Abstract
Background
Traumatic hyphema is the entry of blood into the anterior chamber (the space between the cornea and iris) subsequent to a blow or a projectile striking the eye. Hyphema uncommonly causes permanent loss of vision. Associated trauma (e.g., corneal staining, traumatic cataract, angle recession glaucoma, optic atrophy, etc.) may seriously affect vision. Such complications may lead to permanent impairment of vision. Patients with sickle cell trait/disease may be particularly susceptible to increases of elevated intraocular pressure. If rebleeding occurs, the rates and severity of complications increase.
Objectives
The objective of this review was to assess the effectiveness of various medical interventions in the management of traumatic hyphema.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2010, Issue 6), MEDLINE (January 1950 to June 2010), EMBASE (January 1980 to June 2010), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com) and ClinicalTrials.gov (http://clinicaltrials.gov). We searched the reference lists of identified trial reports to find additional trials. We also searched the ISI Web of Science Social Sciences Citation Index (SSCI) to find studies that cited the identified trials. There were no language or date restrictions in the search for trials. The electronic databases were last searched on 25 June 2010.
Selection criteria
Two authors independently assessed the titles and abstracts of all reports identified by the electronic and manual searches. In this review, we included randomized and quasi-randomized trials that compared various medical interventions to other medical interventions or control groups for the treatment of traumatic hyphema following closed globe trauma. There were no restrictions regarding age, gender, severity of the closed globe trauma or level of visual acuity at the time of enrollment.
Data collection and analysis
Two authors independently extracted the data for the primary and secondary outcomes. We entered and analyzed data using Review Manager (RevMan) 5. We performed meta-analyses using a fixed-effect model and reported dichotomous outcomes as odds ratios and continuous outcomes as mean differences.
Main results
Nineteen randomized and seven quasi-randomized studies with 2,560 participants were included in this review. Interventions included antifibrinolytic agents (oral and systemic aminocaproic acid, tranexamic acid, and aminomethylbenzoic acid), corticosteroids (systemic and topical), cycloplegics, miotics, aspirin, conjugated estrogens, monocular versus bilateral patching, elevation of the head, and bed rest. No intervention had a significant effect on visual acuity whether measured at two weeks or less after the trauma or at longer time periods. The number of days for the primary hyphema to resolve appeared to be longer with the use of aminocaproic acid compared to no use, but was not altered by any other intervention.
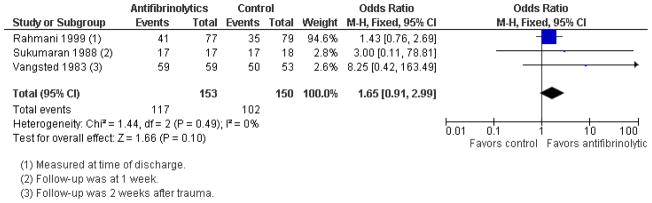
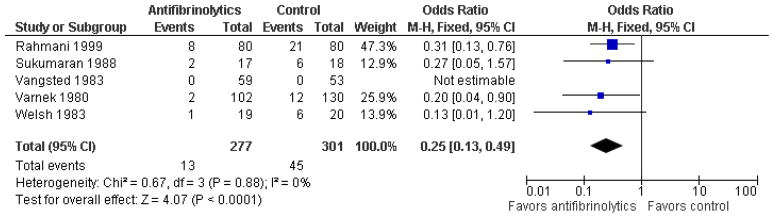
Systemic aminocaproic acid reduced the rate of recurrent hemorrhage (odds ratio (OR) 0.25, 95% confidence interval (CI) 0.11 to 0.5), but a sensitivity analysis omitting studies not using an intention-to-treat (ITT) analysis reduced the strength of the evidence (OR 0.41, 95% CI 0.16 to 1.09). We obtained similar results for topical aminocaproic acid (OR 0.42, 95% CI 0.16 to 1.10). We found tranexamic acid had a significant effect in reducing the rate of secondary hemorrhage (OR 0.25, 95% CI 0.13 to 0.49), as did aminomethylbenzoic acid as reported in a single study (OR 0.07, 95% CI 0.01 to 0.32). The evidence to support an associated reduction in the risk of complications from secondary hemorrhage (i.e., corneal blood staining, peripheral anterior synechiae, elevated intraocular pressure, and development of optic atrophy) by antifibrinolytics was limited by the small number of these events. Use of aminocaproic acid was associated with increased nausea, vomiting, and other adverse events compares with placebo. We found no difference in the number of adverse events with the use of systemic versus topical aminocaproic acid or with standard versus lower drug dose.
The available evidence on usage of corticosteroids, cycloplegics or aspirin in traumatic hyphema was limited due to the small numbers of participants and events in the trials.
We found no difference in effect between a single versus binocular patch nor ambulation versus complete bed rest on the risk of secondary hemorrhage or time to rebleed.
Authors’ conclusions
Traumatic hyphema in the absence of other intraocular injuries, uncommonly leads to permanent loss of vision. Complications resulting from secondary hemorrhage could lead to permanent impairment of vision, especially in patients with sickle cell trait/disease. We found no evidence to show an effect on visual acuity by any of the interventions evaluated in this review. Although evidence is limited, it appears that patients with traumatic hyphema who receive aminocaproic acid or tranexamic acid are less likely to experience secondary hemorrhaging. However, hyphema in patients on aminocaproic acid take longer to clear.
Other than the possible benefits of antifibrinolytic usage to reduce the rate of secondary hemorrhage, the decision to use corticosteroids, cycloplegics, or non-drug interventions (such as binocular patching, bed rest, or head elevation) should remain individualized because no solid scientific evidence supports a benefit. As these multiple interventions are rarely used in isolation, further research to assess the additive effect of these interventions might be of value.
Plain language summary
Medical interventions for traumatic hyphema
Traumatic hyphema is the entry of blood into the space between the cornea and iris following a blow or a projectile striking the eye. Along with the appearance of blood, there may be one or more major injuries to the eye from the trauma, which could result in a significant reduction in vision. In most cases the blood is absorbed, but in some cases there is a secondary hemorrhage (the appearance of fresh blood in the eye after the initial trauma). Complications resulting from secondary hemorrhage include glaucoma, corneal bloodstaining, or damage to the optic nerve. These complications can also result in permanent loss of vision. Nineteen randomized and seven quasi-randomized studies of medical interventions for the treatment of traumatic hyphema were included in this review (2,560 participants in total).
One type of drug often used to treat traumatic hyphema is an antifibrinolytic. Antifibrinolytics, taken either internally or applied as ophthalmic gel, are thought to be effective, because they delay the absorption of the blood clots until complete healing of the damaged blood vessels can take place. This review found that antifibrinolytics did not affect final visual acuity, but did appear to reduce the risk of secondary bleeding. However, patients taking one of the antifibrinolytics, aminocaproic acid, appeared to have more nausea and vomiting compared with control patients. Two other antifibrinolytics, tranexamic acid and aminomethylbenzoic acid, also reduced the risk of secondary hemorrhage, but there was limited information about adverse events. It was unclear whether these medications reduced the complications of secondary hemorrhage (e.g., glaucoma, corneal bloodstaining, and damage to the optic nerve), because few of these events occurred in either the treatment or control groups.
Other interventions evaluated in trials included corticosteroids, taken either internally or applied as eyedrops, estrogens, and other kinds of eyedrops. Because the number of participants was small in these trials, the evidence for any benefit of these drugs is inconclusive. Non-drug interventions that were tested included wearing a patch on one or both eyes, moderate activity versus complete bed rest, and elevation of the head versus lying flat. Again, because the number of participants and events were small, the evidence for a beneficial effect of any of these interventions is inconclusive.
Background
Description of the condition
Introduction
Traumatic hyphema is the entry of blood into the anterior chamber (the space between the cornea and iris) subsequent to a blow or a projectile striking the eye. Apart from the direct consequences of the initial trauma, traumatic hyphema is usually a self-limiting condition that rarely causes permanent loss of vision in the absence of associated damage to the cornea, lens, or optic nerve. Traumatic hyphema is an important clinical entity because of the risks associated with significant initial reduction in vision and because of associated injuries to the tissues of the eye. In young children it can lead to the development of irreversible amblyopia. Complications resulting from secondary hemorrhage, such as glaucoma, corneal bloodstaining or optic atrophy, can lead to permanent impairment of vision, especially if the hyphema is prolonged in association with elevated intraocular pressure (IOP).
Epidemiology
Traumatic hyphema usually is seen in children or young adults with an incidence of approximately two per 10,000 children per year (Wright 2003). Males predominate with a male to female ratio of 3:1 (Crouch 1993). Sports injuries account for 60% of traumatic hyphemas (Crouch 1999).
Presentation and diagnosis
Patients usually present with a sudden decrease or loss of vision following an injury to the eye. The loss of vision depends on the level of hyphema; a patient with a microhyphema occasionally may present with normal vision or with somewhat blurred vision, whereas a patient with a full hyphema may present with almost complete loss of vision. With time, blood in the anterior chamber is forced by gravity to the bottom of the anterior chamber. Subsequently, vision clears gradually unless associated injuries, traumatic uveitis, glaucoma, optic atrophy, or corneal bloodstaining contributes to further losses of vision.
The severity of traumatic hyphema varies from microhyphema, where red blood cells are suspended in the anterior chamber, to a layered hyphema where fresh or clotted blood may be observed grossly in the lower anterior chamber. In a full or total hyphema the entire anterior chamber is filled with blood.
Recurrent hemorrhage, occurring at a rate of 2% to 38% (Walton 2002), increases the time to visual recovery and has been associated with poorer visual outcomes. Secondary hemorrhage typically occurs three to five days after the incident hyphema and may occur due to clot lysis and retraction within the traumatized vessels.
Hyphema in the setting of sickle cell trait/disease appears to be particularly dangerous because the naturally hypoxic and relatively acidotic anterior chamber induces sickling of red blood cells. Sickling in turn prevents normal egress of those blood cells through the trabecular meshwork. Hyphema patients with sickle cell trait/disease may be at a higher risk for elevated IOP (Lai 2001).
The most important sign for diagnosing hyphema is the presence of blood in the anterior chamber assessed by a slit lamp examination. Various grading schemes for hyphema have been proposed. Objective quantification of the level of hyphema is critical, because a sudden increase in the height of a layered hyphema is indicative of ‘rebleed’. Immediate measurement of IOP and a dilated ophthalmoscopic examination (to rule out traumatic retinal tears, dialyses, and detachment) are also indicated at a relatively early time after clearance of hyphema.
Description of the intervention
Management of traumatic hyphema focuses on preventing repeated eye trauma and rebleed, promoting the settling of blood away from the visual axis, controlling traumatic anterior uveitis, and monitoring in order to initiate early prophylaxis or treatment for both secondary glaucoma and corneal bloodstaining. The methods that have been employed to prevent recurrent or iatrogenic trauma include shielding the eye, bed rest, and avoidance of diagnostic interventions such as scleral depression or gonioscopy which could deform the globe. Elevation of the head while sleeping, topical corticosteroids, and cycloplegic medications are mainstays in the management of traumatic hyphema. Hospitalization, once considered essential in order to enforce bed rest, has been questioned and currently is advocated only for patients perceived to be at high risk of rebleed, at risk of noncompliance with bed rest at home, or possibly, with sickle cell trait/disease.
The use of antifibrinolytic agents such as epsilon-aminocaproic acid and tranexamic acid in traumatic hyphema is controversial. They are reported to have potential for reducing the rate of recurrent hemorrhage, but are known to have several possible side effects, such as nausea, vomiting, muscle cramps, conjunctival suffusion, headache, rash, pruritis, dyspnea, toxic confusional states, arrhythmias and systemic hypotension. Epsilon-aminocaproic acid is contraindicated in patients who are pregnant and in patients with coagulopathies or renal diseases; it should be used cautiously in patients with hepatic, cardiovascular or cerebrovascular diseases. A topical gel form of epsilon-aminocaproic acid has not yet received Food and Drug Association (FDA) approval. It appears to have comparable effectiveness, with fewer side effects, as compared with the oral form, and thus might be used on an outpatient basis. Tranexamic acid (Cyclokapron) is reported to be more potent than epsilon-aminocaproic acid and has similar side effects, but with fewer gastric side effects (Rahmani 1999).
Corticosteroids also have been used to treat hyphema and have been reported to be effective (Walton 2002). Investigators have studied both topical and systemic corticosteroids, applying these agents for varying lengths of time with or without other interventions, such as bed rest or cycloplegics. Topical administration of steroids avoids the side effects of systemic corticosteroid use, but it is not known whether topically applied steroids are as effective as systemic steroids in reducing the rate of rebleed. The mechanism of action of corticosteroids is thought to be due to stabilization of the blood-ocular barrier, direct inhibition of fibrinolysis, or reduced inflammation (Walton 2002).
Surgical evacuation of hyphema generally is not needed. In the past, surgical evacuation was often contraindicated due to the possibility of sudden decreases in IOP and increased risk of recurrent hemorrhage (due to decompression of the damaged iris and ciliary body). However, surgical ‘washout’ is advocated in patients with non-clearing hyphema, in whom secondary glaucoma threatens to cause permanent visual loss due to glaucomatous optic neuropathy or to corneal bloodstaining. Surgical washout often is performed (via simple paracentesis) in patients with sickle cell trait because of the increased risk of elevated IOP.
How the intervention might work
The mode of action of medications used to treat traumatic hyphema, especially the antifibrinolytics, is through slowing or inhibiting the resorption of the blood clot within traumatized blood vessels. Aminocaproic acid slows the dissolution of the fibrin blood clot by competing at sites that bind lysine, including lysine sites on tissue plasminogen activator, inhibiting the conversion of plasminogen to plasmin, the enzyme involved in the breakdown of the fibrin clot (Sheppard 2009; Walton 2002). Aminocaproic acid also competitively inhibits the binding of plasmin to the fibrin clot itself. Both of these mechanisms result in slowing the breakdown of the fibrin clot, thus stabilizing it and reducing the risk of secondary hemorrhage. Tranexamic acid also binds to fibrin and is believed to act through a similar mechanism. The action of aminobenzoic acid involves inhibition of fibrinolysis, and estrogens decrease antithrombin activity, both of which result in delays of clot resorption (Westlund 1982). In addition to inhibition of fibrinolysis, corticosteroids are also believed to stabilize the blood-ocular barrier and reduce inflammation.
The goal of most of the other interventions used in the management of traumatic hyphema is to prevent complications from the trauma or from a rebleed, including further trauma, anterior uveitis, secondary glaucoma, optic atrophy, or corneal bloodstaining. These interventions include bed rest and eye patching to prevent further trauma; use of mydriatic or miotic agents to prevent motion of the iris, increased intraocular pressure, or uveitis; corticosteroids to prevent inflammation; and elevation of the head to facilitate settling of the blood in the anterior chamber. Hospitalization facilitates close monitoring of the more severe cases of trauma and/or rebleeding, allowing more timely medical or surgical intervention, if warranted.
Why it is important to do this review
Despite the existence of guidelines for the management of traumatic hyphema (Crouch 1999; Rhee 1999; Sheppard 2009), the safety and effectiveness of various therapeutic modalities such as use of antifibrinolytic agents, their routes of administration, use of corticosteroids and hospitalization are controversial. The evidence for the impact of rebleed on visual outcomes, glaucoma, optic atrophy and bloodstaining is limited. Furthermore, rebleed, which is a surrogate outcome (rather than visual outcome) dominates the published literature on management of traumatic hyphema. It is important to examine the impact of the various antifibrinolytic medications, routes of administration, and dosages used across various populations.
Objectives
The objective of this review was to assess the effectiveness of various medical interventions in the management of traumatic hyphema.
Methods
Criteria for considering studies for this review
Types of studies
This review included randomized and quasi-randomized trials.
Types of participants
We included trials in which the study population consisted of people with traumatic hyphema following closed globe trauma. There were no restrictions regarding age, gender, or severity of the closed globe trauma or level of VA at the time of enrollment.
Types of interventions
We included trials in which:
antifibrinolytic agents (e.g., epsilon-aminocaproic acid, tranexamic acid) or corticosteroids in any form or dosage, with the intention-to-treat or reduce the signs or symptoms of traumatic hyphema, were compared with other treatments, placebo, or no treatment. There was no time limit on the duration of treatment;
bed rest was compared with ambulatory management;
bilateral patching was compared with unilateral or no patching;
outpatient management was compared with inpatient management.
Types of outcome measures
Primary outcomes
The primary outcomes for this review were:
Visual acuity (VA) assessed at short, medium, and long-term follow up, defined respectively as two weeks or less; more than two weeks but within two months, and more than two months from the traumatic event. Visual acuity at resolution of hyphema also was assessed.
Time to resolution of primary hemorrhage (hyphema) defined as the length of time from onset to resolution of hyphema.
Secondary outcomes
Secondary outcomes for this review were sequelae of traumatic hyphema assessed at the time of last study follow-up.
Risk of and time to rebleed, defined as (a) an increase in height of layered hyphema using a biomicroscopic caliper or by any other method or (b) the occurrence of fresh (red) blood in the eye with the existing clot.
Risk of corneal bloodstaining.
Risk of peripheral anterior synechiae (PAS) formation.
Risk of pathological increase in intraocular pressure (IOP) or glaucoma development.
Risk of optic atrophy development.
Adverse effects
We summarized the reported adverse effects related to treatment.
Quality of life measures
In addition to examining the time to hyphema resolution we described available data on other indicators of quality of life, especially time to best visual acuity.
Economic outcomes
We assessed the need for bed rest or hospitalization versus outpatient care. We also compared length of hospital stay as described in the primary reports. No other economic outcomes were reported.
Follow up
There were no restrictions based on length of follow-up.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2010, Issue 6), MEDLINE (January 1950 to June 2010), EMBASE (January 1980 to June 2010), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com) and ClinicalTrials.gov (http://clinicaltrials.gov). There were no language or date restrictions in the search for trials. The electronic databases were last searched on 25 June 2010.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), mRCT (Appendix 4) and ClinicalTrials.gov (Appendix 5).
Searching other resources
We searched the reference lists of identified trial reports to find additional trials. We also searched the ISI Web of Science Social Sciences Citation Index (SSCI) to find studies that have cited the identified trials. We planned to contact the primary investigators of identified trials for details of additional trials, but were unable to do so because most trials were published more than 10 years ago. We did not conduct manual searches of conference proceedings or abstracts specifically for this review.
Data collection and analysis
Selection of studies
Two authors independently assessed the titles and abstracts of all reports identified by the electronic and manual searches as per the ‘Criteria for considering studies for this review’. The abstracts were classified as (a) definitely include, (b) unsure or (c) definitely exclude. Full copies of those classified as (a) or (b) were obtained and re-assessed as per the ‘Criteria for considering studies for this review’. The studies were classified as (1) include, (2) awaiting assessment or (3) exclude. We documented the concordance between authors. Disagreements were resolved by consensus, or by a third author who resolved disagreements between the two authors. We planned to contact authors of studies classified as (2) for clarification of unclear inclusion and exclusion criteria, but were unable to. We excluded studies identified by both authors as (3) from the review and documented them in the table of ‘Characteristics of excluded studies’. We included studies identified as (1) in the review and described them in the table of ‘Characteristics of included studies’. The review authors were unmasked to the reports’ authors, institutions and trial results during this assessment.
Characteristics of excluded studies
| Amirova 1991 | |
| Reason for exclusion | Included non-traumatic hyphema cases in trial and could not determine outcomes in traumatic hyphema cases separately; the method of choosing the control group was not mentioned |
| Anderson 1971 | |
| Reason for exclusion | Not a clinical trial, case reports |
| Berrios 1995 | |
| Reason for exclusion | Review of traumatic hyphema, no original data |
| Bramsen 1977 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Bramsen 1980 | |
| Reason for exclusion | Review of previously published studies, no original data |
| Dralands 1981 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Gastaldi 1970 | |
| Reason for exclusion | Review of treatments for traumatic hyphema, no original data |
| Ghisolfi 1972 | |
| Reason for exclusion | Included non-traumatic hyphema cases in trial and could not determine outcomes in traumatic hyphema cases separately |
| Gilbert 1973 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Gillan 1961 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Goldberg 1960 | |
| Reason for exclusion | Not a clinical trial, cohort study using chart review |
| Gundorova 1985 | |
| Reason for exclusion | Not a clinical trial. There were only 3 patients with post-traumatic hyphema and no obvious control group was defined |
| Heath 1966 | |
| Reason for exclusion | Not a clinical trial, case reports |
| Kotas 1990 | |
| Reason for exclusion | Not a clinical trial, case report |
| Krasnov 1971 | |
| Reason for exclusion | There were only 6 patients with post-traumatic hyphema without surgery or penetrating injuries; patients with different types of glaucoma were classified and treated with glycerin alone or with glycerin and thromboplatin accordingly |
| Latinovic 1981 | |
| Reason for exclusion | Interventional case series, no control group |
| Li 2009 | |
| Reason for exclusion | Not a clinical trial, cohort study |
| Mathis 1987 | |
| Reason for exclusion | Not a clinical trial, case reports |
| Missotten 1977 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Mortensen 1978 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Munoz Negrete 1989 | |
| Reason for exclusion | Interventional case series, no control group |
| Murzin 1966 | |
| Reason for exclusion | Not a clinical trial, appears to be without a control group and the author tested two different drugs in various combinations for various types of bleeds in the eye which occurred at various times before the onset of treatment |
| Ohrstrom 1972 | |
| Reason for exclusion | Not a clinical trial, cohort study |
| Oksala 1967 | |
| Reason for exclusion | Not a clinical trial, cohort study |
| Pierse 1964 | |
| Reason for exclusion | Not a clinical trial, case reports |
| Polychronakos 1967 | |
| Reason for exclusion | Not a clinical trial, case reports |
| Rakusin 1971 | |
| Reason for exclusion | Not eligible, surgical interventions |
| Romano 1986 | |
| Reason for exclusion | Review of steroids for the treatment of traumatic hyphema, no original data |
| Romashchenko 1985 | |
| Reason for exclusion | There were 3 groups of patients with bleeds in the eye: Group 1 was a mix of post- traumatic and post-operative hyphemas (no clear group with post-traumatic hyphemas); the control group was taken from a retrospective study of case notes from 1979 to 1981 and those patients had received an entirely different set of drugs as treatment for their bleeds in the eye |
| Spoor 1990 | |
| Reason for exclusion | Not a clinical trial, cohort study |
| Stepanov 2002 | |
| Reason for exclusion | Not a clinical trial, no control group |
| Surel 1987 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Tartakovskaia 1972 | |
| Reason for exclusion | Not a clinical trial, no control group |
| Uusitalo 1988 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Watkins 1974 | |
| Reason for exclusion | Not a clinical trial, animal study and case reports |
| Welsh 1971 | |
| Reason for exclusion | Not a clinical trial, case reports |
| Williams 1993 | |
| Reason for exclusion | Not a clinical trial, interventional case series |
| Wilson 1990 | |
| Reason for exclusion | Not a clinical trial, cohort study |
| Wright 1964 | |
| Reason for exclusion | Included non-traumatic hyphema cases in trial and could not determine outcomes in traumatic hyphema cases separately |
| Yasuna 1974 | |
| Reason for exclusion | Not a clinical trial, used historical controls |
| Zhou 1982 | |
| Reason for exclusion | Not a clinical trial, groups were selected based on severity of injury |
Characteristics of included studies
| Bedrossian 1974 | ||
| Methods | Study design: Quasi-randomized controlled series Exclusions after allocation: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were assigned. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: Not reported Number allocated: 58 consecutive patients alternately assigned to treatment group after classification based on the size of initial hyphema. Age: Not reported Sex: Not reported Race: Not reported Sickle cell disease: Not reported Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Non-total traumatic hyphema |
|
| Interventions | Cycloplegics (n = 28): 1% atropine ointment Miotics (n = 30): 2% pilocarpine ointment (or eserine ointment) Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Time to resolution of primary hemorrhage Secondary outcomes:
|
|
| Notes | Funding source not reported | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | No | Allocation was not randomized; alternately assigned patients to treatment groups based on the blood level in the anterior chamber. |
| Allocation concealment? | No | Allocation was assigned on an alternate basis. |
| Blinding? Participants |
No | Masking was not reported. |
| Blinding? Personnel and outcome assessors |
No | Masking was not reported. |
| Incomplete outcome data addressed? Primary outcome |
Yes | All participants were analyzed in the group to which they were assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | All participants were analyzed in the group to which they were assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Christianson 1979 | ||
| Methods | Study design: Randomized, double-masked, placebo-controlled clinical trial Exclusions after randomization: None reported Losses to follow-up: None reported Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: Not reported Number randomized: 45 Age: Not reported Sex: Not reported Race: Not reported Sickle cell disease: Not reported Inclusion criteria: Traumatic hyphema Exclusion criteria: Not reported |
|
| Interventions | Treatment (n = 22): Loading dose 75 mg/kg oral aminocaproic acid, followed by 60 mg/kg every 4 hours; length of treatment not reported. Control (n = 23): Placebo, presumably every 4 hours |
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, details not reported Secondary outcomes: Time to resolution of primary hyphema, details not reported |
|
| Notes | Abstract of unpublished study | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Method of randomization not reported. |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
Yes | Authors used a placebo control and stated that the study was double- masked. |
| Blinding? Personnel and outcome assessors |
Yes | Authors used a placebo control and stated that the study was double- masked. |
| Incomplete outcome data addressed? Primary outcome |
Yes | Unclear if number randomized equaled the number reported and analyzed in the abstract, but no exclusions or losses to follow-up were reported. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | Unclear if number randomized equaled the number reported and analyzed in the abstract, but no exclusions or losses to follow-up were reported. |
| Free of selective reporting? | Unclear | Few study details available in the abstract and no full version was published. |
| Free of other bias? | Unclear | Few study details available in the abstract and no full version was published. |
| Crouch 1976 | ||
| Methods | Study design: Randomized, double-masked, placebo-controlled clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: September 1972 to October 1974 Number randomized: 59 Age: 83% of all study participants were between the ages of 6 and 30 years. Sex: 83% of study participants were male. Race: 65% of study participants were black and 35% were white. Sickle cell disease: 8/59 (14%) of all study participants had sickle cell trait. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment (n =32): 100 mg/kg oral aminocaproic acid every 4 hours for 5 days. Control (n = 27): Placebo (200 mL of aromatic elixir (5% glucose, water, and ethanol) in 1,000 mL sterile water) every 4 hours for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed by daily slit lamp examination, and documented by three observers. Secondary outcomes:
|
|
| Notes | Funded by the National Eye Institute, National Institutes of Health | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Yes | Study participants assigned to treatment groups using computerized randomization. |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
Yes | Authors used a placebo control and stated that the study was double- masked. |
| Blinding? Personnel and outcome assessors |
Yes | Authors used a placebo control and stated that the study was double- masked. |
| Incomplete outcome data addressed? Primary outcome |
Yes | There were no exclusions and losses to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | There were no exclusions and losses to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Crouch 1997 | ||
| Methods | Study design: Randomized, double-masked clinical trial Exclusions after randomization: One individual assigned to oral aminocaproic acid and topical placebo excluded based on side-effect of drug (vomiting). Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Sample size was determined to be between 25 and 30 participants in each of the three groups based on alpha of 0.05 and power of 80%. Additional comments: The investigators also studied a control group that did not receive either topical or systemic aminocaproic acid and had refused randomization. We did not include these patients in our analyses. |
|
| Participants | Country: USA Dates: March 1990 to May 1996 Number randomized: 64: 29 assigned to oral aminocaproic acid and topical placebo, and 35 to oral placebo and topical aminocaproic acid. Additional 54 patients included as control group. Age: 72% of study population was younger than 21 years. Sex: 67% of study population was male. Race: 50% of study population was black, 49% was white, and 1% (one participant) was Asian. Sickle cell disease: 2/35 (6%) of participants assigned to topical aminocaproic acid, and 2/29 (7%) of participants assigned to oral aminocaproic acid had sickle cell trait. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: 0.2 ml of 30% aminocaproic acid in 2% carboxymethylene gel applied to inferior fornix every 6 hours plus oral placebo solution every 4 hours, for 5 days. Control: 50 mg/kg oral aminocaproic acid (up to 30 g/day) plus placebo gel every 4 hours, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed by daily slit lamp examination, and documented by a sketch each day. Secondary outcomes:
|
|
| Notes | Funded in part by the Lions Medical Eye Bank and Research Center of Eastern Virginia | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Yes | Study participants assigned to treatment groups using computerized randomization. |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
Yes | Authors used a placebo control and stated that the study was double- masked. Placebo pills were given to the topical group and placebo gel administered to the systemic group to make both regimens similar. |
| Blinding? Personnel and outcome assessors |
Yes | Authors used a placebo control and stated that the study was double- masked. “Data were compiled by observers who did not know what patients were in the treated and untreated control groups.” |
| Incomplete outcome data addressed? Primary outcome |
Yes | One patient was excluded: one individual assigned to oral aminocaproic acid and topical placebo excluded based on side-effect of drug (vomiting). The remaining participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | One patient was excluded: one individual assigned to oral aminocaproic acid and topical placebo excluded based on side-effect of drug (vomiting). The remaining participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Edwards 1973 | ||
| Methods | Study design: Quasi-randomized controlled series Exclusions after allocation: Patients over 20 years old were excluded from the study because of the small number enrolled. Losses to follow-up: None Intention-to-treat: Participants aged 20 and younger were analyzed in the group to which they were assigned. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: 1969 to 1971 Number allocated: 64 consecutive patients alternately assigned to treatment group. Age: Mean was 10 years (up to 20 years) Sex: 61 (95%) men and 3 (5%) women Race: Not reported Sickle cell disease: Not reported Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria: Patients over 20 years of age. |
|
| Interventions | Treatment: Monocular patching (n =35) Control: Binocular patching (n = 29) Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Not reported Secondary outcomes:
|
|
| Notes | Funded by Research to Prevent Blindness Inc., Public Health Service Training Grant, and the National Institutes of Health | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | No | Allocation was not randomized; an independent study director assigned patients to treatment groups on an alternate basis by turning a card. Occasionally the card was not turned each time which led to an uneven number of patients in each group. |
| Allocation concealment? | No | Allocation was assigned on an alternate basis. |
| Blinding? Participants |
No | Masking of patients was not possible with the interventions being studied. |
| Blinding? Personnel and outcome assessors |
Unclear | Authors reported study to be double-masked, although this statement is not clear. The study investigators seldom participated in patient care to allow other examiners with less experience in monocular patching to collect data in hopes of minimizing observation bias. |
| Incomplete outcome data addressed? Primary outcome |
Unclear | Patients over 20 years of age were excluded after allocation to treatment group. |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | Patients over 20 years of age were excluded after allocation to treatment group. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Farber 1991 | ||
| Methods | Study design: Randomized, double-masked clinical trial Exclusions after randomization: 6 participants in the aminocaproic acid group were excluded; 4 were administered prednisone instead of aminocaproic acid (treatment crossover), 1 participant had an unrelated seizure, and 1 developed thrombocytopenia. 1 participant in the prednisone group was administered aminocaproic acid instead of prednisone (treatment crossover) and was excluded. Losses to follow-up: 2 participants in the aminocaproic acid group and 1 participant in the prednisone group withdrew from the study. Intention-to-treat: The participants lost to follow-up or excluded were not included in the analyses and the intention-to-treat principle was not followed in the analyses. Sample size calculations: Not reported Additional comments: The authors noted that there were no secondary hemorrhages in the individuals who had been excluded or withdrew from the study. |
|
| Participants | Country: USA Dates: July 1985 to March 1990 Number randomized: 122: 64 assigned to aminocaproic acid and 58 to prednisone. Age: Mean age in aminocaproic acid group = 23.8 ± 13.8 years (range = 4 to 64 years); mean age in the prednisone group = 23.3 ± 13.4 years (range = 1.5 to 62 years). Sex: 79% of total study population was male. Race: 53% of study population was black, 27% was white, 22% was Hispanic, and 3% was of another ethnic or racial group. Study groups were not balanced by race: there were 57% of blacks and 20% of whites in the aminocaproic acid group compared with 48% of blacks and 25% of whites in the prednisone group. Sickle cell disease: None; excluded Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: 50 mg/kg oral aminocaproic acid (up to 30 g per day) every 4 hours plus 2 doses placebo, for 5 days. Control: 40 mg/day oral prednisone in two doses plus 6 doses placebo; children and adults weighing less than 60 kg were given 0.6 mg/kg/day prednisone, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, recorded daily by slitlamp examination, documented by measuring height in mm and defined as a definite increase in level of presence of “fresh” blood visible over darker clotted blood. Secondary outcomes:
|
|
| Notes | Funded by the National Eye Institute of the National Institutes of Health, Bethesda, Md, and Research to Prevent Blindness | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported. |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
Yes | Authors used a double dummy placebo design and stated that the study was double-masked. |
| Blinding? Personnel and outcome assessors |
Yes | Authors used a double dummy placebo design and stated that the study was double-masked. “All of the treating physicians and nurses were masked to the identity of the treatment.” |
| Incomplete outcome data addressed? Primary outcome |
Unclear | The participants lost to follow-up or excluded were not included in the analyses and the intention to treat principle was not followed in the analyses. |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | The participants lost to follow-up or excluded were not included in the analyses and the intention to treat principle was not followed in the analyses. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Karkhaneh 2003 | ||
| Methods | Study design: Randomized, double-masked clinical trial Exclusions after randomization: None Losses to follow-up: 23 participants lost to follow-up; 4 in the group assigned to cycloplegic drops and topical aminocaproic acid gel, 5 in group assigned to cycloplegic drops and topical placebo gel, and 14 in group assigned to cycloplegic drops only. Intention-to-treat: The participants lost to follow-up were not included in the analyses and the intention to treat principle was not followed in the analyses. Sample size calculations: Not reported |
|
| Participants | Country: Iran Dates: 1998 to 1999 Number randomized: 155: 45 assigned to cycloplegic drops and topical aminocaproic acid gel, 44 to cycloplegic drops and placebo gel, and 66 to cycloplegic drops only. Age: Age range of study population (4 to 30). Sex: 87% of study population (not including those lost to follow-up) was male. Race: Not reported Sickle cell disease: Not reported Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Non-pentrating traumatic hyphema and emergency room outpatient of Farabi Eye Hospital. Exclusion criteria:
|
|
| Interventions | Treatment 1: 2 drops of 25% aminocaproic acid in 2% carboxymethylene gel applied to inferior fornix of affected eye every 6 hours plus homotropine eye drops 3 times per day, for 5 days. Control 1: 2 drops 2% carboxymethylene (placebo) gel applied to inferior fornix of affected eye every 6 hours plus homotropine eye drops 3 times per day, for 5 days. Control 2: Homotropine eye drops 3 times per day, for 5 days. Treatment for all groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit lamp examination for 7 days, and then at day 14. Method for documentation and definition not reported. Secondary outcomes: All measured daily for 7 days and at day 14
|
|
| Notes | Conducted with support from Sina Darou (an ophthalmic pharmaceutical company in Iran), who provided the aminocaproic acid preparation. | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation was not reported |
| Allocation concealment? | Yes | Allocation was concealed from investigators by use of coded bottles. |
| Blinding? Participants |
Unclear | Authors used coded bottles to mask participants for the topical medication, but the group assigned to cycloplegic drops and no topical medication was not masked. |
| Blinding? Personnel and outcome assessors |
Yes | Authors used coded bottles to mask healthcare providers and outcomes assessors. “The ophthalmologist who examined the patients did not know if they were treated or not.” |
| Incomplete outcome data addressed? Primary outcome |
Unclear | The participants lost to follow-up were not included in the analyses and the intention-to-treat principle was not followed in the analyses. There were 23 participants lost to follow-up: 4 in the group assigned to cycloplegic drops and topical aminocaproic acid gel, 5 in group assigned to cycloplegic drops and topical placebo gel, and 14 in group assigned to cycloplegic drops only. |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | The participants lost to follow-up were not included in the analyses and the intention to treat principle was not followed in the analyses. There were 23 participants lost to follow-up: 4 in the group assigned to cycloplegic drops and topical aminocaproic acid gel, 5 in group assigned to cycloplegic drops and topical placebo gel, and 14 in group assigned to cycloplegic drops only. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Unclear | Conducted with support from Sina Darou (an ophthalmic pharmaceutical company in Iran), who provided the aminocaproic acid preparation. |
| Kraft 1987 | ||
| Methods | Study design: Randomized, double-masked clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Not reported |
|
| Participants | Country: Canada Dates: May 1978 to December 1984 Number randomized: 49: 24 assigned to oral aminocaproic acid and 25 to placebo. Age: Age range of study population was 3 to 18 years. Mean age in aminocaproic acid group was 10.6, and in placebo group 11.2. Sex: 73% of study population was male. Race: There were 3 black participants in the aminocaproic acid group and 1 in the placebo group. The ethnicity or race of the other study participants was not reported. Sickle cell disease: None; excluded Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Children with non-penetrating traumatic hyphema treated at the Hospital for Sick Children in Toronto, Canada. Exclusion criteria:
|
|
| Interventions | Treatment: 100 mg/kg oral aminocaproic acid every 4 hours, for 5 days. Control: Placebo every 4 hours, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit lamp examination; documented by two observers and defined as definite increase in amount of blood compared with amount at admission or fresh red blood over darker clotted blood. Secondary outcomes: Outcomes measured daily during hospitalization (up to 5 days), then at 6 weeks, and 3, 6, 12, and 18 months after discharge.
|
|
| Notes | ||
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Yes | Study participants assigned to treatment groups using computerized randomization. |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
Yes | Authors used a placebo control and stated that the study was double- masked. |
| Blinding? Personnel and outcome assessors |
Yes | Authors used a placebo control and stated that the study was double- masked. |
| Incomplete outcome data addressed? Primary outcome |
Yes | There was no loss to follow-up and all participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | There was no loss to follow-up and all participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Kutner 1987 | ||
| Methods | Study design: Randomized, double-masked clinical trial Exclusions after randomization: One participant was excluded from the aminocaproic acid group due to systemic hypotension attributable to the study drug. Losses to follow-up: None Intention-to-treat: The participant excluded from the study was not included in the analyses and the intention to treat principle was not followed in the analyses. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: November 1983 to January 1986 Number randomized: 34: 21 to the aminocaproic acid group and 13 to the placebo group. Age: mean age in the aminocaproic acid group was 18.9±7.7 years and in the placebo group it was 22.8±7.6 years. Sex: 88% of the study population was male. Race: 85% of the study population was white. Sickle cell disease: None; excluded Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Non-penetrating traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: 100 mg/kg oral aminocaproic acid every 4 hours (up to 5 g/dose and 30 g/day), for 5 days. Control: Placebo every 4 hours, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit lamp examination, for 6 days and one week after discharge. Defined as a definite increase in the amount of blood in the anterior chamber compared with that noted on the previous day’s examination. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Yes | Study participants assigned to treatment groups using computerized randomization. |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
Yes | Authors used a placebo control and stated that the study was double- masked. |
| Blinding? Personnel and outcome assessors |
Yes | Authors used a placebo control and stated that the study was double- masked. Assignment codes maintained by a central data evaluator who had no clinical contact with any patient. “Physicians caring for study patients did not have access to the cumulative data until the code was broken.” |
| Incomplete outcome data addressed? Primary outcome |
Unclear | One participant was excluded from the aminocaproic acid group due to systemic hypotension attributable to the study drug. It was reported that this patient did not rebleed. |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | One participant was excluded from the aminocaproic acid group due to systemic hypotension attributable to the study drug. Data for this patient was analyzed until time of study withdrawal. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Liu 2002 | ||
| Methods | Study design: Randomized clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Not reported |
|
| Participants | Country: China Dates: December 1997 to December 2000 Number randomized: 92: 60 to aminomethylbenzoic acid group and 32 to the control group. Age: The mean age of the aminomethylbenzoic acid group was 32.7±11.25 years and that of the control group was 33.4±10.75 years. Sex: 75% of the study population were male. Race: Not reported Sickle cell disease: Not reported Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: 0.5 g oral aminomethylbenzoic acid plus 20 mg oral vitamin B1 3 times a day, for 6 days. For children, the dosage of aminomethylbenzoic acid was modified to “follow age-recommended dose”; the vitamin B1 dosage remained the same. Control: 20 mg oral vitamin B1 3 times a day, for 6 days Treatment for both groups included 0.3% ofloxacin eye drops 4 times a day, for 6 days. |
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, details not reported. Secondary outcomes: Risk of complications and adverse events |
|
| Notes | ||
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported. |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
Unclear | The authors do not state whether masking was used. |
| Blinding? Personnel and outcome assessors |
Unclear | The authors do not state whether masking was used. |
| Incomplete outcome data addressed? Primary outcome |
Yes | No exclusions or loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | No exclusions or loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Unclear | Study outcomes of interest not clearly stated. |
| Free of other bias? | Unclear | Poor description of study methods in publication. |
| Marcus 1988 | ||
| Methods | Study design: Randomized clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Not reported |
|
| Participants | Country: Israel Dates: not reported Number randomized: 51: 23 assigned to aspirin group and 28 to observation. Age: Mean age of study population = 20 years Sex: Not reported Race: Not reported Sickle cell disease: Not reported Author stated that participants were balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: 500 mg aspirin 3 times a day for 5 days. Control: observation Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily. Documented by estimating percentage involvement and plotting diagrammatically; definition not reported. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported. |
| Allocation concealment? | Yes | Allocation was concealed from investigators by use of sequentially numbered envelopes. |
| Blinding? Participants |
No | The study participants were not masked to treatment. No placebo was given to the control group. |
| Blinding? Personnel and outcome assessors |
No | The health care providers were not masked to treatment. No placebo was given to the control group. |
| Incomplete outcome data addressed? Primary outcome |
Yes | No exclusions or loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | No exclusions or loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Unclear | Only report results for secondary hemorrhage. |
| Free of other bias? | Unclear | Poor description of study methods and results in publication. |
| McGetrick 1983 | ||
| Methods | Study design: Randomized, double-masked clinical trial Exclusions after randomization: The chart of 1 participant in the placebo group was “lost” was this participant was excluded. Losses to follow-up: None Intention-to-treat: The excluded participant was not included in the analyses and the intention to treat principle was not followed in the analyses. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: August 1980 to February 1982 Number randomized: 50: 28 assigned to aminocaproic acid and 22 to placebo. Age: 86% of the study population was between the ages of 6 and 40 years. Sex: 81% of the study population was male. Race: 69% of the study population was black, 21% Hispanic and 10% white. Sickle cell disease: None; excluded Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Non-penetrating traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment:100 mg/kg oral aminocaproic acid (up to 5 g per dose and 30 g per day) every 4 hours, for 5 days. Control: Placebo every 4 hours, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit lamp examination. Defined as a definite increase in the amount of blood in the anterior chamber following admission. Secondary outcomes:
|
|
| Notes | Funded by the National Eye Institute, National Institutes of Health, Bethesda, Md and Research to Prevent Blindness, Inc. | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Yes | Study participants assigned to treatment groups using computerized randomization. |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
Yes | Authors used a placebo control and stated that the study was double- masked. |
| Blinding? Personnel and outcome assessors |
Yes | Authors used a placebo control and stated that the study was double- masked. Assignment codes were not broken until the study was terminated. |
| Incomplete outcome data addressed? Primary outcome |
Unclear | The chart of 1 participant in the placebo group was “lost” and this participant was excluded. The excluded participant was not included in the analyses and the intention to treat principle was not followed in the analyses. |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | The chart of 1 participant in the placebo group was “lost” and this participant was excluded. The excluded participant was not included in the analyses and the intention to treat principle was not followed in the analyses. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Palmer 1986 | ||
| Methods | Study design: Randomized, double-masked clinical trial Exclusions after randomization: Two participants were excluded: one from the low dose aminocaproic acid group due to need for surgery and one from the usual dose aminocaproic acid group due to severe hypotension. Losses to follow-up: None Intention-to-treat: The intention-to-treat principle was followed only for analyses of adverse events. The 2 excluded participants were not included in the analyses and the intention to treat principle was not followed in the analyses. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: July 1982 to December 1983 Number randomized: 59: 26 assigned to low dose aminocaproic acid and 33 to standard dose aminocaproic acid. Age: The mean age of the low dose aminocaproic acid group was 20 years (range = 4 to 46 years) and for the standard aminocaproic acid group, it was 22.8 years (range = 3 to 50 years). Sex: 23 (88%) of the low dose aminocaproic acid group and 27 (82%) of the standard aminocaproic acid group was male. Race: There were 13 (50%) black, 7 (27%) white, and 5 (19%) Hispanic in the low dose aminocaproic acid group; the race of the excluded participant was not reported. There were 17 (52%) black, 7 (27%) white, and 9 (21%) Hispanic in the standard dose aminocaproic acid group. Sickle cell disease: None; excluded Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema, including both primary and secondary hemorrhages Exclusion criteria:
|
|
| Interventions | Treatment: Low dose (50 mg/kg) oral aminocaproic acid (up to 5 g per dose or 30 g per day) every 4 hours, for 5 days. Control: Standard dose (100 mg/kg) oral aminocaproic acid (up to 5 g per dose or 30 g per day) every 4 hours, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Incidence of secondary hyphema, assessed daily by slit lamp examination. Documented by level in mm and percentage of anterior chamber filled with blood. Defined as a definite increase in the amount of fresh blood in the anterior chamber over level at admission. Secondary outcomes:
|
|
| Notes | Funded by the National Eye Institute, National Institutes of Health, Bethesda, Md, Research to Prevent Blindness, Inc., and Lederle-Cyanamid Laboratories for serum assays. | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Yes | Assignments determined by computerized randomization in the pharmacy. |
| Allocation concealment? | Yes | Allocation was possibly concealed from investigators by pharmacy preparation of drugs. |
| Blinding? Participants |
Yes | Participants masked by preparation of drugs by pharmacy. “The treating physicians and the patients were not told of the admission dose in order to maintain the double-masked status.” |
| Blinding? Personnel and outcome assessors |
Yes | Healthcare providers and outcomes assessors masked by preparation of drugs by pharmacy. “The treating physicians and the patients were not told of the admission dose in order to maintain the double-masked status.” |
| Incomplete outcome data addressed? Primary outcome |
Unclear | Two participants were excluded: one from the low dose aminocaproic acid group due to need for surgery and one from the standard dose aminocaproic acid group due to severe hypotension. The study authors noted that excluding the patient from the full-dose group did not affect the statistical results. |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | Two participants were excluded: one from the low dose aminocaproic acid group due to need for surgery and one from the standard dose aminocaproic acid group due to severe hypotension. The intention to treat principle was followed only for analyses of adverse events. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Pieramici 2003 | ||
| Methods | Study design: Randomized, double-masked, placebo-controlled clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: 124 study participants based on secondary hemorrhage rate of 15% and 3% in placebo and aminocaproic acid treated participants, respectively, with alpha = 0.05, power = 80%, and one-tailed test of significance; study terminated due to slow enrollment. Notes: Multi-center study with 8 centers. |
|
| Participants | Country: USA Dates: Not reported, although study was conducted over 14 months. Number randomized: 51: 24 assigned to aminocaproic acid and 27 to placebo Age: The mean age of the aminocaproic acid group was 24±4 years (range = 4 to 73 years) and for the placebo group, it was 23±3 years (range = 6 to 48 years). Sex: 21 (88%) of the aminocaproic acid group and 23 (85%) of the placebo group was male. Race: There were 15 (63%) white, 8 (33%) black, and 1 (1%) other in the aminocaproic acid group. There were 13 (48%) white, 11 (41%) black, and 3 (11%) other in the placebo group. Sickle cell disease: 2/24 (8%) of participants assigned to topical aminocaproic acid and 1/27 (4%)% of participants assigned to oral aminocaproic acid had sickle cell trait. Participants appeared to be balanced with respect to baseline characteristics except for race and size of primary hyphema with larger hyphemas found in the placebo group. Inclusion criteria: traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: Following 1 drop of 0.05% proparacaine hydrochloride, 30% aminocaproic acid in 2% gel instilled in inferior fornix every 6 hours, for 5 days. Control: Following 1 drop of 0.05% proparacaine hydrochloride, placebo gel instilled in inferior fornix every 6 hours, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit lamp examination for 7 days; defined as increase in height of hyphema of at least 0.5 mm above darker blood, colour change of blood of at least 0.5 mm, obvious new “trickle” of blood on iris, or reappearance of blood after resolution. Secondary outcomes:
|
|
| Notes | Funded by Orphan Medical Inc., Covance Inc, National Eye Institute, National Institutes of health, Bethesda, Md, and Research to Prevent Blinding | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Yes | Study participants assigned to treatment groups using computerized randomization. |
| Allocation concealment? | Yes | Allocation was concealed from investigators in that treatment assignments were based on a trial number obtained from a contract research organization. |
| Blinding? Participants |
Yes | Authors used a placebo control and stated that the study was double- masked. “The investigators and patients were masked to the treatment arm.” |
| Blinding? Personnel and outcome assessors |
Yes | Authors used a placebo control and stated that the study was double- masked. “The investigators and patients were masked to the treatment arm.” |
| Incomplete outcome data addressed? Primary outcome |
Yes | No exclusions or loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | No exclusions or loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Unclear | “There were a number of protocol violations noted in both study groups.” “During the course of the study, only 8 of the original 13 sites enrolled patients, and at 14 months a total of 51 patients were enrolled overall. The study was terminated at this point by Orphan Medical, the manufacturer, against the advice of the principal investigators, because of slow enrollment.” |
| Rahmani 1999 | ||
| Methods | Study design: Randomized, placebo-controlled clinical trial Exclusions after randomization: 6; 2 participants in the tranexamic acid group, 3 in the prednisone group, and 1 in the placebo group left the hospital before the end of the study and were excluded. Losses to follow-up: none Intention-to-treat: The excluded participants were not included in the analyses and the intention-to-treat principle was not followed in the analyses. Sample size calculations: Not reported |
|
| Participants | Country: Iran Dates: January 1991 to May 1992 Number randomized: 244: 82 assigned to tranexamic acid, 81 assigned to prednisone, and 81 assigned to placebo. Age: Median age in tranexamic acid group was 11 years (range = 1 to 65 years); in the prednisone group, it was 11.5 years (range = 1 to 50 years), and in the placebo group, it was 12 years (range = 1 to 58 years). Sex: 63 (79%) of the tranexamic acid group, 58 (73%) of the prednisone group, and 66 (82%) of the placebo group were male. Race: All study participants were white. Sickle cell disease: Not reported, but all white study population. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment 1: 75 mg/kg oral tranexamic acid per day, divided into 3 doses per day, for 5 days. Treatment 2: 0.75 mg/kg oral prednisolone per day, divided into 2 doses per day, for 5 days. Control: Placebo administered 3 times per day. Treatment for all groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit lamp examination for 5 days. Defined as definite increase in size of level of blood or appearance of fresh blood over darker clotted blood in the anterior chamber. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Yes | Randomization was based on a randomization list. |
| Allocation concealment? | Unclear | Study participants assigned to treatment groups using a randomization list, but not clear whether list was revealed before allocation to individuals enrolling study participants. |
| Blinding? Participants |
Unclear | Participants partially masked in that authors used a placebo control for the tranexamic acid, but not for prednisone. |
| Blinding? Personnel and outcome assessors |
Yes | Healthcare providers partially masked in that authors used a placebo control for the tranexamic acid, but not for prednisone; however, ophthalmologists and outcome assessors were masked. |
| Incomplete outcome data addressed? Primary outcome |
Unclear | Six patients were excluded from the study: 2 participants in the tranexamic acid group, 3 in the prednisone group, and 1 in the placebo group left the hospital before the end of the study and were excluded. The excluded participants were not included in the analyses and the intention to treat principle was not followed in the analyses. |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | Six patients were excluded from the study: 2 participants in the tranexamic acid group, 3 in the prednisone group, and 1 in the placebo group left the hospital before the end of the study and were excluded. The excluded participants were not included in the analyses and the intention to treat principle was not followed in the analyses. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Rakusin 1972 | ||
| Methods | Study design: Quasi-randomized controlled series Exclusions after allocation: 59 patients in the series with large hyphemas underwent surgery and were not included in the analysis. Losses to follow-up: 20 patients were lost to follow-up. Intention-to-treat: All participants were not accounted for in the final analyses, thus intention-to-treat analysis was not followed. Sample size calculations: Not reported |
|
| Participants | Country: South Africa Dates: 1966 to 1969 Number allocated: 390 consecutive patients Age: Not reported Sex: Not reported Sickle cell disease: Not reported Race: 90% African origin and 10% Asiatic origin. Inclusion criteria: Traumatic hyphema Exclusion criteria: Surgical treatment indicated |
|
| Interventions | Series of comparisons based on 6 variable factors:
Excluding the variable factor for each series, all patients received bed rest, single pad over the injured eye, and topical chloramphenicol or chloromycetin. |
|
| Outcomes | Primary outcomes:
|
|
| Notes | Funded by the University of Witwatersrand, the South African Medical Research Council, Leo Laboratories, Mer-National, and Warner Pharmaceutical Co. In the third comparison group, antibiotics versus corticosteroids, 3 patient were assigned to receive neither treatment, but this group was discontinued after all 3 patients developed a mucous conjunctival discharge. |
|
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | No | Method of allocation unclear, not all patients in the series were allocated to the 6 comparisons under study; 59 patients were selected for surgery. Also even and odd patient number allocation is not applicable to comparison with three treatment groups. |
| Allocation concealment? | No | Method of allocation concealment not reported, not randomized. |
| Blinding? Participants |
No | Masking of patients was not possible for some variables (i.e., bed rest and eye patching). Use of placebo for other variables was not mentioned. |
| Blinding? Personnel and outcome assessors |
Unclear | Masking was not reported. |
| Incomplete outcome data addressed? Primary outcome |
Unclear | 79 participants were not included in the analyses and the intention to treat principle was not followed. |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | 79 participants were not included in the analyses and the intention to treat principle was not followed. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Unclear | The primary interventions of interest for this study are not clear. Although the majority of the patients in the series were assigned to one of six conservative treatment comparison groups, 59 recruited patients were selected for surgery. |
| Read 1974 | ||
| Methods | Study design: Quasi-randomized controlled series Exclusions after allocation: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the groups to which they were assigned. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: February 1970 to July 1972 Number allocated: 137 consecutive patients Age: Mean 15.9 years Sex: 108 men and 29 women; 78% male Race: 101 (74%) African-American Sickle cell disease: Not reported Participants were similar in regards to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Medical treatment #1 (n = 66): Bed rest with elevation of head to 30 degrees, bilateral ocular patches and shield over injured eye, and sedation. Medical treatment #2 (n = 71): Moderate ambulatory activity in the hospital, patching and shielding of the traumatized eye only, and no sedation. Eye drops were not administered in either medical treatment regimen. On day 5 patients with remaining major primary or secondary hyphemas (n = 16) were alternately assigned to continue with medical treatment or to receive surgical intervention (ab externo corneal section with clot expression). |
|
| Outcomes | Primary outcome: Not reported Secondary outcomes:
|
|
| Notes | Funded by a grant from the Research to Prevent Blindness, Inc. | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | No | Allocation was not randomized; alternately assigned patients to treatment groups at time of admission. Imbalance in number assigned to each group (66 versus 71) makes it appear alternation was not systematic. |
| Allocation concealment? | No | Allocation was assigned on an alternate basis. |
| Blinding? Participants |
No | Masking of patients was not possible with the interventions being studied. |
| Blinding? Personnel and outcome assessors |
No | All patients were treated by the primary investigator in order to standardize therapy and record results as accurately as possible. |
| Incomplete outcome data addressed? Primary outcome |
Yes | All participants were analyzed in the group to which they were assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | All participants were analyzed in the group to which they were assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | No | A subset of patients with major hyphema on day 5 were alternately allocated to either continue with medical treatment as originally assigned or undergo surgical intervention. Thus the patients that had surgery were censored on day 5 from their medical treatment outcomes. |
| Spaeth 1966 | ||
| Methods | Study design: Randomized, double-masked, placebo-controlled clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: not reported |
|
| Participants | Country: USA Dates: 1963 to 1964 Number randomized: 85: 39 assigned to estrogen and 46 to placebo. Age: Mean age in the estrogen group = 16.2 years (range 2 to 62 years), and in the placebo group, it was 18.9 years (range 0.5 to 65 years). Sex: 80% of the estrogen group and 85% of the placebo group was male. Race: 72% of the estrogen group and 70% of the placebo group was black; remaining study participants were white. Sickle cell disease: Not reported Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: Conjugated estrogen, 5 mg intramuscularly for children < 5 years; 10 mg for children 5 or older but < 10; and 20 mg intravenously for children 10 or older and adults, for 5 days. Control: Placebo, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by “complete ocular examination” for 5 days. Documentation and definition not reported. Secondary outcomes:
|
|
| Notes | Placebo and conjugated estrogen supplied by Ayerst Laboratory | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported |
| Allocation concealment? | Yes | Allocation was concealed from investigators by use of coded bottles. |
| Blinding? Participants |
Yes | Authors used coded bottles to mask participants. “Neither the person administering nor the patient receiving the medications knew whether estrogen or placebo was being given.” |
| Blinding? Personnel and outcome assessors |
Yes | Authors used coded bottles to mask healthcare providers and outcomes assessors. “Neither the person administering nor the patient receiving the medications knew whether estrogen or placebo was being given.” |
| Incomplete outcome data addressed? Primary outcome |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Spoor 1980 | ||
| Methods | Study design: Randomized, double-masked, placebo-controlled clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Not reported |
|
| Participants | Country: USA Dates: September 1975 to December 1977 Number randomized: 43: 23 assigned to prednisone, and 20 to placebo. Age: The mean age of the prednisone group was 20.1 (range = 5 to 61) and that of the placebo group was 21.2 (range 9 to 51). Sex: 16 (70%) of the prednisone group and 16 (80%) of the placebo group were male. Race: There were 14 (61%) white, 6 (26%) Hispanic, and 3 (13%) black in the prednisone group. There were 11 (55%) white, 7 (35%) Hispanic, and 2 (10%) black in the placebo group. Sickle cell disease: Not reported Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: Oral prednisone, 40 mg/day for adults and children > 10 years; 15 mg/day for children between 4 and 10 years; and 10 mg/day for children between 18 months up to 4 years, for 7 days. Control: Lactose placebo capsules administered daily for 7 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily for 7 days, using slit lamp examination, documented by drawings or photography. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported. |
| Allocation concealment? | Yes | Allocation was concealed from investigators by use of encoded capsules prepared by pharmacy. |
| Blinding? Participants |
Yes | Participants by use of encoded capsules prepared by pharmacy. “Neither the doctor nor the patient knew which capsule the patient was receiving until the conclusion of the course of treatment and follow-up.” |
| Blinding? Personnel and outcome assessors |
Yes | Healthcare providers and outcomes assessors by use of encoded capsules prepared by pharmacy. “Neither the doctor nor the patient knew which capsule the patient was receiving until the conclusion of the course of treatment and follow-up.” |
| Incomplete outcome data addressed? Primary outcome |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Sukumaran 1988 | ||
| Methods | Study design: Quasi-randomized controlled series Exclusions after allocation: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were assigned. Sample size calculations: Not reported |
|
| Participants | Country: Malaysia Dates: Not reported Number allocated: 35 consecutive patients Age: 80% below 30 years old Sex: 35 men Race: Not reported Sickle cell disease: Not reported Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment (n =17): 25 mg/kg oral tranexamic acid (cyklokapron) divided into 3 doses for 7 days in addition to routine treatment. Control (n = 18): Routine treatment Routine treatment for both groups included:
|
|
| Outcomes | Primary outcomes:
|
|
| Notes | Funding source not reported | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | No | Method of allocation unclear, not randomized. |
| Allocation concealment? | No | Method of allocation concealment not reported, not randomized. |
| Blinding? Participants |
No | No placebo was used for the control group. |
| Blinding? Personnel and outcome assessors |
Unclear | Masking was not reported. |
| Incomplete outcome data addressed? Primary outcome |
Yes | All participants were analyzed in the group to which they were assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | All participants were analyzed in the group to which they were assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Teboul 1995 | ||
| Methods | Study design: Randomized, double-masked, placebo-controlled clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Authors reported that sample sizes were not calculated because the rate of secondary hemorrhage in children was unknown and that of other populations was too variable to estimate. |
|
| Participants | Country: Canada Dates: November 1987 to February 1994 Number randomized: 94: 48 assigned to aminocaproic acid and 46 to placebo. Age: The mean age of the aminocaproic acid group was 8.2 years, while that of the placebo group was 10.6 years. Sex: 42 (88%) of the aminocaproic acid group, and 39 (85%) of the placebo group were male. Race: 43 (90%) of the aminocaproic acid group, and 42 (91%) of the placebo group was white. Sickle cell disease: None; excluded Participants appeared to be balanced with respect to baseline characteristics, except for mean age where the aminocaproic acid group was younger (8.2 to 10.6 years). Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: 100 mg/kg oral aminocaproic acid every 4 hours (up to 30 g per day), for 5 days. Control: Placebo every 4 hours. for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed by daily slit lamp examination for 5 days; documented by drawing of hyphema with distinction between fresh and clotted blood. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported |
| Allocation concealment? | Yes | Allocation was concealed from investigators by preparation of drugs by pharmacy; statement that investigators were unaware of next treatment assignment. |
| Blinding? Participants |
Yes | Participants by use of medications prepared by pharmacy. |
| Blinding? Personnel and outcome assessors |
Yes | Healthcare providers and outcomes assessors by use of medications prepared by pharmacy. “The double-blind code was not broken until completion of the study.” |
| Incomplete outcome data addressed? Primary outcome |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | “The authors have no proprietary interest in aminocaproic acid or any competing drug.” |
| Vangsted 1983 | ||
| Methods | Study design: Randomized clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Not reported |
|
| Participants | Country: Sweden Dates: November 1978 to May 1981 Number randomized: 112: 59 assigned to tranexamic acid, and 53 to bed rest. Age: The mean age of the tranexamic acid group was 23.5 years (range = 9 to 60), and that of the bed rest group was 23.5 years (range 9 to 67 years). Sex: The ratio of male: female of the study population was 4:1. Race: Not reported. Sickle cell disease: Not reported Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema Exclusion criteria:
|
|
| Interventions | Treatment: 25 mg/kg oral tranexamic acid 3 times a day, for 7 days. Control: Complete bed rest, for 6 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit lamp examination at days 2 and 7. Documentation and definition not reported. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
No | Participants were not masked to treatment assignment (bed rest compared with tranexamic acid). |
| Blinding? Personnel and outcome assessors |
No | Healthcare providers and outcome assessors were not masked to treatment assignment (bed rest compared with tranexamic acid). |
| Incomplete outcome data addressed? Primary outcome |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Varnek 1980 | ||
| Methods | Study design: Quasi-randomized controlled series Exclusions after allocation: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were assigned. Sample size calculations: Not reported |
|
| Participants | Country: Denmark Dates: March 1978 to November 1979 Number allocated: 232 consecutive patients from 4 study centers Age: Mean 24.4 years Sex: 188 men and 44 women; 81% male Race: All were white Sickle cell disease: Not reported, but all white study population. Inclusion criteria:
|
|
| Interventions | Treatment (n =102): 25 mg/kg oral tranexamic acid divided into 3 doses for 6 days. Control (n = 130): Conservative treatment Treatment for both groups included:
|
|
| Outcomes | Primary outcomes:
|
|
| Notes | Funding source not reported Method used to calculate mean visual acuity not reported |
|
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | No | Allocation was not randomized; assigned patients to treatment groups based on date of admission. |
| Allocation concealment? | No | Method of allocation based on even versus odd admission dates. |
| Blinding? Participants |
No | No placebo was used for the control group. |
| Blinding? Personnel and outcome assessors |
No | Masking was not done because of the noticeable delay in resolution time between treatment groups. Tranexamic acid was considered to induce persistence of the primary clot a priori. |
| Incomplete outcome data addressed? Primary outcome |
Yes | All participants were analyzed in the group to which they were assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | All participants were analyzed in the group to which they were assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Welsh 1983 | ||
| Methods | Study design: Randomized, double-masked, placebo-controlled clinical trial Exclusions after randomization: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were randomly assigned. Sample size calculations: Not reported |
|
| Participants | Country: South Africa Dates: Not reported Number randomized: 39: 19 assigned to tranexamic acid, and 20 to placebo. Age: The mean age of the tranexamic acid group was 25.2 years (range = 15 to 38), and that of the placebo group was 25.2 years (range 14 to 52). Sex: 15 (79%) of the tranexamic acid group, and 17 (85%) of the placebo group were male. Race: All study participants were black. Sickle cell disease: Not reported Participants appeared to be balanced with respect to baseline characteristics. Three of 39 patients had a hyphema due to cataract surgery; 2 in the in the tranexamic group and one in the control group. Inclusion criteria: Hyphema; either non-perforated, or if perforated, then the wound was sutured and treated as closed injury. Exclusion criteria:
|
|
| Interventions | Treatment: 3 500 mg tablets of oral tranexamic acid 3 times a day for 7 days, for an overall total of 31.5 g tranexamic acid. Control: 3 tablets of placebo 3 times a day for 7 days Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by visual examination. Documentation and definition not reported. Secondary outcomes:
|
|
| Notes | Tranexamic acid and placebo supplied by Adcock Ingram Laboratories. | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported |
| Allocation concealment? | Yes | Allocation was concealed from investigators by preparation of drugs by pharmacy; statement that investigators were unaware of next treatment assignment. |
| Blinding? Participants |
Yes | Participants by use of medications prepared by pharmacy. “Neither patient nor staff knew which tablet the patient was receiving and the code was broken by the pharmaceutical firm at the end of the trial.” |
| Blinding? Personnel and outcome assessors |
Yes | Healthcare providers and outcomes assessors by use of medications prepared by pharmacy. “Neither patient nor staff knew which tablet the patient was receiving and the code was broken by the pharmaceutical firm at the end of the trial.” |
| Incomplete outcome data addressed? Primary outcome |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | There were no exclusions and no loss to follow-up. All participants were analyzed in the group to which they were randomly assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Unclear | Cyklokapron and placebo tablets were supplied by Adcock Ingram Laboratories. |
| Zetterstrom 1969 | ||
| Methods | Study design: Quasi-randomized controlled series Exclusions after allocation: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were assigned Sample size calculations: Not reported |
|
| Participants | Country: Sweden Dates: September 1967 to September 1968 Number allocated: 117 consecutive patients Age: Mean was 22.0 years (range 5 to 57) Sex: 102 men and 17 women (as reported); 86% male Race: Not reported Sickle cell disease: Not reported Inclusion criteria: Traumatic hyphema Exclusion criteria: Perforation of the eyeball |
|
| Interventions | Treatment (n = 58): Topical atropine with Decadron (cortisone) eye drops five times daily and moderate ambulatory activity within hospital. Control (n = 59): Conservative treatment of complete bed rest without pinhole glasses or simultaneous local therapy. Treatment for both groups included in-patient care until visual acuity in the injured eye was satisfactory, the hyphema was absorbed, and intraocular pressure did not deviate from normal. |
|
| Outcomes | Primary outcomes:
|
|
| Notes | Funding source not reported Method used to calculate mean visual acuity not reported |
|
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | No | Allocation was not randomized; alternately assigned patients to treatment groups based on order of admission. |
| Allocation concealment? | No | Method of allocation based on order of admission. |
| Blinding? Participants |
No | Masking of patients was not possible with the interventions being studied. |
| Blinding? Personnel and outcome assessors |
Unclear | Masking was not reported, but unlikely because of the types of interventions being studied. |
| Incomplete outcome data addressed? Primary outcome |
Yes | All participants were analyzed in the group to which they were assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | All participants were analyzed in the group to which they were assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
| Zi 1999 | ||
| Methods | Study design: Randomized controlled series Exclusions after allocation: None Losses to follow-up: None Intention-to-treat: All participants were analyzed in the group to which they were assigned. Sample size calculations: Not reported |
|
| Participants | Country: China Dates: September 1990 to 1997 Number randomized: 79 patients Age: Mean was 24.5 years (range 7 to 43) Sex: 70 men and 4 women (as reported); 95% male Race: Not reported Sickle cell disease: Not reported Inclusion criteria: Hyphema Exclusion criteria: Not reported |
|
| Interventions | Treatment (n = 39): Alternatively right and left lateral position. Control (n = 35): Semi-reclined position |
|
| Outcomes | Primary outcomes: time to resolution by severity. Secondary outcomes:
|
|
| Notes | Funding source not reported | |
| Risk of bias table | ||
| Item | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear | Randomized, but method of allocation not reported |
| Allocation concealment? | Unclear | Method of allocation concealment not reported. |
| Blinding? Participants |
No | Participants were not masked to treatment assignment (lying either semireclining or on side) |
| Blinding? Personnel and outcome assessors |
No | Healthcare providers and outcome assessors were not masked. |
| Incomplete outcome data addressed? Primary outcome |
Yes | All participants were analyzed in the group to which they were assigned. |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | All participants were analyzed in the group to which they were assigned. |
| Free of selective reporting? | Yes | Reported results for primary and secondary outcomes. |
| Free of other bias? | Yes | |
g: gram
IOP: intraocular pressure
kg: kilogram
l: liter
mg: milligram
micromol: micromole
mL: microliter
mm: millimeter
mmHg: millimeters of mercury
n: number of participants
Data extraction and management
Two authors independently extracted the data for the primary and secondary outcomes onto data collection forms developed by the Cochrane Eyes and Vision Group. We resolved discrepancies by discussion. We attempted to contact primary investigators for missing data, but were unable to. One author entered all data into Review Manager 5 (RevMan) and a second author verified all values.
Assessment of risk of bias in included studies
Two authors assessed the sources of systematic bias in trials according to methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). The following parameters were considered: adequate sequence generation and allocation concealment (selection bias), masking of participants and researchers (performance bias), masking of outcome assessors (detection bias), adequate handling of incomplete data by reporting rates of follow-up and using intention-to-treat analysis (attrition bias), and complete reporting of outcomes (reporting bias). Each of the parameters was graded as yes (low risk of bias), unclear risk of bias, or no (high risk of bias). We documented agreement between authors. We resolved disagreements by consensus, or by a third author. We used masking of participants and care providers as a quality criterion only in interventions where masking was feasible. We contacted authors of trials categorized as ‘unclear risk of bias’ for additional information when contact information for the trial authors could be found. If the study authors did not respond or we were unable to contact the authors, we assigned a grade based on the available information.
Measures of treatment effect
Dichotomous data
For dichotomous outcomes we calculated summary odds ratios with 95% confidence intervals (CIs). We analyzed VA outcomes as dichotomous variables. For each follow-up period with sufficient data, we compared the proportion of patients with VA between 20/20 and 20/40 between treatment and control groups. We analyzed data on the proportion of patients with secondary hemorrhage, corneal bloodstain, peripheral anterior synechiae formation, glaucoma development and optic atrophy development as dichotomous data.
Continuous data
We calculated weighted mean differences for continuous outcomes. We analyzed the time to resolution of primary hemorrhage (hyphema), defined as the length of time from onset to resolution, as a continuous variable. We also analyzed the length of time to rebleed, the duration of hospitalization, and other quality of life and economic outcomes as continuous data.
Ordinal data
We summarized ordinal data qualitatively.
Counts and rate data
We summarized counts and rate data in rate ratios when the event was rare, and as continuous outcome data when the event was more common. We analyzed adverse events data as counts and rates.
Unit of analysis issues
The unit of analysis for this review was the affected eye or eyes of the individual participant.
Dealing with missing data
We contacted authors of included studies to obtain additional data when contact information for the trial authors could be found. When additional data could not be retrieved due to non-response from the authors or because we were unable to contact the authors, we imputed data from what was available in the study report. We reported loss to follow-up for each study when available. We also noted when intention-to-treat analyses were performed.
Assessment of heterogeneity
We tested for statistical heterogeneity using the I2 statistic and examined clinical heterogeneity using forest plots. We considered I2 values greater than 40% to represent statistical heterogeneity between studies.
Assessment of reporting biases
We used funnel plots to assess the possibility of reporting biases when more than three studies were included in a meta-analysis.
Data synthesis
Data analysis followed the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2008). We tested for statistical heterogeneity. When it was not detected and there was no clinical heterogeneity within the trials, we combined the results in a meta-analysis using a random-effects model. We used a fixed-effect model if the number of trials was three or fewer. In cases of statistical or clinical heterogeneity we did not combine study results but presented a tabulated summary.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses according to age, race, presence of sickle cell trait/disease, presenting IOP, and severity of hyphema were planned, but not performed because sufficient numbers of trials were not available. We presented results by subgroup as an additional table.
Sensitivity analysis
We conducted sensitivity analyses to determine the impact of excluding studies of lower methodological quality, unpublished studies, and industry-funded studies.
Results
Description of studies
Results of the search
The electronic literature searches conducted in June 2010 identified 836 potentially relevant references for this review. After duplicate review of the titles and abstracts, we classified 748 references as definitely exclude, 23 as definitely include, and 65 as unsure. Seventeen of the 65 references assessed as unsure were letters or editorials that did not report original data and were excluded. We obtained full-text copies of the 48 remaining references classified as unsure and reviewed them in duplicate. Of those, we excluded 40 and included eight.
A manual search of other resources, including reference lists of included studies and citation index databases, yielded four additional potentially relevant full-text references for this review. Of those four references, we included two and excluded two from this review.
In total, there were 26 studies included as reported in 33 publications and 41 studies excluded that were in 42 publications.
Included studies
The 26 studies included in this review are described in the ‘Characteristics of included studies’ table. Nineteen of the included studies were randomized controlled trials (RCTs), and seven used a quasi-randomized method to assign participants to treatment groups. The review outcomes reported by the included studies are listed in Table 1.
Table 1.
Summary of outcomes* reported by intervention
| Interventions | Primary outcomes | Secondary outcomes | Adverse effects | Duration of hospitalization or quality of life outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visual acuity (VA) | Time to resolution of primary hemorrhage | Secondary hemorrhage | Risk of corneal bloodstaining | Risk of PAS formation | Risk of pathological increase in IOP or glaucoma | Risk of optic atrophy | ||||
| Risk of rebleed | Time to rebleed | |||||||||
| Aminocaproic acid versus placebo | ||||||||||
| Oral aminocaproic acid | ||||||||||
| Christianson 1979 | Not reported | Partially reported** | Risk of rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Crouch 1976 | Long-term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal bloodstaining reported | Partially reported** | Not reported | Risk of optic atrophy reported | Not reported | Not reported |
| Kraft 1987 | Long-term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Persistent increases in IOP reported | Not reported | Adverse effects reported | Not reported |
| Kutner 1987 | Short-term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Persistent increases in IOP reported | Not reported | Adverse effects reported | Not reported |
| McGetrick 1983 | Final VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Not reported | Not reported | Adverse effects reported | Partially reported** |
| Teboul 1995 | Final VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Not reported | Duration of hospitalization reported |
| Topical aminocaproic acid | ||||||||||
| Karkhaneh 2003 | Reported as NS | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Reported as NS | Not reported | Not reported | Not reported |
| Pieramici 2003 | Short-term VA reported | Reported as NS | Risk of rebleed reported | Time to rebleed reported | Not reported | increases in Not reported | Transient Not IOP reported | reported | Adverse effects reported | Not reported |
| Low versus standard dose aminocaproic acid | ||||||||||
| Palmer 1986 | Final VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Duration of hospitalization reported |
| Oral versus topical aminocaproic acid | ||||||||||
| Crouch 1997 | Final VA reported | Not reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal bloodstaining reported | Partially reported** | Not reported | Risk of optic atrophy reported | Adverse effects reported | Not reported |
| Tranexamic acid versus control | ||||||||||
| Rahmani 1999 | Short-term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Duration of hospitalization reported |
| Sukumaran 1988 | Short-term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Vangsted 1983 | Short-term VA reported | Partially reported** | Risk of rebleed reported | No rebleeds occurred | Risk of corneal bloodstaining reported | Not reported | Transient increases in IOP reported | Not reported | Not reported | Duration of hospitalization and days off work reported |
| Varnek 1980 | Partially reported** | Not reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal bloodstaining reported | Not reported | Transient increases in IOP reported | Risk of optic atrophy reported | Not reported | Duration of hospitalization reported |
| Welsh 1983 | Not reported | Partially reported** | Risk of rebleed reported | Not reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Not reported |
| Aminomethylbenzoic acid versus placebo | ||||||||||
| Liu 2002 | Not reported | Not reported | Risk of rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Adverse effects reported | Not reported |
| Corticosteroids versus control | ||||||||||
| Oral corticosteroids | ||||||||||
| Rahmani 1999 | Short-term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Duration of hospitalization reported |
| Spoor 1980 | Final VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal bloodstaining reported | Risk of PAS formation reported | Transient increases in IOP reported | Not reported | Not reported | Not reported |
| Topical corticosteroids | ||||||||||
| Rakusin 1972 | Short-term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Partially reported** | Partially reported** | Not reported | Not reported | Not reported | Not reported |
| Zetterstrom 1969 | Short-term VA reported | Not reported | Risk of rebleed reported | Not reported | Risk of corneal bloodstaining reported | Not reported | Transient increases in IOP reported | Risk of optic atrophy reported | Not reported | Duration of hospitalization reported |
| Oral aminocaproic acid versus oral prednisone | ||||||||||
| Farber 1991 | Short-term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Not reported | Not reported | Reported as NS | Not reported | Not reported | Not reported |
| Conjugated estrogen versus placebo | ||||||||||
| Spaeth 1966 | Partially reported** | Not reported | Risk of rebleed reported | Partially reported** | Risk of corneal bloodstaining reported | Partially reported** | Partially reported** | Not reported | Not reported | Not reported |
| Cycloplegics versus miotics | ||||||||||
| Bedrossian 1974 | Not reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Rakusin 1972 | Short-term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Reported as NS | Reported as NS | Not reported | Not reported | Not reported | Not reported |
| Aspirin versus observation | ||||||||||
| Marcus 1988 | Not reported | Not reported | Risk of rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Monocular versus binocular patching | ||||||||||
| Edwards 1973 | Final VA reported | Not reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal bloodstaining reported | Not reported | Risk of secondary glaucoma reported | Not reported | Not reported | Quality of life outcomes reported |
| Rakusin 1972 | Short-term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Reported as NS | Reported as NS | Not reported | Not reported | Not reported | Not reported |
| Ambulatory versus conservative treatment | ||||||||||
| Rakusin 1972 | Short-term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Reported as NS | Reported as NS | Not reported | Not reported | Not reported | Not reported |
| Read 1974 | Partially reported | Days to resolution reported | Risk of rebleed reported | Partially reported** | Risk of corneal bloodstaining reported | Not reported | Transient increases in IOP reported | Not reported | Not reported | Not reported |
| Elevation of the head versus control | ||||||||||
| Zi 1999 | Not reported | Days to resolution reported | Not reported | Not reported | Not reported | Not reported | Risk of secondary glaucoma reported | Not reported | Not reported | Not reported |
See Types of outcome measures for detailed descriptions of outcomes
Noted as “partially reported” if some information was reported, but it was insufficient for quantitative data analyses
IOP: intraocular pressure
NS: not significant
PAS: peripheral anterior synechiae
All but two of the studies restricted entry to patients with primary traumatic hyphema; Welsh 1971 also included patients with perforated globes that had been sutured and were treated as closed globe injuries, and Palmer 1986 also included some patients with secondary hemorrhage. Most studies included all age groups, although some studies excluded very young children (e.g., less than four or seven years) (Farber 1991; Kutner 1987; Marcus 1988; Pieramici 2003; Vangsted 1983; Welsh 1983), and one study included children only (Kraft 1987). Of studies reporting demographic data, the mean age of study participants ranged from 10 to 32 years, and the proportion of male study participants ranged from 67% to 100%. Studies took place in a number of different countries: two each in Iran, Sweden, China, and South Africa, one each in Denmark, Israel and Malaysia, and the remainder in Canada and the United States. The race of study participants varied by country but many studies reported a high proportion of black study participants, or participants from another minority race or ethnicity.
Three types of antifibrinolytic agents (epsilon-aminocaproic acid (aminocaproic acid), tranexamic acid, and aminomethylbenzoic acid) were investigated in the included studies. Other types of pharmaceuticals investigated by the studies included in this review were corticosteroids; including prednisone, prednisolone, hydrocortisone, and cortisone; conjugated estrogen; aspirin; and topical mydriatics and miotics. Non-pharmaceutical interventions included the use of monocular or binocular patching, eye shields, bed rest, and elevation of the head. The primary outcome for all but two studies was the risk of a secondary hemorrhage.
Aminocaproic acid
Eight studies investigated the use of aminocaproic acid compared with placebo in treating traumatic hyphema: six studies prescribed oral aminocaproic acid (Christianson 1979; Crouch 1976; Kraft 1987; Kutner 1987; McGetrick 1983; Teboul 1995), and two studies prescribed topical aminocaproic acid (Karkhaneh 2003; Pieramici 2003). The dosage of oral aminocaproic acid used in five studies was 100 mg/kg of body weight every four hours for five days (Crouch 1976; Kraft 1987; Kutner 1987 ; McGetrick 1983; Teboul 1995), and the remaining study used a loading dose of 75 mg/kg of body weight, then doses of 60 mg/kg of body weight every four hours, although the length of treatment was not reported (Christianson 1979). In total, the six studies included 331 participants (34 to 94 participants per study); 175 participants were randomized to receive oral aminocaproic acid, and 156 participants were randomized to receive placebo pills. The follow-up periods ranged from the length of hospitalization (typically about one to two weeks) to 3.4 years after discharge.
Two studies evaluated topical aminocaproic acid and included a total of 206 participants. Karkhaneh 2003 had three treatment groups: 45 participants were randomized to receive aminocaproic acid (two drops of 25% aminocaproic acid in 2% carboxymethylene gel applied to the inferior fornix of the affected eye every six hours for five days) plus homatropine eye drops three times per day; 44 participants were randomized to receive placebo gel plus homatropine eye drops; and 66 participants were randomized to receive homatropine eye drops only. The follow-up period for this study was 14 days. In Pieramici 2003, 24 participants were randomized to receive aminocaproic acid (30% aminocaproic acid in 2% gel instilled in the inferior fornix following one drop of 0.05% proparacaine hydrochloride every six hours for five days), and 27 participants were randomized to receive placebo gel applied in the same manner as the intervention group. Participants in this study were managed on an outpatient or inpatient basis and followed for seven days.
One included study compared oral aminocaproic acid with topical aminocaproic acid for the treatment of traumatic hyphema (Crouch 1997). Of 118 participants eligible for inclusion in the study, 64 participants agreed to be randomized to receive either topical aminocaproic acid (0.2 ml of 30% aminocaproic acid in 2% carboxymethylene gel applied to the inferior fornix every six hours plus oral placebo solution every four hours for five days) or oral aminocaproic acid (50 mg/kg of body weight of oral aminocaproic acid, up to 30 g/day, plus placebo gel every four hours for five days). The 54 participants who declined study entry were followed as an untreated control group. The participants in this study were hospitalized and followed for five days.
The last study investigating the use of aminocaproic acid compared low dose oral aminocaproic acid (50 mg/kg, up to 5 g per dose or 30 g per day every four hours for five days) with the standard dose oral aminocaproic acid (100 mg/kg, up to 5 g per dose or 30 g per day every four hours for five days) for the treatment of traumatic hyphema (Palmer 1986). The participants in this study, 26 in the low dose group and 33 in the standard dose group, were followed for the duration of hospitalization.
Tranexamic acid
Five studies investigated the use of oral tranexamic acid compared with a control in treating traumatic hyphema (Rahmani 1999; Sukumaran 1988; Vangsted 1983; Varnek 1980; Welsh 1983). In total, there were 578 participants included in the studies; 277 were assigned to tranexamic acid and 301 to a control intervention. The doses of tranexamic acid administered in these studies varied from 1.75 mg/kg per day for five days to 1.5 g/day for seven days. Participants were followed for five to 12 days. The study using the lowest dose of tranexamic acid assigned 82 participants to 1.75 mg/kg oral tranexamic acid daily for five days, 81 to 0.75 mg/kg prednisone daily for five days, and 81 to daily placebo for five days. All participants were followed for five days (Rahmani 1999). In two studies, participants were assigned to 25 mg/kg tranexamic acid per day for seven days (Sukumaran 1988; Vangsted 1983). In Sukumaran 1988, both the group receiving tranexamic acid (n = 17) and the control group (n = 18) received bilateral patching, bed rest, sedation, analgesics and topical steroid drops from day three through day seven. Both groups were followed for one week. In Vangsted 1983, 59 participants were randomized to receive tranexamic acid, and 53 participants were randomized to receive complete bed rest for six days; follow-up was seven days. Varnek 1980 compared the same dose of tranexamic acid, 25 mg/kg daily for seven days along with hospitalization and bed rest (n = 102), with hospitalization and bed rest alone in the control group (n = 130). Participants were followed for 12 days. In Welsh 1983, 19 participants were randomized to receive the largest dose of tranexamic acid, three 500 mg tablets of oral tranexamic acid three times a day for seven days (for an overall total dose of 31.5 g tranexamic acid), and 20 participants were randomized to receive three tablets of placebo three times a day for seven days.
Aminomethylbenzoic acid
One included study compared oral aminomethylbenzoic acid with placebo for the treatment of traumatic hyphema (Liu 2002). The study, published in Chinese, randomized 60 participants to the intervention group and 32 participants to the placebo group. Participants in the intervention group received 0.5 g oral aminomethylbenzoic acid plus 20 mg oral vitamin B1 three times a day for six days. For children, the dosage of aminomethylbenzoic acid was modified to “follow age-recommended dose”; the vitamin B1 dosage remained the same. Participants in the control group received oral vitamin B1 (20 mg) three times a day for six days. The follow-up period for the study was one week post blood resolution.
Corticosteroids
Four studies examined the use of corticosteroids, two using an oral preparation (Rahmani 1999; Spoor 1980) and two using a topical preparation (Rakusin 1972; Zetterstrom 1969). Spoor 1980 compared oral prednisone with placebo for the treatment of traumatic hyphema; 23 participants were randomized to the treatment group: oral prednisone, 40 mg/day for adults and children over 10 years old; 15 mg/day for children between four and 10 years; and 10 mg/day for children between 18 months and four years, for seven days, and 20 participants were randomized to the control group: lactose placebo capsules administered daily for seven days. All participants were followed for seven days and some for up to six months. The second study consisted of three intervention arms with a total of 244 participants (Rahmani 1999). One arm of the study included 82 participants who received 75 mg/kg oral tranexamic acid per day, divided into three doses per day, for five days. The second arm included 81 participants who received 0.75 mg/kg oral prednisolone per day, divided into two doses per day, for five days. The third group included 81 participants who received placebo administered three times per day. The follow-up period for this study was five days or until discharge. The remaining two studies administered topical corticosteroids. In Zetterstrom 1969, atropine plus corticosteroid eyedrops (Decadron) were administered five times daily in 58 participants, while the control group of 59 participants simply received bed rest. In the fourth study, Rakusin 1972 compared the use of 0.5% hydrocortisone acetate in 13 participants with topical 0.5% chloramphenicol in 21 participants.
Antifibrinolytic agents versus corticosteroids
Two studies compared the use of antifibrinolytic agents to corticosteroids in treating traumatic hyphema. The first study included 122 participants; 64 allocated to receive oral aminocaproic acid and 58 to receive oral prednisone. All were followed through the treatment period (Farber 1991). Those in the aminocaproic acid group received 50 mg/kg oral aminocaproic acid (up to 30 g per day) every four hours plus two doses of placebo for five days. Those in the prednisone group received 40 mg/day of oral prednisone in two doses plus six doses of placebo; children and adults weighing less than 60 kg were given 0.6 mg/kg/day of prednisone for five days. The second study, described above, divided study participants into three groups: oral prednisolone, tranexamic acid, and placebo (Rahmani 1999).
Conjugated estrogen
One included study compared the use of conjugated estrogen with placebo to treat traumatic hyphema (Spaeth 1966). Participants randomized to receive conjugated estrogen were given 5 mg intramuscularly for children less than five years of age; 10 mg for children five years of age but less than 10 years of age; and 20 mg intravenously for children 10 years of age or older and adults, for five days. The 51 participants included in the study were followed for five days or until discharge.
Cycloplegics versus miotics
Two studies compared the use of cycloplegics with miotics. Bedrossian 1974 evaluated 1% atropine ointment in 28 study participants versus 2% pilocarpine (or eserine) ointment in 30 study participants. The participants were treated and followed until the hyphema cleared (one to seven days). Rakusin 1972 examined the effects of 1% homatropine eyedrops in 17 participants, 4% pilocarpine in 17 participants, both homatropine and pilocarpine in 17 participants, and neither agent in 19 participants over a period of one to two weeks.
Aspirin
One included study compared aspirin (500 mg three times a day for five days) with observation for the treatment of traumatic hyphema (Marcus 1988). Of the 51 included participants, 23 were randomized to the aspirin group and 28 to the observation group. All participants were followed for seven days.
Monocular versus binocular patching
Two studies compared monocular versus binocular patching. Edwards 1973 compared monocular patching in 35 participants to binocular patching in 29 participants. Follow-up was from one to seven days. In one of the comparisons conducted by Rakusin 1972, 27 participants wore binocular patching, 26 wore monocular patches, and 10 wore no patch. Follow-up ranged from one to two weeks.
Ambulatory versus conservative treatment
In two studies the test and control interventions consisted of multiple components but could be assessed as treatments allowing moderate activity compared with bed rest. Read 1974 evaluated an intervention that included bed rest with elevation of the head, bilateral patches, an eye shield over the injured eye, and sedation in 66 participants with a comparison intervention comprised of moderate ambulatory activity, patching and shielding of the injured eye only, and no sedation in 71 participants. In the second study, Rakusin 1972 compared bed rest with ambulation in 26 participants each.
Combination and other interventions
In one study (Rakusin 1972) various components of a multiple-component intervention were tested sequentially and separately. Four of these comparisons are described above (i.e., 0.5% hydrocortisone eyedrops versus 0.5% chloramphenicol eyedrops, monocular versus binocular patching, cycloplegics versus miotics, and ambulation versus bed rest). In addition, Rakusin 1972 also presented results on the following comparisons: 1) oral trypsin in 15 participants compared with oral papase in 18 participants or no treatment in 10 participants; and 2) 250 mg acetazolamide in 18 participants compared with 1 ml/kg oral glycerol in 18 participants and no treatment in 10 participants.
The remaining study compared the time to resolution for participants laying flat either on the right or left side versus remaining in a semi-reclined position (that is with the head elevated) (Zi 1999).
Excluded studies
There were 41 excluded studies. The reasons for exclusion are described in the ‘Characteristics of excluded studies’ table. We excluded 31 studies because the study design was not a randomized or controlled clinical trial; five studies because they included non-traumatic hyphema cases and did not report outcomes for traumatic hyphema cases separately; four studies because no original data were presented; and one study because it reported only on a surgical intervention.
Risk of bias in included studies
Allocation
Nineteen of the 26 studies included in the review were RCTs. Seven studies specified using computerized randomization to generate the allocation sequence and one study used a randomization list; these studies were judged as having a low risk of sequence generation bias (Figure 1). Of the 19 included randomized trials, eight reported that allocation concealment was implemented: one study used sealed numbered envelopes, two studies used coded bottles, and five studies maintained the randomization code at a pharmacy or other central study center. The remaining seven studies were controlled clinical trials but did not use randomization to assign participants to treatment. Participants were allocated by alternation for four studies, and by date of admission in one study. The method of allocation was not reported in the remaining two studies.
Figure 1.
Methodological quality summary: review authors’ judgments about each methodological quality item for each included study. Green: low risk of bias; red: high risk of bias; yellow: unclear risk of bias.
Blinding
Twelve of the 19 included RCTs were double-masked (study participants and study investigators), placebo-controlled trials. One study investigating two doses of oral aminocaproic acid was also double-masked (Palmer 1986). Participants and treating physicians were partially masked in two studies in which there was only one placebo-control group for two intervention groups that had different treatment regimens (Karkhaneh 2003; Rahmani 1999). In both of these studies it was noted that the ophthalmologists and outcome assessors were not involved in participant treatment and were masked to the treatment groups. The interventions of interest in two studies precluded masking; the first study compared aspirin three times daily to observation only (Marcus 1988) and the second study compared bed confinement to walking and oral tranexamic acid three times daily (Vangsted 1983). Two studies did not mention whether or not masking occurred (Liu 2002; Zi 1999).
Masking was not possible because of the type of intervention in four of the seven quasi-randomized studies included in this review (Edwards 1973; Rakusin 1972; Read 1974; Zetterstrom 1969), and not reported in one (Bedrossian 1974). Masking was not achieved in the remaining two quasi-randomized studies (Sukumaran 1988; Varnek 1980).
Incomplete outcome data
Attrition rates for included studies were minimal due to the nature of the condition and treatment regimens. Typically, treatment duration for traumatic hyphema at the time the studies were completed comprised one week or less, and hospitalization was frequently implemented. Sixteen of the 26 included studies reported no exclusions or losses to follow-up, and thus used intention-to-treat analyses. Of the ten studies that excluded participants from the analysis, three studies excluded only one or two participants due to an adverse effect of treatment (Crouch 1997; Kutner 1987; Palmer 1986), or treatment failure (Palmer 1986). The remaining seven studies did not conduct intention-to-treat analyses, although all reported the number of exclusions and losses to follow-up.
Selective reporting
The risk of a secondary hemorrhage was reported as a primary outcome in all but four of the included studies; in two studies time to resolution of the hyphema was reported as the primary outcome (Bedrossian 1974; Zi 1999) while in the other two studies secondary hemorrhage was reported as a secondary outcome with no primary outcome identified (Edwards 1973; Read 1974). All investigators except Zi et al., reported results for secondary hemorrhage. There were three included studies in which the risk of reporting bias was unclear; due to the lack of study details available in the abstract, and no full version being published (Christianson 1979), because study outcomes were not clearly stated in the publication (Liu 2002), and because only results for secondary hemorrhage were reported, although visual acuity and IOP were measured throughout the duration of the study (Marcus 1988).
Other potential sources of bias
We detected no other potential sources of bias in seventeen of the included studies. We classified three studies as having an unclear risk of other bias because the publications had poor descriptions of study methods and results (Christianson 1979; Liu 2002; Marcus 1988). In two studies, some participants were selected to receive surgery either at recruitment (Rakusin 1972) or after having been assigned to a treatment group (Read 1974). We classified three studies as having an unclear risk of other bias because they were funded by pharmaceutical companies that either manufactured the drug being investigated in the study or that supplied study drug (Karkhaneh 2003; Pieramici 2003; Welsh 1983).
Effects of interventions
Antifibrinolytics versus control
Aminocaproic acid versus placebo
Visual acuity (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 2.1)
Two of the studies evaluating aminocaproic acid measured long-term visual acuity at nine months or from six months to 2.5 years after discharge (Crouch 1976; Kraft 1987). Neither study found a difference in the proportion of study participants who achieved useful final VA, defined as VA between 20/20 and 20/40 (Analysis 1.1). Kraft 1987 reported that 17 of 24 (70.8%) participants who had been assigned to aminocaproic acid had VA between 20/20 and 20/40, compared with 20 of 25 (80%) participants assigned to placebo. Similar results were reported by Crouch 1976, with 25 of 32 (79%) participants assigned to drug versus 18 of 27 (67%) participants assigned to placebo achieving useful VA. The summary odds ratio (OR) for these two studies indicated no significant difference (OR 1.11, 95% CI 0.47 to 2.61).
No study observed a difference in VA measured at two weeks or less after the hospital admission. At the time of discharge, Kutner 1987 observed VA of 20/40 or better in 14 of 21 (67%) participants in the oral aminocaproic acid treated group and in 10 of 13 (77%) participants in the placebo group. Similarly, Pieramici 2003 reported that 10 of 24 (42%) participants in the topical aminocaproic acid treated group and 13 of 27 (48%) participants in the placebo group had VAs of 20/40 or better seven days after study enrolment. Neither study result was significant (Analysis 1.2; Analysis 2.1). Although Karkhaneh 2003 did not report on the proportion of participants with good VA, they did report that there was no significant difference in VA between topical aminocaproic acid treated participants and placebo treated participants after two weeks of follow-up.
Two additional studies evaluated final VA with the time of measurement including both short and long-term time points ranging from five days to 3.4 years (Teboul 1995) or from zero to nine months (McGetrick 1983). Forty-six of 48 (95.8%) children in the aminocaproic acid group and 44 of 46 (95.6%) children of those in the placebo group had good final VA in Teboul 1995. McGetrick 1983 reported that the number of participants with final VA of 20/40 or better was 22 of 28 (78.6%) in the aminocaproic acid group and 14 of 21 (66.6%) in the placebo group. The summary OR for final VA of 20/40 or better for these two studies was 1.56 (95% CI 0.53 to 4.56; Analysis 1.3).
Time to resolution of primary hemorrhage (Analysis 1.4; Analysis 2.2)
In general, the hyphemas in participants assigned to aminocaproic acid took longer to clear than those in participants assigned to placebo or control groups. Christianson 1979 noted that drug treated hyphemas tended to take longer to clear compared to controls but reported that it was significant only among hyphemas filling more than half of the anterior chamber. Of the five remaining studies using oral aminocaproic acid, the average time to resolution of the primary hemorrhage ranged from 4.1 to 6.7 days in the aminocaproic acid group and 2.4 to 6.3 days in the placebo group among all study participants. Two studies evaluated the time to clear the initial hyphema after excluding participants who rebled (Crouch 1976; Kraft 1987). In both studies the group receiving aminocaproic acid took longer to clear the initial hyphema than the group receiving placebo (4.0 days versus 2.8 days in Crouch 1976 and 5.3 days versus 2.6 days in Kraft 1987). In Kraft 1987 the time to resolution appeared to be associated with initial hyphema severity, with larger initial hyphemas taking longer to resolve. The longer resolution times for drug treated groups were statistically significant as reported in the Kraft and Teboul studies individually; however, there were insufficient data available to perform a meta-analysis. In contrast, in McGetrick 1983 the average time to resolution was longer in the placebo than the aminocaproic acid group.
The mean time to resolution of the primary hemorrhage in participants receiving topical aminocaproic acid in Karkhaneh 2003 was 11.1 days (standard deviation (SD) = 4.7) versus 9.3 days (SD = 4.2) in the participants in the placebo group (P = 0.07). Pieramici 2003 reported no significant difference in time to clearance of the primary hyphema between topical aminocaproic acid treated participants and placebo treated participants. However, these studies included all study participants, including those who had a secondary hemorrhage.
Risk of secondary hemorrhage (Analysis 1.5; Analysis 2.3; Table 8)
Table 8.
Initial hyphema severity
| Study | Severity scale | Reported severity | Secondary hemorrhage | Other outcomes |
|---|---|---|---|---|
| Oral aminocaproic acid versus control | ||||
| Christianson 1979 | NR | NR | NR | Time to resolution of the primary hyphema was significantly longer (P < 0.05) for patients receiving drug in which the hyphema filled more than ½ of the anterior chamber |
| Crouch 1976 | Blood filling < ⅓ of anterior chamber | Reported no statistically significant differences across groups | NR | NR |
| Blood filling ⅓ to ½ of anterior chamber | ||||
| Blood filling > ½ to ¾ of anterior chamber | ||||
| Blood filling > ¾ to total of anterior chamber, but excluded total hyphema | ||||
| Kraft 1987 | Blood filling < ⅓ of anterior chamber | 30/49 (61%) study participants; 13/24 (54%) in drug group; 17/25 (68%) in placebo group | 1/3 (33%) secondary hemorrhage (in placebo group) | Excluding secondary hemorrhages, mean time to resolution of 3.4 days in drug group (range 1 to 11 days); mean time to resolution of 2.2 days in placebo group (range 1 to 4 days) |
| Blood filling ⅓ to ½ of anterior chamber | 14/49 (29%) study participants; 9/24 (37.5%) in drug group; 5/25 (20%) in placebo group | 1/3 (33%) secondary hemorrhage (in drug group) | Excluding secondary hemorrhages, mean time to resolution of 7.1 days in drug group, (range 6 to 9); mean time to resolution of 4.0 days in placebo group, (range 3 to 4) | |
| Blood filling ½ or more of anterior chamber | 5/49 (10%) study participants; 2/24 (8.3%) in drug group; 3/25 (12%) in placebo group | 1/3 (33%) secondary hemorrhage (in drug group) | Excluding secondary hemorrhages, time to resolution of 10 days in drug group: mean of placebo = 4.3 (range 3 to 5) | |
| Kutner 1987 | Mean hyphema height | 2.2 mm (SD 1.7, n = 21) in drug group; 1.7 mm (SD 1.0, n = 13) in placebo group | “all who rebled had initial hyphemas of 15% or less” | NR |
| McGetrick 1983; | Mean hyphema height | 100% (28/28) hyphemas in drug group were < 25% of anterior chamber; 86% (18/21) hyphemas in placebo group were < 25% of anterior chamber | 1 secondary hemorrhage in drug group; 6 secondary hemorrhages in placebo group | NR |
| Teboul 1995 | Blood filling < ⅓ of anterior chamber | 88/94 (94%) study participants; 44/48 (92%) in drug group; 44/46 (96%) in placebo group | 1 secondary hemorrhages in drug group and 2 in placebo group | NR |
| Blood filling ⅓ to ½ of anterior chamber | 6/94 (6%) study participants; 4/48 (8%) in aminocaproic acid group; 2/46 (4%) in placebo group | No rebleeds | NR | |
| Topical aminocaproic acid versus control | ||||
| Karkhaneh 2003 | Blood filling < ¼ of anterior chamber; excluded microscopic hyphemas | 65/80 (81%) study participants; 34/41 (83%) in drug group; 31/39 (79.5%) in placebo group; | Reported no effect of hyphema size on secondary hyphema (RR 0.7, 95% CI 0.2 to 2.5) | NR |
| Blood filled ¼ to ½ of anterior chamber | 14/80 (18%) study participants; 7/41 (17%) in drug group; 7/39 (18%) in placebo group | |||
| blood filling > ½ of anterior chamber; excluded total or blackball hyphemas | 1/80 (1%) study participants; 0/41 in drug group; 1/39 (2.5%) in placebo group | |||
| Pieramici 2003 | Mean hyphema height in mm | 1 mm (SE 0) in drug group (range 0 to 4); 2 mm (SE 0) in placebo group (range 0 to 8) | Size of primary hyphema in 2 participants with secondary hemorrhages in drug group: 0.3 and 1 mm; in 8 participants in the placebo group: 0.8, 0.9, 1, 1.4, 1.8, 2, 2, and 4.5 mm | NR |
| Low versus standard dose of aminocaproic acid | ||||
| Palmer 1986 | Mean hyphema height in mm | 1.7 mm (SD 2.0, range 0.1 to 9.9) in low dose group (n = 25); 1.5 mm (SD 2.2, range 0.1 to 9.9) in standard dose group 1.5 mm in standard dose group (n = 33) | 1 secondary hemorrhage in low dose group; 5 secondary hemorrhages in standard dose group | NR |
| Oral versus topical aminocaproic acid | ||||
| Crouch 1997 | Blood filling < ⅓ of anterior chamber | 44/64 (69%) of study participants | NR | NR |
| Blood filling > ½ to ¾ of anterior chamber | 6/64 (9%) of study participants | |||
| Blood filling > ½ to ¾ of anterior chamber | 8/64 (13%) of study participants | |||
| Blood filling > ¾ to total of anterior chamber | 6/64 (9%) of study participants | |||
| Tranexamic acid versus control | ||||
| Rahmani 1999 | Microscopic, but excluding patients with unlayered microscopic hyphemas | 17/238 (7%) study participants; 6/80 (7%) in aminocaproic acid group; 4/78 (5%) in prednisolone group; 7/80 (9%) in placebo group | 2/43 (5%) secondary hemorrhages | NR |
| Blood filling < ¼ of anterior chamber | 173/238 (72%) study participants; 56/80 (70%) in aminocaproic acid group; 61/78 (78%) in prednisolone group; 56/80 (70%) in placebo group | 30/43 (70%) secondary hemorrhages | ||
| Blood filling ¼ to ½ of anterior chamber | 36/238 (15%) study participants; 13/80 (16%) in aminocaproic acid group; 10/78 (13%) in prednisolone group; 13/80 (16%) in placebo group | 7/43 (16%) secondary hemorrhages | ||
| Blood filling > ½ of anterior chamber; excluded total hyphemas | 12/238 (5%) study participants; 5/80 (6%) in aminocaproic acid group; 3/78 (4%) in prednisolone group; 4/80 (5%) in placebo group | 4/43 (9%) secondary hemorrhages | ||
| Sukumaran 1988 | Hyphema height of 0 to 1 mm | 8/35 ( 23%) study participants; 4/17 (24%) in drug group; 4/18 (22%) in control group | NR | NR |
| Hyphema height of 2 to 3 mm | 12/35 (34%) study participants; 6/17 (35%) in drug group; 6/18 (33%) in control group | |||
| Hyphema height of 4 to 5 mm | 10/35 (29%) study participants; 5/17 (29%) in drug group; 5/18 (28%) in control group | |||
| Hyphema height of 6 to 7 mm | 5/35 (14%) study participants; 2/17 (12%) in drug group; 3/18 (17%) in control group | |||
| Vangsted 1983 | Hyphema height of 1 mm | 10/112 (9%) study participants; 8/59 (14%) in drug group; 2/53 (4%) in control group | NR | NR |
| Hyphema height of 2 mm | 33/112 (29%) study participants; 15/59 (25%) in drug group; 18/53 (34%) in control group | |||
| Hyphema height of 3 mm | 37/112 (33%) study participants; 18/59 (31%) in drug group; 19/53 (36%) in control group | |||
| Hyphema height of 4 mm | 18/112 (16%) study participants; 9/59 (15%) in drug group; 9/53 (17%) in control group | |||
| Hyphema height of 5 mm | 9/112 (8%) study participants; 6/59 (10%) in drug group; 3/53 (6%) in control group | |||
| Hyphema height of 6 mm | 4/112 (4%) study participants; 3/59 (5%) in drug group; 1/53 (2%) in control group | |||
| Hyphema height of 7 mm | None in either group | |||
| Hyphema height of 8 mm | 1/112 (1%) study participants; 0/59 (0%) in drug group; 1/53 (2%) in control group | |||
| Varnek 1980 | Mean hyphema height in mm | 2.0 mm in drug group (n = 102); 2.1 mm in control group (n = 130) | 1.0 mm in 2 study participants in drug group with a secondary hemorrhage; 2.2 mm in 12 study participants in control group with a secondary hemorrhage | NR |
| Welsh 1983 | Mean of proportion of anterior chamber area filled with blood | 68% in drug group (n = 19); 63% in placebo group (n = 20) | NR | NR |
| Aminomethylbenzoic acid versus control | ||||
| Liu 2002 | Blood filling < ⅓ of anterior chamber and level is lower than the inferior boarder of pupil | 47/92 (51%) study participants; 31/60 (52%) in drug group; 16/32 (50%) in control group | NR | NR |
| Blood filling ½ of anterior chamber and level is higher than the inferior border of the pupil, but not exceeding the median line | 30/92 (33%) study participants; 19/60 (32%) in drug group; 11/32 (34%) in control group | |||
| blood filling > ½ of anterior chamber or filling the entire anterior chamber | 15/92 (16%) study participants; 10/60 (17%) in drug group; 5/32 (16%) in control group | |||
| Oral corticosteroids versus control | ||||
| Spoor 1980 | 0 to 33% of anterior chamber area filled with blood | 38/43 (88%) study participants; 21/23 (91%) in prednisone group; 17/20 (85%) in placebo group | 2/4 (50%) secondary hemorrhages |
|
| > 33% to 75% of anterior chamber filled with blood | 5/43 (12%) study participants; 2/23 (9%) in prednisone group; 3/20 (15%) in placebo group | 2/4 (50%) secondary hemorrhages |
|
|
| Rahmani 1999 | See above under “Tranexamic acid versus control” | |||
| Topical corticosteroids | ||||
| Zetterstrom 1969 | Mean hyphema height in mm | 2.5 mm in topical corticosteroid group (n = 58); 3.5 mm in control group (n = 59) | No patient with secondary hemorrhage in topical corticosteroid group; 4 patients with secondary hemorrhage in control group | NR |
| Antifribrinolytics versus oral corticosteroids | ||||
| Farber 1991 | Microscopic | 24/116 (21%) study participants; 11/56 (20%) in aminocaproic acid group; 13/56 (23%) in prednisone group, | 3/8 (38%) secondary hemorrhages; 2 in aminocaproic acid group; 1 in prednisone group | NR |
| Hyphema height of 0.1 to 3.9 mm | 80/116 (69%) study participants; 41/56 (73%) in aminocaproic acid group; 39/56 (70%) in prednisone group | 4/8 (50%) secondary hemorrhages; 1 in aminocaproic acid group; 3 in prednisone group | ||
| Hyphema height of 4.0 to 5.9 mm | 4/116 (3%) study participants; 3/56 (6%) in aminocaproic acid group; 1/56 (2%) in prednisone group | No secondary hemorrhages in either group | ||
| Hyphema height of 6.0 to 11 mm | 2/116 (2%) study participants; 0/56 (0%) in aminocaproic acid group; 2/56 (4%) in prednisone group | No secondary hemorrhages in either group | ||
| Total hyphema | 2/116 (2%) study participants; 1/56 (2%) in aminocaproic acid group; 1/56 (2%) in prednisone group, | 1/8 (12%) secondary hemorrhage; 1 in aminocaproic acid group; none in prednisone group | ||
| Rahmani 1999 | See above under “Tranexamic acid versus control” | |||
| Conjugated estrogens versus control | ||||
| Spaeth 1966 | Blood filling < 20% of anterior chamber | 55/85 (65%) study participants; 28/39 (72%) in estrogen treated group; 27/46 (59%) in control group | 13/20 (65%) secondary hemorrhages; 8 in estrogen group; 5 in control group | NR |
| Blood filling 20% to 40% of anterior chamber | 17/85 (20%) study participants; 5/39 (13%) in estrogen treated group; 12/46 (26%) in control group | 4/20 (20%) secondary hemorrhages; 1 in estrogen group; 3 in control group | ||
| Blood filling 40% to 60% of anterior chamber | 5/85 (6%) study participants; 2/39 (5%) in estrogen treated group; 3/46 (7%) in control group | 1/20 (5%) secondary hemorrhage; none in estrogen group; 1 in control group | ||
| Blood filling 60% to 80% of anterior chamber | 2/85 (2%) study participants; 1/39 (3%) in estrogen treated group; 1/46 (2%) in control group | no secondary hemorrhages in either group | ||
| Blood filling > 80% of anterior chamber | 6/85 (7%) study participants; 3/39 (8%) in estrogen treated group; 3/46 (7%) in control group | 2/20 (10%) secondary hemorrhages; 1 in estrogen group; 1 in control group | ||
| Cycloplegics versus miotics | ||||
| Bedrossian 1974 | Hyphema height of 1 mm | 20/58 (34%) study participants; 10/28 (36%) in the cycloplegic group; 10/30 (33%) in the miotic group | 1/1 (100%) secondary hemorrhage (in cycloplegic group) | Mean time to resolution in cycloplegic group of 1.9 days (SD = 1.4); mean time to resolution in miotic group of 2.5 days (SD= 1) |
| Hyphema height of 2 mm | 22/58 (38%) study participants; 10/28 (36%) in the cycloplegic group; 12/30 (40%) in the miotic group | No secondary hemorrhages in either group | Mean time to resolution in cycloplegic group of 3.3 days (SD = 1.8); mean time to resolution in miotic group of 4.2 days (SD = 1.3) | |
| Hyphema height of 3 mm | 12/58 (21%) study participants; 6/28 (21%) in the cycloplegic group; 6/30 (20%) in the miotic group | No secondary hemorrhages in either group | Mean time to resolution in cycloplegic group of 3.2 days (SD = 1.9); mean time to resolution in miotic group of 4.0 days (SD = 1.1) | |
| Hyphema height of 4 mm | 4/58 (7%) study participants; 2/28 (7%) in the cycloplegic group; 2/30 (7%) in the miotic group | No secondary hemorrhages in either group | Mean time to resolution in cycloplegic group of 2.5 days (1 resolved on day 2 and 1 on day 3); mean time to resolution in miotic group of 4.0 days (1 resolved on day 3 and 1 on day 5) | |
| Aspirin versus no aspirin | ||||
| Marcus 1988 | Reported that “the two groups were comparable with respect to age, cause, and extent of hyphema” and that 2 of 3 eyes with a secondary hemorrhage in the aspirin group (n = 23) had an initial total hyphema, while of the 2 eyes with a secondary hemorrhage in the control group (n = 28), 1 had 30% and 1 had almost total hyphema | NR | ||
| Monocular versus binocular patching | ||||
| Edwards 1973 | Blood filling < ⅓ of anterior chamber | 42/64 (66%) study participants; 21/35 (60%) in the monocular patching group; 21/29 (72%) in the binocular patching group | 7/14 (50%) secondary hemorrhages; 4 in the monocularly treated group; 3 in the binocularly treated group | 62% (13/21) of patients with final visual acuity of 20/50 or better in the monocularly treated group; 71% (15/21) of patients with final visual acuity of 20/50 or better in the binocularly treated group |
| Blood filling ⅓ to ½ of anterior chamber | 14/64 (22%) study participants; 9/35 (26%) in the monocular patching group; 5/29 (17%) in the binocular patching group | 7/14 (50%) secondary hemorrhages; 4 in the monocularly treated group; 3 in the binocularly treated group | 57% (8/14) of patients with final visual acuity of 20/50 or better in the monocularly treated group; 62% (5/8) of patients with final visual acuity of 20/50 or better in the binocularly treated group | |
| Blood filling ½ or more of anterior chamber | 8/64 (12%) study participants; 5/35 (14%) in the monocular patching group; 3/29 (11%) in the binocular patching group | |||
| Ambulatory versus conservative treatment | ||||
| Read 1974 | Blood filling < ⅓ of anterior chamber | 79/137 (58%) study participants; 47/71 (66%) in the ambulatory group; 32/66 (48%) in the conservatively treated group | 16/30 (53%) secondary hemorrhages; 9 in the ambulatory group; 7 in the conservatively treated group | NR |
| Blood filling ⅓ to ½ of anterior chamber | 11/71 (16%) patients in the ambulatory group; 17/66 (26%) or patients in the conservatively treated group | 5/30 (17%) secondary hemorrhages; 4 in the ambulatory group; 1 in the conservatively treated group | ||
| Blood filling ½ but not total anterior chamber | 8/71 (11%) patients in the ambulatory group; 11/66 (17%) or patients in the conservatively treated group | 6/30 (20%) secondary hemorrhages; 3 in the ambulatory group; 3 in the conservatively treated group | ||
| Total hyphema | 5/71 (7%) patients in the ambulatory group; 6/66 (9%) or patients in the conservatively treated group | 3/30 (10%) secondary hemorrhages; 2 in the ambulatory group; 1 in the conservatively treated group | ||
| Elevation of head versus lying flat | ||||
| Zi 1999 | Blood filling < ½ of anterior chamber and level is lower than the inferior boarder of pupil | 36/74 (49%) study participants; 18/35 (51%) with elevation of the head; 18/39 (46%) lying flat | NR | NR |
| Blood filling ½ of anterior chamber and level is higher than the inferior border of the pupil | 19/74 (26%) study participants; 6/35 (17%) with elevation of the head; 13/39 (33%) lying flat | NR | NR | |
| Blood filling > ½ of anterior chamber or filling the entire anterior chamber | 19/74 (26%) study participants; 11/35 (31%) with elevation of the head; 8/39 (21%) lying flat | NR | NR | |
| Other | ||||
| Rakusin 1972 * | Blood filling < ½ of anterior chamber | n = 213 | NR |
|
| Blood filling > ½ of anterior chamber | n = 157 | NR |
|
|
Rakusin 1972 reported severity for entire study population rather than by trials of topical corticosteroids, cycloplegics versus miotics, monocular versus binocular patching, and ambulatory versus conservative treatment. See under “Other”
95% CI: 95% confidence interval
mm: millimeter
n: number of participants
NR: not reported
RR: relative risk
SD: standard deviation
SE: standard error
Data from eight studies, all RCTs comparing aminocaproic acid with placebo, reported results on the risk of secondary hemorrhage (Christianson 1979; Crouch 1976; Karkhaneh 2003; Kraft 1987; Kutner 1987; McGetrick 1983; Pieramici 2003; Teboul 1995). Participants who were assigned to receive aminocaproic acid, either orally or topically, less often experienced a secondary hemorrhage compared with participants receiving placebo. This association was stronger when oral aminocaproic acid was used (OR 0.25, 95% CI 0.11 to 0.57) than when topical aminocaproic acid was used (OR 0.42, 95% CI 0.16 to 1.10; Figure 2; Analysis 2.3). Because an intention-to-treat analysis was not performed in two studies of oral aminocaproic acid, each of which excluded a single participant from analysis (Kutner 1987; McGetrick 1983), we performed a sensitivity analysis to assess the effect of excluding these studies. Excluding these two studies resulted in a non-significant effect of aminocaproic acid (OR 0.41, 95% CI 0.16 to 1.09).
Figure 2.
(Analysis 1.5). Forest plot of comparison: 1 Oral aminocaproic acid versus placebo, outcome: 1.5 Secondary hemorrhage.
Of the six studies comparing oral aminocaproic acid with placebo, four excluded study participants with sickle cell trait (Kraft 1987; Kutner 1987; McGetrick 1983; Teboul 1995). Crouch 1976 reported that eight study participants had sickle cell trait, although the trial investigators do not say to which group these participants were assigned. The one study participant who had a secondary hemorrhage in the aminocaproic acid group and two of the nine participants who had a secondary hemorrhage in the placebo group also had sickle cell trait. Of the eight participants with sickle cell trait, five rebled. Similarly, in the topical aminocaproic acid versus placebo studies, only Pieramici 2003 reported that two study participants in the aminocaproic acid group and one in the placebo group had sickle cell trait but again they did not report on the rebleed rate for participants with sickle cell trait/disease.
Initial hyphema severity was reported in almost all studies. Most investigators reported initial hyphema severity by the proportion of anterior chamber filled with blood or by the height of the hyphema in millimeters. There did not appear to be any overall pattern in the proportion of study participants who had a secondary hemorrhage within groups defined by initial hyphema severity. Some studies reported no effect of initial hyphema size on secondary hemorrhages (Karkhaneh 2003) or that all secondary hemorrhages occurred in initially less severe hyphemas (Kutner 1987; Teboul 1995), while other studies found evidence of a higher proportion of secondary hemorrhages when the initial hyphema was more severe (Kraft 1987).
Time to rebleed (Analysis 1.6; Analysis 2.4)
Five of the six studies that studied oral aminocaproic acid reported data on the time between the initial injury and a secondary hemorrhage. Of the ten participants who had a secondary hemorrhage in Crouch 1976, the single participant in the aminocaproic acid rebled on day one, and the nine placebo treated participants rebled between days two and seven. Of the three participants in Kraft 1987 who experienced a secondary hemorrhage, the two who had received aminocaproic acid had a rebleed on days three and four, and the placebo treated participant rebled on day four. All three participants who rebled in Kutner 1987 were in the placebo group and rebled on day two. In the single aminocaproic acid treated participant who rebled in McGetrick 1983, the secondary hemorrhage occurred on day four, and three of the five participants in the placebo group rebled on day three, one on day five and one on day six. Of the three participants who rebled in Teboul 1995, one rebled on day two (placebo), one rebled on day six (aminocaproic acid), and one rebled on day seven (placebo).
The mean time to rebleed in the five participants receiving topical aminocaproic acid who rebled in Karkhaneh 2003 was 3.2 days (SD = 0.5) versus 3.0 days (SD = 0.8) in the seven participants who rebled in the placebo group (P = 0.18). Pieramici 2003 reported that of the participants in their study who rebled, those receiving topical aminocaproic acid took longer to rebleed (n = 1; day six) compared with those receiving placebo (n = 8; range in days two to six). However, this result was observed after excluding a participant in the drug treated group who had taken aspirin and rebled on day three.
Overall there appeared to be little difference in the time for a secondary hemorrhage to occur although the small numbers of events makes statistical testing unreliable.
Risk of corneal bloodstain (Analysis 1.7; Table 2)
Table 2.
Risk of corneal bloodstaining
| Study | Test intervention | No. with outcome/No. in group | Control intervention | No. with. outcome | Total No./No with outcome |
|---|---|---|---|---|---|
| Aminocaproic acid | |||||
| Crouch 1976 | Oral aminocaproic acid | 0/32 | Placebo | 2/27 | 2/59 |
| Crouch 1997 | Oral aminocaproic acid | 0/29 | Topical aminocaproic acid | 0/35 | 0/64 |
| Tranexamic acid | |||||
| Vangsted 1983 | Tranexamic acid | 0/59 | Bed rest only | 0/53 | 0/112 |
| Varnek 1980 | Tranexamic acid | 1/102 | Conservative treatment | 0/130 | 1/232 |
| Prednisone/cortisone | |||||
| Spoor 1980 | Oral prednisone | NR | Placebo | NR | 1/43 |
| Zetterstrom 1969 | Atropine plus cortisone eye drops | 0/58 | Conservative treatment | 1/59 | 1/117 |
| Estrogen | |||||
| Spaeth 1966 | Estrogen | 2/39 | Placebo | 2/46 | 4/85 |
| Non-drug medical interventions | |||||
| Edwards 1973 | Monocular patching | 1/35 | Binocular patching | 1/29 | 2/64 |
| Read 1974 | Bed rest with elevation of the head, bilateral patches and eye shield | 4/66 | Moderate ambulatory activity, patching and shielding of injured eye | 5/71 | 9/137 |
NR: Not reported
One study examining oral aminocaproic acid reported outcomes for corneal bloodstain (Crouch 1976). Two participants in the placebo treated group who also had secondary hemorrhages required surgery “due to increased intraocular pressure and early corneal bloodstaining.”
Risk of peripheral anterior synechiae (PAS) formation
Crouch 1976 reported that 14 participants experienced PAS formation in the study cohort. Although the difference between groups was reported to be non-significant, the number of participants for each group were not reported.
Risk of glaucoma or elevated intraocular pressure (Analysis 1.8; Analysis 1.9; Analysis 2.5; Table 4)
Table 4.
Risk of elevated intraocular pressure
| Study | Test intervention | No. with outcome/No. in group | Control intervention | No. with outcome | Total No./No. with outcome |
|---|---|---|---|---|---|
| Aminocaproic acid | |||||
| Kraft 1987 | Oral aminocaproic acid | 1/24 | Placebo | 1/25 | 2/49 |
| Kutner 1987 | Oral aminocaproic acid | 1/21 | Placebo | 3/13 | 4/34 |
| Teboul 1995 | Oral aminocaproic acid | 3/48 | Placebo | 3/46 | 6/94 |
| Pieramici 2003 | Topical aminocaproic acid | 2/24 | Placebo | 1/27 | 3/25 |
| Palmer 1986 | Standard dose oral aminocaproic acid | 2/33 | Low dose oral aminocaproic acid | 0/26 | 2/59 |
| Tranexamic acid | |||||
| Vangsted 1983 | Tranexamic acid | 8/59 | Bed rest only | 6/53 | 14/112 |
| Varnek 1980 | Tranexamic acid | 8/102 | Conservative treatment | 7/130 | 15/232 |
| Rahmani 1999 | Tranexamic acid | 12/80 | Placebo | 12/80 | 24/160 |
| Welsh 1983 | Tranexamic acid | 1/19 | Placebo | 2/20 | 3/39 |
| Prednisone/cortisone | |||||
| Spoor 1980 | Oral prednisone | 0/23 | Placebo | 0/20 | 0/43 |
| Rahmani 1999 | Oral prednisone | 9/78 | Placebo | 12/80 | 21/158 |
| Zetterstrom 1969 | Atropine plus cortisone eye drops | 3/58 | Conservative treatment | 2/59 | 5/117 |
| Non-drug medical interventions | |||||
| Edwards 1973 | Monocular patching | 3/35 | Binocular patching | 0/29 | 3/64 |
| Read 1974 | Ambulation | 17/71 | Bed rest | 19/66 | 36/137 |
| Zi 1999 | Lying on right and left lateral position | 7/39 | Lying in semi-reclining position | 8/35 | 15/74 |
NR: Not reported
Three studies reported the number of participants with elevated IOP in oral aminocaproic acid and placebo groups (Kraft 1987; Kutner 1987; Teboul 1995). None of the studies included participants with sickle cell disease/trait. Teboul 1995 reported that six participants (three in each group) developed transient increases in IOP which did not persist following discharge (OR 0.96, 95% CI 0.18 to 5.00). Kraft 1987 reported that two participants (one in each group) had IOP greater than 25 mmHg at follow-up and Kutner 1987 reported that four participants (one in the aminocaproic group and three in the control group) had elevated IOP at time of discharge (summary OR 0.35, 95% CI 0.06 to 1.98) (Analysis 1.8).
One study involving topical aminocaproic acid (Pieramici 2003) reported a non-significant increase in the number of participants using aminocaproic acid who had elevated IOP during the seven day trial compared to participants using placebo (OR 2.36, 95% CI: 0.20 to 27.85). This study enrolled three participants (6%) with sickle cell disease/trait, but it was not clear if any of these participants developed elevated IOP. The other study involving topical aminocaproic acid (Karkhaneh 2003) reported no significant differences in initial or final IOP between treatment groups.
Risk of optic atrophy (Analysis 1.10; Table 5)
Table 5.
Risk of optic atrophy
| Study | Test intervention | No. with outcome/No. in group | Control intervention | No. with outcome | Total No./No. with outcome |
|---|---|---|---|---|---|
| Aminocaproic acid | |||||
| Crouch 1976 | Oral aminocaproic acid | 0/32 | Placebo | 2/27 | 2/59 |
| Crouch 1997 | Oral aminocaproic acid | 0/29 | Topical aminocaproic acid | 0/35 | 0/64 |
| Tranexamic acid | |||||
| Varnek 1980 | Tranexamic acid | 1/102 | Conservative treatment | 0/130 | 1/232 |
| Cortisone | |||||
| Zetterstrom 1969 | Atropine plus cortisone eye drops | 0/58 | Conservative treatment | 1/59 | 1/117 |
| Non-drug medical interventions | |||||
| Read 1974 | Bed rest with elevation of the head, bilateral patches and eye shield | NR | Moderate ambulatory activity, patching and shielding of injured eye | NR | 8/137 |
NR: Not reported
Crouch 1976 reported that two participants (7.4%) in the placebo treated group, and none in the aminocaproic acid group developed optic atrophy. This difference was not statistically significant.
Adverse effects (Analysis 1.11; Table 6; Table 7)
Table 6.
Risk of other ocular events
| Study | Outcome | Test intervention | No. with outcome/No. in group | Control intervention | No. with outcome | Total No./No. with outcome |
|---|---|---|---|---|---|---|
| Aminocaproic acid | ||||||
| Crouch 1997 | Conjunctival/corneal foreign body sensation | Topical aminocaproic acid | 4/35 | Oral aminocaproic acid | 0/29 | 4/64 |
| Transient punctate corneal staining | Same | 3/35 | Same | 0/29 | 3/64 | |
| Tranexamic acid | ||||||
| Varnek 1980 | Vitreous and retinal hemorrhage | Tranexamic acid | 5/102 | Conservative treatment | 5/130 | 10/232 |
| Traumatic cataract | Same | 2/102 | Same | 0/130 | 2/232 | |
| Non-drug medical intervention | ||||||
| Read 1974 | Traumatic cataract | Bed rest with elevation of the head, bilateral patches and eye shield | NR | Moderate ambulatory activity, patching and shielding of injured eye | NR | 8/137 |
| Vitreous hemorrhage | Same | NR | Same | NR | 11/137 | |
| Commotio retinae | Same | NR | Same | NR | 4/137 | |
| Occluded pupil | Same | NR | Same | NR | 2/137 | |
| Optic atrophy with nasalization of optic cup | Same | NR | Same | NR | 4/137 | |
| Optic atrophy without nasalization of optic cup | Same | NR | Same | NR | 8/137 | |
NR: Not reported
Table 7.
Risk of non-ocular adverse effects
| Study ID | Comparison | Type of complication | Results |
|---|---|---|---|
| Aminocaproic acid | |||
| Kraft 1987 | Oral aminocaproic acid versus placebo | Nausea | Drug group: 8 of 24; Placebo group 1 of 25 |
| Kutner 1987 | Oral aminocaproic acid versus placebo | Nausea or vomiting | Drug group: 6 of 21; Placebo group: 0 of 13 |
| Light headedness | Drug group: 7 of 21; Placebo group: 1 of 13 | ||
| Systemic hypotension | Drug group: 4 of 21; Placebo group: 1 of 13 | ||
| Total complications | Drug group: 10 of 21; Placebo group: 1 of 13 | ||
| McGetrick 1983 | Oral aminocaproic acid versus placebo | Nausea or vomiting | Drug group: 6 of 28; Placebo group: 0 of 20 |
| Diarrhea | Drug group: 2 of 28; Placebo group: 0 of 20 | ||
| Muscle cramps | Drug group: 1 of 28; Placebo group: 0 of 20 | ||
| Pieramici 2003 | Topical aminocaproic acid versus placebo | Systemic hypotension | Drug group: 3 of 24; Placebo group: 3 of 27 |
| Crouch 1997 | Oral versus topical aminocaproic acid | Dizziness, nausea, vomiting | Oral group: 5 of 29; Topical group: 1 of 35 |
| Palmer 1986 | Low dose versus standard dose oral aminocaproic acid | Nausea or vomiting | Low dose group: 5 of 25; Standard dose group: 9 of 33 |
| Dizziness and hypotension | Low dose group: 0 of 25; Standard dose group: 5 of 33 | ||
| Syncope | Low dose group: 0 of 25; Standard dose group: 2 of 33 | ||
| Diarrhea | Low dose group: 1 of 25; Standard dose group: 0 of 33 | ||
| Rash or pruritis | Low dose group: 1 of 25; Standard dose group: 2 of 33 | ||
| Hot flashes | Low dose group: 1 of 25; Standard dose group: 0 of 33 | ||
| Dry mouth or nose | Low dose group: 1 of 25; Standard dose group: 0 of 33 | ||
| Farber 1991 | Oral aminocaproic acid versus oral prednisone | Any adverse event | Aminocaproic acid group: 0 of 56; Prednisone group; 0 of 56 |
| Tranexamic acid | |||
| Welsh 1983 | Tranexamic acid versus placebo | Nausea | Drug group: 1 of 19; Placebo group: 0 of 20 |
| Rahmani 1999 | Tranexamic acid versus placebo | Nausea | Drug group: 0 of 80; Placebo group: 0 of 80 |
| Aminomethylbenzoic acid | |||
| Liu 2002 | Oral aminomethylbenzoic acid versus placebo | Nausea and vomiting | Drug group: 7 of 60; Placebo group: NR |
NR: Not reported
Nausea and vomiting occurred significantly more often in participants treated with oral aminocaproic acid than in participants treated with placebo. In three studies (Kraft 1987; Kutner 1987; McGetrick 1983) that reported the occurrence of nausea and vomiting in the drug treated group compared with the placebo group, the summary OR was 11.76 (95% CI 2.59 to 53.46; Analysis 1.11).
In addition, McGetrick 1983 reported that two participants experienced diarrhea and one participant had muscle cramps; all were in the group treated with oral aminocaproic acid. No participants in Kutner 1987 had diarrhea or muscle cramps, but 10 (45%) of the participants in the aminocaproic acid group had at least one complication compared with only one participant (8%) in the placebo group (P < 0.02). Other than nausea and vomiting, complications reported in Kutner 1987 included light-headedness and systemic hypotension. Systemic hypotension was also observed in 13% of participants in the topical aminocaproic acid group versus 11% of participants in the placebo group in Pieramici 2003.
Duration of hospitalization (Analysis 1.12)
The duration of hospitalization was reported by two studies, although not enough details were provided to perform a meta-analysis. McGetrick 1983 reported that the mean duration of hospitalization was 5.7 days for the aminocaproic acid group and 7.3 days for the placebo group. The difference was not statistically significant. This trend was reversed in Teboul 1995, in which the aminocaproic acid group had a longer hospital stay (7.3 days) compared with the placebo group (5.4 days) (P < 0.001).
Low versus standard dose aminocaproic acid
Visual acuity (Analysis 3.1)
Only one study (Palmer 1986) compared low dose (50 mg/kg) with the standard dose (100 mg/kg) of oral aminocaproic acid, so we performed no meta-analyses for any outcome. Although “final” VA was measured, the time from injury to final VA was not reported. Final VAs of 20/40 or better were attained by 16 of 25 (64.0%) participants receiving low dose aminocaproic acid and by 25 of 32 (78.1%) participants receiving standard dose aminocaproic acid. These results were not statistically different (P = 0.24).
Time to resolution of primary hemorrhage (Analysis 3.2)
No significant difference was reported between groups regarding time to resolution of the primary hemorrhage. The mean time for resolution of the primary hemorrhage was 3.1 days (SD = 2.3) in the low dose group and 3.3 days (SD = 1.8) in the standard dose group.
Risk of secondary hemorrhage (Analysis 3.3; Table 8)
The investigators reported that one of 25 (4.0%) eyes receiving low dose aminocaproic acid rebled, and five of 33 (15.2%) eyes receiving the standard dose of aminocaproic acid rebled. These results were not statistically different (P = 0.20). Participants with sickle cell trait were excluded from this study, and there did not appear to be an effect of initial hyphema severity on the rate of secondary hemorrhage.
Time to rebleed (Analysis 3.4)
The one participant who rebled in the low dose group rebled on day four. Of the five participants who rebled in the standard dose group, one did so on day two, two on day three, and two on day six.
Risk of corneal bloodstain
Palmer 1986 did not report this outcome.
Risk of peripheral anterior synechiae formation
Palmer 1986 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure (Analysis 3.5; Table 4)
Two participants in the standard dose group experienced elevated IOP requiring surgical intervention. No elevated IOP was observed in the low dose group however the groups were not statistically different (P = 0.36).
Risk of optic atrophy
Palmer 1986 did not report this outcome.
Adverse effects (Analysis 3.6; Table 7)
There were no significant differences in adverse events reported between groups (Analysis 3.6). Nausea or vomiting was reported in five participants in the low dose group and in nine participants in the standard dose group (P = 0.52). Dizziness and hypotension were reported in five participants in the standard dose group, and syncope was reported in two participants in the standard dose group. Other adverse events in the low dose group included diarrhea and dry mouth or nose, each in a single participant. Rash or pruritis was reported in one participant in the low dose group and in two participants in the standard dose group.
Duration of hospitalization (Analysis 3.7)
The duration of hospitalization was not statistically different between groups. The mean hospital stay was 5.4 days (SD = 1.1) in the low dose group and 5.5 days (SD = 1.4) in the standard dose group (P = 0.76).
Oral versus topical aminocaproic acid
Visual acuity (Analysis 4.1)
Results for final (short-term) VA were reported by Crouch 1997. Final VAs of 20/40 or better were attained by 20 of 29 (85.7%) participants receiving oral aminocaproic acid and by 30 of 35 (69.0%) participants receiving topical aminocaproic acid. These results were not statistically different (P = 0.11).
Time to resolution of primary hemorrhage
Crouch 1997 did not report this outcome.
Risk of secondary hemorrhage (Analysis 4.2)
We did not perform meta-analysis because only one study (Crouch 1997) compared oral with topical aminocaproic acid. The number of secondary hemorrhages was not statistically different between groups: one of 29 (3%) eyes in the oral group versus one of 35 (3%) eyes in the topical group (P = 0.89). Two participants in each of the treatment groups had sickle cell trait, but there was no report on the rate of secondary hemorrhage by this condition nor by initial hyphema severity.
Time to rebleed (Analysis 4.3)
Crouch 1997 reported that the secondary hemorrhage in the participant in the oral aminocaproic acid group occurred on day three and the secondary hemorrhage in the participant in the topical aminocaproic acid group occurred on day five.
Risk of corneal bloodstain (Analysis 4.3; Table 2)
No incident corneal bloodstaining was reported in either the oral or topical aminocaproic acid groups (Crouch 1997).
Risk of peripheral anterior synechiae formation
Crouch 1997 reported that four participants experienced PAS formation, but the number of participants for each group were not reported.
Risk of glaucoma or elevated intraocular pressure
Crouch 1997 did not report this outcome.
Risk of optic atrophy (Analysis 4.5; Table 5)
No incident optic atrophy was reported in either the oral or topical aminocaproic acid groups (Crouch 1997).
Adverse effects (Analysis 4.6; Table 6; Table 7)
There were no significant differences in adverse events reported between groups. Of the 35 participants in the topical aminocaproic acid group, four reported feeling a conjunctival or corneal foreign body sensation, three experienced transient punctate corneal staining, and one had dizziness, nausea, and vomiting on two occasions. Five of the 29 participants in the oral aminocaproic acid group had dizziness, nausea, and vomiting (Analysis 4.6).
Duration of hospitalization
Crouch 1997 did not report this outcome.
Tranexamic acid versus control
Visual acuity (Analysis 5.1)
We analyzed data from five studies reporting results comparing tranexamic acid versus control (Rahmani 1999; Sukumaran 1988; Vangsted 1983; Varnek 1980; Welsh 1983). Three studies were RCTs, and two were quasi-randomized controlled clinical trials. Short-term VA was reported by four of these studies. Visual acuity was measured by Rahmani 1999 at the time of discharge (range five to 15 days); 41 of 77 (57%) participants in the tranexamic acid group had VA of 20/40 or better compared with 35 of 79 (44%) participants in the placebo group. These results were not statistically different (P = 0.23). We did not perform an intention-to-treat analysis however because VA measurements were missing for three excluded participants in the tranexamic acid group, and for one excluded participant in the control group. Sukumaran 1988 reported that all participants had a final VA of 20/30 or better with the exception of one participant in the control group. The time of measurement for final VA was not reported but participants were followed-up for only one week. Vangsted 1983 reported that all 59 participants in the tranexamic acid group had VA between 20/20 and 20/40 two weeks after the initial trauma. In the control group, all 53 participants had VA between 20/20 and 20/50 two weeks after the initial trauma. A meta-analysis of these three studies showed no statistically significant effect of tranexamic acid (OR 1.65, 95% CI 0.91 to 2.99; Figure 3). In addition, Varnek 1980 reported mean VAs of 0.9 in both the tranexamic acid and control treated groups at day five after the trauma. Visual acuity was not reported by Welsh 1983.
Figure 3.
(Analysis 5.1). Forest plot of comparison: 5 Tranexamic acid versus control, outcome: 5.1 Short-term visual acuity from 20/20 to 20/40.
Time to resolution (Analysis 5.2)
Rahmani 1999 found no significant difference for time to primary resolution between groups who received tranexamic acid (mean = 4.0 days, SD = 2.2) versus placebo (mean = 3.7 days, SD = 1.6) after excluding participants who had secondary hemorrhages. Sukumaran 1988 also found no difference in time to resolution between groups, but included study participants with and without secondary hemorrhages in the analysis (tranexamic group; mean = 4.0, SD = 2.4 versus control group; mean = 3.9, SD = 2.4). Although Welsh 1983 did not report time to resolution of the primary hyphema directly, the group estimated the daily rate of improvement in the hyphema by calculating the geometric mean of the percent area of the hyphema remaining at each day following injury. These calculations indicated that tranexamic acid-treated hyphemas cleared faster than those treated with placebo.
Risk of secondary hemorrhage (Analysis 5.3; Table 8)
All five studies reported the risk of a secondary hemorrhage. Using a fixed-effect model, the summary OR comparing oral tranexamic acid to placebo or control was 0.25 (95% CI 0.13 to 0.49). This result was significant with P < 0.05 and no statistical heterogeneity detected (I2 = 0%) (Figure 4).
Figure 4.
(Analysis 5.3). Forest plot of comparison: 5 Tranexamic acid versus control, outcome: 5.3 Secondary hemorrhage.
No study that evaluated tranexamic acid reported on the presence of sickle cell trait. Two of the studies had all white populations, thus it would be unlikely for any study participant to have this condition (Rahmani 1999; Varnek 1980). Although initial hyphema severity was reported by all investigators, only Rahmani 1999 reported the proportion of secondary hemorrhages in groups defined by the severity of the initial hyphema, finding no effect of severity on rebleed rate. Varnek 1980 reported that the initial size of the hyphemas that underwent secondary hemorrhage was 1.0 mm (one secondary hemorrhage) in the study group and 2.2 mm (12 secondary hemorrhages) in the control group.
Time to rebleed (Analysis 5.4)
Three studies reported the time interval between the initial injury and the time of the secondary hemorrhage (Rahmani 1999; Sukumaran 1988; Varnek 1980). In Rahmani 1999, the mean time to rebleed in eight participants who experienced a secondary hemorrhage in the tranexamic acid group was 3.4 days (SD = 0.7) compared with 3.8 days (SD = 1.0) in the 21 participants who rebled in the placebo group. This difference was reported as not significant. In Sukumaran 1988, rebleeding occurred between days two and three in the participants who rebled in either group, and Varnek 1980 reported that the secondary hemorrhage took place at day three in the two study participants in the tranexamic group who experienced this event. The time to rebleed ranged from day two to day seven in the 12 participants who rebled in the control group.
Risk of corneal bloodstain (Analysis 5.5; Table 2)
Two studies reported corneal bloodstaining as an outcome. Vangsted 1983 observed corneal bloodstaining in a single participant in the control group of 53, and Varnek 1980 reported observing no corneal bleeding in either the tranexamic acid treated group or the placebo group.
Risk of peripheral anterior synechiae formation
This outcome was not reported by any study comparing tranexamic acid with control.
Risk of glaucoma or elevated intraocular pressure (Analysis 5.6; Table 4)
Four of the five studies reported the number of participants with transient increases in IOP in each group following the treatment period (Rahmani 1999; Vangsted 1983; Varnek 1980; Welsh 1983). None of the studies reported including participants with sickle cell disease/trait. Rahmani 1999 defined elevated IOP as greater than 21 mmHg during the hospital stay and requiring medical or surgical treatment or both. Vangsted 1983 and Varnek 1980 defined transient elevated IOP as greater than or equal to 25 mmHg. Welsh 1983 did not define IOP by a pressure level but stated that three participants required surgery for elevated IOP. The summary OR was 1.23 (95% CI 0.70 to 2.16) when comparing tranexamic acid with control (Figure 5). In addition, Vangsted 1983 reported no instances of secondary glaucoma.
Figure 5.
(Analysis 5.6). Forest plot of comparison: 5 Tranexamic acid versus control, outcome: 5.6 Incidence of glaucoma or increased IOP.
Risk of optic atrophy (Analysis 5.7; Table 5)
Varnek 1980 reported one incident of optic atrophy in the tranexamic acid treated group and none in the placebo group.
Adverse effects (Analysis 5.8; Table 7)
Welsh 1983 reported that one of 19 participants receiving tranexamic acid complained of nausea. Rahmani 1999 reported that medical staff observed no adverse events in either the drug-treated or control group.
Duration of hospitalization (Analysis 5.9)
Three studies reported on the length of hospitalization (Rahmani 1999; Vangsted 1983; Varnek 1980). The average hospital stay for participants receiving tranexamic acid in Rahmani 1999 was six days (SD = 1.6), and that of participants in the control group was 6.3 days (SD = 1.8). This difference was not significant. Vangsted 1983 reported that the average length of hospitalization for the tranexamic acid group was six days compared with seven days for the control group. The length of hospitalization for the tranexamic acid group in Varnek 1980 was 6.8 days compared to 6.5 days for the control group.
One study reported the average number of days off work (Vangsted 1983). The average period off work for the tranexamic acid group was 17 days compared with 20 days for the control group.
Aminomethylbenzoic acid versus placebo
We performed no meta-analysis because only one study (Liu 2002) compared aminomethylbenzoic acid with placebo.
Visual acuity
Liu 2002 did not report this outcome.
Time to resolution of primary hemorrhage
Liu 2002 did not report this outcome.
Risk of secondary hemorrhage (Analysis 6.1)
Liu 2002 reported that participants treated with oral aminomethylbenzoic acid are less likely to rebleed compared with participants treated with placebo (OR 0.07, 95% CI 0.01 to 0.32).
Time to rebleed
Liu 2002 did not report this outcome.
Risk of corneal bloodstain
Liu 2002 did not report this outcome.
Risk of peripheral anterior synechiae formation
Liu 2002 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
Liu 2002 did not report this outcome.
Risk of optic atrophy
Liu 2002 did not report this outcome.
Adverse events (Table 7)
Of the 60 participants who received oral aminomethylbenzoic acid, seven complained of nausea and vomiting. Adverse events for the placebo group were not reported.
Duration of hospitalization
Liu 2002 did not report this outcome.
Corticosteroids versus control
Visual acuity (Analysis 7.1; Analysis 7.2; Analysis 8.1)
Two studies compared oral corticosteroids with placebo. Visual acuity outcomes between studies could not be combined because they were assessed at different follow-up times and participants were divided by cut points into different levels of VA. Spoor 1980 reported that 21 of 23 (91%) participants in the prednisone group achieved final VA between 20/20 and 20/50 compared to 18 of 20 (90%) participants in the placebo group (P = 0.88). In Rahmani 1999, short-term VA was compared for participants in each treatment group. At time of discharge (range five to 12 days), 40 of 75 (53%) participants in the corticosteroid group had VA of 20/40 or better compared to 35 of 80 (44%) participants in the placebo group. These results were not statistically different (P = 0.23).
Two studies administering topical corticosteroids reported short-term VA. Again, the VA outcomes could not be combined because different cut points were used across studies (Rakusin 1972; Zetterstrom 1969). Rakusin 1972 reported that six of 13 (46%) study participants assigned to corticosteroid eyedrops and 13 of 21 (62%) participants assigned to the control eyedrops achieved short-term VA better than 20/60. Zetterstrom 1969 reported that 56 of 58 (97%) study participants in the corticosteroid group had final VA of 0.9 (between 20/20 and 20/25), and 53 of 59 (90%) in the control group achieved VA better than 0.7 (about 20/30). At discharge, mean VA in the group assigned to corticosteroids was 0.96, compared with 0.91 in the control group.
Time to resolution of primary hemorrhage (Analysis 7.3; Analysis 8.2; Table 8)
In one of the two studies that evaluated oral corticosteroids, Spoor 1980 reported means of 4.4 days and 4.5 days for the resolution of primary hemorrhage in groups receiving prednisone and placebo, respectively. This result remained non-significant when we excluded participants who rebled from the analysis. Spoor 1980 reported that the time to resolution was shorter in hyphemas that were less severe initially. Rahmani 1999 also found no significant difference for time to primary resolution in participants who had not experienced a secondary hemorrhage and were assigned to prednisolone (mean = 3.5 days, SD = 1.8) or placebo (mean = 3.7 days, SD = 1.6). In the one study evaluating topical corticosteroids that measured this outcome, Rakusin 1972 reported that the primary hyphema was resolved within one week in 10 of 13 (77%) study participants assigned to corticosteroid eye drops and in 16 of 21 (76%) participants assigned to the control group.
Risk of secondary hemorrhage (Analysis 7.4; Analysis 8.3; Table 8)
We analyzed data from two studies evaluating systemic corticosteroids and reporting results for the risk of secondary hemorrhage (Rahmani 1999; Spoor 1980). Using a fixed-effect model, the summary OR comparing oral corticosteroids to placebo was 0.61 (95% CI 0.31 to 1.22; Analysis 7.4) however we did not perform an intention-to-treat analysis due to missing data from the exclusion of four participants by Rahmani 1999. A meta-analysis of secondary hemorrhage including data from Rakusin 1972 (topical corticosteroids versus placebo eye drops) and Zetterstrom 1969 (topical corticosteroids versus complete bed rest with no simultaneous local therapy) did not show a statistically significant difference (OR 0.27, 95% CI 0.05 to 1.61; Analysis 8.3).
None of the four studies reported on the presence of sickle cell trait.
Rahmani 1999 observed no effect of initial hyphema severity on the proportion of participants with a secondary hemorrhage, but Spoor 1980 found that there was a lower proportion of secondary hemorrhages in participants with less severe initial hyphemas (2/38 (13%) versus 2/5 (40%) where severity was defined as blood filling ⅓ versus more than ⅓ of the anterior chamber).
Time to rebleed (Analysis 7.5)
In Rahmani 1999, rebleeding occurred an average of 3.2 days (SD = 0.8) from the time of trauma in the 14 participants who rebled in the prednisolone group and 3.8 days (SD = 1.0) in the 21 participants who rebled in the placebo group. This difference was reported as not significant. In Spoor 1980, the mean time to rebleed in three participants who experienced a secondary hemorrhage in the prednisone group was 2.3 days compared with 2.6 days in the four participants who rebled in the placebo group. Like the Rahmani study, this difference was not significant.
Risk of corneal bloodstain (Analysis 7.6; Analysis 8.4; Table 2)
One of 43 participants included in Spoor 1980 experienced corneal bloodstaining. The study group in which the bloodstain occurred was not reported. In Zetterstrom 1969, a single participant in the control group experienced corneal bloodstaining compared with none in the group receiving corticosteroid eyedrops.
Complications of hyphema, including corneal bloodstaining, pigment on endothelium, anterior lens capsule, or vitreous, posterior synechiae, peripheral anterior synechiae, anterior chamber blood clots, and fibrous membrane formation, were documented for all participants in Rakusin 1972. It was reported that 54% of the corticosteroid group had complications compared with 70% of the control group, although this difference was not significant and the risk of corneal bloodstain was not reported separately.
Risk of peripheral anterior synechiae (Analysis 7.7; Table 3)
Table 3.
Risk of peripheral anterior synechiae
| Study | Test intervention | No. with outcome/No. in group | Control intervention | No. with outcome | Total No./No. with outcome |
|---|---|---|---|---|---|
| Aminocaproic acid | |||||
| Crouch 1997 | Oral aminocaproic acid | NR | Topical aminocaproic acid | NR | 4/64 |
| Prednisone | |||||
| Spoor 1980 | Oral prednisone | 0/23 | Placebo | 0/20 | 0/43 |
| Conjugated estrogen | |||||
| Spaeth 1966 | Conjugated estrogens | NR | Placebo | NR | 15/85 |
| Non-drug medical interventions | |||||
| Read 1974 | Bed rest with elevation of the head, bilateral patches and eye shield | NR | Moderate ambulatory activity, patching and shielding of injured eye | NR | 9/137 |
NR: Not reported
Spoor 1980 reported that there was no instance of peripheral anterior synechiae formation in either group.
Risk of glaucoma or elevated intraocular pressure (Analysis 7.8; Analysis 8.5; Table 4)
Rahmani 1999 reported that nine (11.5%) of 78 participants in the prednisolone group and 12 (15%) of 80 participants in the placebo group had an IOP greater than 21 mmHg during hospitalization that required medical treatment, surgical treatment or both. This difference was not significant. Two participants studied by Spoor 1980 had elevated IOP that was controlled by acetazolamide therapy alone; one participant was in the prednisolone group, and one was in the control group. No participant in this cohort had IOP greater than 35 mmHg. Five participants in Zetterstrom 1969 developed “elevated” IOP (undefined); three of 58 in the group assigned to topical corticosteroids and two of 59 in the control group (Analysis 7.8).
Risk of optic atrophy (Analysis 8.6)
One incident of optic atrophy was reported by Zetterstrom 1969 in the group of 58 participants assigned to topical corticosteroid eyedrops.
Adverse effects
Rahmani 1999 reported that medical staff observed no adverse events in either the drug-treated or control groups.
Duration of hospitalization (Analysis 7.9; Analysis 8.7)
In Rahmani 1999, participants treated with prednisolone were hospitalized an average of 5.9 days (SD = 1.4) and participants treated with placebo were hospitalized an average of 6.3 days (SD = 1.8). The mean difference between groups was −0.40 days (95% CI −0.90 to 0.10).
Zetterstrom 1969 reported duration of hospitalization, finding that the mean length of stay for participants assigned to corticosteroid drops was 5.9 days compared with 8.9 days for participants assigned to the control group.
Oral aminocaproic acid versus oral prednisone
Visual acuity (Analysis 9.1)
We performed no meta-analysis because only one study (Farber 1991) compared oral aminocaproic acid to oral prednisone. After five days of hospitalization, 10 of 56 (18%) participants in the aminocaproic acid group had short-term VA of 20/200 or worse compared with seven of 56 (12.5%) participants in the prednisone group. These results were not statistically different (P = 0.43). Likewise, there was no difference in final VA of 20/40 or better between groups (26 of 56 (46%) participants in the aminocaproic acid group and 31 of 56 (55%) participants in the prednisone group).
Time to resolution of primary hyphema
Farber 1991 did not follow the study participants past discharge and so did not report on time to resolution of the primary hyphema. They did report however that “at discharge” (mean time to discharge = five days) 43% of the aminocaproic acid group compared with 75% or the prednisone groups had complete resolution of their hyphema. This difference was statistically significant (P = 0.001).
Risk of secondary hemorrhage (Analysis 9.2; Table 8)
The risk of secondary hemorrhage was equal for both groups; four eyes out of 56 eyes per group (P = 1.00). Study participants with sickle cell trait/disease were excluded from this study. There did not appear to be an influence of initial hyphema severity on rate of secondary hemorrhage.
Time to rebleed
Farber 1991 did not report this outcome.
Risk of corneal bloodstain
Farber 1991 did not report this outcome.
Risk of peripheral anterior synechiae formation
Farber 1991 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
No significant differences were reported for mean IOPs at time of discharge between groups.
Risk of optic atrophy
Farber 1991 did not report this outcome.
Adverse events
Farber 1991 did not report this outcome.
Duration of hospitalization
Farber 1991 did not report this outcome.
Conjugated estrogen versus placebo
Visual acuity
Visual acuity at time of discharge was partially reported by the one study that compared conjugated estrogen to placebo (Spaeth 1966). Among all study participants, 61% had visual acuity better than 6/12, 30% had visual acuity better than 6/60, and 9% had visual acuity 6/60 or worse at time of discharge. These results were not reported by treatment groups.
Time to resolution of primary hyphema
Spaeth 1966 did not report this outcome.
Risk of secondary hemorrhage (Analysis 10.1; Table 8)
It was reported that of 39 estrogen-treated participants, 10 rebled (25.6%) and of 46 placebo-treated participants, 10 rebled (21.7%). These results were not statistically different (P = 0.67).
Spaeth 1966 did not report on the presence of sickle cell trait/disease. The risk of secondary hemorrhage by initial hyphema severity did not appear to differ across severity ratings.
Time to rebleed
The time to rebleed, reported not by treatment group but overall, was on average 3.5 days after injury with a range of one to eight days.
Risk of corneal bloodstain (Analysis 10.2)
In the estrogen-treated group, two of 39 (5%) participants had corneal bloodstaining compared with two of 46 (4%) participants in the placebo-treated group (OR 1.19, 95% CI 0.16 to 8.86).
Risk of peripheral anterior synechiae formation
Fifteen cases of PAS were reported among all study participants; however the number of cases by treatment group were not reported.
Risk of glaucoma or elevated intraocular pressure
Thirteen cases of secondary glaucoma were reported among all study participants; however the number of cases by treatment group were not reported. Four of these thirteen cases occurred prior to secondary hemorrhage.
Risk of optic atrophy
Spaeth 1966 did not report this outcome.
Adverse events
Spaeth 1966 did not report this outcome.
Duration of hospitalization
Spaeth 1966 did not report this outcome.
Cycloplegics versus miotics
Short term visual acuity (Analysis 11.1)
Two studies looked at the effect of cycloplegics compared with miotics (Bedrossian 1974; Rakusin 1972). Rakusin 1972 reported that nine of 17 (53%) participants in the homatropine treated group and 11 of 17 (65%) participants in the pilocarpine treated group had short-term VA better than 20/60. Bedrossian 1974 did not report on VA.
Time to resolution (Analysis 11.2; Table 8)
Bedrossian 1974 reported a longer time to resolution with the pilocarpine-treated group (mean = 3.6 days, SD = 1.3) compared with the atropine treated group (mean = 2.7 days, SD = 1.7). The time to resolution showed a slight increase with increased size of initial hyphema. In Rakusin 1972, there was no significant difference between the proportion of participants with absorption within one week between cycloplegic (12/17) and miotic (13/17) treated groups.
Risk of secondary hemorrhage (Analysis 11.3; Table 8)
In Bedrossian 1974, only one participant experienced a secondary hemorrhage; that participant was in the cycloplegic group and had an initial hyphema height of 1 mm. The single participant with a secondary hemorrhage in Rakusin 1972 was in the group receiving homatropine (Analysis 11.3).
Time to rebleed (Analysis 11.4)
Bedrossian 1974 reported that the time to rebleed in the one individual with a secondary hyphema was two days.
Risk of corneal bloodstain
It was reported that the number of complications of hyphema, including corneal bloodstaining, pigment on endothelium, anterior lens capsule, or vitreous, posterior synechiae, peripheral anterior synechiae, anterior chamber blood clots, and fibrous membrane formation, were similar in all groups in Rakusin 1972.
Risk of peripheral anterior synechiae formation
See previous outcome.
Risk of glaucoma or elevated intraocular pressure
Bedrossian 1974 and Rakusin 1972 did not report this outcome.
Risk of optic atrophy
Bedrossian 1974 and Rakusin 1972 did not report this outcome.
Adverse events
Bedrossian 1974 and Rakusin 1972 did not report this outcome.
Duration of hospitalization
Bedrossian 1974 and Rakusin 1972 did not report this outcome.
Aspirin versus observation
Because only one study (Marcus 1988) compared aspirin to observation, we did not perform a meta-analysis.
Short term visual acuity
Marcus 1988 did not report this outcome.
Time to resolution
Marcus 1988 did not report this outcome.
Secondary hemorrhage (Analysis 12.1)
Marcus 1988 reported that three of 23 (13%) eyes receiving aspirin rebled and two of 28 (7%) eyes receiving observation rebled. These results were not statistically different (P = 0.49). The study investigators reported that two of the three eyes that rebled in the aspirin group initially had a total hyphema, while of the two eyes that rebled in the control group, one had an initial hyphema of 30% and one an “almost total” hyphema.
Time to rebleed
Marcus 1988 did not report this outcome.
Risk of corneal bloodstain
Marcus 1988 did not report this outcome.
Risk of peripheral anterior synechiae formation
Marcus 1988 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
Marcus 1988 did not report this outcome.
Risk of optic atrophy
Marcus 1988 did not report this outcome.
Adverse events
Marcus 1988 did not report this outcome.
Duration of hospitalization
Marcus 1988 did not report this outcome.
Monocular versus binocular patching
Visual acuity (Analysis 13.2; Table 8)
We identified two studies that compared the use of monocular versus binocular patches (Edwards 1973; Rakusin 1972). Rakusin 1972 reported that 22 of 26 (85%) participants in the monocular group compared with 24 of 27 (89%) participants in the binocular group had short-term VA better than 20/60. Edwards 1973 reported that 21 of 26 (81%) participants in the monocular group had VA better than 20/50 compared with 20 of 20 (100%) participants in the binocular group, although the time at which VA was measured was not specified. In the study participants with an initial hyphema filling less than 1/3 of the anterior chamber, 67% (28/42) had VA of 20/50 or better compared with 59% (13/22) of those with more severe hyphemas.
Time to resolution
Rakusin 1972 reported that the primary hyphema was resolved within one week in 22 of 26 (85%) study participants with monocular patching and in 24 of 27 (89%) participants with binocular patching.
Risk of secondary hemorrhage (Analysis 13.3; Table 8)
In Edwards 1973 there were eight participants each with a secondary hemorrhage from both the group with a patch on both eyes (n = 35; 23%) and the group with a patch only on the injured eye (n = 29; 28%). The proportion of secondary hyphemas was greater in study participants with initially more severe hyphemas (32% (seven of 22) versus 17% (seven of 42) for those with an initial hyphema filling less than ⅓ of the anterior chamber versus more). The results from Rakusin 1972 also showed no difference between groups on risk of secondary hemorrhage (one of 26 (3.8%) in the group with a monocular patch and two of 27 (7.4%) in the group with binocular patches) (Analysis 13.3).
Time to rebleed (Analysis 13.4)
A mean of three days between injury and secondary hemorrhage was reported for eight individuals in the group with a monocular patch as well as for eight individuals who had a secondary hemorrhage in the group with binocular patches (Edwards 1973).
Risk of corneal bloodstain (Analysis 13.5; Table 2)
A single individual in each of the two treatment groups experienced corneal bloodstaining in Edwards 1973.
It was reported that the risk of complications of hyphema, including corneal bloodstaining, pigment on endothelium, anterior lens capsule, or vitreous, posterior synechiae, peripheral anterior synechiae, anterior chamber blood clots, and fibrous membrane formation, were similar in both groups in Rakusin 1972.
Risk of peripheral anterior synechiae formation
See previous outcome.
Risk of glaucoma or elevated intraocular pressure (Analysis 13.6; Table 4)
In the study by Edwards et al, three participants in the monocular patching group developed secondary glaucoma while none in the binocular patch developed this condition (Edwards 1973).
Risk of optic atrophy
Edwards 1973 and Rakusin 1972 did not report this outcome.
Adverse events
Edwards 1973 and Rakusin 1972 did not report this outcome.
Duration of hospitalization
Edwards 1973 and Rakusin 1972 did not report this outcome.
Quality of life
Edwards 1973 noted no difference between groups on the “cooperation index”. This index included a number of outcomes including those associated with quality of life (pain, restlessness, activity, and emotional state while in the hospital).
Ambulatory versus conservative treatment
Visual acuity (Analysis 14.1)
Two studies compared ambulatory (that is moderate activity allowed) versus conservative treatment, which comprised bed rest alone (Rakusin 1972) or bed rest with elevation of the head, bilateral ocular patches and a shield over the injured eye (Read 1974). In Read 1974, VA was not reported by treatment group but the authors distinguished between poor VA due to the initial trauma and that due to secondary effects of the hyphema. They stated that poor VA due to hyphema occurred in nine of 71 (13%) participants in the ambulatory group compared with four of 66 (6%) participants in the conservatively treated group. Overall, the proportion of participants with good VA was 104 of 137 (76%) with more participants in the ambulatory group having good VA. In Rakusin 1972, 22 of 26 (85%) study participants had short-term VA better than 20/60 compared with 20 of 26 (77%) study participants in the conservatively treated group.
Time to resolution of primary hyphema (Analysis 14.2)
Read 1974 reported a mean of 5.8 days between the initial injury and resolution of the hyphema in the ambulatory group compared with 5.6 days in the group receiving bed rest. Rakusin 1972, however, observed a significant difference in the speed of reabsorption. The primary hyphema was resolved within one week in 13 of 26 (50%) study participants in the ambulatory group compared with 22 of 26 (85%) study participants in the conservatively treated group.
Risk of secondary hemorrhage (Analysis 14.3; Table 8)
Eighteen of 71 (25%) study participants in the ambulatory group developed a secondary hemorrhage, and 12 of 66 (18%) participants in the group receiving bed rest did so in Read 1974. This difference was not statistically significant. The proportion of study participants with a secondary hemorrhage appeared to be somewhat smaller with more severe initial hyphemas (16 of 30 (53%) versus 14 of 90 (16%) for those with an initial hyphema filling less than ⅓ compared with ⅓ or more of the anterior chamber) (Analysis 14.3).
Time to rebleed
Read 1974 reported that the majority of secondary hemorrhages occurred between day two and day five following injury, although two secondary hemorrhages took place on day seven following the initial injury.
Risk of corneal bloodstain (Analysis 14.4; Table 2)
Nine participants in Read 1974 developed corneal bloodstaining; five of 71 (7%) participants in the ambulatory group and four of 66 (6%) participants in the group receiving bed rest.
It was reported that the risk of complications of hyphema, including corneal bloodstaining, pigment on endothelium, anterior lens capsule, or vitreous, posterior synechiae, peripheral anterior synechiae, anterior chamber blood clots, and fibrous membrane formation, were similar in both groups in Rakusin 1972.
Risk of peripheral anterior synechiae formation
See the previous outcome.
Risk of glaucoma or elevated intraocular pressure (Analysis 14.5; Table 4)
Of the 71 participants in the group that was allowed moderate activity, 17 (23.9%) developed IOP ≥ 25 mmHg while 19 of the 66 (28.8%) participants in the group with bed rest developed this condition during hospitalization in Read 1974.
Risk of optic atrophy
Rakusin 1972 and Read 1974 did not report this outcome.
Adverse events
Rakusin 1972 and Read 1974 did not report this outcome.
Duration of hospitalization
Rakusin 1972 and Read 1974 did not report this outcome.
Elevation of the head versus control
A single study compared elevation of the head by assigning participants to a semi-reclined body position or to laying on their right or left side (Zi 1999).
Visual acuity
Zi 1999 did not report this outcome.
Time to resolution
Time to resolution was compared by level of hyphema. The time to resolution was somewhat shorter for participants with their head elevated compared with those laying flat if the initial hyphema filled up to half of the anterior chamber, but longer if the blood filled more than half (level of blood < ½ of the anterior chamber: 1.7 days (n=18) versus 2.8 days (n = 18); level of blood = ½ of the anterior chamber: 2.2 days (n = 6) versus 3.1 days (n = 13); level of blood more than ½ of anterior chamber: 9.0 days (n =11) versus 8.0 days (n = 8)).
Risk of secondary hemorrhage
Zi 1999 did not report this outcome.
Time to rebleed
Zi 1999 did not report this outcome.
Risk of corneal bloodstain
Zi 1999 did not report this outcome.
Risk of peripheral anterior synechiae formation
Zi 1999 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure (Table 4)
Fifteen study participants developed secondary glaucoma, eight of 35 (23%) in the group in the semi-reclined position and seven of 39 (18%) in the group laying flat (Zi 1999).
Risk of optic atrophy
Zi 1999 did not report this outcome.
Adverse events
Zi 1999 did not report this outcome.
Duration of hospitalization
Zi 1999 did not report this outcome.
Discussion
Summary of main results
This systematic review included 26 studies. Nineteen of the included studies were RCTs, and seven used a quasi-randomized method to assign participants to treatment groups. The primary outcome for all but two studies was the risk of a secondary hemorrhage. The primary outcomes for this review were visual outcome and duration of visible hyphema. Secondary outcomes for this review were sequelae of the traumatic hyphema, including risk of and time to rebleed, risk of corneal blood staining, risk of PAS formation, risk of pathological increase in IOP or glaucoma development, and risk of optic atrophy development.
Antifibrinolytic agents
The use of antifibrinolytic agents, such as aminocaproic acid and tranexamic acid, in traumatic hyphema is controversial because they are reported to reduce the rate of recurrent hemorrhage, albeit at the cost of gastric and other adverse events. We found no effect of any antifibrinolytic agent on VA measured at any time point. Neither oral nor topical aminocaproic acid had any effect on final VA, nor did tranexamic acid. Hyphemas in participants on systemic aminocaproic acid appeared to take a somewhat longer time to clear than those in participants not receiving that intervention, although the numbers are small and conclusions unreliable. As expected, it took less time for hyphemas to clear in study participants who did not have a secondary hemorrhage than in those who experienced a secondary hemorrhage. Antifibrinolytics appeared to prolong the time to resolution in both groups - those who had a rebleed and those who did not - but the evidence available is insufficient to make any firm conclusion about the time for a hyphema to clear in participants treated with an antifibrinolytic.
Oral aminocaproic acid appeared to reduce the risk of a secondary hemorrhage, but in a sensitivity analysis excluding studies that did not adhere to an intention-to-treat analysis we found a non-significant effect of this drug on the rate of rebleeds. Likewise, evidence showing an effect of topical aminocaproic acid on the rate of rebleeds is equivocal; although appearing to reduce the rate of secondary hemorrhage, the number of events is small. Thus, although there is some evidence supporting an effect of aminocaproic acid in reducing the risk of secondary hemorrhage, it appears to be less convincing than reported previously (Walton 2002). There appeared to be little difference in the time for a secondary hemorrhage to occur between patients receiving aminocaproic acid (oral or topical) and controls, but again the evidence is weak due to a small number of incidents. In addition, there appears to be no effect of either oral or topically applied aminocaproic acid on the timing of the rebleed or on the number of events related to the traumatic hyphema itself (that is corneal bloodstaining, PAS formation, elevated IOP, or development of optic atrophy). However, the small number of events renders significance testing unreliable. Unfortunately there was insufficient evidence to conclude whether aminocaproic acid would be beneficial specifically for individuals with sickle cell trait/disease. Whether aminocaproic acid is useful for participants with sickle cell trait/disease is of extreme importance because such patients are at higher risk for elevated IOP (Lai 2001).
Aminocaproic acid is reported to have several side effects including nausea, vomiting, muscle cramps, conjunctival suffusion, headache, rash, pruritis, dyspnea, toxic confusional states, arrhythmias and systemic hypotension. Its use is contraindicated in patients who are pregnant, in patients with coagulopathies or with renal diseases and should be cautiously used in patients with hepatic, cardiovascular or cerebrovascular diseases. There were no statistically significant differences in adverse events reported between oral and topical aminocaproic acid nor between standard versus low doses of aminocaproic acid.
Tranexamic acid was not statistically different from controls in terms of final VA, time of resolution of hemorrhage, time of rebleed or duration of hospitalization. Tranexamic acid is reported to have fewer gastric side effects than aminocaproic acid. A single study compared aminomethylbenzoic acid with placebo, with results suggesting that patients treated with oral aminomethylbenzoic acid are less likely to rebleed compared with patients treated with placebo.
Corticosteroids
Corticosteroids have also been used to treat hyphema; the mechanism of action of corticosteroids is believed to be due to reduced inflammation, stabilization of the blood-ocular barrier or direct inhibition of fibrinolysis, thus preventing secondary rebleeds. The effect of oral corticosteroids was evaluated in two studies (Rahmani 1999; Spoor 1980) and the effect of topical corticosteroids in two others (Rakusin 1972; Zetterstrom 1969). No significant difference in terms of resolution of primary hemorrhage, time of rebleed or increased IOP was found.
A single study compared systemic aminocaproic acid with prednisolone (Farber 1991). This study concluded that at discharge more hyphemas in patients in the prednisolone group had resolved than in patients in the systemic aminocaproic acid group. No other differences were noted between these two agents in this study, although the investigators did not follow the patients following discharge.
Other pharmaceutical interventions
Two studies compared homatropine as a cycloplegic to pilocarpine as a miotic (Bedrossian 1974; Rakusin 1972). A secondary hemorrhage occurred in only one patient in each study. Such small numbers of events makes significance testing unreliable. The traumatic hyphemas took a longer time to resolve in patients receiving pilocarpine. No other outcomes nor other miotics or cycloplegics were studied.
No effect was seen with the use of conjugated estrogens in a single study (Spaeth 1966).
No statistically significant difference was reported in the risk of rebleed in patients who had received aspirin in comparison to those who had not (Marcus 1988).
Non-pharmaceutical interventions
No differences in VA, risk of secondary hemorrhage, or time of rebleed was reported in patients receiving a single versus binocular patch (Edwards 1973; Rakusin 1972).
A single study (Zi 1999) evaluated the effect of raising the head (semi-reclined position) compared with right and left lateral positions alternatively on time of resolution of primary hyphema. The results were inconsistent in that the hyphema resolved sooner when the head was raised for small hyphemas but took longer for larger hyphemas. The time of follow-up was not mentioned, and patients were not masked to treatment assignment of course.
Comparing moderate activity with complete bed rest did not show any statistically significant difference in secondary hemorrhage occurrence, final VA, time to rebleed or time to its resolution (Rakusin 1972; Read 1974). Occurrences of complications (elevated IOP or corneal staining) were also comparable.
Overall completeness and applicability of evidence
Our search strategy was comprehensive. We believe that we identified all or a high proportion of published trials of interventions for hyphema and that our review is reasonably complete.
There were only a few studies, or sometimes only a single study, evaluating a particular intervention. For example, only a single study compared a low dose (50 mg/kg) to the standard dose (100 mg/kg) of oral aminocaproic acid, and a single study compared aminomethylbenzoic acid to placebo (Liu 2002). Comparison of topical corticosteroids versus controls was evaluated in only two studies (Rakusin 1972; Zetterstrom 1969), as was systemic corticosteroids versus control (Rahmani 1999; Spoor 1980). A single study compared aminocaproic acid to prednisolone (Farber 1991), and just one study compared conjugated estrogen to placebo (Spaeth 1966). Comparison of cycloplegic versus miotic usage was completed in only two studies, with both comparing homatropine to pilocarpine (Bedrossian 1974; Rakusin 1972). A single study compared aspirin with control (Marcus 1988). Only two studies discussed the value of monocular versus binocular patching (Edwards 1973; Rakusin 1972), and none compared binocular or monocular patching with no patching. Only one study compared the effect of elevation of the head to control (Zi 1999). These few studies made the application of meta-analytic methods unreliable or impossible for many outcomes.
Another limitation of the validity of some results was the lack of information on patients with sickle cell disease/trait. Two of the studies included in this review reported on the occurrence of secondary hemorrhage in patients with sickle cell trait/disease. Crouch 1976 mentioned that the one study participant who had a secondary hemorrhage in the aminocaproic acid group and two of the nine participants who had a secondary hemorrhage in the placebo group also had sickle cell trait, but they did not say to which group the eight sickle cell trait patients were originally assigned. Pieramici 2003 reported that two study participants in the aminocaproic acid group and one in the placebo group had sickle cell trait but they did not comment on their rebleed rate. The subgroup of patients with sickle cell trait/disease is especially important in that this group has been shown to be at higher risk for elevated IOP (Lai 2001). It has been shown (Goldberg 1979a; Goldberg 1979b; Goldberg 1979c) that even modest elevations in IOP are potentially deleterious in sickle cell disease/trait, and specifically that permanent infarction of the optic nerve with substantial loss of vision can occur in such patients. Careful monitoring of IOP is indicated, and early surgery to decompress the eye is often required.
Quality of the evidence
This systematic review included 26 studies, nineteen of which were RCTs, and seven were quasi-randomized studies. Overall, the risk of bias was higher in the non-randomized studies in that the sequence generation and allocation concealment were inadequate. In many cases the studies were not reported clearly, and in some studies participants were inappropriately excluded from the analyses.
Potential biases in the review process
Many of the studies were published more than 20 years ago, and it was not possible to contact the investigators to obtain missing information. A single review author abstracted data from the foreign language articles.
Agreements and disagreements with other studies or reviews
Our review found some evidence for an effect of aminocaproic acid and tranexamic acid on the risk of secondary hemorrhage. In contrast to most reported reviews, the evidence for a preventive effect of antifibrinolytics on rebleeds was not nearly as strong as that reported in the reviews by Walton (Walton 2002) and Sheppard (Sheppard 2009). However, Walton 2002 included RCTs, controlled clinical trials, and also observational studies, but did not take into account any biases in the individual studies. Sheppard 2009 cited only some of the trials and also included observational studies. In all reviews, no effect of either aminocaproic acid or tranexamic acid was found on VA. Walton 2002 presented a stronger case for the use of corticosteroids for prevention of secondary hemorrhage than we report here or than is reported by Sheppard 2009. Our review agrees with most of the existing literature in that there is little evidence for the use of bilateral patching, topical cycloplegics, sedation, or bed rest, although these interventions often are recommended (Sheppard 2009; Walton 2002).
Authors’ conclusions
Implications for practice
Although evidence is limited, the data suggest that patients with traumatic hyphema who receive aminocaproic acid are less likely to experience secondary hemorrhage than those who do not. Complications resulting from secondary hemorrhage such as glaucoma, corneal blood staining, or optic atrophy can lead to permanent impairment of vision. This systematic review did not identify a significant effect on time to best vision or final VA following hyphema, which are the primary endpoints. Moreover, oral aminocaproic acid was demonstrated to yield significant side effects including gastrointestinal upset and systemic hypotension, and hyphema clears more slowly in patients treated with aminocaproic acid.
Tranexamic acid seems to be as effective as aminocaproic acid in terms of effect on secondary hemorrhage but with fewer gastric side effects. Data from the few studies of the effect of corticosteroids on final VA and risk of secondary hemorrhage in hyphema patients do not support the presumed benefits, though corticosteroid usage may aid in relieving the associated inflammation in such cases.
Taking into consideration the risk of side effects for various potential medical treatments (antifibrinolytic agents, corticosteroids and cycloplegics) without the presence of solid scientific evidence to support their benefit, it might be reasonable to recommend their usage only in those patients with high risk of complications (such as sickle cell trait/disease patients).
Controlled clinical trials comparing non-drug treatment modalities with placebo failed to show a protective effect. We found no convincing evidence of benefit of binocular patching over monocular patching, bed rest over moderate activity, or elevation of the head in a semi-reclined position in the treatment of traumatic hyphema. Given that most of these interventions were used collectively in many of the studies presented, it was not possible to assess the extent to which any of these interventions may have contributed to any reported positive results.
Implications for research
There is insufficient high quality evidence from large RCTs to support the use of corticosteroids or cycloplegics and limited evidence for the use of antifibrinolytics in the treatment of traumatic hyphema. It is possible that topical aminocaproic acid or a lower dose of systemic aminocaproic acid (50 mg/kg instead of 100 mg/kg) may be efficacious in reducing secondary hemorrhage with a potential reduction in the risk of side effects. Future research with such agents aimed at assessing impact on final VA after the resolution of the hyphema, time to achieve final VA, cost, and quality of life (side effects and time lost from school and employment) would be most helpful to guide treatment recommendations. Ongoing or future studies on medical treatment of hyphema should particularly study sickle cell disease/trait patients. Studies with direct comparisons of aminocaproic acid to tranexamic acid do not exist yet, and only one study compared aminocaproic acid with prednisolone. Further research to study the additive effect of non-medical interventions in hyphema management might be of value, because they are not usually used independently of one another.
Summary of findings tables
Additional tables
Acknowledgments
We acknowledge the peer reviewers who made valuable comments on the protocol and manuscript of the systematic review. We thank Dr. Milan Mathew for his contribution to the development of the protocol. We also thank Dr. Tianjing Li for her comments on the protocol and translation of a Chinese language trial report. In addition we thank Kinnar Merchant for translating Russian reports of trials. The Cochrane Eyes and Vision Group created and ran the search strategies for this review.
Sources of support
Internal sources
No sources of support provided
External sources
Contract N-01-EY-2-1003 and Grant 1 U01 EY020522-01, National Eye Institute, National Institutes of Health, USA
Appendices
1 CENTRAL search strategy
-
#1
MeSH descriptor Hyphema
-
#2
hyphem* or hyphaema*
-
#3
MeSH descriptor Anterior Chamber explode all trees with qualifier: IN
-
#4
(#1 OR #2 OR #3)
2 MEDLINE search strategy
randomized controlled trial.pt.
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1–7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
Hyphema/
(hyphem$ or hyphaem$).tw.
[*Anterior Chamber/in [Injuries]]
or/13–15
12 and 16
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
3 EMBASE search strategy
exp randomized controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1–5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12–21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25–28
29 not 10
30 not (11 or 23)
11 or 24 or 31
hyphema/
(hyphem$ or hyphaem$).tw.
or/33–34
32 and 35
4 metaRegister of Controlled Trials search strategy
hyphema or hyphaema
5 ClinicalTrials.gov search strategy
Hyphema OR Hyphaema
Data and analyses
1 Oral aminocaproic acid versus placebo
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 1.1 Long-term visual acuity between 20/20 and 20/40 | 2 | 108 | Odds Ratio(M-H, Fixed, 95% CI) | 1.11[0.47, 2.61] |
| 1.2 Short-term visual acuity from 20/20 to 20/40 | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 1.3 Final visual acuity between 20/20 and 20/40 | 2 | 143 | Odds Ratio(M-H, Fixed, 95% CI) | 1.56[0.53, 4.56] |
| 1.4 Time to resolution of primary hemorrhage (days) | 6 | Other data | No numeric data | |
| 1.5 Risk of secondary hemorrhage | 6 | 330 | Odds Ratio(M-H, Fixed, 95% CI) | 0.25[0.11, 0.57] |
| 1.6 Time to rebleed (days) | 6 | Other data | No numeric data | |
| 1.7 Risk of corneal bloodstain | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 1.8 Risk of glaucoma or elevated IOP | 2 | 83 | Odds Ratio(M-H, Fixed, 95% CI) | 0.35[0.06, 1.98] |
| 1.9 Risk of glaucoma or increases in IOP | 3 | Other data | No numeric data | |
| 1.9.1 Transient increase in IOP | 1 | Other data | No numeric data | |
| 1.9.2 Persistant increase in IOP | 2 | Other data | No numeric data | |
| 1.10 Risk of optic atrophy | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 1.11 Adverse effects: Nausea or vomiting | 3 | 131 | Odds Ratio(M-H, Fixed, 95% CI) | 11.76[2.59, 53.46] |
| 1.12 Duration of hospitalization (days) | 2 | Other data | No numeric data |
2 Topical aminocaproic acid versus placebo
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 2.1 Short-term visual acuity from 20/20 to 20/40 | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 2.2 Time to resolution of primary hemorrhage (days) | 2 | Other data | No numeric data | |
| 2.3 Risk of secondary hemorrhage | 2 | 131 | Odds Ratio(M-H, Fixed, 95% CI) | 0.42[0.16, 1.10] |
| 2.4 Time to rebleed (days) | 2 | Other data | No numeric data | |
| 2.5 Risk of glaucoma or elevated IOP | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals |
3 Low versus standard dose aminocaproic acid
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 3.1 Unspecified time for visual acuity between 20/20 and 20/40 | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.2 Time to resolution of primary hemorrhage (days) | 1 | Mean Difference(IV, Fixed, 95% CI) | No totals | |
| 3.3 Risk of secondary hemorrhage | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.4 Time to rebleed (days) | 1 | Other data | No numeric data | |
| 3.5 Risk of glaucoma or elevated IOP | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.6 Adverse effects | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.6.1 Nausea or vomiting | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.6.2 Dizziness or hypotension | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.6.3 Syncope | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.6.4 Diarrhea | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.6.5 Rash or pruritis | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.6.6 Hot flashes | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.6.7 Dry mouth or nose | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 3.7 Duration of hospitalization (days) | 1 | Mean Difference(IV, Fixed, 95% CI) | No totals |
4 Oral versus topical aminocaproic acid
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 4.1 Short-term visual acuity from 20/20 to 20/40 | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 4.2 Risk of secondary hemorrhage | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 4.3 Time to rebleed (days) | 1 | Other data | No numeric data | |
| 4.4 Risk of corneal bloodstain | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 4.5 Risk of optic atrophy | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 4.6 Adverse effects | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 4.6.1 Conjunctival corneal foreign body sensation | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 4.6.2 Transient punctate corneal staining | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 4.6.3 Dizziness, nausea, vomiting | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals |
5 Tranexamic acid versus control
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 5.1 Short-term visual acuity from 20/20 to 20/40 | 3 | 303 | Odds Ratio(M-H, Fixed, 95% CI) | 1.65[0.91, 2.99] |
| 5.2 Time to resolution of primary hemorrhage (days) | 5 | Other data | No numeric data | |
| 5.3 Risk of secondary hemorrhage | 5 | 578 | Odds Ratio(M-H, Fixed, 95% CI) | 0.25[0.13, 0.49] |
| 5.4 Time to rebleed (days) | 5 | Other data | No numeric data | |
| 5.5 Risk of corneal bloodstain | 2 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 5.6 Risk of glaucoma or elevated IOP | 4 | 543 | Odds Ratio(M-H, Fixed, 95% CI) | 1.23[0.70, 2.16] |
| 5.7 Risk of optic atrophy | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 5.8 Adverse effects: Nausea or vomiting | 2 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 5.9 Duration of hospitalization (days) | 3 | Other data | No numeric data |
6 Aminomethylbenzoic acid versus placebo
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 6.1 Risk of secondary hemorrhage | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals |
7 Oral corticosteroids versus control
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 7.1 Short-term (5 to 14 day) visual acuity from 20/20 to 20/40 | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 7.2 Visual acuity between 20/20 and 20/50 at resolution of hyphema | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 7.3 Time to resolution of primary hemorrhage (days) | 2 | Other data | No numeric data | |
| 7.4 Risk of secondary hemorrhage | 2 | 201 | Odds Ratio(M-H, Fixed, 95% CI) | 0.61[0.31, 1.22] |
| 7.5 Time to rebleed (days) | 2 | Other data | No numeric data | |
| 7.6 Risk of corneal bloodstain | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 7.7 Risk of peripheral anterior synechiae | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 7.8 Risk of glaucoma or elevated IOP | 2 | 201 | Odds Ratio(M-H, Fixed, 95% CI) | 0.75[0.31, 1.81] |
| 7.9 Duration of hospitalization (days) | 1 | Mean Difference(IV, Fixed, 95% CI) | No totals |
8 Topical corticosteroids versus control
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 8.1 Short-term (5 to 14 day) visual acuity from 20/20 to 20/40 | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 8.2 Time to resolution of primary hemorrhage (days) | 1 | Other data | No numeric data | |
| 8.3 Risk of secondary hemorrhage | 2 | 151 | Odds Ratio(M-H, Fixed, 95% CI) | 0.27[0.05, 1.61] |
| 8.4 Risk of corneal bloodstain | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 8.5 Risk of glaucoma or elevated IOP | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 8.6 Risk of optic atrophy | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 8.7 Duration of hospitalization (days) | 1 | Other data | No numeric data |
9 Aminocaproic acid versus prednisone
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 9.1 Short-term (5 to 14 day) visual acuity from 20/20 to 20/40 | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 9.2 Risk of secondary hemorrhage | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 9.3 Adverse effect: any adverse event | 1 | 112 | Odds Ratio(M-H, Fixed, 95% CI) | Not estimable |
10 Conjugated estrogen versus placebo
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 10.1 Risk of secondary hemorrhage | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 10.2 Risk of corneal bloodstain | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals |
11 Cycloplegics versus miotics
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 11.1 Short-term visual acuity | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 11.2 Time to resolution of primary hemorrhage (days) | 1 | Mean Difference(IV, Fixed, 95% CI) | No totals | |
| 11.3 Risk of secondary hemorrhage | 2 | 92 | Odds Ratio(M-H, Fixed, 95% CI) | 1.03[0.14, 7.53] |
| 11.4 Time to rebleed (days) | 1 | Other data | No numeric data |
12 Aspirin versus observation
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 12.1 Risk of secondary hemorrhage | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals |
13 Monocular versus binocular patching
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 13.1 Short-term visual acuity | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 13.2 Variable Time Length “Final’ Visual Acuity | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 13.3 Risk of secondary hemorrhage | 2 | 117 | Odds Ratio(M-H, Fixed, 95% CI) | 0.72[0.26, 2.00] |
| 13.4 Time to rebleed (days) | 1 | Other data | No numeric data | |
| 13.5 Risk of corneal bloodstain | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 13.6 Risk of glaucoma or elevated IOP | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals |
14 Ambulatory versus conservative treatment
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 14.1 Short-term visual acuity | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 14.2 Time to resolution of primary hemorrhage | 1 | Other data | No numeric data | |
| 14.3 Risk of secondary hemorrhage | 2 | 189 | Odds Ratio(M-H, Fixed, 95% CI) | 1.36[0.62, 2.99] |
| 14.4 Risk of corneal bloodstain | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals | |
| 14.5 Risk of glaucoma or elevated IOP | 1 | Odds Ratio(M-H, Fixed, 95% CI) | No totals |
Other data tables
1 Oral aminocaproic acid versus placebo
1.4 Time to resolution of primary hemorrhage (days)
| Study ID | Mean (SD) time to resolution in drug treated group | Number of participants in drug treated group | Mean (SD) time to resolution in control group | Number of participants in control group |
|---|---|---|---|---|
| Christianson 1979 | NR | 22 | NR | 23 |
| Crouch 1976 | 4.1 days (4.0 days in study participants without secondary hemorrhage) | 32 (31 without a secondary hemorrhage) | 3.8 days (2.8 days in study participants without secondary hemorrhage) | 27 (18 without a secondary hemorrhage) |
| Kraft 1987 | 8 days (5.3 days in study participants without secondary hemorrhage) | 24 (22 without a secondary hemorrhage) | 5 days (2.6 days in study participants without a secondary hemorrhage) | 25 (24 without a secondary hemorrhage) |
| Kutner 1987 | 4.8 days in all study participants | 21 (no participant had a secondary hemorrhage | 2.4 days in all study participants | 10 study participants without a secondary hemorrhage |
| McGetrick 1983 | 4.5 days in all study participants | 28 (1 study participant had a secondary hemorrhage) | 6.3 days in all study participants | 21 (7 study participants had a secondary hemorrhage) |
| Teboul 1995 | 6.7 days in all study participants | 48 (1 study participant had a secondary hemorrhage) | 2.6 days in all study participants | 46 (2 study participants had a secondary hemorrhage) |
1.6 Time to rebleed (days)
| Study ID | Number of rebleeds in drug treated group | Time to rebleed in drug treated group | Number of rebleeds in control group | Time to rebleed in control group |
|---|---|---|---|---|
| Christianson 1979 | 2 of 22 | NR | 1 of 23 | NR |
| Crouch 1976 | 1 of 32 | Day 1 | 9 of 27 | Days 2 to 7: 2 on day 2; 2 on day 3; 4 on day 4; and 1 on day 7 |
| Kraft 1987 | 2 of 24 | Days 3 and 4 | 1 of 25 | Day 4 |
| Kutner 1987 | 0 of 21 | NA | 3 of 13 | All rebled on Day 2 |
| McGetrick 1983 | 1 of 28 | Day 4 | 7 of 21 | Days 3 to 6: 5 on day 3; 1 on day 5; and 1 on day 6 |
| Teboul 1995 | 1 of 48 | Day 6 | 2 of 46 | Days 2 and 7 |
1.9 Risk of glaucoma or increases in IOP
1.9.1 Transient increase in IOP
| Study ID | Odds Ratio [95% CI] | Total patients (N) | Definition of outcome | Patients with sickle cell/trait |
|---|---|---|---|---|
| Teboul 1995 | 0.96 [0.18, 5.00] | 94 | Transient IOP greater than 25 mmHg, all patients had normal IOP at discharge (5 days) | None (excluded) |
1.9.2 Persistant increase in IOP
| Study ID | Odds Ratio [95% CI] | Total patients (N) | Definition of outcome | Patients with sickle cell/trait |
|---|---|---|---|---|
| Kraft 1987 | 1.04 [0.06, 17.69] | 49 | IOP greater than 25 mmHg at follow-up (6 weeks to 18 months) | None (excluded) |
| Kutner 1987 | 0.17 [0.02, 1.81] | 34 | Elevated IOP at time of discharge (6 days) | None (excluded) |
1.12 Duration of hospitalization (days)
| Study ID | Mean (SD) duration of hospitalization for drug treated group | Number of participants in drug treated group | Mean (SD) duration of hospitalization in control group | Number of participants in control group |
|---|---|---|---|---|
| McGetrick 1983 | 5.7 days | 28 | 7.3 days | 20 |
| Teboul 1995 | 7.3 days | 48 | 5.4 days | 46 |
2 Topical aminocaproic acid versus placebo
2.2 Time to resolution of primary hemorrhage (days)
| Study ID | Mean (SD) time to resolution in drug treated group | Number of participants in drug treated group | Mean (SD) time to resolution in control group | Number of participants in control group |
|---|---|---|---|---|
| Karkhaneh 2003 | 11.1 (4.7) days | 41 | + Placebo gel: 9.3 (4.2) days No placebo gel: 9.5 (3.9) days |
+ Placebo gel: 39 No placebo gel: 52 |
| Pieramici 2003 | Reported as “no difference between treatment groups” | 24 | Reported as “no difference between treatment groups” | 27 |
2.4 Time to rebleed (days)
| Study ID | Number of rebleeds in drug treated group | Time to rebleed in drug treated group | Number of rebleeds in control group | Time to rebleed in control group |
|---|---|---|---|---|
| Karkhaneh 2003 | 5 of 41 | Days 2 to 4: Mean = 3.2 days; SD = 0.5 | + Placebo gel: 7 of 39 No placebo gel: 8 of 52 |
+ Placebo gel: Mean = 3 days; SD = 0.8 No placebo gel: Mean = 3 days; SD = 0.8 |
| Pieramici 2003 | 2 of 24 | Days 3 and 6 | 8 of 27 | Days 2 to 6: 3 on day 2; 1 on day 3; 2 on day 4; and 2 on day 6 |
3 Low versus standard dose aminocaproic acid
3.4 Time to rebleed (days)
| Study ID | Number of rebleeds in the low dose group | Time to rebleed in the low dose group | Number of rebleeds in the standard dose group | Time to rebleed in the standard dose group |
|---|---|---|---|---|
| Palmer 1986 | 1 of 25 | Day 4 | 5 of 32 | Days 2 to 6: 1 on day 2; 2 on day 3; and 2 on day 6 |
4 Oral versus topical aminocaproic acid
4.3 Time to rebleed (days)
| Study ID | Number of rebleeds in oral treated group | Time to rebleed in oral treated group | Number of rebleeds in topical treated group | Time to rebleed in topical treated group |
|---|---|---|---|---|
| Crouch 1997 | 1 | Day 3 | 1 | Day 5 |
5 Tranexamic acid versus control
5.2 Time to resolution of primary hemorrhage (days)
| Study ID | Mean (SD) time to resolution in drug treated group | Number of participants in drug treated group | Mean (SD) time to resolution in control group | Number of participants in control group |
|---|---|---|---|---|
| Rahmani 1999 | 4.0 (2.2) days in study participants without secondary hemorrhage | 72 | 3.7 (1.6) days in study participants without secondary hemorrhage | 59 |
| Sukumaran 1988 | 4.6 (2.4) days in all study participants | 17 (2 study participants had a secondary hemorrhage) | 3.9 (2.4) days in all study participants | 18 (6 study participants had a secondary hemorrhage) |
| Vangsted 1983 | Reported as delayed | 59 | NR | 53 |
| Varnek 1980 | NR | 102 | NR | 130 |
| Welsh 1983 | NR | 19 | NR | 20 |
5.4 Time to rebleed (days)
| Study ID | Number of rebleeds in drug treated group | Time to rebleed in drug treated group | Number of rebleeds in control group | Time to rebleed in control group |
|---|---|---|---|---|
| Rahmani 1999 | 8 of 80 | Days 2 to 4: Mean = 3.4 days; SD = 0.7 | 21 of 80 | Days 2 to 6: Mean = 3.8 days; SD = 1.0 |
| Sukumaran 1988 | 2 of 17 | Days 2 to 3 | 6 of 18 | Days 2 to 3 |
| Vangsted 1983 | 0 of 59 | NA | 0 of 53 | NA |
| Varnek 1980 | 2 of 102 | Day 3 | 12 of 130 | Days 2 to 7: 5 occurred on Day 4 |
| Welsh 1983 | 1 of 19 | NR | 6 of 20 | NR |
5.9 Duration of hospitalization (days)
| Study ID | Mean (SD) duration of hospitalization for drug treated group | Number of participants in drug treated group | Mean (SD) duration of hospitalization in control group | Number of participants in control group |
|---|---|---|---|---|
| Rahmani 1999 | 6.0 (1.6) days | 80 | 6.3 (1.8) days | 80 |
| Vangsted 1983 | 6 days | 59 | 7 days | 53 |
| Varnek 1980 | 6.8 days | 102 | 6.5 days | 130 (Analysis 8.7) |
7 Oral corticosteroids versus control
7.3 Time to resolution of primary hemorrhage (days)
| Study ID | Time to resolution in drug group | Number of participants in drug group | Time to resolution in control group | Number of participants in control group |
|---|---|---|---|---|
| Rahmani 1999 | 3.5 days (SD = 1.8) in study participants without a secondary hemorrhage | 64 | 3.7 days (SD = 1.6) in study participants without a secondary hemorrhage | 59 |
| Spoor 1980 | 4.45 days (4.01 days in study participants without a secondary hemorrhage) | 23 (20 without a secondary hemorrhage) | 4.48 days (3.60 days in study participants without a secondary hemorrhage) | 20 (16 without a secondary hemorrhage) |
7.5 Time to rebleed (days)
| Study ID | Number of rebleeds in the drug group | Mean time to rebleed in the drug group | Number of rebleeds in the control group | Mean time to rebleed in the control group |
|---|---|---|---|---|
| Rahmani 1999 | 14 of 78 | 3.2 days (SD = 0.8) | 21 of 80 | 3.8 days (SD = 1.0) |
| Spoor 1980 | 3 of 23 | 2.3 days | 4 of 20 | 2.6 days |
8 Topical corticosteroids versus control
8.2 Time to resolution of primary hemorrhage (days)
| Study ID | Time to resolution in drug group | Number of participants in drug group | Time to resolution in control group | Number of participants in control group |
|---|---|---|---|---|
| Rakusin 1972 | 10 resolved within 7 days | 13 (1 study participant had a secondary hemorrhage) | 16 resolved within 7 days | 21 (2 study participants had a secondary hemorrhage) |
8.7 Duration of hospitalization (days)
| Study ID | Mean (SD) duration of hospitalization for drug treated group | Number of participants in drug treated group | Mean (SD) duration of hospitalization in control group | Number of participants in control group |
|---|---|---|---|---|
| Zetterstrom 1969 | 5.9 days (SD not reported) | 58 | 8.9 days (SD not reported) | 59 |
11 Cycloplegics versus miotics
11.4 Time to rebleed (days)
| Study ID | Number of rebleeds in the cycloplegic group | Mean time to rebleed in the cycloplegic group | Number of rebleeds in the miotic group | Mean time to rebleed in the miotic group |
|---|---|---|---|---|
| Bedrossian 1974 | 1 of 28 | 2 days | 0 of 30 | NA |
13 Monocular versus binocular patching
13.4 Time to rebleed (days)
| Study ID | Number of rebleeds in monocular patching group | Time to rebleed in monocular patching group | Number of rebleeds in binocular patching group | Time to rebleed in binocular patching group |
|---|---|---|---|---|
| Edwards 1973 | 8 of 35 | Mean 3 days | 8 of 29 | Mean 3 days |
14 Ambulatory versus conservative treatment
14.2 Time to resolution of primary hemorrhage
| Study ID | Time to resolution in ambulatory group | Number of participants in ambulatory group | Time to resolution in control group | Number of participants in control group |
|---|---|---|---|---|
| Read 1974 | 5.8 days | 5.6 days |
Footnotes
Contributions of authors
Conceiving the review: HS, A-MG
Designing the review: RS, HS
Co-ordinating the review: RS
Undertaking manual searches: RS
Screening search results: RS, KL, A-MG
Organizing retrieval of papers: KL
Screening retrieved papers against inclusion criteria: RS, KL, A-MG
Appraising quality of papers: RS, KL
Abstracting data from papers: RS, KL, A-MG
Writing to authors of papers for additional information: RS, KL
Providing additional data about papers: HS, MG
Data management for the review: RS, KL
Entering data into RevMan: RS, KL
Analysis of data: RS, KL
Interpretation of data: A-MG, HS, MG, RS
Writing the review: RS, KL, A-MG, HS, MG
Performing previous work that was the foundation of current study: A-MG, HS, RS, MG
Declarations of interest
None.
References to studies
Included studies
- Bedrossian 1974.Bedrossian RH. The management of traumatic hyphema. Annals of Ophthalmology. 1974;6(10):1016–8. 1020–1. [PubMed] [Google Scholar]
- Christianson 1979.Christianson MD, Crawford JS. Epsilon aminocaproic acid in the treatment of traumatic hyphema. American Journal of Ophthalmology. 1979;88(4):782. [Google Scholar]
- Crouch 1976.Crouch ER, Jr, Frenkel M. Aminocaproic acid in the treatment of traumatic hyphema. American Journal of Ophthalmology. 1976;81(3):355–60. doi: 10.1016/0002-9394(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Crouch 1997.Crouch ER, Jr, Williams PB, Gray MK, Crouch ER, Chames M. Topical aminocaproic acid in the treatment of traumatic hyphema. Archives of Ophthalmology. 1997;115(9):1106–12. doi: 10.1001/archopht.1997.01100160276001. [DOI] [PubMed] [Google Scholar]
- Edwards 1973.Edwards WC, Layden WE. Monocular versus binocular patching in traumatic hyphema. American Journal of Ophthalmology. 1973;76(3):359–62. doi: 10.1016/0002-9394(73)90491-1. [DOI] [PubMed] [Google Scholar]
- Farber 1991.Farber MD, Fiscella R, Goldberg MF. A randomized clinical trial of Amicar vs. prednisone for the treatment of traumatic hyphema. American Academy of Ophthalmology. 1990:119. doi: 10.1016/s0161-6420(91)32299-1. [DOI] [PubMed] [Google Scholar]; * Farber MD, Fiscella R, Goldberg MF. Aminocaproic acid versus prednisone for the treatment of traumatic hyphema. A randomized clinical trial. Ophthalmology. 1991;98(3):279–86. doi: 10.1016/s0161-6420(91)32299-1. [DOI] [PubMed] [Google Scholar]
- Karkhaneh 2003.Karkhaneh R, Naeeni M, Chams H, Abdollahi M, Mansouri MR. Topical aminocaproic acid to prevent rebleeding in cases of traumatic hyphema. European Journal of Ophthalmology. 2003;13(1):57–61. doi: 10.1177/112067210301300108. [DOI] [PubMed] [Google Scholar]
- Kraft 1987.Kraft SP, Christianson MD, Crawford JS, Wagman RD, Antoszyk JH. Traumatic hyphema in children. Treatment with epsilon-aminocaproic acid. Ophthalmology. 1987;94(10):1232–7. doi: 10.1016/s0161-6420(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Kutner 1987.Kutner B, Fourman S, Brein K, Hobson S, Mrvos D, Sheppard J, et al. Aminocaproic acid reduces the risk of secondary hemorrhage in patients with traumatic hyphema. Archives of Ophthalmology. 1987;105(2):206–8. doi: 10.1001/archopht.1987.01060020060029. [DOI] [PubMed] [Google Scholar]
- Liu 2002.Liu SC, Den JS. The effect of aminomethylbenzoic acid on preventing secondary hyphema. Journal of Injuries and Occupational Diseases of the Eye with Ophthalmic. 2002;24(2):127–8. [Google Scholar]
- Marcus 1988.Marcus M, Biedner B, Lifshitz T, Yassur Y. Aspirin and secondary bleeding after traumatic hyphema. Annals of Ophthalmology. 1988;20(4):157–8. [PubMed] [Google Scholar]
- McGetrick 1983.Anonymous. Aminocaproic acid. Archives of Ophthalmology. 1984;102(6):818, 820–1. doi: 10.1001/archopht.1984.01040030644002. [DOI] [PubMed] [Google Scholar]; * McGetrick JJ, Jampol LM, Goldberg MF, Frenkel M, Fiscella RG. Aminocaproic acid decreases secondary hemorrhage after traumatic hyphema. Archives of Ophthalmology. 1983;101(7):1031–3. doi: 10.1001/archopht.1983.01040020033003. [DOI] [PubMed] [Google Scholar]
- Palmer 1986.Palmer DJ, Goldberg MF, Frenkel M, Fiscella R, Anderson RJ. A comparison of two dose regimens of epsilon aminocaproic acid in the prevention and management of secondary traumatic hyphemas. Ophthalmology. 1986;93(1):102–8. doi: 10.1016/s0161-6420(86)33784-9. [DOI] [PubMed] [Google Scholar]
- Pieramici 2003.Pieramici DJ, Goldberg MF, Melia BM. Topical aminocaproic acid (Caprogel) in the treatment of traumatic hyphema: results of a phase III multicenter placebo controlled trial. Investigative Ophthalmology & Visual Science. 2000;41:ARVO Abstract 1616. [Google Scholar]; * Pieramici DJ, Goldberg MF, Melia M, Fekrat S, Bradford CA, Faulkner A, et al. A phase III, multicenter, randomized, placebo-controlled clinical trial of topical aminocaproic acid (Caprogel) in the management of traumatic hyphema. Ophthalmology. 2003;110(11):2106–12. doi: 10.1016/S0161-6420(03)00866-2. [DOI] [PubMed] [Google Scholar]
- Rahmani 1999.Rahmani B, Jahadi HR, Rajaeefard A. An analysis of risk for secondary hemorrhage in traumatic hyphema. Ophthalmology. 1999;106(2):380–5. doi: 10.1016/S0161-6420(99)90080-5. [DOI] [PubMed] [Google Scholar]; Rahmani B, Jahadi HR, Salour H. Comparison of tranexamic acid and prednisolone in treatment of traumatic hyphema. American Academy of Ophthalmology. 1997:210. doi: 10.1016/S0161-6420(99)90079-9. [DOI] [PubMed] [Google Scholar]; * Rahmani B, Jahadi HR. Comparison of tranexamic acid and prednisolone in the treatment of traumatic hyphema. A randomized clinical trial. Ophthalmology. 1999;106(2):375–9. doi: 10.1016/S0161-6420(99)90079-9. [DOI] [PubMed] [Google Scholar]
- Rakusin 1972.Rakusin W. Traumatic hyphema. American Journal of Ophthalmology. 1972;74(2):284–92. doi: 10.1016/0002-9394(72)90546-6. [DOI] [PubMed] [Google Scholar]
- Read 1974.* Read J, Goldberg MF. Comparison of medical treatment for traumatic hyphema. Transactions of the American Academy of Ophthalmology and Otolaryngology. 1974;78(5):OP799–OP815. [Google Scholar]; Read J. Traumatic hyphema: surgical vs medical management. Annals of Ophthalmology. 1975;7(5):659–62. 664–6, 668–70. [PubMed] [Google Scholar]
- Spaeth 1966.Spaeth GL, Levy PM. Traumatic hyphema: its clinical characteristics and failure of estrogens to alter its course. A double-blind study. American Journal of Ophthalmology. 1966;62(6):1098–106. doi: 10.1016/0002-9394(66)92559-1. [DOI] [PubMed] [Google Scholar]
- Spoor 1980.Spoor TC, Hammer M, Belloso H. Traumatic hyphema. Failure of steroids to alter its course: a double-blind prospective study. Archives of Ophthalmology. 1980;98(1):116–9. doi: 10.1001/archopht.1980.01020030118011. [DOI] [PubMed] [Google Scholar]
- Sukumaran 1988.Sukumaran K. The role of tranexamic acid (Cyklokapron) in the treatment of traumatic hyphaema. Medical Journal of Malaysia. 1988;43(2):155–8. [PubMed] [Google Scholar]
- Teboul 1995.* Teboul BK, Jacob JL, Barsoum-Homsy M, Brunette I, Chevrette L, Milot J, et al. Clinical evaluation of aminocaproic acid for managing traumatic hyphema in children. Ophthalmology. 1995;102(11):1646–53. doi: 10.1016/s0161-6420(95)30814-7. [DOI] [PubMed] [Google Scholar]; Teboul BK, Jacob JL, Barsoum-Homsy M, Brunette I, Chevrette L, Milot J, et al. Clinical evaluation of aminocaproic acid for the management of traumatic hyphema in children. American Academy of Ophthalmology. 1995:175. doi: 10.1016/s0161-6420(95)30814-7. [DOI] [PubMed] [Google Scholar]
- Vangsted 1983.Vangsted P, Nielsen PJ. Tranexamic acid and traumatic hyphaema. A prospective study. Acta Ophthalmologica. 1983;61(3):447–53. doi: 10.1111/j.1755-3768.1983.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Varnek 1980.Varnek L, Dalsgaard C, Hansen A, Klie F. The effect of tranexamic acid on secondary haemorrhage after traumatic hyphaema. Acta Ophthalmologica. 1980;58(5):787–93. doi: 10.1111/j.1755-3768.1980.tb06692.x. [DOI] [PubMed] [Google Scholar]
- Welsh 1983.Welsh N, Schoeman H, Blignaut P. The effect of cyklokapron in traumatic hyphema. A double masked study. South African Archives of Ophthalmology. 1983;10(3–4):67–74. [Google Scholar]
- Zetterstrom 1969.Zetterstrom B. The treatment of contusion of the eye. Acta Ophthalmologica. 1969;47(2):784–91. doi: 10.1111/j.1755-3768.1969.tb08169.x. [DOI] [PubMed] [Google Scholar]
- Zi 1999.Zi QL. The clinical observation of alternatively right and left lateral position in the cases of hyphema. Journal of Nursing Science. 1999;14(5):263–4. [Google Scholar]
Excluded studies
- Amirova 1991.Amirova EKh, Dubilei OV. Experience in the use of streptodecase in the treatment of intraocular hemorrhage. Vestnik Oftalmologii. 1991;107(6):34–6. [PubMed] [Google Scholar]
- Anderson 1971.Anderson TW. Treatment of hyphema with atropinization of contralateral eye. Eye, Ear, Nose & Throat Monthly. 1971;50(7):266–7. [PubMed] [Google Scholar]
- Berrios 1995.Berrios RR, Dreyer EB. Traumatic hyphema. International Ophthalmology Clinics. 1995;35(1):93–103. doi: 10.1097/00004397-199503510-00010. [DOI] [PubMed] [Google Scholar]
- Bramsen 1977.Bramsen T. Traumatic eye hemorrhage (hyphema) treated with the antifibrinolytic preparation tranexamic acid. Ugeskrift for Laeger. 1977;139(24):1422–4. [PubMed] [Google Scholar]
- Bramsen 1980.Bramsen T. The influence of antifibrinolytica on traumatic hyphaema and corneal oedema. Acta Ophthalmologica -Supplementum. 1980;(145):1–53. [PubMed] [Google Scholar]
- Dralands 1981.Dralands L, De Clippeleir L, Missotten L. The prevention of secondary traumatic hyphema. Bulletin de la Société Belge d’Ophtalmologie. 1981;193:77–8. [PubMed] [Google Scholar]
- Gastaldi 1970.Gastaldi TS, Grassi MS. Treatment of the complications of traumatic hyphema [Tratamiento de las complicaciones del hipema traumatico] Archivos De Oftalmologia De Buenos Aires. 1970;45(11):477–83. [PubMed] [Google Scholar]
- Ghisolfi 1972.Ghisolfi A, Manfredini U. Clinical study on a polyenzymic antiinflammatory preparation in some ocular inflammatory conditions [Ricerche cliniche su un antiflogistico polienzimatico in alcune flogosi oculari] Annali di Ottalmologia e Clinica Oculistica. 1972;98(2):69–77. [Google Scholar]
- Gilbert 1973.Gilbert HD, Jensen AD. Atropine in the treatment of traumatic hyphema. Annals of Ophthalmology. 1973;5(12):1297–300. [PubMed] [Google Scholar]
- Gillan 1961.Gillan JG. Treatment and prophyaxis of hyphaema by conjugated estrogens. Transactions of the Canadian Ophthalmological Society. 1961;24:217–22. [PubMed] [Google Scholar]
- Goldberg 1960.Goldberg JL. Conjugated estrogens in the prevention of secondary hyphema after ocular trauma. Archives of Ophthalmology. 1960;63(6):127–30. doi: 10.1001/archopht.1960.00950021003016. [DOI] [PubMed] [Google Scholar]
- Gundorova 1985.Gundorova RA, Romashchenko AD. Immobilized streptokinase (streptodecase) in the treatment of traumatic intra-ocular hemorrhage. Voprosy Meditsinskoi Khimii. 1985;31(4):47–51. [PubMed] [Google Scholar]
- Heath 1966.Heath W. Experiences with urokinase in secondary traumatic hyphaema. Transactions of the Ophthalmological Societies of the United Kingdom. 1966;86:843–5. [PubMed] [Google Scholar]
- Kotas 1990.Kotas R, Neumann D, Ashkenazi I. Epsilon aminocaproic acid for management of traumatic hyphema with large blood clot in the anterior chamber. Harefuah. 1990;119(9):269–70. [PubMed] [Google Scholar]
- Krasnov 1971.Krasnov AM. Beneficial action of glycerine in traumatic hyphema. Vestnik Oftalmologii. 1971;6:43–6. [PubMed] [Google Scholar]
- Latinovic 1981.Latinovic S, Pellegrino N. Glycerin-vitamin C in the treatment of traumatic hyphema [Glicerolo-vitamina C nel trattamento degli ipoemi traumatici] Bollettino Chimico Farmaceutico. 1981;120(3):156–8. [PubMed] [Google Scholar]
- Li 2009.Li Y, Zhu Y. Clinical study of medication for the treatment of traumatic hyphema. International Journal of Ophthalmology. 2009;9(10):2025–6. [Google Scholar]
- Mathis 1987.Mathis A, Malecaze F, Vigne J, Bec P. Treatment of traumatic recurrent hyphema with sodium hyaluronate [Traitement des hyphemas traumatiques recidivants par le hyaluronate de sodium] Bulletin des Sociétés d’Ophtalmologie de France. 1987;87(4):503–4. [PubMed] [Google Scholar]
- Missotten 1977.Missotten L, De Clippeleir L, Van Tornout I, Beenders P. The value of tranexamic acid (cyklokapron) in the prevention of secondary bleeding, a complication of traumatic hyphaemia. Bulletin de la Société Belge d’Ophtalmologie. 1977;179:47–52. [PubMed] [Google Scholar]
- Mortensen 1978.Mortensen KK, Sjolie AK. Secondary hemorrhage following traumatic hyphema. A comparative study of conservative and tranexamic acid treatment. Acta Ophthalmologica. 1978;56(5):763–8. doi: 10.1111/j.1755-3768.1978.tb06640.x. [DOI] [PubMed] [Google Scholar]
- Munoz Negrete 1989.Munoz Negrete FJ, Elosua De Juan I. Prospective study of the efficacy of low dose epsilon aminocaproic acid for the prevention of secondary bleeding after traumatic hyphema. Archivos de la Sociedad Espanola de Oftalmologia. 1989;57(2):105–10. [Google Scholar]
- Murzin 1966.Murzin AA. Experiences with the use of fibrinolysin and trypsin in intraocular hemorrhages [Opyt primeneniia fibrinolizina i tripsina pri vnutriglaznykh krovoizliianiiakh] Vestnik Oftalmologii. 1966;79(3):19–21. [PubMed] [Google Scholar]
- Ohrstrom 1972.Ohrstrom A. Treatment of traumatic hyphaema with corticosteroids and mydriatics. Acta Ophthalmologica. 1972;50(4):549–55. doi: 10.1111/j.1755-3768.1972.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Oksala 1967.Oksala A. Treatment of traumatic hyphaema. British Journal of Ophthalmology. 1967;51(5):315–20. doi: 10.1136/bjo.51.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierse 1964.Pierse D. The use of urokinase in the anterior chamber. Transactions of the Ophthalmological Societies of the United Kingdom. 1964;84:271–4. [PubMed] [Google Scholar]
- Polychronakos 1967.Polychronakos D, Razoglou C. Treatment of total hyphema with fibrinolysin. Ophthalmologica. 1967;154(1):31–8. doi: 10.1159/000305146. [DOI] [PubMed] [Google Scholar]
- Rakusin 1971.Rakusin W. Proceedings: The role of urokinase in the management of traumatic hyphaema. Ophthalmologica. 1973;167(5):373–82. doi: 10.1159/000306977. [DOI] [PubMed] [Google Scholar]; Rakusin W. Urokinase in the management of traumatic hyphaema. British Journal of Ophthalmology. 1971;55(12):826–32. doi: 10.1136/bjo.55.12.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano 1986.Romano PE. Pro steroids for systemic antifibrinolytic treatment for traumatic hyphema. Journal of Pediatric Ophthalmology & Strabismus. 1986;23(2):92–5. doi: 10.3928/0191-3913-19860301-11. [DOI] [PubMed] [Google Scholar]
- Romashchenko 1985.Romashchenko AD, Makarova VP. Experience in treating intraocular hemorrhage using immobilized streptokinase [Opyt lecheniia vnutrioglaznogo krovoizliianiia s pomoshch’iu immobilizovannoi streptokinazy] Oftalmologicheskii Zhurnal. 1985;3:148–51. [PubMed] [Google Scholar]
- Spoor 1990.Spoor TC, Kwitko GM, O’Grady JM, Ramocki JM. Traumatic hyphema in an urban population. American Journal of Ophthalmology. 1990;109(1):23–7. doi: 10.1016/s0002-9394(14)75573-4. [DOI] [PubMed] [Google Scholar]
- Stepanov 2002.Stepanov AV, Bolkvadze ER, Belogurov AA, Tovarova II. Prospects for treating intraocular traumatic hemorrhage using a new fibrinolytic hemase [Vozmozhnosti terapii vnutriglaznykh travmaticheskikh krovoizliianii s pomoshch’iu novogo fibrinolitika gemaza] Vestnik Oftalmologii. 2002;118(5):25–7. [PubMed] [Google Scholar]
- Surel 1987.Surel Z, Cicekdag E. Tranexamic acid treatment in secondary hemorrhage after traumatic hyphema. Turk Oftalmologji Gazetesi. 1987;17(1):49–56. [Google Scholar]
- Tartakovskaia 1972.Tartakovskaia AI, Mikhailova NA. Pathogenetic role of fibrinolysis in recurring intra-ocular hemorrhages and their treatment with sigma-aminocapronic acid [Patogeneticheskaia rol’ fibrinoliza pri retsidiviruiushchikh vnutriglaznykh krovoizliianiikah i ikh terapiia sigma-aminokapronovoi kislotoi] Vestnik Oftalmologii. 1972;1:41–5. [PubMed] [Google Scholar]
- Uusitalo 1988.Uusitalo RJ, Ranta-Kemppainen L, Tarkkanen A. Management of traumatic hyphema in children. An analysis of 340 cases. Archives of Ophthalmology. 1988;106(9):1207–9. doi: 10.1001/archopht.1988.01060140367033. [DOI] [PubMed] [Google Scholar]
- Watkins 1974.Watkins G, Venable HP. E-Aminocaproic acid in traumatic hyphema. Journal of the National Medical Association. 1974;66(6):484–6. [PMC free article] [PubMed] [Google Scholar]
- Welsh 1971.Welsh N. The management of traumatic glaucoma. South African Medical Journal. Suid-Afrikaanse Tydskrif Vir Geneeskunde. 1971;45(44):1250–2. [PubMed] [Google Scholar]
- Williams 1993.Williams C, Laidlaw A, Diamond J, Pollock W, Bloom P. Outpatient management of small traumatic hyphaemas: is it safe? Eye. 1993;7(1):155–7. doi: 10.1038/eye.1993.33. [DOI] [PubMed] [Google Scholar]
- Wilson 1990.Wilson TW, Nelson LB, Jeffers JB, Manley DR. Outpatient management of traumatic microhyphemas. Annals of Ophthalmology. 1990;22(10):366–8. [PubMed] [Google Scholar]
- Wright 1964.Wright JC. Hyphema treated by buccal varidase: a double-blind study. American Journal of Ophthalmology. 1964;58(3):479–82. [PubMed] [Google Scholar]
- Yasuna 1974.Yasuna E. Management of traumatic hyphemas. Archives of Ophthalmology. 1974;91(3):190–1. doi: 10.1001/archopht.1974.03900060198008. [DOI] [PubMed] [Google Scholar]
- Zhou 1982.Zhou FU. Direct current iontherapy with radix pseudoginseng solution for hyphema. Chinese Journal of Ophthalmology [Chung-Hua Yen Ko Tsa Chih] 1982;18(2):83–4. [PubMed] [Google Scholar]
Other references
Additional references
- Crouch 1993.Crouch ER, Jr, Williams PB. Trauma: Ruptures and Bleeding. In: Tasman W, Jaeger EM, editors. Duane’s Clinical Ophthalmology. Vol. 4. Philadelphia, PA: JB Lippincott; 1993. pp. 1–18. [Google Scholar]
- Crouch 1999.Crouch ER, Jr, Crouch ER. Management of traumatic hyphema: therapeutic options. Journal of Pediatric Ophthalmology and Strabismus. 1999;36(5):238. doi: 10.3928/0191-3913-19990901-04. [DOI] [PubMed] [Google Scholar]
- Deeks 2008.Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. The Cochrane Collaboration; 2008. Chapter 9: Analysing data and undertaking meta-analyses. [updated September 2008] Available from www.cochrane-handbook.org. [Google Scholar]
- Glanville 2006.Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Goldberg 1979a.Goldberg MF. Sickled erythrocytes, hyphema, and secondary glaucoma: I. The diagnosis and treatment of sickled erythrocytes in human hyphemas. Ophthalmic Surgery. 1979;10(4):17–31. [PubMed] [Google Scholar]
- Goldberg 1979b.Goldberg MF, Dizon R, Moses VK. Sickled erythrocytes, hyphema, and secondary glaucoma: VI. The relationship between intracameral blood cells and aqueous humor pH, PO2, and PCO2. Ophthalmic Surgery. 1979;10(4):78–88. [PubMed] [Google Scholar]
- Goldberg 1979c.Goldberg MF, Tso MO. Sickled erythrocytes, hyphema, and secondary glaucoma: VII. The passage of sickled erythrocytes out of the anterior chamber of the human and monkey eye: light and electron microscopic studies. Ophthalmic Surgery. 1979;10(4):89–123. [PubMed] [Google Scholar]
- Higgins 2008.Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. The Cochrane Collaboration; 2008. Chapter 8: Assessing risk of bias in included studies. [updated September 2008] Available from www.cochrane-handbook.org. [Google Scholar]
- Lai 2001.Lai JC, Fekrat S, Barrron Y, Goldberg MF. Traumatic hyphema in children: Risk factors for complications. Archives of Ophthalmology. 2001;119(1):64–70. [PubMed] [Google Scholar]
- RevMan.Review Manager (RevMan). Version 5.0 [Computer program] Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2008. [Google Scholar]
- Rhee 1999.Rhee D, Pyfer M. The Wills Eye Manual. Lippincott Williams and Wilkins; 1999. [Google Scholar]
- Sheppard 2009.Sheppard JD, Crouch ER, Williams PB, Crouch ER, Jr, Rastogi S, Garcia-Valenzuela E. [accessed 8 January 2010];Hyphema. Updated: Dec 22, 2009. http://emedicine.medscape.com/1190165.overview.
- Walton 2002.Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of traumatic hyphema. Survey of Ophthalmology. 2002;47(4):297–334. doi: 10.1016/s0039-6257(02)00317-x. [DOI] [PubMed] [Google Scholar]
- Westlund 1982.Westlund LE, Lundén R, Wallén P. Effect of EACA, PAMBA, AMCA and AMBOCA on fibrinolysis induced by streptokinase, urokinase and tissue activator. Haemostasis. 1982;11(4):235–41. doi: 10.1159/000214669. [DOI] [PubMed] [Google Scholar]
- Wright 2003.Wright KW. Pediatric ocular trauma: Pediatric hyphema. In: Wright KW, Spiegel PH, editors. Pediatric Ophthalmology and Strabismus. Vol. 6. Springer; 2003. pp. 79–82. [Google Scholar]