Abstract
Complex cross-talk between endoderm and the microenvironment is an absolute requirement to orchestrate hepatic specification and expansion. In the mouse, the septum transversum and cardiac mesoderm, through secreted BMPs and FGFs, respectively, instruct the adjacent ventral endoderm to become hepatic endoderm. Consecutively, endothelial cells promote expansion of the specified hepatic endoderm. Using a mouse reporter embryonic stem (ES) cell line in which hCD4 and hCD25 were targeted to the Foxa2 and Foxa3 loci, we reconstituted an in vitro culture system in which committed endoderm cells co-expressing hCD4-Foxa2 and hCD25-Foxa3 were isolated, and co-cultured with endothelial cells in the presence of BMP4 and bFGF. In this culture setting, we provide mechanistic evidence that endothelial cells function not only to promote hepatic endoderm expansion, but are also required at an earlier step for hepatic specification, at least in part through regulation of the Wnt and Notch pathways. Activation of Wnt and Notch by chemical or genetic approaches increases endoderm cell numbers but inhibits hepatic specification, and conversely, chemical inhibition of both pathways enhances hepatic specification and reduces proliferation. Using identical co-culture conditions, we defined a similar dependence of endoderm harvested from embryos on endothelial cells to support their growth and hepatic specification. Our findings (1) confirm a conserved role of Wnt repression for mouse hepatic specification, (2) uncover a novel role for Notch repression in the hepatic fate decision, and (3) demonstrate that repression of Wnt and Notch signaling in hepatic endoderm is controlled by the endothelial cell niche.
Keywords: Endoderm, endothelial cells, mouse embryonic stem cells, mouse embryo, Wnt, Notch
INTRODUCTION
The mouse liver develops from the ventral foregut definitive endoderm that is generated from the anterior primitive streak, beginning at approximately day7.5 of gestation 1. The subsequent hepatic specification of the endoderm occurs at day8 and is tightly regulated by complex cell communications 1, 2. In the mouse and chick embryo, cardiac mesoderm and the septum transversum, located in the proximity of the ventral foregut endoderm, induce hepatic gene expression including alpha-fetoprotein (Afp) and albumin through FGF (FGF-1,-2,-8) and BMP (BMP-2,-4,-7) signals, respectively 3–7. Adjacent trunk mesoderm secretes Wnt ligands (Wnt8, Wnt8b, Wnt3, Wnt3a and Wnt1) 8 that instruct the posterior endoderm to inhibit hepatic fate and promote intestinal specification, whereas Wnt antagonists (Dkk1, Frzb1, Crescent and Sfrp5) secreted by the anterior foregut endoderm shut down Wnt signaling allowing hepatic specification 9–12. Following initial specification of the hepatic endoderm, the liver bud development is dependent on signals from adjacent Flk1+/CD31+ endothelial cells 13. In Flk-1 mutant embryos, which lack endothelial cells, hepatic specification occurs, but liver bud morphogenesis fails. Although the requirement of endothelial cells for liver bud expansion is documented, their earlier role in hepatic specification has not been investigated.
The feasibility of generating hepatocytes from ES cells has been well documented in a number of studies 14. The most efficient and reproducible approaches are those that recapitulate in ES cell cultures the appropriate signaling pathways uncovered in the mouse embryo studies. For example, we demonstrated that Activin-A, a TGFβ family member that signals through the same receptor as nodal, efficiently induces endoderm in ES cell differentiation cultures 15. Activin-A-induced endoderm in ES cell cultures can be enriched using the surface receptors CXCR4, cKit, E-cadherin and PDGF receptor-α or through the selection of reporter molecules targeted to the Brachyury, Foxa2, goosecoid, sox17 and Hex loci 16–21. Overall, ES cells have been successfully differentiated to endoderm 22 with further hepatic specification of ES cell-derived endoderm 20, 23–29. We showed that BMP4, in concert with bFGF and Activin-A, is required for hepatic specification of the progenitor for endoderm, defined as cells expressing Foxa2, Brachyury, and cKit, using a reporter ES cell line in which GFP and CD4 were targeted to the Brachyury and Foxa2 loci, respectively 21. In that study, upon hepatic cell development, endothelial cells were always seen surrounding the hepatic colonies, and their presence was associated with hepatic endoderm expansion.
In the present study, we investigated the interactions between endothelial cells and endoderm cells harvested from either ES cell differentiation cultures or from E8.25 mouse embryos. We studied specifically the role of the Wnt pathway, known to instruct endoderm, and the Notch pathway, often associated with cell fate decision in specific organ systems. Notch signaling components are expressed by endothelial cells and have been implicated for instance in cardiac progenitor cell fate 30, 31 or arterial specification of endothelium 32, 33 in vertebrate development. Therefore, we tested the hypothesis that canonical Wnt/β̃catenin and Notch pathways are involved in the early hepatic fate of ES cell-derived endoderm, and that endothelial cells contribute to this fate decision. We demonstrated that the embryonic liver specification is controlled by the adjacent endothelial niche through dual repression of Wnt and Notch pathways.
MATERIALS AND METHODS
ES cell lines
The mouse ES cell line GFP-Bry/CD4-Foxa2/CD25-Foxa3 has been established by targeting GFP into the Brachyury locus, hCD4 into the Foxa2 locus 16 and hCD25 into the Foxa3 locus 34. The tet-inducible Notch1-IC-expressing ES cell line was generated by targeting NICD cDNA into the tet-regulated promoter near the HPRT locus of the AinV/GFP-Bry/CD4-Foxa2 ES cell line 35. The ES line A2lox.sβcat (sβ-cat) contains a stabilized β-catenin gene under control of a doxycyclin-regulated promoter 36.
ES cell differentiation
ES cells were cultured at low density (30,000 cells/ml) to allow embryoid body (EB) formation in serum-free differentiation media (SFD) defined previously 21. Day2-EBs were dissociated and cells (40,000 cells/ml) reaggregated in SFD complemented with Activin-A (75ng/ml). Day5-EBs were dissociated and endoderm cells isolated into the F2+/F3+ or the ENDM1+ fraction. Endoderm cells (90,000 cells) were plated in gelatin-coated 48 well-plate in the presence or absence of D4T cells (10,000 cells) for 3 days in the hepatic media as described 21. All cytokines were purchased from R&D Systems, and the γ secretase inhibitor (L-685,458) from Sigma.
Flow cytometry and cell sorting from ES cell cultures and embryos
Day5-EBs were dissociated with trypsin/EDTA. Cells were stained either with anti-hCD4-APC (Caltag) and anti-hCD25-PE (Caltag) or with ENDM1 antibody (Dr Streeter) followed by anti-rat IgG-APC (Jackson ImmunoResearch). Day8-plated cultures were dissociated and stained with anti-CD31-PE (BD Pharmingen) and anti-hCD4-APC. Embryos were collected in IMDM containing 4.5 ×10−4 M MTG, 0.05% BSA and 2mM Glutamin (staining buffer, SB), dissociated with trypsin/EDTA, stained with ENDM1 antibody and subsequently with an anti-rat IgG-APC (Jackson ImmunoResearch). Cells were either analyzed using a LSRII flow cytometer (Becton Dickinson) or sorted on a Moflo cell sorter (Cytomation Systems). Each experiment required 10 pregnant mice (~100 embryos) to isolate ENDM1+ endoderm cells (200,000 cells). Analysis was done using FlowJo software (Tree Star Inc.).
Quantitative real time-PCR (qPCR)
Total RNA was prepared with the RNeasy micro Kit (Qiagen). RNA was reverse-transcribed into cDNA using the Superscript III First-strand Synthesis System kit (Invitrogen). qPCR was performed with a Roche System (LC480). All experiments were done in triplicate using the Roche SYBR Green master mix. Primer sequences used are listed in Supporting information Table 1. Relative quantification was calculated using the comparative threshold cycle (CT) method and was normalized against the ΔCT of housekeeping gene β-actin. Melting curves for each gene were used to confirm homogeneity of the DNA product.
Immuno-staining in the culture dish
Day8-plated cultures were fixed with 4% paraformaldehyde, permeabilized with triton 0.2%, incubated with the blocking buffer (Dako) and consecutively with anti-Afp (Neomarkers), anti-Foxa2 (Santa Cruz), anti-CD31-biotin (BD Pharmingen), anti-Axin2 (abcam), anti-Sfrp5 (Genway Biotech) antibodies, or with rabbit IgG (for Afp, Axin2 and Sfrp5), goat IgG (for Foxa2) or rat IgG-biotin (for CD31) as a negative control. Cells were incubated with an anti-rabbit whole IgG-Cy3 or -Cy2 (for Afp, Axin2 and Sfrp5, Jackson ImmunoResearch), anti-goat IgG-Cy2 or -Cy3 (for Foxa2, Jackson ImmunoResearch), streptavidin-Cy2 or -Cy3 (for CD31, Jackson ImmunoResearch). The stained cells were visualized using a fluorescent microscope (Leica DMIRB) and images captured using Magnafire software.
Western Blotting
Total protein lysates of isolated day5 F2+/F3+ endoderm cells cultured for 72 hrs alone or co-cultured with D4T endothelial cells were harvested in RIPA buffer with inhibitor cocktails. Total lysates were fractionated on a 12% SDS-polyacrylamide gel and electroblotted on PVDF membrane. Chemiluminescence detection was performed according to manufacturer’s instructions (Millipore Immobilon Western Chemiluminescent HRP substrate). Anti-Afp (Neomarkers), anti-β-tubulin (Abcam) and anti-rabbit IgG-HRP (Amersham Pharmacia) antibodies were used at 1:200, 1:3000 and 1:20,000, respectively.
Statistical analysis
Results are expressed as mean +/− SD. For each group, triplicate wells were analyzed and treatment groups were compared using the t-test analysis. P<0.05 was considered statistically significant, * P<0.05, **P<0.01 and ***P<0.001.
RESULTS
Day5 Foxa2+/Foxa3+ ES cell derived cell population represents a definitive endoderm population devoid of endothelial potential
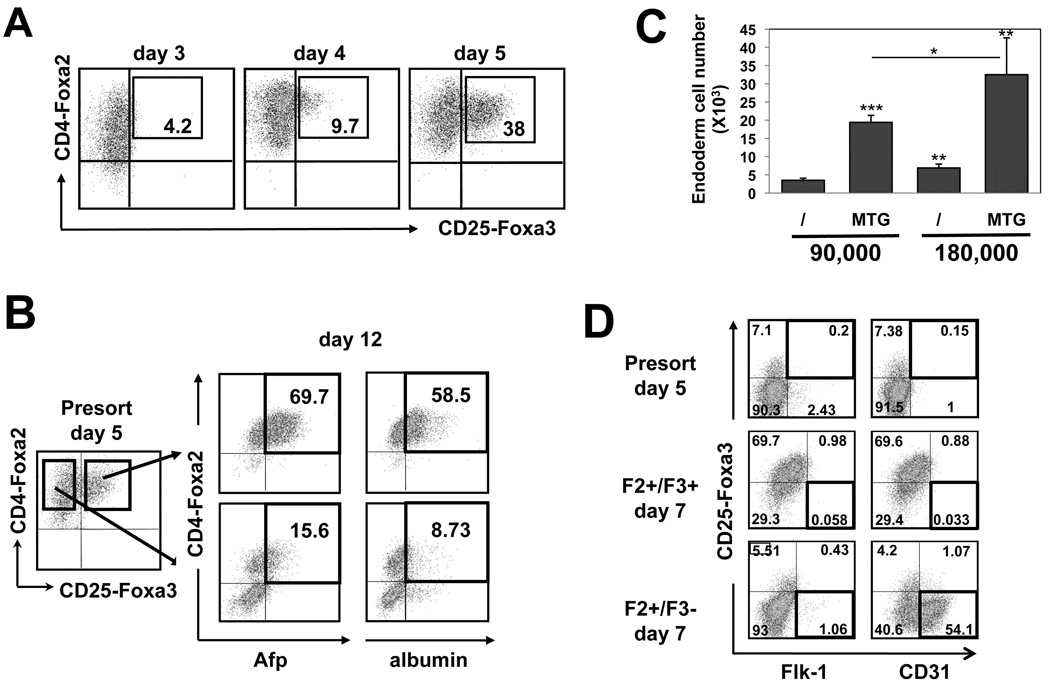
The role of endothelial cells in the hepatic specification and expansion of ES cell-derived endoderm was investigated using a reporter ES cell line in which, in the context of the GFP-Bry/CD4-Foxa2 ES cell line, the hCD25 was targeted to the Foxa3 locus 34. Since Foxa3 is expressed in the gut tube but not in the primitive streak, this cell line enables us to isolate a GFP-Bry−/CD4-Foxa2+/CD25-Foxa3+ population, which was shown to be highly enriched for definitive endoderm cells 34. An endoderm program was induced with Activin-A in EB differentiation cultures as previously described 21. The CD25-Foxa3+ cells developed mainly at day5 (Fig. 1A), and co-expressed CD4-Foxa2. Both CD4-Foxa2+/CD25-Foxa3+ (hereafter referred to as F2+/F3+) and CD4-Foxa2+/CD25-Foxa3− (hereafter referred to as F2+/F3−) fractions were isolated and plated in hepatic media that included BMP-4 and bFGF to allow hepatic specification, as well as HGF, EGF and TGFα to promote hepatic cell expansion 21. Following 2 days of culture, F2+/F3+ endoderm cells grew poorly as assessed by low CD4-Foxa2 endoderm cell number (Fig. 1C). However, addition of the anti-oxidant agent mono-thioglycerol (MTG) to the media and an increase in the number of cells plated promoted sufficient endoderm cell survival and proliferation to analyze their hepatic fate (Fig. 1C). MTG is commonly used in ES cell differentiation cultures to promote cell survival and growth and more recently has been found to promote hepatic specification 21. By day12 of differentiation, virtually all F2+/F3+ cells maintained an endoderm phenotype as indicated by their homogenous expression for CD4-Foxa2 (Fig 1B) and developed up to 60% of hepatic cells defined as CD4-foxa2+/Afp+/albumin+ cells. In contrast, many of the F2+/F3− cells down-regulated expression of CD4-Foxa2 indicating that they lost their endoderm fate, and generated only up to 9% of hepatic cells. Flow cytometry analysis of day5 populations indicated no overlap between the endothelial markers Flk-1 and CD31 and the endoderm marker CD25-Foxa3 (Fig. 1D). Moreover, the day5 F2+/F3+ cells plated for 2 days did not generate Flk-1+ or CD31+ cells, while the day5 F2+/F3− cells gave rise to a large fraction of CD31+ cells (~50%), indicative of potential endothelial cell lineage development strictly from the F2+/F3− population. Altogether, these data demonstrate a high enrichment for definitive endoderm committed cells in the day5 F2+/F3+ population, and that this fraction is devoid of endothelial potential. We therefore used the day5 F2+/F3+ endoderm to examine the role of endothelial cells on the fate of endoderm using in vitro co-cultures.
Fig. 1. Hepatic specification of the day5 endoderm F2+/F3+ population devoid of endothelial potential.
(A) Kinetics of CD4-Foxa2 and CD25-Foxa3 expression between day3 and day5 of differentiation following Activin-A induction at day2. (B) F2+/F3+ and F2+/F3− fractions were isolated from day5 EBs, plated in liver media in the presence of MTG and analyzed for CD4-Foxa2, Afp and albumin expression by flow cytometry at day12 of differentiation. (C) CD4-Foxa2 endoderm cell numbers obtained 2 days following plating of either 90,000 or 180,000 day5 F2+/F3+ cells in the absence or presence of MTG in the media. (D) Expression of CD25-Foxa3, Flk-1, CD31 at day5 of differentiation and at day7 following plating of either the day5 F2+/F3+ or F2+/F3− populations.
D4T endothelial cells promote endoderm survival, growth and hepatic specification
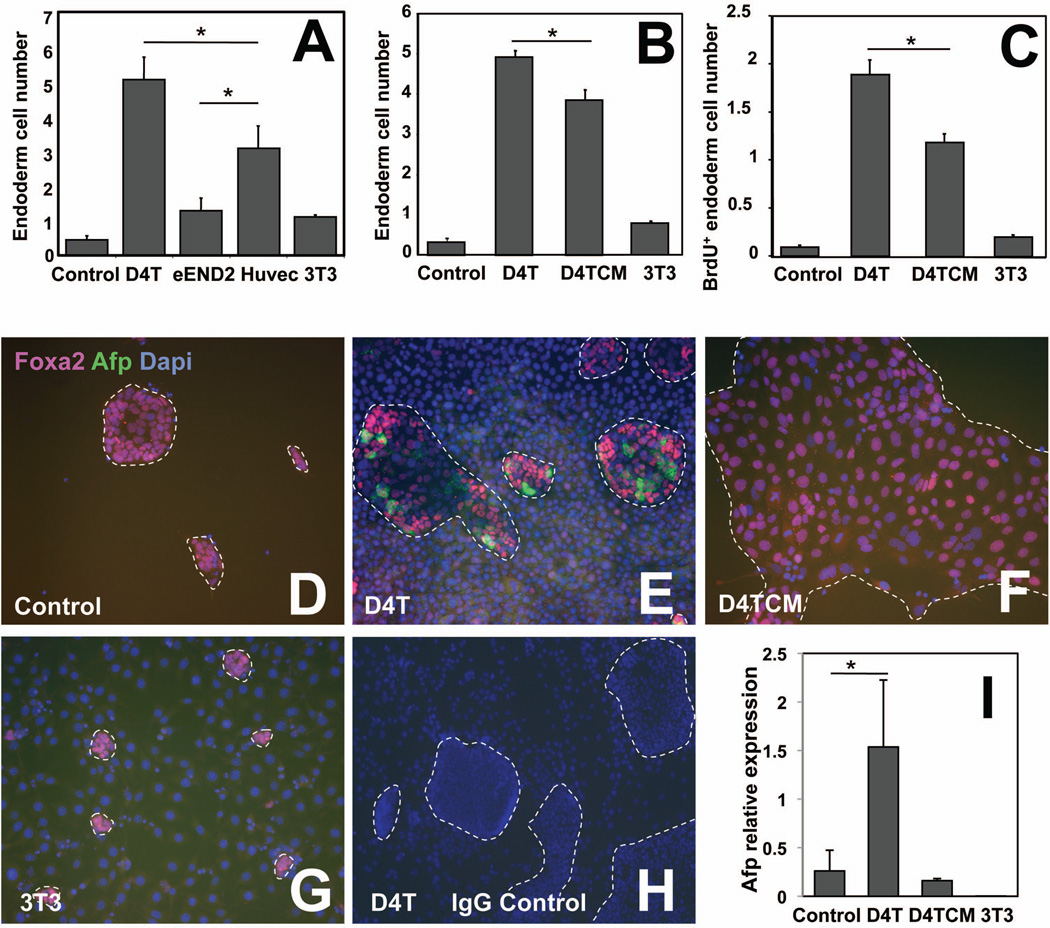
To define the most permissive endothelial cells for their ability to promote ES cell-derived endoderm survival and growth, F2+/F3+ cells were plated for 2 days in the absence of MTG in hepatic media alone (control), with either the ES cell-derived D4T endothelial cells, mouse cavernous hemangiomae endothelial eEND2 cells, primary human umbilical vein endothelial Huvec cells, or 3T3 fibroblastic cells (Fig. 2A). All lines except 3T3 cells express the endothelial markers Flk-1 or KDR (VEGFR-2), PECAM1 (CD31), VE-Cadherin (CD144), and Tie-2 (Supporting information Fig. 1). D4T cells were the most supportive endothelial cells as assessed by the highest number of CD4-Foxa2+ cells. Huvec cells also improved significantly F2+/F3+ endoderm cell survival and growth, while eEND2 cells and 3T3 did not. The inability of the eEND2 endothelial cells and 3T3 fibroblastic cells to support endoderm expansion is correlated to the low (for eEND2 cells) or lack of (for 3T3 cells) expression of Flk-1 cell surface expression that may be relevant to this phenotype. Discrepancies between cell lines were expected as endothelial cells display different functions depending on their locations in the body and their developmental stages 37. D4T cells were originally generated from a day4 ES cell differentiation culture by immortalization with a retrovirus expressing the Polyoma Middle T antigen 38. They represent a very early developmental stage of endothelial cell development well-matched with the early developmental stage of F2+/F3+ endoderm cells. Therefore, we used D4T cells to investigate further the roles of endothelial cells in promoting endoderm survival, growth and hepatic specification (Fig. 2B–H). F2+/F3+ cells did not survive well when plated alone (Fig. 2B,C,D, control), most likely because this highly purified definitive endoderm was separated from supporting cells. However, addition of D4T endothelial cells to endoderm cells promoted survival, growth and hepatic specification of this population, illustrated by increased numbers of CD4-Foxa2+ cells and BrdU incorporation (Fig. 2B,C, D4T), as well as by the presence of Afp protein (Fig. 2E) and high Afp transcript levels (Fig. 2I, D4T). None of these effects were observed in co-cultures with 3T3 cells (Fig. 2B,C,G,I). Conditioned media from D4T cells (D4T CM) allowed endoderm cell survival but not hepatic specification (Fig. 2B,C,F,I), suggesting that direct contacts between endothelial and endoderm cells are required for hepatic specification. Moreover, direct co-cultures were always significantly more efficient in promoting endoderm survival and growth (Fig. 2B,C). These data demonstrate that D4T endothelial cells promote efficiently the survival, growth and hepatic specification of ES cell-derived F2+/F3+ endoderm through direct contacts.
Fig. 2. D4T endothelial cells promote F2+/F3+ endoderm cell expansion and hepatic specification.
(A, B, C) Numbers of endoderm CD4-Foxa2 cells (×104) following 2 days plating in hepatic media in the absence of MTG of day5 isolated F2+/F3+ (90,000 cells) cultured alone (control), or in the presence of 20,000 D4T, eEND2, Huvec or 3T3 cells, or 50% of conditioned media from D4T cells (D4T CM). The % of CD4-Foxa2 endoderm cells, D4T and CD4-Foxa2 proliferative cells were defined by flow cytometry using antibodies against CD4, CD31 and BrdU/CD4, respectively. BrdU was added to each condition 1-hour prior to collecting cells (C). (D–H) Co-immunostaining for Foxa2 (red), Afp (green) and Dapi (blue) at day7 in the culture dish of the 4 condition cultures described in (B,C). Rabbit and goat IgG control is represented in (H). (I) qPCR-based quantitative analysis of Afp transcript levels in the 4 conditions harvested at day7. Expression levels have been adjusted to reflect the proportion of CD4-Foxa2 endoderm cells for each condition. All results are from one representative experiment (n=3). Original magnification: ×100 (D–H).
Wnt/β̃catenin and Notch pathways are involved in the endothelial-endoderm cross-talk that promotes hepatic specification
To test the hypothesis that canonical Wnt/β̃catenin or Notch pathways are involved in the endoderm cell fate, and that endothelial cells contribute to this fate decision, we investigated whether these pathways were perturbed upon co-culture. Transcript levels of components from each pathway were compared between endoderm cells cultured alone and isolated following co-culture with D4T cells, as well as between D4T cultured alone and D4T cells isolated following co-culture with endoderm cells (Fig. 3A). In this setting, isolated F2+/F3+ endoderm cells were co-cultured with D4T cells for 72–90-hours, and were then separated again by FACS using the reporter CD4-Foxa2 to mark endoderm cells and the cell surface marker CD31 to track D4T cells. The purity of the isolated populations was always higher than 95%. In parallel, F2+/F3+ cells and D4T cells were grown alone. The four groups of cells were harvested after 72- or 92-hours as indicated.
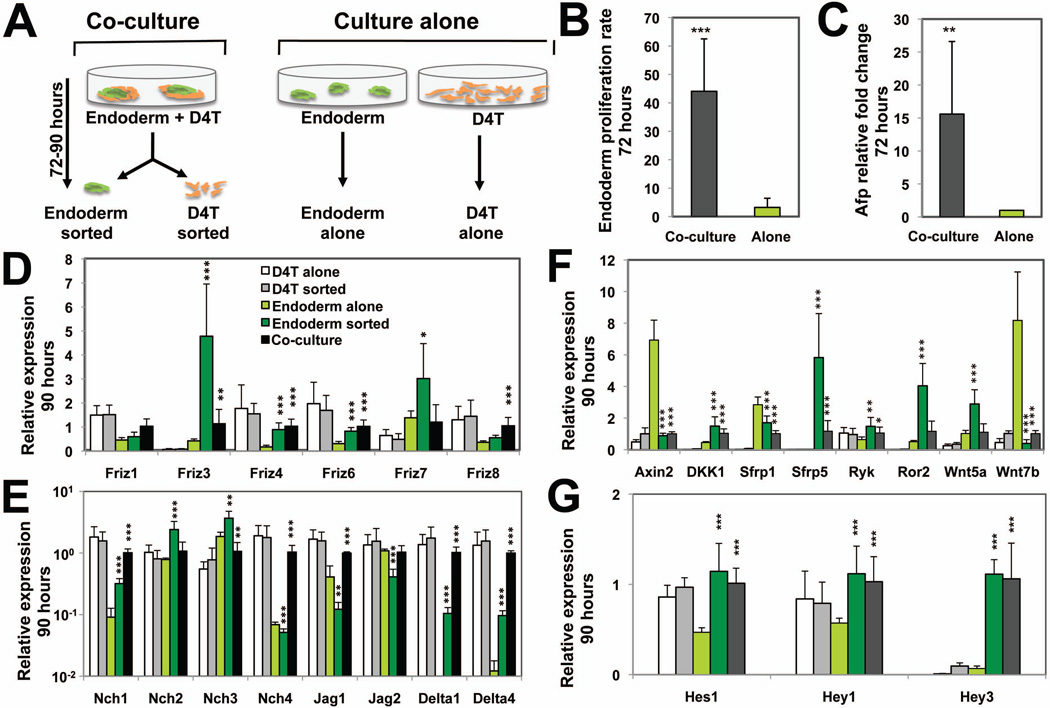
Fig. 3. Co-culture of F2+/F3+ endoderm with D4T cells affects Wnt and Notch signaling pathways.
(A) Isolated day5 F2+/F3+ endoderm and D4T cells were cultured either separately or co-cultured for 72–90-hours in hepatic media and then isolated by FACS. (B) Proliferation rate of CD4-Foxa2 cells following 72-hours plating in hepatic media of day5 F2+/F3+ (90,000 cells) cultured alone (Alone) or in the presence of 10,000 D4T cells (Co-culture). (C) qPCR analysis of Afp transcript fold changes in endoderm cultured alone or co-cultured with D4T cells for 72-hours. Expression levels have been adjusted to reflect the proportion of CD4-Foxa2 endoderm cells for each condition. (D–G) qPCR analysis of Wnt (D,F) and Notch (E,G) signaling components in endoderm or D4T cells cultured alone or co-cultured for 90-hours and subsequently isolated. Errors bars represent the standard deviation of means from 5, 7 or 2 independent experiments in graphs (B), (C) and (D–G), respectively. In graphs (D–G), * represents the significant differences between the “endoderm alone” group and the “endoderm sorted” or “co-culture” groups.
We confirmed that the 72-hours co-culture significantly improved the expansion and hepatic specification of endoderm, compared to when endoderm is cultured alone, assessed by increased proliferation rate (Fig. 3B) and Afp induction (Fig. 3C). The comparison between endoderm cultured alone or co-cultured for 90-hours identified clearly and consistently the upregulation of the Wnt receptors Frizzled 3, 4, 6 and 7 in endoderm cells following co-culture (Fig. 3D, light green versus dark green columns). Also increased upon co-culture were transcript levels for receptors Notch 1, 2 and 3 as well as their ligands Delta1 and 4, while the expression levels for ligands Jagged1 and 2 decreased (Fig. 3E). The co-culture dramatically decreased the levels of the downstream target of canonical Wnt signaling Axin2 39, consistent with increased expression levels for the Wnt inhibitors Dkk1 and Sfrp5, and lower levels of the canonical Wnt ligand Wnt7b, indicating a repression of the Wnt pathway upon co-culture (Fig. 3F). High levels of Sfrp5 transcripts and low levels of Axin2 transcripts in co-cultured endoderm cells were correlated with their protein levels as assessed by immunocytostaining in the culture dish (Supporting information Fig. 2). However, elevated expression levels of the non-canonical Wnt receptors Ryk 40 and Ror2 41 and the β-catenin-independent Wnt5a ligand suggested an activation of the non-canonical Wnt in co-cultured endoderm. Levels of Notch effectors Hes1, Hey1 and Hey3 increased significantly in co-cultured endoderm (Fig. 3G). Interestingly, changes in gene expression levels following co-culture were observed strictly in endoderm cells but not in endothelial cells (white versus light grey columns). Altogether, the global up- and down-regulation of genes related to the Wnt and Notch pathways clearly implicates both these pathways in the endoderm-endothelial cell cross-talk upon endoderm hepatic specification.
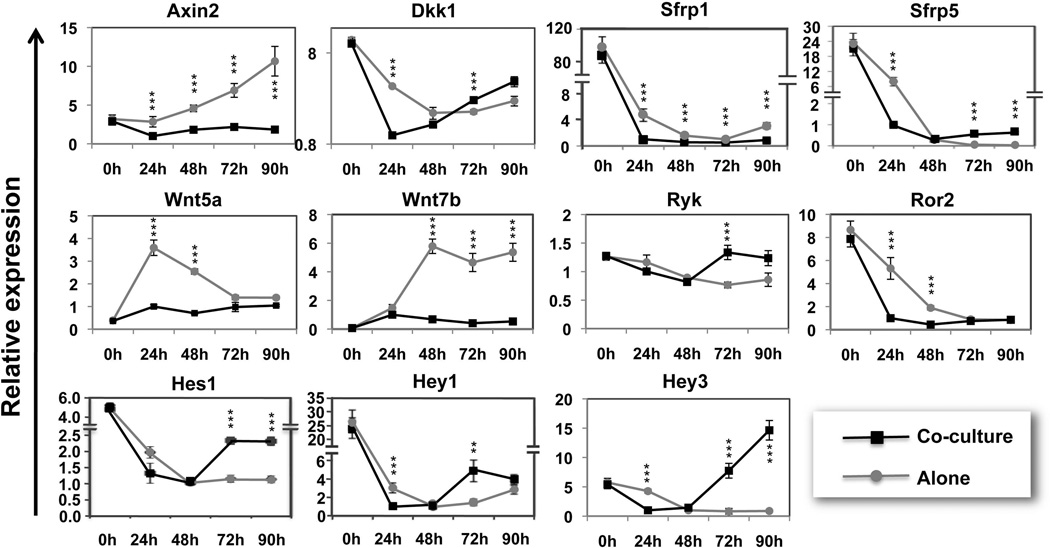
The relative expression levels for components of the Wnt and Notch pathways were then dissected in a temporal manner in endoderm cultured alone or in co-cultures including endoderm and D4T cells (Fig. 4). In the first group (endoderm cultured alone, grey lines), the Wnt pathway was largely activated as indicated by increasing levels of Axin2 and the Wnt ligand Wnt7b, and consistent with decreasing levels of Wnt inhibitors Dkk1, Sfrp1 and Sfrp5. In contrast, the levels of β-catenin-independent Wnt receptors Ryk and Ror2 decreased significantly. The Notch pathway was slowly inhibited, indicated by decreasing levels of the Notch effectors Hes1, Hey1, and Hey3. In the co-culture group (black lines), expression levels were impacted strongly on both signaling pathways. At all time points the Wnt effector Axin2 levels remained low and the expression levels for the inhibitors Dkk1 and Sfrp5 increased with time and were significantly upregulated by 90-hours compared to the levels found in alone cells (1.6- and 32.4- fold increase respectively), suggesting that the co-culture functions to repress the Wnt pathway. The Notch pathway was sharply repressed during the first two days of co-culture, as demonstrated by the rapid decrease of Hes1, Hey1 and Hey3 that were significantly lower following 24-hours of co-culture compared to those levels found in endoderm cultured alone (1.5-, 2.9- and 4.3- fold decrease respectively). However, levels for Hes1 and Hey3 increased by 90-hours of co-culture. Together, these gene expression profiles reveal a rapid repression of Wnt and Notch signaling in the co-cultures that is associated with endoderm hepatic specification.
Fig. 4. Dynamic changes of Wnt and Notch pathways components upon co-culture.
Co-cultured cells (black) or endoderm cultured alone (grey) were harvested at 0, 24, 48, 72 and 90 -hours following plating. Graphs represent relative gene expression levels. These results are from one representative experiment (n=3). * Represent the significant differences that are at least 2-fold different between the co-cultures and endoderm cultured alone for a specific time point.
D4T Endothelial cells support hepatic specification of endoderm from mouse embryos
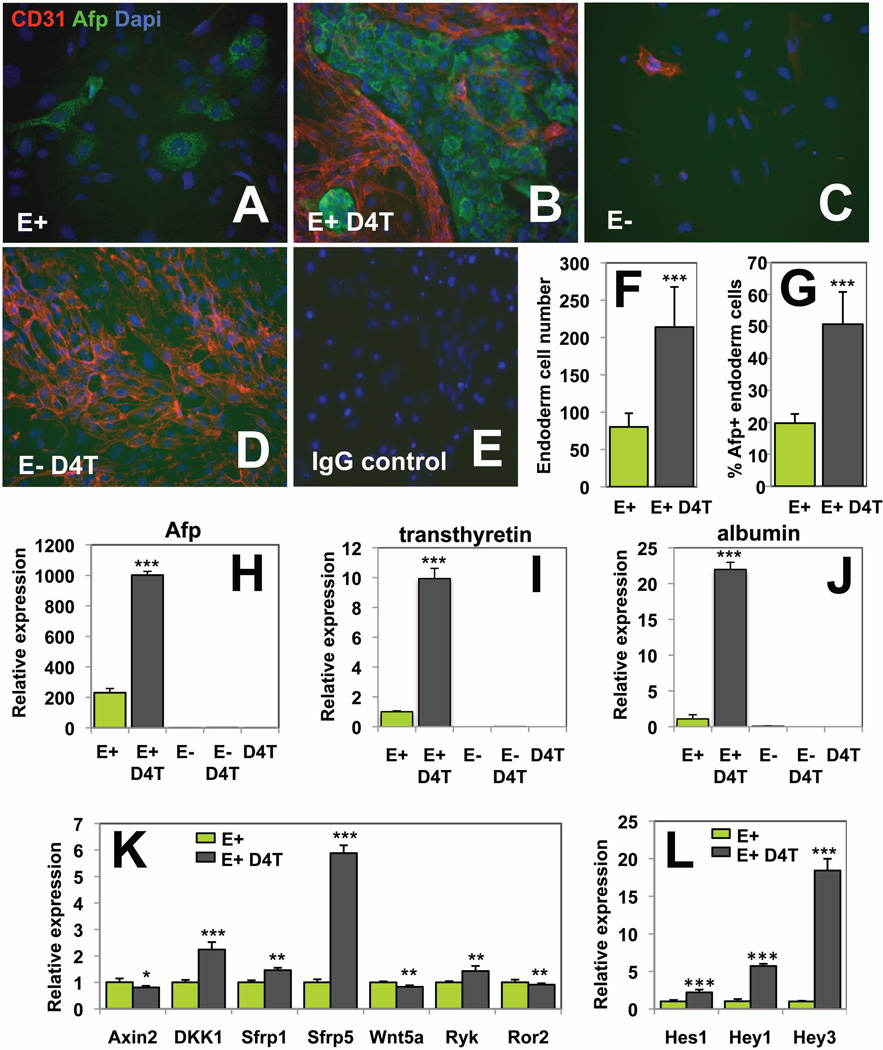
We next investigated whether D4T cells can also induce hepatic specification of endoderm cells harvested from embryos. To isolate mouse definitive endoderm, we used the monoclonal antibody ENDM1 that recognizes a cell surface marker expressed on endoderm from ES cell cultures or early embryos 34. As ENDM1 recognizes also the granular (high SSC parameter) visceral endoderm cells from the dissociated embryos, definitive endoderm cells were purified by gating the cells that specifically displayed a low SSC parameter 34. Kinetic analyses for ENDM1, Flk-1 and CD31 expression were performed on E7.25, E8.25 and E9.25 dissociated embryos, and showed a complete exclusion of Flk-1 or CD31 endothelial markers within the ENDM1+ endoderm fraction (Supporting information Fig. 3). This indicated that SSClow/ENDM1+ cells represent a highly purified definitive endoderm cell population devoid of endothelial cells, which could have affected the co-culture assays. Subsequently, SSClow/ENDM1+ and SSClow/ENDM1− cells were isolated from E8.25 embryos and plated alone or in the presence of D4T endothelial cells for 72-hours. ENDM1+ endoderm cells did not survive well unless co-cultured with D4T cells (Fig. 5A). Upon co-culture, large Afp+ hepatic endoderm colonies developed, as assessed by Afp immunostaining (Fig. 5B). None of the plated ENDM1− cells expressed Afp (Fig. 5C,D), and the ENDM1+ population did not develop CD31+ endothelial cells (Fig. 5A) confirming the high specificity of the ENDM1 antibody for endoderm. By performing morphometric analysis of the immunostained cultures, we found significantly increased endoderm cell numbers when ENDM1+ endoderm cells were co-cultured compared to being cultured alone (Fig. 5F). Approximately 50% of endoderm cells expressed Afp in co-cultures versus 20% when cultured alone (Fig. 5G). These data were supported by the high transcript levels of Afp found in co-culture groups compared to those ENDM1 cells cultured alone (Fig. 5H). Afp levels were undetectable in D4T cells or ENDM1− cells grown alone or with D4T cells. Similarly to Afp expression, transthyretin, a marker for early liver development 42, and albumin transcript levels were highly upregulated upon co-culture (Fig. 5I,J). These findings demonstrate a similar dependence of endoderm harvested from embryos on endothelial cells to support their growth and hepatic specification, as defined with the ES cell-derived endoderm.
Fig. 5. D4T endothelial cells promote expansion and hepatic specification of ENDM1 endoderm cell from E8.25 embryos.
ENDM1+/SSClow (E+) and ENDM1−/SSClow (E−) cells were isolated by FACS from dissociated E8.25 embryos. Both populations were cultured alone (A,C) or co-cultured with D4T cells (B,D,E) for 72-hours in hepatic media. (A–G) Cells were either fixed and immunostained in the dish for Afp (green), CD31 (red) and for Dapi (blue), or (H–J) processed for RNA to evaluate by qPCR relative Afp, transthyretin and albumin expression adjusted to reflect the proportion of endoderm cells in co-cultures. (F) and (G) represent respectively endoderm cell numbers and the % of endoderm cells that expressed Afp, quantified from analysis of 4 to 6 pictures taken from the ENDM1+ cells cultured alone or co-cultured with D4T cells. Endoderm cells were defined as cells whose membrane did not express CD31, and the Afp+ cells were recognized by the Afp green staining. (K,L) qPCR-based quantitative expression of Wnt and Notch signaling components in co-culture or culture alone conditions. (A–E) Original magnification ×200. These data were generated from one representative experiment (n=4). In graphs (F–L), * depict the significant differences between the culture alone (E+, green) and co-cultures (E+ D4T, grey).
We next determined whether Wnt and Notch pathways are involved in endoderm hepatic specification upon 72-hours embryo co-culture, similar to what we found using the ES cell system co-cultures (Fig. 5K,L). The Wnt signaling was downregulated following 72-hours of co-culture indicated by enhanced levels of the Wnt inhibitors Dkk1, Sfrp1 and Sfrp5 and lower levels of the Wnt effector Axin2. Conversely, expression levels for the Notch effectors Hes1, Hey1 and Hey3 were greater indicative of an upregulation of the Notch signaling 72-hours following co-culture. The changes in gene expression patterns between the E8.25 embryo-derived endoderm grown alone or co-cultured for 72-hours were remarkably similar to the changes observed between the ES cell-derived endoderm cells grown for 90-hours alone or co-cultured (comparing endoderm alone in bright green and co-cultures in dark grey shown in Fig. 3F,G and Fig. 5K,L). The common gene profiling patterns included increased expression of Dkk1, Sfrp5, Ryk, Hes1, Hey1, and Hey3, and decreased expression of Axin2 following co-culture. This was consistent with repression of the Wnt signaling and activation of the Notch signaling observed at later time points (Fig. 4) during co-culture of the ES cell-derived endoderm with D4T cells. The resemblance of the Wnt and Notch pathway gene expression patterns in both ES cell- and embryo- derived 72-hours co-cultures suggests strongly that the molecular mechanisms that orchestrate the early endoderm hepatic specification and expansion is endothelial cell-dependent, and requires modulation of both Wnt and Notch activities.
Wnt and Notch pathway repression is required for endothelial cell-dependent endoderm hepatic specification
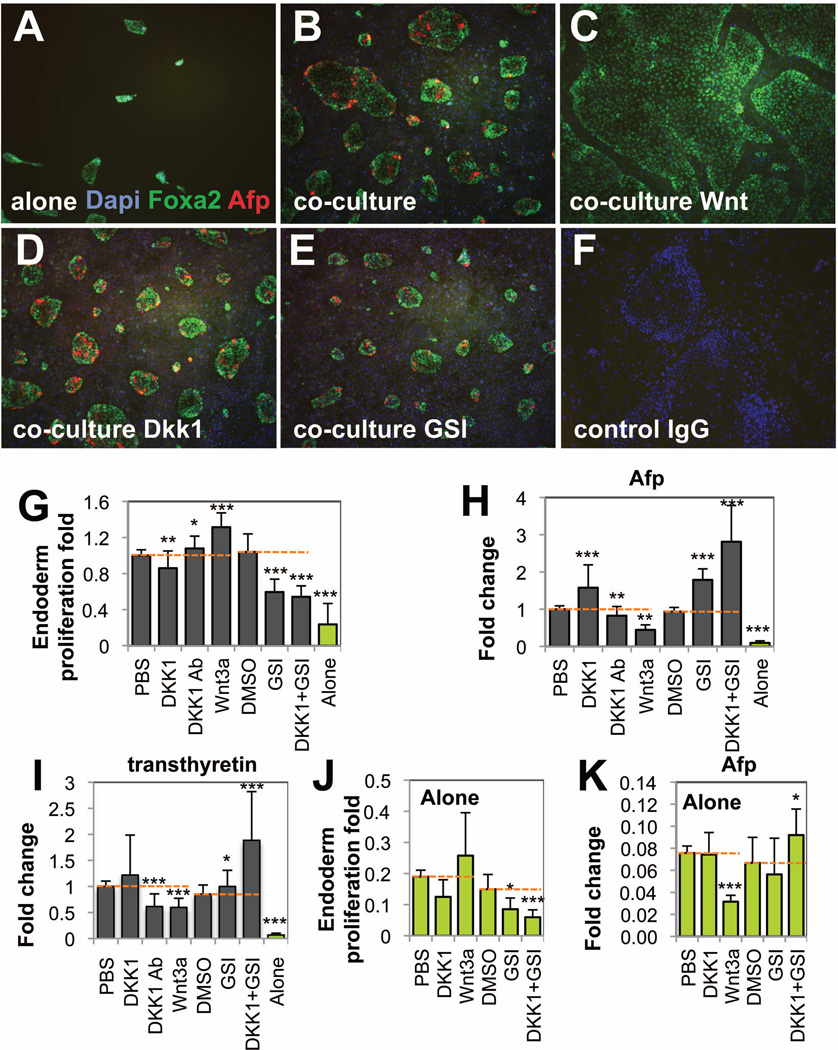
To further demonstrate that both Wnt and Notch are functionally involved in the cross-talk, we manipulated these pathways in endoderm either cultured alone or co-cultured. To manipulate the Wnt/β̃catenin pathway we added agonists (Wnt3a, or a blocking antibody against the antagonist Dkk1) or expressed in endoderm the stabilized β̃catenin (sβ-cat) or we used an antagonist (Dkk1). For the Notch pathway, we used an inhibitor (γ secretase inhibitor, GSI), or an activator through the inducible expression in endoderm cells of the active intracytoplasmic domain of Notch1 (NICD). The manipulations were confirmed in co-cultures by analyses of a time course of effector and inhibitor expression from both pathways, and showed the efficient manipulations of the Wnt and Notch pathways using Wnt3a and GSI and to a lesser extent with Dkk1 (Supporting information Fig. 4). As expected, in the absence of D4T cells, endoderm cells did not survive well, as assessed by small endoderm colonies and low endoderm cell numbers, and did not express Afp and transthyretin (Fig. 6A,G,H,I, Alone). In contrast, in their presence, endoderm colonies expanded greatly and expressed Afp and transthyretin (Fig. 6B,G,H,I, PBS). Addition of Wnt3a to the co-culture increased significantly the size of the endoderm colonies, and conversely down-regulated hepatic specification, assessed by a loss of Afp and transthyretin expression (Fig. 6C,G,H,I). Consistent with these findings, addition of Dkk-1 in the co-culture decreased significantly the size of colonies and increased Afp levels, while inhibiting endogenous Dkk1 with a blocking antibody significantly enhanced endoderm cell numbers and decreased Afp and transthyretin expression (Fig. 6D,G,H,I). Inhibition of the Notch pathway with GSI decreased endoderm cell numbers and enhanced significantly Afp and transthyretin expression (Fig. 6E,G,H,I). When both Wnt and Notch pathways were inhibited with the combination of Dkk1 and GSI, endoderm cell numbers were the lowest and Afp and transthyretin levels the highest compared to the other co-cultures (Fig. 6G,H,I), indicating an additive effect of both repression on hepatic specification. Modulations of Afp following pharmacological manipulations of both Wnt and Notch pathways have been confirmed at the protein levels by western blotting analyses (Supporting information Fig. 5). The co-culture induced dramatically Afp expression (Alone PBS, compared to Co-culture PBS). In co-culture condition, activation of the Wnt pathway (Wnt3a, compared to PBS condition) decreased strongly Afp expression while inhibiting the Notch pathways increased it strongly (GSI, compared to DMSO condition). Dkk1 treatment did not show the expected increase of Afp protein levels indicating that Afp upregulation was only observed at the transcript levels. Analyses of phase contrast microscopy pictures indicated a more compact morphology of co-cultured endoderm cells compared to when grown alone. However no obvious morphology differences have been noticed in endoderm cells nor in D4T cells upon the various culture conditions (Supporting information Fig. 6). Overall, these data support our previous observation that Wnt signaling is shut down and Notch signaling sharply abrogated during the early time point of co-culture-dependent hepatic specification (Fig. 4). In the absence of D4T cells, inhibition of both pathways in endoderm cells with Dkk1 and GSI decreased significantly endoderm proliferation rate and increased modestly but significantly the Afp transcript levels (Fig. 6J,K). Consistent with these data, addition of Wnt3a decreased significantly Afp levels but only induced modest endoderm cell numbers. These data indicated that repression of both Wnt and Notch pathways in endoderm cells in the absence of D4T cells improves hepatic specification and affects negatively endoderm growth (Fig. 6J,K,DKK1+GSI). However the increase of Afp levels was relatively modest compared to the co-culture setting, indicating that the effect of repression of Wnt and Notch signaling on hepatic specification is highly dependent on the endothelial cell niche.
Fig. 6. Modulations of Wnt and Notch pathways affect endoderm expansion and hepatic specification.
Isolated day5 F2+/F3+ endoderm cells were cultured for 72-hours alone (A, G–K green columns), co-cultured with D4T cells (B–F, G–I grey columns) either in the presence of Wnt3a (C,G–K 100ng/ml), Dkk1 (D,G–K 200ng/ml), GSI (E,G–K 4µM), a blocking Dkk-1 antibody (G–I, 30µg/ml), or with the combination of Dkk1 and GSI (G–K). PBS was used as control for Dkk1, Dkk1 antibody and Wnt3a conditions, and DMSO for GSI conditions. Cells were either fixed and immunostained in the dish for Foxa2 (green) and Afp (red) (A–E), harvested, counted and processed for flow cytometry analysis for CD4-Foxa2 and CD31 expression to determine endoderm cell numbers (G,J); or were processed for RNA to evaluate relative Afp or transthyretin expression by qPCR (H,I,K). Graphs represent the means of triplicates of endoderm cell proliferation fold (G,J) or relative expression fold changes for Afp (H,K) and for transthyretin (I) from 3 to 6 independent experiments. * Depict the significant differences between the culture controls (PBS, DMSO) and the treated cultures. (F) Represents the goat and rabbit IgG control for immunostaining. The orange dotted lines represent the control levels. (A–F) Original magnification ×50.
Notch and Wnt pathways need to be repressed in endoderm cells to allow hepatic specification upon co-culture
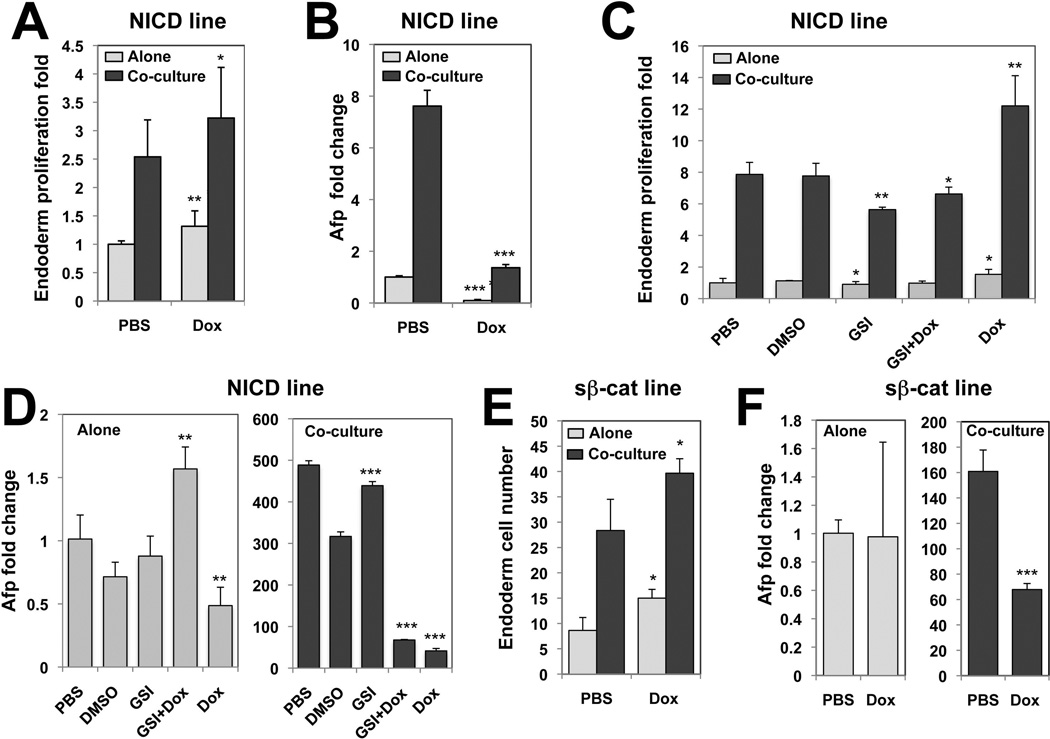
To demonstrate that Notch and Wnt signaling have to be repressed specifically in endoderm cells upon co-culture, ENDM1+ endoderm cells were isolated from the NICD and sβ-cat doxycyclin (Dox) inducible ES cell line differentiation cultures 35, 36. First, we verified that Dox treatment was sufficient to induce in endoderm cells alone increased levels of (1) NICD and the Notch effectors Hey1, and Hey3 (although Hes1 levels were not induced) in the NICD line, and (2) the direct canonical Wnt effectors Axin2 and Dkk1 in the sβ-cat line (Supporting information Fig. 7).
A slight but significant increase in endoderm cell number was observed following 72-hours NICD (Dox) expression in endoderm cells alone or co-cultured (Fig. 7A). The increased endoderm cell numbers in cultures lacking D4T cells never reached those obtained upon co-culture without Dox, indicating that activation of Notch signaling is not sufficient to mimic the endoderm cell proliferation in co-cultures. Importantly Afp transcript levels were strongly diminished in both endoderm co-cultured or cultured alone (Fig. 7B). These data demonstrate that NICD overexpression in endoderm cells abrogates hepatic specification, and supports further the induction of hepatic specification noted previously with the Notch inhibitor GSI. Moreover, Notch signaling needs to be repressed specifically in endoderm cells to allow endothelial cell-dependant hepatic specification. Expression of the Notch effectors Hes1, Hey1 and Hey3 declined sharply within the first 24-hours upon co-culture (seen in Fig. 4) and was increased again by 90-hours of co-culture, suggesting that the dynamic Notch inhibition followed by Notch activation may be required for endoderm hepatic specification. To determine if this is the case, day5 ENDM1+ endoderm cells derived from the NICD ES cell line were treated with GSI for the first 48-hours of co-culture to inhibit Notch signaling and consecutively stimulated with Dox the following 42-hours to induce NICD expression (Fig. 7C,7D, GSI+Dox). The combined treatment (GSI+Dox) maintained low numbers of endoderm cells as found with the GSI condition, suggesting that GSI-treated endoderm cells were no longer competent to proliferate in response to Notch activation. Most importantly, the combined treatment did not improve Afp expression, but rather decreased it significantly compared to the control DMSO condition. These findings demonstrate that Notch signaling needs to be inhibited at all times in endoderm cells upon co-culture to allow hepatic specification, and suggest that the increase of Notch effector levels seen during later time points upon co-culture does not contribute to hepatic specification.
Fig. 7. Notch and Wnt pathway repression in endoderm cells is required for endothelial cell-dependent endoderm hepatic specification.
NICD (A–D) or sβ-cat (E,F) Dox inducible ES cell lines were used to generate day5 ENDM1+ endoderm cells. (A,B,E,F) Day5 ENDM1+ endoderm cells were isolated and plated alone (grey) or co-cultured with D4T cells (black) for 72-hours in the presence or absence of Dox (2µg/ml). (C,D) ENDM1+ endoderm cells were cultured alone or co-cultured with D4T cells for 90-hours in the presence of PBS, DMSO, GSI for 90-hours (GSI), GSI for 48-hours and then Dox for the following 42-hours (GSI+Dox) or with Dox for 90-hours (Dox). (A,C) Represent endoderm proliferation fold from triplicate from 3 experiments and (E) the endoderm cell number from one representative experiment (n=3). (B,D,F) The relative Afp expression of each group harvested 72- or 90- hours following plating assessed by qPCR. (B) Is from triplicates of 3 experiments and (D,F) are a representative experiments (n=3). (D,F) Identical scales are used for both graphs. * Depict the significant differences between the Dox treated and untreated groups (A,B,E,F), or between the pharmacologically treated and control (PBS, DMSO) groups (C,D).
Overexpression of the stabilized form of β-catenin after Dox treatment promoted endoderm cell proliferation in endoderm cells cultured alone and in co-cultures, while decreased levels of Afp were found in co-cultures, supporting the data obtained with the previous modulations of Wnt signaling. However induction of sβ-catenin in endoderm alone did not affect Afp levels, as was found following Wnt3a treatment (Fig. 6K), most likely due to a more moderate activation of the canonical pathway, indicated by only a 1.5-fold increase of Axin2 levels following Dox stimulation compared with 3.5-fold following Wnt3a treatment (Supporting information Fig. 4A and Fig. 7B).
The genetic manipulation of the Wnt and Notch signaling specifically in endoderm cells supports the data from the pathway pharmacological manipulation experiments. Altogether the two approaches indicate a trend for induced hepatic specification and decreased endoderm cell proliferation by inhibition of both pathways in isolated or co-cultured endoderm. The effects on endoderm proliferation rate were similar regardless of the culture context (endoderm alone or co-cultured), while the effects of the manipulation on hepatic specification were more profound on co-cultures than isolated endoderm. This indicates that we cannot recapitulate fully the activity of endothelial cells on liver specification by blocking Wnt and Notch pathways in endoderm alone, and supports further the essential role of the endothelial niche in this process.
DISCUSSION
In this study, we used a reporter ES cell line that allows isolation of highly purified endoderm committed cells (F2+/F3+) that are completely devoid of endothelial potential. We were able to probe early events of hepatic specification upon co-culture with D4T endothelial cells representing a very early developmental stage of endothelial cells. Our data indicate that endothelial cells function not only as inducers for hepatic cell expansion but also earlier for the hepatic endoderm fate decision. This observation is novel and differs from previous studies showing that in the absence of Flk1+ endothelial cells in the Flk1LacZ/LacZ deficient mice, endoderm cells underwent hepatic specification. One explanation for this discrepancy relies on the precise characterization of endothelial cell development in the Flk1LacZ/LacZ deficient mouse model. In the mutant embryos, many LacZ positive cells that initiated Flk1 expression were detected in many intra-embryonic sites, although organized LacZ+ blood vessels could not be observed 43. This observation indicated that early progenitors for endothelial cells were present in this mouse model but did not complete their differentiation program to produce mature functional vessels. It is possible that these early progenitors were present long enough at the vicinity of the anterior ventral endoderm to induce hepatic fate, but underwent rapid cell death as they were missing the Flk-1 receptor survival cue. LacZ staining of earlier embryos prior to hepatic gene expression would be very informative to address this point. We confirmed the role of endothelial cells in hepatic specification and hepatic endoderm expansion using ENDM1+ endoderm cells isolated from E8.25 embryos upon co-culture with the D4T endothelial cells.
There is growing evidence that the canonical Wnt signaling pathway patterns the anterior and posterior endoderm in the mouse, Xenopus and zebrafish embryos 9–12, 44. In gastrula and early-somite stage Xenopus embryos, Wnt/β̃catenin activity must be repressed in the anterior endoderm to maintain foregut identity and allow liver and pancreas development 9. Consistent with this work, we showed that addition of Wnt3a or induction of β-catenin expression in F2+/F3+ endoderm cells in the presence of D4T cells allows expansion of endoderm or endoderm derivatives, while preventing hepatic specification. Moreover, inhibition of canonical Wnt signaling by addition of the inhibitor Dkk1 increased Afp transcripts levels, whereas a blocking antibody against Dkk1 decreased these levels. Similar results were found using ENDM1+ endoderm cells harvested from E8.25 embryos. Hepatic specification of ENDM1+ cells was significantly improved following co-culture with D4T endothelial cells, and was associated with inhibition of the Wnt pathway indicated by higher levels of the Wnt inhibitors Dkk1, Sfrp1 and Sfrp5. In line with our data, Sfrp5 has been recently reported to coordinate foregut hepatic and pancreatic specification and morphogenesis by antagonizing Wnt11 signaling in Xenopus embryos 10. Altogether, the co-culture experiments using endoderm cells isolated from ES cell culture or harvested from the E8.25 embryos demonstrated that the canonical Wnt activities must be repressed to allow the endothelial cell-dependent hepatic specification.
Although long recognized in the human and mouse developing liver as a crucial biliary duct inducer from the hepatoblast, the common progenitor for hepatocytes and biliary duct cells, the role of Notch in early hepatic endoderm fate decision was unclear 45–48. This lack of documentation may be mostly due to the high redundancy of the multiple receptors, ligands and target genes and because of the embryonic lethality of mice null for Notch signaling components 49. By modulating Notch activity in co-culture, we showed that inhibiting Notch with the chemical GSI enhances Afp expression in endoderm cells, while overexpression of NICD in endoderm cells blocked it completely. We further demonstrated that Notch activity needs to be inhibited at all times in endoderm cells upon co-culture to allow hepatic specification, even though expression of some Notch effectors increased by 72-hours of co-culture. This is the first evidence indicating that the Notch pathway is essential for early hepatic fate decision.
Previous studies have shown that Wnt and Notch can function synergistically or antagonistically according to the identity of progenitors or the developmental stage of the cells 50. In liver development, antagonistic effects of Wnt and Notch pathways have been well documented for the fate decision of the hepatoblast to hepatocytes or biliary duct cells. Activity of the β̃catenin-dependent Wnt pathway is required for hepatoblast proliferation and further maturation 51–54, whereas Notch signaling induces differentiation and morphogenesis of biliary ducts 46–48, 55, 56. In the co-culture system used in this study, we identified additive effects of the repression of both Wnt and Notch pathways to instruct endoderm hepatic specification in the presence of endothelial cells. In line with these data, activation of each pathway in co-culture by either genetic or pharmacological means abrogated hepatic specification. Inversely, dual repression of both Wnt and Notch by addition of Dkk1 and GSI on endoderm cells alone induced a significant but modest hepatic specification. Altogether, the repression of Wnt and Notch signaling is required for a hepatic fate decision and is highly dependent on the endothelial cell niche.
CONCLUSION
In summary, we uncovered the novel role for endothelial cells in early hepatic specification of endoderm harvested either from ES cell differentiation cultures or the mouse embryo. The endothelial cell niche functions to induce hepatic specification through dual repression of Wnt and Notch signaling, highlighting further the crucial time-wise coordination of molecular pathways to allow proper developmental processes such as liver development. The approach used in this study reinforces the power of the ES cell system combined with in vivo animal studies to dissect developmental processes, and to ultimately generate functional ES cell-derived hepatic cells clinically relevant for liver cell therapy.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Dr. Phil Streeter for providing us with the ENDM1 antibody and Dr Kenneth Murphy for the sβ-cat ES cell line. We would like to thank the Gouon-Evans laboratory members for their helpful discussions as well as Drs Todd Evans and Michael Rendl for the critical readings of the manuscript. We are grateful to Drs. Jianlong Wang and Junjun Ding for western blotting advice and technical help.
Financial support: National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK068041-01A1 and R01DK087867-01 to VGE).
Footnotes
Author contribution summary: Songyan Han: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript. Noelle Dziedzic: conception. Paul Gadue: provision of the Bry-GFP/CD4-Foxa/Cd25-Foxa3 mouse ES cell line, final approval of manuscript. Gordon Keller: design, data interpretation, manuscript writing, final approval of manuscript. Valerie Gouon-Evans: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflict of interest.
REFERENCES
- 1.Zaret KS. Liver specification and early morphogenesis. Mech Dev. 2000;92:83–88. doi: 10.1016/s0925-4773(99)00326-3. [DOI] [PubMed] [Google Scholar]
- 2.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 4.Jung J, Zheng M, Goldfarb M, et al. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 5.Rossi JM, Dunn NR, Hogan BL, et al. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Yatskievych TA, Baker RK, et al. Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol. 2004;268:312–326. doi: 10.1016/j.ydbio.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Kemp C, Willems E, Abdo S, et al. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- 9.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Rankin SA, Sinner D, et al. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leyns L, Bouwmeester T, Kim SH, et al. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilcher KE, Krieg PA. Expression of the Wnt inhibitor, sFRP5, in the gut endoderm of Xenopus. Gene Expr Patterns. 2002;2:369–372. doi: 10.1016/s1567-133x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Yoshitomi H, Rossant J, et al. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 14.Snykers S, De Kock J, Rogiers V, et al. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577–605. doi: 10.1634/stemcells.2008-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo A, Shinozaki K, Shannon JM, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 16.Gadue P, Huber TL, Paddison PJ, et al. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasunaga M, Tada S, Torikai-Nishikawa S, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 18.D'Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 19.Tada S, Era T, Furusawa C, et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- 20.Morrison GM, Oikonomopoulou I, Migueles RP, et al. Anterior definitive endoderm from ESCs reveals a role for FGF signaling. Cell Stem Cell. 2008;3:402–415. doi: 10.1016/j.stem.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Gouon-Evans V, Boussemart L, Gadue P, et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- 22.Soto-Gutierrez A, Navarro-Alvarez N, Caballero-Corbalan J, et al. Endoderm induction for hepatic and pancreatic differentiation of ES cells. Acta Med Okayama. 2008;62:63–68. doi: 10.18926/AMO/30961. [DOI] [PubMed] [Google Scholar]
- 23.Cai J, Zhao Y, Liu Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 24.Basma H, Soto-Gutierrez A, Yannam GR, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heo J, Factor VM, Uren T, et al. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology. 2006;44:1478–1486. doi: 10.1002/hep.21441. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal S, Holton KL, Lanza R. Efficient Differentiation of Functional Hepatocytes from Human Embryonic Stem Cells. Stem Cells. 2008 doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 28.Duan Y, Catana A, Meng Y, et al. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- 29.Hay DC, Fletcher J, Payne C, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci U S A. 2008;105:12301–12306. doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen VC, Stull R, Joo D, et al. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat Biotechnol. 2008;26:1169–1178. doi: 10.1038/nbt.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boni A, Urbanek K, Nascimbene A, et al. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Hirashima M. Regulation of endothelial cell differentiation and arterial specification by VEGF and Notch signaling. Anat Sci Int. 2009 doi: 10.1007/s12565-009-0026-1. [DOI] [PubMed] [Google Scholar]
- 33.Fischer A, Schumacher N, Maier M, et al. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadue P, Gouon-Evans V, Cheng X, et al. The Generation of Monoclonal Antibodies Specific for Cell Surface Molecules Expressed on Early Mouse Endoderm. Stem Cells. 2009 doi: 10.1002/stem.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng X, Huber TL, Chen VC, et al. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development. 2008;135:3447–3458. doi: 10.1242/dev.025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsley RC, Gill JG, Kyba M, et al. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 37.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol. 1997;17:1193–1202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 39.Jho EH, Zhang T, Domon C, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu W, Yamamoto V, Ortega B, et al. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makover A, Soprano DR, Wyatt ML, et al. An in situ-hybridization study of the localization of retinol-binding protein and transthyretin messenger RNAs during fetal development in the rat. Differentiation. 1989;40:17–25. doi: 10.1111/j.1432-0436.1989.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 43.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Krinks M, Lin K, et al. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- 45.Alagille D. Liver transplantation in children--indications in cholestatic states. Transplant Proc. 1987;19:3242–3248. [PubMed] [Google Scholar]
- 46.Zong Y, Panikkar A, Xu J, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kodama Y, Hijikata M, Kageyama R, et al. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 50.Hayward P, Kalmar T, Arias AM. Wnt/Notch signalling and information processing during development. Development. 2008;135:411–424. doi: 10.1242/dev.000505. [DOI] [PubMed] [Google Scholar]
- 51.Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Decaens T, Godard C, de Reynies A, et al. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology. 2008;47:247–258. doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- 53.Tan X, Yuan Y, Zeng G, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monga SP. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165–3174. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 56.Geisler F, Nagl F, Mazur PK, et al. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.