Overview
In 1999 the initial approved clinical indication for inhaled nitric oxide (iNO) issued by the Food and Drug Administration was limited to: …treatment of term and near-term (>34 weeks) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension. At the time, anticipation was high that iNO would receive subsequent clearance as a therapeutic intervention for a variety of pediatric and adult respiratory conditions. Thirteen years, multiple clinical trials, and millions of dollars later the FDA-approved indication for iNO is the same as it was last century. This has been a particularly disappointing outcome with respect to bronchopulmonary dysplasia (BPD). It was predicted that iNO mediated improvements in neonatal oxygenation would hasten resolution of acute respiratory distress and thus lead to a significant decline in BPD incidence rates. Instead a series of well-designed multi-center clinical trials have at best demonstrated inconsistent equivocal benefits of iNO therapy to prevent or treat BPD.
Inhaled NO is not a panacea for pulmonary pathologies. But this is not to say future respiratory therapies directed towards NO bioactivity will not confer therapeutic benefit in the prevention or treatment of BPD; it is just that the agents may be in a different form than a free radical gas. A growing understanding of endogenous NO biology is helping to explain how and when exogenous NO may confer benefit or harm; this knowledge is also helping to identify new better-targeted NO-based therapies. In this review we will attempt to place in context results of the BPD clinical trials that employed iNO in the preterm population, consider the biologic basis for novel NO therapeutics, and identify possible future directions for NO-focused clinical and basic research in developmental lung disease.
The Clinical Problem
Concomitant in the 1960s with the development of ventilatory strategies to oxygenate newborns in respiratory distress was the recognition that these interventions could produce lung pathology.1 The original characterization of BPD was based on chest radiographic findings (cysts and pulmonary fibrosis) and a need for prolonged oxygen support resulting from oxygen toxicity and mechanical trauma; it typically applied to neonates >30 weeks’ post-menstrual age, the lower age threshold for survival at the time. In the ensuing decades, as additional interventions were developed to survive distressed neonates (most notably surfactant and antenatal steroid therapy), the etiology of BPD has evolved such that it is currently viewed as a disease of prematurity.
The concept of BPD resulting from lung injury (old definition) has been replaced with the view that it arises from aberrant development of immature lungs exposed to the ex uterine milieu (new definition). This is borne-out by epidemiological data. In today’s neonatal intensive care environment rarely would the average patient in Northway’s cohort (1,900 g at 33 weeks’ gestation) develop BPD. Instead the disease is concentrated amongst what are now considered low and very-low birth weight but viable populations. From an overall incidence of 20% for infants weighing less than 1,500 g, the BPD rate climbs to 30% when the birth weight is between 750 and 1,000 g and to 50% for infants born weighing less than 750 g.2 The sickest infants are now surviving but with lung dysfunction keeping the overall incidence rate essentially constant.
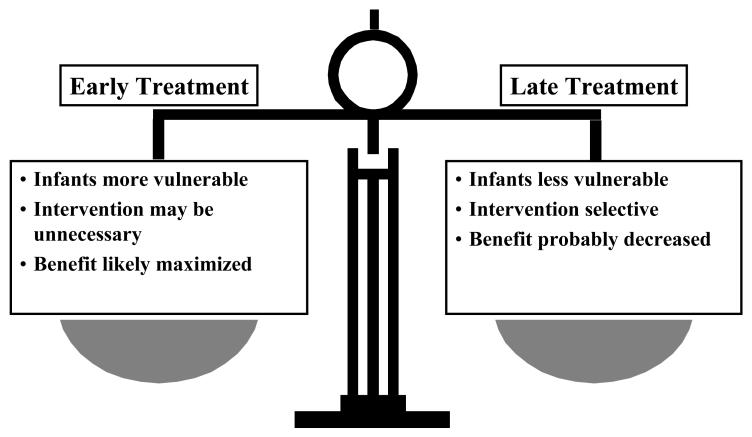
BPD has a multi-factorial etiology through a combination of hyperoxia and baro- or volutrauma superimposed upon a structurally and biochemically immature lung. The acute lung injury in the vulnerable infant results in airway remodeling, decreased alveolarization, alveolar simplification, and lung fibrosis.3,4 Many other factors are contributory, notably antenatal and postnatal infection and inflammation, along with nutritional deficiencies. In addition, BPD may well have a genetic component.5 One of the major therapeutic challenges is to identify which specific pathophysiologic process or processes to target. An even greater challenge is to determine the appropriate timing of any therapy to optimize potential benefit and minimize both unnecessary treatment and untoward side effects [Fig. 1].
Figure 1.
The pros and cons of early versus late therapeutic intervention in the course of BPD.
BPD continues to be the major pulmonary morbidity of prematurity6,7 with annual care costs in the United States estimated to be upwards of $26 billion.8 Equally important is the emerging concept that BPD may be a chronic condition. Following their initial care, 50% of low and very low birth weight patients will be re-hospitalized for respiratory distress during early childhood. And while some lung parameters can normalize in later childhood, exacerbated regression in function may well occur as these individuals age. When Northway et al. re-assessed their original (pre-surfactant era) patients most had some degree of airway obstruction, airway hyper-reactivity, and/or hyperinflation.9 This finding was replicated in a different cohort of BPD survivors who have undergone serial pulmonary testing into mid-adulthood;10 at their most recent testing (mean age of 38) these individuals exhibited “increasing static pulmonary hyperinflation with age indicative of bronchiolar dysfunction or early emphysematous changes.”
Unfortunately, longitudinal assessments of BPD survivors who received surfactant are scant so it remains unclear the full scope of adult respiratory disease that can be traced to aberrant neonatal lung development and/or overly aggressive therapeutic intervention. Nonetheless, the possibility of chronic pulmonary pathology adds impetus to finding effective therapies to prevent and treat BPD.
Pre-Clinical Support for Nitric Oxide Gas in Neonatal Lung Disease
The development of iNO for persistent pulmonary hypertension of the newborn followed a course of positive pre-clinical11 and case series12,13 results that led to multi-center clinical trials.14-16 Pre-clinical results from animal models of BPD have also demonstrated benefits with iNO therapy.
In a premature baboon preparation, inhalation of 5 ppm of NO during the first 14 days of life was compared to a standard ventilatory arm;17 animals in both cohorts were administered surfactant. At the end of the study, animals in the iNO group had improved pulmonary function (increased compliance and decreased expiratory resistance) compared to baboons in the control arm. Continuous iNO therapy was also associated with preservation of lung growth, normalization of elastin deposition, and stimulation of secondary crest development. The authors proposed that iNO corrected dysfunctions in NO-mediated biosynthetic pathways and suggested that this dysfunction contributes to the pathogenesis of BPD. Dysregulation of NO activities in the lungs have also been observed in rodents18 and were again found to be responsive to iNO.19
Positive results were also seen in sheep. Chronically ventilated preterm lambs received 5-15 ppm iNO for 21 days; comparisons were again made to animals who received standard ventilatory support.20 Lung development was measured by radial alveolar counts and lung capillary surface density – parameters that were significantly better in the iNO treatment group. In addition, the treated lambs had markedly lower expiratory resistance with a significant decrease in airway smooth muscle mass. Inhaled NO also produced decreases in pulmonary vascular resistance and pulmonary arterial smooth muscle but these benefits were modest compared to the other end-points.
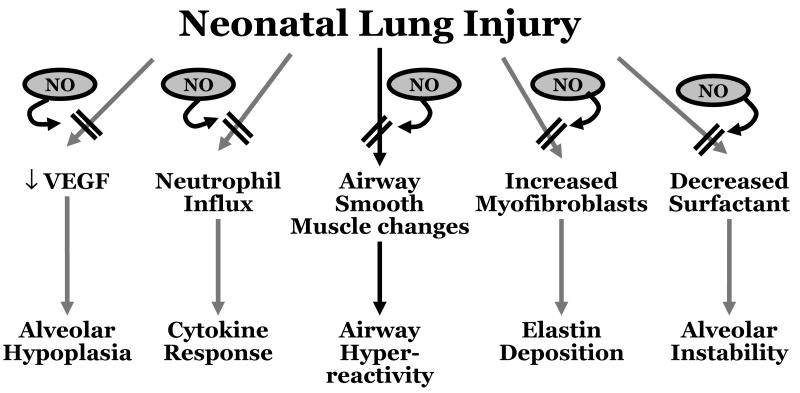
The pre-clinical data point to an impairment of endogenous NO signaling in neonatal lung injury, an impairment that can be addressed with iNO [Fig. 2]. Unfortunately, as detailed in the next section, these positive animal findings have not translated into clear clinical benefits of iNO therapy for human preterm neonates.
Figure 2.
Neonatal lung injury may trigger a diversity of cellular pathways with adverse effects on lung development. Based on available animal data nitric oxide appeared to have the potential to block these pathways and downstream consequences.
Clinical Trial Results For Use of Inhaled Nitric Oxide In Preterm Infants
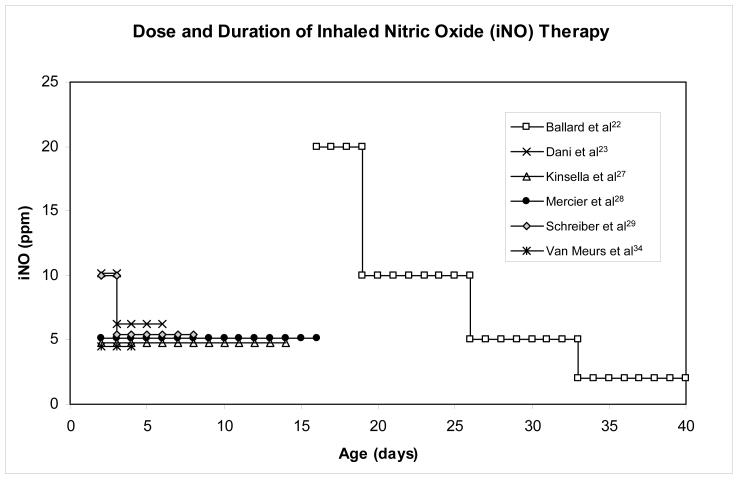
Fourteen randomized controlled clinical trials21-34 have been conducted to test the ability of iNO to reduce mortality and/or the incidence of BPD in preterm infants (a total study population of 3,430). The methodology, dosage, and duration of iNO treatment, as well as timing of the intervention have varied amongst these trials [as demonstrated in Fig. 3] leading to conflicting and equivocal results [Table 1; the reader is referred to the individual reports or the manuscripts describing the group analyses for detailed descriptions of the methodological differences]. In addition to the conclusions reached by the individual clinical research teams, the current study population of over 3,400 has allowed for post-hoc testing of pooled results.
Figure 3.
Graphic representation of the temporal relationship in the median initiation, dose, and duration of inhaled NO treatment among 6 major trials. Graphic is an adaptation of original by Truog WE. Inhaled nitric oxide for the prevention of bronchopulmonary dysplasia. Expert opinion on pharmacotherapy 2007;8:1505-13.
TABLE 1.
Study Protocols of Randomized Controlled Trials using inhaled NO (iNO) in Preterm Infants
| Author (Year) |
Number | Gestational Age (weeks) |
Age at Enrollment |
Birth Weight (gm) |
Start→Titration (Max) iNO, ppm |
Weaning Protocol | Planned Duration of iNO |
RR of Death or BPD (95% CI)a |
|---|---|---|---|---|---|---|---|---|
| Subhedar et al32 (1997) |
42 | <32 | >4 d | -- | 20→5 (20)b | Attempt at 72 h; iNO stopped with extubation |
72 h | 1.04 (0.92, 1.19) |
| Franco-Belgian Collaborative21 (1999) | 85 | <33 | <7 d | -- | 10→5 (20)b | Practitioner’s discretion; 5 ppm and then off |
-- | 0.84 (0.54, 1.31) |
| Kinsella et al27 (1999) | 80 | ≤34 | ≤7 d | -- | 5→0 (5)b | Attempt at 7 d; iNO stopped with extubation |
7-14 d | 0.85 (0.70, 1.03) |
| Srisuparp et al30 (2002) |
34 | <32 | <3 d | <2000 | 20→5 (20)b | Down to 5 ppm over 24-48 h. iNO stopped with extubation |
Max: 7 d | BPD rates not reported |
| Schreiber et al29 (2003) | 207 | <34 | <3 d | <2000 | 10→5 (10) | 10 ppm for 1 d, 5 ppm for 6 d, then off; iNO stopped 1 hr before extubation |
7 d | 0.76 (0.60, 0.97) |
| Van Meurs et al34 (2005) |
420 | <34 | >4 h | 401-1500 | 5→10 (10) | Attempt at 10-14 h; iNO stopped if no response at 10 ppm or with extubation |
Max: 14 d | 0.99 (0.90, 1.09) |
| Field et al24 (2005) | 108 | <34 | <28 d | -- | 5→40 (40)b | iNO doses doubled until response achieved to a max of 40 ppm; iNO stopped before extubation |
48 h to 3 d |
0.98 (0.87, 1.12) |
| Hascoet et al25 (2005) |
145 | <32 | 6-48 h | -- | 5→10 (10)b | iNO stopped when a/AO2 >0.22 or with extubation |
Median: 28 h | 1.04 (0.77, 1.41) |
| Dani et al23 (2006) | 40 | <30 | <7 d | -- | 10→6 (10) | 10 ppm for 4 h, then 6 ppm. Attempt at 72 h; iNO stopped with extubation |
Mean: 4.1 d | 0.56 (0.35, 0.88) |
| Kinsella et al26 (2006) | 793 | ≤34 | <48 h | 500-1250 | 5 (5) | None; iNO stopped with extubation |
Max: 21 d | 0.95 (0.87, 1.03) |
| Ballard et al22 (2006) |
582 | <32 | 7-21 d | 500-1250 | 20→2 (20) | 20 ppm for 48-96 h, then decreased to 10, 5, and 2 ppm at weekly intervals. Continued for duration of therapy regardless of respiratory support |
Min: 24 d | 0.89 (0.78, 1.02) |
| Van Meurs et al33 (2007) |
29 | <34 | >4 h | >1500 | 5→10 (10)b | Attempt at 10-14h. iNO stopped if no response at 10 ppm or with extubation |
Max: 14 d | 0.83 (0.43, 1.62) |
| Su and Chen31 (2008) | 65 | <32 | Mean: 2.4- 2.5 d |
<1500 | 5→20 (20)b | Attempt at 6 h. | Mean: 4.9 d | 0.79 (0.51, 1.21) |
| Mercier et al28 (2010) | 800 | 24-28 6/7 | <24 h | >500 | 5 (5) | None; iNO continued for ≥7 d, then discontinued with extubation for maximum of 21 days |
7-21 d | 0.98 (0.81, 1.18) |
|
| ||||||||
| Total (14 RCT) | 3430 | ≤34 | Birth to 27 d | 401-2000 | Start 5-20 (Max 5-40) | -- | <24 h to 24 d | -- |
Risk Ratio (RR) and 95% Confidence Intervals (CI) reported as calculated in Barrington and Finer’s Cochrane Review35
Changes to iNO dose made based on physiologic response in patient
In a 2010 Cochrane review,35 Barrington and Finer determined that the wide variations in patient age, illness severity, and control group mortalities precluded pooling and analyzing the results as a single data set. Instead they organized the fourteen clinical trials into cohorts based upon the entry criteria and the timing of iNO administration. Three distinct groups were identified:
Nine of the trials21,23-25,27,30,31,33,34 were defined as early rescue treatment (<3 days of life) based on oxygenation criteria
Three of the trials26,28,29 were given the designation of routine early use for intubated infants (<3 days of life)
Two of the trials22,32 were defined as later use (>3 days of life) for infants at elevated risk for BPD
There were trends towards improved outcomes with iNO treatment most notably in Group 2 where the effect size (typical relative risk of 0.93 with 95% confidence interval of 0.86 to 1.01) approached significance – but they remained only trends. Analyses found no statistically significant effect of iNO on rates of BPD or mortality, however, it is interesting to note that one large multicenter trial employing later use, longer duration, and higher initial dose did document benefit.22 In addition, the authors found no effect of iNO on the incidence of neurological impairment and there were no clear effects on the frequency or severity of intra-ventricular hemorrhage. Barrington and Finer summarized their findings this way: “iNO as rescue therapy for the very ill preterm infant does not appear to be effective. Early routine use of iNO in preterm infants with respiratory disease does not affect serious brain injury or improve survival without BPD. Later use of iNO to prevent BPD might be effective, but requires further study.”
Using different methodology, Askie et al. performed a meta-analysis on twelve23-30,32-34,36 of the fourteen trials for which individual patient data (IPD) was available.37 The authors tested multiple sub-groups, which can be linked under three categories:
Therapeutic
starting dose and duration of iNO treatment, antenatal or postnatal steroid use, administration of surfactant, and ventilation mode at randomization.
Demographic
gestational age at birth, postnatal age at time of randomization, birth weight, multiplicity, and ethnicity.
Pathology
severity of hypoxemia (in terms of oxygen index), presence of a patent ductus arteriosus, and pulmonary hypertension.
In patients of multiple births, the researchers also analyzed for correlations between siblings.
Out of a study population of 3,298 infants, the incidence of mortality or chronic lung disease was 59% in the iNO treatment group and 61% in the control cohort for a relative risk of 0.96 with a 95% confidence interval of 0.92 to 1.01 (p = 0.11). The incidence of neurologic injury was also similar at 25% and 23% for iNO treated infants and controls, respectively (relative risk of 1.12 and 95% confidence interval of 0.98 to 1.28; p = 0.9). In addition, iNO was found to have no statistically significant benefit when tested with respect to the demographic variables.
In trials that started with an iNO dose >5 ppm there was a 9% absolute risk reduction in the composite outcome of death or BPD (relative risk of 0.83 and 95% confidence interval of 0.74-0.95). However, translating this positive finding into a therapeutic recommendation is not straight forward for two reasons:
The relevant low dose trials, while starting at <5 ppm, subsequently exceeded this concentration during the course of therapy because iNO dosing was based on (and titrated to) the patient’s response
The relevant higher dose trials used protocols that reduced the iNO dose over the course of therapy
The authors’ conclusions: “…[R]esults of this IPD meta-analysis of all available worldwide data indicate that routine use of iNO for treatment of respiratory failure in preterm infants cannot be recommended. The use of a higher starting dose might be associated with improved outcome, but because there were differences in the designs of the trials included in the analyses, it requires further examination.”
A third group analysis of the available iNO data was conducted by Donohue et al.38 These authors combined the results of the fourteen randomized trials with those from seven follow-up assessments39-46 and one observational study.47 Their meta-analyses were broken down into short-term and long-term outcomes:
Short-Term Outcomes
Survival/Death in the NICU
BPD at 36 weeks’ post-menstruation age
Death or BPD Composite at 36 weeks’ post-menstruation age
Brain Injury
Patent Ductus Arteriosus
Sepsis
Necrotizing Enterocolitis
Retinopathy of Prematurity
Pulmonary Hemorrhage
Air Leak
Long-Term Outcomes
Survival/Death after NICU Discharge
Cerebral Palsy
Mental Development Index
Neurodevelopmental Impairment
The authors reported one positive finding in that there was no evidence treatment of preterm infants with iNO influences the incidence rates for other complications of prematurity. Unfortunately the same lack of influence was found to be true for the beneficial parameters. Inhaled NO therapy did not alter the incidence of:
NICU mortality rate (risk ratio of 0.97 with 95% confidence interval at 0.82 - 1.15);
BPD in survivors at 36 weeks (risk ratio of 0.93 with 95% confidence interval at 0.86 -1.003);
Cerebral Palsy (risk ratio of 1.36 with 95% confidence interval at 0.88 - 2.10)
Neurodevelopmental Impairment (risk ratio of 0.91 with 95% confidence interval at 0.77 - 1.12); nor
Cognitive Impairment (risk ratio of 0.72 with 95% confidence interval at 0.35 - 1.45).
A small difference was identified in the composite outcome of death or BPD at 36 weeks post-menstrual age that favored iNO therapy over the control group (risk ratio of 0.93 with 95% confidence interval at 0.87 - 0.99). However, this 7% reduction in death or BPD was not enough for the authors to accept iNO as a viable therapy for preterm infants: “There is currently no evidence to support the use of iNO in preterm infants with respiratory failure outside the context of rigorously conducted randomized clinical trials.” And while some have criticized this conclusion,48 Donohue et al.’s findings38 are certainly consistent with the results of the other two analyses.
The National Institutes of Health Weighs-In
National Institutes of Health (NIH) Consensus and State-of-the-Science Statements are prepared by independent panels of health professionals and public representatives. The goal of such efforts is to provide health care workers, patients, and the general public with a responsible (presumably free of bias) assessment of currently available data regarding a particular medical condition, practice, or therapy. Such statements are an independent report of the panel; they are not considered policy statements of the NIH or the Federal Government.
To assess the risks and benefits of treating premature infants with iNO, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Heart, Lung, and Blood Institute, and the NIH Office of Medical Applications of Research convened a Consensus Development Panel that met between the 27th and 29th of October 2010.8 The 16-member Consensus Development Panel was comprised of physicians and caregivers active in the areas of Neonatology, Pediatric Pulmonology and Neurology, and Perinatal Epidemiology. An additional 18 experts from relevant medical fields (many of whom had been involved in one or more of the iNO preterm infant clinical trials) participated by presenting data to the panel and the conference attendees.
To develop the consensus statement the panel was charged with answering six questions:
Does iNO therapy increase survival and/or reduce the occurrence or severity of BPD among premature infants who receive respiratory support?
Are there short-term risks of iNO therapy among premature infants who receive respiratory support?
Are there effects of iNO therapy on long-term pulmonary and/or neurodevelopmental outcomes among premature infants who receive respiratory support?
Does the effect of iNO therapy on BDP and/or death or neurodevelopmental impairment vary across subpopulations of premature infants?
Does the effect of iNO therapy on BPD and/or death or neurodevelopmental impairment vary by timing of initiation, mode of delivery, dose and duration, or concurrent therapies?
What are the future research directions needed to better understand the risks, benefits, and alternatives to iNO therapy for premature infants who receive respiratory support?
In attempting to answer these questions the panel generated a 5-part consensus statement:
The available evidence does not support use of iNO in early-routine, early-rescue, or later-rescue regimens in the care of premature infants of <34 weeks’ gestation who require respiratory support.
There are rare clinical situations, including pulmonary hypertension or hypoplasia, that have been inadequately studied in which iNO may have benefit in infants of <34 weeks’ gestation. In such situations, clinicians should communicate with families regarding the current evidence on its risks and benefits as well as remaining uncertainties.
Future research should seek to understand the gap between benefits on lung development and function in infants at high risk of BPD suggested by basic research and animal studies and the results of clinical trials to date.
Predefined subgroup and post-hoc analyses of previous trials showing potential benefit of iNO have generated hypotheses for future research for clinical trials. Previous strategies shown to be ineffective are discouraged unless new evidence emerges. Future trials should attempt to quantify the individual effects of each of these treatment-related variables (timing, dose, and duration), ideally by randomly assigning them separately.
On the basis of assessment of currently available data, hospitals, clinicians, and the pharmaceutical industry should avoid marketing iNO for premature infants of <34 weeks’ gestation.
Taken together, it is obvious iNO is not a panacea for preterm infants. At the same time, given the vital roles NO plays in lung development and oxygen delivery, it is hard to rationalize why it has been so difficult to demonstrate therapeutic benefit in these patients. In this regard, the answer may lie not so much in how or when to start iNO therapy as in how administration of NO as a nitrogen monoxide radical differs from how the body generates and deploys endogenous NO bioactivity.
Biology of Nitric Oxide and S-Nitrosylation
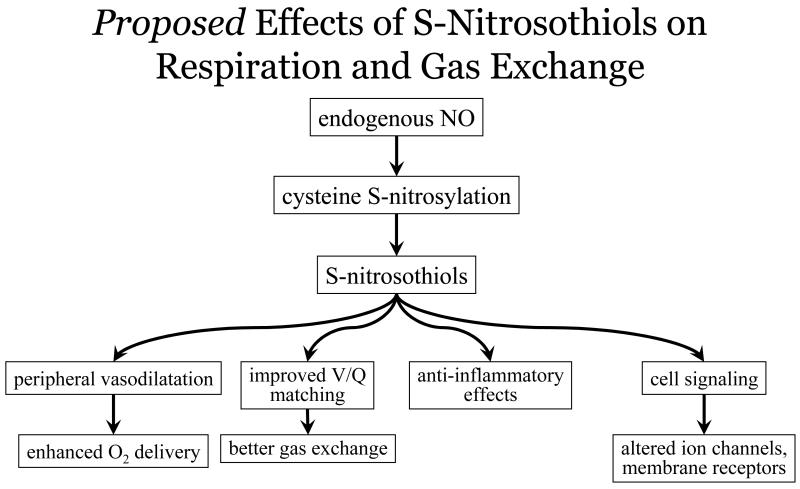
Over the last two decades there has been great interest in and delineation of the multiple roles NO has in cellular signaling, inflammation, growth and differentiation, and metabolism. Nitric oxide-based signaling classically involves the binding of NO to hemes in soluble guanylate cyclase (sGC) to increase cyclic guanosine monophosphate (cGMP). This pathway explains how NO bioactivity derived from the endothelium produces vascular relaxation (and how iNO may chiefly act). However it is now recognized that a majority of the effects of NO on cellular signaling are elicited by S-nitrosylation49 – the ubiquitous modification of cysteine thiol side chains to produce S-nitrosothiols (SNOs) [Fig. 4]. Rather than mediate cellular signaling that involves post-translational modifications, protein hemes appear to promote the requisite redox chemistry of NO. Thousands of proteins have been identified as targets of S-nitrosylation where activity can increase or decrease in response to the addition (or removal) of the NO group.50
Figure 4.
The multiple roles of endogenous NO bioactivity in affecting respiratory and gas exchange via S-Nitrosothiol mediated signaling.
The breadth of cellular activities regulated by S-nitrosylation is reminiscent of phosphorylation. This includes the developing lung and encompasses maturational changes in lung parenchymal, vascular, and airway structures.51 It is important to recognize that in vivo SNO homeostasis is a balance between S-nitrosylation and denitrosylation, and that both the addition and removal of NO are key components in the transduction of SNO-based signaling. It is also important to appreciate NO/SNO regulation is tied to oxygenation. “Nitroso-redox balance” refers to the interplay between reactive oxygen species (ROS) and NO at critical regulatory cysteine thiols. Irreversible thiol oxidation of cysteine residues by ROS can lead to losses in S-nitrosylation control of protein function. The balance between NO bioactivity and ROS production plays a pivotal role in cellular and organ function.52-55
By direct analogy to dysregulated phosphorylation, aberrant S-nitrosylation is increasingly acknowledged as a causal factor in disease.56,57 Typically these states are associated with hypo- or hyper-nitrosylation of key proteins that play essential roles in disease (patho)physiology. In a number of acute and chronic conditions where SNO levels are altered administration of an S-nitrosylating agent or an exogenous SNO was determined to be beneficial.58-63 A few aspects of protein S-nitrosylation are highlighted in the following section. For more in-depth coverage, the reader is referred to several excellent reviews that have been published in the last few years.56,64-68
S-Nitrosothiols in the Pulmonary System
The major sources/producers of endogenous NO are the three NO synthases: neuronal NOS (nNOS, NOS1), inducible NOS (iNOS, NOS2), and endothelial NOS (eNOS, NOS3). All three isoforms are expressed and active throughout the lungs. In many areas, NOS is co-localized with its target protein(s), providing for tight control of activity under normal physiologic conditions. SNOs have integral roles in respiratory biology from regulating pulmonary vascular tone to ventilation perfusion matching to central control of breathing.64 Dysregulation of SNO homeostasis is a common theme in a number of lung diseases including cystic fibrosis,69,70 asthma,71,72 and pulmonary arterial hypertension (PAH)73 among others.
SNOs and Oxygenation
Hemoglobin (Hb) is the prototypical S-nitrosylated protein in that it can deploy NO bioactivity as the red blood cells (RBCs) transit the circulatory system.74,75 In this setting, NO binds to heme iron of deoxy, T-state Hb mainly in the venous circulation to generate HbFeNO.76,77 In reponse to oxygenation within the lungs and the transition of Hb from T-state to R-state, the NO group can transfer to the cysteine residue at position 93 on the Hb β chain to form SNO-Hb. The transition from high to low oxygen tension in the arterial periphery promotes the release of SNO-based vasodilatory activity from the RBCs.78,79 Under normal physiological conditions, tissue pO2 is low and falls further with local increases in metabolism. Thus, the release of vasodilatory NO bioactivity by RBCs sub-serves the graded increases in blood flow that are coupled to progressive decreases in Hb oxygen saturation.80
The uptake of oxygen by RBCs within the lungs is similarly controlled to match flow to ventilation, with SNO-Hb entering the lung positioned to influence ventilation-perfusion matching.80 Local hypoxic pulmonary vasoconstriction occurs to divert blood to better oxygenated areas within the lungs; NO trapping by Hb is an important contributor to this vasoconstriction.81,82 In essence, the binding and release of NO bioactivity in the form of SNOs is a central component of the physiologic response to local hypoxia.81
The re-conceptualization of the respiratory cycle as a three-gas system75 (NO, oxygen, and carbon dioxide), provides the explanation for why increasing blood oxygen content can fail to improve tissue oxygenation.83 Tissue blood flow not blood oxygenation is the primary determinant of oxygen delivery.74,81
Tissue perfusion is primarily regulated by hypoxic vasodilation, which couples metabolic demand to local blood flow.84-86 Work by Guyton, Saltin, and Stamler have identified the RBC as the principal transducer of hypoxic vasodilation.87-89 Both acute and chronic reductions in oxygenation produce profound declines in circulating NO bioactivity. In healthy human volunteers a 60 min exposure to hypobaric hypoxia (0.56 atmospheres) reduced circulating RBC SNO-Hb levels by ~ 80%.90 This response helps to rationalize the reductions in systemic vascular resistance that occur with acute hypoxia (i.e., SNO unloading in the hypoxic periphery), as well as the exaggerated increases in pulmonary vascular resistance and pulmonary arterial pressure that are produced by SNO-depleted RBCs.
SNOs and Inflammation
SNOs have broad-based anti-inflammatory actions, from inhibition of toll-like receptor (TLR) signaling, to regulating expression of various cytokines91-93 and anti-inflammatory mediators94,95 to modulating a myriad of signal transduction pathways including the mitogen-activated protein (MAP) kinases. Diverse mechanisms have been proposed to account for these actions56 including attenuation of nuclear factor κB (NF-κB) p50-p65 activity.96,97 Under basal conditions, upstream S-nitrosylation acts to reduce the amount of inflammatory interleukins (IL-1β, IL-6, etc) and chemokines while enhancing the anti-inflammatory interleukins such as IL-10. Protein S-nitrosylation by NO generated from NOS2 (iNOS) can be pro-inflammatory but it occurs downstream of TLR activation, i.e. it first requires a loss of SNO regulatory inhibition of TLR-mediated signaling and/or NOS2 expression.56 In addition, S-nitrosylation has been found to regulate the activities of other mediators of lung inflammation including surfactant protein D,98 c-Jun NH2-terminal kinase 1,99 and cyclooxygenase 2.100
SNOs and Cell Signalling
A broad range of membrane receptors and ion channels have their activity and/or expression regulated by S-nitrosylation. These include but are not limited to G-protein coupled receptors, voltage gated potassium and sodium channels, L-type calcium channels, intra-cellular second messengers such as G-protein and tyrosine kinases, and intra-cellular regulators of receptor function such as β-arrestin and dynamin. Again, co-localization is important. For instance NOS isoforms associate directly with the ion channels or with neighboring scaffolding proteins that places the NO source in close proximity to the channel. NOSs can thereby selectively S-nitrosylate critical thiols to influence channel activity. In addition to effecting post-translational protein modification within cells, S-nitrosylation also has major regulator roles and control over the other methods of post-translational modification (phosphorylation, acetylation, ubiquitylation, sumoylation, etc). 67
Endogenous NO production both acts upon and is acted upon by factors that regulate the growth and development of the lung, notably vascular endothelial growth factor (VEGF) and hypoxia inducible factor-1 alpha (HIF-1α). Other factors regulated by S-nitrosylation (either directly or through upstream effectors) include angiopoietins, metalloproteinases, intra-cellular adhesion molecules, transforming growth factor-β1, tumor necrosis factor-α, caspases, lactoferrin, and endothelin-1. While S-nitrosylation control has not been demonstrated to occur within the lungs for all of this disparate group it is noteworthy that a large number of these factors (along with the inflammatory mediators identified earlier) have been proposed as potential bio-markers for diagnosing and assessing the severity of BPD.101
Relevance for Lung Development
Human lung development can be viewed as progressing through five stages; each stage is identified by the appearance, growth, and differentiation of various structures in the airway and pulmonary vasculature.102
Stage 1
Embryonic (between weeks 3 and 7 of gestation) – budding of the foregut endodermal epithelium into the adjacent primitive mesoderm.
Stage 2
Pseudoglandular (weeks 7 through 17 of gestation) – repeated dichotomous branching of epithelial-lined airways.
Stage 3
Canalicular (weeks 17 through 27) – differentiation of the alveolar cells in concert with proliferation of the vasculature and epithelial thinning to start forming functional gas-exchange units.
Stage 4
Saccular (weeks 28 through 36 of gestation) – additional branching and lengthening of the acinar tubules and buds into thin-walled alveolar saccules and ducts.
Stage 5
Alveolar (from week 36 into the postnatal period) – extensive proliferation of the alveolar ducts and alveoli.
Pre-term infants at high risk for BPD are bridging Stages 3 and 4, with viability dictated by the presence of functional gas exchange units. Surfactant therapy (along with antenatal steroids) has increased survival by acting to accelerate mesenchyme thinning thus improving gas diffusion and exchange as well as initiating endogenous surfactant production. However, while this intervention improves survival it may not impact the development of BPD. Lungs obtained at autopsy from preterm infants with severe BPD showed the same pattern of alveolar simplification and interstitial fibrosis immaterial of whether or not the subjects had received surfactant before death.103
It is important to appreciate that except for the latter parts of Stage 5 (i.e., postnatal) pulmonary development occurs under conditions of low oxygen tension104 and high pulmonary vascular resistance with reduced blood flow (<20% of ventricular output).105 It is under these conditions that the growth factors linked to low oxygen are optimized to direct lung development and vascularization – and from this perspective even room air (FiO2 of 21%) can be considered hyperoxic. As a result, when the immature lung is exposed to air following preterm delivery hypoxic growth factors (VEGF, HIF-1α, platelet derived growth factor) decline and factors that limit cell growth and alveolarization (transforming growth factors α and β, connective tissue growth factor, etc) are subsequently over-expressed and thus act to arrest pulmonary development early in Stage 4.106
Aberrant Protein S-Nitrosylation in The Immature Lung
The three NOS isoforms are present in fetal pulmonary tissues by the time of ex uterine viability (>24 weeks’ gestation).107,108 In the healthy developing lung, NOS1 (nNOS) is mostly associated with large blood vessels and with the lining of the airway. NOS1 can also be found in smaller blood vessels but not generally in the lung parenchyma. NOS3 (eNOS) is mostly localized to the pulmonary epithelium. There is only a modest amount of inducible NOS2 (iNOS) found in healthy lung and it is mostly limited to the epithelium in large airways.
As noted earlier, dysregulation of NO bioactivity in the mature lung is a common theme for a number of pulmonary diseases.69-73 There is also clear evidence that pulmonary SNO homeostasis and protein S-nitrosylation are disrupted in the immature lung with BPD. Within autopsied lungs of infants who died of severe BPD there is a significant increase in protein nitrosylation109 as well as significant increases in the levels of NOS2;108 both consistent with a loss of upstream SNO control of inflammation. Others have reported significant amounts of nitrotyrosine (a stable marker of nitrosative stress) distributed throughout the lungs of infants with chronic lung disease of prematurity with the amount directly correlating with disease severity107 (however these authors did not identify differences in NOS levels).
Consistent with dysregulation of SNO homeostasis levels of pro-angiogenic factors activated by S-nitrosylation (notably VEGF) are reduced110-112 while levels of anti-angiogenic agents repressed by S-nitrosylation (e.g. TGF-β, endoglin) are elevated113,114 in lung tissues and tracheal aspirates of infants with BPD. A similar effect is seen with endothelial colony-forming cells (ECFC) where hyperoxia significantly disrupts VEGF-NO signaling leading to impaired growth – a disruption that can be corrected by addition of exogenous VEGF and NO115. Of note, ECFCs from the cord blood of premature infants are significantly more sensitive to hyperoxic disruption of VEGF-NO activity than cells from term infants.
Other medical manipulations conducted on preterm infants (independent of ventilator strategies) may also impact SNO-homeostasis and contribute to BPD; chief among these is blood transfusion. Upwards of 80% of extremely preterm infants will receive RBCs during their stay in the NICU116 typically to correct anemia resulting from multiple phlebotomies.117 Administration of RBCs has been identified as a risk factor for developing BPD,118,119 with relative risk coincident with the number of transfusions.120 The processing and storage of blood leads to significant depletion of SNO-Hb such that banked RBCs have reduced ability to effect hypoxic vasodilation121 – this defect is directly linked to the impaired ability of banked blood to increase tissue oxygenation. As a result, infused SNO-Hb depleted RBCs can act as overall sinks for NO bioactivity leading to disruptions in oxygen uptake within the lungs and reduced oxygen delivery in the periphery.122 In this setting, transfusion can be additive to (rather than corrective of) the other disrupters of pulmonary SNO homeostasis.
Based on the preceding information resolution of aberrant S-nitrosylation provides an attractive therapeutic target for BPD prevention and/or amelioration. There is significant support for the postulate that S-nitrosylating therapy could ameliorate a number of the inflammatory and other injurious cellular processes that adversely effect ex uterine development of the premature lung. What is not so clear is whether or not restoration/supplementation of S-nitrosylation is best accomplished with iNO.
Why lno Should Work for bpd (And Why It Might Not)
Nitric oxide dilates pulmonary resistance vessels by primarily acting on sGC to increase cGMP levels.123 Inhaled NO was originally viewed as a selective pulmonary vasodilator because of its rapid metabolism by Hb as RBCs transit the lung. This view is now changing as various studies indicate iNO can exert peripheral effects on blood flow in addition to the oxygenation benefit gained by improved ventilation-perfusion matching.124-130 This list includes a study involving 8 infants (0-38 months) in acute respiratory distress who exhibited increases in microcirculatory blood flow 60 min after starting iNO therapy at 20 ppm.129 These blood flow effects of iNO appear to occur via SNO-based mechanisms as reflected by increases in the concentrations of circulating SNO-Hb and other SNO moieties.126,130,131
This triple combination of reducing pulmonary resistance, increasing blood oxygenation, and peripheral improvements in end-organ blood flow should, on the surface, make iNO an ideal agent to improve the physiologic status of preterm infants. However, there are a number of factors that alone or in combination can reduce the therapeutic efficacy of NO gas:
There are several pathways by which iNO can generate SNO-Hb (and other SNOs) but these reactions are not efficient. This entails higher dosages and/or longer exposure periods to iNO (although this could account for the dose-related trend for improvement noted by Askie et al.37). Note that in the pediatric study by Top et al. microcirculatory blood flow increased with an iNO dose of 20 ppm.129
Nitric oxide gas has a predilection to react with oxygen to generate higher-order tissue damaging nitrogen oxides (NOx), including peroxy-nitrite. The generation of NOx by iNO is enhanced in the presence of supplemental oxygen and at higher iNO doses.
RBC Hb interacts with iNO to form met-Hb. Fetal Hb is much more sensitive to oxidation than adult Hb and preterm infants have significantly less met-Hb reductase.132
To effect relaxation in pulmonary vessels iNO needs to interact with sGC. Currently it is not clear how much functional sGC is present in the immature lung nor is it certain that there is sufficient reserve activity for iNO to increase formation of cGMP.
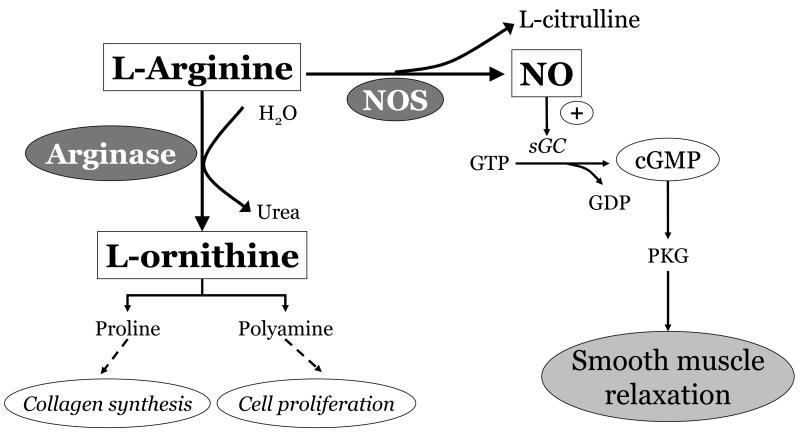
Hyperoxia and/or inflammation can lead to increases in phosphodiesterase activity, which in turn will result in faster breakdown of cGMP.133 Hyperoxia has also been shown to increase arginase expression and activity which diverts the substrate arginine from nitric oxide synthase134 [Fig. 5].
Discontinuation of iNO therapy can induce rebound pulmonary hypertension.
The impact of these limitations probably varies based on the patient’s physiologic status and developmental state. Collectively though they point to the need for alternatives to iNO. One obvious alternative is to identify S-nitrosylating agents that are more efficient than NO gas in correcting dysregulations in SNO homeostasis.
Figure 5.
Arginine serves as a common substrate for the enzymes NOS and arginase. Upregulation of arginase may divert arginine away from NOS, resulting in deficient NO/cGMP signaling and potential detrimental lung effects.
Potential Sources of Exogenous no/sno Bioactivity
Organic nitrates and organic nitrites can S-nitrosylate proteins.135 However, the intravenous use of such NO-donor compounds to treat preterm infants is limited by non-specific vasodilation and significant systemic hypotension can impair oxygenation by reducing flow to the right heart. In addition, most of these agents also impair hypoxic vasoconstriction that can further disrupt ventilation-perfusion matching resulting in enhanced blood flow to poorly-ventilated lung areas. Drug efficacy is also limited by tolerance development and accumulation of toxic metabolites (e.g., cyaongen/cyanide radical from sodium nitroprusside).
Inhalation of aerosolized formulations of various organic nitrates and nitrites can relax the pulmonary vasculature136 but they have seen little use in Neonatology; this despite some agents’ long history in adult therapy (e.g., inhaled amyl nitrite has been used to treat angina since the 1860s137). Administration of sodium nitroprusside through the ventilation circuit was reported to improve oxygenation status of 10 critically ill neonates (between 28 and 40 weeks’ gestational age).138 At the same time, the report suggests the infants desaturated when drug administration ceased (the manuscript does not provide outcome information). In addition, only two infants were less than 33 weeks, which makes predicting effects in a preterm cohort impracticable. In a study involving older children (9 to 53 months) with congenital heart disease and pulmonary arterial hypertension inhaled nitroglycerin acutely decreased pulmonary arterial pressure.139 Extrapolating this finding to preterm care in the NICU is difficult. The exposure time was brief as the drug was administered during a diagnostic right heart catheterization precluding the collection of data on effect duration or appearance of tachyphylaxis.
Outside of potential financial savings it is unclear if inhalation of an organic nitrate/nitrite currently in clinical use would offer appreciable benefit over iNO, especially since the aerosolized forms that have been tested (nitroglycerin and sodium nitroprusside) can still lower systemic blood pressure after inhalation.140 The consummate nitrosylating agent would work via S-nitrosylation mechanisms to improve oxygenation, stimulate alveolar growth and differentiation, and reduce inflammation. It would preferentially react with thiols, be biocompatible (both drug and metabolites), resist decomposition in a gaseous medium, be unreactive with oxygen, and yet be highly volatile so that it can be inhaled to act in the lungs. One agent that meets these criteria is ethyl nitrite
Ethyl Nitrite as an Exogenous S-Nitrosylating Agent
Ethyl nitrite (ENO) is a low-molecular-weight (75.07), colorless organic nitrite with a density of 0.9. It can be stored as a liquid, but with a low boiling point (16.5 to 17° C) it is volatile at room temperature, which allows for inhalation or other gaseous routes of delivery. Upon exposure to biologic media (including blood) ENO preferentially react with thiols to form stable adducts with endocrine activity. Further, it does not form toxic NOx when mixed with oxygen.141 ENO – like SNO-Hb itself142 – has potent systemic (blood flow-increasing) as well as pulmonary vascular effects. A series of pre-clinical translational studies with this agent demonstrated that ENO:
are highly selective for thiols
can rapidly restore RBC SNO-Hb levels141
produces SNOs, which are known to increase circulating stem cells143
effectively attenuates pneumoperitoneum-induced reductions in splanchnic blood flow and insufflation-induced markers of tissue injury144,145
improves outcome in a mouse model of subarachnoid hemorrhage146
reduces both hyperoxic60 and immunologic61 pulmonary inflammatory responses and associated pulmonary cell damage in rodent models of lung injury
ENO has been tested clinically in two high risk patient populations. In the first trial, ENO was administered by ventilator to infants with persistent pulmonary hypertension of the newborn (the 7 patients ranged between 38 and 40 weeks gestational age).147 The drug produced sustained dose-dependent improvements in postductal arterial oxygen saturation and in systemic hemodynamics. There was no evidence of rebound pulmonary hypertension when drug administration was abruptly terminated after 4 hours of exposure.
The second trial was conducted on adults with pulmonary hypertension.73 These patients were found to have low amounts of circulating SNO-Hb, which negatively impacted their RBCs’ ability to elicit hypoxic vasodilation. In addition, RBC-SNO levels were inversely correlated with pulmonary artery pressure. Inhaled ENO produced immediate salutary effects: pulmonary arterial pressure and pulmonary vascular resistance declined, and arterial pO2 increased. These effects were accompanied by increases in SNO-Hb. Moreover, ENO corrected the impairments in RBC hypoxic vasodilatory activity. This is notable because studies in large animals have demonstrated that effects of ENO are mediated primarily via RBCs (and not through direct effects of ENO in the lungs).
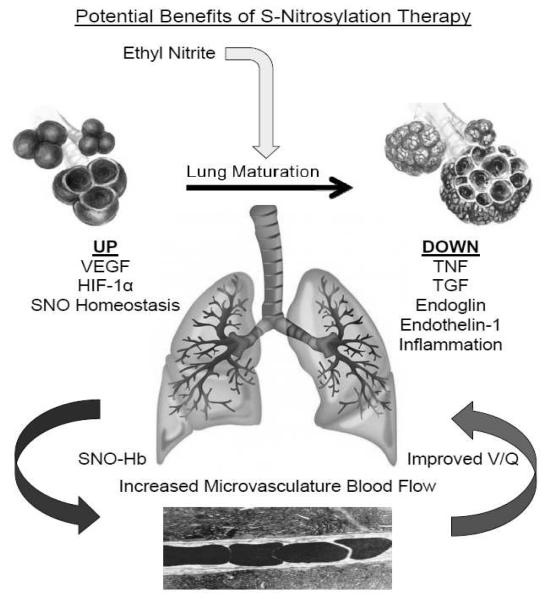
As of this writing no preterm infant has been administered ENO but the results compiled to date are supportive for conducting a clinical trial for preventing or ameliorating BPD in this patient population. In addition, it is reasonable to predict that ENO therapy could confer multiple benefits by addressing other organ injuries of prematurity that are believed to result from impaired blood flow and oxygen delivery (e.g., acute kidney injury, necrotizing enterocolitis, periventricular leukomalacia; administration of ENO can correct flow deficiencies to multiple internal organs) 145 [Fig. 6]. We note again that ENO’s mechanism of action differs fundamentally from the major action of iNO (or phophodiesterase inhibitors). These latter agents act mainly on the sGC pathway within the pulmonary vasculature and as such have limited ability to increase SNO-Hb (i.e., improve peripheral oxygen delivery) or to correct other aspects of SNO-based signaling that are disrupted in the immature lung.
Figure 6.
Potential mechanisms whereby ethyl nitrite might enhance lung maturation and improve ventilation/perfusion matching..
Conclusion
Over the last decade there has been remarkable interest in the potential ability of inhaled NO to decrease the incidence or severity of BPD. As we have detailed, this has been driven by a solid body of experimental data primarily in animal models, documenting a diversity of biologically based beneficial effects on lung development. Fortunately, these encouraging experimental data were followed up by a considerable number of well designed, randomized, clinical trials, blinded for experimental versus control groups. As a result of these clinical trials, initial enthusiasm has been greatly diminished by a series of negative results. Systematic meta-analysis of the available data is complicated by the variable study designs. Furthermore, there is no currently available biomarker to indicate potential for selective benefit.
Further studies addressing the combination of patient population, dose, duration, and timing of inhaled NO exposure are needed. These studies need to be appropriately powered to detect an effect on BPD and/or mortality as well as monitor for adverse outcomes of treatment. At the time of this writing, there are four active clinical trials148-151 underway that will add to the field’s growing fund of knowledge and hopefully identify a dosing regimen most beneficial in this at risk population of preterm infants. Nonetheless, based on available data, it was possible to convene a consensus conference in late 2010 to provide therapeutic guidelines from leading clinical and basic investigators in the field.8 Their primary conclusion is that apart from occasional instances of pulmonary hypertension or hypoplasia, routine or rescue use of inhaled NO cannot be recommended at this time in preterm infants with respiratory failure. As for preterm infants with BPD, while promising results derived from basic studies have not been realized, future investigation of the role of NO in lung development should proceed.
KEY POINTS.
Nitric oxide plays a role in cellular signaling, inflammation, growth and differentiation, and metabolism and has specifically shown an ability to decrease pulmonary vascular resistance.
Animal models of the major pulmonary morbidity of prematurity, bronchopulmonary dysplasia (BPD), have shown that inhaled nitric oxide (iNO) improved pulmonary function, promoted lung development, and prevented much of the pathology associated with BPD.
Multiple clinical trials have examined the use of iNO in preterm infants to prevent BPD; despite differing protocols, iNO has not proved to be of clear, unequivocal benefit in this at-risk population.
In 2010, the NIH Consensus Development Conference provided therapeutic guidelines for the use of iNO; their primary conclusion is that apart from occasional instances of pulmonary hypertension or hypoplasia, routine or rescue use of iNO cannot be recommended at this time in preterm infants with respiratory failure.
Much of the bioactivity of NO is mitigated through S-nitrosylation of proteins, and this may infact be a better therapeutic target.
Inhaled ethyl nitrite could be a superior therapeutic agent than iNO because it more efficiently S-nitrosylates proteins, does not create potentially harmful by-products like peroxy-nitrite, and improves tissue blood flow and oxygen delivery.
Future investigations of the role NO plays in lung development and pulmonary function are needed.
Acknowledgments
Supported in part by:
NIH Grant HL 56470 [Martin]
NIH Grants R01HL095463, R01HL091876, and UL1RR024989, and by DARPA Contract N66001-10-C-2015 [Reynolds]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Northway WH, Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–68. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147, e1–8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.D’Angio CT, Maniscalco WM. Bronchopulmonary dysplasia in preterm infants: pathophysiology and management strategies. Paediatric drugs. 2004;6:303–30. doi: 10.2165/00148581-200406050-00004. [DOI] [PubMed] [Google Scholar]
- 4.Gien J, Kinsella JP. Pathogenesis and treatment of bronchopulmonary dysplasia. Current opinion in pediatrics. 2011;23:305–13. doi: 10.1097/MOP.0b013e328346577f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancalari E, Polin RA. The newborn lung. 1st ed Saunders/Elsevier; Philadelphia: 2008. [Google Scholar]
- 6.Baraldi E, Filippone M. Chronic lung disease after premature birth. The New England journal of medicine. 2007;357:1946–55. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 7.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Resp Crit Care. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 8.Cole FS, Alleyne C, Barks JD, et al. NIH Consensus Development Conference statement: inhaled nitric-oxide therapy for premature infants. Pediatrics. 2011;127:363–9. doi: 10.1542/peds.2010-3507. [DOI] [PubMed] [Google Scholar]
- 9.Northway WH, Jr., Moss RB, Carlisle KB, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323:1793–9. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 10.Trachsel D, Brutsche MH, Hug-Batschelet H, Hammer J. Progressive static pulmonary hyperinflation in survivors of severe bronchopulmonary dysplasia by mid-adulthood. Thorax. 2011 doi: 10.1136/thoraxjnl-2011-200695. [DOI] [PubMed] [Google Scholar]
- 11.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–47. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JD, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:818–9. doi: 10.1016/0140-6736(92)92686-a. [DOI] [PubMed] [Google Scholar]
- 13.Kinsella JP, Neish SR, Shaffer E, Abman SH. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:819–20. doi: 10.1016/0140-6736(92)92687-b. [DOI] [PubMed] [Google Scholar]
- 14.The Neonatal Inhaled Nitric Oxide Study Group Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. The New England journal of medicine. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 15.Clark RH, Kueser TJ, Walker MW, et al. Clinical Inhaled Nitric Oxide Research Group Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. The New England journal of medicine. 2000;342:469–74. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 16.Davidson D, Barefield ES, Kattwinkel J, et al. The I-NO/PPHN Study Group Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. Pediatrics. 1998;101:325–34. doi: 10.1542/peds.101.3.325. [DOI] [PubMed] [Google Scholar]
- 17.McCurnin DC, Pierce RA, Chang LY, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. American journal of physiology Lung cellular and molecular physiology. 2005;288:L450–9. doi: 10.1152/ajplung.00347.2004. [DOI] [PubMed] [Google Scholar]
- 18.Iben SC, Dreshaj IA, Farver CF, Haxhiu MA, Martin RJ. Role of endogenous nitric oxide in hyperoxia-induced airway hyperreactivity in maturing rats. Journal of applied physiology. 2000;89:1205–12. doi: 10.1152/jappl.2000.89.3.1205. [DOI] [PubMed] [Google Scholar]
- 19.Lin YJ, Markham NE, Balasubramaniam V, et al. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatric research. 2005;58:22–9. doi: 10.1203/01.PDR.0000163378.94837.3E. [DOI] [PubMed] [Google Scholar]
- 20.Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Resp Crit Care. 2005;172:899–906. doi: 10.1164/rccm.200503-384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Franco-Belgium Collaborative NO Trial Group Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial. Lancet. 1999;354:1066–71. [PubMed] [Google Scholar]
- 22.Ballard RA, Truog WE, Cnaan A, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. The New England journal of medicine. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 23.Dani C, Bertini G, Pezzati M, Filippi L, Cecchi A, Rubaltelli FF. Inhaled nitric oxide in very preterm infants with severe respiratory distress syndrome. Acta paediatrica. 2006;95:1116–23. doi: 10.1080/08035250600702594. [DOI] [PubMed] [Google Scholar]
- 24.Field D, Elbourne D, Truesdale A, et al. Neonatal Ventilation With Inhaled Nitric Oxide Versus Ventilatory Support Without Inhaled Nitric Oxide for Preterm Infants With Severe Respiratory Failure: the INNOVO multicentre randomised controlled trial (ISRCTN 17821339) Pediatrics. 2005;115:926–36. doi: 10.1542/peds.2004-1209. [DOI] [PubMed] [Google Scholar]
- 25.Hascoet JM, Fresson J, Claris O, et al. The safety and efficacy of nitric oxide therapy in premature infants. The Journal of pediatrics. 2005;146:318–23. doi: 10.1016/j.jpeds.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Kinsella JP, Cutter GR, Walsh WF, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. The New England journal of medicine. 2006;355:354–64. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 27.Kinsella JP, Walsh WF, Bose CL, et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet. 1999;354:1061–5. doi: 10.1016/s0140-6736(99)03558-8. [DOI] [PubMed] [Google Scholar]
- 28.Mercier JC, Hummler H, Durrmeyer X, et al. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet. 2010;376:346–54. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. The New England journal of medicine. 2003;349:2099–107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 30.Srisuparp P, Heitschmidt M, Schreiber MD. Inhaled nitric oxide therapy in premature infants with mild to moderate respiratory distress syndrome. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2002;85(Suppl 2):S469–78. [PubMed] [Google Scholar]
- 31.Su PH, Chen JY. Inhaled nitric oxide in the management of preterm infants with severe respiratory failure. J Perinatol. 2008;28:112–6. doi: 10.1038/sj.jp.7211881. [DOI] [PubMed] [Google Scholar]
- 32.Subhedar NV, Ryan SW, Shaw NJ. Open randomised controlled trial of inhaled nitric oxide and early dexamethasone in high risk preterm infants. Archives of disease in childhood Fetal and neonatal edition. 1997;77:F185–90. doi: 10.1136/fn.77.3.f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Meurs KP, Hintz SR, Ehrenkranz RA, et al. Inhaled nitric oxide in infants >1500 g and <34 weeks gestation with severe respiratory failure. J Perinatol. 2007;27:347–52. doi: 10.1038/sj.jp.7211690. [DOI] [PubMed] [Google Scholar]
- 34.Van Meurs KP, Wright LL, Ehrenkranz RA, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. The New England journal of medicine. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 35.Barrington KJ, Finer N. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane database of systematic reviews. 2010:CD000509. doi: 10.1002/14651858.CD000509.pub4. [DOI] [PubMed] [Google Scholar]
- 36.Ballard PL, Merrill JD, Truog WE, et al. Surfactant function and composition in premature infants treated with inhaled nitric oxide. Pediatrics. 2007;120:346–53. doi: 10.1542/peds.2007-0095. [DOI] [PubMed] [Google Scholar]
- 37.Askie LM, Ballard RA, Cutter GR, et al. Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials. Pediatrics. 2011;128:729–39. doi: 10.1542/peds.2010-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donohue PK, Gilmore MM, Cristofalo E, et al. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics. 2011;127:e414–22. doi: 10.1542/peds.2010-3428. [DOI] [PubMed] [Google Scholar]
- 39.Hibbs AM, Walsh MC, Martin RJ, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. J Pediatr. 2008;153:525–9. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh MC, Hibbs AM, Martin CR, et al. Two-year neurodevelopmental outcomes of ventilated preterm infants treated with inhaled nitric oxide. J Pediatr. 2010;156:556–61. e1. doi: 10.1016/j.jpeds.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson RS, Clermont G, Kinsella JP, et al. Clinical and Economic Effects of iNO in Premature Newborns With Respiratory Failure at 1 Year. Pediatrics. 2009;124:1333–43. doi: 10.1542/peds.2009-0114. [DOI] [PubMed] [Google Scholar]
- 42.Hamon I, Fresson J, Nicolas MB, Buchweiller MC, Franck P, Hascoet JM. Early inhaled nitric oxide improves oxidative balance in very preterm infants. Pediatr Res. 2005;57:637–43. doi: 10.1203/01.PDR.0000156507.03879.19. [DOI] [PubMed] [Google Scholar]
- 43.Huddy CL, Bennett CC, Hardy P, et al. The INNOVO multicentre randomised controlled trial: neonatal ventilation with inhaled nitric oxide versus ventilatory support without nitric oxide for severe respiratory failure in preterm infants: follow up at 4-5 years. Arch Dis Child Fetal Neonatal Ed. 2008;93:F430–5. doi: 10.1136/adc.2007.129353. [DOI] [PubMed] [Google Scholar]
- 44.Hintz SR, Van Meurs KP, Perritt R, et al. Neurodevelopmental outcomes of premature infants with severe respiratory failure enrolled in a randomized controlled trial of inhaled nitric oxide. J Pediatr. 2007;151:16–22. e1–3. doi: 10.1016/j.jpeds.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med. 2005;353:23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- 46.Bennett AJ, Shaw NJ, Gregg JE, Subhedar NV. Neurodevelopmental outcome in high-risk preterm infants treated with inhaled nitric oxide. Acta Paediatr. 2001;90:573–6. [PubMed] [Google Scholar]
- 47.Tanaka Y, Hayashi T, Kitajima H, Sumi K, Fujimura M. Inhaled nitric oxide therapy decreases the risk of cerebral palsy in preterm infants with persistent pulmonary hypertension of the newborn. Pediatrics. 2007;119:1159–64. doi: 10.1542/peds.2006-2269. [DOI] [PubMed] [Google Scholar]
- 48.Steinhorn RH, Shaul PW, deRegnier RA, Kennedy KA. Inhaled nitric oxide and bronchopulmonary dysplasia. Pediatrics. 2011;128:e255–6. doi: 10.1542/peds.2011-1270A. author reply e6-7. [DOI] [PubMed] [Google Scholar]
- 49.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–46. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hess DT, Foster MW, Stamler JS. Assays for S-nitrosothiols and S-nitrosylated proteins and mechanistic insights into cardioprotection. Circulation. 2009;120:190–3. doi: 10.1161/CIRCULATIONAHA.109.876607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin RJ, Walsh MC. Inhaled nitric oxide for preterm infants--who benefits? The New England journal of medicine. 2005;353:82–4. doi: 10.1056/NEJMe058124. [DOI] [PubMed] [Google Scholar]
- 52.Khan SA, Lee K, Minhas KM, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–8. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saavedra WF, Paolocci N, St John ME, et al. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 54.Landmesser U, Spiekermann S, Dikalov S, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–8. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 55.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–83. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 56.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei W, Li B, Hanes MA, Kakar S, Chen X, Liu L. S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci Transl Med. 2010;2:19ra3. doi: 10.1126/scitranslmed.3000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prasad R, Giri S, Nath N, Singh I, Singh AK. GSNO attenuates EAE disease by S-nitrosylation-mediated modulation of endothelial-monocyte interactions. Glia. 2007;55:65–77. doi: 10.1002/glia.20436. [DOI] [PubMed] [Google Scholar]
- 59.Savidge TC, Newman P, Pothoulakis C, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–58. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 60.Auten RL, Mason SN, Whorton MH, et al. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med. 2007;176:291–9. doi: 10.1164/rccm.200605-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marshall HE, Potts EN, Kelleher ZT, Stamler JS, Foster WM, Auten RL. Protection from lipopolysaccharide-induced lung injury by augmentation of airway S-nitrosothiols. Am J Respir Crit Care Med. 2009;180:11–8. doi: 10.1164/rccm.200807-1186OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 62.Que LG, Liu L, Yan Y, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–21. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med. 2002;165:922–6. doi: 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- 64.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med. 2006;173:1186–93. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daaka Y. S-nitrosylation-regulated GPCR signaling. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seth D, Stamler JS. The SNO-proteome: causation and classifications. Curr Opin Chem Biol. 2011;15:129–36. doi: 10.1016/j.cbpa.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hess DT, Stamler JS. Regulation by S-Nitrosylation of Protein Post-translational Modification. J Biol Chem. 2012;287:4411–8. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schulman IH, Hare JM. Regulation of cardiovascular cellular processes by S-nitrosylation. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grasemann H, Gaston B, Fang K, Paul K, Ratjen F. Decreased levels of nitrosothiols in the lower airways of patients with cystic fibrosis and normal pulmonary function. J Pediatr. 1999;135:770–2. doi: 10.1016/s0022-3476(99)70101-0. [DOI] [PubMed] [Google Scholar]
- 70.Zaman K, Carraro S, Doherty J, et al. S-nitrosylating agents: a novel class of compounds that increase cystic fibrosis transmembrane conductance regulator expression and maturation in epithelial cells. Mol Pharmacol. 2006;70:1435–42. doi: 10.1124/mol.106.023242. [DOI] [PubMed] [Google Scholar]
- 71.Gaston B, Sears S, Woods J, et al. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet. 1998;351:1317–9. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- 72.Que LG, Yang Z, Stamler JS, Lugogo NL, Kraft M. S-nitrosoglutathione reductase: an important regulator in human asthma. Am J Respir Crit Care Med. 2009;180:226–31. doi: 10.1164/rccm.200901-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMahon TJ, Ahearn GS, Moya MP, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102:14801–6. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 75.Doctor A, Stamler JS. Comprehensive Physiology. John Wiley & Sons, Inc.; 2011. Nitric Oxide Transport in Blood: A Third Gas in the Respiratory Cycle. [DOI] [PubMed] [Google Scholar]
- 76.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel DJ. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci U S A. 2003;100:461–6. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A. 2006;103:8366–71. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pezacki JP, Ship NJ, Kluger R. Release of nitric oxide from S-nitrosohemoglobin. Electron transfer as a response to deoxygenation. J Am Chem Soc. 2001;123:4615–6. doi: 10.1021/ja015716o. [DOI] [PubMed] [Google Scholar]
- 79.McMahon TJ, Stone AE, Bonaventura J, Singel DJ, Stamler JS. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. Journal of Biological Chemistry. 2000;275:16738–45. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 80.Doctor A, Platt R, Sheram ML, et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci U S A. 2005;102:5709–14. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doctor A, Stamler JS. NO Transport in Blood: a third gas in the respiratory cycle. Comprehensive Physiology. doi: 10.1002/cphy.c090009. in press. [DOI] [PubMed] [Google Scholar]
- 82.Deem S, Min JH, Moulding JD, Eveland R, Swenson ER. Red blood cells prevent inhibition of hypoxic pulmonary vasoconstriction by nitrite in isolated, perfused rat lungs. Am J Physiol Heart Circ Physiol. 2007;292:H963–70. doi: 10.1152/ajpheart.00812.2006. [DOI] [PubMed] [Google Scholar]
- 83.Hodges AN, Delaney S, Lecomte JM, Lacroix VJ, Montgomery DL. Effect of hyperbaric oxygen on oxygen uptake and measurements in the blood and tissues in a normobaric environment. Br J Sports Med. 2003;37:516–20. doi: 10.1136/bjsm.37.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970;27:669–78. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- 85.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med. 2009;15:452–60. doi: 10.1016/j.molmed.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Intaglietta M, Johnson PC, Winslow RM. Microvascular and tissue oxygen distribution. Cardiovasc Res. 1996;32:632–43. [PubMed] [Google Scholar]
- 87.Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol. 1999;276:H438–45. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- 88.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–55. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McMahon TJ, Moon RE, Luschinger BP, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–7. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 91.Schroeder RA, Cai C, Kuo PC. Endotoxin-mediated nitric oxide synthesis inhibits IL-1beta gene transcription in ANA-1 murine macrophages. Am J Physiol. 1999;277:C523–30. doi: 10.1152/ajpcell.1999.277.3.C523. [DOI] [PubMed] [Google Scholar]
- 92.Xiong H, Zhu C, Li F, et al. Inhibition of interleukin-12 p40 transcription and NF-kappaB activation by nitric oxide in murine macrophages and dendritic cells. J Biol Chem. 2004;279:10776–83. doi: 10.1074/jbc.M313416200. [DOI] [PubMed] [Google Scholar]
- 93.Into T, Inomata M, Nakashima M, Shibata K, Hacker H, Matsushita K. Regulation of MyD88-dependent signaling events by S nitrosylation retards toll-like receptor signal transduction and initiation of acute-phase immune responses. Mol Cell Biol. 2008;28:1338–47. doi: 10.1128/MCB.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.del Fresno C, Gomez-Garcia L, Caveda L, et al. Nitric oxide activates the expression of IRAK-M via the release of TNF-alpha in human monocytes. Nitric Oxide. 2004;10:213–20. doi: 10.1016/j.niox.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez-Leon MC, Soares-Schanoski A, del Fresno C, et al. Nitric oxide induces SOCS-1 expression in human monocytes in a TNF-alpha-dependent manner. J Endotoxin Res. 2006;12:296–306. doi: 10.1179/096805106X118843. [DOI] [PubMed] [Google Scholar]
- 96.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–93. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 97.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282:30667–72. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 98.Guo CJ, Atochina-Vasserman EN, Abramova E, et al. S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol. 2008;6:e266. doi: 10.1371/journal.pbio.0060266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park HS, Huh SH, Kim MS, Lee SH, Choi EJ. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc Natl Acad Sci U S A. 2000;97:14382–7. doi: 10.1073/pnas.97.26.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–70. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 101.Thompson A, Bhandari V. Pulmonary Biomarkers of Bronchopulmonary Dysplasia. Biomark Insights. 2008;3:361–73. doi: 10.4137/bmi.s834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schwarz MA, Cleaver OB. Development of the Pulmonary Endothelium in Development of the Pulmonary Circulation: Vasculogenesis and Angiogenesis. In: Voelkel NF, Rounds S, editors. The Pulmonary Endothelium: Function in Health and Disease. Vol. 24. John Wiley & Sons; Hoboken, NJ: 2009. [Google Scholar]
- 103.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–7. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 104.Pardi G, Cetin I, Marconi AM, et al. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med. 1993;328:692–6. doi: 10.1056/NEJM199303113281004. [DOI] [PubMed] [Google Scholar]
- 105.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90:1291–335. doi: 10.1152/physrev.00032.2009. [DOI] [PubMed] [Google Scholar]
- 106.Ahlfeld SK, Conway SJ. Aberrant signaling pathways of the lung mesenchyme and their contributions to the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2012;94:3–15. doi: 10.1002/bdra.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sheffield M, Mabry S, Thibeault DW, Truog WE. Pulmonary nitric oxide synthases and nitrotyrosine: findings during lung development and in chronic lung disease of prematurity. Pediatrics. 2006;118:1056–64. doi: 10.1542/peds.2006-0195. [DOI] [PubMed] [Google Scholar]
- 108.Davis CW, Gonzales LW, Ballard RA, Ballard PL, Guo C, Gow AJ. Expression of nitric oxide synthases and endogenous NO metabolism in bronchopulmonary dysplasia. Pediatr Pulmonol. 2008;43:703–9. doi: 10.1002/ppul.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gow AJ, Chen Q, Hess DT, Day BJ, Ischiropoulos H, Stamler JS. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J Biol Chem. 2002;277:9637–40. doi: 10.1074/jbc.C100746200. [DOI] [PubMed] [Google Scholar]
- 110.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol. 2010;661:323–35. doi: 10.1007/978-1-60761-500-2_21. [DOI] [PubMed] [Google Scholar]
- 111.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–80. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 112.Hasan J, Beharry KD, Valencia AM, Strauss A, Modanlou HD. Soluble vascular endothelial growth factor receptor 1 in tracheal aspirate fluid of preterm neonates at birth may be predictive of bronchopulmonary dysplasia/chronic lung disease. Pediatrics. 2009;123:1541–7. doi: 10.1542/peds.2008-1670. [DOI] [PubMed] [Google Scholar]
- 113.Popova AP, Bozyk PD, Bentley JK, et al. Isolation of tracheal aspirate mesenchymal stromal cells predicts bronchopulmonary dysplasia. Pediatrics. 2010;126:e1127–33. doi: 10.1542/peds.2009-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Paepe ME, Patel C, Tsai A, Gundavarapu S, Mao Q. Endoglin (CD105) up-regulation in pulmonary microvasculature of ventilated preterm infants. Am J Respir Crit Care Med. 2008;178:180–7. doi: 10.1164/rccm.200608-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fujinaga H, Baker CD, Ryan SL, et al. Hyperoxia disrupts vascular endothelial growth factor-nitric oxide signaling and decreases growth of endothelial colony-forming cells from preterm infants. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1160–9. doi: 10.1152/ajplung.00234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ohls RK. Transfusions in the Preterm Infant. Neoreviews. 2007;8:e377–e86. [Google Scholar]
- 117.Nunes dos Santos AlM, Trindade CEP. Red Blood Cell Transfusions in the Neonate. Neoreviews. 2011;12:e13–e9. [Google Scholar]
- 118.Cooke RW, Drury JA, Yoxall CW, James C. Blood transfusion and chronic lung disease in preterm infants. Eur J Pediatr. 1997;156:47–50. doi: 10.1007/s004310050551. [DOI] [PubMed] [Google Scholar]
- 119.Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 2009;155:331–37. e1. doi: 10.1016/j.jpeds.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang H, Fang J, Su H, Chen M. Risk factors for bronchopulmonary dysplasia in neonates born at </= 1500 g (1999-2009) Pediatr Int. 2011;53:915–20. doi: 10.1111/j.1442-200X.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- 121.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reynolds JD, Hess DT, Stamler JS. The transfusion problem: role of aberrant S-nitrosylation. Transfusion. 2011;51:852–8. doi: 10.1111/j.1537-2995.2011.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. The New England journal of medicine. 2006;355:2003–11. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 124.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci U S A. 2008;105:11430–5. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fox-Robichaud A, Payne D, Hasan SU, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ibrahim YI, Ninnis JR, Hopper AO, et al. Inhaled nitric oxide therapy increases blood nitrite, nitrate, and s-nitrosohemoglobin concentrations in infants with pulmonary hypertension. J Pediatr. 2012;160:245–51. doi: 10.1016/j.jpeds.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minamishima S, Kida K, Tokuda K, et al. Inhaled nitric oxide improves outcomes after successful cardiopulmonary resuscitation in mice. Circulation. 2011;124:1645–53. doi: 10.1161/CIRCULATIONAHA.111.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ng ES, Jourd’heuil D, McCord JM, et al. Enhanced S-nitroso-albumin formation from inhaled NO during ischemia/reperfusion. Circ Res. 2004;94:559–65. doi: 10.1161/01.RES.0000117771.63140.D6. [DOI] [PubMed] [Google Scholar]
- 129.Top AP, Ince C, Schouwenberg PH, Tibboel D. Inhaled nitric oxide improves systemic microcirculation in infants with hypoxemic respiratory failure. Pediatr Crit Care Med. 2011;12:e271–4. doi: 10.1097/PCC.0b013e31820ac0b3. [DOI] [PubMed] [Google Scholar]
- 130.Terpolilli NA, Kim SW, Thal SC, et al. Inhalation of Nitric Oxide Prevents Ischemic Brain Damage in Experimental Stroke by Selective Dilatation of Collateral Arterioles. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.253419. [DOI] [PubMed] [Google Scholar]
- 131.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006;3:153–60. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wind M, Stern A. Comparison of human adult and fetal hemoglobin: aminophenol-induced methemoglobin formation. Experientia. 1977;33:1500–1. doi: 10.1007/BF01918834. [DOI] [PubMed] [Google Scholar]
- 133.Farrow KN, Groh BS, Schumacker PT, et al. Hyperoia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008;102:226–33. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ali NKM, Jafri A, Sopi RB, Prakash YS, et al. Role of arginase in impairing relaxation of lung parenchyma of hyperoxia-exposed neonatal rats. Neonatology. 2012;101:106–15. doi: 10.1159/000329540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Janero DR, Bryan NS, Saijo F, et al. Differential nitros(yl)ation of blood and tissue constituents during glyceryl trinitrate biotransformation in vivo. Proc Natl Acad Sci U S A. 2004;101:16958–63. doi: 10.1073/pnas.0406075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schutte H, Grimminger F, Otterbein J, et al. Efficiency of aerosolized nitric oxide donor drugs to achieve sustained pulmonary vasodilation. J Pharmacol Exp Ther. 1997;282:985–94. [PubMed] [Google Scholar]
- 137.Brunton TL. On the Employment of Nitrite of Amyl in the Collapse of Cholera. Br Med J. 1872;1:42–5. doi: 10.1136/bmj.1.576.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Palhares DB, Figueiredo CS, Moura AJ. Endotracheal inhalatory sodium nitroprusside in severely hypoxic newborns. J Perinat Med. 1998;26:219–24. doi: 10.1515/jpme.1998.26.3.219. [DOI] [PubMed] [Google Scholar]
- 139.Goyal P, Kiran U, Chauhan S, Juneja R, Choudhary M. Efficacy of nitroglycerin inhalation in reducing pulmonary arterial hypertension in children with congenital heart disease. Br J Anaesth. 2006;97:208–14. doi: 10.1093/bja/ael112. [DOI] [PubMed] [Google Scholar]
- 140.Bando M, Ishii Y, Kitamura S, Ohno S. Effects of inhalation of nitroglycerin on hypoxic pulmonary vasoconstriction. Respiration. 1998;65:63–70. doi: 10.1159/000029228. [DOI] [PubMed] [Google Scholar]
- 141.Moya MP, Gow AJ, McMahon TJ, et al. S-nitrosothiol repletion by an inhaled gas regulates pulmonary function. Proc Natl Acad Sci U S A. 2001;98:5792–7. doi: 10.1073/pnas.091109498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–6. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 143.Lima B, Lam GK, Xie L, et al. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A. 2009;106:6297–302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ali NA, Eubanks WS, Stamler JS, et al. A method to attenuate pneumoperitoneum-induced reductions in splanchnic blood flow. Ann Surg. 2005;241:256–61. doi: 10.1097/01.sla.0000153034.54128.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shimazutsu K, Uemura K, Auten KM, et al. Inclusion of a nitric oxide congener in the insufflation gas repletes S-nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin Transl Sci. 2009;2:405–12. doi: 10.1111/j.1752-8062.2009.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sheng H, Reynolds JD, Auten RL, et al. Pharmacologically augmented S-nitrosylated hemoglobin improves recovery from murine subarachnoid hemorrhage. Stroke. 2011;42:471–6. doi: 10.1161/STROKEAHA.110.600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moya MP, Gow AJ, Califf RM, Goldberg RN, Stamler JS. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet. 2002;360:141–3. doi: 10.1016/S0140-6736(02)09385-6. [DOI] [PubMed] [Google Scholar]
- 148. [Accessed December 16, 2011];Inhaled nitric oxide and neuroprotection in premature infants (NOVA2) at http://clinicaltrials.gov/ct2/show/NCT00515281.
- 149. [Accessed December 16, 2011];Examining the use of non-invasive inhaled nitric oxide to reduce chronic lung disease in premature newborns. at http://clinicaltrials.gov/ct2/show/NCT00955487.
- 150. [Accessed December 16, 2011];Inhaled nitric oxide (INO) for the prevention of bronchopulmonary dysplasia (BPD) in preterm infants. at http://clinicaltrials.gov/ct2/show/NCT00931632.
- 151. [Accessed December 16, 2011];Trial of late surfactant to prevent BPD: a pilot study in ventilated preterm neonates receiving inhaled nitric oxide (TOLSURF Pilot) at http://clinicaltrials.gov/ct2/show/NCT00569530.
- 152.Truog WE. Inhaled nitric oxide for the prevention of bronchopulmonary dysplasia. Expert opinion on pharmacotherapy. 2007;8:1505–13. doi: 10.1517/14656566.8.10.1505. [DOI] [PubMed] [Google Scholar]