Abstract
Twenty-five patients with chemotherapy refractory cancer were treated with a fully serotype 3-based oncolytic adenovirus Ad3-hTERT-E1A. In mice, Ad3 induced higher amounts of cytokines but less liver damage than Ad5 or Ad5/3. In humans, the only grade 3 adverse reactions were self-limiting cytopenias and generally the safety profile resembled Ad5-based oncolytic viruses. Patients that had been previously treated with Ad5 viruses presented longer lasting lymphocytopenia but no median increase in Ad3-specific T-cells in blood, suggesting immunological activity against antigens other than Ad3 hexon. Frequent alterations in antitumor T-cells in blood were seen regardless of previous virus exposure. Neutralizing antibodies against Ad3 increased in all patients, whereas Ad5 neutralizing antibodies remained stable. Treatment with Ad3-hTERT-E1A resulted in re-emergence of Ad5 viruses from previous treatments into blood and vice versa. Signs of possible efficacy were seen in 11/15 (73%) patients evaluable for tumor markers, four of which were treated only intravenously. Particularly promising results were seen in breast cancer patients and especially those receiving concomitant trastuzumab. Taken together, Ad3-hTERT-E1A seems safe for further clinical testing or development of armed versions. It offers an immunologically attractive alternative, with possible pharmacodynamic differences and a different receptor compared to Ad5.
Introduction
Gene therapy is an emerging field with an increasing number of positive clinical trials. Cancer has been the most common disease addressed due to unmet clinical need and serotype 5 adenoviruses have been the most common vector. Oncolytic viruses are modified so that they selectively replicate in cancer cells and cause cell lysis. Also, it has become evident that the role of the immune system is important in two ways. It may hinder the spread and replication of the virus but if tumor-associated immunological tolerance can be broken by oncolysis-mediated danger signals, it also helps to fight the cancer.1,2,3,4 The immune system has many effective ways for resisting tumor development, all of which have to be overcome by tumors in situ. Thus, the capacity for immunoevasion is considered as a new hallmark of cancer.5 Cancer cell lysis and inflammation caused by oncolytic virus can help present tumor-associated antigens (TAA) to immunological cells in the context of “danger signals” which can then result in reactivation of immune responses against the tumor.6 Therefore, oncolytic viruses can be regarded as personalized anticancer vaccines.
One evident problem with serotype 5 adenovirus (Ad5) is that it uses coxsackie- and adenovirus receptor (CAR)—known to be downregulated in advanced tumors—as a primary entry receptor.7 In contrast, a receptor that would be regularly present in most tumors would be optimal. The entry receptor(s) for adenovirus 3 has been debated but it seems to be amply present also in advanced tumors, resulting in generation of Ad5/3 chimeric viruses,8,9,10,11 which are serotype 5 except for the knob of the fiber which is from serotype 3. These viruses were shown to enhance the transduction of CAR low cells.12 However, a modification in the knob region does not overcome immune responses against other components of the virus13 and thus the notion of an oncolytic virus based completely on a different serotype is attractive.14
Exposure to adenovirus typically results in high-neutralizing antibody titers (NAbs) but also other elements of the immune system can recognize the virus, causing a problem for virus spreading within tumor masses and especially in the context of hematological dissemination to distant tumors. Therefore, in the context of patients with high pre-existing immunity against serotype 5 adenovirus, due to either wild-type virus infection or subsequent to oncolytic virus treatment, a different serotype virus would be appealing from an immunological perspective.14
A recent publication suggests that desmoglein 2 is a primary attachment receptor for Ad3.15 This receptor is associated with the tight junctions and binding of Ad3 initiates a cascade resembling epithelial to mesenchymal transition (EMT) and resulting in transient opening of tight junctions between epithelial cancer cells. This increases access to many important receptors trapped in this area (e.g., Her2/neu, EGFR, CAR), offering rationale for potential synergy with other cancer treatments (trastuzumab, cetuximab, Ad5).16 It was shown that Ad5/3 is capable of binding to this receptor, but its ability to open the tight junction is compromised presumably due to the long fiber of the serotype 5 virus. Another interesting feature of Ad3 is that it produces a million-fold excess of virus-like particles slightly smaller than the Ad3 virus.17 These particles consist of the viral surface proteins but do not contain the genetic material inside and are thought to be important in the opening of the epithelial junctions. In addition to desmoglein 2, Ad3 might use also other receptors such as CD46.18
To render Ad3-hTERT-E1A selectively oncolytic, the endogenous E1A promoter was replaced by a human telomerase (hTERT) promoter enabling efficient replication of the virus only in cell with high-telomerase activity—another hallmark of cancer.5 This virus was then tested in vitro and in vivo and was found to behave differently than Ad5 or Ad5/3 viruses. In vitro the virus was found slower in tumor cell killing, but in vivo the virus was always found to be at least as potent as serotype 5 or 5/3 viruses.14 The virus retained its oncolytic potency in the presence of anti-Ad5 neutralizing antibodies. Here, we report murine biodistribution and toxicity studies, followed by analysis of safety, efficacy, virological, and immunological analysis of patients treated with Ad3-hTERT-E1A.
Results
Preclinical
Ad3 biodistribution. Ad3 biodistribution assay at 6 hours following intravenous injection to immunocompetent mice (Supplementary Figure S1, N = 5) indicated high (>60 viral particles (VP)/β-actin) virus amount in lung, spleen, liver, blood clot, and bone marrow. These results are similar to previously reports with Ad5 and Ad5/3 and thus the biodistribution of Ad3 in rodents resembles that of Ad5 and Ad5/39,12,19,20,21 All other organs had low (<10 VP/β-actin) amounts of virus. Without heparin, 106 VP/ml was found in the clot and only 2 × 104 VP/ml in the serum. When analyzing heparin tubes 3 × 105 VP/ml was found from the plasma and only 4 × 103 VP/ml from the erythrocytes. These findings indicate that most of the virus in mouse blood is found from the peripheral blood mononuclear cells (PBMC) and platelets.
Acute immunological toxicity. Acute immunological toxicity was accessed by injecting immunocompetent mice intravenously and collecting blood samples 6 hours later (Supplementary Figure S2). Ad3 elicited higher Rantes, tumor necrosis factor-α, and interleukin-6 proinflammatory cytokines than Ad5 or Ad5/3 control viruses suggesting that Ad3 can activate mouse macrophages and T-cells. However, the levels of these cytokines were well below concentrations associated with toxicity.22,23,24
Toxicity assays. Toxicity assays performed in immune competent mice (N = 5 per group) indicated that Ad3wt and Ad3-hTERT-E1A were less toxic than Ad5wt or Ad5/3delta24 (Supplementary Figure S3). At 72 hours, mice in the latter two groups appeared ill and livers were macroscopically yellow. No signs of toxicity were seen in any other organs examined (heart, lung, intestine, kidney, spleen, pancreas, brain, testicle, and muscle) in mice treated with Ad3-hTERT-E1A, Ad3wt, or phosphate-buffered saline. Ad5wt showed 36-fold and Ad5/3delta24 showed 176-fold higher liver enzymes than mock. Other groups displayed only minor elevations in liver enzymes (Ad3wt and Ad3-hTERT-E1A threefold, Ad5/3-hTERT-E1A fivefold) compared to the mock group. Analysis of other blood values showed nonspecific thrombocytopenia but no changes in leukocytes or other parameters.
Clinical
Baseline characteristics. Baseline characteristics of the 25 patients treated with Ad3-hTERT-E1A can be seen in Table 1. The patients had a wide variety of cancer types and were heavily pretreated with a median of three chemotherapy regimens. About half of them had been previously treated with serotype 5 oncolytic adenovirus while the other half received Ad3-hTERT-E1A as a first oncolytic virus treatment. Patient by patient cancer treatments can be found from Supplementary Table S2.
Table 1. Patient characteristics.

Adverse reactions. Adverse reactions are described in Table 2. No serious adverse events leading to patient hospitalization were encountered and the only severe events were asymptomatic and self-limiting hematological findings. Therefore, no dose-limiting toxicity was seen. Typically, the patients suffered from mild flu-like symptoms: chills, fatigue, fever, and nausea were present in about half of the patients, as was injection site (=tumor) pain. Almost all patients displayed lymphocytopenia and some showed minor elevation of aspartate aminotransferase. A transient decrease in hemoglobin was also seen in many patients. If the patient had a grade × symptom before treatment and it worsened to grade Y, it was scored as grade Y (not Y-X). For example, the hemoglobin of patient P297 was 100 g/l before treatment and 79 g/l the day after treatment and was thus scored as grade 3. A decrease in hemoglobin has been consistently reported in patients treated with oncolytic adenovirus25,26 and may be a general phenomenon related to virus treatment but not to binding of the virus to erythrocytes directly since this was not seen in our patients (Supplementary Table S1). However, many cancer patients had baseline anemia caused by the cancer and dilution of blood from the 3,000 ml of fluids the patients received on the treatment day may also have contributed to hemoglobin readings. Few patients reported also mild diarrhea. No correlation of adverse reactions with e.g. previous serotype 5 treatments, virus dose, or route of administration was found.
Table 2. Adverse Reactions.

Patients first treated with Ad5 and then with Ad3 experienced prolonged lymphocytopenia (P <0.05 between Ad5 pretreated and naive patients), which was not seen in patients that received Ad3 as a first treatment, nor was it seen when these Ad3-treated patients received Ad5 as a second treatment (Figure 1). In attempt to dissect reasons for the lymphocytopenia we assessed antiviral and antitumor T-cells in the blood of the patients (Figure 1b,c). Before Ad3-hTERT-E1A treatment, we saw some T-cell activation against serotype 3 hexon in Ad5 pretreated patients (median 15 spot-forming units), but none in naive patients, perhaps suggesting some crossreactivity between T-cell epitopes.27 Interestingly, after Ad3-hTERT-E1A treatment median antiviral T-cells in the Ad5 pretreated group remained stable (13 SPU) whereas T-cells in the non-pretreated group showed an increase (median 79 spot-forming units). On a patient level, there was variation in the former group whereas all patients showed an increase in the latter. With regard to TAAs, we used survivin and one other TAA mix (selected individually for each tumor on the basis of a literature search) for each patient. While changes in the overall activity of T-cells were seen in most patients, suggesting immunological activation, we could not see any clear correlation with Ad5 pretreatment.
Figure 1.
White blood cell changes in treated patients. (a) Ad5 pretreated patients get longer lasting lymphocytopenia after Ad3-hTERT-E1A treatment P < 0.05. Please note that Ad3-hTERT-E1A to Ad3 pretreated patient consists from only one patient and thus little conclusions can be drown from this patient. (b) Ad3 hexon enzyme-linked immunosorbent spot (ELISPOT) of peripheral blood mononuclear cells. Ad5 pretreated patients have lymphocytes that are activated when stimulated by serotype 3 hexon [median 15 spot-forming units (SFU)], this is not seen with non-pretreated patients (median 2 SFU, P = 0.06). After Ad3-hTERT-E1A treatment the lymphocytes of the pretreated patients do not show big changes against serotype 3 hexon (median 13 SFU), whereas the non-pretreated patients show high activity (median 79 SFU, P = 0.10). (a–b) A long-lasting lymphocytopenia is generated when patients are primed by Ad5 and then treated by Ad3 but the immune reaction is not against serotype 3 hexon. Unstimulated background is subtracted. (c) Tumor-associated antigen (TAA) ELISPOT of peripheral blood mononuclear cells. Clear change in lymphocyte behavior is seen after Ad3-hTERT-E1A treatment. No clear correlation with the pretreated or non-pretreated is seen. No prestimulation or clonal expansion of PBMCs was done in these assays and thus the results indicate the actual frequency of these cells in blood. Survivin was analyzed from all patients. SSX2 was analyzed from S171, T181, O14, S119, H309. CEA from H305, K260, V264, K253, K304, R218, R8, and PSA from P284. Unstimulated is shown. Bars mean + SD.
Ad3-hTERT-E1A was detected in blood for weeks (Table 3). Patients that received >1012 VP had a median of 5,800 VP/ml in blood the following day and 8/9 patients were positive at 3 and 6 weeks. Patients that received <1012 VP had a median of <125 VP/ml in the blood the following day, but 2/6 patients were still detected positive at 3 and 6 weeks. Roughly 10 times more virus was usually detected from the clot, compared with serum. This finding however displayed considerable variance and sometimes even with the same patient more virus was detected from the serum than from the clot. No clear correlation between pretreatment status, neutralizing antibody titer and the amount of virus in blood could be seen.
Table 3. Virus amounts in blood and neutralizing antibody titers.
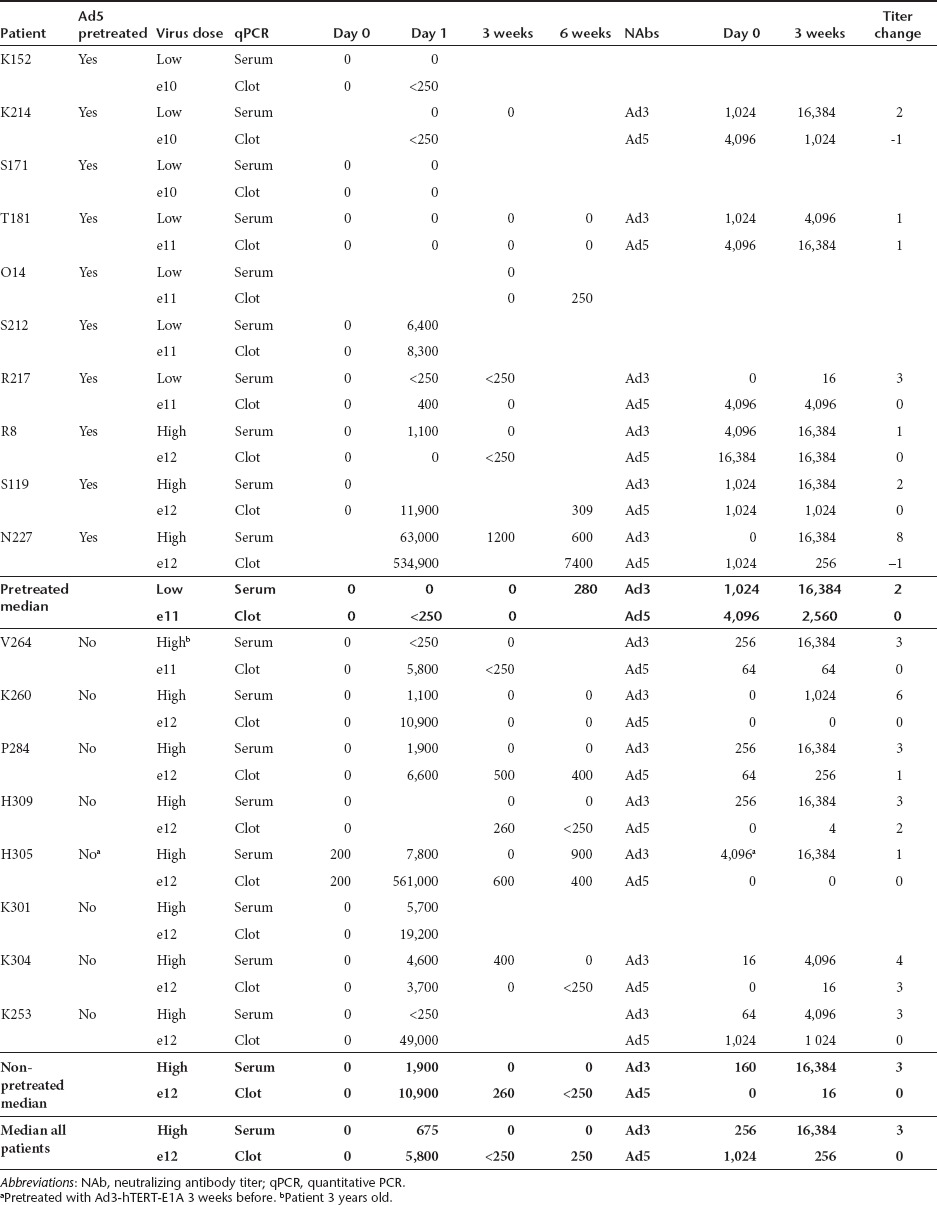
Neutralizing antibody assay. Neutralizing antibody assay was performed separately against serotype 3 and 5. Interestingly most patients had Ad3 NAbs already before treatment (median 256) suggesting previous natural Ad3wt infection. As expected, no significant difference was found between the serotype 5 pretreated and non-pretreated patients with regard to baseline Ad3 NAbs. In contrast, patients previously treated with Ad5 had higher Ad5 NAbs (P < 0.05). Proving complete lack of cross-neutralization, median Ad5 NAb titer did not increase as a result of the Ad3-hTERT-E1A treatment while the Ad3 NAb titer became a median of 64-fold higher.
Blood samples of the first two patients treated with a low dose (1010 VP) of Ad3-hTERT-E1A were taken at 10 minutes, 2, 6, and 20 hours (Supplementary Table S1) and different blood compartments were analyzed. While most of the virus seemed to be cleared from the blood in minutes, no virus was ever detected in red blood cells. At 10-minute virus was detected in PBMC, plasma and platelet compartments while later it was mainly seen in PBMCs and plasma.
Objective evidence of possible antitumor activity (stable disease or better in RECIST, positron emission tomography (PET) criteria or tumor markers) were seen in 15 out of 23 (= 65%) evaluable patients. Most patients (22/25) received a serial treatment (three different viruses at a 3-week interval followed by imaging with the same method as done at baseline) and thus were not radiological evaluable for Ad3-hTERT-E1A effect only (Table 4). Of the 16 patients that could be evaluated for Ad3-hTERT-E1A only, 12 (75%) showed disease control. In 15 cases tumor markers were measured immediately before and 3 weeks after treatment while patient S171 was imaged with computer tomography (CT) immediately before and 1 month after the virus.
Table 4. Patients treated with Ad3-hTERT-E1A.

11 out of 15 patients (= 73%) analyzed for tumor markers had signs of antitumor activity. Two complete responses (mCR) were noted via normalization of CEA and CA15-3 in patients R217 and R263, respectively. Patient K260 had a high CEA level of 854 before treatment and was after treatment graded as a partial response (mPR) due to a drop to 460. CA12-5 of R218 had previously been elevated and changes had correlated with treatment efficacy. However, before treatment with Ad3-hTERT-E1A, her CA12-5 was within normal limits but decreased further (15–>10) and this was therefore scored as a partial marker response (mPR). H309 was graded as minor response (mMR) in CA19-9. Patients O14, R8, R277, K236, P297 were graded stable disease (mSD) and rest as progressive disease (mPD).
Correlation between objective signs of antitumor activity and survival. If we focus on patients that survived <100 days (N = 4), all evaluable cases had progressive disease. In contrast, all patients that survived over 300 days (N = 8) showed objective signs of antitumor activity in efficacy evaluations. Obviously larger patient numbers are needed to reliably study possible surrogate endpoints. Nevertheless, another tantalizing finding is that 5 out of 6 evaluable patients that received 100% of the virus through the intravenous route featured disease control.
Median survival. Median survival for patients that had any objective sign of antitumor activity (N = 15) is 295 days while for nonresponders (N = 8) it is 108 days (P < 0.001). When taking only the marker responders (N = 11, mSD or better) into account the median survival is 255 days and for nonresponders (N = 4) it is 110 days, (P < 0.05). No clear correlation between WHO, pretreatment status, virus dose, route of administration, and the patient responses or survival was observed.
Patient examples
All five breast cancer patients showed objective evidence of possible anticancer activity (markers: mCR 2, mPR 1, mSD 2, Table 4). No other tumor types yielded such provocative data but the number of patients was smaller for many of them. Two patients who received concurrent trastuzumab showed a drop in tumor markers (CEA 11–>5 and CA12-5 15–>10, and the former was graded as mCR) after the administration of the Ad3-hTERT-E1A virus. This was the first virus treatment these patients had ever received and both had progressed on trastuzumab previously. Both patients received a fully intravenous high dose of Ad3-hTERT-E1A and experienced prolonged survival (both alive at 310 and 630 days, respectively). Of all of the 25 patients treated with Ad3-hTERT-E1A, only these two received trastuzumab as a concominant therapy so no conclusions can be drawn but the data is compatible with an additive effect between Ad3 and trastuzumab as proposed in preclinical studies.15
For patient S171 long-term radiological follow up was possible without confounding from other treatments. The patient had metastatic malignant fibrous histocytoma and had been heavily pretreated with multiple operations and chemotherapeutics, but the disease was progressing before virus therapy. He was initially treated with five Ad5-based treatments over 6 months but CT scans suggested progression. He was then treated with a single round of Ad3-hTERT-E1A and CT scans were taken before, 1 month and 4 months after the Ad3-hTERT-E1A treatment (Figure 2). Reduction of the largest injected tumor was seen and the overall situation was stable according to RECIST. At 4 months, the injected tumors were still stable (N = 5, –0.4%) but noninjected tumors had grown and progressive disease was diagnosed.
Figure 2.
Example of imaging result and survival graph of patients. (a) Sarcoma patient that was treated with a single dose of Ad3-hTERT-E1A and followed by CT. (b) Cumulative survival of Ad3-hTERT-E1A-treated patients.
Patient N227 showed high blood virus titers for up to 6 weeks suggesting emphatic replication. The patient was a 3-year-old female with neuroblastoma. Before virus treatment, she had received seven rounds of chemotherapy and radiation therapy but the NSE tumor marker was rising and bone marrow immunofluorescence showed more malignant cells than before. Progressive disease was therefore present and she was treated first with an Ad5-based oncolytic adenovirus. After administration of Ad3-hTERT-E1A (her second oncolytic virus), high-virus titer was found in blood on day 1 (Table 3). On day 3, 310,000 VP/ml from serum and 261,000 VP/ml from blood clot were detected suggesting replication of the virus. Virus was detected at 3 weeks and even at 6 weeks 600 and 7,400 VP/ml were present in the serum and clot, respectively. At 6 weeks bone marrow aspirate immunofluorescence and biopsy were performed and found free of malignant cells, in contrast to the samples taken before oncolytic virus treatment. Tumor marker NSE—progressing prior to therapy—decreased from 25–21 in 3 weeks which was graded as mMR.
Patient H305 is a 58-year-old male with pancreatic cancer who had been operated a year before and multiple lines of chemotherapy had been tried. He received Ad3-hTERT-E1A as a first and also as a second virus treatment three weeks later. Low but persistent virus was still seen in the serum before the second treatment. Before the third treatment [Ad5-based Icovir-7 (ref. 28)] no Ad3-hTERT-E1A could be detected from serum but 3 and 19 days later Ad3-hTERT-E1A re-emerged with titers of 210 and 900 VP/ml, respectively (Supplementary Figure S4).
Ad3-hTERT-E1A increased in six out of nine evaluable patients in serum and/or clot after Ad5 treatment (K304, H305, N227, O14, S171, H309). Motivated by this finding we also assessed if Ad3-hTERT-E1A treatment would result in re-emergence of serotype 5 virus into blood. Interestingly, five out of the seven evaluable patients previously treated with an Ad5-based virus showed reappearance of the Ad5 virus to the clot and/or serum after Ad3-hTERT-E1A treatment (K214, S212, R217, S119, N227).
Discussion
In biodistribution assays in mice at 6 hours Ad3 was still present in blood. High amounts of proinflammatory cytokines were seen suggesting that serotype 3 is potent in activating T-lymphocytes (RANTES, interleukin-6) and macrophages (tumor necrosis factor-α, interleukin-6). Activation of multiple proinflammatory cytokines has been suggested to correlate with cytolytic function and in vivo activity.29,30 Whether serotype 3 truly is more immunogenic in mice or whether the higher amount of cytokines is due to the prolonged presence in blood due to lack of high-avidity entry receptors, resulting in eventual uptake by e.g., macrophages, are interesting subjects for further study.
According to the murine toxicology results at 72 hours, serotype 3 seems to be safer than serotype 5 or 5/3 viruses. When comparing Ad5/3delta24 and Ad5/3-hTERT-E1A, which are identical except for the E1 region, the hTERT promoter seems to protect the liver from damage, perhaps through downregulation of E1A expression in nontarget tissues, as the selectivity of delta-24 type viruses occurs post-E1A-expression. This finding has relevance for Ad3-hTERT-E1 as the same promoter and virus design are used. Taking the mouse cytokine and toxicity data together with our previous data14 and adding the finding that Ad3,16 in contrary to Ad5 (ref. 31) and Ad5/3 (ref. 32), does not seem to bind into erythrocytes in mice (Supplementary Figure S1) or in patients (Supplementary Table S1) it seems that Ad5/3 resembles more Ad5 than Ad3 from the point of view of toxicity in mice and binding to blood cells.
In oncolytic virus-treated patients, blood lymphocytes tend to drop to one-third of the pretreatment value in 1 day (Figure 1 and previous reports25,26,33). One explanation for this is that these lymphocytes traffic to lymph nodes, spleen and to the tumor where the virus is injected. Thus, “lymphocytopenia” in blood could reflect redistribution of cells to tissues where they are needed for mounting an immune response, and could in fact constitute a surrogate for immunological activity, as reported in mice or in humans treated with another immunotherapeutic.34,35,36 When measuring lymphocytes 3 weeks later they are normally back to pretreatment values25,26,33 but this was not the case with Ad3-hTERT-E1A-treated patients that had received previous Ad5 treatments (Figure 1a). These patients had some T-cells reactive against Ad3-hTERT-E1A already before treatment, but the median amount in blood did not change after the treatment (Figure 1b). When antitumor T-cells were analyzed, changes compatible with trafficking to tumor or clonal replication were seen in many patients. Taken together, these finding raise the possibility that “priming” advanced cancer patients with Ad5 viruses and then “boosting” with Ad3 leads to immunological changes sufficiently prominent to be seen even in blood lymphocyte counts and that the effect is not only against the Ad3 virus (Figure 1a–c). However, the right place to assess this would be the tumor, not the blood, which is important to keep in mind for future clinical approaches.
We did not see a trend between an increase in antitumor T-cells and treatment benefits. This may relate to (i) the blood being the wrong place to look at antitumor T-cells, (ii) us not knowing what the most relevant epitope is for each patient, (iii) the lack of surrogate endpoints for treatment benefits (the biggest impact of immunotherapy will ultimately be on survival but a randomized setting would be needed to measure this. Tumor size is not a good endpoint due to inflammatory swelling), (iv) a possible disconnect between induction of antitumor immunity and clinical benefit, perhaps mediated by immunosuppression, as proposed in the cancer vaccine field.37
Preclinical studies have indicated that despite the presence of viral DNA in cancer cells, in vivo cell lysis and antitumor effect may be disabled. This phenomenon is not well understood but proposals implicate induction of antiviral interferon pathways38 or intratumoral antibodies.39 These phenomena might relate also to attenuation of cell-to-cell spread of the virus. Therefore, reactivation of virus residing dormant in tumors could result in therapeutic effects. In six out of nine patients Ad3-hTERT-E1A increased in serum and/or clot after Ad5 treatment. This suggests reactivation of replication in cancer cells or destruction of cancer cells containing Ad3 viruses or possibly both. In theory, Ad3—which is only 67% similar to Ad5 on the genomic level40—might have mechanisms for counteracting resistance mechanisms resulting in reactivation of the oncolytic effect. Alternatively, the high local concentration of virus resulting from intratumoral injection could overwhelm antiviral mechanisms. To investigate whether this finding was specific for Ad3 we analyzed Ad5 quantitative PCR before and after Ad3-hTERT-E1A treatment in patients who had previously been treated with an Ad5 virus and made the same observation suggesting a general phenomenon.
For effective transduction of distant metastases extended seroprevalance might be beneficial. If the dose was over 1012 VP ample virus was usually detected in blood on the following day and it often persisted for at least 6 weeks. No correlation between dose and adverse reactions was seen. As dose-limiting toxicity was not seen either, even higher doses than 4 × 1012 VP might be considered.
Seven patients were safely treated by an intravenous injection (without intratumoral delivery) of Ad3-hTERT-E1A. Six were evaluable to efficacy and five of them showed signs of possible benefit. If this is confirmed in larger patient populations, one explanation could be that a large virus dose can overwhelm NAbs and other antiviral immunity. After treatment the NAb titer however increased exponentially and such response might compromise further systemic treatments with the same virus unless the virus is able to e.g., hitchhike on cells to avoid NAbs.32 Alternatively, if opsonization by NAbs is a dynamic process, some virus might nevertheless have access to target receptors despite high NAb titers. When treating intratumorally NAbs are probably not an issue, but changing the serotype might nevertheless give an additive boost to the immune system (Figure 1).
Roughly two thirds of the patients show some kind of objective evidence of possible antitumor activity. Trying to adopt RECIST or PET response criteria to oncolytic virus treatments likely underestimates the actual antitumor effect because of inflammation and tumor swelling, resulting in false diagnosis of tumor progression.41 With regard to tumor markers, virus-induced S-phase can increase marker peptide production.42 Following lysis, these proteins are released into blood, and therefore the amount of marker peptide in blood might no longer correlate with the number of viable tumor cells. Also increased metabolism due to virus infection might cause stronger PET signals, especially as activated lymphocytes preferentially use glucose for fuel, and PET imaging is based on uptake of labeled glucose. However, these evaluation criteria may not be completely useless as tumor control with these methods was associated with a survival benefit in our patient series.
In summary, Ad3-hTERT-E1A seems safe for treatment of cancer patients and therefore further clinical development seems indicated. Of note, the patients reported here were treated in a personalized therapy scheme and thus it would be important to confirm and expand the data in trials featuring more homogeneous patient populations. Immunologically the virus differs from serotype 5 viruses and changing the serotype might reactivate replication of the original treatment virus. Also, the ability of Ad3 to use desmoglein 2, found abundantly in advanced tumors, as a primary entry receptor, could be a benefit over CAR binding viruses. Combining serotype 3 viruses with trastuzumab, anti-EGFR or CAR dependent viruses is appealing due to the ability of Ad3 to open intercellular tight junctions. To further enhance the immunological effects of the virus arming with immunostimulatory molecules such as GM-CSF or CD40L might be beneficial. The apparently good safety of Ad3-hTERT-E1A provides a good starting point for development of such viruses.
Materials and Methods
Treatment protocol. Twenty-five cancer patients with advanced solid tumors, progressing after routine therapies, were treated. Patients with other severe diseases, brain tumors, severe thrombocytopenia (<½ lower normal limit) or clearly elevated liver enzymes (>3× upper normal limit) were not treated. All patients signed written informed consent. Ad3-hTERT-E1A14 was manufactured by Oncos Therapeutics (Helsinki, Finland). It was administered intravenously and usually also in ultrasound guidance in a personalized scheme (Table 4). Time-lapse dose escalation from 1010 VP was utilized to maximize patient safety but minimize delays in enrolling new patients at potentially more effective higher doses. Dose could be escalated when sufficient time (typically 2 weeks) had lapsed (and relevant safety information collected) from the treatment of the first patient at that dose. Most patients were treated in a serial manner—three treatments with different viruses, every three weeks and many were also treated with concomitant low-dose cyclophosphamide43 and/or low-dose pulse temozolomide.44 This Advanced Therapy Access Program (ATAP) is regulated by Finnish Medicines Agency FIMEA as determined by EC/1394/2007, Good Clinical Practice and the Declaration of Helsinki. Side effects were graded according to CTCAE v.3.0. Patients were monitored overnight at the clinic and then as outpatients for 28d for adverse events. Survival and late adverse events were followed ad infinitum.
Human interferon-γ enzyme-linked immunosorbent spot. PBMCs were isolated from the blood by Percoll (Sigma, St Louis, MO) gradient. Cells were frozen in CTL-CryoABCTM serum-free media (Cellular Technology, Cleveland, OH). Enzyme-linked immunosorbent spot Pro was performed as instructed in the manufacturers (Mabtech, Nacka strand, Sweden) protocol. 200,000 PBMCs per well was used. For adenovirus enzyme-linked immunosorbent spot, cells were stimulated with HAdV-3 Hexon protein (ProImmune, Oxford, UK). For tumor enzyme-linked immunosorbent spot we used the following peptides: Survivin (BIRC5_PONAB), SSX2, CEA or PSA (ProImmune). PBMCs of each patient were stimulated with survivin and one rationally chosen peptide mix. No prestimulation or clonal expansion of PBMCs was done in this assay and thus the results indicate the actual frequency of these cells in blood.
NAb. A549 (ATCC, Manassas, VA) cells were seeded at 1 × 104 cells per well on 96-well plates and cultured overnight in Dulbecco's modified Eagle medium without fetal calf serum. Next day, human serum samples were incubated at 56 °C for 90 minutes to inactivate complement, and a fourfold dilution series (1:1–1:16 384) was prepared in serum-free Dulbecco's modified Eagle medium45. Ad5-luc8 and Ad3-luc45 was mixed with serum dilutions and incubated at room temperature for 30 minutes. Thereafter, cells in duplicates were infected with 100 VP per cell in 50 µl of mix, and 100 µl of growth medium with 10% fetal calf serum was added 1 hour later. Twenty four hours postinfection, cells were lysed and luciferase activity was measured with Luciferase Assay System (Promega, Madison, WI) using TopCount luminometer (PerkinElmer, Waltham, MA). Luciferase readings were plotted relative to gene transfer achieved with Ad5luc1 or Ad3luc1 alone. The NAb titer was determined as the lowest degree of dilution that blocked gene transfer >80%.
Evaluation of antitumor efficacy. CT and/or PET imaging was done before and after a serial treatment (three virus injections) or after a single treatment (S171). RECIST1.1 criteria are: PR (>30% reduction in the sum of tumor diameters), SD (no response/progression), PD (>20% increase25). In addition MR was scored to indicate 12–29% reduction. For tumor markers the same percentages were used. For PET the following criteria were used 25: PR (≥30% decrease in summed SUVmax, up to five lesions counted, max. 2/organ), MR (10–29% decrease in summed SUVmax), SD (–9 to 29% change in summed SUVmax), PD (≥30% increase in summed SUVmax or ≥2 cm PET positive new lesion, except local lymph nodes whose signal might indicate immune reaction).
The following experiments are described in Supplementary Materials and Methods: Ad3-hTERT-EIA qPCR, biodistribution and cytokine experiments, toxicity experiment and the Ad3-hTERT-E1A biodistribution.
SUPPLEMENTARY MATERIAL Figure S1. Ad3 biodistribution in mice. Figure S2. Cytokines. Figure S3. Ad3 toxicity in mice. Figure S4. Reappearance of Ad3-hTERT-E1A in serum after Ad5 treatment. Table S1. For the first two patients treated with Ad3-hTERT-E1A blood samples were taken as indicated to get preliminary information about the in vivo human biodistribution of the virus. Table S2. Previous cancer treatments of the patients. Materials and Methods.
Acknowledgments
We thank the National Graduate School of Clinical Investigation (CLIGS, University of Helsinki, Finland) for financial support. Minna Oksanen, Sirkka-Liisa Holm, Gerd Bauerschmitz (CGTG, University of Helsinki, Helsinki, Finland), Silvio Hemmi (University of Zurich, Zurich, Switzerland), Maija Lappalainen (HUCH, Finland) for expert advice and help and Ari Ristimäki (Department of Pathology, HUCH, Finland) for histological analysis. We thank Saila Eksymä-Sillman, Marina Rosliakova and other personnel at International Comprehensive Cancer Center Docrates, and at Eira Hospital, Helsinki, Finland. Mikael von Euler (Oncos Therapeutics) is thanked for critical commentary. Akseli Hemminki is K. Albin Johansson Research Professor of the Foundation for the Finnish Cancer Institute. O.H. is shareholder in Oncos Therapeutics. A.H. is shareholder in and consultant for Oncos Therapeutics. The work has been done in Helsinki, Finland.
Supplementary Material
Ad3 biodistribution in mice.
Cytokines.
Ad3 toxicity in mice.
Reappearance of Ad3-hTERT-E1A in serum after Ad5 treatment.
For the first two patients treated with Ad3-hTERT-E1A blood samples were taken as indicated to get preliminary information about the in vivo human biodistribution of the virus.
Previous cancer treatments of the patients.
REFERENCES
- Edukulla R, Ramakrishna E, Woller N, Mundt B, Knocke S, Gürlevik E.et al. (2009Antitumoral immune response by recruitment and expansion of dendritic cells in tumors infected with telomerase-dependent oncolytic viruses Cancer Res 691448–1458. [DOI] [PubMed] [Google Scholar]
- Gürlevik E, Woller N, Strüver N, Schache P, Kloos A, Manns MP.et al. (2010Selectivity of oncolytic viral replication prevents antiviral immune response and toxicity, but does not improve antitumoral immunity Mol Ther 181972–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt KM, Ni S, Tieu AT., and, Lieber A. Assessment of a combined, adenovirus-mediated oncolytic and immunostimulatory tumor therapy. Cancer Res. 2005;65:4343–4352. doi: 10.1158/0008-5472.CAN-04-3527. [DOI] [PubMed] [Google Scholar]
- Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H.et al. (2006Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect Gene Ther 131010–1020. [DOI] [PubMed] [Google Scholar]
- Hanahan D., and, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Mellman I, Coukos G., and, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva A., and, Hemminki A. Modified adenoviruses for cancer gene therapy. Int J Cancer. 2004;110:475–480. doi: 10.1002/ijc.20129. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ.et al. (2002Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells Clin Cancer Res 8275–280. [PubMed] [Google Scholar]
- Kangasniemi L, Kiviluoto T, Kanerva A, Raki M, Ranki T, Sarkioja M.et al. (2006Infectivity-enhanced adenoviruses deliver efficacy in clinical samples and orthotopic models of disseminated gastric cancer Clin Cancer Res 123137–3144. [DOI] [PubMed] [Google Scholar]
- Tsuruta Y, Pereboeva L, Glasgow JN, Rein DT, Kawakami Y, Alvarez RD.et al. (2007A mosaic fiber adenovirus serotype 5 vector containing reovirus sigma 1 and adenovirus serotype 3 knob fibers increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway Clin Cancer Res 132777–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Ulasov IV, Han Y, Tyler MA, Zhu ZB., and, Lesniak MS. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–160. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM.et al. (2002Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses Mol Ther 5695–704. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Wang M, Desmond RA, Strong TV, Alvarez RD., and, Curiel DT. Serum and ascites neutralizing antibodies in ovarian cancer patients treated with intraperitoneal adenoviral gene therapy. Hum Gene Ther. 2002;13:1505–1514. doi: 10.1089/10430340260185139. [DOI] [PubMed] [Google Scholar]
- Hemminki O, Bauerschmitz G, Hemmi S, Lavilla-Alonso S, Diaconu I, Guse K.et al. (2011Oncolytic adenovirus based on serotype 3 Cancer Gene Ther 18288–296. [DOI] [PubMed] [Google Scholar]
- Wang H, Li ZY, Liu Y, Persson J, Beyer I, Möller T.et al. (2011Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14 Nat Med 1796–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li Z, Yumul R, Lara S, Hemminki A, Fender P.et al. (2011Multimerization of adenovirus serotype 3 fiber knob domains is required for efficient binding of virus to desmoglein 2 and subsequent opening of epithelial junctions J Virol 856390–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fender P, Boussaid A, Mezin P., and, Chroboczek J. Synthesis, cellular localization, and quantification of penton-dodecahedron in serotype 3 adenovirus-infected cells. Virology. 2005;340:167–173. doi: 10.1016/j.virol.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Trinh HV, Lesage G, Chennamparampil V, Vollenweider B, Burckhardt CJ, Schauer S.et al. (2012Avidity binding of human adenovirus serotypes 3 and 7 to the membrane cofactor CD46 triggers infection J Virol 861623–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Ryan MJ, Estep JE, Miniard BM, Rudge TL, Peggins JO.et al. (2011A new generation of serotype chimeric infectivity-enhanced conditionally replicative adenovirals: the safety profile of ad5/3-?24 in advance of a phase I clinical trial in ovarian cancer patients Hum Gene Ther 22821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkioja M, Kanerva A, Salo J, Kangasniemi L, Eriksson M, Raki M.et al. (2006Noninvasive imaging for evaluation of the systemic delivery of capsid-modified adenoviruses in an orthotopic model of advanced lung cancer Cancer 1071578–1588. [DOI] [PubMed] [Google Scholar]
- Ranki T, Särkioja M, Hakkarainen T, von Smitten K, Kanerva A., and, Hemminki A. Systemic efficacy of oncolytic adenoviruses in imagable orthotopic models of hormone refractory metastatic breast cancer. Int J Cancer. 2007;121:165–174. doi: 10.1002/ijc.22627. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M., and, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Morral N, O'Neal WK, Rice K, Leland MM, Piedra PA, Aguilar-Córdova E.et al. (2002Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons Hum Gene Ther 13143–154. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Seiler MP, Mane V, Brunetti-Pierri N, Clarke C, Bertin TK.et al. (2007Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors Mol Ther 15378–385. [DOI] [PubMed] [Google Scholar]
- Pesonen S, Diaconu I, Cerullo V, Escutenaire S, Raki M, Kangasniemi L.et al. (2012Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors Int J Cancer 1301937–1947. [DOI] [PubMed] [Google Scholar]
- Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I.et al. (2010Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF Mol Ther 181874–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Sili U, Vanin EF, Jewell AM, Xie W, Vignali D.et al. (2004Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells Blood 1042432–2440. [DOI] [PubMed] [Google Scholar]
- Nokisalmi P, Pesonen S, Escutenaire S, Särkioja M, Raki M, Cerullo V.et al. (2010Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors Clin Cancer Res 163035–3043. [DOI] [PubMed] [Google Scholar]
- Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL., and, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G, Bédard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP.et al. (2008Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells J Virol 8210017–10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle RC, Di Y, Cerny AM, Sonnen AF, Sim RB, Green NK.et al. (2009Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1 Blood 1131909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escutenaire S, Cerullo V, Diaconu I, Ahtiainen L, Hannuksela P, Oksanen M.et al. (2011In vivo and in vitro distribution of type 5 and fiber-modified oncolytic adenoviruses in human blood compartments Ann Med 43151–163. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M.et al. (2010Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients Cancer Res 704297–4309. [DOI] [PubMed] [Google Scholar]
- Kanerva, A. Philadelphia; 2012. American Society of Gene and Cell Therapy. [Google Scholar]
- Chen J, Zajac AJ, McPherson SA, Hsu HC, Yang P, Wu Q.et al. (2005Primary adenovirus-specific cytotoxic T lymphocyte response occurs after viral clearance and liver enzyme elevation Gene Ther 121079–1088. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH.et al. (2010Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates J Clin Oncol 283167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M, Callahan MK., and, Wolchok JD. Beyond cancer vaccines: a reason for future optimism with immunomodulatory therapy. Cancer J. 2011;17:372–378. doi: 10.1097/PPO.0b013e31823261db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liikanen I, Monsurrò V, Ahtiainen L, Raki M, Hakkarainen T, Diaconu I.et al. (2011Induction of interferon pathways mediates in vivo resistance to oncolytic adenovirus Mol Ther 191858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar D, Spencer JF, Toth K., and, Wold WS. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirena D, Ruzsics Z, Schaffner W, Greber UF., and, Hemmi S. The nucleotide sequence and a first generation gene transfer vector of species B human adenovirus serotype 3. Virology. 2005;343:283–298. doi: 10.1016/j.virol.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid TR, Freeman S, Post L, McCormick F., and, Sze DY. Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Ther. 2005;12:673–681. doi: 10.1038/sj.cgt.7700819. [DOI] [PubMed] [Google Scholar]
- Pesonen S, Nokisalmi P, Escutenaire S, Särkioja M, Raki M, Cerullo V.et al. (2010Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors Gene Ther 17892–904. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A.et al. (2011Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus Mol Ther 191737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liikanen, IAL, Kangasniemi, L, Cerullo, V, Nokisalmi, P, Escutenaire, S, Diaconu, I.et al. (2011Treatment of cancer with oncolytic adenovirus combined with temozolomide, an autophagy inducer Mol Ther
- Fleischli C, Sirena D, Lesage G, Havenga MJ, Cattaneo R, Greber UF.et al. (2007Species B adenovirus serotypes 3, 7, 11 and 35 share similar binding sites on the membrane cofactor protein CD46 receptor J Gen Virol 88Pt 112925–2934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ad3 biodistribution in mice.
Cytokines.
Ad3 toxicity in mice.
Reappearance of Ad3-hTERT-E1A in serum after Ad5 treatment.
For the first two patients treated with Ad3-hTERT-E1A blood samples were taken as indicated to get preliminary information about the in vivo human biodistribution of the virus.
Previous cancer treatments of the patients.




