Abstract
Exhaustion of CD8+ T cells and upregulation of programmed death 1 (PD-1), a negative regulator of T cell activation, are characteristic features of individuals chronically infected with human immunodeficiency virus type 1. In a previous study, we showed in mice that a dendritic cell-directed lentiviral vector (DCLV) system encoding the human immunodeficiency virus (HIV)-1 Gag protein was an efficient vaccine modality to induce a durable Gag-specific T cell immune response. In this study, we demonstrate that blocking of the PD-1/PD-1 ligand (PD-L) inhibitory signal via an anti-PD-L1 antibody generated an enhanced HIV-1 Gag-specific CD8+ immune response following both a single round of DCLV immunization and a homologous prime/boost regimen. The prime/boost regimen combined with PD-L1 blockade generated very high levels of Gag-specific CD8+ T cells comprising several valuable features: improved ability to produce multiple cytokines, responding to a broader range of Gag-derived epitopes, and long-lasting memory. This enhanced cellular immune response generated by DCLV immunization combined with anti-PD-L1 blockade correlated with improved viral control following challenge with Gag-expressing vaccinia virus. Taken together, our studies offer evidence to support the use of PD-1/PD-L1 blockade as an adjuvant modality to enhance antigen-specific immune responses elicited by T cell-based immunizations such as DCLV.
Introduction
The immune system fails to control such chronic infections as human immunodeficiency virus (HIV), hepatitis C virus, and hepatitis B virus, and in the case of HIV and hepatitis C virus, developing effective vaccines has been extremely difficult. One of the main causes of this failure may stem from the fact that in these patients, continuous antigenic stimulation leads to defects in cytotoxic T lymphocytes (CTLs) activation.1 For example, exhaustion of virus-specific CD8+ T cells was first reported in lymphocytic choriomeningitis virus infection and has been observed in other chronic infections as well, including HIV, simian immunodeficiency virus, hepatitis B virus, and hepatitis C virus models.1,2,3,4 One signature of exhausted CD8+ T cells is their upregulation of certain inhibitory molecules. Programmed death 1 (PD-1), a transmembrane immunoreceptor of CD28 family, has been identified as one of these key negative regulators of T cell functions.5
PD-1 is expressed by a range of activated immune cells, including CD8+ and CD4+ T cells, B cells, and natural killer cells.6 It binds to two known ligands, PD-1 ligand (PD-L1) and PD-2 ligand (PD-L2), with PD-L1 more widely expressed on T cells, B cells, dendritic cells (DCs), and macrophages.7 A number of studies have provided specific evidence that PD-1 upregulation and increased engagement to its ligands are correlated with the reduced ability of T cells to undergo activation, proliferation, and cytokine production.8,9,10 Based on these data, several groups have suggested that blocking the PD-1-mediated pathway may restore the function of exhausted antigen-specific CD8+ T cells and may therefore provide a therapeutic means to alleviate these infections.11,12,13 Toward this goal, Ha et al. showed in an lymphocytic choriomeningitis virus infected mouse model that therapeutic vaccination in combination with PD-L1 blockade enhanced the antigen-specific CD8+ T cell response and promoted viral clearance.13 Similarly, in a simian immunodeficiency virus infected macaque study, Velu et al. observed expansion of simian immunodeficiency virus specific polyfunctional CD8+ T cells, as well as B cell proliferation and an increase in antibody titer following treatment with PD-L1 blockade.12 Relevant to HIV infection, PD-1 expression level is positively associated with HIV-specific CD8+ T cell impairment and plasma viremia10 and CD4 effector functions can be restored in vitro by PD-L1 blockade.14 Therefore, PD-1 treatment could potentially be used to enhance the immune response in patients with HIV,15 or in combination with vaccines to improve their efficacy.
DCs are professional antigen presenting cells that are responsible for mounting and modulating the adaptive immune response.16 Various forms of potent DC-based vaccines have been developed, such as targeting specific DC populations,16 involvement of molecular adjuvants,17,18,19 and administration of molecules to inhibit signaling involved in attenuating DC activation.20 In our previous studies, we developed a lentiviral vector enveloped with a Sindbis virus-derived glycoprotein engineered to be specific for DCs.21 In this study, we have evaluated a combinatorial vaccine strategy that combines a dendritic cell-directed lentiviral vector (DCLV)-based vaccine with blockade of the PD-1/PD-L1 pathway. We injected an antibody specific to mouse PD-L1 (αPD-L1) that has been shown to effectively suppresses the interaction between PD-L1 and its cognate receptor PD-1.13,22 When applied with the primary phase of immunization delivered by DCLV encoding HIV-1 Gag (DCLV-Gag), or with a homologous boosting immunization, αPD-L1 significantly enhanced the generation of Gag-specific and cytokine-producing CD8+ T cells. The responding Gag-specific CD8+ T cells from prime/boost regimens displayed an increased ability to produce multiple cytokines, a broader T cell repertoire capable of recognizing multiple Gag epitopes, and an enhanced capacity to control virus infection following a vaccinia virus challenge.
Results
PD-L1 blockade increases the frequency of Gag-specific CD8+ T cells elicited by DCLV immunization
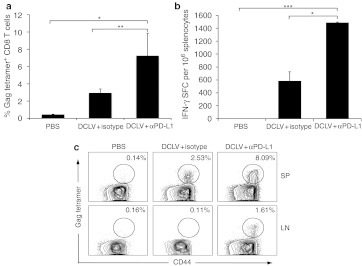
To examine the potential role of PD-1/PD-L1 pathway on eliciting Gag-specific T cell immunity, we first investigated the effect of in vivo blockade of the PD-L1 molecule on the generation of T cell immune responses from a DCLV-based vaccine for HIV-1 Gag. BALB/c mice were immunized with 5 × 106 transduction units (TU) of DCLV-Gag, in the presence of anti-PD-L1 blocking antibody (αPD-L1), or an isotype antibody as a control. Two weeks after vaccination, cells from the spleen and lymph nodes (LNs) were collected for tetramer analysis of Gag-specific CD8+ T cell responses. In spleens, consistent with our previous study,23 DCLV immunization alone induced potent expansion of Gag-specific CD8+ T cells (~3% of total CD8+ cells) (Figure 1a,c). Notably, in vivo PD-L1 blockade by αPD-L1 resulted in a marked increase in the Gag-specific CD8+ T cell frequency (~7% of total CD8+ cells) (Figure 1a,c). An enzyme-linked immunosorbent spot (ELISPOT) assay with the Gag-epitope peptide showed that more IFN-γ-secreting cells were present in the group with PD-L1 blockade (1,500 versus 600 spot-forming cells (SFC)/106 cells, P < 0.05, Figure 1b). In LNs, which are the reservoir of memory T cells, Gag-specific CD8+ T cells were minimally detectable in mice immunized with DCLV alone. However, a substantial number of Gag-specific T cells (~1.6%) were detected in LN of mice immunized with DCLV along with αPD-L1 (Figure 1c). These data show that PD-L1 blockade can increase the generation of antigen-specific CD8+ T cells induced by DCLV-based vaccination.
Figure 1.
In vivo PD-L1 blockade in combination with DCLV immunization increased the frequency of HIV-1 Gag-specific CD8+ T cells. Two weeks after DCLV-Gag vaccination and treatment with either αPD-L1 or isotype antibody, (a) the number of Gag-specific CD8+ T cells in splenocytes was measured by H2-Kd-AMQMLKETI-PE tetramer and CD44 staining, and (b) frequency of IFN-γ-producing cells was assessed by ELISPOT. (c) Representative FACS plots showing the Gag tetramer+ CD8+ T cell population in spleen (SP) and lymph nodes (LN). Each group consisted of four mice. P values were calculated using one-way Anova followed by a Bonferroni nonparametric posttest (ns, not significant; ***P < 0.001; **P < 0.01; *P < 0.05). The data are representative of two independent experiments. DCLV, dendritic cell-directed lentiviral vector; ELISPOT, enzyme-linked immunosorbent spot; HIV, human immunodeficiency virus; LN, lumph node; PD-L1, programmed death 1 ligand; SP, spleen.
PD-L1 blockade enhances DCLV-based booster immunization for generating Gag-specific CD8+ T cell immunity
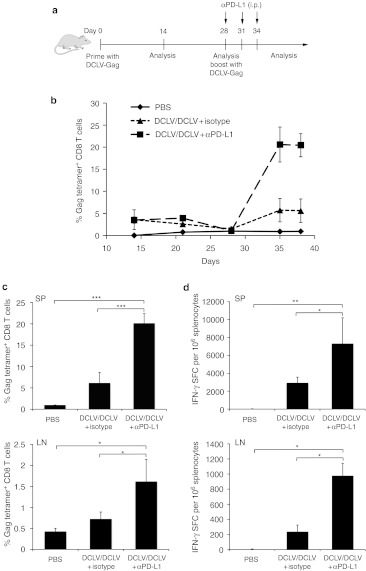
In our previous study, we showed that DCLV immunization could significantly improve a primary antigen-specific memory immune response when administered as a booster immunization following the primary DCLV vaccination.23 Having shown that PD-L1 blockade could improve the CD8+ T cells response in a single immunization regimen (above), we hypothesized that a prime/boost regimen could further improve the T cell response when combined with PD-L1 blockade. To test this hypothesis, BALB/c mice were primed with a footpad injection of DCLV-Gag, followed by a booster injection consisting of a second dose of DCLV-Gag with either αPD-L1 or an isotype antibody treatment. Mice vaccinated with phosphate-buffered saline (PBS) at all injection points were used as controls. To assess the immune response, blood samples from all groups were obtained at multiple time points as indicated, and Gag-tetramer staining were performed on peripheral blood mononuclear cells (PBMC) (Figure 2a). In agreement with our previous study,23 the initial immunization reached a peak response at week two (~3% Gag-tetramer+ CD8+ T cells), and the boost injection greatly increased the number of antigen-specific T cells (Figure 2b). While a similar level of Gag-specific CD8+ T cell responses was observed in both groups after the prime vaccination, a boost DCLV-Gag immunization with PD-L1 blockade exhibited a much greater increase than that observed without blockade (to 20 versus 6% CD8+ tetramer+ T cells, Figure 2b).
Figure 2.
DCLV immunization combined with PD-L1 blockade efficiently boosted primary Gag-specific T cell immunity. (a) Schematic representation of immunization and analysis protocol. (b–d) BALB/c mice were primed with 5 × 106 transduction units of DCLV-Gag, and boosted with DCLV-Gag in conjugation with αPD-L1 (▪) or isotype antibody (▴) treatment three times; the control group was primed and boosted with PBS (♦). (b) Frequencies of Gag tetramer+ CD8+ T cells were determined in PBMC samples from different groups at the indicated time points. Ten days post-boost immunization, T lymphocytes from spleen and lymph node cells were evaluated for (c) Gag-specific CD8+ T cell expansion by tetramer staining and (d) IFN-γ secretion by ELISPOT. P values were calculated using one-way Anova followed by a Bonferroni nonparametric posttest (ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001); n = 4 mice per group. The data are representative of two independent experiments. DCLV, dendritic cell-directed lentiviral vector; ELISPOT, enzyme-linked immunosorbent spot; LN, lumph node; PBMC, peripheral blood mononuclear cell; PBS, phosphate-buffered saline; PD-L1, programmed death 1 ligand; SFC, spot-forming cells; SP, spleen.
Ten days after boost injection, cells from spleens and LNs were assayed by Gag-tetramer staining and IFN-γ ELISPOT, as described in the previous section. In spleen cells, mice immunized with PD-L1 blockade showed a significant advantage in numbers of antigen-specific CD8+ T cells (20 versus 6%, P < 0.001) and a large increase in IFN-γ-producing CD8+ T cells (7,800 versus 2,500 SFC/million cells, P < 0.05) (Figure 2c,d, upper). Analysis of DCs in spleens showed a slightly higher activation of DCs after αPD-L1 treatment (Supplementary Figure S1 online). In LNs, PD-L1 blockade also led to more antigen-specific CD8+ T cells (1.5% versus 0.6%, P < 0.05) and IFN-γ-producing CD8+ T cells (980 versus 240 SFC/million cells, P < 0.05), compared with those without blockade (Figure 2c,d, bottom).
DCLV prime/boost regimen combined with PD-L1 blockade elicits multifunctional CD8+ T cell responses
Increasing evidence points to the correlation of polyfunctional HIV-specific CD8+ T cells frequencies with delay of disease progression.24,25 Since blocking the PD1/PD-L1 pathway has been shown to be beneficial to restoration of CD8+ T cell function, we next sought to test whether the PD-L1 blockade could enhance the production of polyfunctional Gag-specific CD8+ T cells. Therefore, we characterized the functional repertoire of antigen-specific CD8+ T cells generated by different vaccination protocols, in term of their ability to produce cytokines. Spleen cells were harvested 10 days post-boost immunization from mice vaccinated with DCLV prime/DCLV boost with the treatment of either an αPD-L1 or an isotype antibody, followed by restimulation with a library of overlapping HIV-1 Gag peptides. A polychromatic flow cytometry assay was performed on the restimulated spleen cells to simultaneously assess the production of three cytokines (IFN-γ, IL-2, and TNF-α). The pattern (%) of cytokine-secreting cells in both groups was similar, with high levels of IFN-γ secretion, followed by TNF-α, and much lower levels of IL-2 (Figure 3b). PD-L1 blockade in conjunction with DCLV prime/boost regimen increased the total frequency of IFN-γ+ (from 4.5 to 8.7%) and TNF-α+ (from 1.4 to 3.5%) cells within the CD8+ T cell population, whereas it had a slight effect on the frequency of cells producing IL-2 (from 0.7 to 1.1%) (Figure 3b). Generally, compared with the prime/boost alone, PD-L1 blockade produced larger subsets of Gag-specific CD8+ T cells that secreted more than one cytokine simultaneously. The number of CD8+ T cells producing three cytokines from αPD-L1-treated mice was almost four times that of the isotype control group (Figure 3c). On average, mice treated by PD-L1 blockade generated more Gag-specific CD8+ T cells capable of producing both IFN-γ and TNF-α cytokines than those without blockade (1.84 versus 0.67%, Figure 3a,c). These results demonstrate a significant polyfunctional advantage of the cellular immune response that results from prime/boost vaccination with PD-L1 blockade over that without blockade.
Figure 3.
Multifunctionality of CD8+ T cell immune responses induced by DCLV prime/DCLV boost in combination with PD-L1 blockade. Splenocytes harvested from DCLV/DCLV + αPD-L1, DCLV/DCLV + isotype, and the PBS control groups of BALB/c mice at day 10 post-boost injection were in vitro restimulated with the HIV-1 Gag peptide pools (or phorbol 12-myriatate 13-acetate/Ionomycin as a control) for 6 hours. (a) Representative flow cytometry plots show the distribution of Gag-specific cytokine-secreting CD8+ T cells as a percentage of total CD8+ T cells 10 days after the boost injection. (b) The percentages of cytokine-secreting CD8+ T cells are shown. (c) Cytokine coexpression subsets are expressed as a percentage of total CD8+ T cells. Each group consisted of four mice. The P values are calculated using one-way Anova followed by a Bonferroni nonparametric posttest (ns, not significant; *P < 0.05; **P < 0.001; ***P < 0.001). The data are representative of two independent experiments. DCLV, dendritic cell-directed lentiviral vector; HIV, human immunodeficiency virus; PBS, phosphate-buffered saline; PD-L1, programmed death 1 ligand.
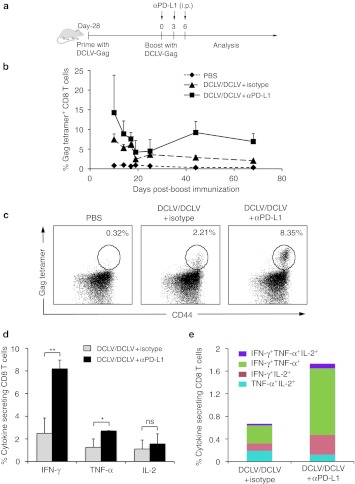
DCLV-based prime/boost vaccination combined with PD-L1 blockade induces long-term immunity
To assess the persistence of the Gag-specific immune response, we conducted a separate experiment consisting of three groups of mice that received the same vaccine modalities as in Figure 2, but were analyzed 2 months after the boost injection (Figure 4a). The Gag-specific CD8+ T cell responses in blood samples from each group were longitudinally monitored. The numbers of Gag-specific CD8+ T cells in PBMC samples of both groups decreased during the first 3 weeks and reached a stable value through day 68, with an ultimate frequency of ~7% of tetramer-positive CD8+ T cells from αPD-L1-treated mice and ~2% from nontreated mice (Figure 4b). At day 68 post-boost immunization, spleens were harvested and subjected to tetramer and cytokine staining, as described above. Consistent with the PBMC results, the frequency of Gag tetramer-positive CD8+ T cells in the αPD-L1-treated mice at day 68 had declined, but was still maintained at a considerable frequency, 8.4% (Figure 4c). In contrast, only 2.2% of Gag-specific CD8+ T cells were observed in nontreated mice (Figure 4c). Upon stimulation with the HIV-1 Gag peptide pools, the number of cytokine-secreting CD8+ T cells in the PD-L1 blockade group was greater than that of the group without blockade at this longer time point (Figure 4d). Moreover, the Gag-specific CD8+ T cells in the PD-L1 blockade group maintained their polyfunctionality better with an average of 1.2% IFN-γ+/TNF-α+ (versus 0.3%), and 0.4% IFN-γ+/IL-2+ cells (versus 0.2%, Figure 4e). The above findings confirmed that PD-L1 blockade leads to better preservation of the number and polyfunctionality of HIV-1-specific CD8+ T cells.
Figure 4.
DCLV prime/DCLV boost in combination with PD-L1 blockade generated prolonged immunity. (a) Schematic representation of experimental procedures. (b) The percentage of Gag tetramer-positive CD8+ T cells in PBMC was determined at the indicated time points. Day 0 denotes the date that mice (n = 4) were boosted with DCLV-Gag plus αPD-L1 or isotype antibody treatment. At day 68 post-boost immunization, splenocytes were harvested from different groups of mice for analysis. (c) Representative FACS plots for measuring the population of tetramer-positive CD8+ T cells. (d) The percentages of cytokine-secreting CD8+ T cells upon restimulation with HIV-1 Gag peptide pools are shown. (e) Cytokine coexpression subsets are expressed as a percentage of total CD8+ T cells. P values were calculated using one-way Anova followed by a Bonferroni nonparametric posttest (ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001). The data are representative of two independent experiments. DCLV, dendritic cell-directed lentiviral vector; HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell; PBS, phosphate-buffered saline; PD-L1, programmed death 1 ligand, SFC, spot-forming cells.
DCLV-based prime/boost vaccination combined with PD-L1 blockade induces a broad HIV-1 Gag-specific T cell response
Due to the genetic diversity of HIV-1 in nature, inducing a T cell response against multiple epitopes is a desirable feature of an acquired immune deficiency syndrome (AIDS) vaccine.26,27 Previously, we showed that a DCLV vaccine could lead to the proliferation of T cells that responded to several epitopes by utilizing a cross-linked peptide matrix of multiple Gag epitopes.23 We next hypothesized that PD-L1 blockade could further broaden that response. To test this, a library of 123 peptides covering the entire repertoire of epitopes for the HIV-1 B Gag sequence was divided into 23 pools named P1–P23. Each 15 amino acid-long single peptide was present in two independent peptide pools (Figure 5a). The splenocytes from animals immunized with PBS, DCLV prime/DCLV boost + αPD-L1, or DCLV prime/DCLV boost + isotype antibody were isolated 10 days post-boost injection, exposed to each of the 23 peptide pools, and then assessed by IFN-γ ELISPOT assay. As the mean readout of T cells from PBS mice was less than 160 SFC/million, we defined a cutoff value of 800 SFC/million (five times background readout) as a threshold for measuring a positive response. We found that mice immunized with DCLV plus PD-L1 blockade positively responded to 10 peptide pools (P4, P5, P6, P7, P8, P15, P16, P17, P22, and P23), whereas the mice without PD-L1 blockade positively responded to only four pools (P4, P5, P16, and P17) (Figure 5a,b). Moreover, we noticed that the overall T cell response elicited by vaccination with PD-L1 blockade included greater frequencies of antigen-specific T cells than that from the prime/boost only. The aforementioned results indicate that PD-L1 blockade with DCLV prime/boost strategy induced the activation of a set of CD8+ T cells that could respond to a broader array of epitopes.
Figure 5.
DCLV prime/DCLV boost in combination with PD-L1 blockade broadened the profile of vaccine-specific T cell responses. Spleen cells harvested from DCLV/DCLV + αPD-L1, DCLV/DCLV + isotype, or the control group of BALB/c mice were pooled, stimulated in vitro with one of peptide pools for 18–24 hours, and assayed by IFN-γ ELISPOT. (a) Distribution of 123 15-mer peptides spanning the entire HIV-1 clade B Gag sequence into 23 pools (P1–P23) (left). Positively reactive peptide pools of DCLV/DCLV + αPD-L1 (coloring) and DCLV/DCLV + isotype (shading) groups are indicated (left) and summarized (right). (b) Number of spot-forming cells (SFC) produced by stimulation with each of the 23 pools. A peptide pool was regarded as a positive group when its ELISPOT readout was five times higher than the mean readout of the control group. Each group consisted of four mice. P values were calculated using one-way Anova followed by a Bonferroni nonparametric posttest (ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001). The data are representative of two independent experiments. DCLV, dendritic cell-directed lentiviral vector; ELISPOT, enzyme-linked immunosorbent spot; HIV, human immunodeficiency virus; PBS, phosphate-buffered saline; PD-L1, programmed death 1 ligand.
DCLV-based vaccination combined with PD-L1 blockade provides better protection against vaccinia virus challenge
Having shown that PD-L1 blockade could enhance the characteristics of a CD8+ T cell response, we hypothesized that this blockade could also provide better protection against infection by a pathogen. A recombinant vaccinia virus carrying the full length of HIV-1 Gag gene (vv-Gag) was used as a challenge model. These viruses do not incorporate Gag proteins, but present Gag-derived peptides on the surface of infected cells.18,28 Three groups of mice (unvaccinated mice, mice vaccinated with the DCLV-based prime/boost protocol alone, or DCLV-based prime/boost with PD-L1 blockade) were challenged intraperitoneally with vv-Gag (107 PFU) 2 weeks after last vaccination. At day 7 following challenge, mice treated with either vaccination regimen developed a significantly lower level of vaccine virus titer in ovaries as compared with the unvaccinated group (6 × 104 or 2 × 102 PFU/ml versus 6 × 107 PFU/ml; P < 0.001, Figure 6). PD-L1 blockade afforded a nearly 300-fold greater reduction of vaccinia virus titer compared with that without PD-L1 blockade. Furthermore, in two of the six mice treated with PD-L1 blockade, viral load declined to a nearly undetectable level. These data show that the stronger immunogenicity engendered by PD-L1 blockade correlates to a stronger antiviral response during vaccinia virus challenge.
Figure 6.
Immunization delivered by DCLV prime/DCLV boost in combination with PD-L1 blockade protects animals from recombinant vaccinia virus (vv) challenge. BALB/c mice were immunized with PBS, DCLV-Gag prime/DCLV-Gag boost alone, or DCLV-Gag prime/DCLV-Gag boost along with PD-L1 blockade. Two weeks following the boost immunization, all the groups were challenged with 107 PFU of vv-Gag. Vaccinia virus titers in paired ovaries harvested 7 days after challenge were determined on CV-1 cells. Titers from each mouse are shown as data points, and the mean value for each group is shown with a horizontal line. P values were calculated using one-way Anova followed by a Bonferroni nonparametric posttest (ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001). The data are representative of two independent experiments. DCLV, dendritic cell-directed lentiviral vector; PBS, phosphate-buffered saline; PD-L1, programmed death 1 ligand.
Discussion
The cellular immune response is of pivotal importance because the effector T cells are the guards through which the immune system eliminates virus-infected cells and controls viral infection.29 An ideal AIDS vaccine should elicit sufficient CTLs to kill all HIV-infected cells, but traditional vaccine strategies have failed to accomplish this feat. Many strategies to enhance the traditional vaccine response have been tested towards achieving this AIDS vaccine goal. For example, elevated vaccination efficiency was seen when immunizations were combined with either cytokines/chemokines (IL-2, IL-7, IL-15, MIP-1α, and MIP-3β) capable of regulating survival,30 expansion or trafficking of immune cells,28,30,31 or reagents that can activate positive T cell receptor costimulatory molecules (CD80, CD40).32 Recent studies also suggested that blocking the signaling of certain negative costimulatory molecules in T cells could restore cellular immunity.20 In this study, we demonstrated that in vivo blockade of the negative costimulatory PD1/PD-L1 pathway combined with DCLV-based immunization either at the primary phase or at the boost phase could markedly enhance antigen-specific CD8+ T cell responses. The PD-L1 blockade, combined with prime/boost immunization, improved the quality and function of Gag-specific T cells, prolonged CD8+ T cell responses, and facilitated vaccine-induced protection against virus challenge.
We first studied a single dose of DCLV immunization to highlight the role of PD1/PD-L1 in priming a T cell response. In contrast to previous work, which mostly tested PD-L1 blockade in a therapeutic setting to stimulate one's own antiviral immune response,13 the research presented here focuses on PD-L1 blockade with DCLV immunization to induce and stimulate the primary T cell responses in the prophylactic setting. When delivered at both the priming and boosting stage, αPD-L1 resulted in enhanced expansion of Gag-specific CD8+ T cells, as well as increased production of IFN-γ by these cells, which is crucial for inducing Th1 immunity.12,28 One of the most striking differences we observed between the blockaded and non-blockaded groups was the number of antigen-specific CD8+ T cells present in LNs. Without αPD-L1 treatment, Gag-specific CTLs were nearly undetectable, whereas blocking the PD1/PD-L1 pathway generated a substantially larger number of Gag-specific CD8+ T cells (Figures 1 and 2). In an HIV infection, lymphoid tissues are the main sites where viruses replicate; therefore, maintenance of virus-specific CD8+ T cells in lymphoid organs is of great importance.33 These findings suggest that such a blockade may be especially desirable in a vaccine to control HIV infection. We also found that after PD-L1 blockade, Gag-specific CD8+ T cells (including central memory and effector memory populations) exhibited lower levels of PD-1 expression (Supplementary Figure S2b online), suggesting a lower extent of T cell exhaustion.
Cytokines play a prominent role in retaining effector function and memory of CD8+ T cells, and providing essential help to antibody production and killer cells.29 Polyfunctionality of T cells correlates with the reduction of viremia and clinical nonprogression in HIV-infected patients.25 As shown in Figure 3, when the CD8+ T cells extracted from prime/boost regimens were stimulated with the HIV-1 Gag peptide pool, the most dramatic difference in cytokine production by αPD-L1 treatment was the enhanced number of IFN-γ+/TNF-α+ double producers, which is in agreement with the findings for lymphocytic choriomeningitis virus specific CD8+ T cells in a therapeutic vaccine study,13 and with HIV-specific CTL analysis performed on HIV-infected patients.33 In the αPD-L1-treated group, a measurable increased frequency of IFN-γ+/IL2+ coproducers was observed, which is characteristic of a population displaying a cytolytic function with proliferative ability.6 To assess whether the CTLs induced by the prophylactic vaccine were well maintained,34 we performed kinetic studies of vaccine-specific T cells circulating in the blood. We noticed extensive numbers of antigen-specific memory CD8+ T cells in the αPD-L1 group 2 months post-boost immunization. Moreover, these vaccine-induced CD8+ T cells retained their polyfunctionality, with more IFN-γ+/IL2+ producers and less IFN-γ+/TNF-α+ producers, which is presumably due to the immune system undergoing contraction and developing memory.29 This is supported by the results that more long-term central memory T cells were generated after αPD-L1 treatment (Supplementary Figure S2 online)
One desirable feature of an AIDS vaccine is to generate CTL that react with multiple targets on the virus or virus-infected cells.27 To query the ability of our vaccine to achieve this kind of broad response, we tested the activated CD8+ T cells against peptide pools containing an array of peptides.35 Our results showed that the most three-potent responsive pools in both groups contain the well-characterized dominant peptide sequence (AMQMLKETI). Both the diversification and magnitude of immunogens recognized by DCLV/DCLV with PD-L1 blockade was greater than that by DCLV/DCLV alone. Therefore, DCLV vaccine administration with PD-L1 blockade generated CTLs responding to a wider pool of peptides.
The vaccine delivered by DCLV/DCLV along with αPD-L1 resulted in a better control of viral load in mice challenged with a Gag-encoding vaccinia virus. This superior vaccine-induced protection is presumably due to larger numbers of Gag-specific CTLs fighting against virus-producing cells.28,31 It is likely that PD1/PD-L1 blockade increased the number of virus-specific CD8+ T cells through the regulation of survival and differentiation ability of these cells,33 which is supported by the evidence that Gag-specific CD8+ cells enhanced the proliferation capability (Supplementary Figure S3 online), following the boost vaccination with PD-L1 blockade.
Beside its immunosuppressive role, the PD-1 molecule is also an immunoregulatory receptor. It has been shown to be involved in regulating T cell tolerance and autoreactive B cells.36,37 There is evidence showing that PD-1 mRNA is highly expressed in CD4+ CD25+ regulatory T cells and anergic T cells, and PD-1-deficient mice exhibit augmentation of autoantibodies to heart tissue and develop dilated cardiomyopathy.38,39 Therefore, the dose and frequency of αPD-L1 treatments with vaccine modalities need to be carefully evaluated to maintain a balanced CTL response.40 PD1/PD-L pathways may regulate CD4+ T cells and B cells in distinct manners from CD8+ T cells. One critical next step is to evaluate the role of PD-L1 blockade in the immune responses generated by other cell types, and to investigate the potential of combing PD-L1 blockade with other therapies (such as adoptive T cell transfer) to benefit AIDS treatment.
Materials and Methods
Mice and vaccination procedure. Six- to 8-week-old female BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). All animal procedures were performed in accordance with the guidelines set by the National Institute of Health and the University of Southern California on the Care and Use of Animals. For the single dose DCLV vaccination protocol, 5 × 106 TU of DCLV-Gag was given through footpad injection along with administration of rat anti-mouse PD-L1 antibody (clone 10F.9G2; BioXcell, West Lebanon, NH) or rat IgG2b isotype antibody control. For the DCLV-Gag prime/DCLV-Gag boost with PD-L1 blockade vaccinations, mice were primed with the DCLV-Gag (5 × 106 TU) via footpad injection, and 4 weeks thereafter, they were boosted with the same vector and dose via subcutaneous injection (at the base of tail) in combination with the treatment of anti-mouse PD-L1 or isotype control antibodies. For the antibody treatment, 200 µg of anti-mouse PD-L1 or isotype control antibodies were administered intraperitoneally every 3 days with totaling three times, starting on the day of vector immunizaiton.
Lentiviral vector production. The plasmid encoding the DC-targeted envelope SVGmu was constructed as previously described.21 FUW-Gag was constructed by insertion of the cDNA of a HIV-1 subtype B Gag into the lentiviral backbone plasmid FUW downstream of the human ubiquitin C promoter.23 To efficiently produce this lentiviral vector (termed DCLV-Gag), human embryonic 293T cells were seeded at 18 million per 15-cm tissue culture dish (BD Biosciences, San Jose, CA) and grown for 18–20 hours. 293T cells were then cotransfected with the lentiviral backbone plasmid FUW-Gag, the SVGmu-encoding envelope plasmid, and the packaging plasmids (pMDLg/pRRE and pRSV-Rev) using a standard calcium phosphate precipitation protocol. The viral supernatant was harvested twice at both 48 and 72 hour posttransfection, combined and filtered through a 0.45-µm filter (Corning, NY). The concentrated viral pellets were obtained after ultracentrifugation of the viral supernatants at 50,000g for 90 minutes, and were then resuspended in an appropriate volume of cold HBSS for in vivo study.
Gag peptide and peptide pools. The immunodominant H2-Kd-restricted CD8+ T cell epitope (AMQMLKETI, aa 197-205) is located in the p24 portion of the Gag protein. This peptide was synthesized (GenScript, Piscataway, NJ) and dissolved in dimethyl sulfoxide at 8 mg/ml. The Gag peptide libraries contain 123 overlapping 15-mer peptides, and span the entire HIV-1 subtype B Gag sequence. Individual peptides in the libraries were dissolved in dimethyl sulfoxide at 10 mg/ml, and stored at −80 ºC. The HIV-1 Gag libraries were divided into 23 pools of 11 to 12 peptides as illustrated (Figure 5a).
Tetramer staining. The phycoerythrin (PE)-conjugated major histocompatibility complex (MHC) class I tetramer H2-Kd-AMQMLKETI was obtained from Beckman Coulter (Fullerton, CA). Spleen cells and LN cells were harvested from vaccinated and control mice. In some experiments, blood was drawn from the retro-orbital venous plexus of mice periodically. Following lysis buffer treatment to remove red blood cells, the surface staining was performed by blocking the Fcγ receptors of cells with an anti-mouse CD16/CD32 antibody (clone 2.4G2, BD Biosciences), followed by incubating the cells with tetramer in conjunction with FITC-anti-CD44 and PE-Cy5-anti-CD8 (BD Biosciences) antibodies. The cells were washed and resuspended in PBS. Samples were analyzed using either the FACSort or the FACSCalibur instrument (BD Biosciences).
To calculate frequency of Gag-tetramer+ CD8+ T cells within the total CD8+ population, gating was performed on CD8+ T cells, and the percentage of Gag-tetramer+ CD44+ T cells was calculated.
Intracellular cytokine staining. Splenocytes (1 × 106/sample) were cultured for 5 hours at 37 °C in a 96-well round-bottom plate in Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO), 10 U/ml of penicillin, 100 µg/ml of streptomycin, and 2 mmol/l glutamine. The HIV-1 Gag peptide (AMQMLKETI) (4 µg/ml) was added to stimulate T cells for 6 hours, and GolgiPlug (0.67 µl/ml) was supplemented in the culture to accumulate intracellular cytokines. After washing, restimulated cells were incubated with anti-mouse CD16/CD32 antibody, followed by surface stained with anti-mouse CD8 and anti-mouse CD4 antibodies. Cells were then permeabilized in 100 µl of Cytofix/Cytoperm solution (BD Bioscience) at 4 °C for 20 minutes, washed with Perm/Wash buffer (BD Bioscience), and stained with PE-conjugated anti-mouse IFN-γ at 4 °C for 30 minutes. The flow cytometry analysis was carried out using the FACSort instrument from BD Biosciences.
Multiparameter intracellular cytokine staining. Spleen cells (1 × 106/sample) were stimulated with the HIV-1 Gag peptide libraries (2.5 µg/ml for each peptide) in the presence of costimulatory anti-CD28 antibody (2 µg/ml, BD Biosciences) and anti-CD49d (2 µg/ml, BD Biosciences) for 6 hours at 37 °C in a 96-well round-bottom plate. Brefeldin A (BFA, Sigma) was added (10 µg/ml) for the last 4 hours to inhibit cytokine exporting. The multiparameter intracellular cytokine staining procedure was similar to the procedure of above intracellular cytokine staining, except that the cells were stained with the following surface monoclonal antibodies: anti-CD4-PerCP, anti-CD8-APC-Cy7, anti-CD3-Alexa488, and intracellular monoclonal antibodies: anti-IL-2-PE, anti-IFN-γ-APC, and anti-TNF-α-PE-Cy7. The data was acquired on a BD LSR II flow cytometry (BD Biosciences). All of the staining antibodies were purchased from BD Bioscience.
IFN-γ enzyme-linked immunospot assay. The ELISPOT was conducted with the Millipore IFN-γ ELISPOT kit (Billerica, MA) according to the manufacturer's instruction. Briefly, 96-well MultiScreen-IP plates were coated with 100 µl/well of anti-mouse IFN-γ antibody (10 µg/ml in PBS) and stored overnight at 4 ºC. The plate was decanted and blocked with Roswell Park Memorial Institute medium containing 10% fetal bovine serum at 37 ºC for 2 hours. Splenocytes from mice were plated at 1 × 105 cells/well in 150 µl complete medium with addition of HIV-1 Gag single peptide (2 µg/ml) or pools of peptides (at a final concentration of 3 µg/ml for each peptide) for this assay. After 18 hours incubation at 37 ºC, plates were washed, and incubated with 100 µl/well of biotinylated anti-IFN-γ detection antibody (0.5 µg/ml, BD Biosciences) for 2 hours at room temperature. Plates were further washed and incubated with the 1,000-fold-diluted streptavidin-alkaline phosphate conjugate for 45 minutes at room temperature. After a final extensive wash, spots were identified by addition of BCIP/NBT plus substrate, and the number of IFN-γ producing cells was quantified by an Zeiss ELISPOT reader.
Proliferation assay. Cells (2 × 105/well) were cultured in 96-well round-bottom plates, and were incubated with or without stimuli (either HIV-1 Gag dominant peptide, or Gag peptide pools) for 40 hours. Wells were then pulsed with [3H]-thymidine (1 µCi/well) for 12 hours, and [3H]-thymidine incorporation was measured.
Vaccinia virus and challenge of mice. Vaccinia virus encoding Gag (vv-Gag, NIH AIDS Research & Reference Reagent Program, Rockville, MD, contributed by Dr D Kuritzkes) was propagated in CV-1 cells, and the virus titer was evaluated by the standard plaque forming assay on CV-1 cells. Groups of BALB/c mice received DCLV-Gag prime followed by DCLV-Gag boost with or without PD-L1 blockade vaccinations. Two weeks after the boost immunization, mice were challenged with 107 PFU of vv-Gag intraperitoneally. The animals were inspected daily to monitor the body weight for 7 days. On day 7 after challenge, mice were killed, and the ovaries were harvested, disrupted by homogenization. Vaccinia virus titers in ovaries were evaluated by plating serial log dilutions of extracts on a density of 5 × 105 CV-1 indicator cells for 48 hours. After 2 days, the media was removed, CV-1 cell monolayer was stained with 0.1% crystal violet (Sigma), and vaccinia virus plaques were quantified.
Statistical analysis. Statistical analysis was performed using one-way Anova followed by a Bonferroni nonparametric posttest. P values <0.05 were considered significant. The results were generated by GraphPad Prism version 5.0 software.
SUPPLEMENTARY MATERIAL Figure S1. Phenotypic analysis of dendritic cells after vaccination and PD-L1 blockade. Figure S2. Phenotypic changes in Gag-specific CD8+ T cells after PD-L1 blockade. Figure S3. Enhanced proliferation capability of Gag-specific CD8+ T cells after PD-L1 blockade.
Acknowledgments
We thank Gary Nabel for providing reagents and Larry Corey for an initial discussion of the breadth of antigen responses. This research was generously supported by grants from the National Institute of Health, the Bill and Melinda Gates Foundation, and the California HIV/AIDS Research Program.
Supplementary Materials
Phenotypic analysis of dendritic cells after vaccination and PD-L1 blockade.
Phenotypic changes in Gag-specific CD8+ T cells after PD-L1 blockade.
Enhanced proliferation capability of Gag-specific CD8+ T cells after PD-L1 blockade.
REFERENCES
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B.et al. (2006Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction Nat Med 121198–1202. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V.et al. (2007SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection Blood 110928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y.et al. (2007PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors Blood 1094671–4678. [DOI] [PubMed] [Google Scholar]
- Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, González-Praetorius A.et al. (2011Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1(+)/CD127(−) HCV-specific CD8(+) cells targeting the virus in chronic hepatitis C virus infection Cell Immunol 269104–114. [DOI] [PubMed] [Google Scholar]
- Khoury SJ., and, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529–538. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- Ha SJ, West EE, Araki K, Smith KA., and, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol Rev. 2008;223:317–333. doi: 10.1111/j.1600-065X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F.et al. (2005In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses J Exp Med 2011531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A.et al. (2007Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction J Immunol 1791979–1987. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S.et al. (2006PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression Nature 443350–354. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH.et al. (2006Restoring function in exhausted CD8 T cells during chronic viral infection Nature 439682–687. [DOI] [PubMed] [Google Scholar]
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L.et al. (2009Enhancing SIV-specific immunity in vivo by PD-1 blockade Nature 458206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH.et al. (2008Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection J Exp Med 205543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA.et al. (2011Responsiveness of HIV-specific CD4 T cells to PD-1 blockade Blood 118965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatangay BJ., and, Rinaldo CR. PD-1 blockade: A promising immunotherapy for HIV. Cellscience. 2009;5:61–65. [PMC free article] [PubMed] [Google Scholar]
- Trumpfheller C, Finke JS, López CB, Moran TM, Moltedo B, Soares H.et al. (2006Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine J Exp Med 203607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod MK, McKee AS, David A, Wang J, Mason R, Kappler JW.et al. (2011Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells Proc Natl Acad Sci USA 1087914–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Arruda LB, Chikhlikar PR, August JT., and, Marques ET. DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response. Immunology. 2004;112:126–133. doi: 10.1111/j.1365-2567.2004.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H.et al. (2004In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination J Exp Med 199815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA., and, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L.et al. (2008Engineered lentivector targeting of dendritic cells for in vivo immunization Nat Biotechnol 26326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gajewski TF., and, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B, Yang L, Yang H, Hu B, Baltimore D., and, Wang P. HIV-1 Gag-specific immunity induced by a lentivector-based vaccine directed to dendritic cells. Proc Natl Acad Sci USA. 2009;106:20382–20387. doi: 10.1073/pnas.0911742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA.et al. (2008Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys J Virol 824844–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Schmitz JE, Buzby AP, Barker BR, Rao SS, Xu L.et al. (2006Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge J Virol 8010950–10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Kong WP., and, Nabel GJ. Enhanced breadth of CD4 T-cell immunity by DNA prime and adenovirus boost immunization to human immunodeficiency virus Env and Gag immunogens. J Virol. 2005;79:8024–8031. doi: 10.1128/JVI.79.13.8024-8031.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z., and, Nabel GJ. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J Virol. 2003;77:12764–12772. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Liu S., and, Leong KW. Effects of MIP-1 alpha, MIP-3 alpha, and MIP-3 beta on the induction of HIV Gag-specific immune response with DNA vaccines. Mol Ther. 2007;15:1007–1015. doi: 10.1038/mt.sj.6300129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT., and, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Colombetti S, Lévy F., and, Chapatte L. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood. 2009;113:6629–6637. doi: 10.1182/blood-2008-05-155309. [DOI] [PubMed] [Google Scholar]
- Sumida SM, McKay PF, Truitt DM, Kishko MG, Arthur JC, Seaman MS.et al. (2004Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines J Clin Invest 1141334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC.et al. (2006PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection J Exp Med 2032281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang J, Mi Z, Robbins P., and, Falo LD., Jr Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174:3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- Arruda LB, Sim D, Chikhlikar PR, Maciel M, Jr, Akasaki K, August JT.et al. (2006Dendritic cell-lysosomal-associated membrane protein (LAMP) and LAMP-1-HIV-1 gag chimeras have distinct cellular trafficking pathways and prime T and B cell responses to a diverse repertoire of epitopes J Immunol 1772265–2275. [DOI] [PubMed] [Google Scholar]
- Fagarasan S., and, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X.et al. (2011The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo J Immunol 1871113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafian N., and, Khoury SJ. T cell costimulatory pathways: blockade for autoimmunity. Expert Opin Biol Ther. 2003;3:227–236. doi: 10.1517/14712598.3.2.227. [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Wherry EJ, Ahmed R., and, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- Shin EC., and, Rehermann B. Taking the brake off T cells in chronic viral infection. Nat Med. 2006;12:276–277. doi: 10.1038/nm0306-276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypic analysis of dendritic cells after vaccination and PD-L1 blockade.
Phenotypic changes in Gag-specific CD8+ T cells after PD-L1 blockade.
Enhanced proliferation capability of Gag-specific CD8+ T cells after PD-L1 blockade.