Abstract
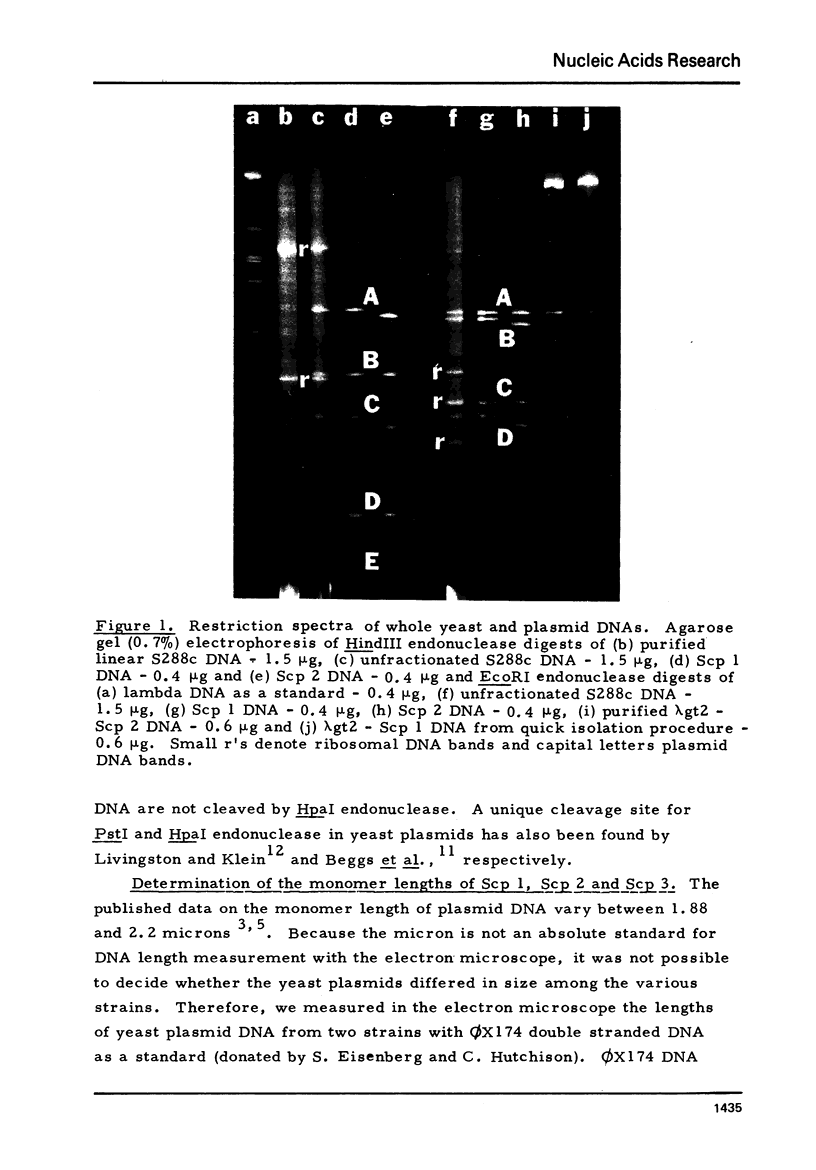
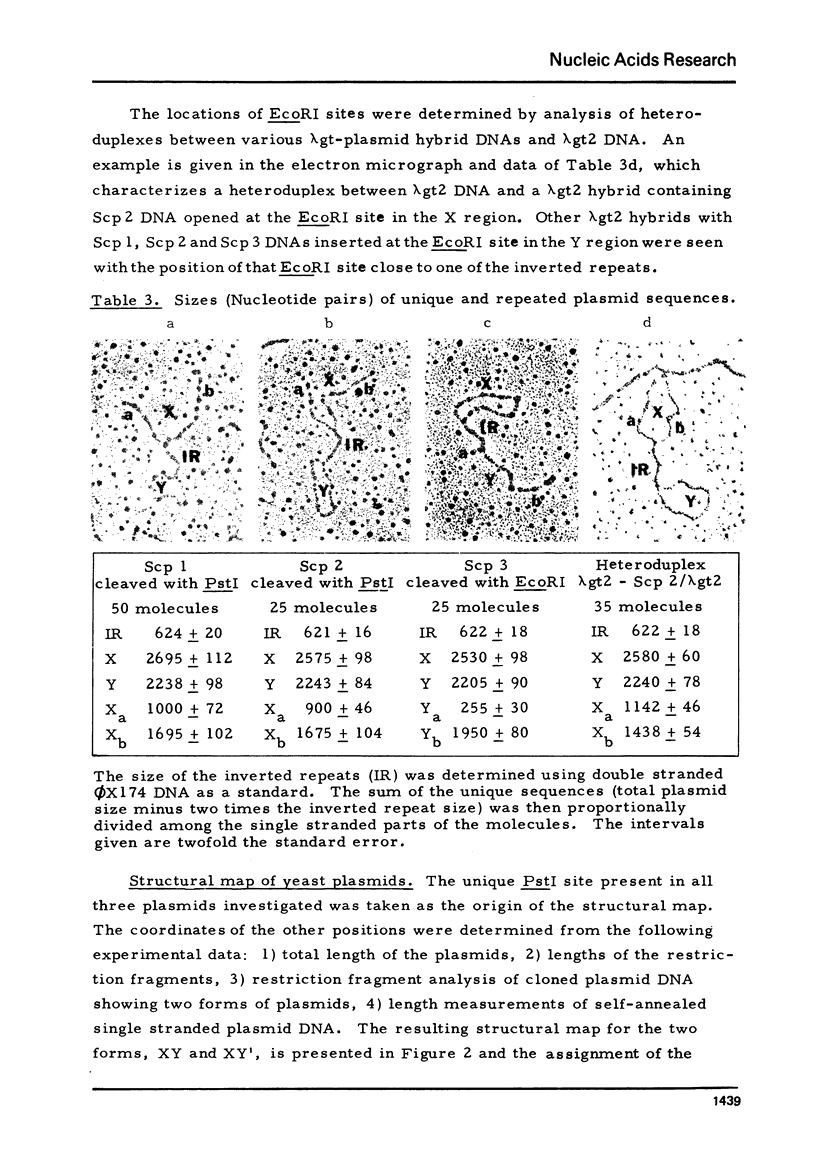
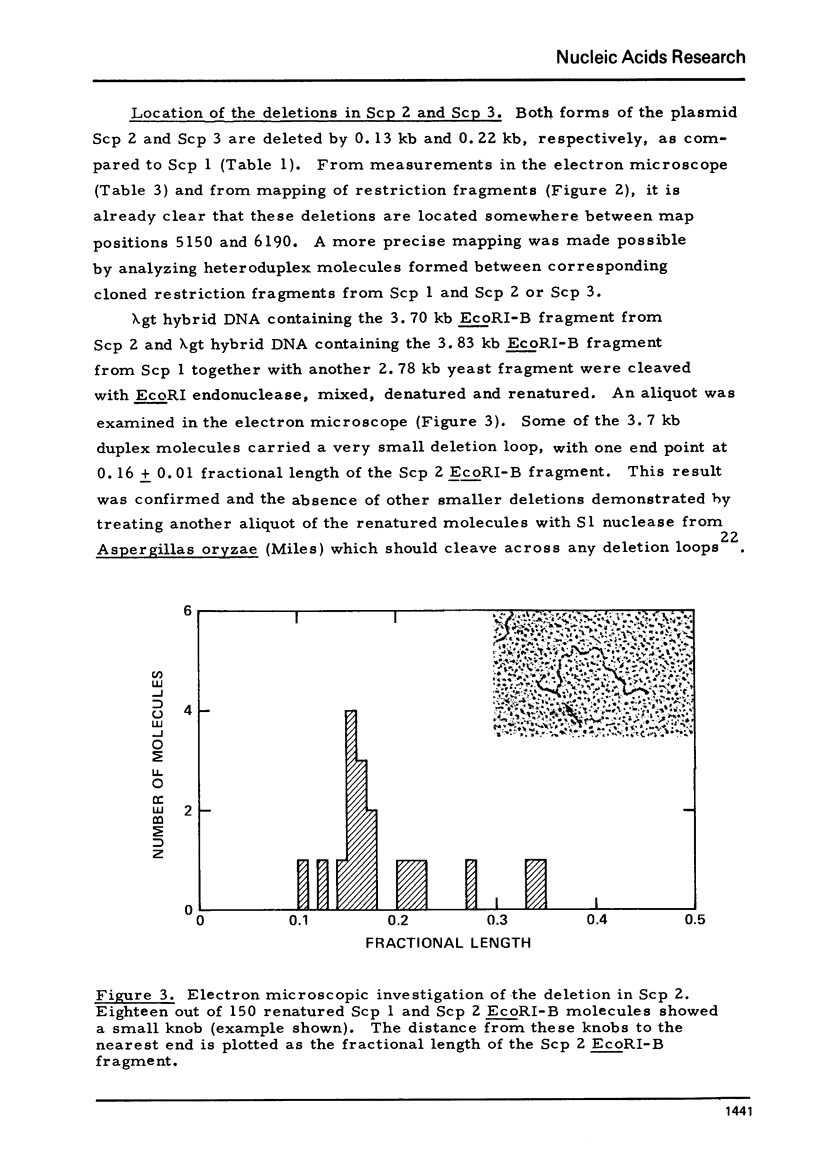
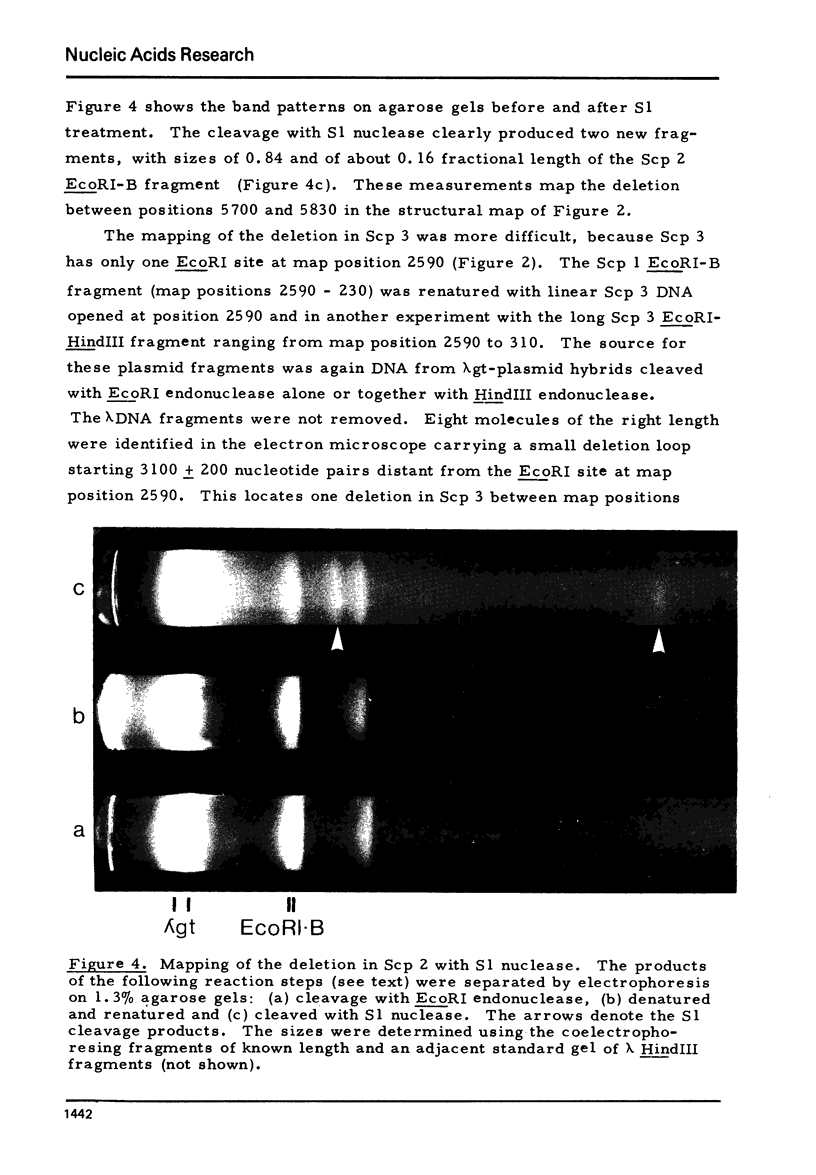
Plasmid DNAs from six strains of Saccharomyces cerevisiae were compared. Three different plasmids were found, designated Scp 1, Scp 2 and Scp 3, with monomer lengths of 6.19, 6.06 and 5.97 kilobases as referenced to sequenced phiX174 DNA. DNA from each of the plasmids was inserted into a lambda vector DNA. Hybrid phage containing inserted DNA of the desired size were enriched by genetic selection and their DNAs analysed by rapid techniques. All three plasmids share the same organization, two unique sequences separated by two inverted repeats, and share basically the same DNA sequences. Scp 2 and Scp 3 differ from Scp 1 by missing a unique HpaI site and by having small overlapping deletions in the same region. The HpaI site in Scp 1 is, therefore, in a nonessential region and suitable for insertion of foreign DNA in the potential use of the yeast plasmid as a vector. Hybridization of labelled cloned plasmid DNA to restriction fragments of linear yeast DNA separated on agarose gels showed that the plasmid DNA was not stably integrated into the yeast chromosomal DNA.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak A. L., Christiansen C., Christiansen G. Circular, repetitive DNA in yeast. Biochim Biophys Acta. 1972 May 29;269(3):527–530. doi: 10.1016/0005-2787(72)90144-x. [DOI] [PubMed] [Google Scholar]
- Beggs J. D., Guerineau M., Atkins J. F. A map of the restriction targets in yeast 2 micron plasmid DNA cloned on bacteriophage lambda. Mol Gen Genet. 1976 Nov 17;148(3):287–294. doi: 10.1007/BF00332903. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Panasenko S. M., Lehman I. R., Davis R. W. In vitro construction of bacteriophage lambda carrying segments of the Escherichia coli chromosome: selection of hybrids containing the gene for DNA ligase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3416–3420. doi: 10.1073/pnas.72.9.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D. Isolation of circular DNA from a mitochondrial fraction from yeast. Proc Natl Acad Sci U S A. 1972 Feb;69(2):388–392. doi: 10.1073/pnas.69.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Localization and quantification of circular DNA in yeast. Eur J Biochem. 1974 Jan 16;41(2):359–365. doi: 10.1111/j.1432-1033.1974.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Size distribution of circular DNA from petite-mutant yeast lacking rho DNA. Eur J Biochem. 1973 Jan 15;32(2):263–267. doi: 10.1111/j.1432-1033.1973.tb02606.x. [DOI] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., MacCosham V., Baldwin R. L. Tandem genetic duplications in phage lambda. III. The frequency of duplication mutants in two derivatives of phage lambda is independent of known recombination systems. J Mol Biol. 1975 Jan 15;91(2):133–146. doi: 10.1016/0022-2836(75)90154-0. [DOI] [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Paoletti C., Slonimski P. Characterization of a new class of circular DNA molecules in yeast. Biochem Biophys Res Commun. 1971 Feb 5;42(3):550–557. doi: 10.1016/0006-291x(71)90406-2. [DOI] [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Slonimski P. P. Circular DNA of a yeast episome with two inverted repeats: structural analysis by a restriction enzyme and electron microscopy. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3030–3034. doi: 10.1073/pnas.73.9.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Biochemical genetics of yeast. Annu Rev Genet. 1970;4:373–396. doi: 10.1146/annurev.ge.04.120170.002105. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Hollenberg C. P., Degelmann A., Kustermann-Kuhn B., Royer H. D. Characterization of 2-mum DNA of Saccharomyces cerevisiae by restriction fragment analysis and integration in an Escherichia coli plasmid. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2072–2076. doi: 10.1073/pnas.73.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobban P. E., Kaiser A. D. Enzymatic end-to end joining of DNA molecules. J Mol Biol. 1973 Aug 15;78(3):453–471. doi: 10.1016/0022-2836(73)90468-3. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Williamson D. H. Replicating circular DNA molecules in yeast. Cell. 1975 Mar;4(3):249–253. doi: 10.1016/0092-8674(75)90172-5. [DOI] [PubMed] [Google Scholar]
- Scandella D., Arber W. Phage lambda DNA injection into Escherichia coli pel- mutants is restored by mutations in phage genes V or H. Virology. 1976 Jan;69(1):206–215. doi: 10.1016/0042-6822(76)90207-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. H., Stevens B. J., Sanghavi P., Rabinowitz M. Mitochondrial-satellite and circular DNA filaments in yeast. Science. 1967 Jun 2;156(3779):1234–1237. doi: 10.1126/science.156.3779.1234. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]