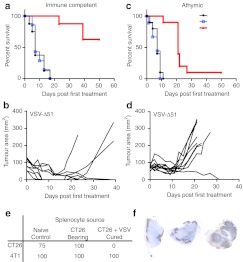
Many OV platforms have been observed to induce antitumor immune responses.17,18,19,20 We examined the role of the T cell compartment in oncolytic VSV-Δ51 treatment of cancer. A VSV-sensitive clone of colon carcinoma tumors (CT26.LacZ) was established in immunocompetent and athymic nude mice. When tumors were palpable, mice were treated with six intravenous (i.v.) doses of VSV-Δ51-GFP, UV-inactivated VSV, or phosphate-buffered saline (PBS). In the immune-competent mice, only those treated with VSV-Δ51-GFP had measurable responses, with 60% of the mice demonstrating complete tumor clearance (Figure 1a,b). The athymic nude mice initially responded to VSV treatment, demonstrating stable tumor sizes, but showed marginal long-term efficacy, with only 1 out of 10 mice having a durable response (Figure 1c,d). This suggests that the T-cell compartment is required for long-term tumor eradication following systemic VSV therapy in this model.
Figure 1.
Vesicular stomatitis virus (VSV) treatment induces a potent antitumor immune response, on which treatment is dependent. (a,b) Balb/C or (c,d) athymic nude mice were injected subcutaneously with CT26.LacZ cells. Immune-competent Balb/C mice were treated starting on day 14 post-tumor implantation and nude mice were treated on day 10 to reflect a slightly faster onset of tumor development. Mice were injected six times with 5 × 108 plaque-forming unit (pfu) of VSV-Δ51-GFP intravenous (i.v.) or equivalent amount of UV-inactivated VSV-Δ51 or phosphate-buffered saline (PBS). (a) Kaplan–Meier survival analysis of VSV-Δ51-GFP treatment in Balb/C mice. N = 8 per group. Statistical significance verified by the log rank test, where P < 0.0001. (b) Tumor area growth over time plotted only for VSV-Δ51-GFP-treated mice. (c) Kaplan–Meier survival analysis of VSV-Δ51-GFP treatment in nude mice. N = 10 for each group. Statistical significance verified by the log rank test, where P < 0.0001. (d) Tumor area growth over time plotted only for VSV-Δ51-GFP-treated mice. (e) Splenocytes were harvested from either naive mice, CT26.LacZ tumor-bearing mice, or CT26.LacZ tumor-bearing mice cured with six doses of VSV-Δ51-GFP. These splenocytes were injected i.v. into naive Balb/C mice, which were challenged subcutaneously 48 hours later with CT26.LacZ cells and 4T1 cells on the contralateral flank. (f) Balb/C mice-bearing CT26.LacZ subcutaneous tumors were injected i.v. with 5 × 108 plaque-forming unit (pfu) of VSV-Δ51. Two days later, mice were euthanized; tumors were harvested, and frozen. Sections were stained by immunohistochemistry (IHC) for VSV.
Subsequently, immune-competent mice demonstrating long-term complete responses were used as splenocyte donors in an adoptive cell transfer. Naive immune-competent mice that received splenocytes from VSV-treated and cured mice were not susceptible to CT26.LacZ tumor growth, but were susceptible to syngeneic 4T1 growth (Figure 1e). Splenocytes from naive mice and CT26.LacZ tumor-bearing untreated mice were not able to protect against subsequent tumor challenge. Therefore, a specific and long-lived antitumor immune response is generated through treatment with oncolytic VSV.
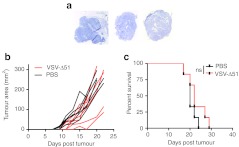
UV-inactivated VSV was not able to induce any efficacy in the CT26 subcutaneous model (Figure 1a). This leads us to reason that VSV replication in the tumor cells is required for immune stimulation. CT26.LacZ tumors are very sensitive to VSV and demonstrate robust infection by immunohistochemistry at 24 hours following i.v. administration (Figure 1f). Conversely, B16-F10 cells do not demonstrate any VSV replication in i.v.-treated tumors (Figure 2a) and B16-F10 tumor-bearing mice have no response to VSV-treatment (Figure 2b,c), further demonstrating the importance of replication in efficacy. Previous research by Breitbach et al.8 demonstrates that after i.v. administration, UV-inactivated VSV is undetectable in tumor sections using the methods described in the current manuscript. The viral proteins found in the tumor sections in Figures 1 and 2 must result from productive virus replication and spread and not simply tumor-specific accumulation of viral particles.
Figure 2.
Vesicular stomatitis virus (VSV) replication is poor in B16-F10 tumors and leads to no efficacy. (a) C57BL/6 mice-bearing B16-F10 subcutaneous tumors were injected intravenously (i.v.) with 5 × 108 plaque-forming unit (pfu) of VSV-Δ51. Two days later, mice were euthanized, and tumors were harvested and frozen. Sections were stained by immunohistochemistry (IHC) for VSV. (b) C57BL/6 mice-bearing B16-F10 subcutaneous tumors were injected three times a week starting on day 6 for a total of six doses of VSV-Δ51 i.v. Tumor area growth over time plotted for PBS (in black) and VSV-Δ51 treated (in red). N = 6 per group. (c) Kaplan–Meier survival analysis with statistics examined by log rank test where P > 0.2.
VSV infection is a potent immune stimulator in a prophylactic ICV
We have so far demonstrated that VSV replication in a permissive tumor can elicit a therapeutic antitumor T-cell response. We postulated whether we could generate a sufficiently robust therapeutic response in VSV-resistant B16-F10 cells by infecting them ex vivo and presenting this cocktail as an ICV. This would bypass the necessity for in vivo replication to mount an antitumor immune response. Though B16-F10 cells are not readily permissive to VSV following i.v. delivery, we can achieve complete infection by infecting the cells in vitro at a high multiplicity of infection (Supplementary Figure S1a).
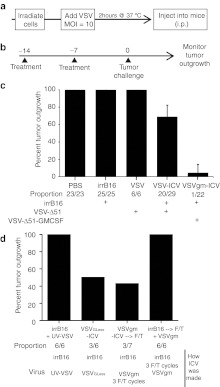
As a means of determining the immunogenicity of such a vaccine, γ-irradiated tumor cells were infected and assessed for their ability to provide protection against a future tumor challenge (Figure 3a). This VSV-ICV was administered intraperitoneally (i.p.) to mice on days 0 and 7, with a tumor challenge on day 14 (Figure 3b).
Figure 3.
Vesicular stomatitis virus (VSV) acts as a potent adjuvant in a prophylactic B16-F10 infected cell vaccine. (a) Schematic representing preparation of infected cell vaccine (ICV). (b) Prophylactic ICV treatment timeline in days. (c–d) C57BL/6 mice were immunized with various control or vaccine preparations according to the timeline in (b). They were then challenged with 1 × 105 B16-F10 cells subcutaneously and tumor outgrowth was monitored. (c) Shown is the weighted mean + weighted standard deviation of final tumor outgrowth for each group, averaged from results from multiple experiments. The total number of mice tested, with the fraction exhibiting tumor growth is listed below the graph. (d) Shown is the percent outgrowth from the one experiment in which that condition was tested.
The immunization of mice with γ-irradiated B16-F10 cells infected with VSV-Δ51-GFP was able to completely protect 30% of mice tested (9 protected/29) from later live cell challenge (Figure 3c). Control groups immunized with PBS or γ-irradiated B16-F10 cells demonstrate complete susceptibility to the tumor challenge. These results were also verified in a different mouse strain with the parental CT26.wt cell line (Supplementary Figure S2). Like the B16-F10 cells, and unlike the clone CT26.LacZ, the parental CT26.wt cells are not permissive to in vivo VSV infection.
GM-CSF expression by VSV enhances immune activation by the VSV-ICV
GM-CSF is a potent immunostimulating cytokine able to increase monocyte and macrophage migration and activation.21 To increase the immune stimulation properties of our vaccine, GM-CSF was cloned into the VSV-Δ51 genome and expression was confirmed by western blot (data not shown). The ICV made with VSV-Δ51-GMCSF (VSVgm-ICV) prevented B16-F10 tumor engraftment in over 95% of mice tested (21 protected/22) (Figure 3c). Due to the heightened efficacy of this approach, we chose the VSV-Δ51-GMCSF virus for further characterization. The VSV-Δ51-GMCSF virus was tested as a direct oncolytic alongside VSV-Δ51-GFP in the B16-F10 subcutaneous model and it demonstrated no increased efficacy (Supplementary Figure S1b).
Replication beyond the infected cells of the vaccine is not required for full ICV efficacy
We examined whether virus replication and spread or tumor cell integrity were important for ICV efficacy. UV-inactivated VSV lacks the ability to express gene products and was unable to confer any protection (Figure 3d). G-Less VSV is a recombinant that lacks the gene encoding the glycoprotein, but is grown in cells expressing VSV G. This virus infects cells and expresses N, M, L, and P genes. It can package new virions, though these are not infectious.22 This virus was able to protect the same proportion of mice as the VSV-ICV in this experiment (Supplementary Figure S3). These viruses are compared to VSV-Δ51-GFP because neither UV-inactivated nor G-Less virus expresses GM-CSF. To determine the importance of cellular integrity to the efficacy of the vaccine, vaccine preparations were attempted in two other methods. γ-Irradiated B16-F10 cells were first freeze/thawed multiple times before being mixed with VSV-Δ51-GMCSF (irrB16 –> F/T + VSVgm). Compared to the regular VSVgm-ICV, this preparation was not able to protect any of the six mice treated. Alternatively, the VSVgm-ICV was made as per usual but freeze/thawed multiple times before injection (VSVgm-ICV –> F/T). This preparation protected only four out of seven mice.
Taken together, these results indicate that in two VSV-resistant cancer models tumor cells infected with VSV-Δ51 can stimulate an antitumor immune response that is capable of protecting mice from a later tumor challenge. In addition, the expression of GM-CSF from infected cells greatly increased the immunization capabilities of the ICV in the B16-F10 model. Interestingly, it seems that cellular integrity is important in conferring immunological protection from this vaccine but virus only needs basal replication within the cells constituting the vaccine, as demonstrated by the VSVGLess-ICV. Whether it is simply transcription or genome replication that is required is not presently clear.
VSVgm-ICV induces rapid innate immune activation
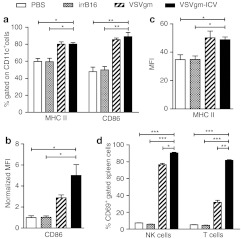
We next examined the activation of early innate cells following VSVgm-ICV treatment. Splenocytes were harvested at 24 hours post-treatment and dendritic cells (DCs) evaluated for markers of activation. Mice treated with either VSVgm alone or VSVgm-ICV had a higher proportion of activated DCs. This is demonstrated by a higher frequency of cells expressing MHC II and CD86, as well as higher expression levels of these activation markers (Figure 4a–c).
Figure 4.
The VSVgm-infected cell vaccine (ICV) leads to dendritic cell and lymphocyte early activation in the spleen within 24 hours of vaccination. C57BL/6 mice were immunized with the VSVgm-ICV or relevant controls i.p. and euthanized 24 hours later. Splenocytes were stained and examined by flow cytometry for dendritic cell markers of activation. (a) Percent of CD11c+ cells that express MHC II and/or CD86. (b) Mean fluorescence intensity of MHC II staining on CD11c+ cells. (c) Mean fluorescence intensity of CD86 staining on CD11c+ cells normalized to phosphate-buffered saline (PBS) levels. N = 3 mice per group, except for VSVgm-ICV that had four mice. (d) C57BL/6 mice were immunized with the VSVgm-ICV or relevant controls i.p. and euthanized 15 hours later. Splenocytes were stained and examined by flow cytometry for NK and T cells markers in addition to CD69. Percent of indicated cells that express CD69. All data presented as mean + SEM with three mice per group. P values, *P < 0.05, **P < 0.005, *** P ≤ 0.0001.
In addition, splenic lymphocytes were examined for early activation through CD69 expression early after treatment with the VSVgm-ICV. CD69 is a marker of early lymphocyte activation and is not found on naive lymphocyte populations.23,24 Lymphocytes from VSVgm-ICV-treated mice demonstrate dramatically higher degrees of early activation than control animals (Figure 4d). In keeping with this finding, at 24 hours post-treatment, a higher frequency of blood NK cells from VSVgm or VSVgm-ICV-treated mice express IFNγ and more of the cytokine is expressed per cell (Supplementary Figure S4a,b). However, not surprisingly, NK cells are no longer expressing IFNγ in the blood on the day of tumor challenge (Supplementary Figure S4c,d).
VSVgm-ICV increases tumor infiltration by activated T and NK cells
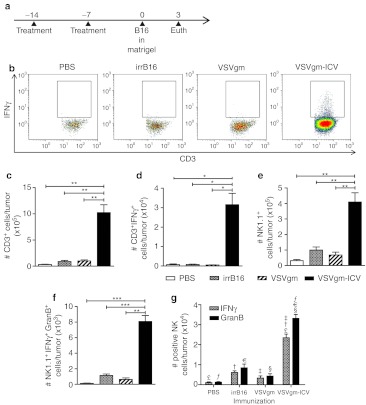
To understand what cell types are responsible for tumor rejection in the B16-F10 model following VSVgm-ICV treatment, we implanted the challenge flank tumor in matrigel, thereby allowing us to easily resect and disaggregate the tumor (Figure 5a). Mice were injected with Brefeldin A 6 hours before tumor harvest. This allows us to determine the expression profiles of tumor infiltrating cells while they are in the tumor environment. We determined that T cells are 10 times more numerous in the tumor following vaccination with the VSVgm-ICV than with irradiated cells alone or VSVgm (Figure 5c). This difference is even larger when compared to the PBS-treated mice, with 30 times more T cells in the treated tumor. Indeed, over 8% of the tumor cellular content is T cells, equal to a ratio of one T cell for every 12.5 tumor cells (Supplementary Figure S5a). Importantly, there is also a much greater number of CD3+ IFNγ+ cells in the tumor following VSVgm-ICV than in any control group (Figure 5b,d and Supplementary Figure S5b).
Figure 5.
Prophylactic immunization with the VSVgm-infected cell vaccine (ICV) leads to robust activated T cell and NK cell infiltration of the challenge tumor. (a) C57BL/6 mice were prophylactically immunized as described earlier with VSVgm-ICV or controls. B16-F10 cells in matrigel were subcutaneously injected on day 0. On day 3, mice were injected intravenously (i.v.) with brefeldin A, then 6 hours later were euthanized. Tumors were resected on day 3 for enzymatic disaggregation and flow cytometric analysis. (b) Representative dot plots demonstrating CD3+ cells expressing IFNγ. (c) The average total number of CD3+ cells per tumor in each group. (d) The average total number of CD3+ IFNγ+ cells per tumor in each group. (e) The average total number of NK1.1+ cells per tumor in each group. (f) The average total number of NK1.1+IFNγ+GranzymeB+ cells per tumor in each group. (g) The total number of NK1.1+IFNγ+ cells and NK1.1+GranzymeB+ cells per tumor in each group. P values, £, †, ‡, ∫, §, and  are all P ≤ 0.005. All data are presented as mean + SEM with five mice per group. P values, * P < 0.05, ** P < 0.005, *** P ≤ 0.0005.
are all P ≤ 0.005. All data are presented as mean + SEM with five mice per group. P values, * P < 0.05, ** P < 0.005, *** P ≤ 0.0005.
In addition to a significant increase in T cells in the challenge tumor, VSVgm-ICV-immunized mice have 4–13-fold more NK cells (Figure 5e). Importantly, there are more NK cells producing either IFNγ or Granzyme B (Figure 5g), and there are more NK cells expressing both IFNγ and Granzyme B (Figure 5f).
A VSVgm-ICV reduces tumor burden in the therapeutic setting
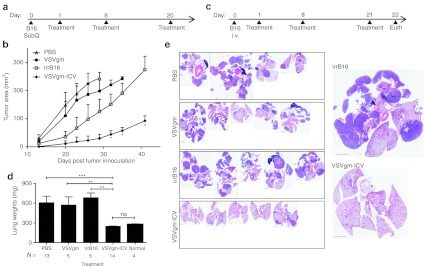
Having demonstrated that a VSVgm-ICV can protect mice from a tumor challenge, we sought to examine the vaccine's potency in more relevant therapeutic models, through the treatment of mice that have already been inoculated with tumors. C57BL/6 mice bearing B16-F10 subcutaneous tumors were treated i.p. with VSVgm-ICV, irrB16, VSVgm, or PBS control (Figure 6a). Animals treated with the VSVgm-ICV had a dramatic delay in tumor growth (Figure 6b). In contrast, treatment with oncolytic VSV-Δ51-GMCSF had similar tumor growth to PBS-treated animals. Treatment with γ-irradiated B16-F10 cells led to marginally delayed tumor growth compared to the other control groups, though this is not statistically significant.
Figure 6.
Treatment with the VSVgm-infected cell vaccine (ICV) can significantly impact tumor growth in subcutaneous and systemic models. (a) C57BL/6 mice were implanted with B16-F10 subcutaneous tumors and treated according to the presented timeline. (b) Tumor area was monitored and is shown in days following tumor implantation as mean + SEM with five mice per group, except for the VSVgm only group that had four. (c) C57BL/6 mice were injected intravenously (i.v.) with B16-F10 cells and treated according to presented timeline with the VSVgm-ICV or controls. Mice were euthanized on day 22, and their lungs were weighed and fixed in 10% formalin. Lungs were then sliced and analyzed by hematoxylin and eosin (H&E) staining. (d) Lung weights shown are pooled from two separate experiments and presented as mean + SEM with variance analysis by Mann–Whitney test. Normal lungs are those from mice that have not received any lung tumors or treatments. (e) Hematoxylin and eosin (H&E) staining of all mice in one experiment with representative sections demonstrating tumor burden at endpoint. All lungs are on the same scale, with black bar indicating 2 mm. A higher magnification of a representative vaccine and control-treated lung are presented on the right. One phosphate-buffered saline (PBS) mouse had the heart buried in tumor, and so the organ could not be removed. A few control mice had tumors in their thymus and so these organs were kept in the H&Es and weights. P values, * P < 0.05, ** P < 0.005, ***P ≤ 0.0001.
A systemic dissemination model was also undertaken to examine the effectiveness of this vaccine. Mice were given B16-F10 cells i.v., leading to tumor seeding mostly in the lung, though macroscopic tumors can also occur in the thymus, kidneys, and ovaries. Treatments were initiated the following day and all mice were euthanized on day 22 to examine tumor burden (Figure 6c). Treatment with VSVgm-ICV demonstrated undetectable tumor burden at the time of sacrifice in 80% of mice and no other tumors were found in any of the animals (Figure 6e,d). In contrast, control-treated mice demonstrated heavy tumor burden: 3 PBS-treated mice, 1 VSVgm-treated mouse, and 1 irrB16-treated mouse had large growths in locations other than the lung. Another PBS-treated mouse was found dead before scheduled euthanizing. Lung weights demonstrated that the VSVgm-ICV-treated mice had a much lower tumor burden than controls, identical to non-tumor–bearing mouse lungs. As a more stringent test of the VSVgm-ICV's therapeutic potential, treatments were started on days 3 or 4 after tumor seeding. In both cases two of four VSVgm-ICV-treated mice had no visible lung tumors at the time of sacrifice, whereas all irrB16-treated mice had significant tumor burden (Supplementary Figure S6). Treatments beginning later than day 4 were not attempted and so it remains to be seen if efficacy can be achieved while delaying treatments further.
A spontaneous model of ovarian cancer also demonstrated therapeutic benefit from the VSVgm-ICV (Supplementary Figure S7). These transgenic mice develop spontaneous bilateral ovarian tumors driven by the SV40 TAg.25 The vaccine was made with the 6048R cell line that had been previously established from one such tumor. Though normal ovary weights were not quantified, one VSVgm-ICV-treated mouse had normal appearing ovaries and these weighed 0.05 g in total.
These results highlight the potency of this vaccine platform; able to initiate antitumor immune responses that can single-handedly slow the progression of highly aggressive and VSV-resistant tumors.
Discussion
Several recent studies have reported the very important role the immune system plays in tumor clearance. Indeed the quantity and quality of CD8+ T cells found in the tumor is one of the strongest favorable prognostic markers in many cancer types.25 Not surprisingly, cancers evolve multiple mechanisms of immune evasion and suppression.26
OVs are emerging as promising clinical candidates that target tumors at multiple fronts. Importantly, many have been observed to stimulate antitumor immune responses when replicating in permissive tumors.10 However, not all tumors are permissive to these viruses. We sought to optimize and test an OV vaccine that could be used with all tumor types, regardless of in vivo permissivity; harnessing the antitumor immune response generated when an immunogenic virus replicates in tumor cells.
We observed that the efficacy obtained with VSV-Δ51 in the permissive CT26.LacZ colon cancer model is largely dependent on an intact T-cell compartment and that mice cured with this OV treatment generate a robust antitumor immune response (Figure 1). However, this efficacy does not translate to tumor models that are resistant to the viral doses achieved in systemic delivery of VSV (Figure 2). We propose that the deficit in efficacy due to the lack of in vivo replication could be overcome by infecting γ-irradiated tumor cells in vitro, and then injecting this ICV into the mouse. Indeed an ICV using VSV-Δ51-GFP was able to protect 30% of mice from future B16-F10 tumor challenge in a prophylactic setting (Figure 3c). Interestingly, cloning the cytokine GM-CSF into the viral genome greatly increased the potency of the ICV. The VSVgm-ICV protects 95% of mice from future tumor challenge. GM-CSF enhances the recruitement and activation of antigen presenting cells.21 However, further studies are required to fully elucidate the role of GM-CSF in this vaccine.
Though UV-inactivated VSV does not lead to sufficient immune stimulation, a G-Less VSV was able to recapitulate the tumor protection achieved with fully replication competent virus (Figure 3d). Therefore a basal level of viral transcription/replication is required, though it need not replicate beyond the initially infected cells that constitute the vaccine. We also observed a requirement for cellular integrity, thus, it is reasonable to hypothesize that this vaccine does not simply present viral danger signals in the context of tumor antigens. Instead, we speculate that viral infection of cells initiates critical immunogenic processes that, coupled with tumor-associated antigens, lead to robust immune activation. In addition, viral infection of an intact cell is quite immunogically relevant, offering persistent toll-like receptor ligation required for a robust immune response.27
Treatment with the VSVgm-ICV leads to rapid innate immune activation seen in the spleen and blood (Figure 4, and Supplementary Figure S4). In many cases, VSVgm leads to the same level of early immune activation as does the vaccine. VSV injected i.p. will productively infect the first cells it encounters, thereby initiating similar immune activation due to viral infection. However, no antitumor immune responses were detected at late timepoints with VSVgm alone (Figures 5 and 6) and importantly no auto-immune sequelae have ever been observed with VSVgm treatment, whether i.p. or i.v. (data not shown).
Though the VSVgm-ICV is demonstrated to activate NK cells 24 hours after prophylactic vaccination, they do not likely play a role in challenge tumor rejection as tumor implantation occurs after NK cells have returned to baseline (Supplementary Figure S4). Importantly, NK cell activation following VSVgm-ICV should have a significant role in a therapeutic setting, through the early debulking of the existing tumor and through the induction of inflammation at the tumor site. Though seemingly related to the vaccination, we believe that the NK cell infiltration and activation observed in the challenge tumor following VSVgm-ICV is in fact a consequence of activated T cell infiltration (Figure 5). Previous research indicates that T cells can activate NK cells in this manner.28 NK cells have been demonstrated to be important mediators of early tumor debulking and in cytokine secretion, which further amplifies Th1 responses.29,30,31 Certainly, the large quantity and activated nature of the T cells observed infiltrating the B16-F10 challenge tumor only 3 days after implantation indicates that the VSVgm-ICV initiates an effective Th1 T cell response.
The activity of this vaccine is highlighted by its impact in therapeutic models of cancer (Figure 6 and Supplementary Figures S6 and S7). Importantly, therapy could be delayed to 4 days after systemic dissemination, while still providing a therapeutic benefit. In some cases, the vaccine is delivered in a completely separate anatomical compartment and yet leads to significant tumor clearance. Further studies will focus on better understanding the critical immunological components that lead to this efficacy.
The concept of using virally infected cells as a cancer vaccine has been previously investigated in both mouse models and human patients32,33,34,35 with some success, though few have investigated the immunological basis for this efficacy. Clinical trials using NDV-infected autologous and allogeneic melanoma cells demonstrated impressive 10 and 15-year survival data.36,37 However, many of these approaches used inactivated virus, replication-defective, or non-lytic strains. Of note, Livingston et al. used wild-type VSV to infect melanoma cell lines to create a vaccine, though observed very limited responses. However, in this case the infected cells were swelled, homogenized, enucleated, and the virus UV-inactivated before treatment.38 The results we have presented in this manuscript suggest that intact cells and replication competent lytic virus is much more immunogenic. We used an oncolytic strain of VSV so as to minimize toxicity, while allowing us to keep actively, yet locally, replicating virus as part of the vaccine. In addition, in virus-permissive tumor models, there might be an added benefit of tumor debulking and local inflammation in the tumor microenvironment provided by the OV.
Though other immunotherapies have also achieved therapeutic efficacy in the B16-F10 tumor model, the VSVgm-ICV achieves this while requiring no previous knowledge about the relevant tumor antigens15 or the immunosuppressive mechanisms employed by the tumor. Importantly, the ICV is relatively simple to prepare, requiring no long-term ex vivo manipulations.39,40
The ICV platform would be best coupled to a debulking treatment that might also stimulate the immune system. Local tumor irradiation may help with tumor debulking and has been demonstrated to increase inflammation in the tumor environment,41 leading to enhanced immunotherapeutic responses.42,43 An ideal scenario might include first surgically removing the tumor, using this tumor bulk to create the VSV-ICV, and then treating the patient to reduce metastatic recurrence.
The ICV is a promising immunotherapeutic platform that achieves the stimulation of both innate and adaptive immune cells. The potency of the ICV is highlighted by the significant impact it has on the progression of an aggressive and immunosuppressive tumor. In addition, the use of autologous tumor leads to a personalized vaccine that can potentially present the full range of a patient's unique tumor antigens. Recently, Castle et al.44 have shown that the B16-F10 tumor cell line has acquired over 500 somatic mutations that could, in principle, encode numerous novel immunogenic epitopes. Despite this, γ-irradiated B16-F10 cells, on their own, are ineffective in stimulating antitumor immunity, probably due to the lack of danger signals. Here, we show that infection of B16-F10 cells makes them a very potent vaccine platform that has the capacity to induce both a protective and therapeutic immune response. Since the B16-F10 cell line expresses a vast array of potential neo-antigens, perhaps many of these could now be made visible to the immune system when presented as an ICV. It is possible that because of this, the ICV has the potential to induce a broadly active T-cell response against a spectrum of neo-antigens. Currently, we have no data to support this notion, however studies are underway to determine the number and nature of mutant epitopes that the cellular immune system recognizes in B16-F10 cells following infected cell vaccination. It remains possible that our ICV approach simply focuses a robust response on a single or limited number of tumor antigens.
Materials and Methods
Cell lines and mice. CT26.WT and CT26.LacZ (also known as CT26.CL25) colon carcinoma, 4T1 breast cancer, and B16-F10 melanoma cells were purchased from the American Type Culture Collection (Manassas, VA) and the B16-F10.LacZ were a gift from Dr Ann F Chambers. All were cultured in HyQ Dulbecco's modified Eagle's medium (high glucose) (HyClone, Logan, UT) supplemented with 10% fetal calf serum (CanSera, Etobicoke, Ontario, Canada). 6048R cells (gift from Dr Vanderhyden) were grown in αMEM with 10% fetal bovine serum, 2.08 µg/ml epidermal growth factor (R&D Systems, Minneapolis, MN), 1× of ITSS (Roche, Montreal, CA), gentamicin, and penicillin/streptomycin (Invitrogen, Burlington, CA).
Female 6-week-old Balb/C, C57BL/6, and CD1 nude mice were purchased from Charles River Laboratories (Wilmington, MA). Female 8-week-old FVB/N MISIIRTAg transgenic mice (line tg4568—a gift from Dr Vanderhyden) were generated using the transgene described by Connolly et al.45 These mice develop bilateral ovarian tumors of epithelial origin with full penetrance and typically endpoint at 14 weeks of age. All experiments were conducted with the approval of the University of Ottawa Animal Care and Veterinary Service. Tumor Area is calculated by multiplying the width by the length of the tumor.
Virus. VSV-Δ51-GFP and VSV-Δ51-GMCSF were grown in Vero cells and purified by centrifugation or sucrose gradient banding and centrifugation. VSV-GLess was grown on 293G cells. Virus stocks were aliquoted in PBS, kept at –80 °C, used once, and then discarded. VSV-Δ51-GMCSF was cloned using PCR primers to murine GM-CSF and amplified off the pcDNA4.1-GMCSF vector. GM-CSF was cloned into the VSV-Δ51 vector at the XhoI and NheI sites between the G and L genes.
Direct treatment model and immunohistochemistry. Subcutaneous tumors were established by injecting 3 × 105 CT26.LacZ or B16-F10 cells in PBS on the hind flank of the mouse. Tumors were allowed to grow until palpable, six treatments were then administered i.v. for 2 weeks, every monday, wednesday, and friday, unless otherwise stated. VSV-Δ51 was used at 5 × 108 plaque-forming unit (pfu)/100 µl. To analyze VSV replication in CT26.LacZ and B16-F10 tumors following i.v. delivery, Balb/c or C57BL/6 mice were implanted with tumors subcutaneously and tumors were allowed to grow until reaching a sufficient size to dissect. Mice were then injected i.v. with 5 × 108 pfu/100 µl. Forty eight hours after injection, mice were euthanized, tumors were excised, and frozen in Shandon Cryomatrix freezing medium (TermoElectron, Waltham, MA) in liquid nitrogen. Five microgram sections were stained by immunohistochemistry with rabbit anti-serum raised against VSV (gift of Dr Earl Brown) at a 1/5,000 dilution for 30 min. Secondary antibody and ABC reagents were used as directed from the Vectastain ABC kit and Horseradish peroxidase activity was assessed using a Diaminobenzene-HRP kit (KPL Biosciences, Guelph, Ontario, Canada). Nuclei were counterstained with hematoxylin. Images were obtained using an Epson Perfection 2450 Photo Scanner.
Rechallenge and splenocyte transfer. Mice were treated as in the direct oncolysis model with six doses of VSV at 5 × 108 pfu/100 µl i.v. Once tumors were palpable. Mice that had complete responses were kept for at least 3 months to ensure long-term responses. Splenocytes were harvested and purified by Lympholyte-M gradient from mice that were naive, had a tumor but received no treatment, or cured by VSV treatment. 5 × 107 of these isolated splenocytes were transferred to naive mice i.v., and these mice were then challenged 48 hours later with 3 × 105 CT26.LacZ cells on the right hind flank or 4T1 cells on the left flank. Tumor outgrowth was monitored.
ICV. Tumor cells were harvested from tissue culture and aliquoted in Eppendorf tubes at 2 × 107 cells/200 µl in PBS. These were γ-irradiated for 30Gy (CT26.wt), 45Gy (6048R), or 60Gy (B16-F10) in a Pantak HF320 X-Ray machine. Virus or PBS was added to the tubes at 2 × 108 pfu in 200 µl of PBS and incubated at 37 °C for 2 hours. The mixture was then injected in mice, 100 µl i.p.; therefore giving each mouse 5 × 106 γ-irradiated cells and 5 × 107 pfu of virus per dose. For Figure 3f, the “irrB16 → F/T + VSVgm” sample was γ-irradiated, then subjected to 3 freeze/thaw cycles in a dry ice bath and 42 °C water bath. Cells were then mixed with VSVgm before injection into the animal. Conversely, for the “VSVgm-ICV → F/T” sample, the ICV was made as usual and following the 2 hour infection the mixture was subjected to 3 freeze/thaw cycles before injection as detailed above. In the prophylactic model, mice were immunized on days –14 and –7, and then challenged with 1 × 105 live tumor cells subcutaneously on day 0. For the therapeutic model, mice were given 1 × 105 B16-F10 cells subcutaneously on day 0 or 7 × 104 B16-F10 cells i.v., and then vaccinated on days 1, 8, and 20 i.p. For the 3 and 4 day B16-F10 i.v. model, mice were treated on days 3, 10, and 22 or 4, 11, and 23, and then euthanized on day 28 to determine lung tumor burden.
In subcutaneous models, tumor measurements were determined with callipers until end point was reached. In i.v. model, endpoint was reached when mouse demonstrated severe respiratory distress, had a mass larger than 15 mm, or predetermined experimental endpoint was reached. Lungs were removed and fixed in 10% formalin for at least 3 days. These were then blotted dry and weighed. Lungs were then paraffin embedded and slices were analyzed by hematoxylin and eosin staining. Pictures were taken on the Aperio ScanScope (Axiovision Technologies, Toronto, Ontario, Canada) and analyzed using Aperio ImageScope software (Axiovision Technologies, Toronto, Ontario, Canada).
Flow cytometry. Spleens and blood were harvested from mice at indicated timepoints, red blood cells were lysed using ACK lysis buffer, and resuspended in RPMI + 10% fetal bovine serum. For examination of DC maturation, cells were stained with cell surface antibodies for CD11c-PE-Cy7 (clone N418; eBioscience), CD86/B7-1 (clone GL1; eBioscience, San Diego, CA), and MHC class II-FITC (clone M5/114.15.2l eBioscience). For early lymphocyte activation, splenocytes were stained with CD3-PerCP (clone 17A2; R&D Systems), DX5-PE (BD Bioscience), and CD69-FITC (clone H1.2F3; BD Biosciences). All flow cytometry was performed on a Beckman Coulter CyAn and data analyzed with Kaluza v1.1 software. For the examination of NK cell activation splenocytes were restimulated for 1.5 hours with PMA and ionomycin, during the last hour GolgiPlug (BD Biosciences) was added. These cells were then stained with CD3-PerCP (clone 17A2; R&D Systems), DX5-PE, Granzyme-B-PE-Cy7 (clone 16G6; eBioscience), and IFNγ-FITC (clone XMG1.2; eBiosciences) and examined by flow cytometry.
Examination of cellular infiltrate of matrigel challenge tumor. Following the regular prophylactic immunization schedule mice were challenged with 3 × 105 B16-F10 cells resuspended in 300 µl of matrigel (BD Biosciences). 6 hours before euthanasia, mice were treated i.v. with 0.25 mg Brefeldin A (Sigma, Oakville, Canada) as previously published.46 Mice were euthanized and matrigel plugs were excised from the flank and disaggregated using a cocktail of collagenase type IV (Cooper Biomedical, Malvern, PA), Dispase, and DNase I (Invitrogen) resuspended in HBSS. This mixture was then washed and stained with surface antibodies: anti-CD3-PE (clone 17A2; BD Biosciences) or anti-NK1.1-PE (clone PK136; BD Biosciences). Cells were then permeabilized and fixed (BD Cytofix/Cytoperm; BD Biosciences) and stained with intracellular antibodies: IFNγ-FITC (clone XMG1.2; eBiosciences) and Granzyme B-PE-Cy7 (16G6; eBioscience).
Statistical analysis. All statistical analyses were determined using GraphPad Prism 5.0 software. Where applicable, data are presented as mean + SEM and significance of variance was determined by T-test with Welch's correction, unless otherwise stated.
SUPPLEMENTARY MATERIAL Figure S1. VSV infection of B16-F10 cells at a high MOI can overcome replication issues. Figure S2. VSV acts as a potent adjuvant in a prophylactic CT26.wt-infected cell vaccine. Figure S3. The VSVGLess-ICV performs identically as the VSV-ICV. Figure S4. Prophylactic immunization with the VSVgm-ICV leads to early NK cell activation; however activation is not maintained until tumor challenge. Figure S5. A significant increase in the proportion of T and NK cells is observed within the challenge tumor. Figure S6. Treatment with the VSVgm-ICV reduces tumor burden even when treatment is delayed to day 3 or 4 after tumor inoculation. Figure S7. The VSVgm-ICV has therapeutic efficacy in the MISIIRTAg spontaneous ovarian cancer model.
Acknowledgments
C.G.L. and J.L.R. are supported by CIHR Doctoral awards: Frederick Banting and Charles Best Canada Graduate Scholarship. J.C.B. is supported by the Terry Fox Foundation, the Ontario Institute for Cancer Research, the Ottawa Regional Cancer Foundation, the Ottawa Hospital Foundation, and the Canadian Institute for Health Research. The authors declared no conflict of interest.
Supplementary Material
VSV infection of B16-F10 cells at a high MOI can overcome replication issues.
VSV acts as a potent adjuvant in a prophylactic CT26.wt-infected cell vaccine.
The VSVGLess-ICV performs identically as the VSV-ICV.
Prophylactic immunization with the VSVgm-ICV leads to early NK cell activation; however activation is not maintained until tumor challenge.
A significant increase in the proportion of T and NK cells is observed within the challenge tumor.
Treatment with the VSVgm-ICV reduces tumor burden even when treatment is delayed to day 3 or 4 after tumor inoculation.
The VSVgm-ICV has therapeutic efficacy in the MISIIRTAg spontaneous ovarian cancer model.
REFERENCES
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P.et al. (2008Core signaling pathways in human pancreatic cancers revealed by global genomic analyses Science 3211801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpizar YA, Chain B, Collins MK, Greenwood J, Katz D, Stauss HJ.et al. (2011Ten years of progress in vaccination against cancer: the need to counteract cancer evasion by dual targeting in future therapies Cancer Immunol Immunother 601127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer K, Gottfried E, Kreutz M., and, Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60:425–431. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand PH, Nuti M, Colcher D., and, Schlom J. Definition of antigenic heterogeneity and modulation among human mammary carcinoma cell populations using monoclonal antibodies to tumor-associated antigens. Cancer Res. 1983;43:728–735. [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E.et al. (2012Intratumor heterogeneity and branched evolution revealed by multiregion sequencing N Engl J Med 366883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Hepp F, Sommer HL., and, Pantel K. Tumor-antigen heterogeneity of disseminated breast cancer cells: implications for immunotherapy of minimal residual disease. Int J Cancer. 1999;84:1–5. doi: 10.1002/(sici)1097-0215(19990219)84:1<1::aid-ijc1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ.et al. (2011Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans Nature 47799–102. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA.et al. (2007Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow Mol Ther 151686–1693. [DOI] [PubMed] [Google Scholar]
- Liu TC, Hwang T, Park BH, Bell J., and, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- Melcher A, Parato K, Rooney CM., and, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther. 2011;19:1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS., and, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S.et al. (2003VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents Cancer Cell 4263–275. [DOI] [PubMed] [Google Scholar]
- Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD.et al. (2007Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity Mol Ther 15123–130. [DOI] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J.et al. (2007Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus Cancer Res 672840–2848. [DOI] [PubMed] [Google Scholar]
- Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E.et al. (2010Potentiating cancer immunotherapy using an oncolytic virus Mol Ther 181430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle BW, Boudreau JE, Lichty BD, Brunellière J, Stephenson K, Koshy S.et al. (2009Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus Mol Ther 171814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T.et al. (2008Tumor infection by oncolytic reovirus primes adaptive antitumor immunity Clin Cancer Res 147358–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F., and, Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- Gujar SA, Marcato P, Pan D., and, Lee PW. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Mol Cancer Ther. 2010;9:2924–2933. doi: 10.1158/1535-7163.MCT-10-0590. [DOI] [PubMed] [Google Scholar]
- Sobol PT, Boudreau JE, Stephenson K, Wan Y, Lichty BD., and, Mossman KL. Adaptive antiviral immunity is a determinant of the therapeutic success of oncolytic virotherapy. Mol Ther. 2011;19:335–344. doi: 10.1038/mt.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivendran S, Glodny B, Pan M, Merad M., and, Saenger Y. Melanoma immunotherapy. Mt Sinai J Med. 2010;77:620–642. doi: 10.1002/msj.20215. [DOI] [PubMed] [Google Scholar]
- Roberts A, Buonocore L, Price R, Forman J., and, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. 1999;73:3723–3732. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey WB, Lowdell MW, Marti GE, Abbasi F, Zenger V, King KM.et al. (2007CD69 expression as an index of T-cell function: assay standardization, validation and use in monitoring immune recovery Cytotherapy 9123–132. [DOI] [PubMed] [Google Scholar]
- Pitsios C, Dimitrakopoulou A, Tsalimalma K, Kordossis T., and, Choremi-Papadopoulou H. Expression of CD69 on T-cell subsets in HIV-1 disease. Scand J Clin Lab Invest. 2008;68:233–241. doi: 10.1080/00365510701630227. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C.et al. (2006Type, density, and location of immune cells within human colorectal tumors predict clinical outcome Science 3131960–1964. [DOI] [PubMed] [Google Scholar]
- Stewart TJ., and, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27:5894–5903. doi: 10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- Yang Y, Huang CT, Huang X., and, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M.et al. (2003CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity Blood 1013052–3057. [DOI] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL.et al. (2011Innate or adaptive immunity? The example of natural killer cells Science 33144–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Hayakawa Y, Takeda K., and, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A.et al. (2004Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming Nat Immunol 51260–1265. [DOI] [PubMed] [Google Scholar]
- Heicappell R, Schirrmacher V, von Hoegen P, Ahlert T., and, Appelhans B. Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. I. Parameters for optimal therapeutic effects. Int J Cancer. 1986;37:569–577. doi: 10.1002/ijc.2910370416. [DOI] [PubMed] [Google Scholar]
- Bohle W, Schlag P, Liebrich W, Hohenberger P, Manasterski M, Möller P.et al. (1990Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumor-cell vaccine. First clinical results with tumor-cell vaccines modified with live but avirulent Newcastle disease virus Cancer 661517–1523. [DOI] [PubMed] [Google Scholar]
- Liebrich W, Schlag P, Manasterski M, Lehner B, Stöhr M, Möller P.et al. (1991In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients Eur J Cancer 27703–710. [DOI] [PubMed] [Google Scholar]
- Sivanandham M, Shaw P, Bernik SF, Paoletti E., and, Wallack MK. Colon cancer cell vaccine prepared with replication-deficient vaccinia viruses encoding B7.1 and interleukin-2 induce antitumor response in syngeneic mice. Cancer Immunol Immunother. 1998;46:261–267. doi: 10.1007/s002620050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel WA., and, Murray DR. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med Oncol Tumor Pharmacother. 1992;9:169–171. doi: 10.1007/BF02987752. [DOI] [PubMed] [Google Scholar]
- Batliwalla FM, Bateman BA, Serrano D, Murray D, Macphail S, Maino VC.et al. (1998A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire Mol Med 4783–794. [PMC free article] [PubMed] [Google Scholar]
- Livingston PO, Albino AP, Chung TJ, Real FX, Houghton AN, Oettgen HF.et al. (1985Serological response of melanoma patients to vaccines prepared from VSV lysates of autologous and allogeneic cultured melanoma cells Cancer 55713–720. [DOI] [PubMed] [Google Scholar]
- Kottke T, Errington F, Pulido J, Galivo F, Thompson J, Wongthida P.et al. (2011Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors Nat Med 17854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Diaz RM, Kaluza K, Pulido J, Galivo F, Wongthida P.et al. (2008Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer Mol Ther 161910–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Bhardwaj N, McBride WH., and, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J., and, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P.et al. (2005Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer Clin Cancer Res 113353–3362. [DOI] [PubMed] [Google Scholar]
- Castle JC, Kreiter S, Diekmann J, Löwer M, van de Roemer N, de Graaf J.et al. (2012Exploiting the mutanome for tumor vaccination Cancer Res 721081–1091. [DOI] [PubMed] [Google Scholar]
- Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X.et al. (2003Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer Cancer Res 631389–1397. [PubMed] [Google Scholar]
- Foster B, Prussin C, Liu F, Whitmire JK., and, Whitton JL. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol. 2007;Chapter 6:Unit 6.24. doi: 10.1002/0471142735.im0624s78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VSV infection of B16-F10 cells at a high MOI can overcome replication issues.
VSV acts as a potent adjuvant in a prophylactic CT26.wt-infected cell vaccine.
The VSVGLess-ICV performs identically as the VSV-ICV.
Prophylactic immunization with the VSVgm-ICV leads to early NK cell activation; however activation is not maintained until tumor challenge.
A significant increase in the proportion of T and NK cells is observed within the challenge tumor.
Treatment with the VSVgm-ICV reduces tumor burden even when treatment is delayed to day 3 or 4 after tumor inoculation.
The VSVgm-ICV has therapeutic efficacy in the MISIIRTAg spontaneous ovarian cancer model.