Abstract
Neuropeptide S (NPS) is a recently discovered neuropeptide that increases arousal and wakefulness while decreasing anxiety-like behavior. Here, we used a self-administration paradigm to demonstrate that intracerebroventricular infusion of NPS reinstates extinguished cocaine-seeking behavior in a dose-dependent manner in mice. The highest dose of NPS (0.45 nm) increased active lever pressing in the absence of cocaine to levels that were equivalent to those observed during self-administration. In addition, we examined the role of the corticotropin-releasing factor receptor 1 (CRF1) in this behavior as well as locomotor stimulation and anxiolysis. CRF1 knock-out mice did not respond to either the locomotor stimulant or cocaine reinstatement effects of NPS, but still responded to its anxiolytic effect. The CRF1 antagonist antalarmin also blocked the increase in active lever responding in the reinstatement model and the locomotor activating properties of NPS without affecting its anxiolytic actions. Our results suggest that NPS receptors may be an important target for drug abuse research and treatment and that CRF1 mediates the cocaine-seeking and locomotor stimulant effects of NPS, but not its effects on anxiety-like behavior.
Introduction
Neuropeptide S (NPS) is a recently discovered neuropeptide that has intriguing behavioral and neuroanatomical properties. Intracerebroventricular infusions of NPS (0.1–1 nm) increase locomotor activity and wakefulness, and decrease anxiety-like behavior within the first hour after injection, making this the only neuropeptide described thus far with both locomotor-activating and anxiety-reducing properties (Xu et al., 2004; Reinscheid and Xu, 2005a,b; Reinscheid et al., 2005; Leonard et al., 2008). Prepro-NPS mRNA is expressed discretely in a few brain areas, with strongest expression in the peri-locus ceruleus, principle sensory 5 nucleus, and the lateral parabrachial nucleus of the brainstem. NPS receptor mRNA is widely distributed and most abundant in the amygdala, thalamus, hypothalamus, and cortex.
Because of the effects of NPS on locomotion and anxiety-like behavior, it is particularly interesting that NPS is colocalized with corticotropin-releasing factor (CRF) in the lateral parabrachial nucleus (Xu et al., 2007). However, whereas both NPS and CRF can increase activity levels, NPS decreases and CRF increases anxiety-like behavior (Smith et al., 1998; Lowry and Moore, 2006). In addition, NPS receptors have been localized to several of the same regions of the amygdala and extended amygdala (Xu et al., 2007) implicated in drug abuse and addiction, and several studies have shown that the CRF system plays an important role in these phenomena (Rivier and Vale, 1987; Sarnyai et al., 1992; Koob and Le Moal, 1997; Bruijnzeel and Gold, 2005; Steckler and Dautzenberg, 2006; Specio et al., 2008). Shared localization and domains of behavioral action suggest that NPS may be important in behaviors relevant to stress and drug abuse and that it may interact in some way with the CRF system to achieve these effects.
Relapse to drug taking in abstinent addicts is a major problem in drug addiction. A rodent model of relapse that has been used quite extensively to study this phenomenon involves training animals to self-administer a drug, then removing the drug and extinguishing the self-administration behavior, and finally examining the effect of various manipulations on the reinstatement of responding still in the absence of drug (Shaham et al., 2003; Bossert et al., 2005). Stress, drug-related environmental cues, or renewed contact with the drug itself can induce reinstatement of extinguished drug-seeking behavior in experimental animals and relapse in humans (de Wit and Stewart, 1981; Jaffe et al., 1989; Carter and Tiffany, 1999; Sinha et al., 2000). CRF administration has been shown to reinstate cocaine seeking (Erb et al., 2006) and CRF1 antagonists block the reinstating effects of cocaine priming and stressor exposure (Shaham et al., 1998; Erb et al., 2001). Here, we examined the ability of NPS to reinstate cocaine-seeking behavior and investigated CRF1 receptor involvement in NPS effects on locomotor activity, anxiety-like behavior, and the reinstatement of cocaine-seeking behavior, using both genetic and pharmacologic approaches.
Materials and Methods
Animals.
Adult male C57BL/6J, CRF1 knock-out (KO) mice and their littermate wild-type (WT) mice (C57BL/6J × 129/SvJ background) (Smith et al., 1998) maintained by The Scripps Research Institute (TSRI) were used. Mice were housed individually after surgery under reversed 12 h light/dark cycle conditions. Water and food were available ad libitum and all experiments occurred during the animals' active phase. All aspects of this research were in accordance with the guidelines established by the United States Department of Agriculture and the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of TSRI.
Jugular catheter implantation surgery.
For intravenous self-administration testing, mice were placed under general anesthesia with an isoflurane vapor mixture and surgically implanted with chronic jugular catheters (for detailed explanation of the methods, see Roberts et al., 1997; Thomsen and Caine, 2007). Briefly, incisions were made in the midscapular region as well as anteromedial to the right forearm above the external right jugular vein. A catheter made from SILASTIC tubing [inner diameter (i.d.), 0.3 mm; outer diameter (o.d.), 0.64 mm; 7 mm length] affixed to a infusion/mounting assembly was passed subcutaneously from the dorsal to the ventral incision, and the tubing was inserted into and gently tied with suture thread to the jugular vein. The dorsal incision was closed and the exit point was then capped with a tygon stopper. Catheter patency was verified by administering 0.02 ml of sodium brevital (5 mg/ml; Monarch Pharmaceuticals) 24–48 h after surgery and again at the completion of cocaine self-administration (i.e., before extinction sessions).
Intracerebroventricular surgery and injections.
Between 1 and 24 h after jugular catheterization, mice were anesthetized with an isoflurane vapor mixture and secured in a Kopf stereotaxic instrument fitted with a mouse adapter. A 27 gauge, 7.5-mm-long stainless-steel cannula was lowered within 1 mm of the lateral ventricle (anteroposterior, −0.1; dorsoventral, −1.5; mediolateral, ±1.0) (Paxinos and Franklin, 2001) and anchored to the skull with 1.6 mm stainless-steel screws and dental cement. Thirty gauge dummy cannulae were kept inside the guide cannula when not in use.
For each intracerebroventricular injection, a 33 gauge stainless-steel injector made 1 mm longer than the guide cannula was inserted. This was attached to tubing (i.d., 0.01 inch; o.d., 0.03 inch), and 2 μl of solution was allowed to flow into the ventricle by gravity (rate controlled to ∼30 s per infusion by manipulating the height of the tubing). The injector was left in place for an additional 60 s to prevent efflux of the infusion material. The mouse was returned to its home cage for 20 min before testing.
Successful gravity flow implies proper cannulae placement; however, a subset of the mice had additional verification by dye injection 6 weeks after surgery, and in all cases placements were in the lateral ventricles.
Self-administration training and testing.
Mice were trained to self-administer cocaine using a two lever operant paradigm. Experimental sessions were conducted in mouse operant conditioning chambers (ENV-307W; MED Associates) located within ventilated sound attenuation chambers. Each chamber was equipped with two levers, above which were cue lights. Mice were trained to self-administer cocaine hydrochloride (0.5 mg/kg; 15 μl infusion) in daily (6 d per week) 1 h sessions. A fixed ratio 1 (FR 1) schedule of reinforcement with a 33 s time-out period was used. Responses on the active lever resulted in a simultaneous activation of the infusion pump and the stimulus light above the active lever. Responses on the inactive lever and during the time-out period were recorded, but had no programmed consequences. Intravenous drug infusions were delivered by a software-operated infusion pump (Razel Scientific Instruments) placed outside the sound-attenuating box, through a liquid swivel and a syringe.
The criteria for successful acquisition of cocaine self-administration were as follows: (1) >10 infusions per session, (2) 70% or more of the pressing occurring on the active (cocaine-associated) lever, and (3) variation of pressing on the active lever ≤25% across 3 consecutive days.
Extinction sessions were initiated once each mouse met the acquisition criteria. The mouse was connected to the infusion line and allowed access to the levers; however, responses had no programmed consequence. Extinction sessions were conducted 6 d per week until the mice reached the criterion of ≤30% of baseline cocaine responding across 2 consecutive days. Mice that did not reach this criterion within 40 d were removed from the study.
After the extinction criterion was met, mice were injected intracerebroventricularly with saline followed by increasing concentrations of NPS (0.1, 0.2, 0.45 nm) and saline again to test for possible reinstatement of lever-pressing behavior. Each test was separated by at least three normal extinction sessions and was both immediately preceded by and immediately followed by an extinction test day. Reinstatement conditions were exactly the same as during extinction. The highest dose of NPS was used in separate studies designed to examine the role of the CRF1 system in NPS-induced reinstatement. This was examined pharmacologically in C57BL/6J mice using the CRF1 antagonist, antalarmin, and genetically using CRF1 KO and WT mice. In these subsequent experiments, mice received treatments in a counterbalanced order, again separated by at least three normal extinction sessions.
Locomotor activity test.
Locomotor activity was measured for 30 min in polycarbonate cages (42 × 22 × 20 cm) placed into frames (25.5 × 47 cm) mounted with photocell beams 2 cm above the bottom of the cage (San Diego Instruments). These beams allowed for the recording of horizontal ambulatory behavior. This test is repeatable and therefore was performed using a within-subjects approach.
Light/dark transfer test.
The light/dark transfer procedure has been used to assess anxiety-like behavior in mice by capitalizing on the conflict between exploration of a novel environment and the avoidance of a brightly lit open field (Crawley, 2007). The apparatus was a rectangular box made of Plexiglas divided by a partition into two environments. One compartment (14.5 × 27 × 26.5 cm) was dark (8–16 lux), and the other compartment (28.5 × 27 × 26.5 cm) was highly illuminated (400–600 lux). The compartments were connected by an opening (7.5 × 7.5 cm) located at floor level in the center of the partition. The time spent in the light compartment and the number of transitions between the dark and light compartments were recorded over the 5 min test session. This test is not repeatable and therefore was performed using a between-subjects approach.
Marble burying test.
Selective inhibition of object-burying behavior over a fixed period of time in rodents has been proposed as test for anxiolytics (Njung'e and Handley, 1991). This test involved placing each mouse in a polycarbonate cage (29 × 18 × 12 cm) containing 20 marbles (1.5 cm diameter) evenly spaced on 5-cm-deep rodent bedding. Thirty minutes later, the number marbles covered at least two-thirds was counted. This test is not repeatable and therefore was performed using a between-subjects approach.
Drugs.
Cocaine-HCl (National Institute on Drug Abuse) was dissolved in sterile physiological saline (0.9%). NPS (also dissolved in saline) was a generous gift from Rainer Reinscheid (University of California, Irvine, Irvine, CA) and custom synthesized by a commercial supplier (Advanced Chemtech). A stock solution of antalarmin (Sigma-Aldrich) was prepared by dissolving the drug in cremaphor/ethanol at 2:1 to a concentration of 30 mg/ml. This solution was kept at 4°C and was diluted in saline immediately before subcutaneous injection. Antalarmin (30 mg/kg; 0.01 ml/g) or the identical cremaphor/ethanol/saline combination (vehicle) was administered 1 h before the intracerebroventricular NPS/saline infusions.
Results
NPS reinstates cocaine-seeking behavior via a CRF1-dependent mechanism
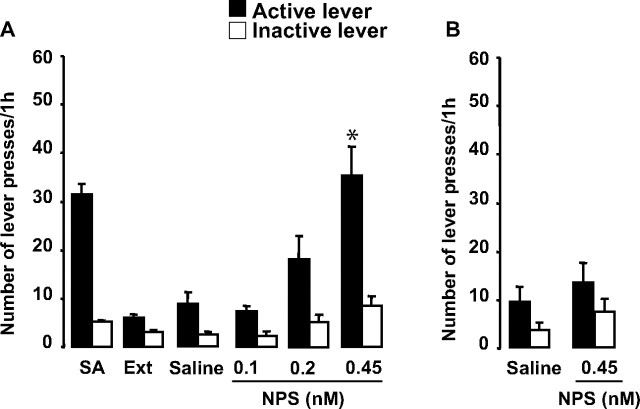
Approximately 12% of the mice intended for self-administration studies were removed because of failure to meet acquisition criteria (5%) or failure to meet the extinction criteria within 40 d (7%). Eight C57BL/6J mice completed the experimental sequence in this first study, having patent catheters and achieving the cocaine self-administration acquisition criteria (in 3.9 ± 0.3 d) and extinction criterion (in 14.9 ± 2.2 d). Active and inactive lever pressing was analyzed using ANOVA with condition as the repeated measure. Conditions included SA (mean of the 3 last days of self-administration), Ext (mean of the 2 last days of extinction), and the saline and NPS administrations. There were no differences between the first and second saline administration in responding; therefore, these data were collapsed. There was an overall effect of condition on active (F(5,35) = 17.3; p < 0.0001) and inactive (F(5,35) = 5.9; p < 0.001) lever pressing (Fig. 1A). The a priori comparisons of importance were between extinction levels of responding and responding after saline, and responding after saline and the three doses of NPS. Therefore, for each ANOVA, four paired t tests were performed and considered significant at p < 0.0125 (Bonferroni's correction). The highest dose of NPS significantly increased lever pressing relative to saline conditions (p = 0.002). NPS did not significantly increase lever pressing on the inactive lever; however, there was a trend for the highest dose (p = 0.03). Responding returned to extinction levels on the day after NPS (between 2 and 12 responses in the mice across all reinstatement experiments and conditions within each experiment).
Figure 1.
A, Intracerebroventricular administration of NPS induced cocaine-seeking behavior. SA, Mean of the 3 last days of self-administration; Ext, mean of the 2 last days of extinction. The highest dose of NPS significantly increased lever pressing in the absence of cocaine (N = 8; p = 0.002). B, The highest dose of NPS did not increase lever pressing in drug-naive mice (N = 6). The data are represented as means ± SEM. Statistical significance was calculated by ANOVA and Bonferroni's corrections were made for the subsequent pairwise comparisons such that significance was set at p < 0.0125. *Significant versus saline.
To determine whether the small effect of NPS on inactive lever pressing was dependent on having cocaine previously associated with the other lever (i.e., mice slightly increased inactive lever pressing because of a renewed interest in obtaining cocaine vs simply because of the stimulatory effect of NPS), six naive mice never receiving cocaine were given the highest NPS dose and allowed access to the operant boxes (Fig. 1B). Under these conditions, NPS did not increase lever-pressing behavior (active lever: F(1,5) = 0.4, p = 0.5; inactive lever: F(1,5) = 1.5, p = 0.3). These results indicate that NPS reinstates cocaine-seeking behavior.
To study the role of CRF1 in NPS effects on cocaine-seeking behavior, we examined its actions when given after the CRF1 antagonist, antalarmin, and when administered to CRF1 KO mice. C57BL/6J (N = 9) were pretreated with 30 mg/kg antalarmin (Steckler and Dautzenberg, 2006) or vehicle before testing for reinstatement with NPS (0.45 nm) or saline. All mice received all four combinations of treatments separated by at least three standard extinction sessions. Active and inactive lever pressing was analyzed using a 2 × 2 factorial ANOVA with the factors pretreatment (vehicle, antalarmin) and treatment (saline, NPS). There were overall effects of pretreatment (F(1,32) = 22.7; p < 0.0001), treatment (F(1,32) = 59.5; p < 0.0001), and an interaction between pretreatment and treatment (F(1,32) = 23.3; p < 0.0001) on active lever pressing (Fig. 2A). Additional analysis of this interaction revealed that NPS increased active lever pressing after vehicle pretreatment, but not after antalarmin pretreatment. The was also a smaller overall effect of treatment on inactive lever pressing (F(1,32) = 6.9; p < 0.05). The a priori comparisons of importance were between extinction levels of responding and responding after vehicle/saline and between the other three combinations of pretreatment/treatment and vehicle/saline. Therefore, four paired t tests were performed and considered significant at p < 0.0125 (Bonferroni's correction). There were no individual conditions that increased inactive lever pressing over extinction levels. The vehicle/NPS combination significantly increased active lever pressing (p < 0.0001), whereas no other combination did, indicating that antalarmin blocked the effect of NPS to reinstate lever pressing.
Figure 2.
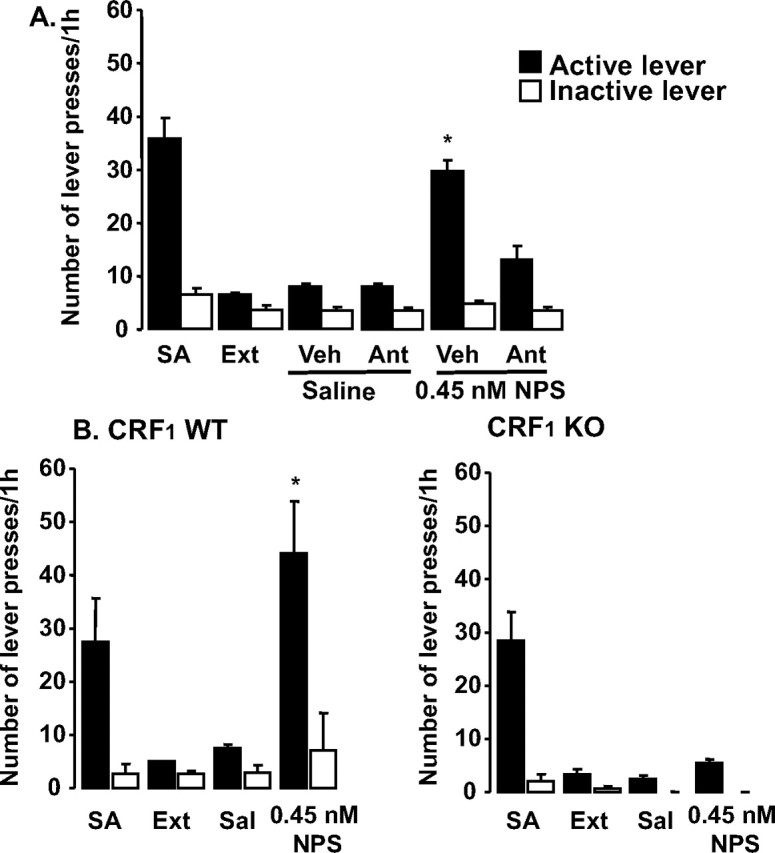
NPS effects on cocaine reinstatement are mediated by CRF1. SA, Mean of the 3 last days of self-administration; Ext, mean of the 2 last days of extinction; Sal, saline injection. A, B, Lever pressing in C57BL/6J mice pretreated with vehicle or antalarmin followed by saline or NPS (N = 9) (A) and in WT and CRF1 KO mice treated with saline or NPS (N = 12) (B). The data are represented as means ± SEM. Statistical significance was calculated by ANOVA and Bonferroni's corrections were made for the subsequent pairwise comparisons such that significance was set at p < 0.0125. *Significant versus vehicle/saline.
CRF1 KO and WT (N = 12) reached acquisition criteria in 5.25 ± 0.55 and 7.0 ± 1.22 d, respectively, and the extinction criterion in 18.0 ± 4.0 and 9.0 ± 2.1 d, respectively. Mice were then treated with 0.45 nm NPS or saline, separated by at least three standard extinction sessions. Active and inactive lever pressing was analyzed using ANOVA with condition as the repeated measure. There was an overall effect of genotype (F(1,4) = 30.7; p < 0.01), condition (F(3,12) = 9.1; p < 0.01), and a significant genotype by condition interaction (F(3,12) = 4.1; p < 0.05) on active lever pressing (Fig. 2A). There were no significant effects on inactive lever pressing. The a priori comparisons of importance were between extinction levels of responding and responding after saline and NPS in CRF1 KO and WT mice. Therefore, four paired t tests were performed and considered significant at p < 0.0125 (Bonferroni's correction). NPS significantly increased active lever pressing in WT mice (p < 0.01), but not in KO mice, indicating that knocking out CRF1 abolished the effect of NPS to reinstate lever pressing. Together, NPS induces cocaine-seeking behavior using this reinstatement model, and, based on both pharmacologic and genetic results, this effect appears to be CRF1 dependent.
NPS increases locomotor activity through a CRF1-dependent mechanism
The goal of this experiment was to examine the role of CRF1 in the locomotor effects of NPS by examining its effects when given after CRF1 antagonism and when administered to CRF1 KO and WT mice.
C57BL/6J mice were pretreated with the CRF1 antagonist, antalarmin, or vehicle (N = 39), and then administered 0.45 nm NPS before being tested in the locomotor activity boxes (Fig. 3A). A two-way ANOVA with factors pretreatment (vehicle, antalarmin) and treatment (saline, NPS) was performed for activity levels, and significant effects of pretreatment (F(1,12) = 10.5; p < 0.01), treatment (F(1,23) = 20.2; p < 0.001), and an interaction between these (F(1,23) = 5.9; p < 0.05) were detected. Post hoc analysis of this interaction revealed a significant effect of NPS on activity (p < 0.001), which was not present in mice pretreated with antalarmin (p = 0.2).
Figure 3.
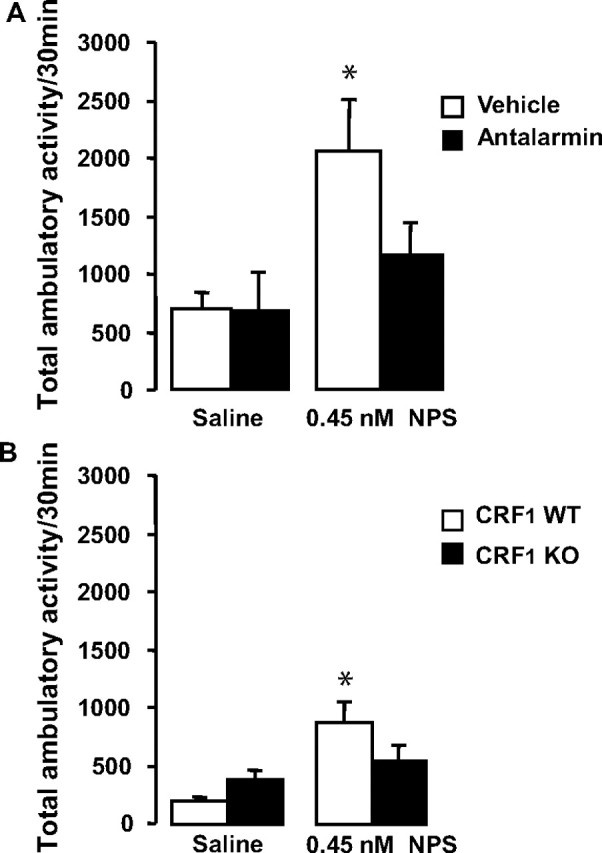
NPS increases locomotor activity through a CRF1-dependent mechanism. A, B, Locomotor activity in response to NPS after pretreatment with vehicle or antalarmin in C57BL/6J mice (N = 39) (A) and in CRF1 WT and KO mice (N = 27) (B). The data are represented as means ± SEM. Statistical significance was calculated by ANOVA and Bonferroni's corrections were made for the subsequent pairwise comparisons such that significance was set at p < 0.016. *Significant versus vehicle/saline.
Locomotor activity was analyzed in CRF1 KO and WT mice (N = 27) after administration of 0.45 nm NPS (Fig. 3B). A two-way ANOVA with the between-subject factor genotype and within-subject factor treatment was performed for activity levels, and significant effects of treatment (F(1,5) = 6.5; p < 0.01) and an interaction between genotype and treatment (F(1,5) = 6.6; p < 0.05) were detected. Post hoc analysis of this interaction revealed a significant effect of NPS in WT mice (p < 0.05), but not in KO mice. Together, the results of this study showed that CRF1 antagonism (pharmacological and genetic) inhibited the locomotor stimulating effects of NPS.
NPS decreases anxiety-like behavior through a CRF1-independent mechanism
The goal of this experiment was to examine the role of CRF1 in the anxiolytic-like effects of NPS by examining its effects when given after a CRF1 antagonist and when administered to CRF1 KO and WT mice. C57BL/6J mice (a subset of the mice used in the locomotor study above; N = 16) were treated with either vehicle/saline, vehicle/NPS, antalarmin/saline, or antalarmin/NPS, and then tested in the light/dark transfer test (Fig. 4A). Two-way ANOVA with between-subjects factors pretreatment (vehicle or antalarmin) and treatment (saline or NPS) revealed a significant effect of treatment on the time spent in the light compartment (F(1,12) = 5.9; p < 0.05) and the number of transitions between the dark and light compartments (F(1,12) = 17.7; p < 0.01). NPS increased both measures and there were no effects of pretreatment, suggesting that NPS decreased anxiety-like behavior regardless of pretreatment with antalarmin.
Figure 4.
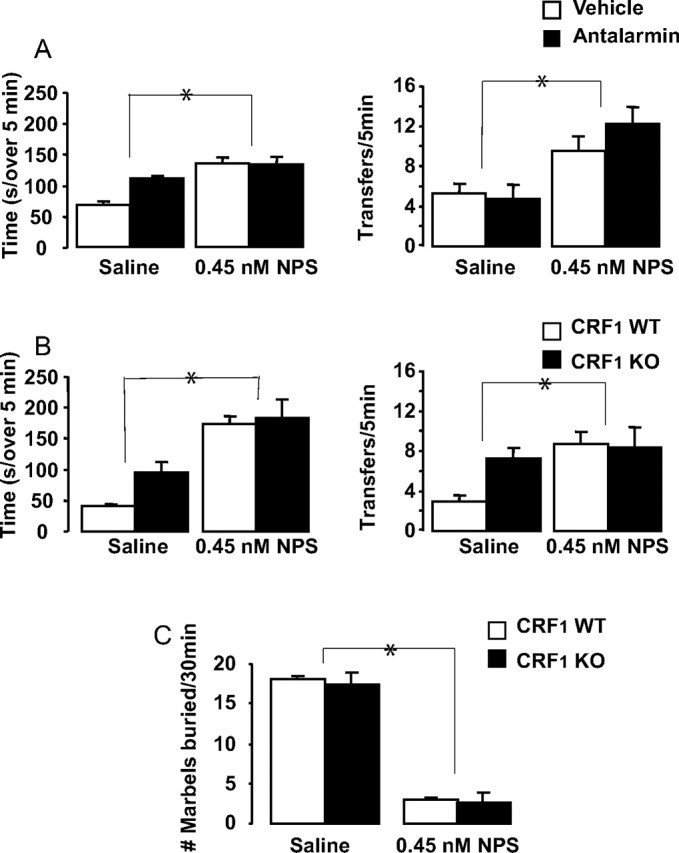
NPS decreases anxiety-like behavior through a CRF1-independent mechanism. A, B, Light/dark transfer behaviors in response to NPS after pretreatment with vehicle or antalarmin in C57BL/6J mice (N = 16) (A) and in CRF1 WT and KO mice (N = 20) (B). The left panels show the time (in seconds) spent in the light compartment, and the right panels show the number of transitions the mice made between the dark and light compartments of the apparatus. C, Marbles buried after NPS in CRF1 WT and KO mice. The data are represented as means ± SEM. Statistical significance was calculated by two-way between-subjects ANOVA. *Indicates overall significant effect of treatment (saline vs NPS).
Light/dark transfer behavior was analyzed in CRF1 KO and WT mice (a subset of the mice used in the locomotor study above; N = 20) after administration of saline or 0.45 nm NPS (Fig. 4B). Two-way ANOVA with between-subjects factors genotype (WT or KO) and treatment (saline or NPS) revealed a significant effect of treatment on the time spent in the light compartment (F(1,16) = 23.1; p < 0.001) and the number of transitions between the dark and light compartments (F(1,16) = 6.5; p < 0.05). NPS increased both measures, suggesting that NPS decreased anxiety-like behavior regardless of genotype.
Although there were no statistically significant differences in light/dark transfer behavior between CRF1 KO and WT mice, we know that these mice differ in measures of activity and anxiety-like behavior involving exploration (Smith et al., 1998) and there is a hint of this in the present results. Therefore, a separate group of CRF1 KO and WT mice (N = 8) were tested for marble-burying behavior after treatment with 0.45 nm NPS (Fig. 4C). Two-way ANOVA with between-subjects factors genotype and treatment revealed a significant effect of treatment only (F(1,8) = 208.4; p < 0.0001). In this case, we were able to examine the anxiolytic effect of NPS independent of increased activity levels (indeed, decreased anxiety in this test assumes a restriction of the behavior of burying). NPS produced significant anxiolysis in both WT and CRF1 KO mice. Together, these results suggest that the anxiolytic effect of NPS is independent of CRF1 function.
Discussion
NPS is particularly intriguing with respect to the localization of a portion of its receptors to the amygdala and its effects on arousal, suggesting a possible involvement in drug abuse and dependence. Relapse to drug use is one of the hallmarks of dependence and is devastating among cocaine users. We found that NPS administration reinstates cocaine-seeking behavior in mice. In addition, our results suggest that NPS acts via CRF1 to reinstate cocaine-seeking behavior and to increase arousal. Pharmacologic inhibition or the genetic deletion of this receptor blocks the effects of NPS on relapse and arousal. These results support a stress system-dependent mechanism for NPS in behavioral activation and drug-seeking behavior.
It is worthwhile mentioning the similarity of NPS and hypocretin systems. Both neuropeptides modulate sleep and arousal, and reinstate cocaine-seeking behavior, although the effects of hypocretin last ∼2–3 h (Boutrel et al., 2005; Pañeda et al., 2005), whereas NPS effects are more robust within the first hour after injection (Xu et al., 2004). We found common mediators for the effects of these two neuropeptides on reinstatement and arousal, CRF and CRF1, but additional experiments are needed to determine whether NPS is mediating the effects of stress on reinstatement as hypocretin appears to (Boutrel et al., 2005) or whether it represents a novel reinstatement mechanism.
NPS was shown previously to have both wake-promoting and anxiety-reducing properties (Xu et al., 2004). This is an unusual combination of behavioral effects and it is puzzling that a compound that has anxiety-decreasing properties may, in fact, increase relapse potential. On the contrary, stress has been shown in several instances to increase stimulant self-administration (Kreek, 1996; Piazza and Le Moal, 1996) and to induce reinstatement of drug-seeking behavior (Sinha et al., 2000). However, it was shown that the anxiolytic drug diazepam increases cocaine self-administration in a mouse strain that typically does not take this drug (David et al., 2001). These authors suggested that there may be opposing influences of stress/state anxiety and emotional reactivity/trait anxiety on stimulant-seeking behavior. Taken further, reinstatement may be induced by increases in stress and decreases in emotional reactivity.
The results of this study suggest that NPS works through independent pathways to affect locomotor activity/drug-seeking behavior and anxiety-like behavior. This is supported by recent results of Jüngling et al. (2008), who found using a fear conditioning paradigm that NPS infused into the amygdala reduced anxiety-like behavior without increasing locomotor activity. Therefore, although stress systems have been implicated in drug-seeking behavior (Kreek, 1996; Piazza and Le Moal, 1996; Sinha et al., 2000; David et al., 2001), the drug-seeking effects of NPS may be more closely related to its effects on activity, as these effects both appear to require CRF1 function.
A caveat of the present study is that NPS effects on the reinstatement of responding to alternative reinforcers have not been investigated. It is possible that NPS might reinstate seeking behavior toward other drugs of abuse and/or nondrug reinforcers. Interestingly, however, it recently was found that NPS inhibited morphine-induced conditioned place preference, suggesting a blockade of its rewarding effects (Li et al., 2009). The expression of preference was blocked by NPS even when given after morphine conditioning, suggesting that NPS did not “reinstate” preference in the absence of drug, but rather appeared to inhibit it. This is interesting as it suggests that NPS effects may be reinforcer specific.
Our results demonstrate that CRF1 KO mice self-administer cocaine. The rate of self-administration and the amount of cocaine taken did not differ from their WT littermates. CRF1 KO mice showed trends toward both decreased anxiety-like behavior in the light/dark transfer test and increased locomotor activity levels. These results were expected and in accordance with the already described behavioral responses to CRF (Smith et al., 1998; Lowry and Moore, 2006). Even with these baseline genotypic differences, it was clear that NPS effects on locomotor activity were inhibited and that its effects on anxiety-like behavior were not inhibited in KO mice. The marble-burying test allowed for the examination of anxiolysis under conditions in which genotype differences were not detected and in which increased activity levels did not correlate with decreased anxiety-like behavior (i.e., decreased burying was indicative of decreased anxiety-like behavior). Again, the anxiolytic effects of NPS were unaffected by lack of the CRF1 receptor.
In conclusion, this study shows that NPS reinstates previously extinguished cocaine-seeking behavior, likely through CRF1 receptors. CRF1 receptors also appear to mediate the arousing, but not the anxiolytic, actions of NPS.
Footnotes
This work was supported by National Institutes of Health Grants NS057096 (A.J.R.) and MH58543 (L.d.L.). We thank George F. Koob for his useful suggestions on a previous draft of this manuscript, and Coree Levy, Kathleen Chu, and Lisa Underwood for excellent technical assistance.
References
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice. Ed 2. Hoboken, NJ: Wiley-Interscience; 2007. What's wrong with my mouse? [Google Scholar]
- David V, Gold LH, Koob GF, Cazala P. Anxiogenic-like effects limit rewarding effects of cocaine in BALB/cByJ mice. Neuropsychopharmacology. 2001;24:300–318. doi: 10.1016/S0893-133X(00)00205-0. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl) 2006;187:112–120. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdale. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Cocaine, dopamine and the endogenous opioid system. J Addict Dis. 1996;15:73–96. doi: 10.1300/J069v15n04_05. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, Malberg JE, Beyer CE, Schechter LE, Rosenzweig-Lipson S, Ring RH. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;197:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- Li W, Gao YH, Chang M, Peng YL, Yao J, Han RW, Wang R. Neuropeptide S inhibits the acquisition and the expression of conditioned place preference to morphine in mice. Peptides. 2009;30:234–240. doi: 10.1016/j.peptides.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Moore FL. Regulation of behavioral responses by corticotropin-releasing factor. Gen Comp Endocrinol. 2006;146:19–27. doi: 10.1016/j.ygcen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Evaluation of marble-burying behaviour as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Pañeda C, Winsky-Sommerer R, Boutrel B, de Lecea L. The corticotropin-releasing factor-hypocretin connection: implications in stress response and addiction. Drugs News Perspect. 2005;18:250–255. doi: 10.1358/dnp.2005.18.4.908659. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL. Neuropeptide S as a novel arousal promoting peptide transmitter. FEBS J. 2005a;272:5689–5693. doi: 10.1111/j.1742-4658.2005.04982.x. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL. Neuropeptide S and its receptor: a newly deorphanized G protein-coupled receptor system. Neuroscientist. 2005b;11:532–538. doi: 10.1177/1073858405276405. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Civelli O. Neuropeptide S: a new player in the modulation of arousal and anxiety. Mol Interv. 2005;5:42–46. doi: 10.1124/mi5.1.8. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422:403–406. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Polis IY, Gold LH. Intravenous self-administration of heroin, cocaine, and the combination in BALB/c mice. Eur J Pharm. 1997;326:119–125. doi: 10.1016/s0014-2999(97)85405-2. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Höhn J, Szabó G, Penke B. Critical role of endogenous corticotropin-releasing factor (CRF) in the mediation of the behavioral action of cocaine in rats. Life Sci. 1992;51:2019–2024. doi: 10.1016/0024-3205(92)90151-e. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Dautzenberg FM. Corticotropin-releasing factor receptor antagonists in affective disorders and drug dependence—an update. CNS Neurol Disord Drug Targets. 2006;5:147–165. doi: 10.2174/187152706776359619. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Intravenous drug self-administration in mice: practical considerations. Behav Genet. 2007;37:101–118. doi: 10.1007/s10519-006-9097-0. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]