Abstract
NK cell expression and use of the IL-2Rα chain (CD25), required for the high affinity IL-2R, remain poorly understood. The studies reported here demonstrate that infections with MCMV, but not lymphocytic choriomeningitis virus, induce CD25 on NK cells along with high levels of IL-12 and IL-18. The cytokines act ex vivo to elevate CD25 levels, and IL-12 along with the IL-12R and STAT4, but not the NK activating receptor Ly49H, are required for peak induction in vivo. All examined NK cell populations are driven into proliferation and incorporate BrdU in response to high, but only those from MCMV infection respond to low ex vivo concentrations of IL-2. Numbers of NK cells are reduced during MCMV infection by IL-2 neutralization. Thus, a link between innate and adaptive immunity is established by which composition of innate cytokine responses sets up to promote NK cell use of a factor supporting adaptive responses.
INTRODUCTION
NK cells of the innate immune system express the IL-2/15Rβ (CD122) and common γ (CD132) chains used as receptor components for multiple cytokines and sufficient to stimulate proliferation in response to high concentrations of IL-2. Formation of the high affinity IL-2R, however, also requires expression of the IL-2Rα (p55) chain, identified as CD25. Starting with early studies in human and mouse, the conditions supporting NK cell expression and use of the CD25 chain have remained elusive (1–5). IL-2 is best characterized as an adaptive cytokine produced by T cells to support T cell proliferation, with induction of high affinity IL-2Rs in response to TCR stimulation and IL-2 exposure being critical in the selection of T cell subsets for expansion (6).
NK cells are stimulated into blastogenesis and proliferation under a variety of conditions in vivo (1, 7, 8). During early infections of mice with either murine CMV (MCMV) 4 or lymphocytic choriomeningitis virus (LCMV), type 1 IFNs, i.e., IFNα/β, promote NK cell blastogenic responses at least in part by inducing IL-15, and polyinosinic-polycytidylic acid, a chemical inducer of type 1 IFNs, as well as administered type 1 IFNs do the same in vivo (1, 7, 9). NK cells elicited through this pathway fail to express CD25 (IL-2Rα), have IL-2-independent in vivo responses, and only respond to high IL-2 concentrations ex vivo (10). Facilitated by expression of an activating receptor with specificity for a virus-induced ligand, Ly49H, NK cell responses to MCMV infection can extend into periods overlapping adaptive immunity (4, 11, 12).
The studies reported here investigated MCMV infections to demonstrate that NK cells induced under these conditions express CD25, acquire the ability to respond to low dose IL-2 concentration ex vivo, and use IL-2 for in vivo proliferation. The CD25 molecule is uniquely induced to high levels during MCMV but not LCMV infection, and dependent on IL-12, IL-12R and the STAT4 signaling molecule but Ly49H independent. Thus, the enigma concerning a pathway to expression and use of the high affinity IL-2R on NK cells is resolved. More importantly, a novel cytokine-dependent mechanism for CD25 induction and a major new link between innate and adaptive immunity are identified.
MATERIALS and METHODS
Mice
C57BL/6 (B6) mice were from Taconic Farms, and B6 IL-12Rβ2−/− (13) were originally obtained for breeding, and RAG1-mutated mice with controls were, from Jackson Laboratory. Mice bred and housed under specific pathogen free conditions in our facility were B6 STAT4−/− (originally obtained from M. Kaplan, Indiana Univ. School of Medicine)(14), and B6 Ly49H−/− mice (12). Mice were used at 6-to-14 wks of age. The Institutional Animal Care and Use Committee approved the experiments.
In vivo manipulation
Mice were infected i.p. with either 5,000 or 50,000 PFU of salivary gland-derived Smith MCMV from American Type Culture Collection (12) or with 4×104 PFUs of Armstrong LCMV strain clone E350 (15, 16). For IL-2 blocking, 250μg of each of two anti-IL-2 antibodies (S4B6 and JES1A12; BioXcell) was administered i.p. Mice received injections at D4 and D2 prior to harvest. For IL-12/23 blocking, 750μg of an antibody directed against the common p40 chain of mouse IL-12/23 (clone C17.8; BioXcell) was administered i.p 4h before infection. For controls, equal amounts of isotype-matched antibodies (BioXcell) or rat IgG (Sigma) were used.
Flow cytometric analyses
Splenic leukocytes were incubated for 20min in 20% FBS-PBS with 2.4G2 antibody. NK cells were identified as NK1.1+TCRβ− populations. Under these conditions, there are no relevant differences in the NK cell subsets identified as TCRβ- as compared to CD3-(6). Cell surface staining was performed using antibodies directed against: PE-CD25 (clone PC61.5), PE-CD122, PE-CD132, PerCP-NK1.1, FITC-TCRβ, allophycocyanin (APC)-Ly49H, PerCP5.5-TCRβ, APC-CD8a, PE-NK1.1, PE-CD49b, and APC-CD49b (BD Biosciences or eBioscience). For the BrdU detection, cells were fixed and permeabilized with Cytofix/Perm (BD Biosciences), treated with DNAse I (Sigma-Aldrich), and stained with FITC-anti-BrdU antibody (clone 3D4; BD Bioscience). Samples were acquired using a FACSCalibur (BD Bioscience). Data were analyzed with FlowJo (Tree Star) software.
Ex vivo cell manipulations
Sensitivity to IL-2-induced proliferation was evaluated using total splenic leukocytes at 3×105 cells (data not shown) or NK cells, enriched to 50–70% NK1.1+TCR-β − using DX5 positive selection with magnetic beads (Miltenyi Biotec), at 1×105 cells per well in 96-well plates. Concentrations of rIL-2 (0, 0.4, 2, 10, 100, 500, 2500 U/ml) were added. After 40h, cells were incubated with BrdU at 20 μM for an additional 2h. Both rhIL-2 (17×106 U/mg protein from Chiron) and rmIL-2 (10×106 U/mg protein from eBioscience) (data not shown) were tested with similar effective concentrations. The BrdU incorporating NK cell proportions in total and enriched populations were similar. For evaluating CD25 induction on NK1.1+TCRβ − cells, 5×105 total splenic leukocytes isolated from uninfected B6 mice were stimulated in 96-well plates with type I IFN (rmIFNα 1,000U/ml, PBL or rmIFNβ 1,000 U/ml Biogen), rhIL-2 (10U/ml and 500U/ml, Chiron), rmIL-6 (100ng/ml, R&D Systems), rmIL-12 (1pg/ml to 10ng/ml, eBioscience), rmIL-18 (1pg/ml to 10ng/ml, eBioscience), rmTNFα (100ng/ml, R&D Systems), or rmIFNγ (100ng/ml, eBioscience) for 24h.
Statistical analysis
Results are means ± SE. Statistical significance of differences was determined by unpaired two-tailed student’s t test. P values. For the comparison of results across the IL-2 titration, a paired test was used.
RESULTS and DISCUSSION
Expression and function of CD25 on NK cells
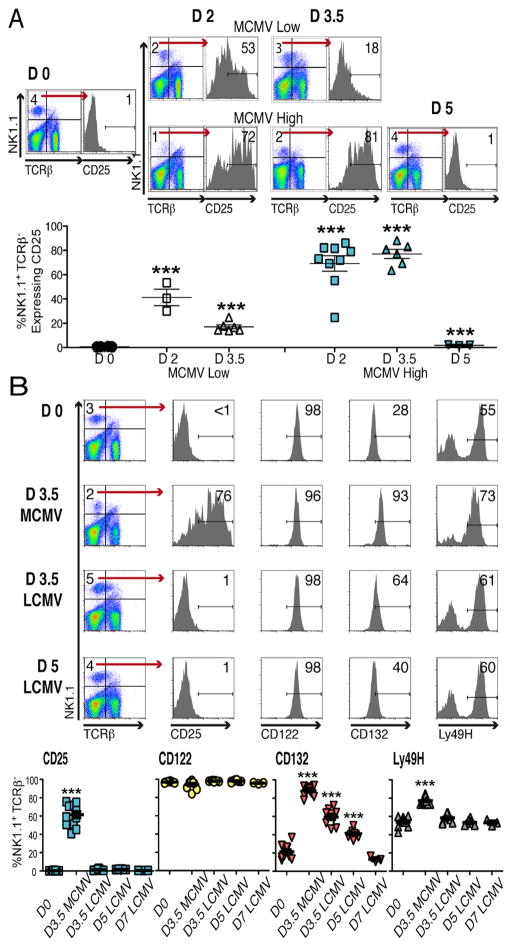
Splenic populations, from B6 mice that were uninfected (D0) or MCMV infected with low, 5,000, or high, 50,000, PFU doses, at 2 (D2), 3.5 (D3.5) and 5 (D5) days prior to harvest, were prepared for analysis. The NK cells were identified as NK1.1+TCRβ−. The CD25 chain was induced to detectable levels during MCMV infection with up to 86% of NK cells having peak expression at D3.5 (Fig. 1A).
Figure 1. IL-2 receptor expression on NK cells.
Mice, B6, were infected with either 5,000 or 50,000 PFU of MCMV, or 4×104 PFU of LCMV. Flow cytometry was used, identifying NK cells within total splenic leukocytes by gating on NK1.1+TCRβ − cells. (A) CD25 expression on D0, D2, D3.5 and D5 of low and high dose MCMV infections are shown. Representative samples from individual mice are given as flow plots. Composites, with data from 6 separate experiments, present symbols for results from individual mice. (B) Evaluated expression of CD25, CD122, CD132, along with the Ly49H activating molecules, is shown. Representative samples prepared from individual mice that were D0, D3.5 high dose MCMV infected, and D3.5 or D5 LCMV infected are given as flow plots. Composites, with data from 3 different experiments including the conditions shown along with studies of D7 LCMV infection, present symbols for results from individual mice. Total numbers of mice examined were from 10-to-13 with the exception of D5 LCMV with 8 and the D7 LCMV with 3. Means ± SE are shown as bars with error spread. P values ≤0.0001 are noted with ***.
All three chains of the high affinity IL-2R were examined using cells from D0 or D3.5 high dose MCMV-infected mice (Fig. 1B). Although MCMV infection was required to induce detectable CD25, cells from D0 and infected mice expressed CD122 and CD132. The levels of CD122 remained relatively constant, whereas CD132 was elevated on D3.5. As expected, the proportions of Ly49H+ cells were increased on D3.5. The effects of LCMV infection were evaluated on D3.5, D5, and D7 after i.p. infection with 4×104 PFU. Detectable CD25 expression was not induced, and CD122 expression was consistently observed after LCMV infection. On D3.5 and D5, CD132 expression was at levels intermediate to D0 and D3.5 of MCMV infection (Fig. 1B).
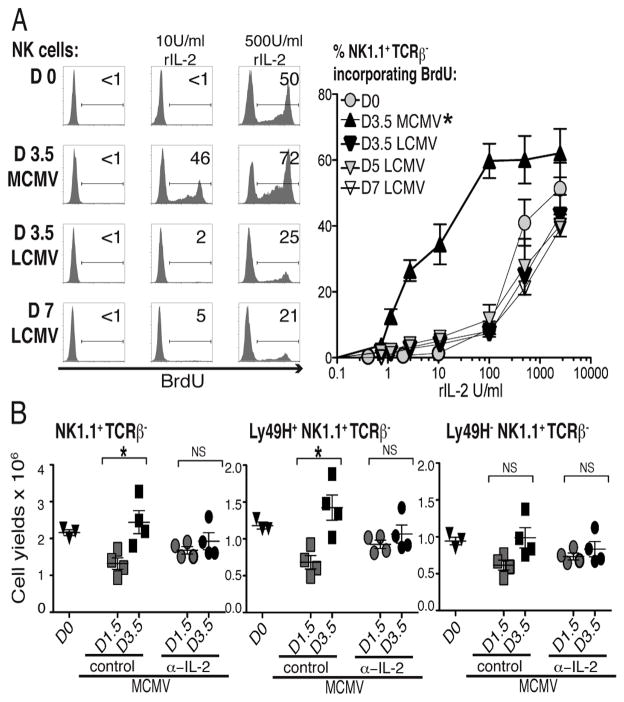
Although cells expressing CD122 and CD132 can proliferate in response to high IL-2 concentrations, e.g., >100 U/ml, the CD25 accessory chain is required to induce proliferation in response to low IL-2 concentrations, e.g., ≤10 U/ml (17). To determine the functional consequences associated with the various levels of IL-2R chains, NK cells were enriched from D0, D3.5 MCMV, D3.5 LCMV, and D5 or D7 LCMV infection, and responsiveness to various rIL-2 concentrations, for incorporation of the DNA precursor molecule, BrdU, was evaluated ex vivo. The cells were exposed to IL-2 for 40h with BrdU added for the last 2h. Approximately 20-to-50% of NK cells prepared from D0, D3.5, D5 or D7 LCMV infection but >70% of those from D3.5 of MCMV infection were BrdU+ in response to 500U/ml of rIL-2 (Fig. 2A). Only those prepared from the D3.5 MCMV infection incorporated BrdU in response to 10U/ml of rIL-2. Titration studies demonstrated that as compared to the other NK cell populations, the overall concentrations of IL-2 required to stimulate BrdU incorporation of NK cells from D3.5 of MCMV infection were 100-fold lower (Fig. 2A).
Figure 2. Sensitivity of NK cells to IL-2-induced proliferation.
(A) Enriched NK cells from D0 mice and mice infected with high dose MCMV or LCMV on indicated days after infection were stimulated with rIL-2. BrdU incorporation in NK1.1+TCR-β − NK cells was measured. Representative flow cytometry data from stimulation with two doses of rIL-2 (10U/ml and 500U/ml) are shown on left. Summarizes of rIL-2 titrations are on right. These data were compiled from 3 independent experiments for D0, D3.5 MCMV, and D3.5 LCMV; 2 for D5 LCMV; and 1 for D7 LCMV. *The differences between the D3.5 MCMV NK cell responses to all others had P values <0.005. (B) In vivo use of IL-2 was evaluated by blocking IL-2 during infection. The absolute numbers of NK cell (NK1.1+TCRβ −), Ly49H+ NK cells, and Ly49H- yields from spleens of mice either receiving 500 ug of control isotyped-matched Ab or 250 ug each of two α-IL-2 Abs were compared on D1.5 and/or D3.5 after MCMV infection with 5,000 PFU. Each symbol indicates the results from an individual mouse, with 4 per group in each infected group. Means ± SE are shown with error. P values <0.05 are noted with *, and <0.01 with **. Non-significant is denoted as ns.
A role for IL-2 in supporting endogenous NK cell proliferation during MCMV infection was evaluated by neutralizing the factor in vivo. Combinations of antibodies directed against different parts of IL-2 were used. Control antibody-treated mice received equal concentrations of isotype-matched antibody. Splenic NK cells yields were assessed at D1.5 and 3.5 after low dose MCMV infection. In comparison to D0, both the control and anti-IL-2-treated mice had decreases in NK cell numbers on D1.5 (Fig. 2B). These recovered and were elevated on D3.5 of infection in the control, but not the anti-IL-2-treated, group. The Ly49H proportions within the NK cell subsets were preferentially increasing during this period, and the IL-2-dependent effects were predominantly found in this subset. Thus, during MCMV infection, NK cells are uniquely induced to express the CD25 chain, form the high affinity receptor for IL-2 and respond to low doses of IL-2 ex vivo, and IL-2 supported their proliferation in vivo.
Mechanism for CD25 induction
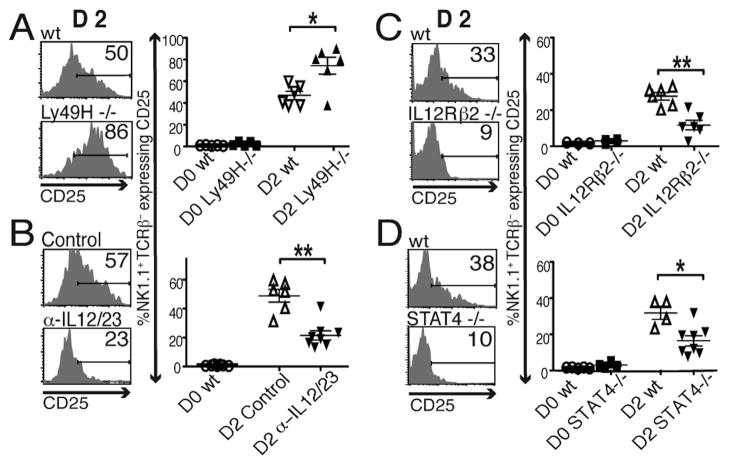
Because both Ly49H and TCR receptors use overlapping signaling pathways and TCR signaling leads to IL-2R induction, experiments were carried out evaluating the Ly49H contribution to CD25 induction on NK cells. Wild type (wt) control B6 mice and B6 mice rendered Ly49H deficient as a result of genetic mutation (Ly49H−/−) were either D0 or D2 infected with 5,000 PFU of MCMV and examined for the contribution made by Ly49H to CD25 induction. The proportions of NK cells expressing CD25 reached 80% after infection of Ly49H−/− mice (Fig. 3A). Thus, the activating molecule was not required.
Figure 3. In vivo requirements for CD25 induction.
The expression of CD25 was evaluated, on NK1.1+TCRβ − NK cells, in mice at D0 and D2 of MCMV infection with 5,000 PFU. CD25 expression was compared on NK cells from wt and Ly49H−/− mice (A), from mice either receiving control Ab or α-IL-12/23 Ab at 4h before MCMV infection (B), from wt and IL-12Rβ2−/− mice (C), and from wt and STAT4−/− mice (D). Representative flow plots from individual mice are shown. Composites of results from individual animals are given on the right. Data for panels A, B, and C were assembled from 2 independent experiments with total mice examined on D2 being 6 to 8. The data for panel D are from 1 experiment with 5 to 8 mice per group. Statistically significant differences between groups are indicated (*p<0.01, **p<0.001).
The major cytokines during early LCMV infection are type 1 IFNs, and circulating levels of biologically active IL-12p70 heterodimer and processed IL-18, are low to undetectable (15, 18). In contrast, MCMV infection induces IL-12 and IL-18 as well as type 1 IFNs (18–20). In these studies, the mean ± SE serum levels of IL-12p70 were undetectable during LCMV but reached 789 ± 92 pg/ml at D1.5 during high dose MCMV infection. The role of endogenous IL-12 in the induction of CD25 was evaluated in B6 mice treated with control antibodies or antibodies neutralizing IL-12 as a result of binding to the p40 chain, anti-IL-12/23 (Fig. 3B), and in B6 wt as compared to IL-12 unresponsiveness, i.e., mutated in the IL-12Rβ2 chain specific for the cytokine, mice (Fig. 3C). Both approaches resulted in profound inhibition of CD25 induction on NK cells at D2 of MCMV infection, with less than half of the populations identified as receptor positive, and these expressing much lower levels.
The STAT4 is the major signaling molecule from the IL-12R. Experiments carried out in B6 wt and STAT4-deficient, i.e. STAT4−/−, mice (Fig. 3D) demonstrated that STAT4 was critical for maximal CD25 induction with the proportions of and intensities on expressing cells dramatically reduced in its absence. Thus, IL-12, the IL-12R and STAT4 provide a pathway for CD25 induction in vivo during MCMV infection.
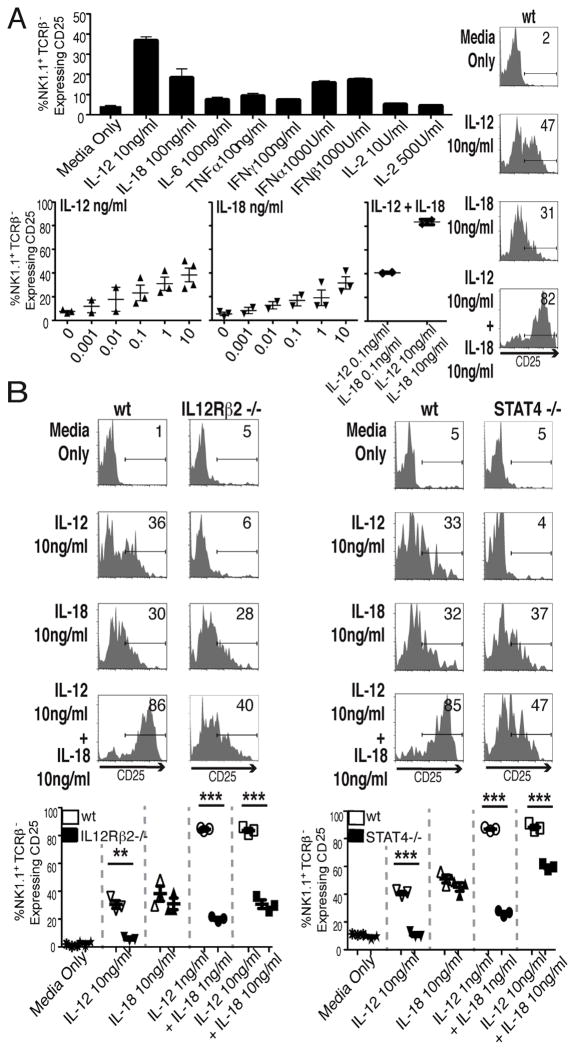
To evaluate effects ex vivo and separate induction from any viral effects, splenic leukocytes, from uninfected B6 mice, were cultured with media control or various cytokines overnight. The populations were then harvested and stained for NK1.1, TCRβ, and CD25. The cytokines tested included the innate cytokines induced at early times after MCMV infection (18–20) and IL-2 (Fig. 4A). IL-12 was the strongest inducer of CD25. Titration studies showed that IL-12 alone induced a plateau of approximately 40% of NK cells expressing CD25 at 100 pg/ml to 1 ng/ml, whereas IL-18 added alone induced a plateau response reaching approximately 30% expressing cells at 10 ng/ml. When added together, there was a synergistic interaction with over 80% of the NK cells induced to express high levels of CD25 (Fig. 4B). The responses were independent of T cells because similar results were obtained with NK cells from T and B cell-deficient RAG1-mutant mice (data not shown). Thus, CD25 induction on NK cells can be achieved without viral infection by addition of innate cytokines and is T cell independent.
Figure 4. Cytokine induction of CD25 ex vivo.
Splenic leukocytes from naïve mice were incubated with indicated cytokines. CD25 expression on NK cells (NK1.1+TCR-β −) was evaluated after 24h stimulation. (A) Responses to a panel of cytokines at the indicated doses. The results are means from 2 separate experiments. Effects of titrated ranges of IL-12, IL-18, or combinations of IL-12 and IL-18 on CD25 induction were determined after 24h stimulation. Each symbol indicates the percentage response from an individual experiment. Experiments were repeated 3 to 4 times. Representative flow cytometric plots from gated NK cells are provided for panel A studies. Mean ± SE of 3 independent experiments for A & B are shown in summaries. CD25 expression on NK cells from wt and IL12Rβ2−/− mice or from wt and STAT4−/− mice (B) after 24h stimulation with individual and combination of IL-12 and IL-18 concentrations are shown. Representative results with gated NK cells are given in histograms. Data are summarized with each symbol representing the value from individual mice. Total numbers of mice for each group were 3. Statistically significant differences between groups are indicated (**p<0.001, ***p<0.0001).
To evaluate the roles for the IL-12R and STAT4 ex vivo, populations were taken from B6 wt, IL-12Rβ2−/−, and STAT4−/− mice and stimulated with the cytokines (Fig. 4C). All of the CD25 induction mediated by IL-12, including any synergism with IL-18, was ablated using IL-12Rβ2−/− populations, and all of the effects mediated by IL-12 alone were ablated in STAT4-negative populations. There was also a significantly reduced interaction between IL-18 and IL-12. Thus, the IL-12 through STAT4 pathway to NK cell CD25 expression can be mechanistically differentiated in culture.
In summary, these studies conclusively demonstrate that under the appropriate, but not all, conditions NK cell express CD25 and a high affinity IL-2R, and define a new link between innate and adaptive immunity. The pathway to induction is shown to be through IL-12 but Ly49H independent. A lack of requirement for the activating receptor separates the response from that elicited through TCRs on T cells, but previous work from our group has shown that NK cells are unique in their basal readiness for IL-12 responsiveness and high STAT4 expression (16, 21, 22). Thus, they are equipped prior to infection to respond to the innate cytokine with responsiveness to an adaptive cytokine. The results help explain a number of observations concerning NK cells, including the importance of IL-12 and IL-18 in supporting expansion of Ly49H NK cells during MCMV infection (23), the ability to use IL-12 and IL-18 in culture to derive populations promoting long-term mouse NK cells (24), the role of IL-12 and STAT4 in supporting the development of long-lasting “memory” NK cells during MCMV infection (25), and responsiveness of human NK cell subsets to IL-2 for IFNγ secretion (26). They also help to bring understanding of the human and mouse systems closer together by demonstrating how the reported response of a human NK cell subset to IL-12 exposure with CD25 expression (5) works in the mouse during endogenous infections. Contrasting the two species suggests interesting avenues for further investigation to reconcile apparent differences including evaluating STAT4 levels in human NK cell subsets relative to their IL-12 responsiveness for CD25 expression, and the IL-2 role in mouse NK cell IFNγ production at the later times associated with extended MCMV infection (12).
There are likely to be other factors in place with overlapping, alternative or augmenting functions for supporting NK cell proliferation. Although the induction of CD25 was seen in response to both low and high dose MCMV infection in these studies, the requirement for IL-12 in vivo was best demonstrated during low dose infection. Type 1 IFNs have the potential to alternatively elicit CD25 because the receptors for these factors are also expressed on NK cells and because they can signal basally through STAT4. This can explain the low level CD25 induction on NK cells observed when IFNα or IFNβ was evaluated in culture with populations from uninfected mice (Fig. 4A). However, the pathway from type 1 IFNs to STAT4 is tightly regulated because the cytokines concurrently induce STAT1 and once elevated, this molecule acts to block type 1 IFN access to STAT4 (21, 22). In contrast, IL-12 use of STAT4 is sustained in NK cells in the context of MCMV infection for IFNγ induction (9) and as reported here, for CD25 induction.
In terms of the IL-2 role in proliferation, the results presented here on NK cell yields following IL-2 neutralization in vivo were also best demonstrated during low dose infection and during the period from cell loss at D1.5 to NK Ly49H cell recovery on D3.5 of infection (Fig. 2B). They are not consistent with the reported lack of an IL-2 role for Ly49H NK cell BrdU incorporation during a 3 hr pulse prior to harvest at D4 of MCMV infection (4). The other studies, however, used high dose infection and failed to measure total cell yields. In addition, they evaluated the role for IL-2 exclusively under conditions of genetic IL-2 deficiency, which is associated with confounding variables because of the development of an autoimmune syndrome (27). IL-15 is induced by type 1 IFNs and does alternatively contribute to early NK cell proliferation in vivo (1, 7, 9). An example of an augmenting pathway is the suggested role for signaling through the Ly49H adaptor molecule in promoting NK cell proliferative responses to IL-15 (4).
In closing, the most remarkable aspects of the work reported here are that there is an innate cytokine-dependent pathway to CD25 expression uniquely expressed on NK cells, independent of activating receptor engagement, present in some but not all infections. Why sustain a pathway for NK cell responsiveness to IL-2 for use only some of the time? In contrast to the relatively non-cytopathic LCMV infection with pathology, when present, induced by adaptive immune responses, MCMV is cytopathic and stimulates innate cytokines with potential for life-threatening disease. Thus, there is a critical need for limiting MCMV burdens at times prior to fully developed adaptive effector functions. The IL-12 induction of IL-2 responsiveness suggest the possibility that when necessary, the immune system has evolved a mechanism for sustaining the effector lymphocytes of innate immunity, NK cells, through the periods required for development of adaptive effector defense mechanisms.
Acknowledgments
We thank Drs. O. Boyman of the Univ. Hospital Zurich for advice.
Footnotes
Abbreviations: murine CMV, MCMV; lymphocytic choriomeningitis virus, LCMV; allophycocyanin, APC
References
- 1.Biron CA, Young HA, Kasaian MT. Interleukin 2-induced proliferation of murine natural killer cells in vivo. J Exp Med. 1990;171:173–188. doi: 10.1084/jem.171.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990;171:1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caligiuri MA, Murray C, Robertson MJ, Wang E, Cochran K, Cameron C, Schow P, Ross ME, Klumpp TR, Soiffer RJ, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91:123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French AR, Sjolin H, Kim S, Koka R, Yang L, Young DA, Cerboni C, Tomasello E, Ma A, Vivier E, Karre K, Yokoyama WM. DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections. J Immunol. 2006;177:4981–4990. doi: 10.4049/jimmunol.177.8.4981. [DOI] [PubMed] [Google Scholar]
- 5.Fu X, Liu Y, Li L, Li Q, Qiao D, Wang H, Lao S, Fan Y, Wu C. Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO-natural killer cells following stimulation with interleukin-12. Immunology. 2011;134:41–49. doi: 10.1111/j.1365-2567.2011.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 7.Biron CA, Welsh RM. Blastogenesis of natural killer cells during viral infection in vivo. J Immunol. 1982;129:2788–2795. [PubMed] [Google Scholar]
- 8.Santoni A, Piccoli M, Ortaldo JR, Mason L, Wiltrout RH, Herberman RB. Changes in number and density of large granular lymphocytes upon in vivo augmentation of mouse natural killer activity. J Immunol. 1985;134:2799–2810. [PubMed] [Google Scholar]
- 9.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 10.Kasaian MT, Biron CA. Cyclosporin A inhibition of interleukin 2 gene expression, but not natural killer cell proliferation, after interferon induction in vivo. J Exp Med. 1990;171:745–762. doi: 10.1084/jem.171.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009;206:2235–2251. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Wang X, Gadina M, O’Shea JJ, Presky DH, Magram J. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol. 2000;165:6221–6228. doi: 10.4049/jimmunol.165.11.6221. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 15.Pien GC, Nguyen KB, Malmgaard L, Satoskar AR, Biron CA. A unique mechanism for innate cytokine promotion of T cell responses to viral infections. J Immunol. 2002;169:5827–5837. doi: 10.4049/jimmunol.169.10.5827. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen KB, Cousens LP, Doughty LA, Pien GC, Durbin JE, Biron CA. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat Immunol. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 17.Wang HM, Smith KA. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987;166:1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 19.Ruzek MC, Miller AH, Opal SM, Pearce BD, Biron CA. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 21.Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack EA, Kallal LE, Demers DA, Biron CA. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. M Bio. 2011;2:e00169–11. doi: 10.1128/mBio.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 24.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 27.Horak I, Lohler J, Ma A, Smith KA. Interleukin-2 deficient mice: a new model to study autoimmunity and self-tolerance. Immunol Rev. 1995;148:35–44. doi: 10.1111/j.1600-065x.1995.tb00092.x. [DOI] [PubMed] [Google Scholar]