Abstract
Peyer's patches constitute both an inductive immune site and an enteropathogen invasion route. Peyer's patch mucosae from porcine jejunum were mounted in Ussing chambers, and either Salmonella choleraesuis vaccine strain SC-54 or non-pathogenic rodent and porcine E. coli strains contacted the Peyer's patch mucosa for 90 min. Internalized bacteria were quantified by a gentamicin resistance assay. Monodansylcadaverine (300 µM, luminal addition), an inhibitor of clathrin-mediated endocytosis, significantly inhibited internalization of both E. coli strains relative to tissues untreated with the inhibitor; internalization of SC-54 was unaffected. The actin-disrupting agent cytochalasin D (10 µM, luminal addition), inhibited internalization of pig-adapted E. coli but not that of rodent-adapted E. coli or SC-54. Internalization of SC-54 and non-pathogenic E. coli in Peyer's patches appears to occur through different cellular routes.
Keywords: Salmonella choleraesuis, Escherichia coli, mucosal immunity, enteropathogen, endocytosis, clathrin
I. Introduction
By disseminating immunological information from the gut lumen to mucosal surfaces throughout the body, discrete Peyer’s patch follicles in the small intestine constitute an inductive site for mucosal immunity. The uptake of antigens and other macromolecules involves the adherence of luminal material to the follicle-associated epithelium (FAE), and its subsequent capture and transcellular transport by endosomes (Neutra, 1998). In addition to their role in mucosal immunity, Peyer’s patches serve as portals for rapid intestinal invasion by a number of pathogenic microorganisms (Vasquez-Torres and Fang, 2000). Salmonella (Jepson and Clark, 2001), Yersinia (Autenrieth and Firsching, 1996), and Shigella (Sansonetti et al., 1996) are among the bacterial species that have been shown to gain access to the intestinal submucosa through macropinocytosis into the FAE. On the other hand, Listeria monocytogenes internalizes in Peyer’s patches through clathrin-mediated endocytosis (Velge et al., 1997).
Jejunal Peyer's patches from swine internalize significant amounts of yeast (Beier and Gebert, 1998) as well as macromolecules such as ferritin (Liebler et al., 1995) and horseradish peroxidase (Keljo and Hamilton, 1983). They also internalize Salmonella choleraesuis and E. coli O157:H7 (Green et al., 2003). The present study was designed to test the hypothesis that the internalization processes for pathogenic salmonellae and non-pathogenic E. coli in Peyer’s patches are different. Therefore, we examined and compared the effects of the actin-disrupting drug cytochalasin D and monodansylcadaverine, an inhibitor of clathrin-mediated endocytosis, on the internalization of the enteropathogenic S. choleraesuis vaccine strain SC-54 and both rodent- and swine-adapted commensal E. coli strains in jejunal Peyer's patches from juvenile pigs.
2. Materials and methods
2.1. Drugs
Cytochalasin D was obtained from Calbiochem (San Diego, CA), and initially solubilized in chloroform. Monodansylcadaverine (MDC) was obtained from Sigma Chemical Co. (St. Louis, MO) and initially dissolved in glacial acetic acid. Serial dilutions of both stock solutions were made in distilled water.
2.2. Animals and tissue preparation
Jejunal Peyer's patches were isolated from outbred Yorkshire-Landrace pigs of either sex that were 5 to 9 weeks old and weighed between 10 and 18 kg. Animals had continuous access to water and nonmedicated pig feed and were not fasted prior to sacrifice. They were anesthetized with tiletamine hydrochloride-zolazepam (Telazol®; 8 mg/kg, i.m. injection; Fort Dodge Laboratories, Fort Dodge, IA) in combination with xylazine (8 mg/kg), and subsequently euthanized with Beuthanasia®-D Special (0.5 ml/kg, i.v. injection; Schering-Plough Animal Health, Union, NJ) in accordance with approved University of Minnesota IACUC protocols. Each jejunal Peyer's patch was stripped of its underlying smooth muscle coats and the remaining mucosa with attached submucosa was mounted in Ussing flux chambers (2 cm2 area). Mucosal sheets were bathed on their luminal and contraluminal aspects with a physiological saline solution similar in composition to porcine extracellular fluid (composition in mM: NaCl, 130; KCl, 6; CaCl2, 3; MgCl2, 0.7; NaHCO3, 20; NaH2PO4, 0.29; and Na2HPO4, 1.3); this buffer solution was maintained at porcine core temperature (39° C) and aerated with 95% O2/5% CO2 by gas lift.
2.3. Bacterial cultures and assessment of intracellular bacterial uptake
A mucosa-associated strain (lab code #3) of porcine non-O157 E. coli was obtained by plating homogenized colonic mucosa from normal pigs onto Fluorocult agar (EM Science, Gibbstown, NJ) supplemented with 100 µg/ml streptomycin sulfate to isolate E. coli strains that were resistant to this antibiotic drug. The selective isolation and differentiation capabilities of Fluorocult medium for Enterobacteriaceae, especially E. coli O157:H7, which are achieved by a combination of fluorogenic and chromogenic substrates, have been well described to identify relevant bacteria from a variety of sources (15). Presumptive colonies of E. coli that did not have the appearance of E. coli O157:H7 were randomly chosen from Fluorocult plates following overnight incubation and were streaked onto Luria-Bertani (LB) agar plates supplemented with 100 µg/ml streptomycin. Following 24 h incubation at 37° C, individual colonies were picked from these plates and their identities confirmed as Escherichia coli using the API-20E Enteric Identification System (BioMerieux, Hazelwood, MO). Colonies were further determined to represent non-O157 E. coli with the use of an E. coli O157 latex agglutination-based diagnostic test kit (Oxoid, Ogdensburg, NY).
On the day of each experiment, an inoculum of the porcine commensal E. coli strain, a non-pathogenic, streptomycin-resistant E. coli strain M-21 (Wells et al., 1994), or Salmonella choleraesuis strain SC-54 (Roof and Doitchinoff, 1995) was grown to mid-log phase in Luria-Bertani (LB) medium at 37° C in a humidified 5% CO2 atmosphere. All bacteria were sensitive to gentamicin. Following reservation of aliquots for subsequent quantification by spread-plating, bacterial inocula were added to the luminal aspect of Peyer's patches to achieve an initial bacterial load of 168. 9 ± 17.4 × 104, 41.1 ± 8.3 ×104, and 28.5 ± 3.1 ×104 CFU/ml for pig and rodent E. coli and SC-54, respectively. Bacteria remained in contact with the mucosal aspect of Peyer’s patches for 90 min. After each experiment, tissues were incubated at 37° under 5% CO2 in PBS containing 100 µg/ml gentamicin for 80 min to eliminate extracellular bacteria (Elsinghorst, 1994). They were subsequently homogenized, serially diluted, and spread-plated on differential and selective media for porcine E. coli (Fluorocult agar containing 1 mg/ml streptomycin sulfate; EM Science, Gibbstown, NJ), M-21 (MacConkey agar containing 1 mg/ml streptomycin sulfate; Becton Dickinson, Sparks, MD) and SC-54 (XLD agar; Becton Dickinson, Sparks, MD). Some tissues from each animal were not mounted in Ussing chambers or exposed to bacteria, but were nevertheless homogenized and cultured for indigenous bacteria to control for any pre-existing Salmonella infections or the presence of any gentamicin-resistant microorganisms. Drugs were added to the luminal bathing medium 15 min prior to (rodent-adapted E. coli, SC-54) or at the time of (porcine E. coli) bacterial inoculation; in control experiments, drug solvents were added to 10 ml bathing reservoirs in a volume of 10 µl.
2.5. Data analysis
Bacterial counts (CFU/g tissue ) recovered from tissues were converted to log10 values and compared to bacterial counts obtained in control tissues from the same animal that were exposed to luminal bacteria, but not inhibitors. Comparisons between control and treatment means were made by a paired, two-tailed t test. Comparisons of a control mean with multiple treatment means were made by one-way analysis of variance followed by Tukey’s test. Statistical analyses of data were performed using the PRISM computer software program (Version 3.0; GraphPad Software, Inc., San Diego, CA). The limit for statistical significance was set at P < 0.05.
3. Results
3.1. Effects of monodansylcadaverine (MDC) on bacterial internalization
MDC is an inhibitor of transglutaminase activity that is associated with clathrin-dependent endocytosis (Levitzki et al., 1980). Luminal addition of 300 µM MDC inhibited Peyer's patch internalization of both strains of E. coli, but not S. choleraesuis (Fig. 1). MDC had no effect on either Isc (data not shown) or Gt (Table 1). There were no significant changes in bacterial internalization or electrical parameters in tissues exposed to glacial acetic acid (10 µl/10 ml reservoir), which was used to solubilize MDC (data not shown).
Fig. 1.
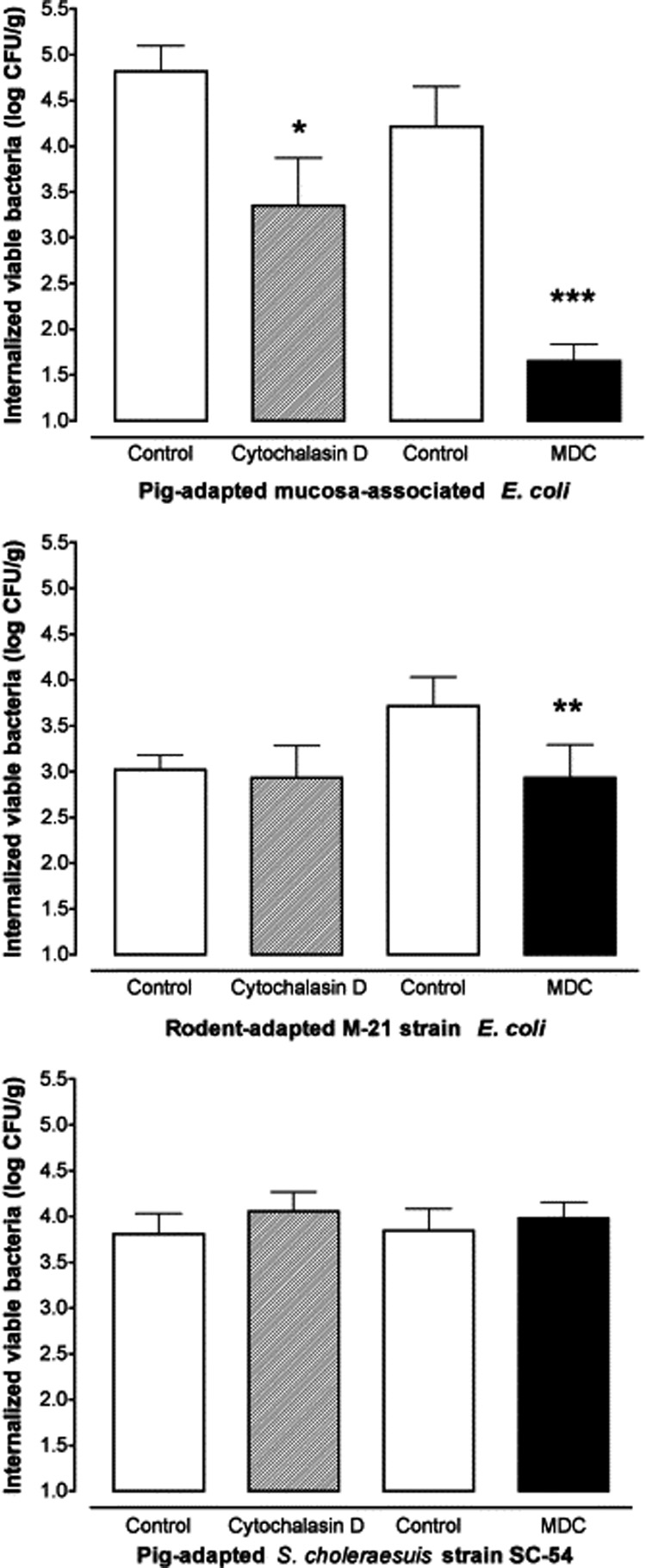
The effect of cytochalasin D and monodansylcadaverine (MDC) on bacterial internalization of pig-adapted E. coli (top), rodent E. coli strain M-21 (middle) and S. choleraesuis SC-54 (bottom) in porcine jejunal Peyer's patches. Tissues were pretreated with either 10 µM cytochalasin D or 300 µM MDC in the luminal bathing medium prior to 90 min exposure to luminal bacteria. Mean internalization of porcine E. coli, rodent E. coli M-21, or SC-54 in control tissues not treated with cytochalasin was 4.82 ± 0.28 (6 tissues/6 pigs), 3.02 ± 0.16 (6 tissues/6 pigs) and 3.81 ± 0.23 log10 CFU/g (8 tissues/8 pigs), respectively. Mean internalization of porcine E. coli, rodent E. coli M-21, or SC-54 in control tissues not treated with MDC was 4.22 ± 0.43 (6 tissues/6 pigs), 3.71 ± 0.32 (8 tissues/8 pigs) and 3.85 ± 0.24 log10 CFU/g (9 tissues/9 pigs), respectively. *P < 0.05, **P < 0.01 and ***P < 0.005 vs. control mean; two-tailed, paired t-test.
Table 1.
Tissue conductance (Gt) in monodansylcadaverine (MDC)-treated or untreated Peyer's patches at different time points before (t = 0 and 15 min) and during (t = 30 and 105 min) mucosal exposure to S. choleraesuis SC-54 or E. coli M-21
| Treatment | Gt t=0 (mS/cm2) | Gt t=15 (mS/cm2) | Gt t=30 (mS/cm2) | Gt t=105 (mS/cm2) | n/N |
|---|---|---|---|---|---|
| Control/SC-54 | 13 ± 1 | 13 ± 1 | 14 ± 1 | 13 ± 1 | 28/18 |
| MDC/SC-54 | 16 ± 1 | 18 ± 2 | 16 ± 2 | 13 ± 2 | 21/9 |
| Control/M-21 | 16 ± 1 | 16 ± 2 | 12 ± 2 | 14 ± 1 | 41/14 |
| MDC/M-21 | 16 ± 2 | 15 ± 2 | 14 ± 2 | 12 ± 2 | 17/8 |
Data are expressed as mean ± standard error of the mean.
3.2. Effects of cytochalasin D and other actin inhibitors on bacterial internalization
Cytochalasin D caps the barbed end of actin filaments to prevent elongation of filamentous (F) actin, severs actin filaments, and sequesters actin monomers (Spector et al., 1999). Pretreatment of Peyer's patches with 10 µM cytochalasin D in the luminal bathing medium significantly decreased internalization of the porcine-adapted E. coli strain relative to control tissues that were not treated with cytochalasin (Fig. 1, top). Internalization of E. coli M-21 and S. choleraesuis were not significantly different between control tissues and those exposed to cytochalasin D (Fig. 1, middle and bottom).
S. choleraesuis internalization was not affected by pretreatment of Peyer’s patches with jasplakinolide (1 µM, luminal addition) which induces actin polymerization (3.73 ± 0.18 and 3.84 ± 0.33 log10 CFU/g in control and jasplakinolide -treated tissues, n = 6 tissue pairs, P = 0.80). It was also unaffected by latrunculin A (1 µM, luminal addition) which binds to and sequesters monomeric globular (G) actin to prevent actin filament formation and increases the rate of actin depolymerization (3.69 ± 0.21 and 4.09 ± 0.24 log10 CFU/g in control and latrunculin-treated tissues, n = 7 tissue pairs, P = 0.17).
3.3. Effects of MDC and cytochalasin D on mucosal electrical parameters
At the luminal concentrations used, neither MDC nor cytochalasin had significant effects on Isc (data not shown) or Gt (Table 1). In contrast, the calcium-chelating agent EDTA, which impairs epithelial barrier function (Quan et al., 1998), did not significantly change Gt from a resting mean value of 20.5 ± 2.1 mS/cm2 to 22.1 ± 1.9 mS/cm2 measured within 3 min after its luminal addition at a concentration of 20 mM (P = 0.21, df 10, paired t test).
4. Discussion
Peyer’s patches constitute a well known route of entry and colonization for Salmonella in the intestinal tract (Jepson and Clark, 2001). The S. enterica serovar choleraesuis avirulent strain SC-54 used in the present investigation produces a mild enteritis in swine, suggesting that it can invade submucosal regions of the intestine (Roof and Doitchinoff, 1995). Several strains of E. coli have been reported to adhere to the Peyer’s patch mucosa, but to our knowledge, few if any studies have reported their apparent internalization into Peyer’s patches with a gentamicin-resistance assay. MDC did not alter the recovery of viable SC-54 from gentamicin-treated Peyer’s patches, but significantly decreased that of pig- and rodent-adapted strains of E. coli used in this investigation. By disrupting the formation of clathrin-coated vesicles, MDC interferes with one endocytotic route in Peyer’s patches (Brodsky et al., 2001). In some strains of pathogenic E. coli, the afimbrial adhesin protein D mediates bacterial internalization into HeLa cells through a clathrin-dependent mechanism (Jouve et al., 1997). It is possible that the E. coli strains examined in the present study express similar adhesins. Polyamines contributing to mucosal integrity are incorporated into porcine jejunal enterocytes by a transglutaminase-catalyzed process (M’Rabet-Touil et al., 1995), and it is possible that MDC-induced inhibition of E. coli internalization might be a consequence of impaired polyamine metabolism leading to changes in FAE function.
The epithelial cytoskeleton plays a crucial role in the internalization of enteropathogens, including Salmonella (Jeng and Welch, 2001; Finlay and Brumell, 2000). For example, cytochalasin D inhibits internalization of S. choleraesuis into MDCK cell monolayers (Finlay and Falkow, 1988). On the other hand, Wells et al. (1998) has reported that cytochalasin D likewise augments S. typhimurium uptake into HT-29 and Caco-2 colonic epithelial cell monolayers. This effect was attributed to an increase in the epithelial surface area available for pathogen uptake, as inferred from substantial decreases in transepithelial electrical resistance (or increases in its reciprocal parameter, Gt). The mechanisms underlying Salmonella internalization have not been studied previously in intact Peyer’s patches. Cytochalasin D had no effect on the internalization of SC-54 or the non-pathogenic rodent E. coli strain M-21, but internalization of the swine-adapted E. coli strain was markedly decreased. In isolated, muscle-stripped sheets of porcine ileal mucosa, we have observed that cytochalasin D at 10 µM significantly decreases S. typhimurium internalization (K.L. Schreiber and D.R. Brown, unpublished observations). This result implies that the mechanisms by which porcine-adapted E. coli and S. choleraesuis internalize in intact Peyer’s patches are fundamentally different. Moreover, there may be a difference in the sensitivity of porcine- and rodent-adapted E. coli strains to cytochalasin D. The visualization of drug actions on intracellular actin dynamics are clearly needed to confirm the mechanisms involved in E. coli and SC-54 internalization in intact Peyer's patches.
There has been a considerable amount of interest in using salmonellae as vaccine vectors capable of targeting the FAE of Peyer’s patches, a key inductive site for mucosal immunity (Kaiserlian and Etchart, 1999). These bacterially-based vectors are being investigated in animals and humans for their ability to either confer protection against S. typhi or induce humoral immune responses to heterologous antigens (Mastroeni et al., 2001). Identification of the cellular routes by which salmonellae and other enteric bacteria become associated with Peyer’s patches will enhance our understanding of the pathogenesis of enteric infections and aid in the development of more effective mucosal vaccines.
Acknowledgements
This work was supported in part by National Institutes of Health grants R01 DA-10200 and T32 DA-007239.
The authors thank Dr. Carol L. Wells (Dept. of Laboratory Medicine and Pathology, University of Minnesota) for generously providing the M-21 strain of E. coli for this investigation, Dr. Sanford Weisberg (University of Minnesota Statistical Consulting Service) for expert advice on data analysis, and Lisa Price for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Autenrieth IB, Firsching R. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- Beier R, Gebert A. Kinetics of particle uptake in the domes of Peyer’s patches. Am. J. Physiol. 1998;275:G130–G137. doi: 10.1152/ajpgi.1998.275.1.G130. [DOI] [PubMed] [Google Scholar]
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: Formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Elsinghorst EA. Measurement of invasion by gentamicin resistance. Meth. Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- Finlay BB, Brumell JH. Salmonella interactions with host cells: in vitro to in vivo. Phil. Trans. Roy. Soc. Lond. [B] 2000;355:623–631. doi: 10.1098/rstb.2000.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BB, Gumbiner B, Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J. Cell. Biol. 1988;107:221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BT, Lyte M, Kulkarni-Narla A, Brown DR. Neuromodulation of enteropathogen internalization in Peyer’s patches from porcine jejunum. J. Neuroimmunol. 2003;141:74–82. doi: 10.1016/s0165-5728(03)00225-x. [DOI] [PubMed] [Google Scholar]
- Jeng RL, Welch MD. Cytoskeleton: actin and endocytosis – no longer the weakest link. Curr. Biol. 2001;11:R691–R694. doi: 10.1016/s0960-9822(01)00410-9. [DOI] [PubMed] [Google Scholar]
- Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect. 2001;3:1183–1190. doi: 10.1016/s1286-4579(01)01478-2. [DOI] [PubMed] [Google Scholar]
- Jouve M, Garcia MI, Courcoux P, Labigne A, Gounon P, Le Bouguenec C. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect Immun. 1997;65:4082–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserlian D, Etchart N. Entry sites for oral vaccines and drugs: A role for M cells, enterocytes and dendritic cell? Sem. Immunol. 1999;11:217–227. doi: 10.1006/smim.1999.0177. [DOI] [PubMed] [Google Scholar]
- Keljo D, Hamilton JR. Quantitative determination of macromolecular transport rate across intestinal Peyer’s patches. Am. J. Physiol. 1983;244:G637–G644. doi: 10.1152/ajpgi.1983.244.6.G637. [DOI] [PubMed] [Google Scholar]
- Levitzki A, Willingham M, Pastan I. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc. Natl. Acad. Sci. U.S.A. 1980;77:2706–2710. doi: 10.1073/pnas.77.5.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebler EM, Lemke C, Pohlenz JF. Ultrastructural study of the uptake of ferritin by M cells in the follicle-associated epithelium in the small and large intestines of pigs. Am. J. Vet. Res. 1995;56:725–730. [PubMed] [Google Scholar]
- Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: immune responses and vaccines. Vet. J. 2001;161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- M'Rabet-Touil H, Blachier F, Hellio N, Robert V, Cherbuy C, Darcy-Vrillon B, Duee PH. Transglutaminase activity in enterocytes isolated from pig jejunum. Mol. Cell. Biochem. 1995;146:49–54. doi: 10.1007/BF00926881. [DOI] [PubMed] [Google Scholar]
- Neutra MR. Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol. 1998;274:G785–G791. doi: 10.1152/ajpgi.1998.274.5.G785. [DOI] [PubMed] [Google Scholar]
- Quan YS, Hattori K, Lundborg E, Fujita T, Murakami M, Muranishi S, Yamamoto A. Effectiveness and toxicity screening of various absorption enhancers using Caco-2 cell monolayers. Biol Pharm Bull. 1998;21:615–620. doi: 10.1248/bpb.21.615. [DOI] [PubMed] [Google Scholar]
- Roof MB, Doitchinoff DD. Safety, efficacy, and duration of immunity induced in swine by use of an avirulent live Salmonella choleraesuis-containing vaccine. Am. J. Vet. Res. 1995;56:39–44. [PubMed] [Google Scholar]
- Sansonetti PJ, Arondel J, Cantey JR, Prevost MC, Huerre M. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect. Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I, Braet F, Shochet NR, Bubb MR. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microscopy Res. Techn. 1999;47:18–37. doi: 10.1002/(SICI)1097-0029(19991001)47:1<18::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Velge P, Bottreau E, Van-Langendonck N, Kaeffer B. Cell proliferation enhances entry of Listeria monocytogenes into intestinal epithelial cells by two proliferation-dependent entry pathways. J. Med. Microbiol. 1997;46:681–692. doi: 10.1099/00222615-46-8-681. [DOI] [PubMed] [Google Scholar]
- Wells CL, Jechorek RP, Olmstead SB, Erlandsen SL. Bacterial translocation in cultured enterocytes: magnitude, specificity, and electron microscopic observations of endocytosis. Shock. 1994;1:443–451. [PubMed] [Google Scholar]
- Wells CL, van de Westerlo EMA, Jechorek RP, Haines HM, Erlandsen SL. Cytochalasin-induced actin disruption of polarized enterocytes can augment internalization of bacteria. Infect. Immun. 1998;66:2410–2419. doi: 10.1128/iai.66.6.2410-2419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


