Abstract
Granulocyte–macrophage colony-stimulating factor (GMCSF) and MCP3 (aka CCL7) exert complementary, nonoverlapping, proimmune effects on responsive lymphoid and myeloid cells. We hypothesized that a synthetic cytokine linking GMCSF to MCP3 (hereafter GMME3) as part of a single polypeptide would acquire novel, therapeutically desirable immunomodulatory properties. We demonstrate that GMME3 has enhanced CC-chemokine receptor (CCR)–mediated intracellular Ca++ mobilization with selective effects on the CD21hiCD24hi CD1.dhi subset of splenic B cells inducing substantial interleukin 10 (IL10) production. We demonstrate that BGMME3 exert their suppressive effect through an IL10-mediated inhibition of antigen presentation. More importantly, BGMME3 inhibit the reactivation of encephalomyelitis (EAE)-derived or TGFβ/IL6 differentiated Th17 cells by altering their polarization toward a Th1 or Th2 phenotype. The secretion of interferon-γ (IFNγ) and IL4 in turn inhibits IL17 production. The adoptive transfer of BGMME3, but not IL10–/– BGMME3 cells, to mice symptomatic with experimental autoimmune encephalitis significantly improves their disease score and inhibits lymphoid infiltration into the central nervous system (CNS). We propose that designed CCR modulators such as GMME3, allows for conversion of naive B-cells to a novel suppressor phenotype allowing for the personalized cell therapy of autoimmune ailments.
Introduction
B cells can influence the development of autoimmunity in both pathogenic and protective ways. Self-reactive B cells can produce pathogenic tissue damaging auto-antibodies against self-antigens in systemic lupus erythematosus.1 In addition to antibody-mediated mechanisms, activated B cells can facilitate T cell-mediated inflammation through antigen presentation, costimulation and cytokine production. In fact, emerging clinical data demonstrated that the efficacy of B cell-depletion therapy in T-cell mediated autoimmune diseases such as multiple sclerosis and type 1 diabetes is independent of the levels of circulating autoantibody.1,2 A number of B-cell subsets with varying and overlapping phenotypes have been shown to possess regulatory function primarily via the production of IL10 in certain T-cell driven inflammatory responses. For example, CD1.dhiCD5+ splenic B cells are capable of suppressing central nervous system (CNS) and colonic inflammation.3 In collagen-induced arthritis, transitional 2 (T2)—marginal zone (MZ) (T2-MZ) B cells have also been identified to regulate inflammation.4,5 It has been speculated that B-cells conditioned in an inflammatory environment acquire a regulatory phenotype.6,7 In line with this thought, methods of ex vivo generation of IL10-producing regulatory B cells have been developed including CD40 monoclonal antibody and B cell-activating factor activation of naive B cells.4,8
As part of our group's strategy to develop novel therapeutic immunomodulators, we have designed fusion proteins consisting of two functional distinct cytokines as a single polypeptide chain: a fusokine. We have previously demonstrated that fusokines borne of the coupling of granulocyte–macrophage colony-stimulating factor (GMCSF) and common γ-chain interleukins (hereafter GIFTs) acquire biological properties distinct from the predicted summation.9 Structurally, N-terminal GMCSF has been shown to enhance protein production, increase plasma half-life of the fusion, and alter receptor binding. We have also demonstrated that fusokines combining GMCSF and a CC-chemokine can lead to unheralded biological activities.10,11,12 While CC-chemokines initiate a lymphomyeloid chemotatic response by binding to cognate G-protein-coupled receptor (GPCR) to sustain an inflammatory response, we have demonstrated that fusing GMCSF to the N terminus of monocyte chemoattractant protein 1 had novel proapoptotic effects on CC-chemokine receptor 2 (CCR2)-expressing lymphomyeloid cells, inducing the reversal of symptoms in murine models rheumatoid arthritis and encephalomyelitis (EAE) by directly depleting the mice of Th17 cells.11 In this study, we demonstrate that coupling GMCSF to CC-chemokine MCP3 (hereafter GMME3) generates a GPCR hyperagonist ligand. B cells stimulated ex vivo with GMME3 (BGMME3) upregulate IL10 production. BGMME3 can modulate the progression of experimental autoimmune EAE, a murine model of multiple sclerosis, characterized by T-cell driven pathological inflammation and demyelination in the CNS. Upon adoptive transfer, BGMME3 attenuate disease progression and inhibit immune infiltrate to the CNS. We further characterized BGMME3 regulatory capacity: they directly inhibit macrophages to prime CD4+ T cells via IL10, and skew the polarization of encephalitogenic Th17 cells to a Th1 or Th2 profile. Subsequent interferon-γ (IFNγ) and IL4 production suppressed IL17 expression. This finding suggests a role for hyperactive B cells in the network of Th cells plasticity and differentiation. Thus, our current work has identified a novel strategy to generate immune suppressive B cells ex vivo with therapeutic potential and provided insight to previously unrecognized link between chemokine receptor biology and suppressor B-cell potentiality.
Results
Fusokine GMME3 structure and expression
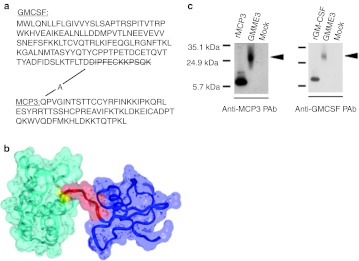
Human MCP3 cDNA was amplified by PCR and subsequently cloned in frame to a 3′ truncated derivative of mouse GMCSF cDNA. The nucleotides encoding for the last 12 amino acid at the carboxyl terminal of GMCSF were deleted to generate a single polypeptide which encodes for a 207 amino acid polypeptide chain—hereafter GMME3 (Figure 1a). From in silico structural homology modeling of GMME3 and solvent accessible surface analysis, N-terminal GMCSF was predicted to be permissive for the interaction of C-terminal MCP3 with its receptor (Figure 1b). Denaturing immunoblotting using conditioned media derived from human embryonic kidney (HEK) 293 cells transfected with the GMME3 transgene demonstrated that the fusokine was expressed, secreted extracellularly, and recognized by both anti-GMCSF and anti-MCP3 antibodies at the predicted molecular weight of 24 kDa (Figure 1c).
Figure 1.
Generation of the fusion GMME3. (a) Amino acid sequence of GMME3. Schematic representation of the fusion GMME3 showing granulocyte–macrophage colony-stimulating factor (GMCSF) at the N-terminus linked to C-terminal domain MCP3 by alanine. (b) Solvent accessible surface and ribbon representation of the GMME3 structural model predicted by homology modeling (GMCSF in cyan ribbon, linker A in yellow ribbon, the first six residues of MCP3 in red ribbon and the remaining residues of MCP3 in blue ribbon) (c) Denatured immunoblotting using supernatant derived from GMME3 or mock-transfected 293 cells. Recombinant murine GMCSF or recombinant human MCP3 were used as controls. The blot was probed with polyclonal goat anti-MCP3 and anti-GMCSF antibody to detect fusion protein secretion.
GMME3 induces a B-regulatory cell phenotype
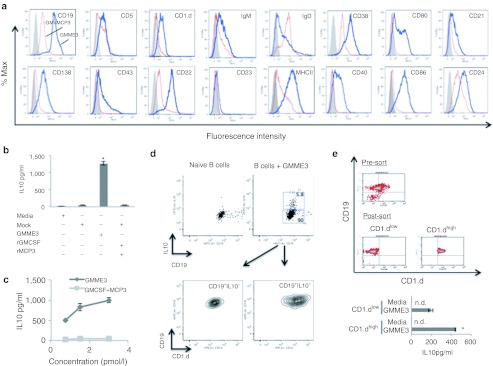
The in vitro treatment of unfractionated splenic lymphocytes with GMME3 led to the selective survival of CD19+ B cells (data not shown). Consequently, we analyzed the phenotype of GMME3 stimulated CD19+ B cells (hereafter BGMME3) and found they expressed CD19, CD1.d, CD5, CD21, CD22, CD24, CD38, but not IgD (Figure 2a). BGMME3 also acquired expression of major histocompatibility complex II (MHCII), CD80, and CD86. After 72 hours of culture, ELISAs were performed on the supernatant of BGMME3 to measure cytokine production. We demonstrated that compared to controls, GMME3 significantly upregulated the production of IL10 (Figure 2b), but not IL4, IL6, tumor necrosis factor α (TNFα), IFNγ, or TGFβ (data not shown). The GMME3-mediated IL10 production was observed in a dose-dependent manner, whereas increasing dose of GMCSF and MCP3 did not lead to significant IL10 induction (Figure 2c). It is conceivable that GMME3 selectively activate a subset of B cell with a regulatory phenotype to secrete IL10. We therefore performed intracellular IL10 staining along with CD1.d, a known marker for regulatory B cells and demonstrated that the IL10+ population of BGMME3 had a CD19+CD1.dhi phenotype (Figure 2d). We subsequently cultured sorted CD19+CD1.dhi or CD19+CD1.dlow B cells with GMME3 or media alone. CD19+CD1.dhi B-cell subset is significantly more responsive to GMME3-mediated IL10 induction (Figure 2e). Taken together, our data suggest GMME3 preferentially activate a subset of B cells marked by high expression of CD1.d to produce IL10.
Figure 2.
Selective activation of B10 cells by GMME3. (a) Immunophenotype of GMME3-activated (thick line) or granulocyte–macrophage colony-stimulating factor (GMCSF) + MCP3 activated (thin line) B cells. Ex vivo manipulated B cells were stained with the indicated antibodies and surface expression was analyzed by FCAS. (b) Interleukin 10 (IL10) ELISA of GMME3-activated-B cell culture supernatant in vitro for 72 hours. CD19+ B cells were purified from C57B/6 splenocytes and then cultured ex vivo with GMME3 or conditioned media from mock-transfected cells with or without recombinant GMCSF and MCP3 for 3 days. Results represent the mean value of three independent tests ± SD; *P < 0.05. (c) Dose-dependent effect of GMME3-mediated IL10 induction. CD19+ B cells were cultured with increasing dose of GMME3 for 3 days. (d) Flow cytometric analysis of IL10-producing B cells treated with GMME3 ex vivo for 3 days. PMA, ionomycin, and brefeldin A were added to culture 6 hours before immunostaining. (e) CD19+CD1.dhi sorted B cells were more responsive to GMME3-mediated IL10 induction. Negatively purified B cells were sorted into CD1.dhi and CD1.dlow population. Sorted cells were cultured in the presence or absence of GMME3. IL10 production in the supernatant was measured by ELISA after 3 days. Results shown represent the mean value of three independent tests ± SD; *P < 0.05.
The cellular biochemical response to GMME3
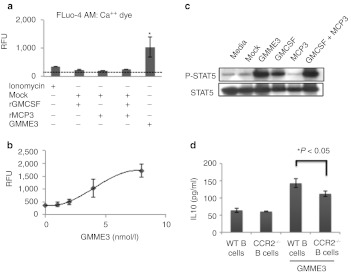
We next investigated the biochemical effect of GMME3 on purified splenic B cells. Since cellular Ca++ influx is downstream of multiple chemotactic pathways, we measured the fluorescence of Fluo-4 dye-loaded B cells upon GMME3 or cytokine control treatment. At 0.4 nmol/l —the concentration where IL10 production from splenic B cells was readily induced—GMME3 led to significantly greater Ca++ influx as compared to GMCSF, MCP3 alone or in combination with GMCSF in a dose-dependent manner (Figure 3a,b). To investigate the signaling response derived from the GMCSF domain of GMME3, we utilized the RAW264.7 macrophage cell line, expressing both α- and β-subunits of the GMCSF receptor. STAT5 phosphorylation (pSTAT5) was used as a measure of the potency of GMCSF-mediated signaling.13 Immunoblotting of RAW264.7 stimulated with GMME3 or cytokine controls showed that GMME3 induced comparable pSTAT5 to GMCSF or GMCSF and MCP3. MCP3 alone did not lead to pSTAT5 activation in RAW264.7 cells (Figure 3c). This observation fits with our previous work, where we have found that N-terminal GMCSF of the fusion often does not acquire enhanced signaling property but altered the binding of C-terminal moiety to its receptor. Our cell biochemical data suggested that fusing GMCSF at the N-terminus of MCP3 did not lead to enhanced GMCSFR-mediated response but profoundly increases the signaling potency of the MCP3 domain. Therefore, we hypothesize that the gain of function (IL10 induction) was primarily derived from the MCP3 signaling moiety. To test this hypothesis, we cultured B cells derived from WT or CCR2-deficient mice with GMME3 and showed that CCR2-deficient B cells have significantly blunted responsiveness to GMME3-mediated IL10 induction (Figure 3d).
Figure 3.
Biochemical characterization of GMME3. (a) GMME3-mediated Ca++ mobilization in CD19+ primary B cells. B cells were isolated from mice spleen and loaded with Fluo-4 AM dye indicator. 1.25 × 105 per well of B cells in 96 well plates were stimulated with 0.4 nmol/l of GMME3, granulocyte–macrophage colony-stimulating factor (GMCSF), MCP3, GMCSF + MCP3 or ionomycin as a positive control to induce Ca++ release. *P < 0.05. (b) Dose-dependent responsiveness of GMME3-mediated Ca++release. B cells were stimulated with 0.1, 0.2, 0.4, or 0.8 nmol/l of GMME3 and the corresponding fluorescence increase was plotted. RFU denotes resonance fluorescence unit. Results shown were the average of three independent tests ± SD. (c) N-terminal domain GMCSF of GMME3 does not lead to enhanced GMCSFR signaling compared to the combination of rGMCSF and rMCP3. Functional activity of GMCSF was assessed by STAT5 phosphorylation of RAW247.6 cells upon stimulation with GMME3 or controls. (d) B cells derived from wild type (WT) or CC-chemokine receptor 2 (CCR2)-deficient mice were treated ex vivo with GMME3. Interleukin 10 (IL10) production was measured by ELISA. *P < 0.05
BGMME3 attenuates EAE progression in an IL10-dependent manner
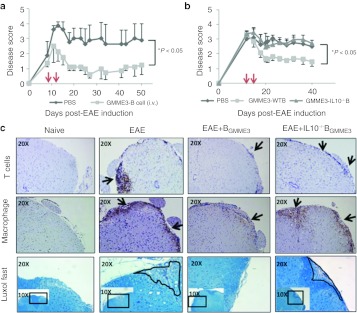
IL10-producing B cells have been demonstrated to possess potent anti-inflammatory properties in vivo.14,15 Indeed, the adoptive transfer of IL10-producing B cells led to protective phenotypes in mouse models of colitis, arthritis and multiple sclerosis.3,4,5,8,16,17,18 We next assessed whether ex vivo generated BGMME3 cells could alter EAE disease progression. Nine days following induction, EAE mice were stratified to reach an average group disease score of 1.5 and each group (n = 4–6) received two intravenous doses of 1 × 106 BGMME3 or phosphate-buffered saline (PBS). BGMME3 treatment significantly reduced disease severity (by an average score of 1.8), characterized by improved gait function and hind limb strength whereas PBS-treated EAE mice remained symptom-ridden (Figure 4a). The injection of 1 × 106 naive splenic B cells had no significant effect (Supplementary Figure S1).
Figure 4.
BGMME3 suppress encephalomyelitis (EAE) disease progression via interleukin 10 (IL10). (a–b) Wild-type C57/B6 mice were MOG—immunized at day 0. Two doses of 1 × 106 wild type (WT) BGMME during the onset of disease reduced the disease severity, but not PBS-treated nor IL10–/– BGMME3—treated EAE mice. (c) Spinal cord histopathology. Spinal cord of EAE mice were removed, processed and stained for CD3, MCA497R, and Luxol-fast blue, which indicate T cells, macrophages infiltrate and demyelination, respectively. Arrows indicate immune infiltrate and circles indicate areas of demyelination. Compared with EAE (PBS treated), or EAE+IL10–/– BGMME3-treated mice, EAE mice treated with BGMME3 exhibit less immune cell infiltrate and areas of demyelination. Similar results were obtained from at least three different mice.
We next investigated whether IL10 played a role in BGMME3-mediated anti-inflammatory properties in vivo. EAE mice were stratified so that each group had an average score of 3. The first infusion of wild-type (WT) BGMME3 or IL10–/– BGMME3 or PBS were given at day 15 and repeated at day 20. The administration of WT BGMME3 at day 15 and 20 modulated the disease course, and we also showed that IL10 was required for BGMME3's effect as the administration of IL10–/– BGMME3 in vivo led to no beneficial effect (Figure 4b). The therapeutic potential of BGMME3 in EAE was also confirmed immunohistologically where we observed a marked reduction in macrophages and T cells infiltrating into the spinal cord of treated EAE mice. Microscopic examination of spinal cords of IL10–/– BGMME3-treated EAE mice, on the other hand, showed comparable levels of infiltration as in the EAE control mice. The extent of demyelination was assessed by Luxol fast staining, which demonstrated that BGMME3, but not IL10–/– BGMME3 infusion protected EAE mice from myelin loss, reflecting the functional improvement of the treated mice (Figure 4c).
Macrophages exposed to BGMME3 hypoactivate CD4+ T cells
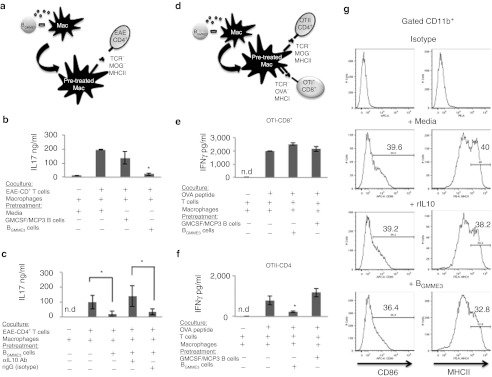
To investigate how the in vivo administration of BGMME3 can inhibit CNS inflammation, we cultured BGMME3 with other immune cell types known to play a role in EAE pathogenesis in order to study its bystander effect. Myeloid antigen-presenting cells (APCs) have been implicated in the pathogenesis of EAE19 and we speculated that BGMME3 may inhibit this function. To test this hypothesis, we cocultured BGMME3 with antigen-presenting murine macrophages for 48 hours and subsequently interrogated their ability to activate antigen-specific responder T-lymphocytes. In a first series of experiments, macrophages were cocultured with BGMME3 and were primed with MOG35–55 peptide and incubated with purified CD4+ T cells derived from EAE mice (Figure 5a). Media-treated macrophages efficiently activated MOG-specific CD4+ T cells to produce IL17, whereas macrophages previously exposed to BGMME3 showed impaired capacity to trigger IL17 production by CD4+ T cells. This inhibitory effect cannot be recapitulated by using control-(GMCSF+MCP3)-activated B cells (Figure 5b). We speculated that the modulation on antigen presentation was IL10 dependent. To test this hypothesis, we cocultured BGMME3 with murine macrophages for 48 hours in the presence of IL10 neutralizing antibody or isotype control. Macrophages were then washed, pulsed with MOG35–55 peptide and incubated with EAE-derived CD4+ T cells. Culture supernatant was analyzed for IL17 production after 72 hours. Here, we showed that IL10 neutralization significantly blocked BGMME3 inhibitory effect on antigen presentation and restored IL17 production back to the level comparable to naive macrophages (Figure 5c).
Figure 5.
BGMME3 inhibits antigen presentation via interleukin 10 (IL10). (a) Schematic representation of the in vitro antigen presentation assay. Peritoneal macrophages were pre-incubated with BGMME3 and subsequently pulsed with MOG 35–55 to reactivate CD4+T cells derived from encephalomyelitis (EAE). We then measured (b) IL17 production from responder EAE-CD4+T cells. (c) IL17 production by in vitro reactivated EAE-CD4+ T cells to BGMME3–pretreated—macrophages in the presence of IL10 neutralizing antibody or isotype. (d) Schematic representation of the in vitro antigen presentation. BGMME3–pretreated–macrophages were OVA-pulsed and cocultured with OTII-CD4+ T cells or OTI-CD8+ T cells. (e) Interferon-γ (IFNγ) production from responding OTI-CD8+ T cells to OVA-pulsed macrophages. (f) IFNγ production from responding OTII-CD4+ T cells, reactivated in vitro by OVA-pulsed macrophages. Results represent the mean value of three independent experiments ± SD; *P < 0.05. (g) Phenotypic analysis of peritoneal macrophages incubated with recombinant IL10 or BGMME3 for 48 hours before immunostaining for the indicated antibodies.
To analyze whether BGMME3 distinctively modulated MHC class I- or class II-restricted antigen presentation by macrophages, we used T cells derived from OTI or OTII transgenic mice as responder cells in an antigen presentation assay. We isolated macrophages and incubated them with BGMME3 or control B cells for 48 hours. After extensive wash, the pretreated macrophages were then primed with soluble recombinant OVA and coincubated with CD4+ T cells or CD8+ T cells derived from OT-II or OT-I transgenic mice with TCR recognizing OVA peptide in the context of MHC-class II or MHC-class I, respectively (Figure 5d). The IFNγ production from responder CD4+ T cells was significantly reduced in the BGMME3-pretreated macrophage coculture (Figure 5f); however, their capacity to induce MHC-class-I-specific response was intact as BGMME3-pretreated macrophages stimulate comparable amounts of IFNγ production from OTI-CD8+ T cells to control-treated macrophages (Figure 5e). Immunophenotyping demonstrated that peritoneal macrophages treated with BGMME3 or recombinant IL10 downregulated MHCII and CD86 (Figure 5g); the expression of MHCI, CD80, and B7H1 remained unchanged (data not shown). Taken together, our data demonstrated that BGMME3 suppressed MHC class II-specific antigen presentation from professional APCs in an IL10-dependent manner.
BGMME3 inhibits Th17 and augments Th1 effector T cells
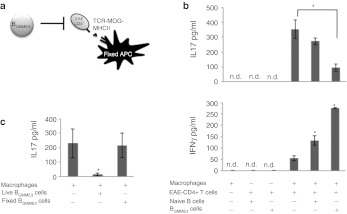
We investigated the direct effects of BGMME3 on encephalitogenic CD4+ effector cells by incubating CD4+ T cells from MOG35–55-immunized mice and incubating them with antigen-pulsed fixed APCs in the presence of BGMME3 or naive B cells (Figure 6a). After 48 hours of coculture, we assessed CD4+ T cell reactivation by measuring IFNγ and IL17 production. We found that CD4+ T cells derived from mice with severe acute EAE (disease score > 3) produced predominantly IL17, demonstrating that MOG-specific CD4+ T cells are biased toward a Th17 response during active disease. The addition of BGMME3, but not naive B cells, significantly lowered Th17 production but increased IFNγ production. Naive B cells, though unable to suppress Th17 response, also induced a slight increase in IFNγ production (Figure 6b). This suggested strongly that BGMME3 had distinct effect on IL17 production from T cells. The coculture of EAE-CD4+ T cells and BGMME3 in the absence of MOG-pulsed APCs did not lead to detectable IL17 or IFNγ production (Figure 6b). These results suggested that antigen re-encounter was required to re-stimulate effector T cells and BGMME3 act as third party bystanders to modulate effector T cell response upon antigen re-stimulation. To determine whether a direct cell–cell contact or secreted factor(s) expressed by BGMME3 was responsible for the observed altered phenotype of Th17 cells, we fixed BGMME3 with paraformaldehyde so they would be metabolically inert, yet present all surface molecules. We showed that a secreted factor was essential for BGMME3-mediated inhibition on Th17 cells as paraformaldehyde-fixation abolished the suppressive effect on IL17 production (Figure 6c).
Figure 6.
BGMME3 inhibit Th17 cells and augment Th1 response. (a) Schematic diagram of the antigen presentation assay. Encephalomyelitis (EAE)-CD4+T cells were reactivated by paraformaldehyde-fixed antigen-presenting cells (APCs) with BGMME3 or control-manipulated B cells introduced as third party. (b) Interleukin 17 (IL17) and interferon-γ (IFNγ) production of EAE-CD4+ cells reactivated in vitro by MOG35–55-pulsed paraformaldehyde (PFA)-fixed APC in the presence of BGMME3 or controls. (c) IL17 production from MOG 35–55 reactivated EAE-CD4+ T cells in the presence of live or PFA-fixed BGMME3. Results are representative of the average of three independent experiments ± SD; *P < 0.05.
The conversion of Th17 cells by BGMME3 is mediated by IL4 and IFNγ
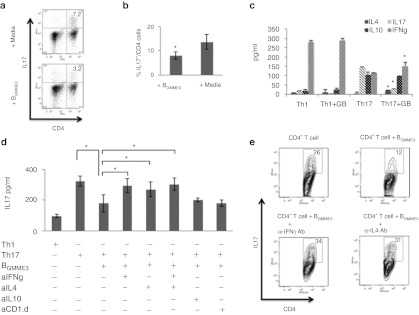
In an attempt to understand the mechanism by which BGMME3 downregulate a Th17 response, we first differentiated naive CD4+ T cells in WT splenocytes in vitro with CD3/CD28 beads under Th17 polarizing conditions. We then introduced BGMME3 into the coculture after 4 days. Similar to MOG-specific CD4+ T cells derived from EAE mice during active disease, in vitro differentiated T cells in the presence of TGF-β and IL6 showed significant IL17 expression (12% of total CD4+ population) and coincubation with BGMME3 led to a significant reduction of the frequency of CD4+IL17+ cells (8% of total CD4+) (Figure 7a,b). In fact, culturing Th1 (CD3/CD28 activated) or Th17 (CD3/CD28 activated with TGF-β and IL6) in the presence or absence of BGMME3 revealed that BGMME3 significantly changed the cytokine secretion profile of Th17 cells, promoting the production of IFNγ and IL4 (Figure 7c). BGMME3 did not appear to have an effect on Th1 effector function. In the neutralizing assay, IL17 expression was restored after IFNγ and IL4 neutralization whereas IL10 and CD1.d neutralization had no effect on BGMME3-mediated inhibition (Figure 7d, e). Taken together, our data suggest BGMME3 selectively polarized Th17 cells toward Th1 (IFNγ) or Th2 (IL4) phenotype, which subsequently antagonized IL17 secretion.
Figure 7.
Suppression of Th17 Cells by BGMME3 is interleukin 4 (IL4) and IFNY dependent. (a) Splenocytes were derived from wild type (WT) (C57B/6) mice and cultured with anti-CD3/CD28 beads in the presence of TGF-β (25 ng/ml) and IL6 (5 ng/ml) for 4 days. The polarized splenocytes were then cocultured with media alone or with BGMME3 for 48 hours. Recovered cells were analyzed for CD4 and IL17 after PMA-ionomycin stimulation for 5 hours. (b) Cumulative data as mean ± SD for frequencies of IL17-positive CD4+ T cell as analyzed in a. (c) CD4+ T cells differentiated in Th1 polarizing condition (CD3/CD28 activation) or Th17 polarizing condition (CD3/CD28 activation with TGF-β and IL6) were subsequently cultured in the presence or absence of BGMME3. Culture supernatant were analyzed for IL4, IL10, IL17, and interferon-γ (IFNγ) production by ELISA. (d) The supernatant of CD4+ T cells differentiated in Th17 polarizing condition cocultured in the presence of BGMME3 with 5 ng/ml of anti-IFNγ, anti-IL4, anti-IL10, or anti-CD1.d IgG Ab was analyzed for IL17 secretion by ELISA. Results are representative of the average of two independent experiments ± SD. (e) Recovered cells were analyzed for CD4 and IL17 after PMA-ionomycin for 5 hours. *P < 0.05.
Discussion
We have previously shown that MCP3 signaling has a profound impact on B-cell biology. Specifically, antagonistic signaling by MCP3 leads to an inhibition of immunoglobulin production and expansion of plasmablasts.20 It has also been observed that CCR2, a putative target CC-chemokine receptor for MCP3, was expressed at higher levels on immature B cells but downregulated at the mature B-cell stage. This regulated expression of CCR2 contributes to B-cell homing and maturation.21 Indeed, CC-chemokine receptor expression and GPCR signaling provide important signals in peripheral B-cell subset development. For example, the development of MZ, T2, and B1a subsets depends on the intactness of Gα2 signaling and the subsequent activation of phosphoinositide 3-kinase cascade. B cells derived from Gα2–/– mice not only exhibit quantitative deficiency in the frequency of MZ, T2, and B1a B cell in the periphery but also qualitative deficiency in their ability to upregulate IL10 production.22,23 Since GMME3 induces significant Ca++ mobilization (Figure 3a), it is conceivable that GMME3 acts on CCR2-expressing transitional (immature) B-cell subset to enhance the development and characteristics of MZ and T2 B cells through Gα2 activation. In this study, we demonstrated that CCR2 at least in part mediated GMME3 signaling, but far from being the only possible pathway given the multitude of MCP3 receptors (Figure 3d). The identification of the relative contribution of different receptors that GMME3 utilize will require additional investigation. We have previously shown that the fusion of N-terminal quadrapeptide truncated monocyte chemoattractant protein 1 to GMCSF (aka GMME1) acquired novel proapoptotic effects on CCR2-expressing lymphoid cells.11 Intriguingly, we observed here that the MCP3 fusokine GMME3 had no significant proapoptotic effect (Supplementary Figure S2). Rather, an important distinguishing feature between GMME1 and GMME3 is that the former coupled an antagonistic cleaved variant of monocyte chemoattractant protein 1 (lacking the first four N-terminal amino acids) to GMCSF, while GMME3-coupled full-length uncleaved MCP3 to GMCSF. We speculate that the GPCR response to MCP3 fusokine variants may be markedly distinct to that observed here. Overall, our work has provided evidence that modulating GPCR signaling can have a substantial impact on B-cell biology and their acquisition of a suppressor phenotype. These results parallel our previously published finding that naive CD19+ splenic B cells can be converted by a common γc IL15 derivative (GIFT15) to CD1.d IL10+ Bregs.17 The sum of these observations suggest that B cells are poised for conversion to a Breg suppressor phenotype through nonoverlapping signal transduction pathways including GPCR and common γc receptor signaling mediated by engineered ligands such as GMME3 and GIFT15. Our immunophenotype data suggests that BGMME3 share many markers with the in vitro activated Bregs, such as CD1.dhi, CD21hi, CD38hi, and CD19hi (Figure 2a). Furthermore, the changes in antigen presentation markers also correlate with the phenotype of ex vivo CD40 ligand-stimulated or B cell-activating factor-induced IL10+ B cells.4,8 The CD19hiCD5+CD1.d+ phenotype corresponds to the endogenous B regulatory cell subset and CD21hiCD23low are markers of MZ B cells.
B cells with regulatory capacity have been identified in a variety of mouse models of autoimmune diseases. CD19–/– mice exhibit exaggerated response in T-cell driven inflammation and exhibit exacerbated disease progression upon autoantigen challenge for EAE.15,18,24 In contrast, overexpression of CD19 (hCD19Tg) via transgenic approach has been shown to expand B cells with regulatory capacity. The adoptive transfer of hCD19Tg B cells normalized T-cell dependent inflammation. In this study, we demonstrated that ex vivo manipulation of autologous B cells based on GPCR modulation provided a viable means to generate immune suppressor cells with beneficial effect in the murine model of MS. Compared to the transgenic approach, fusokine manipulation of autologous B cells has greater translational potential and is clinically feasible as a form of cellular therapy to control the exaggerated inflammation in autoimmune disorders. We may also consider the potential utility of direct administration of GMME3 protein to subjects with the intent of in vivo genesis of BGMME3 cells. However, the theoretical concerns regarding off-target effects and possible immunogenicity of neoantigens harbored by GMME3 remain to be defined.
We demonstrated both in vitro and in vivo that IL10 production is crucial for BGMME3-mediated anti-inflammatory effect, akin to how endogenous or ex vivo induced Breg function by producing IL10.15 We have demonstrated that IL10 produced by BGMME3 is necessary to suppress macrophage APC function. It is well established in the literature that IL10 has a profound inhibitory effect on macrophage APC function, including the downregulation of MHC class II molecules, costimulation markers, and proinflammatory cytokines.25,26,27 While we demonstrated that the IL10 effect was primarily on antigen presentation, we also demonstrated BGMME3 can have a profound effect on the antigen-driven effector CD4+ response by skewing them away from a Th17 pathway toward a Th1 phenotype (Figure 6b). This observation seems paradoxical in light of the beneficial effect of BGMME3 in EAE, where both Th1 and Th17 cells are considered pathogenic.28 However, this paradigm has been challenged by the findings where the disruption of the classical Th1-IFNγ pathway, either by neutralizing IFNγ or IL12, greatly exacerbates the severity of EAE, whereas blocking Th17 development by neutralizing IL23, confers significant protection.29 Moreover, the adoptive transfer of CNS reactive Th17 cells, but not Th1 cells, can transfer EAE from one mouse to the next.29 It has similarly been demonstrated that the intactness of Th1 response dictates the responsiveness of IFNβ treatment in MS patients, where IFNβ uses IFNγ signaling pathway to inhibit Th17 inflammation.28 In fact, it appears that the different lineage T helper cells do not represent terminal differentiation states for effector CD4+ T cells. There is considerable late developmental plasticity and they can regulate the effector function through cytokine production and transcription factors in a mutually antagonizing fashion.30,31 Committed CD4+IL17+ cells can be converted to CD4+IFNγ+ in a STAT4 and T-bet dependent manner.30 Consistent with these findings, our data suggest that adoptive transfer of BGMME3 led to an attenuation of CNS inflammation by their direct inhibition of Th17 cells and enhancement of Th1-IFNγ pathway (Figure 6b). B cells are capable to regulate T-cell mediated immunity via the secretion of a number of polarizing cytokines.32 We demonstrated that a secreted factor was responsible for the inhibition of IL17 production (Figure 6c), and IFNγ and IL4 signaling were necessary in the conversion of EAE-derived or TFGβ/IL6-differentiated Th17 cells, but not IL10 or CD1.d (Figure 7d). BGMME3 appeared to be most potent in modulating the secretome phenotype of Th17 cells, compared to Th1 lineage (Figure 7c). To our knowledge, this is the first report that demonstrates that B cells manipulated ex vivo can influence the late lineage differentiation of T helper cells and their effector function. The precise role of BGMME3 in the intricate network of Th cell differentiation warrants further investigation. Our data suggest Th17 cells are most susceptible to be converted to an IFNγ-secreting Th1 or IL4-secreting Th2 phenotype by hyperactive B cells stimulated via the GPCR pathway.
Taken together, our work has provided evidence that N-terminal fusion with GMCSF allowed MCP3 to hyperactivate its receptor and that GPCR-driven signaling can have a substantial impact on B-cell biology. This observation opens the door for the development of biological agents such as GMME3 for cellular immunotherapy of autoimmune diseases.
Materials and Methods
Animals and reagents. WT female C57Bl/6 (B6) mice, age-matched IL10-deficient mice (B6.129P2-Il10tml/CgnJ), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I), C57BL/6-Tg(TcraTcrb)425Cbn/J (OT-II), ccr2-deficient mice (B6.129S4-Ccr2tm1Ifc/J) used experimentally were between the age of 6–8 weeks, purchased from The Jackson Laboratory (Bar Harbor ME). C57Bl/6 retired breeder mice were purchased from Harlan Laboratories (Indianapolis, IN). Recombinant human MCP3 (rCCL7), recombinant murine GMCSF, and their antibodies were purchased from R&D Systems (Minneapolis MN). Dulbecco's modified Eagle's medium, RPMI-1640, fetal bovine serum and penicillin/streptomycin were purchased from Wisent Technologies (Rocklin, CA). Cell separations were performed using EasySep kits according to the manufacturer's instruction (StemCell Technologies, Vancouver, British Columbia, Canada).
Fusokine generation and protein modeling. To clone the fusion transgene GMME3, mouse GMCSF cDNA was truncated of 3′ DNA sequence encoding the last 12 C-terminal amino acid sequence DIPFECKKPSQK and was cloned in frame to full-length human MCP3 cDNA (Invivogen, San Diego, CA) to generate GMME3 fusion transgene. HEK 293 cells were transiently transfected with GMME3 transgene using PolyFect (Qiagen, Mississauga, Ontario, Canada); serum-free Dulbecco's modified Eagle's medium supernatant was collected after 48 hours and concentrated using Amicon centrifugation columns (Millipore, Cambridge, Ontario, Canada). Western blot analysis and cytokine ELISA (GMCSF and MCP3) were used to confirm the expression and concentration of GMME3. Concentrated conditioned media were used in all in vitro assays. HEK 293 T cells transfected in the same manner in the absence of the plasmid containing the fusion transgene were used as mock-transfected control. The structural model of GMME3 was built by homology modeling using MODELLER 9v3 (University of California at San Francisco).1 Crystal structure of human GMCSF (PDB entry: 2 gmf) and MCP3 (PDB entry: 1 ncv) were used as the templates for homology modeling. 200 structural models of GMME3 were generated and the one with lowest objective function was selected for further analysis.
Western blotting. For the detection of GMME3, concentrated conditioned media from mock (PolyFect alone) or GMME3-transfected HEK 293 cells were denatured at 90 °C for 5 minutes and separated on 4–20% SDS-PAGE gel (Thermo Scientific, Pittsburg, PA). Immunoblotting was performed with antihuman MCP3 (CCL7) antibody and anti-mouse GMCSF antibody according to manufacturer's guideline. For the study of the GMME3 biochemical response, 1–2 × 106 RAW264.7 cells or mouse spleen-derived primary B cells were stimulated with GMME3, or cytokine controls in RPMI media for 20 minutes. Cell lysates were prepared by using cell lytic M supplemented with protease inhibitor and phosphatase inhibitor according to manufacturer's instruction. Antiphosphorylated or total STAT5 antibody (Cell Signaling Technology, Danvers, MA) was used in immunoblotting.
Fluo-4 calcium influx assay. To study the biochemical response of GMME3 on B cells, splenocytes were collected from naive C57/B6 mice. B cells were subsequently purified using mouse B-cell enrichment kit (StemCell Technologies). The purity was routinely >90%. GMME3-mediated Ca++ influx in purified B cells was tested using the Fluo-4 NW Calcium assay kit accounting to manufacturer's instruction (Invitrogen). Briefly, B cells were resuspended in assay buffer (1 × HBSS, 20 nmol/l HEPES) at 1.5 × 106 cells /ml. Fluo-4 dye and probenecid were loaded to 125,000 cells/well of 96 well-late. B cells with Fluo-4 dye mix were then incubated at 37 °C for 30 minutes followed by room temperature for an additional 30 minutes. For Ca++ influx analysis, GMME3 or cytokine combination was used to stimulate fluo-4-loaded B cells. The fluorescence response (494 nmol/l /516 nmol/l) was measured in FL600 microplate fluorescence reader (BioTek, Winooski, VT) and analyzed by KC4 software (BioTek).
Cell culture. HEK 293 cell line was cultured in Dulbecco's modified Eagle's medium (Wisent Technologies) supplemented with 10% fetal bovine serum (Wisent Technologies) and 100 U/ml of penicillin/streptomycin (Wisent Technologies). RAW 264.7 cell line was purchased from ATCC (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 100 U/ml of penicillin/streptomycin. Primary mouse splenocytes were cultured in RPMI supplemented with 2 mmol/l L-glutamine, 1 mmol/l HEPES, 1 mmol/l sodium pyruvate, 0.05 mmol/l β-mercaptoethanol with 10% fetal bovine serum. Spleen-derived B cells were cultured in complete splenocyte medium.
To generate BGMME3, We placed purified B cells derived from the whole splenocyte population of retired breeder mice in culture in complete medium supplemented with GMME3 (10 ng/ml) or cytokine controls supplemented with conditioned media derived from mock-transfected HEK 293 T cells for 4 days.
Flow cytometry and intracellular staining. Cells to be analyzed by fluorescence-activated cell sorter were harvested and resuspended in 10 × 106 cells /ml. Samples were blocked with anti-FcRmAb 2.4G2 for 30 minutes and subsequently stained with fluorochrome-conjugated monoclonal antibody in Ca2+Mg2+ free PBS with 2% fetal bovine serum for 30 minutes. Cells were washed twice with staining buffer and resuspended in 1% paraformaldehyde before analysis. Intracellular staining was performed with Cytofix/Cytoperm Kit (BD, San Diego, CA) according to manufacturer's instruction. All fluorescence-activated cell sorter antibodies were purchased from BD Pharmingen (San Diego, CA).
In vitro antigen presentation assay. Peritoneal macrophages were collected from lavage of the C57B/6 retired breeder peritoneal cavity with 12 ml RPMI. Cells were cultured in 24-well plates and washed after 16 hours. Plastic adherent-macrophages were cultured in RPMI supplemented with 1 mg/ml of MOG35–55 peptide (Sheldon Biotech Center, McGill University) or chicken ovalbumin (Sigma-Aldrich, Oakville, Ontario, Canada) overnight. CD4+ T cells were purified from the spleens of EAE mice with active disease or OT-II transgenic mice. CD8+ T cells were purified from the spleen of OT-I transgenic mice. 5 × 105 purified T cells were cultured with antigen-pulsed, 1% paraformaldehyde-fixed macrophages in complete splenocyte medium. Alternatively, peritoneal-macrophages were pretreated with 105 GMME3-activated B cells (BGMME3) for 48 hours. OTII-CD4+ or OT-I-CD8+ T cells were subsequently added to pretreated antigen-pulsed macrophages. Culture supernatant was collected after 72 hours and inflammatory cytokine IFN-γ or IL17 were measured by ELISA when appropriate.
Mouse Th17 cell differentiation. Single cell suspension of whole spleen from WT C57B/6 was generated by mechanical disruption. Splenocytes were stimulated with T activator CD3/CD28 dynabeads (Invitrogen) according to the manufacture's instruction. Under Th17 polarizing condition, cells were stimulated with the addition of 5 ng/ml of recombinant TGF-β and 20 ng/ml of recombinant IL6 (R&D Systems). Differentiated splenocytes were subsequently cultured with GMME3-activated B cells (BGMME3) in the presence of neutralizing antibodies (BD) where indicated. Supernatant of the coculture was analyzed for IL17 production by ELISA and recovered cells were analyzed by flow cytometry for CD4 and IL17 coexpression.
Experimental autoimmune EAE induction and BGMME3 adoptive cell transfer. The induction of EAE was described previously.17 Briefly, we inject C57B/6 mice subcutaneously at the base of the tail with 1 mg/ml of purified synthetic peptides of MOG35–55 (Sheldon Biotech Center, McGill University) emulsified in complete Freud adjuvant (Cedarlane Laboratories, Burlington, NC) at equal volume. On day 0 and day 2, we also injected mice intraperitoneally with 150 ng of pertussis toxin (Sigma-Aldrich). The disease progression was monitored every 2 days according to the following score: 0, no disease; 1, floppy tail; 2, hind limb weakness; 3, partial hind limb paralysis; 4, Complete hind limb paralysis; 5, moribund state. Animal protocols were approved by the McGill University Animal Care Committee. For the treatment of EAE mice, 1 × 106 ex vivo GMME3-activated B cells was given on day 10 after MOG-immunization. The injection was repeated 7 days later. PBS, naive B cells, GMME3-activated IL10–/– B cells were used as controls.
Histological analysis. To analyze the extent of inflammation in the CNS, mice were perfused with PBS before the removal of spinal cord. For H&E staining, sections were fixed, embedded and cut. MCA497R and CD3 were used in immunohistology staining for recognizing F4/80+macrophage and T cell infiltrate, respectively. Luxol-fast blue was used for the visualization of demyelination.
Statistical analysis. P values were calculated by paired Student's t-test (Excel) and significance was defined as P < 0.05. Data are reported as mean ± SD or SEM as indicated.
SUPPLEMENTARY MATERIAL Figure S1. Untreated B cells derived from wild-type C57B/6 failed to induce inhibit the disease progression of EAE. Figure S2. GMME3 has no proapoptotic capacity.
Acknowledgments
We thank Dr Nikolaus Heveker and Yamina A. Berchiche for advice on the study of GMME3 and CCR2 receptor interaction. This work is supported by Canadian Institute of Health Research (CIHR) operating grant MOP-15017. J.H. is a recipient of a CIHR-funded MD-PhD scholarship and J.G. is a Georgia Cancer Coalition Distinguished Cancer Scholar. The authors declared no conflict of interest.
Supplementary Material
Untreated B cells derived from wild-type C57B/6 failed to induce inhibit the disease progression of EAE.
GMME3 has no proapoptotic capacity.
REFERENCES
- Allman D., and, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund FE., and, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS., and, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA.et al. (2009Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice J Immunol 1823492–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR.et al. (2007Novel suppressive function of transitional 2 B cells in experimental arthritis J Immunol 1787868–7878. [DOI] [PubMed] [Google Scholar]
- Gray M, Miles K, Salter D, Gray D., and, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Matsushita T, Tsubata T., and, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ.et al. (2010Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells J Immunol 1843321–3325. [DOI] [PubMed] [Google Scholar]
- Williams P., and, Galipeau J. GMCSF-interleukin fusion cytokines induce novel immune effectors that can serve as biopharmaceuticals for treatment of autoimmunity and cancer. J Intern Med. 2011;269:74–84. doi: 10.1111/j.1365-2796.2010.02314.x. [DOI] [PubMed] [Google Scholar]
- Rafei M, Berchiche YA, Birman E, Boivin MN, Young YK, Wu JH.et al. (2009An engineered GM-CSF-CCL2 fusokine is a potent inhibitor of CCR2-driven inflammation as demonstrated in a murine model of inflammatory arthritis J Immunol 1831759–1766. [DOI] [PubMed] [Google Scholar]
- Rafei M, Campeau PM, Wu JH, Birman E, Forner K, Boivin MN.et al. (2009Selective inhibition of CCR2 expressing lymphomyeloid cells in experimental autoimmune encephalomyelitis by a GM-CSF-MCP1 fusokine J Immunol 1822620–2627. [DOI] [PubMed] [Google Scholar]
- Rafei M., and, Galipeau J. A CCL2-based fusokine as a novel biopharmaceutical for the treatment of CCR2-driven autoimmune diseases. Crit Rev Immunol. 2010;30:449–461. doi: 10.1615/critrevimmunol.v30.i5.40. [DOI] [PubMed] [Google Scholar]
- Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW.et al. (2009The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease Blood 1141289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz JD, Yanaba K., and, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D., and, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M., and, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafei M, Hsieh J, Zehntner S, Li M, Forner K, Birman E.et al. (2009A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties Nat Med 151038–1045. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M., and, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Hanisch UK., and, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E.et al. (2008Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction Blood 1124991–4998. [DOI] [PubMed] [Google Scholar]
- Flaishon L, Becker-Herman S, Hart G, Levo Y, Kuziel WA., and, Shachar I. Expression of the chemokine receptor CCR2 on immature B cells negatively regulates their cytoskeletal rearrangement and migration. Blood. 2004;104:933–941. doi: 10.1182/blood-2003-11-4013. [DOI] [PubMed] [Google Scholar]
- Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A.et al. (2002A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation J Exp Med 196753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwadi H, Wei B, Schrage M, Spicher K, Su TT, Birnbaumer L.et al. (2003B cell developmental requirement for the G alpha i2 gene J Immunol 1701707–1715. [DOI] [PubMed] [Google Scholar]
- Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C.et al. (1991Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression J Exp Med 174915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW.et al. (1991IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells J Immunol 1463444–3451. [PubMed] [Google Scholar]
- Koppelman B, Neefjes JJ, de Vries JE., and, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–871. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P.et al. (2010T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis Nat Med 16406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD.et al. (2005IL-23 drives a pathogenic T cell population that induces autoimmune inflammation J Exp Med 201233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO.et al. (2009Late developmental plasticity in the T helper 17 lineage Immunity 3092–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H., and, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM.et al. (2000Reciprocal regulation of polarized cytokine production by effector B and T cells Nat Immunol 1475–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Untreated B cells derived from wild-type C57B/6 failed to induce inhibit the disease progression of EAE.
GMME3 has no proapoptotic capacity.