Abstract
A series of bis-pyridinium cyclophane analogs designed as conformationally-restricted bis-quaternary ammonium compounds were evaluated for their affinity for the blood-brain barrier (BBB) choline transporter. All the cyclophanes investigated exhibited high affinity compared to choline. Of these compounds, N, N’-(1, 10-decanediyl)3, 3’-(1, 9-decadiyn-1, 10-diyl)-bis-pyridinium diiodide (5c) and N, N’-(1, 9-nonanediyl)3, 3’-(1, 9-decadiyn-1, 10-diyl)-bis-pyridinium dibromide (5b) exhibited highest affinity with Ki values of 0.8 µM and 1.4 µM, respectively, and constitute some of the most potent BBB choline transporter ligands reported.
Keywords: Blood-brain barrier choline transporter, Quaternary ammonium, Nicotinic acetylcholine receptor
The blood-brain barrier (BBB) is comprised of brain capillary endothelial cells connected by tight cell membrane junctions that circumferentially surround the cell margin, and thus, form a continuous cell membrane, which is essentially impermeable to hydrophilic compounds.1,2 In this respect, the BBB restricts over 98% of all potential neurotherapeutic molecules from entering the central nervous system (CNS).1 Thus, the ability to design compounds which readily penetrate the BBB would be beneficial in pharmacotherapies for many neurological disorders.2,3 Despite this general brain bioavailability issue, it is surprising that research efforts focused on solving this problem are lacking. A potential solution is the utilization of endogenous transporter proteins located at the BBB, the function of which is to shuttle polar nutrients and other endogenous molecules into the CNS from the periphery.1,3 Such active transport brain vector mechanisms for drug delivery may offer new treatment strategies for neurological pathologies and diseases.3 This approach has been successfully employed in early studies on the active transport of polar molecules such as 2-amino-3-(3,4-dihydroxyphenyl)2-methylpropanoic acid (alpha-methyl DOPA) and the chemotherapeutic agent (±)-2-amino-7-bis-[(2-chloroethyl)amino]1,2,3,4-tetrahydro-2-naphthoic acid (D,L-NAM), into the CNS from the periphery via the large neutral amino-acid transporter.4,5
The choline transporter at the BBB has been suggested as a brain drug delivery vector for quaternary ammonium compounds targeting the CNS.2, 3 The BBB choline transporter efficiently delivers the highly polar and positively charged choline molecule from the periphery to the CNS. Choline is a precursor of the neurotransmitter acetylcholine and other essential constituents of cell membranes, such as phosphatidylcholine and sphingomyelin.1 The carrier-mediated transport of choline has been demonstrated in vivo and in vitro to primarily account for total brain uptake of choline.2a,3 The BBB choline transporter has an anionic binding area that accommodates positively charged quaternary ammonium moieties as well as simple cations, such as tetramethylammonium ion.3 Thus, the BBB choline transporter could be a portal for the delivery of pharmacologically relevant concentrations of positively-charged compounds, which normally exhibit restricted permeation by passive diffusion across the BBB. In this respect, the BBB choline transporter has been shown to be responsible for the intracellular transport of the quaternary ammonium ellipticines that are cytotoxic to isolated human brain tumor cells,6 and a nitrogen mustard alkylating agent in lymphoblasts.7
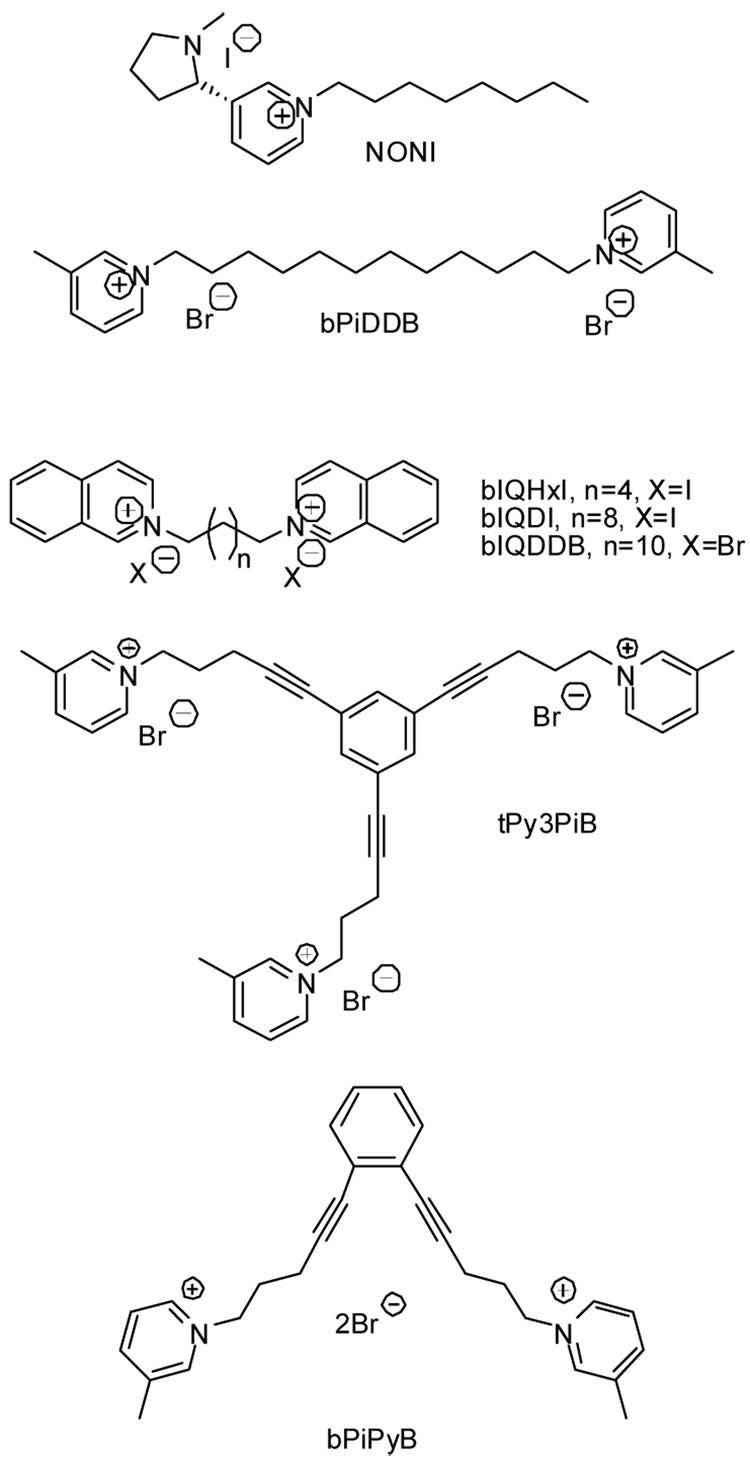
In our continuing effort to develop potent and selective antagonists of nicotinic acetylcholine receptors (nAChRs) as smoking cessation agents,8,9 we have identified a number of quaternary ammonium compounds, such as N-n-octylnicotinium iodide (NONI, Figure 1)10, N,N’-(1,12-dodecandiyl)-bis-pyridinium dibromide (bPiDDB),11a–11d and 1,3,5-tri-{5-[1-(3-picolinium]-pent-1-yn-1-yl}benzene tribromide (tPy3PiB) as potential clinical candidates.12 Given the high polarity and hydrophilicity of such quaternary ammonium compounds, the potential of NONI, bPiDDB and tPy3PiB to penetrate the BBB by passive diffusion is low. However, NONI, bPiDDB and tPy3PiB all have good affinity for the BBB choline transporter with Ki values of 49 µM, 36 µM and 60 µM, respectively; these values are comparable to that of choline (Km ≈ 45 µM). It is important to note that NONI and bPiDDB have both been shown to enter the brain through active transport via the BBB choline transporter.11b,13 This current study evaluates the affinity of some novel, conformationally restricted bis-quaternary ammonium cyclophane analogs for the BBB choline transporter as drug delivery vectors for incorporating into the molecular design of quaternary ammonium drug candidates that target therapeutically relevant sites in the CNS.
Figure 1.
Structure of some mono-, bis- and tris-quaternary ammonium compounds which act as potent nAChR antagonists.
Apart from bPiDDB, our initial studies have also identified a series of additional bis-quaternary ammonium analogs with good affinity for the BBB choline transporter.14 Structurally, these analogs are composed of two quaternary ammonium head groups linked through an n-alkyl linker of different carbon chain lengths. Due to the flexibility of the linker, these bis-quaternary ammonium analogs can adopt a large number of conformations. Based upon COMFA modeling, a specific conformation of these bis-quaternary ammonium analogs was suggested to be favorable for binding to the BBB choline transporter.13 In this current study, we have synthesized a series of novel conformationally-restricted bis-quaternary ammonium analogs and have determined their affinity for the BBB choline transporter. The information obtained from this study may have utility in the design of a new generation of pharmacologically active bis-quaternary ammonium molecules possessing good brain bioavailability.
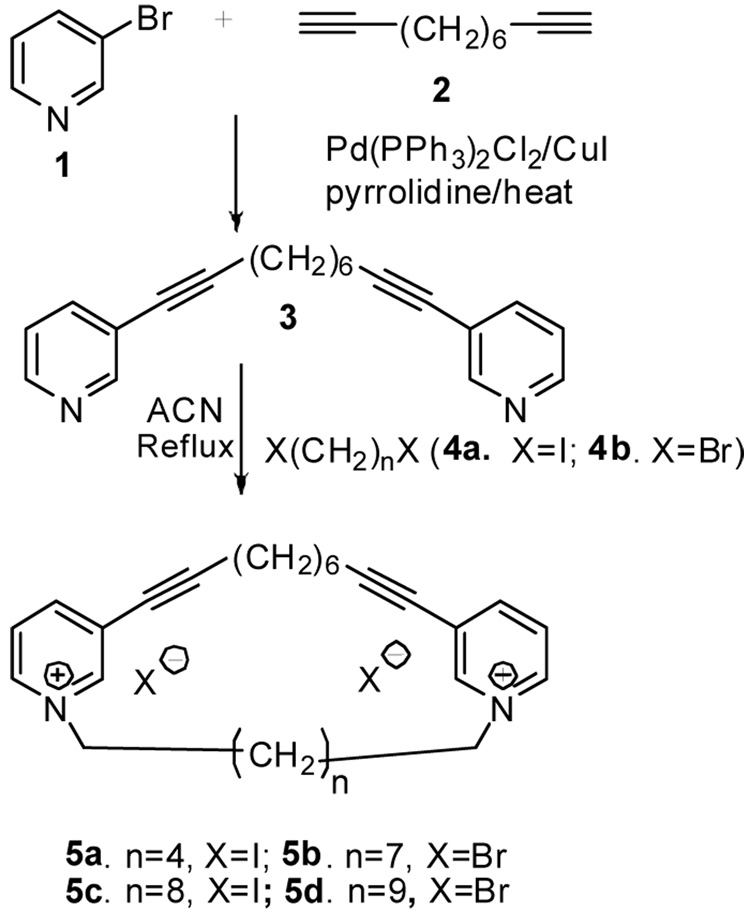
The bis-pyridinium cyclophane compounds designed for this study were synthesized as illustrated in Figure 2. The dipyridino compound 3 is novel, and was prepared through Sonagashira coupling of 3-bromopyridine (1) with 1,9-decadiyne (2) in pyrrolidine in the presence of bis-(triphenylphosphine)palladium(II) chloride and cuprous iodide as catalyst. The conversion of 3 to the desired bis-pyridinium cyclophanes 5a–5d was carried out in acetonitrile at reflux. After initial quaternization of the dipyridine intermediate 3 at one of the pyridine N-atoms, the second quaternization can occur via either intramolecular quaternization (cyclization), or via intermolecular quaternization with another molecule of 3. We found that the desired cyclization product 5 was best obtained utilizing the appropriate diiodo precursor 4 under high dilution conditions (<5 mM), which suppressed the formation of by-products arising from intermolecular reactions. However, under these high dilution conditions, the reaction was extremely slow, and even after 7 days, the reaction did not reach completion. As expected, the reaction rate was even slower when the dibromides 4b (n = 7) and 4d (n = 9) were utilized to prepare 5b and 5d. The presence of polymeric by-products is also problematic in the purification of the cyclophane products, and necessitates costly and time-consuming reverse-phase preparative HPLC purification. It was also observed that the shorter the length of the dihaloalkane 4, the more difficult was the intramolecular cyclization reaction. The general procedure for the preparation of compounds 5a – 5d are provided below.15 Compounds 5a –5d were obtained in good purity and were all fully characterized by 1H- and 13C-NMR, and mass spectroscopic analysis.
Figure 2.
Synthesis of bis-pyridinium cyclophane compounds 5a – 5d.
The affinity of the above bis-pyridinium compounds for the BBB choline transporter was determined by evaluation of their ability to inhibit [3H]choline uptake into brain. These assays were conducted using the in situ rat brain perfusion method, as modified by Allen and Smith.16 Inhibition coefficients (Ki, concentration of analogue inhibiting 50% of [3H]-choline uptake into brain) were determined using a single inhibitor concentration, as previously described.13 Ki values were compared by ANOVA followed by Bonferroni's multiple comparisons test to determine inhibition of [3H]-choline uptake.
Our previous studies have shown that the BBB choline transporter distributes the mono-quaternary ammonium compound NONI into the CNS.11 NONI has previously been shown to inhibit nicotine-evoked dopamine release in rat striatal slices,10,17 which suggested the potential for these compounds as a treatment for smoking cession and other CNS disorders. We have recently shown that more polar, di-cationic bis-quaternary ammonium compounds can also serve as potent ligands for dopamine-releasing neuronal nicotinic receptors.8,9 For example, bPiDDB potently and selectively inhibits nAChRs mediating nicotine-evoked [3H]dopamine release 11a and decreases nicotine self-administration.11f Moreover, bPiDDB has been shown to utilize the BBB choline transporter for ~90% of its permeation into brain.11b This current study was initiated to further explore this drug-transporter interaction, in order to better understand how the BBB choline transporter can serve as a vector for the delivery of other CNS-active bis-quaternary ammonium compounds.
In the absence of a crystal structure of the BBB choline transporter, the design of potential ligands utilizing QSAR studies with existing structural leads is a valuable method for exploring the SAR of these ligands, and for understanding the topological features of the transporter binding site. Thus, bis-pyridinium cyclophanes (5a–5d) were designed as novel bis-quaternary ammonium analogs with restricted conformation. All these cyclophane analogs were evaluated for their ability to interact with the BBB choline transporter and to inhibit [3H]-choline uptake into rat brain. The results are summarized in Table 1.
Table 1.
Affinity of choline, NONI, bis-pyridinium compounds, and cyclophanes 5a-5d for the BBB choline transporter.
Overall, all the bis-pyridinium cyclophanes synthesized (5a–5d) exhibited excellent affinity for the BBB choline transporter. These results suggest that, compared to mono-quaternary ammonium ligands such as NONI, bis-quaternary ammonium compounds have higher affinities for the choline transporter. This is consistent with other studies on non-cyclic bis-azaaromatic ammonium compounds, which also exhibit high affinities for the BBB choline transporter.14 Compared with choline, all the bis-pyridinium cyclophanes in this study and bis-quaternary ammonium compounds from previous studies demonstrated higher or comparable affinities for the BBB choline transporter, although they lack the terminal hydroxyl group present in the choline molecule. These results may indicate that the gain in affinity for the BBB choline transporter upon addition of a second quaternary ammonium moiety is enough to compensate for the loss in affinity that results from the removal of the hydroxyl group in the choline molecule.
Moreover, this study also provides insights about the preferred conformation of bis-quaternary ammonium compounds for their interaction with the BBB choline transporter binding site. For an open chain bis-quaternary ammonium compound, it is reasonable to speculate that after one of the cationic moieties of the bis-quaternary ammonium ligand is anchored to the choline binding site, the second cationic moiety may then bind to another adjacent anionic binding site on the transporter, which is different from the choline binding site. Because the linker moieties in these bis-quaternary ammonium compounds are highly flexible, these ligands can adopt a large number of conformations when interacting with the transporter binding site. For example, in two possible extreme cases, the ligand may bind in a fully extended conformation, where the two cationic moieties are furthest apart, or in a conformation where the cationic groups are close to each other. The bis-pyridinium cyclophanes 5 are designed as conformationally-restrained versions of non-cyclic bis-quaternary compounds such as bPiDDB. There are two alkyl linkers connecting the two pyridine moieties in these molecules, the linker attached to the C-3 positions of the two pyridine rings is kept constant in all the cyclophanes structures (5a–5d), while the second linker attached to the nitrogen atoms of the two pyridine rings are varied in length in order to mimic the different alkyl chain lengths in the non-cyclic bis-quaternary ammonium analogs. These structural changes restrict the conformational flexibility of the molecule and constrains the molecules into “folded” conformations with variable distances between the two quaternary ammonium centers.
The presence of a linker unit containing two acetylene moieties conjugated with the pyridine ring was designed to further restrict molecular flexibility. Thus, the increased affinity of the bis-pyridinium cyclophanes 5a–5d compared to the affinities of the corresponding non-cyclic analogs may be attributed to the conformational features in these molecules that are favored for interaction with BBB choline transporter binding site.
In this respect, the length of the linker unit connecting the quaternary ammonium centers may also play an important role in the interaction of these compounds with the BBB choline transporter binding site. As shown in Table 1, both 5b and 5c, which are tethered with 9 and 10-carbon linkers, respectively, at the pyridinium N-atom, exhibited the highest affinity ever reported for a BBB choline transporter ligand, while compounds with longer N-N linker units (i.e. 5d) or shorter N-N linker units (i.e. 5a) both exhibit decreased affinity. Interestingly in our previous study, the noncyclic bis-analog, bIQDI, where two isoquinolinium moieties are tethered with a 10-carbon N-N linker, also exhibit high affinity for the BBB choline transporter compared to analogs that incorporate shorter and longer N-N carbon linkers into their structures (i.e. bIQHxI and bIQDDB, respectively). These results suggest that the optimum distance between the two quaternary ammonium nitrogen atoms for binding to the BBB choline transporter reflects a substrate conformation that corresponds with a “folded” conformation of the non-cyclic bis-quaternary ammonium molecule The presence of two different linkers in the cyclophane analogs may also enhance the interaction of these molecules with the BBB choline transporter. All these observation suggest that the bis-quaternary ammonium cyclophanes can serve as a novel template in identifying potent ligands with high affinity for the BBB choline transporter. Importantly, we have recently reported that the bis-azaaromatic quaternary ammonium compound bPiPyB (Fig. 1), which represents a conformationally restricted, “folded” analog of the bPiDDB molecule, has affinity for α4β2 nAChRs19 and inhibits α4/6β4 subunit combinations of nAChRs generated from a Xenopus oocyte expression system.20 Thus, the design of bis-quaternary ammonium nAChR antagonist molecules with conformational properties that allow facilitated transport into the CNS may be feasible.
In conclusion, a novel group of bis-pyridinium cyclophanes incorporating one fixed, relatively rigid linker and a second more conformationally flexible linker of varying carbon chain length has been synthesized and evaluated for affinity for the BBB choline transporter. Excellent affinity for the BBB choline transporter has been observed in these cyclophane molecules. To the best of our knowledge, 5c and 5b exhibit the highest affinity for the BBB choline transporter ever reported for compounds of this structural class. These results suggest that bis-quaternary ammonium cyclophanes may serve as a novel template for the discovery of potent, highly polar, cationic CNS agents with high affinity for the BBB choline transporter. This information also indicates that a rational incorporation of a specific structural moiety into highly hydrophilic, cationic CNS agents can confer high BBB choline transporter affinity on the molecule, affording more potential for the drug to access the CNS and achieve therapeutically relevant brain concentrations.
Acknowledgement
This work was supported by National Institute of Health grant U19 DA017548.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Pardridge WM. Nat. Rev. Drug Discov. 2002;1:131. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]; (b) Davson H, Segal MB. Physiology of the CSF and Blood-Brain Barriers. Boca Raton, FL: CRC Press, Inc.; 1996. [Google Scholar]
- 2.(a) Allen DD, Lockman PR. Life Sci. 2003;73:1609. doi: 10.1016/s0024-3205(03)00504-6. [DOI] [PubMed] [Google Scholar]; (b) Ohtsuki S, Terasaki T. Pharm. Res. 2007;24:1745. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]; (c) de Boer AG, Gaillard PJ. Clin. Pharmacokinet. 2007;46:553. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]; (d) Ecker GF, Noe CR. Curr. Med. Chem. 2004;11:1617. doi: 10.2174/0929867043365071. [DOI] [PubMed] [Google Scholar]
- 3.Lockman PR, Allen DD. Drug Devel. Indust. Pharm. 2002;28:749. doi: 10.1081/ddc-120005622. [DOI] [PubMed] [Google Scholar]
- 4.Smith QR. Adv. Exp. Med. Biol. 1993;331:83. doi: 10.1007/978-1-4615-2920-0_14. [DOI] [PubMed] [Google Scholar]
- 5.Takata Y, Vistica DT, Greig NH, Purdon D, Rapoport SI, Smith QR. Cancer Res. 1992;52:2191. [PubMed] [Google Scholar]
- 6.Vistica DT, Kenney S, Hursey ML, Boyd MR. Biochem. Biophys. Res. Commun. 1994;200:1762. doi: 10.1006/bbrc.1994.1657. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg GJ, Begleiter A. Pharmacol. Ther. 1980;8:237. doi: 10.1016/0163-7258(80)90048-0. [DOI] [PubMed] [Google Scholar]
- 8.Crooks PA, Ayers JT, Xu R, Sumithran SP, Grinevich VP, Wilkins LH, Deaciuc AG, Allen DD, Dwoskin LP. Bioorg. Med. Chem. Lett. 2004;14:1869. doi: 10.1016/j.bmcl.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 9.Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA. Bioorg. Med. Chem. Lett. 2004;14:1863. doi: 10.1016/j.bmcl.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins LHJR, Grinevich VP, Ayers JT, Crooks PA, Dwoskin LP. J. Pharmcol. Exp. Ther. 2003;304:400. doi: 10.1124/jpet.102.043349. [DOI] [PubMed] [Google Scholar]
- 11.(a) Dwoskin LP, Wooters TE, Sumithran SP, Siripurapu KB, Joyce BM, Lockman PR, Manda VK, Ayers JT, Zhang Z, Deaciuc AG, McIntosh JM, Crooks PA, Bardo MT. J. Pharmacol. Exp. Ther. 2008 doi: 10.1124/jpet.108.136630. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lockman PR, Manda VK, Geldenhuys WJ, Mittapalli RK, Thomas F, Albayati ZF, Crooks PA, Dwoskin LP, Allen DD. J. Pharmacol. Exp. Ther. 2008;324:244. doi: 10.1124/jpet.107.130906. [DOI] [PubMed] [Google Scholar]; (c) Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Neuropharmacology. 2007;52:755. doi: 10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]; (d) Rahman S, Zhang Z, Papke RL, Crooks PA, Dwoskin LP, Bardo MT. Br. J. Pharmacol. 2008;153:792. doi: 10.1038/sj.bjp.0707612. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wilkins JRLH, Haubner A, Ayers JT, Crooks PA, Dwoskin LP. J. Pharmacol. Exp. Ther. 2002;301:1088. doi: 10.1124/jpet.301.3.1088. [DOI] [PubMed] [Google Scholar]; (f) Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Psychopharmacology. 2006;184:426. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- 12.Zheng G, Sumithran SP, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg. Med. Chem. Lett. 2007;17:6701. doi: 10.1016/j.bmcl.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen DD, Lockman PR, Roder KE, Dwoskin LP, Crooks PA. J. Pharmacol. Exp. Ther. 2003;304:1268. doi: 10.1124/jpet.102.045856. [DOI] [PubMed] [Google Scholar]
- 14.Geldenhuys WJ, Lockman PR, Nguyen TH, Van der Schyf CJ, Crooks PA, Dwoskin LP, Allen DD. Bioorg. Med. Chem. 2005;13:4253. doi: 10.1016/j.bmc.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 15.General procedure for the preparation of bis-pyridinium cyclophanes: the key intermediate, 3,3’-(1,9-decadiyn-1,10-diyl)-bis-pyridine (3), was prepared by mixing 1,9-decandiyne (2) (2.8 g, 20.9 mmol) and 3-bromopyridine (1) (50 mmol) in pyrrolidine followed by the addition of Pd(PPh3)2Cl2 (50 mg) and CuI (50 mg). The mixture was heated to 60–70°C for 2 h. The solvent was removed in vacuum and the residue was subjected to slica gel chromatography. 3-Bromopyridine was eluted from the column with hexane and ethyl acetate (10:1); the product 3 was eluted subsequently from the column with hexane and ethyl acetate (2:1) and the solvent evaporated to afford an oil (5.1 g, 85%):. 1H NMR δ ppm 8.59 (d, J = 1.5 Hz, 2H), 8.43 (dd, J = 1.8 Hz, J = 5.1 Hz, 2H), 7.62 (dt, J = 1.8 Hz, J = 7.8 Hz, 2H), 7.15 (ddd, J = 0.9 Hz, J = 4.8 Hz, J = 7.8 Hz, 2H), 2.40 (t, J = 6.9 Hz, 4H), 1.59-1.63 (m, 4H), 1.46-1.49 (m, 4H). Compound 3 and a molar equivalent of an appropriate dihaloalkane 4 were mixed in acetonitrile to afford a concentration of approximately 1 mM. The mixture was refluxed for 7 days. The solvent was removed in vacuo after cooling, and the resulting residue was taken up in water (20 mL) and diethyl ether (50 mL). The aqueous layer was extracted extensively with diethyl ether (5 × 50 mL) to remove the starting materials. Removal of most of the water from the aqueous layer afforded a residue which was dissolved in methanol (10 mL). Evaporation of methanol/water under vacuum followed by drying of the resulting residue under high vacuum afforded the bis-pyridinium cyclophane as a solid glass).N,N’-(1,6-hexanediyl)3,3’-(1,9-decadiyn-1,10-diyl)-bis-pyridinium diiodide (5a). 1H NMR (300 MHz, D2O) δ ppm 8.76 (s, 2H), 8.56 (d, J = 6.0 Hz, 2H), 8.29 (d, J = 8.4 Hz, 2H), 7.81 (dd, J = 6.3 Hz, 8.1 Hz, 2H), 4.40 (t, J = 7.5 Hz, 4H), 2.39 (t, J = 6.3 Hz, 4H), 1.85-1.84 (m, 4H), 1.49-1.52 (m, 4H), 1.38-1.42 (m, 4H), 1.18-1.22 (m, 4H). MALDI-TOFMS. m/z 499 (M-I) (Calcd For C26H32IN2+, 499.45).N,N’-(1,9-Nonanediyl)3,3’-(1,9-decadiyn-1,10-diyl)-bis-pyridinium dibromide (5b). 1H NMR (300 MHz, D2O), δ ppm 10.39 (s, 2H), 9.52 (d, J = 5.7 Hz, 2H), 8.18 (d, J = 7.5 Hz, 2H), 8.00 (m, 2H), 5.06 (t, J = 7.2 Hz, 4H), 2.49 (t, J = 6.3 Hz, 4H), 2.08-2.18 (br, 4H), 1.60-1.80 (br, 12H), 1.42-1.56 (br, 8H), 1.37-1.40 (br, 2H). MALDI-TOFMS. m/z 493 , 495, (M-Br), (Calcd for C29H38BrN2+, 493.22, 495.22).N,N’-(1,10-decanediyl)3,3’-(1,9-decadiyn-1,10-diyl)-bis-pyridinium diiodide (5c). 1H NMR (300 MHz, D2O), δ ppm 10.11 (s, 2H), 9.37 (d, J = 6.3 Hz, 2H), 8.22 (dt, J= 7.8 Hz, J = 1.2 Hz, 2H), 8.03 (dd, J = 6.3 Hz, J = 7.8 Hz, 2H), 4.99 (t, J=8.1 Hz, 4H), 2.49 (t, J = 6.6 Hz, 4H), 2.15-2.17 (m, 4H), 1.38-1.75 (m, 24H). MALDI-TOFMS. 555 m/z (M-I). Calcd for C30H40IN2+, 555.22).N,N’-(1,11-undecanediyl)3,3’-(1,9-decadiyn-1,10-diyl)-bis-pyridinium dibromide (5d). 1H NMR (300 MHz, D2O), δ ppm 9.92 (s, 2H), 9.48 (d, J = 7.8 Hz, 2H), 8.22 (d, J = 8.1 Hz, 2H), 8.05 (m, 2H), 5.08 (br, 4H), 2.49 (t, J = 6.3 Hz, 4H), 2.05-2.15 (br, 4H), 1.70-1.82 (br, 12H), 1.55-1.65 (br, 4H), 1.45-1.55 (br, 4H), 1.30-1.45 (m,8H). MALDI-TOFMS. m/z 521, 523 (M-Br), Calcd for C31H42BrN2+, 521.25, 523.25).
- 16.Allen DD, Smith QR. J. Neurochem. 2001;76:1032. doi: 10.1046/j.1471-4159.2001.00093.x. [DOI] [PubMed] [Google Scholar]; Lockman PR, Mumper RJ, Allen DD. J. Neurochem. 2003;86:627. doi: 10.1046/j.1471-4159.2003.01888.x. [DOI] [PubMed] [Google Scholar]
- 17.Sumithran SP, Crooks PA, Xu R, Zhu J, Deaciuc AG, Wilkins LH, Dwoskin LP. AAPS J. 2005;7(1):E201. doi: 10.1208/aapsj070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geldenhuys WJ, Lockman PR, McAfee JH, Fitzpatrick KT, Van der Schyf CJ, Allen DD. Bioorg. Med. Chem. Lett. 2004;14:3085. doi: 10.1016/j.bmcl.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Zheng G, Zhang Z, Pivavarchyk M, Deaciuc GD, Dwoskin LP, Crooks PA. Bioorg. Med. Chem. Lett. 2007;17:6734. doi: 10.1016/j.bmcl.2007.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papke RL, Dwoskin LP, Crooks PA, Zheng G, Zhang Z, McIntosh JM, Stokes C. Neuropharmacology. 2008;54:1189. doi: 10.1016/j.neuropharm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]