Abstract
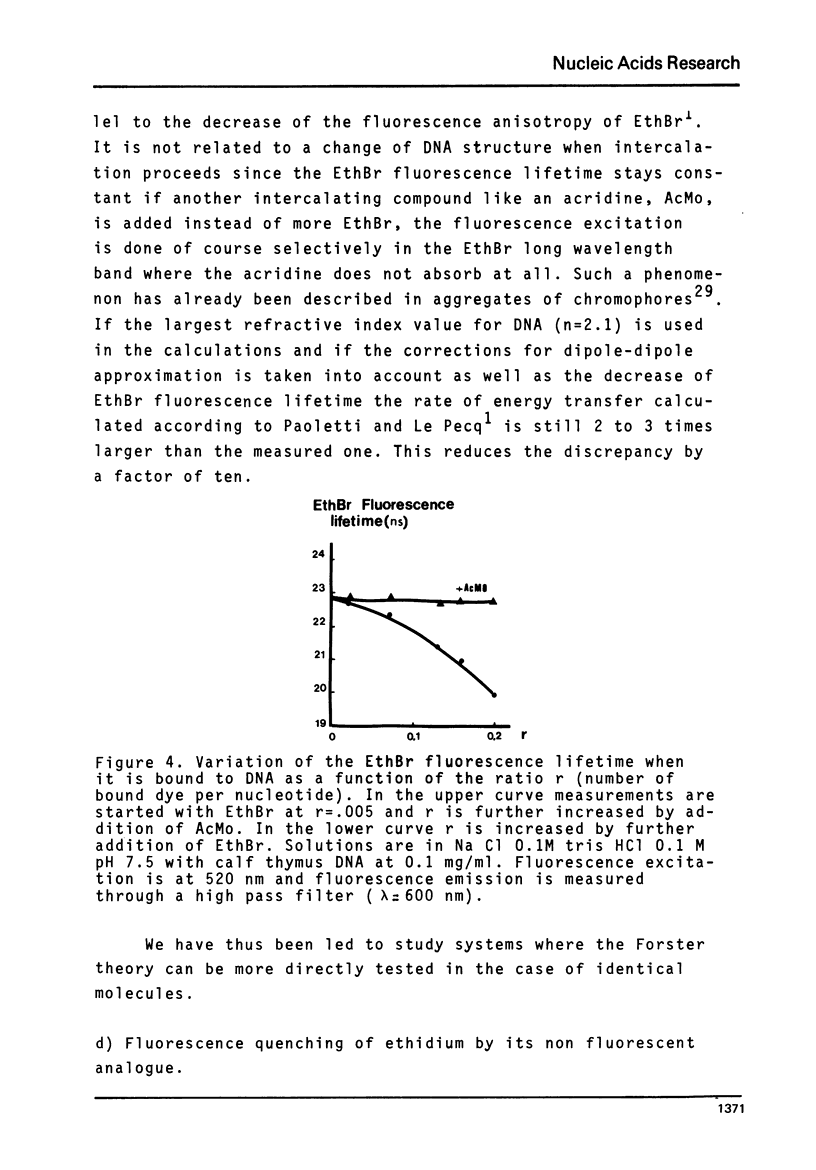
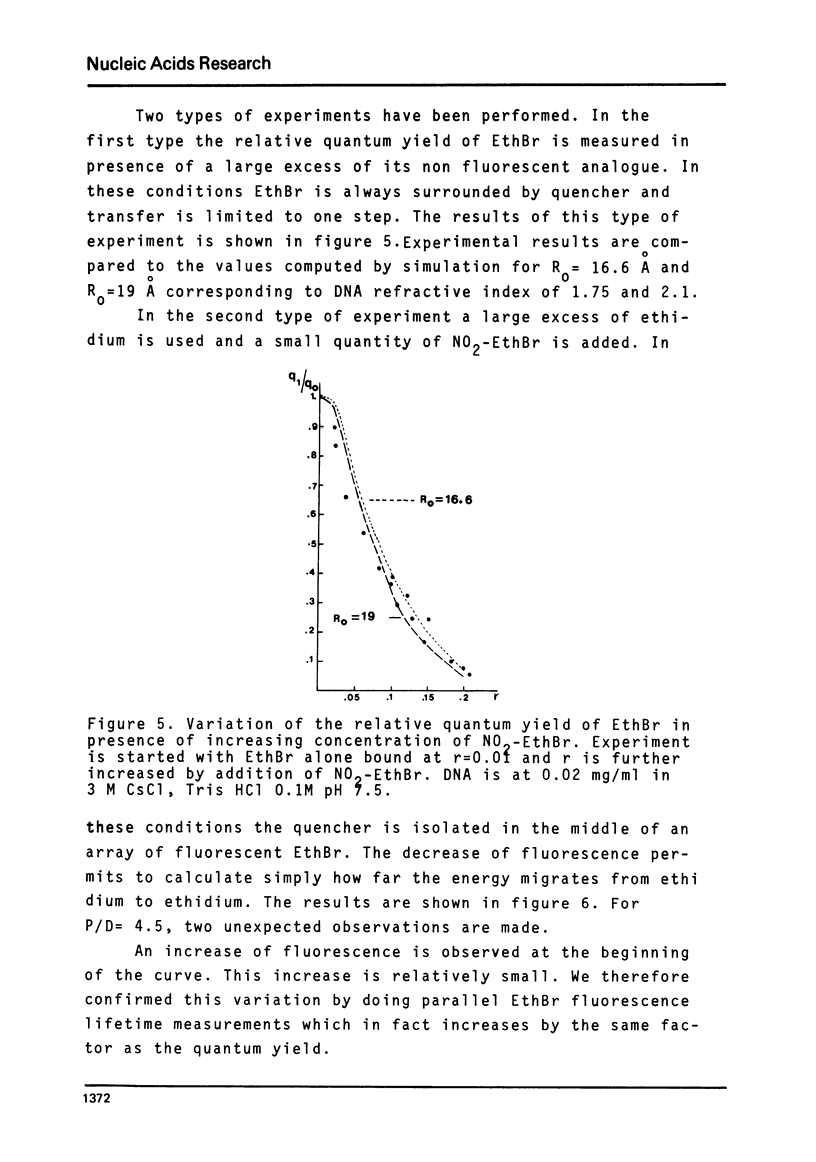
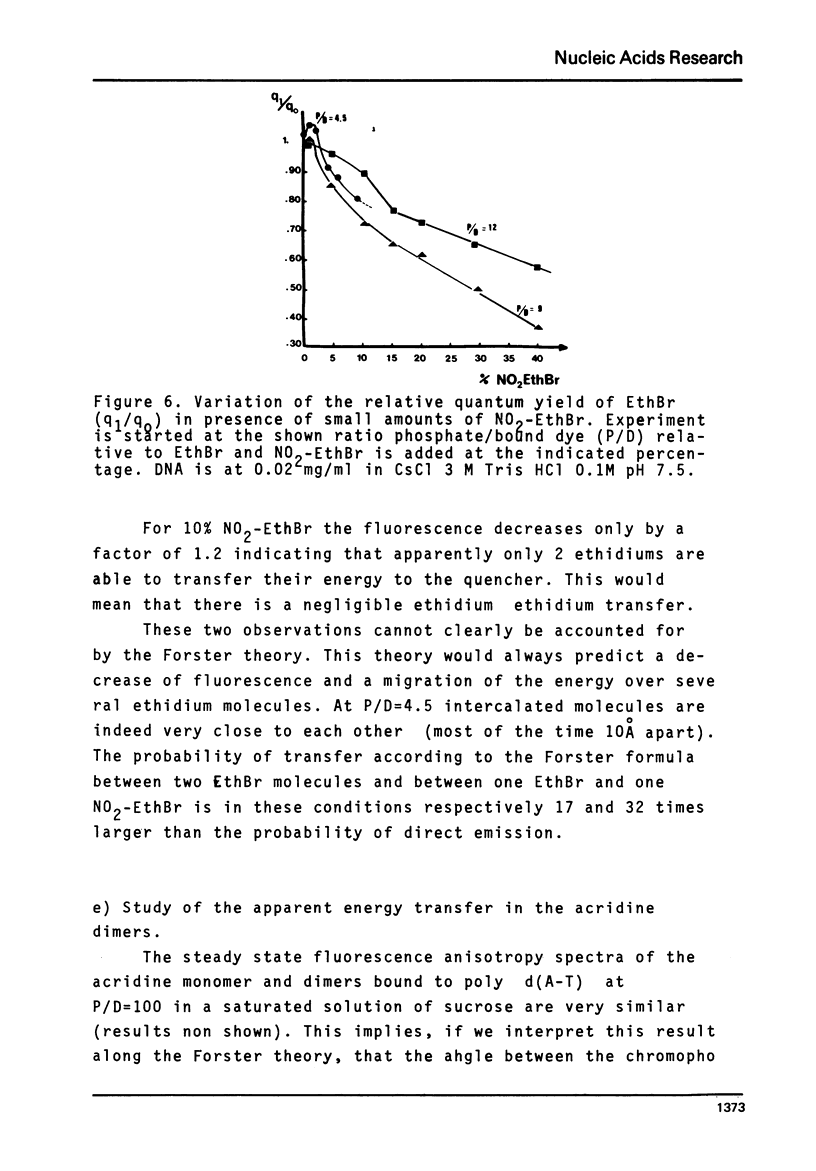
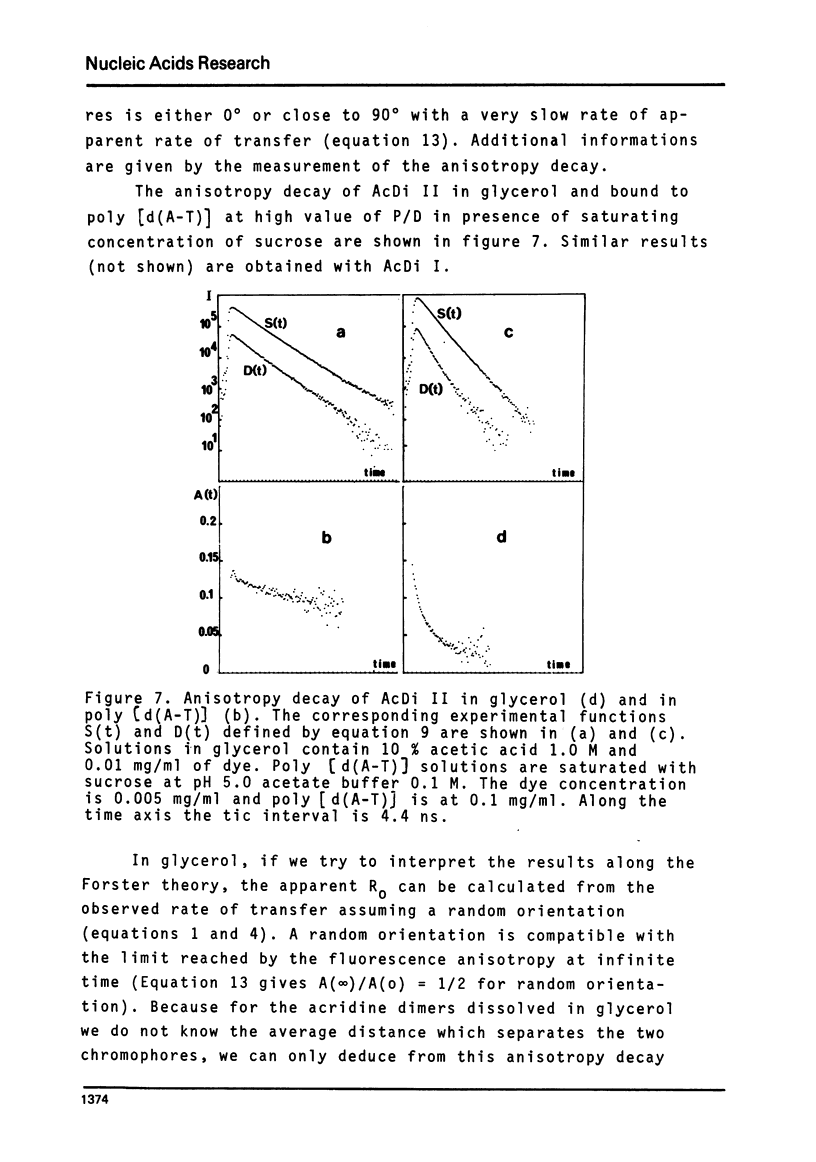
The rate of energy transfer between DNA intercalated ethidium cations calculated by Paoletti and Le Pecq1 using the Forster theory differs from the measured one by a factor of twenty two, if the proper geometrical factors are taken into account. By changing some of the parameters used in the calculation, the discrepancy can be reduced but not eliminated. This led us to the study of other systems where experimental and calculated results can be more directly compared. The apparent rate of energy transfer between ethidium and one of its non fluorescent analogues and between various pairs of intercalated chromophores has been studied. The fluorescence anisotropy decay of acridine dimers in glycerol or bisintercalated in DNA has been measured. These studies show that the Forster theory of energy transfer does not apply to the case of identical chromophores when they are relatively close to each other.
Full text
PDF



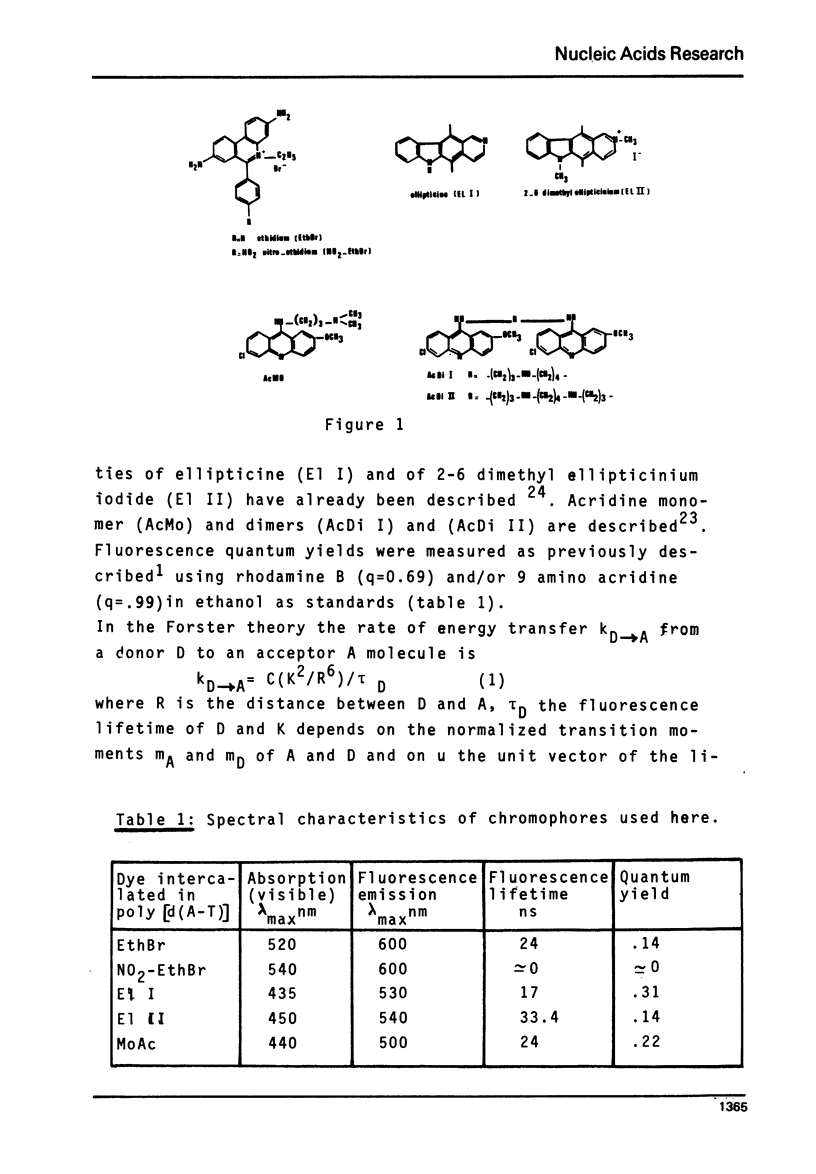
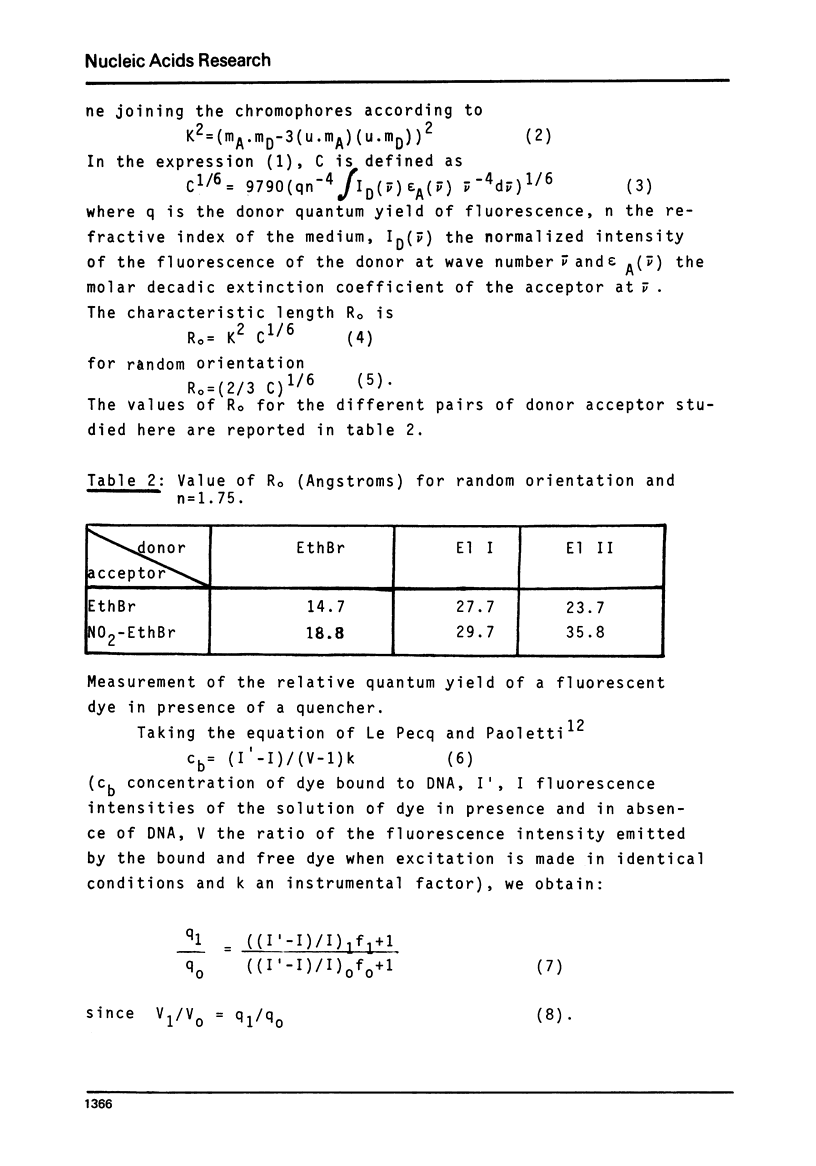


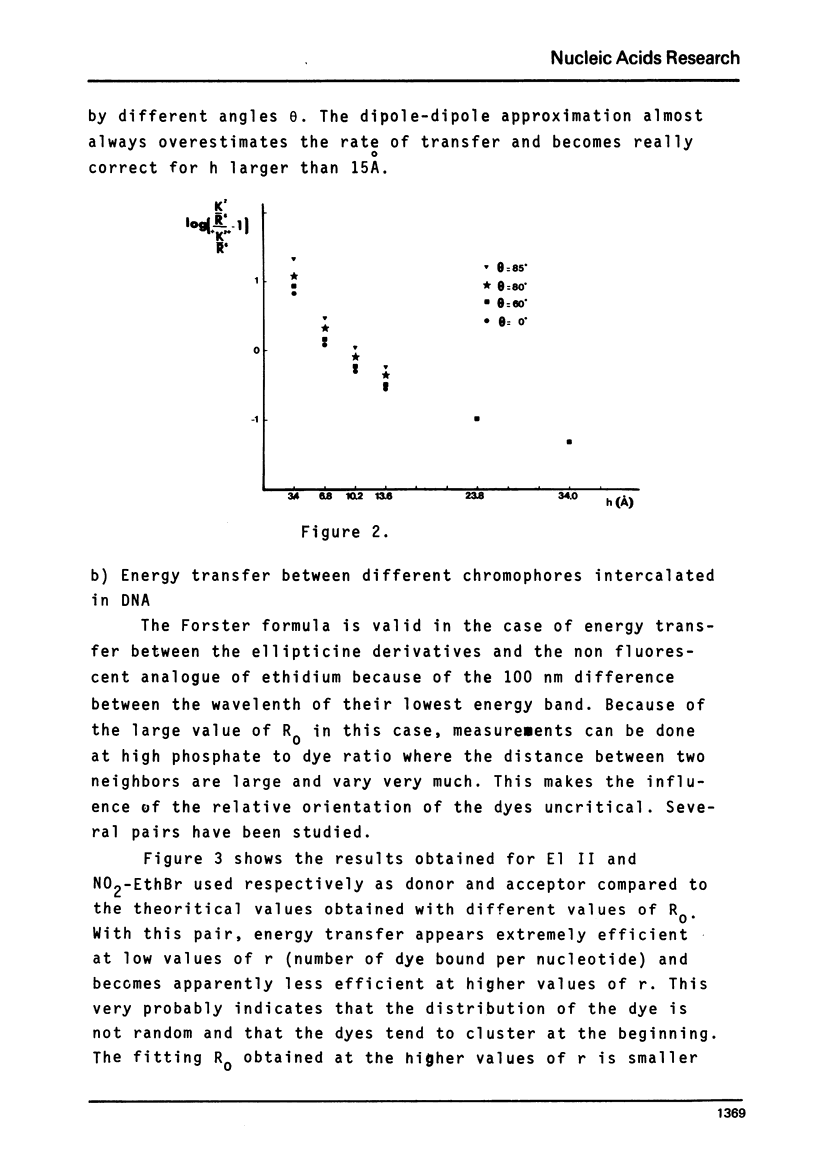
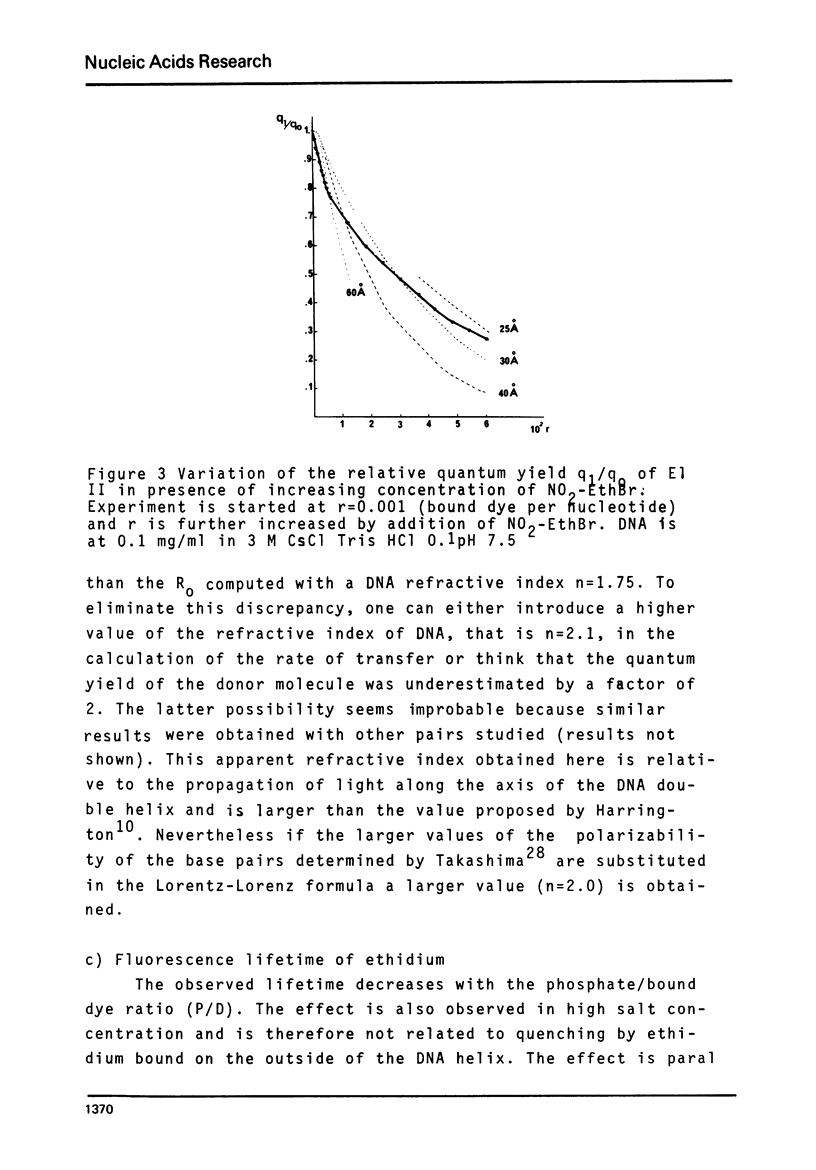




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. Interaction of closed circular DNA with intercalative dyes. II. The free energy of superhelix formation in SV40 DNA. J Mol Biol. 1970 Feb 14;47(3):419–435. doi: 10.1016/0022-2836(70)90312-8. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Crothers D. M. Calculation of binding isotherms for heterogenous polymers. Biopolymers. 1968 Apr;6(4):575–584. doi: 10.1002/bip.1968.360060411. [DOI] [PubMed] [Google Scholar]
- Genest D., Wahl P., Auchet J. C. The fluorescence anisotropy decay due to energy transfers occuring in the ethidium bromide-DNA complex. Determination of the deformation angle of the DNA helix. Biophys Chem. 1974 Apr;1(4):266–278. doi: 10.1016/0301-4622(74)80013-x. [DOI] [PubMed] [Google Scholar]
- Giacomoni P. U., Le Bret M. Electronic structure of ethidium bromide. FEBS Lett. 1973 Feb 1;29(3):227–230. doi: 10.1016/0014-5793(73)80025-0. [DOI] [PubMed] [Google Scholar]
- Harrington R. E. The flow birefringence of persistence length deoxyribonucleic acid. Hydrodynamic properties, optical anisotropy, and hydration shell anistropy. J Am Chem Soc. 1970 Nov 18;92(23):6957–6964. doi: 10.1021/ja00726a038. [DOI] [PubMed] [Google Scholar]
- Haugland R. P., Yguerabide J., Stryer L. Dependence of the kinetics of singlet-singlet energy transfer on spectral overlap. Proc Natl Acad Sci U S A. 1969 May;63(1):23–30. doi: 10.1073/pnas.63.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHA M. ENERGY TRANSFER MECHANISMS AND THE MOLECULAR EXCITON MODEL FOR MOLECULAR AGGREGATES. Radiat Res. 1963 Sep;20:55–70. [PubMed] [Google Scholar]
- Krugh T. R., Reinhardt C. G. Evidence for sequence preferences in the intercalative binding of ethidium bromide to dinucleoside monophosphates. J Mol Biol. 1975 Sep 15;97(2):133–162. doi: 10.1016/s0022-2836(75)80031-3. [DOI] [PubMed] [Google Scholar]
- LATT S. A., CHEUNG H. T., BLOUT E. R. ENERGY TRANSFER. A SYSTEM WITH RELATIVELY FIXED DONOR-ACCEPTOR SEPARATION. J Am Chem Soc. 1965 Mar 5;87:995–1003. doi: 10.1021/ja01083a011. [DOI] [PubMed] [Google Scholar]
- Le Pecq J. B., Le Bret M., Barbet J., Roques B. DNA polyintercalating drugs: DNA binding of diacridine derivatives. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2915–2919. doi: 10.1073/pnas.72.8.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pecq J. B., Nguyen-Dat-Xuong, Gosse C., Paoletti C. A new antitumoral agent: 9-hydroxyellipticine. Possibility of a rational design of anticancerous drugs in the series of DNA intercalating drugs. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5078–5082. doi: 10.1073/pnas.71.12.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Paoletti J., Le Pecq J. B. Resonance energy transfer between ethidium bromide molecules bound to nucleic acids. Does intercalation wind or unwind the DNA helix? J Mol Biol. 1971 Jul 14;59(1):43–62. doi: 10.1016/0022-2836(71)90412-8. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S. Optical anisotropy of synthetic polynucleotides. II. Calculation of pi-electron polarizability of uracil and adenine. Biopolymers. 1969 Aug;8(2):199–216. doi: 10.1002/bip.1969.360080207. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Jain S. C., Sobell H. M. X-ray crystallographic visualization of drug-nucleic acid intercalative binding: structure of an ethidium-dinucleoside monophosphate crystalline complex, Ethidium: 5-iodouridylyl (3'-5') adenosine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):628–632. doi: 10.1073/pnas.72.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Weber G., Shinitzky M. Failure of Energy Transfer between Identical Aromatic Molecules on Excitation at the Long Wave Edge of the Absorption Spectrum. Proc Natl Acad Sci U S A. 1970 Apr;65(4):823–830. doi: 10.1073/pnas.65.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yguerabide J. Nanosecond fluorescence spectroscopy of macromolecules. Methods Enzymol. 1972;26:498–578. doi: 10.1016/s0076-6879(72)26026-8. [DOI] [PubMed] [Google Scholar]


