Abstract
Alzheimer’s disease (AD) is characterized by progressive declines in cognitive function and ability to carry out activities of daily living; and the emergence and worsening of behavioral/neuropsychiatric symptoms. While there is no cure for AD, non-pharmacologic interventions and medications that modulate neurotransmission can slow symptomatic progression. Medical foods may also be useful as adjuncts to pharmacologic agents in AD. Medium chain triglycerides aimed at improving cerebral metabolism significantly improve Alzheimer’s Disease Assessment Scale-Cognitive scores when added to ongoing pharmacotherapy in patients with mild-to-moderate AD. Combination of interventions, such as non-pharmacologic treatments, pharmacotherapy, and medical foods, with complementary mechanisms of action may provide a rational approach that may result in maximum preservation of cognitive function in patients with AD.
Keywords: biomarker, FDG-PET, ketone body, medical food
Introduction
Alzheimer’s disease (AD) is the most common neurologic condition affecting the elderly in the United States. Results from the Centers for Disease Control suggest that 5.3 million Americans of all ages had AD in 2011 [1, 201, 202]. Alzheimer’s disease is the sixth-leading cause of all deaths in the United States with 74,632 deaths attributed to AD in 2009 [203]. The cost associated with AD is currently high and is expected grow. Medicare and Medicaid payments for services to beneficiaries with AD were $123 billion in 2010 [201]. The cost of AD care in the United States is expected exceed $1 trillion by 2050 [1].
This paper briefly reviews AD pathology, strengths and weaknesses of current and emerging treatments, and suggests a polymodal approach to patient management.
Natural History of Untreated AD
Pathology in AD
Three major hypotheses have been put forward to link changes in the brains of AD patients and disease symptoms. These are the cholinergic hypothesis, the amyloid β cascade hypothesis, and abnormal phosphorylation of the tau protein [2,3]. The cholinergic hypothesis suggests that a dysfunctional cholinergic system is the primary cause of the cognitive impairment and memory loss characteristic of AD. Brains from AD patients show degeneration of cholinergic neurons of the basal forebrain and a decline in cholinergic markers in the cerebral cortex [2]. The amyloid cascade hypothesis suggests that neurodegeneration in AD is triggered by abnormal processing of amyloid precursor protein. It has been suggested that inflammation in the vicinity of plaques comprised of amyloid β results in the death of nearby neurons, leading to AD symptoms [3]. The tau hypothesis suggests that accumulation of hyperphosphorylated tau as paired helical filaments within neurofibrillary tangles and in neuritic processes is linked to neuronal dysfunction and death [3, 4].
Cerebral hypometabolism is also involved in AD. A 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) study carried out by deLeon and colleagues and published in 1983 demonstrated significant 21–28% regional reductions in glucose utilization in AD patients [5]. It has been suggested that deficient energy metabolism may change the overall oxidative microenvironment for neurons in AD and that this altered environment could result in alterations in mitochondrial enzymes and glucose metabolism [6].
It has also been shown that brain fatty acid profiles are altered in the brains of patients with AD. Results from the Memory and Aging Project indicated the concentrations of docosahexanoic acid in phosphatidylserine of mid-frontal and superior temporal cortices were lower in patients with AD versus those without any cognitive impairment [7]. It has also been shown folate levels in the cerebrospinal fluid (CSF) of patients with AD are significantly lower than those of age-matched normal controls [8].
Homocysteine has also received considerable attention as a contributor to AD pathology. Homocysteine is an amino acid that becomes elevated in the presence of inadequate folate, vitamin B-12, or vitamin B-6 [9]; and elevated homocysteine is an independent predictor of the development of dementia and AD [10]. Results from one study indicated that declines in constructional praxis, measured by spatial copying, were significantly associated with higher plasma homocysteine and lower folate, and vitamins B-6 and B-12 levels [9].
Progression in AD
Alzheimer’s disease is characterized by progressive declines in cognitive function and ability to carry out activities of daily living (ADL); and the emergence and worsening of behavioral/neuropsychiatric symptoms [11].
Cognition
Before diagnosis of AD, individuals may have memory complaints, which represent periods of subjective cognitive impairment (SCI) [12, 13] or mild cognitive impairment (MCI); and longitudinal evaluation has indicated that changes in visuospatial skills may be apparent 3 years prior to AD diagnosis [14].
Neuropsychiatric Symptoms
Almost all patients with AD are affected by neuropsychiatric symptoms at some point during their illness, and in some cases, such symptoms may occur before diagnosis of dementia [15]. In a sample of 50 consecutive outpatients with mild, moderate, or severe AD, 88% had psychopathology. Apathy is an important neuropsychiatric symptom and it occurred in 72%, agitation occurred in 60%, followed by anxiety in 48%, irritability in 42%, dysphoria in 38%, aberrant motor behavior in 38%, and disinhibition in 36% [16].
Activities of Daily Living
Instrumental ADL include the integration of task initiation, planning, and performance [17]. Evaluation of 471 women with mild-to-moderate AD indicated that 80.7% were impaired in doing grocery shopping, 76.0% in taking medication, 72.2% in preparing meals, 41.4% in traveling on public transportation even when assisted, 40.6% in managing purchases, 30.1% in laundering small items, 14.2% in participating in some housekeeping tasks, and 11.0% in answering the telephone [18]. Impairment in ADL is a significant predictor of placement in a long-term care facility in patients with AD [19].
Factors Influencing Disease Progression
A recent systematic review of results from both randomized controlled trials and observational studies indicated that the APOE ε4 genotype, low plasma selenium, depression, diabetes mellitus, metabolic syndrome, and tobacco use were all associated with more rapid cognitive decline in AD [20]. Cognitive training, vegetable intake, Mediterranean diet, intake of omega-3 fatty acids, physical activity, and non-cognitive, non-physical leisure activities were all associated with slower decline [20]. Vascular factors, such as hypertension and hypercholesterolemia, have not been found to be significantly associated with disease progression [20–22], but there is evidence that other factors, such as ingestion of antioxidant nutrients, fish, and B-vitamins, may be associated with some preservation of cognitive function [23].
It has been hypothesized that individuals with greater cognitive reserve (i.e., alternative cognitive processing approaches or compensatory brain networks) are better able to withstand AD-related pathology without developing symptoms of dementia [24, 25]. This hypothesis was tested in 161 subjects who were not demented and 37 with AD who were evaluated for cognitive function and uptake of N-methyl-[11C] 2-(4′-methylaminophenyl)-6-hydroybenzothiazole ([11C]-Pittsburgh Compound-B [PIB]), a marker whose levels are positively correlated with years of education and negatively correlated with symptom severity in AD patients. Study results showed that cognitive performance was predicted by the interaction of [11C]-PIB uptake and years of education, such that performance on these measures improved with increasing education for participants with elevated PIB uptake [24].
Screening for and Diagnosis of AD in Office Practice
Screening
The use of brief cognitive tests is insufficient for the differential diagnosis of AD [26], but they may be employed for identification of individuals who should undergo additional evaluation. Many of these evaluations are also useful for monitoring response to therapy in AD. These tests can be divided into two general types: performance- and informant-based tests.
Performance Tests
MMSE
The MMSE includes 30 questions (orientation, recall, reverse spelling or serial subtraction, learning, oral language, reading, writing, and construction) and it changes at a rate of approximately 3 points per year in a typical AD patient. It is relatively insensitive to mild changes in well-educated individuals and is not useful in advanced dementia. It also lacks tests of executive function [27,204].
Clock Drawing Test (CDT)
The CDT is a simple test to assess cognitive or visuospatial impairment. The patient is asked to draw the face of a clock with all of the numbers and then draw the hands set to a certain time. It is best used in combination with other cognitive assessment instruments [27,204].
Mini-Cog
The Mini-Cog test is a three-item 3-minute instrument developed as a screen for cognitive impairment in older adults in the primary care setting. The Mini-Cog has an administration time about one-half of that for the MMSE and it is less affected by subject ethnicity, language, and education, and can detect a variety of different dementias [28–30].
Montreal Cognitive Assessment (MoCA)
The MoCA is a brief 30-question test that takes about 10 minutes to complete. It assesses cognitive abilities, including orientation, short-term memory, executive function, language abilities, and visuospatial ability. It includes a CDT and a test of executive function known as Trails B [31–33].
Informant Tests
AD8
The AD8 is a brief dementia screening interview validated against clinical and cognitive evaluations [34]. Assessment of a sample of 257 subjects indicated that individuals with AD8 scores positive for dementia had higher rates of abnormal PIB binding (P<0.001) and CSF AD biomarkers (P<0.001) versus those with AD8 scores not indicative of dementia [35].
Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)
The IQCODE was developed from a study of 39 interview questions assessing changes in memory and intelligence in older people [36]. A short 16-question version of the IQCODE has been developed and found to provide results that are highly correlated (r=0.98) with the full version [37].
Biomarkers in AD
Markers of Cerebral Metabolism
Cerebral metabolism associated with regionally specific default activity may predispose cortical regions to AD-related changes, including amyloid deposition, metabolic disruption, and atrophy. It has been suggested that these cortical regions may be part of a network with the medial temporal lobe whose disruption contributes to memory impairment [38]. This hypothesis is based in part on the observation that AD is associated with progressive, region specific, declines in the cerebral glucose metabolism [5]. Hypometabolism in patients with AD is most notable in the posterior cingulate, parietal, temporal, and prefrontal cortices [39, 40]; and it occurs early in AD [41].
Cerebrospinal Fluid Markers
Development of CSF markers has focused largely on amyloid β and tau protein. Accumulation of amyloid β peptides in amyloid plaques is a hallmark of AD [42]. CSF levels of amyloid β42 are reduced in AD, even in early and preclinical stages of the disease. A low CSF level of amyloid β42 is a well-validated marker of cortical amyloid deposition (as assessed by in vivo amyloid imaging with PIB) [42]. Levels of CSF total tau and phosphorylated tau increase in AD and accelerate during later disease stages, concomitant with neurofibrillary tangle formation and synapse and neuron loss [43].
Current Guidance for Biomarkers in AD
Guidance on biomarkers from the National Institute on Aging-Alzheimer’s Association states that although both amyloid β deposition and elevated tau/phosphorylated tau are hallmarks of AD, alterations in these proteins are also seen in other neurological disorders [44]. This group has divided biomarkers into two major categories: 1) amyloid β accumulation (abnormal tracer retention on amyloid PET imaging and low CSF amyloid β42); and (2) biomarkers associated with neuronal degeneration or injury, which include elevated CSF tau (both total and phosphorylated tau); decreased FDG uptake on PET in a specific topographic pattern involving the temporoparietal cortex; and atrophy on structural magnetic resonance imaging (MRI) in a specific topographic pattern involving medial, basal, and lateral temporal lobes and medial and lateral parietal cortices [44].
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) is an ongoing, longitudinal, multicenter study designed to develop clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of AD [45]. This group has carried out multiple projects aimed at achieving its overall goal. A paper published by Trojanowski et al has synthesized results from this initiative related to the timing of appearance of different biomarkers associated with AD. This analysis indicated that amyloid β biomarkers become abnormal first, followed by changes in neurodegenerative biomarkers including CSF tau, FDG-PET, and MRI [46]. Another recent publication from this group demonstrated that a combination of FDG-PET and MRI significantly improved detection of AD and differentiation from frontotemporal lobar degeneration [47]. The ADNI has also identified a set of MRI features in the medial temporal lobe that are effective for the identification of prodromal AD [48].
Criteria for Differential Diagnosis of AD
The National Institute on Aging and the Alzheimer’s Association have supported a revision of the 1984 diagnostic criteria for AD dementia with the aim of providing flexible criteria useful for general healthcare providers without access to neuropsychological testing, advanced imaging, and CSF measures; and specialists involved in research and/or clinical trials with access to such tools [49]. The National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease have also provided new recommendations for the diagnosis MCI. The core criteria for a diagnosis of this condition include concern regarding a change in cognition, impairment in one or more cognitive domains, preservation of independence and functional abilities, and no evidence of dementia [50]. This group has also defined a staging framework for preclinical AD based on biomarker analysis carried out under the aegis of the ADNI. These three stages are: Stage 1) asymptomatic cerebral amyloidosis characterized by PET or CSF positive for amyloid β; Stage 2) asymptomatic amyloidosis plus “downstream” neurodegeneration (indicated by tau, FDG-PET, or structural MRI); and Stage 3) amyloidosis plus neuronal injury and subtle cognitive/behavioral decline [51]
Treatment of AD
Non-pharmacologic Interventions
Olazaran and colleagues have recently systematically reviewed the efficacy of non-pharmacologic interventions for AD. This review included 179 randomized controlled trials and 29 different types of interventions [52]. Specific interventions that demonstrated efficacy in improving cognitive function in at least one study included cognitive training, behavioral interventions, physical exercise, music, reminiscence, and recreation therapy. Those demonstrating efficacy for improving ADLs included cognitive training, behavioral interventions, reminiscence, ADL training, multisensory stimulation, and muscle relaxation. Behavioral improvements were observed with interventions that included cognitive training, behavioral interventions, music, reminiscence, massage and touch, recreation therapy, use of light, multisensory stimulation, and support and psychotherapy [52].
Consideration of the results from this analysis prompted the conclusion that non-pharmacologic interventions for patients with AD have multiple advantages, including a general absence of side effects and the potential for individualization based on patient symptoms. It was also suggested that these interventions may provide greater benefit in improving quality of life and patient well-being than medications. However, it was also emphasized that non-pharmacologic interventions should be considered as a complement to rather than a substitute for pharmacotherapy [52].
Pharmacotherapy
All current treatments for AD are symptomatic. They do not provide detectable and sustained improvements in cognitive function above baseline and their benefits are not maintained if treatment is terminated. Despite these limitations, the symptomatic benefit achieved with these treatments is clinically important and they are widely used in AD patients [53]. Two classes of pharmacologic agents approved for treatment of AD are cholinesterase inhibitors (ChEIs) and an N-methyl-D-aspartate (NMDA) receptor antagonist [54–56].
Long-term Benefits of Current Treatments
While results from numerous clinical trials have demonstrated short-term benefits with the above-listed agents [53], there is less information about the long-term effects of these interventions. This information is particularly important in designing treatment regimens since most AD patients will require therapy for a number of years.
Results from a study of 794 outpatients treated with a ChEI and followed for a mean of 36.9 months indicated that after 3 months’ treatment, MMSE scores remained stable (responders) in 60% and improved (increase of 3 or more points - good responders) in 15%. After 15 months, the percentage of “good responders” decreased to 7%, while after 15, 27, and 39 months the percentage of responders progressively declined to 40%, 30% and 8%, respectively. Onset of behavioral disturbances was associated with significant worsening of both cognition and function [57].
Long-term ChEI treatment has also been evaluated in a placebo-controlled study of donepezil in 565 outpatients with mild-to-moderate AD who were followed for 3 years and 36 weeks [58]. Study results indicated no significant differences in the rates of institutionalism between donepezil and placebo at 1 year (9% versus 14%; P=0.15) or at 3 years (42% versus 44%; P=0.4). After 12 weeks and until the end of the trial, the Bristol ADL scores of donepezil-treated patients were statistically significantly better than those for placebo (average +1.0 point; P=0.0004). Similarly, MMSE scores were modestly, but statistically significantly higher, in donepezil- versus placebo-treated patients (average 0.8 points; P=0.001). According to the authors, the clinical significance of these findings is questionable [58]. Results from a smaller-scale study of 40 AD patients treated with donepezil for ≥3 years indicated significant decreases in MMSE scores over the study period (P<0.01) [59].
The Swedish Alzheimer Treatment Study followed 435 patients with AD who were treated with donepezil and followed for 3 years [60]. Patients were assessed with MMSE, ADAS-cog, global rating (Clinician’s Interview-Based Impression of Change [CIBIC]) and Instrumental ADLs at baseline and every 6 months. After 3 years, the mean change from baseline in MMSE-score was 3.8 points and the ADAS-cog rise was 8.2 points. These results were described as being better than expected in untreated historical cohorts. However, after 3 years, only 38% of the initial cohort remained in the study and only 30% of them were unchanged or improved in the CIBIC global assessment [60].
A 132-week, open-label extension study assessed the long-term efficacy of donepezil in 579 patients with mild-to-moderate AD who had previously participated in a 24-week double-blind placebo-controlled trial [61]. After 6 weeks of open-label treatment with donepezil 5 mg/day, mean ADAS-cog improved by approximately 2 points, while after 12 weeks of open-label treatment (with a majority of patients receiving 10 mg/day), the mean ADAS-cog score was 1 point better than that at the end of the placebo washout period. Scores then declined gradually over the remainder of the study. Mean changes in Clinical Dementia Rating scale Sum of Boxes (CDR-SB) scores showed slight improvement over the first 12 weeks of open-label treatment and then also declined [61].
Results from 133 patients who completed a total of 254 weeks of treatment with donepezil indicated that during the first 6–9 months of the study, mean ADAS-cog and CDR-SB scores improved from baseline. After this time, scores gradually deteriorated. Overall, the decline was less than that estimated for untreated patients [62]. Similarly, results from an open-label extension of 2 United States phase III, double-blind, placebo-controlled clinical trials of donepezil in 763 AD patients indicated that this treatment was effective for treatment of mild to moderately severe AD for up to 144 weeks [63].
Four-year follow-up of 88 patients treated with donepezil indicated that 64.7% remained on treatment beyond 6 months, 57.9% beyond 1 year and 12.5% beyond 4 years. It was also noted that 56% remained alive at 4 years (almost twice the number predicted); and that mean MMSE scores among patients remaining on treatment did not deteriorate over 4 years [64].
There is very little information about the long-term efficacy of memantine in patients with AD. Results from a 26-week study of memantine versus in 181 patients with moderate-to-severe AD indicated that those who received memantine had significantly better scores on the ADL and cognitive endpoints compared to placebo. Borderline significance was found for global functioning (CIBIC-plus score). This trial was followed by a 24-week open-label extension in which all patients received memantine. Patients who switched to memantine treatment from their previous placebo therapy experienced a significant benefit in all main efficacy assessments (functional, global, and cognitive) relative to their mean rate of decline with placebo. For the patients who were randomized to memantine treatment during the double-blind phase, the clinically relevant benefits observed during the first part of the trial also appeared to be maintained during the extension [65]. It is important to note that memantine is not effective in patients with mild AD. A recent meta-analysis of results from clinical trials has indicated that there is no evidence to support the use of memantine in mild AD (MMSE score 20–23) and only limited evidence to support its use in patients with moderate AD (MMSE score 10–19) [66]. These results are consistent with the fact that memantine is approved only for the treatment of patients with moderate-to-severe AD [67].
Results from a long-term study of patients treated with a ChEI plus memantine have suggested greater benefit than ChEI monotherapy. This study compared the combination of a ChEI with memantine versus ChEI alone and no treatment in 382 subjects with probable AD and showed that combination treatment resulted in significantly lower mean annualized rates of deterioration in the Blessed Dementia Scale and ADL scores versus ChEI alone or no treatment (both P<0.001) [68]. The benefit seen with combination treatment was sustained for several years.
Results from a 1-year observational study of 140 AD patients showed further that addition of memantine to treatment with a ChEI significantly extended time to nursing home admission [69]. It is important to note that there are theoretical concerns about the long-term use of memantine in patients with AD as well as employment of the drug in combination with donepezil. Results from studies in rats carried out by Creeley et al have indicated memantine treatment may result in neurotoxicity (an intra-cytoplasmic vacuole reaction) and that this effect is exacerbated when it is given along with donepezil [70]. Administration of 10–20 mg/day memantine to mice for 6 months has also been shown to result in a significant increase in degenerating axons [71].
A major unmet need in AD is new potentially disease-modifying therapies. There have been no new FDA-approved treatments for AD since 2003 despite an intensive research effort. This has prompted increased attention to other alternatives that may be added on to existing pharmacologic agents in patients with AD.
Medical Foods
The United States Food and Drug Administration has defined a medical food as a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation. Medical foods are distinguished from the broader category of foods for special dietary use and from foods that make health claims by the requirement that medical foods be intended to meet distinctive nutritional requirements of a disease or condition, used under medical supervision and intended for the specific dietary management of a disease or condition [205].
Ketone Bodies
As noted above, the brains of patients with AD display early, progressive, region specific, declines in the cerebral metabolic rate of glucose [39, 41]. Normally, the main energy substrate for the brain is glucose. However, in certain situations, such as extended fasting, the liver produces ketone bodies that serve as an alternative energy source for extrahepatic tissues, including the brain [72–74]. Ketone bodies are an efficient cellular fuel [75–76]; and results from preclinical studies have suggested that induced ketosis may be beneficial in AD [77, 78]. A small study that included 23 older adults with MCI who received either a high carbohydrate or ketogenic diet for 6 weeks indicated improved verbal memory performance for the latter group (P=0.01). Results from this study also indicated that ketone levels were positively correlated with memory performance (P= 0.04) [79]. While this intervention has promise, it may be difficult to maintain patients on ketogenic diets for extended periods.
Use of a medical food provides an alternative to a low-carbohydrate diet for raising levels of ketone bodies. Administration of medium chain triglycerides (MCTs) that increase blood levels of ketone bodies has been shown to be beneficial for the treatment of patients with mild-to-moderate AD [80]. The efficacy and safety of MCTs for the treatment of mild-to-moderate AD have been assessed in a multicenter, randomized, double-blind, placebo-controlled study of 152 outpatients with MMSE scores between 14 and 24 at screening [80]. Subjects were randomized to 90 days of treatment with either MCTs or placebo followed by a 2-week washout. All subjects were allowed to continue on stable concomitant AD treatments (80% were taking a ChEI and/or memantine) [80]. Treatment-associated changes from baseline in ADAS-Cog scores were analyzed in subgroups of patients based on APOE ε4 genotype. Per protocol and dosage-compliant patients lacking the APOE ε4 allele and administered MCTs demonstrated a significant difference from placebo in ADAS-Cog at both days 45 and 90 (both P<0.05) [80]. While this study was well controlled, it is still limited by a relatively short duration; and it is not clear whether long-term administration of MCTs will have sustained clinical benefit in patients with AD.
Cerefolin NAC
This medical food contains folate, vitamin B6, alpha-tocopherol, S-adenosyl methionine, N-acetyl cysteine, and acetyl-L-carnitine. Meta-analysis of results reported in a small number of studies carried out to date has provided no consistent evidence that folic acid, with or without vitamin B-12, positively affects cognitive function in healthy or cognitively impaired elderly individuals [81]. It has also been shown that high-dose vitamin B-6, vitamin B-12, and folate supplementation do not significantly slow cognitive decline in patients with AD [82]
Souvenaid
Souvenaid includes omega-3 fatty acids, uridine, and choline. Results from a 12-week, randomized, double-blind controlled trial that included 225 patients with AD indicated significant improvement in a delayed verbal recall task for patients treated with this preparation versus controls (P=0.021). However, there were no between-group differences for Modified ADAS-Cog and other outcome scores, including Clinician Interview Based Impression of Change plus Caregiver Input, 12-item Neuropsychiatric Inventory, Alzheimer’s disease Cooperative Study-ADL, and Quality of Life in Alzheimer’s Disease [83]. As for the other medical foods considered in this section, available results provide no information about the long-term benefit of souvenaid.
Dietary Supplements
Coconut Oil
Coconut oil is proposed as another source of ketone bodies that has been touted as an effective treatment for AD in popular print and electronic media. However, to date, there have been no controlled studies of coconut oil in patients with AD.
Omega-3 Fatty Acids
Docosahexaenoic acid (DHA) is the most abundant long-chain polyunsaturated fatty acid in the brain and results from epidemiological studies suggest that consumption of DHA may be associated with a reduced incidence of AD. However, a randomized, double-blind, placebo-controlled trial of DHA supplementation (2 g/day) versus placebo in 402 patients with mild-to-moderate AD did not support this relationship [84].
INM-176
INM-176, a dietary supplement, is a plant extract from Angelica gigas nakai that inhibits amyloid β production [206]. The effects of INM 176 versus placebo on cognitive function have been evaluated in 92 elderly Korean patients with cognitive impairment (Korean-MMSE score <25) in a 12-week trial. Study results showed that INM-176 was significantly superior to placebo for total error scores on the ADAS-Cog [85]. The short-term study provides no information about the long-term benefit of INM 176 in the treatment of AD.
Emerging Therapies for AD
A large number of agents with many different mechanisms of action are in various stages of development for the treatment of AD. Agents in phase III development for the treatment of AD (Table 1) include thalidomide, intravenous immune globulins (IVIG), solanezumab, bapineuzumab, and SK-PC-B70M [206]. Thalidomide is an angiogenesis inhibitor and its potential utility in AD is based on observations of increased expression of pro-angiogenic growth factors, such as vascular endothelial growth factor, in regions with senile plaques [86]. Treatment with IVIG, solanezumab, or bapineuzumab in patients with AD is aimed at increasing clearance of amyloid β [87]. SK-PC-B70M is an oleanolic-glycoside saponin-enriched fraction derived from the root of Pulsatilla koreana with neuroprotective effects against amyloid β [206].
Table 1.
| Agent | Mechanism of Action |
|---|---|
| Thalidomide | Tumor necrosis factor and angiogenesis inhibitor |
| Passive Immunotherapy | |
| IVIG | Decreases amyloid β |
| Solanezumab | Humanized anti-amyloid β monoclonal antibody |
| Bapineuzumab | Humanized anti-amyloid β monoclonal antibody |
| PF-04360365 | Anti-amyloid β monoclonal antibody |
| R1450 | Fully human anti-amyloid β monoclonal antibody |
| GSK933766A | Anti-amyloid β monoclonal antibody |
| Active Immunotherapy | |
| ACC-001 | Amyloid β amino-terminal conjugate |
| CAD-105 | Amyloid β1–5 coupled to Qb virus-like particles |
| Affitope | Amyloid β amino-terminal mimotope ± adjuvant |
| V950 | Amyloid β amino-terminal peptides conjugated to ISCO-MATRIX® |
| UB311 | Amyloid β1–14 using UBITh® |
| γ-secretase inhibitors | |
| LY2811376 | |
| BACE-1 inhibitors | |
| CTS-21166 | |
| GSK188909 | |
| Other | |
| SK-PC-B70M | Oleanolic-glycoside saponin-enriched fraction derived from the root of Pulsatilla koreana with neuroprotective effect against amyloid β |
Bapineuzumab, solanezumab, and IVIG are passive immunotherapeutic approaches to the treatment of AD. A phase II trial of bapineuzumab that included 234 AD patients who received this antibody or placebo for 78 weeks indicated no significant differences between treatments for effects on ADAS-Cog. However, an exploratory analysis showed potential treatment differences (P<0.05, unadjusted for multiple comparisons) on this measure for patients who completed the study and were APOE ε4 noncarriers [88]. A phase II study of solanezumab in 52 patients with mild-to-moderate AD and 16 healthy volunteers indicated that the antibody increased amyloid β in blood, reflecting increased clearance from the brain [89]. An open-label dose-ranging study of IVIG in 8 mild AD patients indicated that plasma amyloid β concentrations increased transiently after each infusion and that CSF levels of this protein decreased significantly at 6 months. MMSE scores increased by an average of 2.5 points after 6 months [90]. No clinical results have been published for SK-PC-B70M or thalidomide.
Vaccination strategies (active immunotherapy) are also in development for the treatment of AD. Amyloid β1–42 peptide immunotherapy resulted in significant toxicity and autoimmune responses and newer approaches are aimed at identifying new classes of safe amyloid β fragments [91]. ACC-001 is a short amino-terminal amyloid β1–6 fragment that is derived from the N-terminal B cell epitope of amyloid β. It is currently being evaluated in a phase II study [207] Other vaccines based on amyloid β fragments are currently in development (Table 1).
γ-secretase is the pivotal enzyme involved in the generation of amyloid β and a number of different γ-secretase inhibitors have been developed and evaluated as treatment for the treatment of AD. Agents evaluated in phase III trials to date have been limited by toxicity or poor efficacy. Nevertheless, new more potent compounds with enhanced brain penetration and which have been modified to decrease toxicity are currently in development [92] β-site amyloid precursor protein-cleaving enzyme 1 (BACE-1) catalyzes the rate-limiting step in the production of amyloid β and inhibition of this enzyme is a major target for drug development in AD [93]. Some orally effective BACE-1 inhibitors have been developed and at least one (CTS-21166) has entered a human clinical trial [94].
Conclusions and Future Perspective
AD is a progressive disease with patient, caregiver, and societal burdens that all rise as disease severity increases. Current treatments for AD are symptomatic. They slow disease progression versus no treatment, but this benefit appears to wane over time. There is a significant requirement for new treatments that can be used in conjunction with current pharmacotherapies to further slow or even arrest/reverse the progressive cognitive and behavioral declines characteristic of AD.
In the absence of agents with proven efficacy for modifying disease in patients with AD, it is reasonable to suggest that combining symptomatic interventions with complementary mechanisms of action may be the best approach to managing individuals with this disease. Combining mechanisms may afford optimal approaches to address cognitive, functional and behavioral deficits without necessarily increasing adverse effects. This approach is similar to addressing symptomatic treatments for other chronic diseases including hypertension, diabetes, and heart disease. The available literature supports that a combination of validated non-pharmacologic interventions, approved medications, and a medical food containing MCTs may be useful in patients with mild-to-moderate AD. Results from Atri and colleagues have supported the efficacy of combination therapy with memantine and a ChEI in patients with AD [68]; and it has also been shown that combination of non-pharmacologic intervention with a ChEI was superior to drug alone [95]. Addition of MCTs to treatment in patients already receiving pharmacotherapy has also been shown to improve cognitive function [80]. Combination of all of these elements with complementary mechanisms of action (Figure 1) is a strategy that may be considered for the treatment of patients with mild-to-moderate AD.
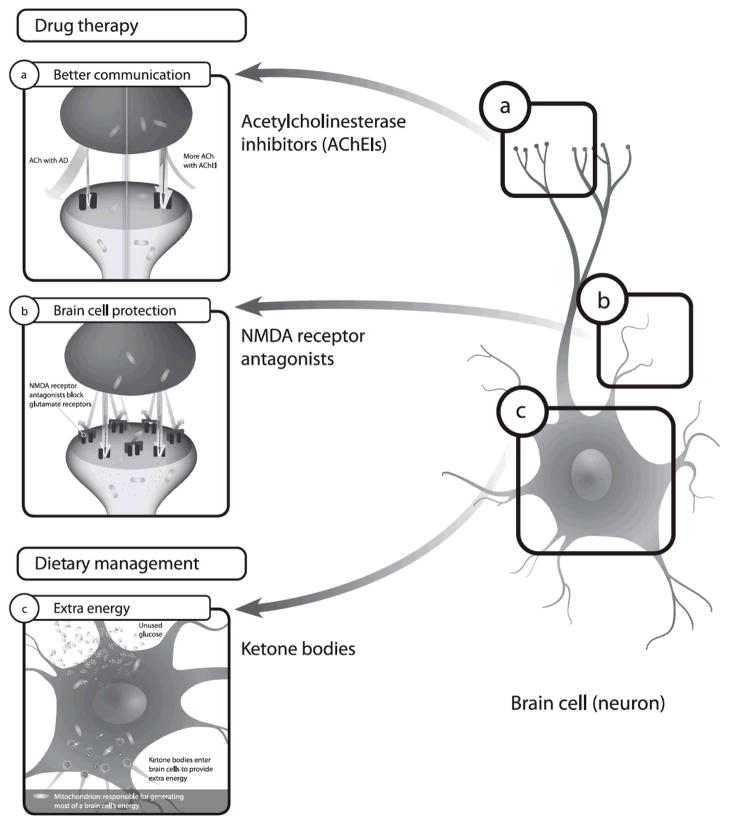
Figure 1. Complementary mechanisms of action for ChEI, memantine, and MCTs in the treatment of AD [54,67,80,98,99].
AChE inhibitors are believed to correct the cholinergic deficit seen in AD in which there is a loss of ACh producing neurons in the brain. These agents increase ACh concentrations by blocking the action of AChE which catalyzes the hydrolysis of acetylcholine into choline and acetic acid. Memantine is a low-to-moderate affinity noncompetitive N-methyl-D-aspartic acid (NMDA) receptor antagonist. It inhibits the prolonged influx of Ca2+ ions that forms the basis of neuronal excitotoxicity that may be involved in AD. It should be noted that memantine is approved only for the treatment of moderate-to-severe AD. Ketone bodies are an efficient fuel for cells and may provide an alternative additional energy source for neurons in patients with AD.
Practice Points.
Current treatments for AD are symptomatic and they do not change disease progression.
There is no cure for AD, but several different types of non-pharmacologic interventions have been shown to have significant benefit.
Pharmacotherapy with a cholinesterase inhibitor or NMDA receptor antagonists (in moderate-to-severe disease) has also been shown to be effective for the treatment of AD.
Therapy aimed at improving cerebral metabolism has significant benefit in patients with mild-to-moderate AD.
A reasonable approach to treatment for patients with mild-to-moderate AD may be combination of appropriate non-pharmacologic interventions, pharmacotherapy, and a medical food.
Combination of interventions with complementary mechanisms of action may result in maximum preservation of cognitive function in patients with AD.
Acknowledgments
none
Footnotes
Disclosure of Commercial and Non-commercial Interests: none
References
- 1.Alzheimer’s Association. 2010 Alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6(2):158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Schindowski K, Belarbi K, Buée L. Neurotrophic factors in Alzheimer’s disease: role of axonal transport. Genes Brain Behav. 2008;7(Suppl 1):43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet. 2010;19(R1):R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golde TE, Petrucelli L, Lewis J. Targeting Abeta and tau in Alzheimer’s disease, an early interim report. Exp Neurol. 2010;223(2):252–266. doi: 10.1016/j.expneurol.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.de Leon MJ, Ferris SH, George AE, et al. Positron emission tomographic studies of aging and Alzheimer disease. AJNR Am J Neuroradiol. 1983;4(3):568–571. First FDG-PET paper to document decreased cerebral glucose metabolism in AD. [PMC free article] [PubMed] [Google Scholar]
- 6.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunnane SC, Schneider JA, Tangney C, et al. Plasma and Brain Fatty Acid Profiles in Mild Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis. 2012 doi: 10.3233/JAD-2012-110629. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smach MA, Jacob N, Golmard JL, et al. Folate and homocysteine in the cerebrospinal fluid of patients with Alzheimer’s disease or dementia: a case control study. Eur Neurol. 2011;65(5):270–278. doi: 10.1159/000326301. [DOI] [PubMed] [Google Scholar]
- 9.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., 3rd High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82(3):627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 10.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82(3):636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 11.Bergvall N, Brinck P, Eek D, et al. Relative importance of patient disease indicators on informal care and caregiver burden in Alzheimer’s disease. Int Psychogeriatr. 2011;23(1):73–85. doi: 10.1017/S1041610210000785. [DOI] [PubMed] [Google Scholar]
- 12.Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr. 2008;20(1):1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 13.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66(10):1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauthier S, Cummings J, Ballard C, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010;22(3):346–372. doi: 10.1017/S1041610209991505. [DOI] [PubMed] [Google Scholar]
- 16.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 17.Nadkarni NK, Levy-Cooperman N, Black SE. Functional correlates of instrumental activities of daily living in mild Alzheimer’s disease. Neurobiol Aging. 2012;33(1):53–60. doi: 10.1016/j.neurobiolaging.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechowski L, de Stampa M, Denis B, et al. Patterns of loss of abilities in instrumental activities of daily living in Alzheimer’s disease: the REAL cohort study. Dement Geriatr Cogn Disord. 2008;25(1):46–53. doi: 10.1159/000111150. [DOI] [PubMed] [Google Scholar]
- 19.Hatoum HT, Thomas SK, Lin SJ, Lane R, Bullock R. Predicting time to nursing home placement based on activities of daily living scores--a modelling analysis using data on Alzheimer’s disease patients receiving rivastigmine or donepezil. J Med Econ. 2009;12(2):98–103. doi: 10.3111/13696990903004039. [DOI] [PubMed] [Google Scholar]
- 20.Williams JW, Plassman BL, Burke J, Holsinger T, Benjamin S. Agency for Healthcare Research and Quality. U.S. Department of Health and Human Services; Apr, 2010. [Accessed April 25, 2011]. Preventing Alzheimer’s disease and cognitive decline. AHRQ Publication No. 10-E005. [Google Scholar]
- 21.Palmer K, Lupo F, Perri R, et al. Predicting disease progression in Alzheimer’s disease: The role of neuropsychiatric syndromes on functional and cognitive decline. J Alzheimers Dis. 2010;24(1):35–45. doi: 10.3233/JAD-2010-101836. [DOI] [PubMed] [Google Scholar]
- 22.Musicco M, Palmer K, Salamone G, et al. Predictors of progression of cognitive decline in Alzheimer’s disease: the role of vascular and sociodemographic factors. J Neurol. 2009;256(8):1288–1295. doi: 10.1007/s00415-009-5116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris MC. The role of nutrition in Alzheimer’s disease: epidemiological evidence. Eur J Neurol. 2009;16(Suppl 1):1–7. doi: 10.1111/j.1468-1331.2009.02735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roe CM, Mintun MA, Ghoshal N, et al. Alzheimer disease identification using amyloid imaging and reserve variables: proof of concept. Neurology. 2010;75(1):42–48. doi: 10.1212/WNL.0b013e3181e620f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matioli MN, Caramelli P. Limitations in differentiating vascular dementia from Alzheimer’s disease with brief cognitive tests. Arq Neuropsiquiatr. 2010;68(2):185–188. doi: 10.1590/s0004-282x2010000200006. [DOI] [PubMed] [Google Scholar]
- 27.Cummings JL. Behavioral and neuropsychiatric outcomes in Alzheimer’s disease. CNS Spectr. 2005;10(11 Suppl 18):22–25. doi: 10.1017/s1092852900014206. [DOI] [PubMed] [Google Scholar]
- 28.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. J Am Geriatr Soc. 2005;53(5):871–874. doi: 10.1111/j.1532-5415.2005.53269.x. [DOI] [PubMed] [Google Scholar]
- 30.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Improving identification of cognitive impairment in primary care. Int J Geriatr Psychiatry. 2006;21(4):349–355. doi: 10.1002/gps.1470. [DOI] [PubMed] [Google Scholar]
- 31.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007;52(5):329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 33.Zadikoff C, Fox SH, Tang Wai DF, et al. A comparison of the Mini Mental State Exam to the Montreal Cognitive Assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23(2):297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 34.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 35.Galvin JE, Fagan AM, Holtzman DM, Mintun MA, Morris JC. Relationship of dementia screening tests with biomarkers of Alzheimer’s disease. Brain. 2010;133(11):3290–3300. doi: 10.1093/brain/awq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorm AF, Korten AE. Assessment of cognitive decline in the elderly by informant interview. Br J Psychiatry. 1988;152(2):209–213. doi: 10.1192/bjp.152.2.209. [DOI] [PubMed] [Google Scholar]
- 37.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychological Medicine. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 38.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosconi L, Brys M, Glodzik-Sobanska L, De Santi S, Rusinek H, de Leon MJ. Early detection of Alzheimer’s disease using neuroimaging. Exp Gerontol. 2007;42(1–2):129–138. doi: 10.1016/j.exger.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Mistur R, Mosconi L, Santi SD, et al. Current challenges for the early detection of alzheimer’s disease: brain imaging and CSF Studies. J Clin Neurol. 2009;5(4):153–166. doi: 10.3988/jcn.2009.5.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wray S, Noble W. Linking amyloid and tau pathology in Alzheimer’s disease: the role of membrane cholesterol in Abeta-mediated tau toxicity. J Neurosci. 2009;29(31):9665–9667. doi: 10.1523/JNEUROSCI.2234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metcalfe MJ, Figueiredo-Pereira ME. Relationship between tau pathology and neuroinflammation in Alzheimer’s disease. Mt Sinai J Med. 2010;77(1):50–58. doi: 10.1002/msj.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trojanowski JQ, Vandeerstichele H, Korecka M, et al. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6(3):230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dukart J, Mueller K, Horstmann A, et al. Combined evaluation of FDG-PET and MRI improves detection and differentiation of dementia. PloS One. 2011;6(3):e18111. doi: 10.1371/journal.pone.0018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chincarini A, Bosco P, Calvini P, et al. Local MRI analysis approach in the diagnosis of early and prodromal Alzheimer’s disease. Neuroimage. 2011;58(2):469–480. doi: 10.1016/j.neuroimage.2011.05.083. [DOI] [PubMed] [Google Scholar]
- 49*.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. Most recent diagnostic guidelines for AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. Most recent diagnostic guidelines for MCI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. New proposed criteria for researchers to investigate preclinical AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olazarán J, Reisberg B, Clare L, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161–178. doi: 10.1159/000316119. [DOI] [PubMed] [Google Scholar]
- 53.Shah S, Reichman WE. Treatment of Alzheimer’s disease across the spectrum of severity. Clin Interv Aging. 2006;1(2):131–142. doi: 10.2147/ciia.2006.1.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gauthier S, Scheltens P. Can we do better in developing new drugs for Alzheimer’s disease? Alzheimers Dement. 2009;5(6):489–491. doi: 10.1016/j.jalz.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Ihl R. Anti-dementia drugs for dementia syndromes-just unkept promises? MMW Fortschr Med. 2006;2:24–26. [PubMed] [Google Scholar]
- 56.Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 57.Sinforiani E, Zucchella C, Pasotti C, Bartolo M, Nappi G. Report of ten years’ activity in an Alzheimer’s disease assessment unit. Aging Clin Exp Res. 2009;21(4–5):365–368. doi: 10.1007/BF03324930. [DOI] [PubMed] [Google Scholar]
- 58.Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet. 2004;363(9427):2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 59.Kanaya K, Abe S, Sakai M, Fujii H, Iwamoto T. Changes in cognitive functions of patients with dementia of the Alzheimer type following long-term administration of donepezil hydrochloride: relating to changes attributable to differences in apolipoprotein E phenotype. Geriatr Gerontol Int. 2010;10(1):25–31. doi: 10.1111/j.1447-0594.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- 60.Wallin AK, Andreasen N, Eriksson S, et al. Donepezil in Alzheimer’s disease: what to expect after 3 years of treatment in a routine clinical setting. Dement Geriatr Cogn Disord. 2007;23(3):150–160. doi: 10.1159/000098052. [DOI] [PubMed] [Google Scholar]
- 61.Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open-label, multicentre study in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(8):806–812. doi: 10.1002/gps.1746. [DOI] [PubMed] [Google Scholar]
- 62.Rogers SL, Doody RS, Pratt RD, Ieni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: final analysis of a US multicentre open-label study. Eur Neuropsychopharmacol. 2000;10(3):195–203. doi: 10.1016/s0924-977x(00)00067-5. [DOI] [PubMed] [Google Scholar]
- 63.Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD Donepezil Study Group. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58(3):427–433. doi: 10.1001/archneur.58.3.427. [DOI] [PubMed] [Google Scholar]
- 64.Lyle S, Grizzell M, Willmott S, Benbow S, Clark M, Jolley D. Treatment of a whole population sample of Alzheimer’s disease with donepezil over a 4-year period: lessons learned. Dement Geriatr Cogn Disord. 2008;25(3):226–231. doi: 10.1159/000114450. [DOI] [PubMed] [Google Scholar]
- 65.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63(1):49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 66.Schneider LS, Dagerman KS, Higgins JP, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 68(8):991–998. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- 67.Namenda. Prescribing Information. 2011. [Google Scholar]
- 68**.Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209–221. doi: 10.1097/WAD.0b013e31816653bc. Paper demonstrating the long-term benefit of combination pharmacotherapy in patients with AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez OL, Becker JT, Wahed AS, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80(6):600–607. doi: 10.1136/jnnp.2008.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Creeley CE, Wozniak DF, Nardi A, Farber NB, Olney JW. Donepezil markedly potentiates memantine neurotoxicity in the adult rat brain. Neurobiol Aging. 2008;29(2):153–167. doi: 10.1016/j.neurobiolaging.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong H, Yuede CM, Coughlan C, Lewis B, Csernansky JG. Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2008;33(13):3226–3236. doi: 10.1038/npp.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr Metab (Lond) 2005;2:30. doi: 10.1186/1743-7075-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sann L, Divry P, Lasne Y, Ruitton A. Effect of oral lipid administration on glucose homeostasis in small-for-gestational-age infants. Acta Paediatr Scand. 1982;71(6):923–927. doi: 10.1111/j.1651-2227.1982.tb09550.x. [DOI] [PubMed] [Google Scholar]
- 74.Décombaz J, Arnaud MJ, Milon H, et al. Energy metabolism of medium-chain triglycerides versus carbohydrates during exercise. Eur J Appl Physiol Occup Physiol. 1983;52(1):9–14. doi: 10.1007/BF00429018. [DOI] [PubMed] [Google Scholar]
- 75.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Effect of hyperketonemia and hyperlacticacidemia on symptoms, cognitive dysfunction, and counterregulatory hormone responses during hypoglycemia in normal humans. Diabetes. 1994;43(11):1311–1317. doi: 10.2337/diab.43.11.1311. [DOI] [PubMed] [Google Scholar]
- 76.Hasselbalch SG, Madsen PL, Hageman LP, et al. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol. 1996;270(5 Pt 1):E746–E751. doi: 10.1152/ajpendo.1996.270.5.E746. [DOI] [PubMed] [Google Scholar]
- 77.Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-betahydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci USA. 2000;97(10):5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab (Lond) 2005;2:28. doi: 10.1186/1743-7075-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33(2):425.e19–e27. doi: 10.1016/j.neurobiolaging.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80**.Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. Controlled clinical trial demonstrating the efficacy of MCTs in mild-to-moderate AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. (4):CD004514. doi: 10.1002/14651858.CD004514.pub2. [DOI] [PubMed] [Google Scholar]
- 82.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scheltens P, Kamphuis PJ, Verhey FR, et al. Efficacy of a medical food in mild Alzheimer’s disease: a randomized, controlled trial. Alzheimers Dement. 2010;6(1):1–10.e1. doi: 10.1016/j.jalz.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304(17):1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim JH, Koh SK, Koh HJ, et al. A Three Month Placebo-Controlled Clinical Trial of INM 176 in the Old Aged Subjects with Memory Impairment. J Korean Neuropsychiatr Assoc. 2003;42:254–262. [Google Scholar]
- 86.De Filippis D, Cipriano M, Esposito G, Scuderi C, Steardo L, Iuvone T. Are anti-angiogenic drugs useful in neurodegenerative disorders? CNS Neurol Disord Drug Targets. 2010;9(6):807–812. doi: 10.2174/187152710793237485. [DOI] [PubMed] [Google Scholar]
- 87.Panza F, Frisardi V, Imbimbo BP, Seripa D, Solfrizzi V, Pilotto A. Monoclonal antibodies against β-amyloid (Aβ) for the treatment of Alzheimer’s disease: the Aβ target at a crossroads. Expert Opin Biol Ther. 2011;11(6):679–686. doi: 10.1517/14712598.2011.579099. [DOI] [PubMed] [Google Scholar]
- 88.Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siemers ER, Friedrich S, Dean RA, et al. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol. 2010;33(2):67–73. doi: 10.1097/WNF.0b013e3181cb577a. [DOI] [PubMed] [Google Scholar]
- 90.Relkin NR, Szabo P, Adamiak B, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30(11):1728–1736. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 91.Verdoliva A, Rivieccio V, Rossi M. Simplified β-amyloid peptides for safer Alzheimer’s vaccines development. Hum Vaccin. 2010;6(11):936–947. doi: 10.4161/hv.6.11.13034. [DOI] [PubMed] [Google Scholar]
- 92.Imbimbo BP, Giardina GA. γ-secretase inhibitors and modulators for the treatment of Alzheimer’s disease: disappointments and hopes. Curr Top Med Chem. 2011;11(12):1555–1570. doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- 93.Klaver DW, Wilce MC, Cui H, Hung AC, Gasperini R, Foa L, Small DH. Is BACE1 a suitable therapeutic target for the treatment of Alzheimer’s disease? Current strategies and future directions. Biol Chem. 2010;391(8):849–859. doi: 10.1515/BC.2010.089. [DOI] [PubMed] [Google Scholar]
- 94.Luo X, Yan R. Inhibition of BACE1 for therapeutic use in Alzheimer’s disease. Int J Clin Exp Pathol. 2010;3(6):618–28. [PMC free article] [PubMed] [Google Scholar]
- 95**.Chapman SB, Weiner MF, Rackley A, Hynan LS, Zientz J. Effects of cognitive-communication stimulation for Alzheimer’s disease patients treated with donepezil. J Speech Lang Hear Res. 2004;47(5):1149–1163. doi: 10.1044/1092-4388(2004/085). Clinical trial demonstrating the benefit of combining pharmacologic and nonpharmacologic treatments in patients with AD. [DOI] [PubMed] [Google Scholar]
- 96.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6(2):108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.May PC, Dean RA, Lowe SL, et al. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31(46):16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woodruff-Pak DS, Tobia MJ, Jiao X, Beck KD, Servatius RJ. Preclinical investigation of the functional effects of memantine and memantine combined with galantamine or donepezil. Neuropsychopharmacology. 2007;32(6):1284–1294. doi: 10.1038/sj.npp.1301259. [DOI] [PubMed] [Google Scholar]
- 99.Hogan DB. Progress update: Pharmacological treatment of Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3(5):569–578. [PMC free article] [PubMed] [Google Scholar]
Websites
- 201.Centers for Disease Control. [Accessed April 25, 2011];Alzheimer’s disease. 2011 Available at: http://www.cdc.gov/aging/aginginfo/alzheimers.htm.pdf.
- 202.Alzheimer’s Association. [Accessed April 25, 2011];Alzheimer’s Disease Facts and Figures. 2011 Available at: http://www.alz.org/downloads/Facts_Figures_2011.pdf.
- 203.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. National Vital Statistics Reports. [Accessed April 25, 2011];Deaths: Preliminary data for 2009. 2011 Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdf. [PubMed]
- 204.Alzheimers Research Forum. [Accessed April 25, 2011];2009 Available at: http://www.alzforum.org/dis/dia/tes/neuropsychological.asp.
- 205.FDA U.S. Food and Drug Administration. [Accessed 4 July, 2011];Guidance for industry: Frequently asked questions about medical foods. 2007 Available at: http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/MedicalFoods/ucm054048.htm.
- 206.ClinicalTrials.gov. [Accessed April 25, 2011];2011 Available at: http://clinicaltrials.gov/
- 207.ClinicalTrials.gov. [Accessed April 25, 2011];Safety, tolerability, and immunogenicity study of ACC-001 in Japanese subjects with mild to moderate Alzheimer’s Disease. 2011 Available at: http://clinicaltrials.gov/ct2/show/NCT00959192?term=NCT00959192&rank=1.